Abstract
The vertebrate telencephalic lobes consist of the pallium (dorsal) and subpallium (ventral). The subpallium gives rise to the basal ganglia, encompassing the pallidum and striatum. The development of this region is believed to depend on Foxg1/Foxg1a functions in both mice and zebrafish. This study aims to elucidate the genetic regulatory network controlled by foxg1a in subpallium development using zebrafish as a model. The expression gradient of foxg1a within the developing telencephalon was examined semi‐quantitatively in initial investigations. Utilizing the CRISPR/Cas9 technique, we subsequently established a foxg1a mutant line and observed the resultant phenotypes. Morphological assessment revealed that foxg1a mutants exhibit a thin telencephalon together with a misshapen preoptic area (POA). Notably, accumulation of apoptotic cells was identified in this region. In mutants at 24 h postfertilization, the expression of pallium markers expanded ventrally, while that of subpallium markers was markedly suppressed. Concurrently, the expression of fgf8a, vax2, and six3b was shifted ventrally, causing anomalous expression in regions typical of POA formation in wild‐type embryos. Consequently, the foxg1a mutation led to expansion of the pallium and disrupted the subpallium and POA. This highlights a pivotal role of foxg1a in directing the dorsoventral patterning of the telencephalon, particularly in subpallium differentiation, mirroring observations in mice. Additionally, reduced expression of neural progenitor maintenance genes was detected in mutants, suggesting the necessity of foxg1a in preserving neural progenitors. Collectively, these findings underscore evolutionarily conserved functions of foxg1 in the development of the subpallium in vertebrate embryos.
Keywords: CRISPR/Cas9, foxg1/foxg1a , pallium, subpallium, zebrafish
A zebrafish foxg1a mutant line was established and the phenotypes were analyzed, showing the pivotal role of foxg1a in directing the dorsoventral patterning of the telencephalon and its necessity in preserving neural progenitors. Collectively, these findings underscore evolutionarily conserved functions of foxg1 in the development of the subpallium in vertebrate embryos.
1. INTRODUCTION
The telencephalon exhibits highly developed anatomical and functional structure in vertebrates, playing a pivotal role in numerous fundamental information processing tasks. In humans, it sits at the apex of the central nervous system and orchestrates advanced brain functions such as emotion, consciousness, recognition, and conception (Hébert & Fishell, 2008; Nadarajah & Parnavelas, 2002; Rubenstein et al., 1998). Anatomically and functionally, the telencephalon is composed of dorsal and ventral areas (Mueller & Wullimann, 2009). The pallium, found in the dorsal area, mediates sensory reception, voluntary movement, and various higher‐order brain activities. Conversely, the ventral subpallium gives rise to the basal ganglia and basal forebrain, whose functions are well documented in mammals (Rapoport, 1990). Specifically, the globus pallidum and striatum within the basal ganglia coordinate movements in conjunction with the cerebellum. The basal nuclei of Meynert, located within the basal forebrain, facilitate cholinergic innervation; their degeneration is associated with the cognitive impairments observed in conditions such as Parkinson's and Alzheimer's diseases. Furthermore, the septum, another constituent of the basal forebrain, modulates anxiety and fear responses via its interaction with the medial habenula nucleus.
During the nascent stages of development in vertebrates, the telencephalon primordium emerges in the anterior lateral portion of the neural plate, subsequently maturing in the anterior dorsal section of the neural tube (Aboitiz & Montiel, 2007; Hébert & Fishell, 2008; Rubenstein et al., 1998). This anlage is patterned along its anteroposterior and dorsoventral axes. In the subsequent stages, spatially appropriate neural progenitor cells are specified and undergo a series of processes including proliferation, differentiation, migration, and axonal elongation. The culmination of these processes results in the formation of a sophisticated, higher‐order structure with elaborate neural circuits.
The telencephalic developmental process varies among vertebrates. In many vertebrates, especially in mammals, bilateral hemispheres arise from the dorsoanterior walls of the neural tube via evagination. In mammals, the pallium is characterized by a six‐layered neocortex, a feature absent in birds, reptiles, and amphibians (Fernandez et al., 1998; Shimizu, 2007). In stark contrast, teleost fish, such as zebrafish (Danio rerio), experience a complex two‐step dynamic morphogenetic event in the pallium: the deep ventricular recess is formed first between the telencephalon and diencephalon, which is followed by anterior expansion of the pallial ventricular zone (VZ), resulting in an eversion where the lateral sides of the alar plate are positioned outward (Folgueira et al., 2012; Mueller et al., 2011). However, irrespective of these pronounced morphological disparities, recent molecular and developmental studies suggest that the fundamental blueprint regulating telencephalon development remains conserved across vertebrates (Ganz et al., 2012; Mueller et al., 2011).
The dorsoventral patterning of the telencephalon is determined by the recognized roles of various secreted factors. BMP and Wnt control the development of the pallium, while Sonic hedgehog (Shh) and FGF are fundamental in subpallium formation. Multiple transcription factor genes operate in a concerted manner under the control of these secretory signals. The emx genes, regulated by Wnt and BMP signaling, play pivotal roles in the formation of the pallium (Cecchi et al., 2000; Hébert & Fishell, 2008). Within the subpallium, Shh signaling is initially relayed to Gli transcription factors via the membrane receptors Patched and Smoothened (Marigo, Davey et al., 1996; Marigo, Johnson et al., 1996). Foxg1, a member of the Forkhead family of transcription factors, is a major regulatory molecule cooperating with the Wnt/BMP and Shh signaling pathways (Hébert & Fishell, 2008). Positioned upstream in the genetic hierarchy, Foxg1 orchestrates telencephalon development, in particular its dorsoventral patterning, by specifying the subpallium.
In mice, Foxg1 expression is initiated in the anterior neural plate at the early somite stages, expanding laterally and posteriorly. By E9.5, Foxg1 is expressed in diverse ectodermal tissues of the head, such as the telencephalon (excluding the dorsal midline), optic vesicle, otocyst vesicle, and olfactory placode (Dou et al., 1999; Duggan et al., 2008; Pauley et al., 2006). Remarkably, Foxg1 mutant mice showed a diminished telencephalon at E12.5 and suffered postnatal mortality. These mutants notably lack a defined subpallium, and the ganglionic eminence was virtually absent (Dou et al., 1999; Xuan et al., 1995). Such embryonic anomalies were characterized by accelerated neuronal differentiation and attenuated progenitor cell proliferation (Hanashima et al., 2002; Martynoga et al., 2005). Thus, mouse Foxg1 plays dual pivotal roles in early telencephalon development: (1) subpallium specification and (2) modulation of progenitor cell proliferation and neuronal differentiation. Foxg1 functions at subsequent stages have been explored by conditional knockout (KO) studies, highlighting its involvement in corticogenesis in the pallium, specifically in the demarcation of various cortical neuron subtypes and axogenesis (Hanashima et al., 2004; Hou et al., 2019; Liu et al., 2022). Moreover, Foxg1 modulates the development of the cortical hem, an essential signaling hub for cortical and hippocampal patterning (Godbole et al., 2018), as well as the formation of the epithalamus (Liu et al., 2018), dentate gyrus (Han et al., 2018; Tian et al., 2012), and preoptic area (POA) (Du et al., 2019). In humans, FOXG1 has been implicated in a broad spectrum of congenital brain anomalies, collectively termed Foxg1 syndrome. This encompasses conditions such as the congenital variant of Rett syndrome, microcephaly, infantile spasms, and autism spectrum disorder. Furthermore, association between FOXG1 and schizophrenia has been reported (Hou et al., 2020).
As mentioned above, the roles of Foxg1 in telencephalon development, particularly in its dorsoventral patterning, are well established in mice. However, it is unclear how this gene interacts with the other regulatory genes to orchestrate their downstream gene cascade in the formation of this complex structure. To decipher these intricate gene interactions, using other animal model systems with advantages in genetics will be invaluable. Furthermore, such knowledge will elucidate the common mechanisms shared among vertebrates as well as species‐specific variations.
Indeed, foxg1 has been identified in other vertebrates, such as Xenopus and zebrafish (Bourguignon et al., 1998; Zhao et al., 2009). In Xenopus embryos, foxg1 is also expressed in the anterior neural plate (Bourguignon et al., 1998). In zebrafish, while four paralogs have been identified (foxg1a, foxg1b, foxg1c, and foxg1d), only foxg1a is expressed in the telencephalon. It is first expressed in the anterior neural plate at the bud stage and subsequently in the telencephalon, optic vesicles (Toresson et al., 1998), nasal retina (Picker et al., 2009), olfactory placode (Duggan et al., 2008), and inner ear (Pauley et al., 2006). At 24 h postfertilization (hpf), foxg1a was described briefly to display a ventral‐high and dorsal‐low expression gradient (Danesin et al., 2009; Liu et al., 2013; Toresson et al., 1998). In zebrafish, Six3 promotes ventral telencephalic fates through transient regulation of foxg1a expression (Carlin et al., 2012). Indeed, knockdown (KD) of foxg1a using morpholino oligos (MOs) led to underdevelopment of the telencephalon, subpallium reduction, and pallium expansion (Danesin et al., 2009). This observation aligns with gene disruption studies in mice, suggesting the conserved basic function of foxg1 among vertebrates.
Nevertheless, MO‐mediated KD can elicit non‐specific (off‐target) effects and/or result in incomplete functional inhibition. Given its transient nature, MOs are less suitable for analyzing gene functions in later development (Pauli et al., 2015). When gene redundancy poses challenges, multiple KDs are feasible, but this approach remains limited due to the inherent constraints of the KD method. Therefore, genetic investigations using mutants are indispensable for deeper understanding of the regulatory mechanisms of telencephalon development in vertebrates.
In the current study, we created foxg1a loss‐of‐function mutants in zebrafish using the CRISPR/Cas9 technique and assessed the phenotypes. Our findings reinforce the idea that the roles of foxg1 are fundamentally conserved among vertebrates. Additionally, we discerned that the development of the POA strongly depends on foxg1a.
2. MATERIALS AND METHODS
2.1. Animals
Adult zebrafish (D. rerio, RW line) were maintained at 26–27°C under a 14‐h light/10‐h dark cycle. Embryos were raised at 28.5°C until reaching appropriate stages. Morphological features and time point (hpf) were used to stage embryos (Kimmel et al., 1995). To prevent pigment formation, 0.2 mM 1‐phenyl‐2‐thiourea (Nacalai Tesque) was employed. All experiments using live fish were conducted in compliance with the protocols approved by the Committee for Animal Care and Use of Saitama University.
2.2. Gene disruption by the CRISPR/Cas9 method
Guide RNAs (gRNAs) were designed using CRISPRdirect (http://crispr.dbcls.jp/) against the N‐terminal region in the coding sequence of foxg1a (5′‐CCGAAGCCGTGCAAAGCGACAAC‐3′; underline, protospacer adjacent motif [PAM]), which was synthesized using the CUGA7 gRNA Synthesis Kit (Nippon Gene) according to the manufacturer's protocol (cf. Figure 4). The gRNA was co‐injected with Cas9 protein (Nippon Gene) into fertilized eggs. Disruption of the target sequence was confirmed in a portion of injected embryos by the heteroduplex mobility (HMA) method (Ota et al., 2013) (Table S1). The remaining embryos thus obtained were raised to maturity and crossed with wild‐type fish, and the offspring were used to establish mutant lines.
FIGURE 4.
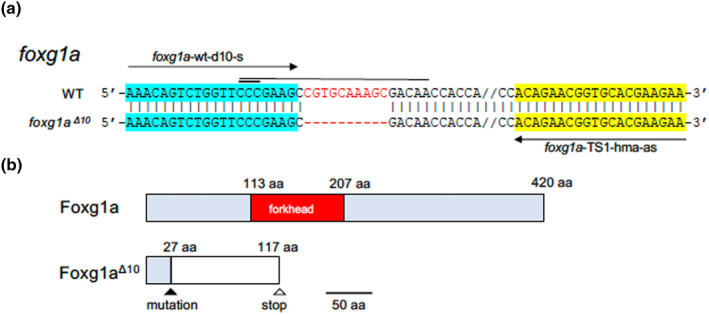
Mutations introduced into foxg1a. (a) The sequence around the site of mutation, introduced into foxg1a by the CRISPR/Cas9 method, is shown, with the wild‐type sequence at the top and the mutated sequence at the bottom. Hyphens show introduced nucleotide deletions, and double dashes show the sequences omitted for convenience's sake. A horizontal thin line and thick line show the target sequence and PAM sequence, respectively. Horizontal arrows show the sequences of primers used for HMA, which are also highlighted. (b) Schematic views of the wild‐type and mutant products of foxg1a. Light‐blue boxes show the wild‐type coding sequences, but for the functional domain that is shown in red instead, whereas the white box shows the nonsense open reading frame generated as a result of the frameshift mutation.
2.3. Direct sequencing of mutated sites
The mutated sites were amplified by PCR and sequenced using appropriate primers (Table S1) and an ABI3130 Capillary DNA Sequencer (Applied Biosystems).
2.4. Genotyping of mutants
Genotyping of mutants was conducted by the HMA method to first discriminate between heterozygotes and wild type/homozygotes. As wild‐type and homozygote individuals gave rise to indistinguishable single bands, their PCR products were mixed with equal amounts of those from wild‐type individuals and treated with another thermal cycle; wild‐type‐derived PCR products again generated a single band, whereas mutant‐derived band gave rise to shifted bands, thus allowing for identification of the genotypes. Actually, it turned out to be possible to discriminate between wild type and mutants simply by the length of the PCR products (Table S1).
2.5. Staining of embryos
Whole‐mount in situ hybridization (WISH) was carried out as described previously (Kikuta et al., 2003). To detect apoptotic cells, live embryos were dechorionated and incubated at 28.5°C for 1 h in PBS containing 2 μg/mL acridine orange (AO, Wako), washed with PBS twice, allowed to further develop for 30 min, and then observed under a fluorescence stereomicroscope as described below.
2.6. Microscopic observation
Live and stained embryos were observed under a fluorescence stereomicroscope (MZFLIII, Leica), and images were captured using a cooled CCD camera (DFC 300 FX, Leica) and Leica Application Suite Version 3.3.1 (Leica).
2.7. Image analysis by ImageJ software
Gene expression staining was quantified with ImageJ software (https://imagej.nih.gov/ij/) with regard to shape, area, and intensity of staining. The expression of given genes in selected regions of experimental embryos was compared to that in control embryos. When measuring the expression gradient, staining intensities along given lines were quantified by the “plot profile” command (see Figures 3 and 6 and their legends for details). Statistical analyses of the data from ImageJ quantification, including one‐way analysis of variance, F‐tests, and two‐sample t‐tests, were conducted using Excel (Microsoft).
FIGURE 3.
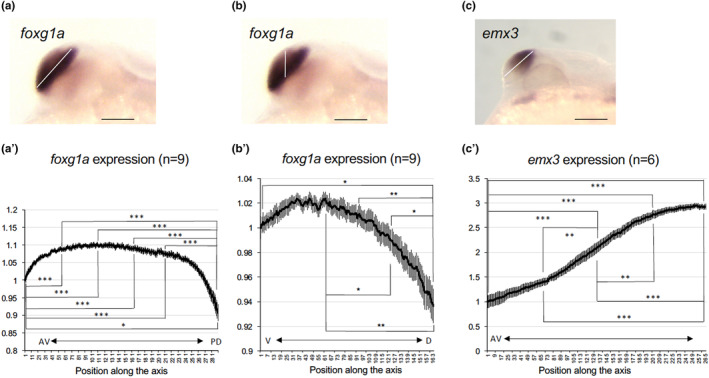
Graded expression of foxg1a and emx3 in the developing telencephalon of wild‐type embryos. Expression intensity of foxg1a (a, b, a′, b′) and emx3 (c, c′) in the telencephalon was quantified at 24 hpf along its major axis (anteroventral end to posterodorsal end, white line in (a) and (c)) or vertical axis (ventral to dorsal, white line in (b)) by ImageJ (a'‐c'). The staining intensities shown in the graph are the average values of nine embryos (foxg1a) or six embryos (emx3). Average values relative to those at the anteroventral/ventral‐most ends are shown along the ordinate with error bars showing SEMs. The abscissa shows positions along the axis set for each quantification. AV, anteroventral; PD, posterodorsal; V, ventral; D, dorsal. *p < .05; **p < .01; ***p < .001.
FIGURE 6.
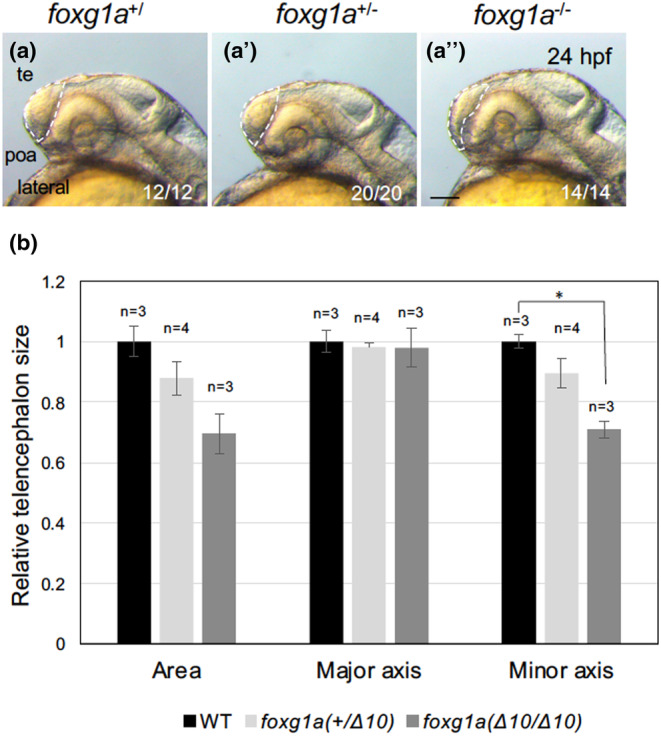
Quantification of the size of the telencephalon in foxg1a mutants. The sizes of the telencephalon were quantified using ImageJ. The telencephalic region was selected by the segmented line tool for each genotype as shown in (a–a″) (white dashed lines), and the area was quantified (b). (a–a″) Lateral views of the head, with anterior to the left and dorsal to the top. The embryos in (a) and (a″) correspond to those in Figure 3a, b. poa, preoptic area; te, telencephalon. Scale bar, 100 μm. (b) Four clutches were quantified independently, resulting in similar results, and representative data are shown. Average values for respective genotypes are shown relative to those for the wild‐type embryos with error bars showing SEMs and the numbers of embryos scored in this experiment. *p < .05. Numbers at the bottom right in (a–a″) indicate the total numbers of embryos showing the features shown and the total numbers of scored embryos with respective genotypes, respectively, all of which are combined numbers from the four independent experiments.
3. RESULTS
3.1. Expression profiles of telencephalic genes during the course of development
Numerous regulatory genes are recognized for their expression in the developing telencephalon (Mueller & Wullimann, 2009; Wullimann, 2009), but the precise timing of their expression onset remains unclear in zebrafish. Initially, we directly compared their appearance in the anterior neural keel (ANK), which later gives rise to the telencephalon (Figure 1). six3b, sfrp1a, and zic1 were activated at 90% epiboly, followed by emx3 at the bud stage. By the two‐somite stage (2‐ss), neurog1, foxg1a, and fgf8a were expressed, and sfrp5 and sp8a expression began at the 5‐ss. The expression pattern of foxg1a continued to be monitored up to the hatching stage (Figure 2), verifying its presence in the ANK and the entire developing telencephalon, including the POA, after the early somite stages.
FIGURE 1.
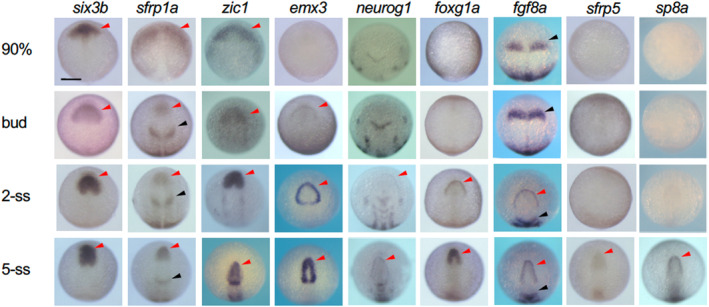
Expression of genes involved in establishment of the anterior neural border and early telencephalon development. The expression of regulatory genes from late gastrulation to early somite stages were stained by WISH. ANK and MHB are shown with red and black arrowheads. Scale bar, 100 μm.
FIGURE 2.
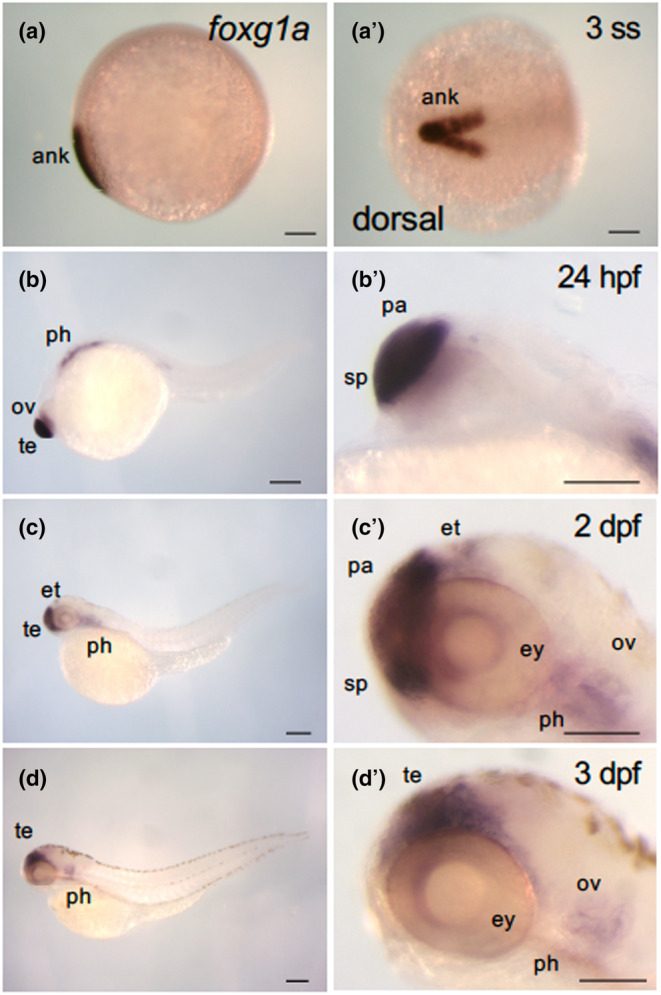
Dynamic expression of foxg1a during the course of zebrafish development. Expression of foxg1a was examined by WISH in wild‐type embryos from the early somite stage through 3 dpf. (a–d, b′–d′) Lateral views of the entire bodies (a–d) and heads (b′–d′) with anterior to the left, dorsal to the top. (a′) Dorsal view, with anterior to the left. foxg1a expression was detected in the anterior neural keel from the two‐somite stage (2‐ss; Figure 1a, a′). At 24 hpf, foxg1a expression was detected in the telencephalon, including the preoptic area, optic vesicle, and pharyngeal arch. At 2 dpf, foxg1a expression was detected in the telencephalon, eyes, epithalamus, and pharyngeal arches. At 3 dpf, foxg1a expression was also found in the telencephalon, eyes, and pharyngeal arches. ank, anterior neural keel; ov, optic vesicle; pa, pallium; ph, pharyngeal arch; sp, subpallium; te, telencephalon. Scale bar, 100 μm.
Earlier observations suggested a ventral‐high to dorsal‐low gradient in foxg1a expression, findings that required clarity. For validation, the staining intensity of foxg1a expression in the telencephalon across the ventroanterior‐to‐dorsoposterior (major) axis and the dorsoventral (vertical) axis of the telencephalon was quantified separately using ImageJ (Figure 3). In both evaluations, graded expression (ventral‐high and dorsal‐low) was quantitatively confirmed. Notably, the gradient across the vertical axis appeared more pronounced (Figure 3a, a′, b, b′). We also analyzed the expression of emx3 in the telencephalon, implicated in pallium formation (Viktorin et al., 2009). Its expression was uniform after 2 days postfertilization (dpf), but exhibited a dorsoposterior‐high to ventroanterior‐low gradient at 24 hpf (data not shown). Expression quantification using ImageJ confirmed this gradient (Figure 3c, c′).
3.2. Establishment of foxg1a mutant fish
To delve deeper into the genetic implications of foxg1a, we aimed to disrupt this gene by the CRISPR/Cas9 technique. A gRNA targeting a position 71 bp downstream of the ATG codon was designed and co‐injected into fertilized eggs together with the Cas9 protein. This resulted in a 10‐bp deletion in the N‐terminal region (Figure 4a, b), which was integrated into the germline. Owing to the subsequent frameshift, the mutated gene product (foxg1a Δ10 ) lacked the protein region following the 27th amino acid, including the forkhead domain, rendering the mutation functionally null.
3.3. Lethality of foxg1a mutation
Offspring from heterozygotic crosses predominantly developed normally until 4 dpf (data not shown). Thereafter, 29% of the larvae that appeared normal at 4 dpf (n = 200) gradually deteriorated and occasionally died by 9 dpf, while the remaining larvae displayed normal morphology. Of the 128 survivors, 64 larvae were randomly chosen and genotyped for the foxg1a mutation. This identified 33% of larvae as wild type and 67% as heterozygotes, with no homozygotes (Table 1). This suggests that the foxg1a homozygotes died between 4 dpf and 9 dpf. The wild‐type‐to‐heterozygote ratio among the normal larvae was approximately 1:2, indicating that most heterozygotes developed normally up to 9 dpf at least. In fact, heterozygotes matured into normal adults and exhibited fertility.
TABLE 1.
Genotyping of live embryos obtained by heterozygotic crosses of foxg1a mutants.
| Stage | Embryos (n) | Genotype (%) | ||
|---|---|---|---|---|
| +/+ | +/− | −/− | ||
| 8 dpf a | 64 | 33% | 67% | 0% |
About a quarter (26%) of larvae that looked apparently normal at 4 dpf died by 8 dpf. All surviving embryos at this stage were morphologically normal (n = 128). In total, 64 embryos were randomly sampled and genotyped.
3.4. Telencephalic morphological aberrations in foxg1a mutants
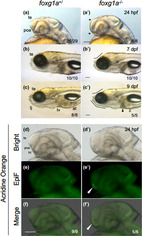
foxg1a homozygous larvae exhibited lethality between 4 dpf and 9 dpf. Close examination at 24 hpf revealed that the telencephalon, although discernible, was thin and underdeveloped (Figure 5a, a′). ImageJ quantification disclosed a significant reduction in the size of the telencephalon in homozygotes compared with wild‐type embryos (29% reduction; Figure 6a–a‴, b). The boundary between the telencephalon and hypothalamus, where the POA forms, appeared malformed (Figure 5a, a′, Figure 6a–a″). In addition, the optic vesicles, including the optic recess region and the lens, were severely deformed (Affaticati et al., 2015). The underdevelopment of the telencephalon was accentuated at 7 dpf and at 9 dpf, close to the time of death (Figure 5b–c′). At 9 dpf, manifestations such as mandibular hypoplasia and absence of gastrointestinal contents were noted (Figure 5c, c′). This confirms the indispensability of foxg1a for telencephalon development in zebrafish and suggests a role of foxg1a in mandibular formation.
FIGURE 5.
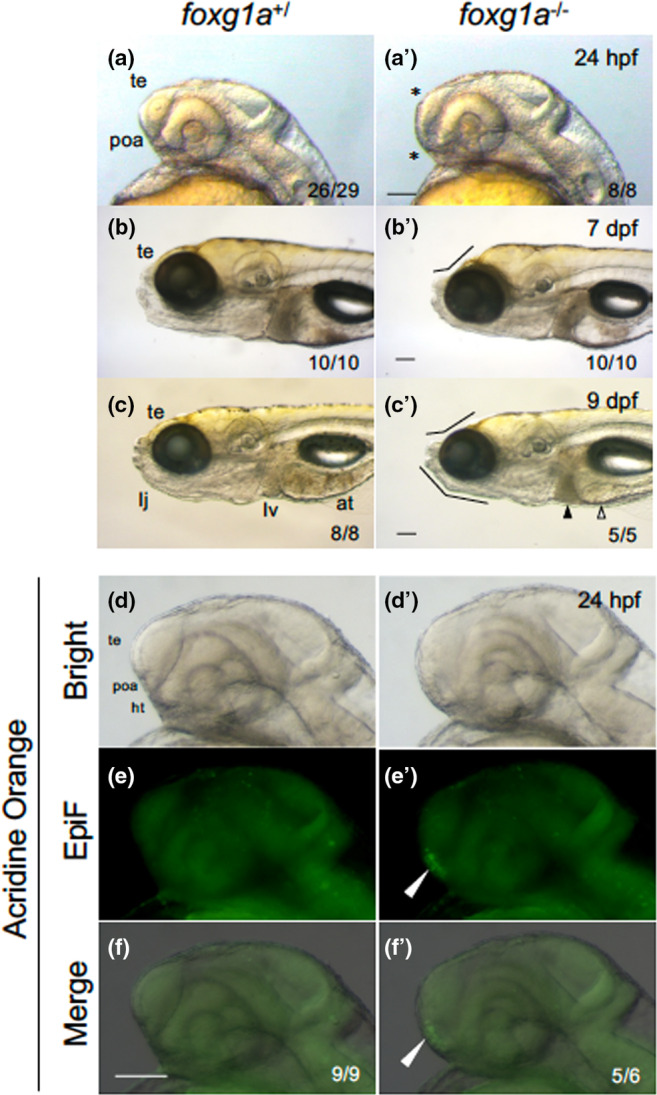
Morphological defects in foxg1a mutant fish. (a–c, a′–c′) Morphology of the heads of embryos/larvae obtained by heterozygotic mating of foxg1a mutant fish (foxg1a +/∆10 ) was observed at 24 hpf (a, a′), 7 dpf (b, b′), and 9 dpf (c, c′), followed by genotyping. For each stage, wild‐type fish and homozygotes (−/−) are shown. In fact, heterozygotes were indistinguishable from wild‐type embryos and are not shown. In homozygotes, the telencephalon was thinner and the POA region was deformed at 24 hpf (asterisks, a′). At 7 and 9 dpf, depression of the anterodorsal head and reduced lower jaws were observed (flexed lines, b′, c′), cell death was observed in the liver (solid triangle), and the alimentary tract was often empty (open triangle). (d–f, d′–f′) Embryos obtained by heterozygotic mating of foxg1a mutant fish (foxg1a +/Δ10 ) were examined by acridine orange (AO) staining at 24 hpf and then genotyped. Wild type and homozygotes are shown on the left and right, respectively. Heterozygotes were indistinguishable from wild‐type embryos and are not shown. Lateral views of the head, with anterior to the left and dorsal to the top. Bright‐field images, epifluorescence images (EpiF), and merged images are shown from top to bottom. On the bottom right are shown the numbers of embryos with the shown phenotypes and the numbers of embryos with the shown genotypes, respectively. at, alimentary tract; ht, hypothalamus; poa, preoptic area; te, telencephalon. Scale bar, 100 μm.
Given the deformation and size reduction of the telencephalon in foxg1a mutants, we assessed cell death in these mutants by AO staining. At 24 hpf, there was a notable upsurge in cell death, particularly in the POA of homozygotes (Figure 5d–f′), suggesting the necessity of foxg1a for POA cell survival. Importantly, the surge in cell death was observed exclusively at 24 hpf, as no abnormal cell death was seen from 2 dpf to 7 dpf in foxg1a mutants (data not shown).
3.5. Dorsoventral patterning of the telencephalon was affected in foxg1a mutants
In foxg1a homozygotes, the telencephalon formed, albeit with morphological deformities. To delineate regionalization of the developing telencephalon, we assessed the progeny of heterozygotic foxg1a mutant crosses at 24 hpf using WISH. This analysis evaluated the expression of critical genes orchestrating telencephalic development, followed by genotyping. The expression of foxg1a in homozygotes remained indistinguishable from that in wild‐type embryos, refuting the possibility of an autoregulatory feedback mechanism (Figure 7a, a′). We then assessed the expression of pallium markers and subpallium markers. Pallial expression of emx1 was downregulated in homozygotes (Figure 7b, b′), whereas emx3 expression in the pallium (Viktorin et al., 2009) anomalously expanded into the subpallium in homozygotes (Figure 7c, c′). Expression quantification using ImageJ established that the area and major axis of the emx3 region within the telencephalon were significantly increased in homozygotes (by 89% and 61%, respectively; Figure 8). Expression of tbr1b in immature neural progenitor cells in the pallium (Englund et al., 2005; Miyake et al., 2017) was enhanced in the medial (ventricular zone) and ventral regions in homozygotes (Figure 7d–e′). The expression of neurog1 in differentiating neural progenitor cells in the pallium (Miyake et al., 2017) also expanded ventrally within the telencephalon (Figure 7f, f′), but its expression in the diencephalon was unchanged. In contrast, neurod1 expression in the pallium and olfactory placode was decreased (Figure 7g–h′). Thus, in general, foxg1a mutants displayed ventral expansion of the pallium, with some genes (emx1 and neurod1) demonstrating different regulation from the aforementioned pallium‐generating genes.
FIGURE 7.
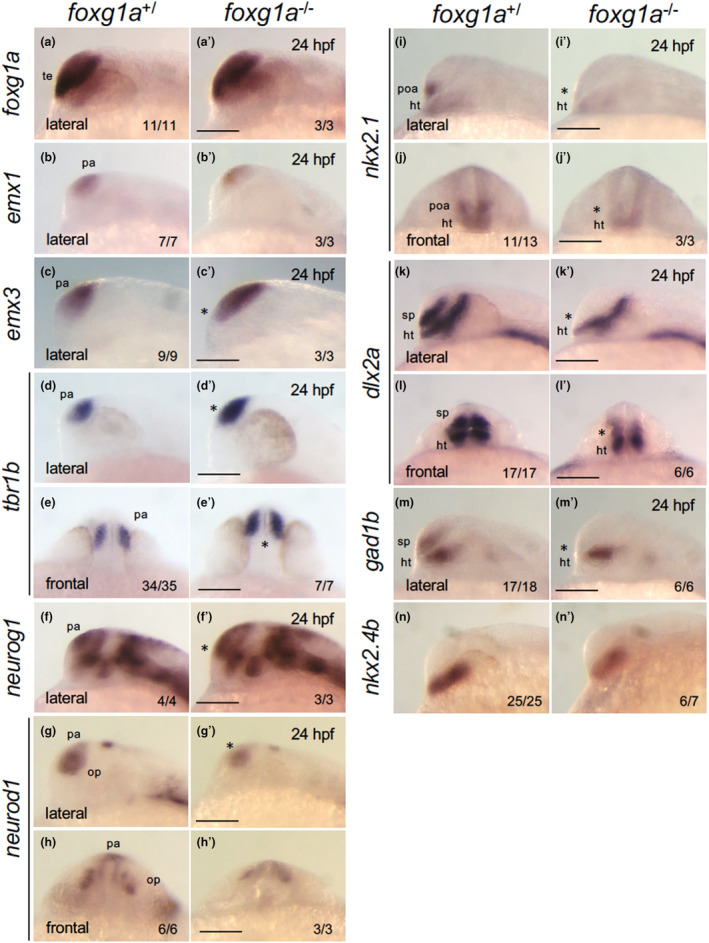
Expression of genes involved in dorsoventral patterning of the telencephalon in foxg1a mutants at 24 hpf. The expression of foxg1a, pallium markers (emx1, emx3, tbr1b, neurog1, neurod1), a POA/medial ganglionic eminence/hypothalamus marker (nkx2.1), and subpallium markers (dlx2a, gad1b) was examined by WISH in foxg1a mutants at 24 hpf, followed by genotyping. For each gene, there was no difference between +/+ and +/− embryos, and the data for wild type/heterozygous (+/) and for homozygotes (−/−) are shown on the left and right, respectively. The numbers of embryos with the indicated patterns and those of embryos with the indicated genotypes are shown on the bottom right. Asterisks indicate abnormal expression. Lateral views of the head with anterior to the left and dorsal to the top, except for (e, e′, h, h′, j, j′, l, l′), where frontal views of the head are shown with dorsal to the top. ht, hypothalamus; op, olfactory placode; pa, pallium; poa, preoptic area; sp, subpallium; te, telencephalon. Scale bar, 100 μm.
FIGURE 8.
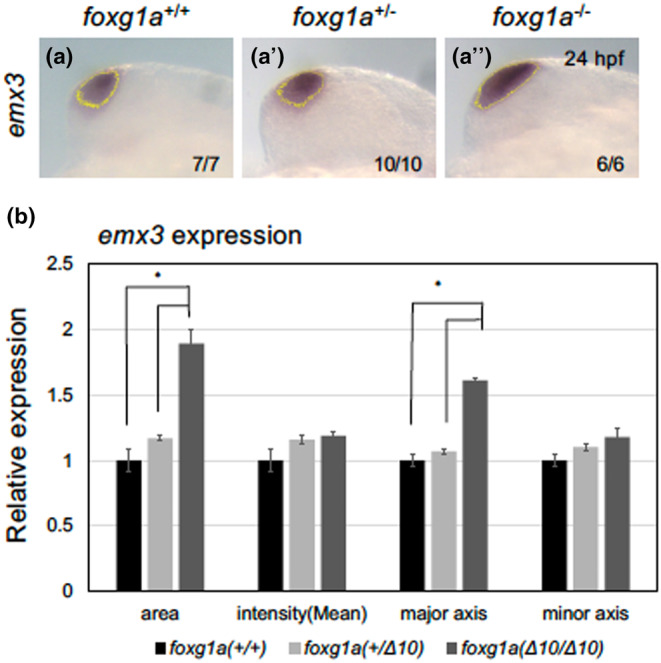
Semi‐quantitative analysis of emx3 expression in foxg1a mutants. The emx3 expression pattern in the telencephalon was quantified by ImageJ. After selecting stained regions based on a brightness threshold (a–a‴, yellow line), the areas, staining intensities, major axes, and minor axes were quantified for respective genotypes. (a–a‴) Wild type (+/+), a heterozygous mutant (+/−), and a homozygous mutant (−/−) are shown in this order from left to right. At the bottom right are shown the numbers of embryos with the indicated patterns and those of embryos with the indicated genotypes. The embryos in (a) and (a”) correspond to those in Figure 5c, c′. Lateral views of the head with anterior to the top. Scale bar, 100 μm. (b) Quantification of the emx3 expression patterns. Average values for each genotype are shown relative to the average for wild‐type embryos, with error bars showing SEMs. *p < .05.
Conversely, nkx2.1 expression (Manoli & Driever, 2014) was entirely absent in the subpallium/POA, but remained in the hypothalamus (Figure 7i–j′). Expression of dlx2a (Nery et al., 2003) and gad1b (Mueller et al., 2008), a GABAergic neuronal marker, was absent in the subpallium/POA but remained in other regions including the prethalamus in the diencephalon of homozygotes (Figure 7k–m′). Of note, the expression of nkx2.4b, which is expressed specifically in the hypothalamus, not in the POA, was only weakly reduced (Figure 7n, n′). These observations suggest that foxg1a mutants are devoid of both the subpallium and the POA.
In foxg1a mutants, we further assessed the expression of six3b, fgf8a, and vax2, which are expressed in distinct regions of the forebrain. Notably, almost all heterozygotes and homozygotes exhibited downregulated expression in the anterior‐most telencephalon and ectopic expression in the POA region, where no expression is typically observed (Figure 9). This anomaly was more significant in homozygotes for all three genes. These findings are consistent with the observed ventral expansion of the pallium and the disappearance of the POA at the telencephalon–hypothalamus boundary in foxg1a mutants. These together suggest an essential role of zebrafish foxg1a in POA development. Of note, milder, but significant phenotypes were observed in heterozygotes, implying a dose‐dependent function of foxg1a at least in this region, a phenomenon also noted for human FOXG1 (Hettige & Ernst, 2019).
FIGURE 9.
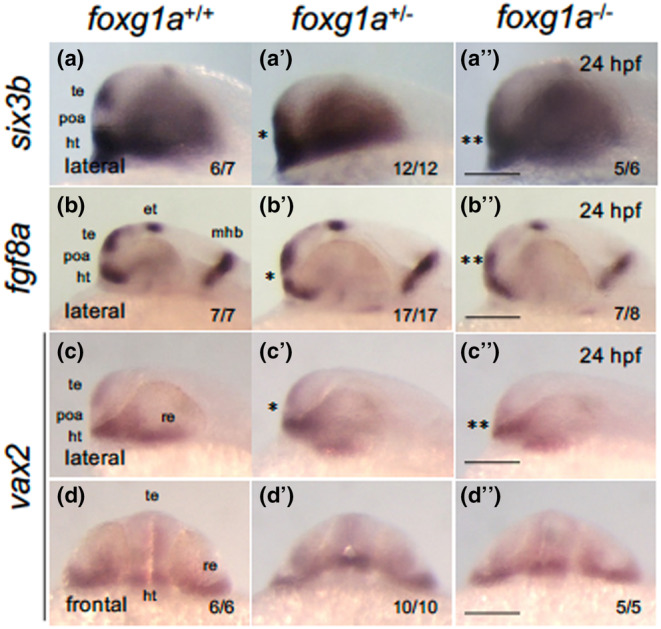
Expression of forebrain‐forming genes in foxg1a mutants at 24 hpf. (a–d″) Expression of anterior forebrain markers (six3b, fgf8a, vax2) was examined by WISH in foxg1a mutants at 24 hpf, followed by genotyping. Wild‐type embryos, heterozygotes, and homozygotes are shown from left to right for each gene. Asterisks indicate abnormal expression, with more asterisks indicating more severe anomalies. (a–c, a′–c′, a″–c″) Lateral views of the head. Left is anterior, top is dorsal. (d, d″, d‴) Front view of the head. Top is dorsal. In the lower right are shown the numbers of embryos with the indicated expression and the numbers of assessed embryos. ht, hypothalamus; et, epithalamus; mhb, midbrain–hindbrain boundary; poa, preoptic area; re, retina; te, telencephalon. Scale bar, 100 μm.
Analyses conducted at 24 hpf revealed that foxg1a plays a pivotal role in the ventral patterning of the telencephalon. Given that foxg1a expression is first observed in the ANK after the end of gastrulation (Figure 1) (Bielen & Houart, 2012), we also evaluated emx3 and six3b expression at the bud stage and the 3‐ss in foxg1a mutants. However, no discernible abnormalities were detected (Figure 10), suggesting that the role of foxg1a in telencephalon development might not be in the initial specification but rather in the progression of regionalization.
FIGURE 10.
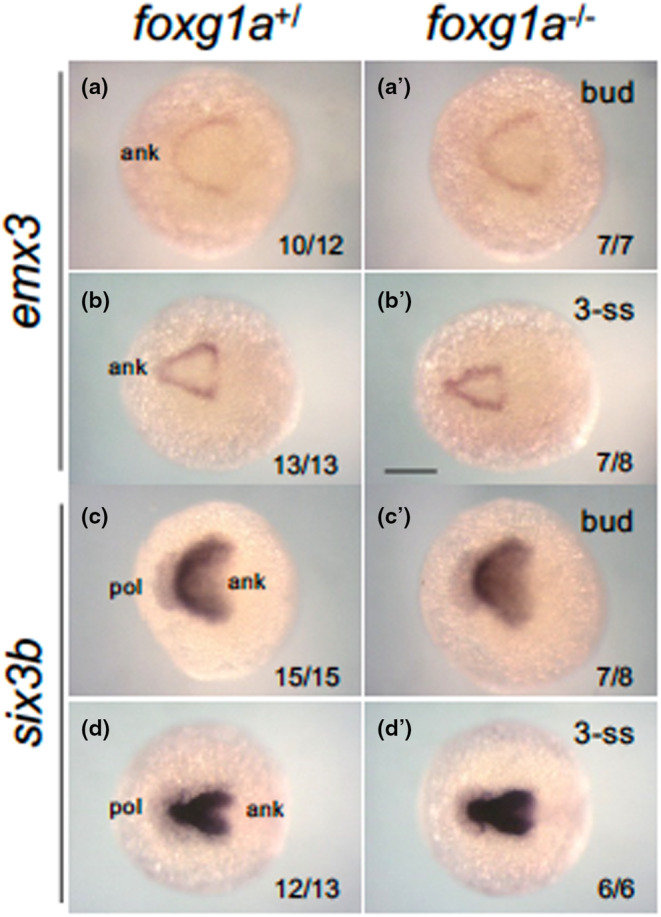
Expression of forebrain‐forming genes in foxg1a mutants at the tailbud and early somitogenesis stage. Expression of anterior forebrain markers (emx3, six3b) was examined by WISH in foxg1a mutants, followed by genotyping. Wild‐type embryos including heterozygotes (left) and homozygotes (right) are shown. For each gene, the upper row and bottom row show embryos at the bud stage (a, a', c, c') and the 3‐ss (b, b', d, d'), respectively. Dorsal views with the anterior side to the left. In the lower right are shown the numbers of embryos with the indicated expression and the numbers of assessed embryos. ank, anterior neural keel; pol, pollster. Scale bar, 100 μm.
3.6. Reduction of the neural progenitor pool in foxg1a mutants
In murine studies, Foxg1 was shown to enhance progenitor cell proliferation, playing a vital role in maintaining neural progenitor cells (Martynoga et al., 2005). Given this, we assessed the expression of pax6a (Englund et al., 2005), ascl1a (Castro et al., 2011), and nr2f1a (Bertrand et al., 2007; Chowdhury et al., 2024) at 24 hpf. These genes are expressed in neural progenitor cells within the telencephalon. As anticipated, their expression was evident around the telencephalic ventricular zone in both wild‐type and heterozygous embryos. However, their expression was notably suppressed in the homozygotes (Figure 11a–f′). This indicates a reduced neural progenitor pool in foxg1a mutants, confirming that zebrafish foxg1a is also required for preserving neural progenitor cells.
FIGURE 11.
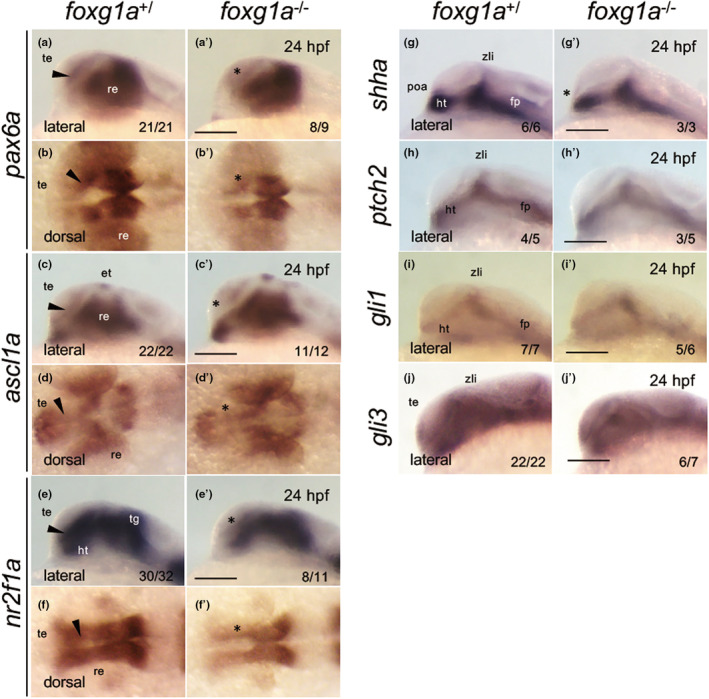
Expression of genes involved in neurogenesis and Shh signaling in foxg1a mutants. The expression of neurogenesis‐related genes (a–f′) and Shh signaling‐related genes (g–j′) was examined by WISH in foxg1a mutants at 24 hpf, followed by genotyping. Wild‐type embryos including heterozygotes (left) and homozygotes (right) are shown for each gene. Wild‐type and heterozygous embryos showed indistinguishable expression patterns. Black arrowheads indicate the telencephalic ventricular zone. The numbers on the lower right indicate the numbers of embryos with the indicated expression patterns and the numbers of total assessed embryos. Asterisks indicate abnormal expression. Lateral views, anterior to the left and dorsal to the top; dorsal views, anterior to the left. et, epithalamus; fp, floor plate; ht, hypothalamus; poa, preoptic area; re, retina; te, telencephalon; tg, tegmentum; zli, zona limitans intrathalamica. Scale bar, 100 μm.
3.7. Expression of Shh signaling‐related genes in foxg1a mutants
In mouse embryos, the Shh signaling pathway functions upstream of Foxg1 to trigger the gene regulatory network that orchestrates telencephalon formation (Hébert & Fishell, 2008). Therefore, we evaluated the expression of genes associated with Shh signaling at 24 hpf. Expression of shha in the hypothalamus, zona limitans intrathalamica (ZLI), and floor plate (Ericson et al., 1995) was consistently observed in both wild‐type and heterozygous embryos, yet was reduced specifically in the POA region of homozygotes (Figure 11g, g′), consistent with the disruption of this signaling center. In contrast, the expression patterns of ptch2, gli1, and gli3 were essentially indistinguishable across the hypothalamus and ZLI irrespective of genotype (Figure 11h–j′), implying that Shh signaling itself remains intact in foxg1a mutants.
4. DISCUSSION
4.1. Expression of the Fox family transcription factor gene foxg1 in vertebrate embryos
In mouse embryos, Foxg1 expression commences at the anterior neural ridge at the 1–3‐ss and is observed at E9.5 in multiple regions, including the telencephalon, POA, hypothalamus, optic vesicles, otic vesicles, and olfactory placodes (Dou et al., 1999; Duggan et al., 2008; Hernández‐Bejarano et al., 2015; Pauley et al., 2006). Among the four paralogs in zebrafish, only foxg1a is expressed in the telencephalon. It was reported to be first expressed in the ANK at the bud stage (Liu et al., 2013), and its expression then pervaded the telencephalon with a ventral‐high dorsal‐low gradient (Toresson et al., 1998). The expression of zebrafish foxg1a was also found in the POA and optic vesicles (Affaticati et al., 2015; Duggan et al., 2008), although it was absent in the hypothalamus, unlike its mouse counterpart.
In our study, we verified that zebrafish foxg1a expression in the ANK begins by the 2‐ss, slightly later than previously reported, following the expression of early ANK markers such as six3b, sfrp1a, zic1, and emx3. The subsequent sequential expression possibly indicates a genetic cascade that defines the ANK region, eventually leading to telencephalon specification. By 24 hpf, foxg1a expression was confirmed in the telencephalon and in the POA. Importantly, we semi‐quantitatively confirmed the foxg1a expression gradient in the telencephalon for the first time in zebrafish, to the best of our knowledge, further showing similarities to mouse Foxg1 expression. At subsequent stages, foxg1a continued to be expressed in the telencephalon, but the expression pattern became complex with no clear polarity, suggesting its dynamic regulation during late telencephalon formation.
4.2. Establishment of a zebrafish foxg1a mutant strain
In human studies, FOXG1 is implicated in various neurodevelopmental disorders, suggesting its involvement in the advanced stages of brain development. However, its functions in early telencephalon development are ambiguous. Functional assessments in mice elucidated the roles of Foxg1 in the development of the subpallium, eyes, and inner ears (Pauley et al., 2006). Furthermore, Foxg1 has been implicated in neocortex layer formation (Toma et al., 2014), maintenance of neural progenitors, and neuronal differentiation in the pallium (Martynoga et al., 2005).
In zebrafish, disruption of orthologs of human schizophrenia‐related genes resulted in forebrain regression in foxg1a mutants, although no detailed analysis has been conducted (Thyme et al., 2019). KD of foxg1a resulted in a thinner telencephalon, shrinkage of the subpallium, and an enlarged pallium (Danesin et al., 2009), similar to findings in mice. However, as already mentioned, the limitations of MO‐mediated KD experiments are well documented. Addressing these issues, our study established a foxg1a mutant strain to determine foxg1a functions genetically. Comparing the roles of zebrafish foxg1a, as outlined in our study, with those of mouse Foxg1 offers a promising avenue to understand the shared and unique aspects of telencephalon development across vertebrates.
In the current study, we established a foxg1a mutant line, characterized by a frameshift in the N‐terminal sequence (foxg1a Δ10 ). Notably, homozygotes exhibited significant impairments and were non‐viable by 9 dpf, underscoring the compromised function of foxg1a. Additionally, as elaborated below, the manifested phenotypes mirrored those reported in prior KD studies for early stages (Danesin et al., 2009). The late lethality of foxg1a Δ10 , absent in early KD experiments, suggests the indispensable role of foxg1a in advanced developmental stages and survival. The precise cause of mortality remains unknown; however, observed lower jaw anomalies, lack of intestinal contents, and potential foraging activity impairments could be contributory factors. In mice, Foxg1 homozygous mutants die shortly after birth, potentially due to respiratory complications (Dou et al., 1999). Considering that zebrafish rely on cutaneous respiration during early development (Stainier, 2001), transitioning to gill respiration after 3 dpf (Kimmel et al., 1995), impaired respiratory function in foxg1a homozygotes is plausible. A comprehensive investigation is warranted to elucidate the exact cause of death.
Conversely, heterozygotes showed neither survival nor morphological anomalies, and reproductive capabilities persisted in adult heterozygotes, similar to observations in mice (Dou et al., 1999). While certain FOXG1 syndromes result from haploinsufficiency (Hou et al., 2020), maintaining two copies of the foxg1a gene is not vital for individual growth and survival. However, under specific conditions, such as during POA formation (Figure 7), many of the heterozygotes showed weak molecular defects, hinting at a potential dosage dependency similar to that of human FOXG1 (Hou et al., 2020). This observation necessitates further exploration.
4.3. Hypoplasia of the telencephalon in foxg1a mutant zebrafish
Given the expression of foxg1a in the ANK during the early somite stages (this work) (Liu et al., 2013), telencephalon defects may manifest well before the observed mortality after 4 dpf. Indeed, we documented telencephalon and POA hypoplasia at 24 hpf, which confirmed the brief descriptions in the prior KD study (Danesin et al., 2009) and preliminary brain defect observation provided in the large‐scale KO screening (Thyme et al., 2019). Analogous early‐stage telencephalon abnormalities were also noted in mice (Martynoga et al., 2005). Future studies should address the anomalies in the foxg1a mutant telencephalon with regard to its cytoarchitecture and neuronogenesis.
4.4. Abnormal dorsoventral patterning of the telencephalon in foxg1a mutant embryos
In mice, Foxg1 is implicated in the dorsoventral patterning of the telencephalon, playing a critical role in subpallium formation. Specifically, Foxg1 KO mice displayed ventrally expanded expression of pallial markers (Emx2, Pax3, and Pax6), with disrupted expression of Dlx, Nkx2.1, Gsx2, and Ascl1 in the subpallium (Dou et al., 1999; Martynoga et al., 2005). Similarly, foxg1a KD zebrafish embryos exhibited ventrally expanded expression of pallial markers, such as emx3 and tbr1b, while dlx2 and nkx2.1 expression in the subpallium disappeared (Danesin et al., 2009).
In our study, upon analyzing dorsoventral markers in zebrafish foxg1a mutants, we observed ventral expansion of pallium markers (emx3, tbr1b, neurog1). Conversely, the expression of nkx2.1, dlx2a, and gad1b in the pallidum and POA was absent. Moreover, six3b, fgf8a, and vax2 showed downregulation in the anterior telencephalon and ectopic upregulation in the POA region. Evidently, as in mice, the telencephalon of foxg1a mutants exhibits dorsalization, characterized by an enlarged pallium and an apparent absence of the subpallium. Our findings underscore the essential role of foxg1a in telencephalic dorsoventral patterning, particularly in subpallium development. Thus, this role appears evolutionarily conserved among vertebrates. As Foxg1 is considered a transcriptional repressor (Das et al., 2014), increased gene expression in mutants might be a direct consequence of Foxg1 absence, while decreased expression may stem from other transcriptional regulatory pathways.
Interestingly, certain pallium markers, such as emx1 and neurod1, demonstrated downregulation, suggesting distinct regulatory interactions with foxg1a compared with other pallium‐forming genes. This emphasizes the intricate regulatory network underpinning telencephalon formation. The observed fgf8a downregulation in the anterior telencephalon mirrors findings in mouse mutants (Martynoga et al., 2005), reinforcing the hypothesis of mutual dependence between Fgf8 and Foxg1 in guiding telencephalon development in mice (Hébert & Fishell, 2008).
4.5. Roles of foxg1a in brain formation during early somitogenesis
In zebrafish embryos, neurulation and its regionalization along the anteroposterior and dorsoventral axes occur during somitogenesis. Primary axogenesis begins around 16 hpf (Ross et al., 1992), and the telencephalon becomes morphologically apparent at 18 hpf (Kimmel et al., 1995). While our focus was primarily on 24 hpf foxg1a mutants, during the late somitogenesis stage, foxg1a expression commences in ANK around the bud stage (Liu et al., 2013), suggesting its functional relevance during early somitogenesis.
Therefore, we examined the expression of emx3 and six3b, which were affected in foxg1a mutants at 24 hpf in the current study, during early somitogenesis. However, their expression remained unaffected, suggesting a role of foxg1a in later differentiation rather than in anterior brain specification. Our ongoing research aims to pinpoint the critical stage at which this gene plays a fundamental role in subsequent brain development.
4.6. Abnormalities in neural progenitor cell pools in foxg1a mutant embryos
Mouse Foxg1 has been postulated to maintain progenitor pools and mediate neuronal differentiation in the telencephalon. Specifically, the telencephalon of Foxg1 homozygotes displayed delayed cell proliferation combined with accelerated neurogenesis after the onset of neural development at E10.5 (Martynoga et al., 2005). To determine whether foxg1a has analogous functions in zebrafish, we analyzed the expression of pax6a, ascl1a, and nr2f1a in the telencephalon of foxg1a mutants at 24 hpf. Our results indicated attenuated expression of these genes in the ventricular zone, suggesting a pivotal role of foxg1a in maintaining neural progenitor cells. Concurrently, it is worth noting that Foxg1 directly represses Nr2f1 to regulate sensory cortex wiring in mice (Hou et al., 2019), implying a potential context‐dependent role of foxg1.
4.7. Expression of Shh signaling‐related genes in foxg1a mutant embryos
In mice, Foxg1 functions downstream of Shh signaling to regulate dorsoventral patterning in the telencephalon (Hébert & Fishell, 2008; Manuel et al., 2010). Similarly, KD studies in zebrafish have demonstrated that Shh regulates foxg1a (Danesin et al., 2009). However, in our current research, contrary to the KD findings, shha expression was consistently observed in the hypothalamus and ZLI. Interestingly, shha was specifically downregulated in the POA in homozygotes, aligning with the observed deformation in this region. Hence, the observed dorsoventral patterning defects in the telencephalon of foxg1a mutants might arise from two concurrent mechanisms: (1) a cell‐autonomous effect stemming directly from the absence of foxg1a function and (2) non‐cell autonomous effects attributed to the absence of the signaling center, POA. Importantly, normal expression of ptch2, gli1, and gli3 was observed in mutants, inferring that Shh signaling remains functional even in the absence of foxg1a.
4.8. Cell death in the POA region induced by the absence of Foxg1a
foxg1 has been identified to maintain cell survival across diverse contexts. In cochlear hair cell‐like mouse OC‐1 cells, KD of Foxg1 resulted in the accumulation of reactive oxygen species, leading to apoptosis (He et al., 2021). Conversely, in cerebellar granule cells undergoing apoptosis, Foxg1 was downregulated. Ectopic expression of Foxg1 inhibited neuronal cell death, while its suppression had the opposite effect (Dastidar et al., 2011). However, in mouse Foxg1 mutants, apoptosis in the anterior telencephalon was decreased (Martynoga et al., 2005), and a foxg1a KD study in zebrafish did not reveal apoptotic cells (Danesin et al., 2009).
In the current study, we stained dying cells with AO at 24 hpf in foxg1a mutants to determine if morphological defects in the telencephalon might be a consequence of aberrant apoptosis. No abnormal cell death was identified in the telencephalon proper, suggesting that the hypoplasia and subpallium defects of the telencephalon in these mutants potentially stemmed from the abovementioned reduction in the progenitor cell pool, although the involvement of cell death is still possible and requires more sensitive and specific evaluation. Additionally, the status of cell proliferation within the telencephalon also needs to be examined.
Interestingly, increased cell death was observed specificall in the area including the POA in foxg1a mutants. The observed deformation, anomalous gene expression patterns, and loss of shha‐expressing cells in the POA can likely be attributed to this aberrant cell death. Our findings suggest that foxg1a plays a role in maintaining the POA, which is among the mechanisms regulating subpallium development. Notably, the cell death observed in the POA region of foxg1a mutants was transient, evident at 24 hpf but not after 2 dpf. This could potentially explain the absence of observed apoptosis in prior foxg1a KD studies (Danesin et al., 2009). Of note, such anomaly in the POA could also explain the deformation of the optic recess region observed in foxg1a mutants (Affaticati et al., 2015).
This observation contrasts with the decreased apoptosis observed in the anterior telencephalon of mouse Foxg1 mutants. A potential explanation lies in the expression patterns of fgf8a in zebrafish mutants. Mouse mutants displayed attenuated Fgf8 expression in the anterior telencephalon, potentially leading to apoptosis (Martynoga et al., 2005). Moreover, conditional KO and overexpression of Fgf8 in the mouse forebrain were found to increase apoptosis, while apoptosis was decreased in the forebrain of Fgf8 hypomorphs, showing that Fgf8 dosage is a determining factor (Storm et al., 2003). In zebrafish, in the absence of functional foxg1a, apoptosis was induced in the POA region where fgf8a was ectopically activated. The divergent effects of foxg1/foxg1a loss on fgf8/fgf8a remain elusive but may be linked to dose‐dependent transcriptional regulation by Foxg1/Foxg1a. Regardless of the underlying mechanism, foxg1/foxg1a might mediate cell survival by modulating Fgf8 levels, thereby contributing to telencephalon development.
Our results highlight the multifaceted role of foxg1a in telencephalon development, encompassing the determination of dorsoventral patterning, maintenance of neural progenitor cells, and regulation of cell survival.
4.9. Possible role of foxg1 genes in the endocrine system
In the developing POA, robust expression of Foxg1/foxg1a has been observed in both mice and zebrafish (Affaticati et al., 2015; Dou et al., 1999), highlighting a potential role of foxg1 in POA development. Indeed, as mentioned above regarding the developing POA region, foxg1a disruption caused morphological defects, specific occurrence of cell death, anomalous gene expression patterns (downregulation of nkx2.1, dlx2a, and gad1b and ectopic upregulation of six3b, fgf8a, and vax2), and loss of shha‐expressing cells.
Given the importance of the POA in homeostasis, it is of importance to reveal the roles foxg1a plays in POA formation. Abrogation of the expression of nkx2.1 is likely at least one of the causes of the POA defects observed in foxg1a mutants, since nkx2.1, not nkx2.4a/b, is required for POA development (Manoli & Driever, 2014). Direct functions of foxg1a in POA development cannot be excluded and remain to be addressed. On the other hand, conditional KO experiments in mice at E12.5 suggested that Foxg1 functions upstream of Dbx1 to restrict the POA (Du et al., 2019). The apparent discrepancy may be due to species differences and/or the stage‐specific roles of Foxg1 during development.
Foxg1 is expressed in the developing hypothalamus in mice (Dou et al., 1999), but foxg1a is not in zebrafish (Danesin et al., 2009). In addition, the expression of regulatory genes integral to diencephalon formation, including neurog1, dlx2a, gad1b, nkx2.1, vax1, and nkx2.4, remained predominantly unaltered in foxg1a mutants. Therefore, it is unlikely that foxg1a is involved in the development of the diencephalon, and other foxg1 paralogs possibly play the roles typically associated with mouse Foxg1 in the zebrafish hypothalamus. Notably, of the four paralogs, foxg1c is expressed in the diencephalon (Zhao et al., 2009).
5. CONCLUSION
Our analysis of zebrafish foxg1a mutants demonstrated that, similar to mouse Foxg1, zebrafish foxg1a is essential for telencephalic growth, the dorsoventral patterning of the telencephalon, and the maintenance of neural progenitor cells. Additionally, our findings suggest that foxg1a contributes to POA maintenance, in part, by suppressing cell death.
AUTHOR CONTRIBUTIONS
KU and KY initiated the project, designed the experiments, and wrote the manuscript. KT played an important role in the start of mutagenesis. GC and KN contributed to mutant analyses. YK conducted part of the expression analysis. All other experiments were performed by KU.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Supporting information
Table S1. Oligonucleotides used in the current study.
ACKNOWLEDGMENTS
We thank the lab members for helpful discussion and technical supports. This work was partially supported by JSPS KAKENHI to K.Y. (Nos. 24240059, 26440114, 18K06242, and 21K06182).
Umeda, K. , Tanaka, K. , Chowdhury, G. , Nasu, K. , Kuroyanagi, Y. , & Yamasu, K. (2024). Evolutionarily conserved roles of foxg1a in the developing subpallium of zebrafish embryos. Development, Growth & Differentiation, 66(3), 219–234. 10.1111/dgd.12917
Communicating Editor: ICHIRO MASAI
REFERENCES
- Aboitiz, F. , & Montiel, J. (2007). Co‐option of signaling mechanisms from neural induction to telencephalic patterning. Reviews in the Neurosciences, 18, 311–342. [DOI] [PubMed] [Google Scholar]
- Affaticati, P. , Yamamoto, K. , Rizzi, B. , Bureau, C. , Peyriéras, N. , Pasqualini, C. , Demarque, M. , & Vernier, P. (2015). Identification of the optic recess region as a morphogenetic entity in the zebrafish forebrain. Scientific Reports, 5, 8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand, S. , Thisse, B. , Tavares, R. , Sachs, L. , Chaumot, A. , Bardet, P. L. , Escrivà, H. , Duffraisse, M. , Marchand, O. , Safi, R. , Thisse, C. , & Laudet, V. (2007). Unexpected novel relational links uncovered by extensive developmental profiling of nuclear receptor expression. PLoS Genetics, 3, e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielen, H. , & Houart, C. (2012). BMP signaling protects telencephalic fate by repressing eye identity and its Cxcr4‐dependent morphogenesis. Developmental Cell, 23, 812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon, C. , Li, J. , & Papalopulu, N. (1998). XBF‐1, a winged helix transcrition factor with dual activity, has a role in positioning neurogenesis in xenopus competent ectoderm. Development, 125, 4889–4900. [DOI] [PubMed] [Google Scholar]
- Carlin, D. , Sepich, D. , Grover, V. K. , Cooper, M. K. , Solnica‐Krezel, L. , & Inbal, A. (2012). Six3 cooperates with hedgehog signaling to specify ventral telencephalon by promoting early expression of Foxg1a and repressing Wnt signaling. Development, 139, 2614–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, D. S. , Martynoga, B. , Parras, C. , Ramesh, V. , Pacary, E. , Johnston, C. , Drechsel, D. , Lebel‐Potter, M. , Garcia, L. G. , Hunt, C. , Dolle, D. , Bithell, A. , Ettwiller, L. , Buckley, N. , & Guillemot, F. (2011). A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome‐wide characterization of its targets. Genes & Development, 25, 930–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi, C. , Mallamaci, A. , & Boncinelli, E. (2000). Otx and Emx homeobox genes in brain development. The International Journal of Developmental Biology, 44, 663–668. [PubMed] [Google Scholar]
- Chowdhury, G. , Umeda, K. , Ohyanagi, T. , Nasu, K. , & Yamasu, K. (2024). Involvement of nr2f genes in brain regionalization and eye development during early zebrafish development. Development, Growth & Differentiation. 10.1111/dgd.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesin, C. , Peres, J. N. , Johansson, M. , Snowden, V. , Cording, A. , Papalopulu, N. , & Houart, C. (2009). Integration of telencephalic Wnt and hedgehog signaling center activities by Foxg1. Developmental Cell, 16, 576–587. [DOI] [PubMed] [Google Scholar]
- Das, D. K. , Jadhav, V. , Ghattargi, V. C. , & Udani, V. (2014). Novel mutation in forkhead box G1 (FOXG1) gene in an Indian patient with Rett syndrome. Gene, 538, 109–112. [DOI] [PubMed] [Google Scholar]
- Dastidar, S. G. , Landrieu, P. M. , & D'mello, S. R. (2011). FoxG1 promotes the survival of postmitotic neurons. The Journal of Neuroscience, 31, 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou, C. L. , Li, S. , & Lai, E. (1999). Dual role of brain factor‐1 in regulating growth and patterning of the cerebral hemispheres. Cerebral Cortex, 9, 543–550. [DOI] [PubMed] [Google Scholar]
- Du, A. , Wu, X. , Chen, H. , Bai, Q.‐R. , Han, X. , Liu, B. , Zhang, X. , Ding, Z. , Shen, Q. , & Zhao, C. (2019). Foxg1 directly represses Dbx1 to confine the POA and subsequently regulate ventral telencephalic patterning. Cerebral Cortex, 29, 4968–4981. [DOI] [PubMed] [Google Scholar]
- Duggan, C. D. , Demaria, S. , Baudhuin, A. , Stafford, D. , & Ngai, J. (2008). Foxg1 is required for development of the vertebrate olfactory system. The Journal of Neuroscience, 28, 5229–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund, C. , Fink, A. , Lau, C. , Pham, D. , Daza, R. A. M. , Bulfone, A. , Kowalczyk, T. , & Hevner, R. F. (2005). Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. The Journal of Neuroscience, 25, 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson, J. , Muhr, J. , Placzek, M. , Lints, T. , Jessell, T. M. , & Edlund, T. (1995). Sonic hedgehog induces the differentiation of ventral forebrain neurons: A common signal for ventral patterning within the neural tube. Cell, 81, 747–756. [DOI] [PubMed] [Google Scholar]
- Fernandez, A. A. , Pieau, C. , Reperant, J. , Boncinelli, E. , & Wassef, M. (1998). Expression of the Emx‐1 and Dlx‐1 homeobox genes define three molecularly distinct domains in the telencephalon of mouse, chick, turtle and frog embryos: Implications for the evolution of telencephalic subdivisions in amniotes. Development, 125, 2099–2111. [DOI] [PubMed] [Google Scholar]
- Folgueira, M. , Bayley, P. , Navratilova, P. , Becker, T. S. , Wilson, S. W. , & Clarke, J. D. (2012). Morphogenesis underlying the development of the everted teleost telencephalon. Neural Development, 7, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz, J. , Kaslin, J. , Freudenreich, D. , Machate, A. , Geffarth, M. , & Brand, M. (2012). Subdivisions of the adult zebrafish subpallium by molecular marker analysis. The Journal of Comparative Neurology, 520, 633–655. [DOI] [PubMed] [Google Scholar]
- Godbole, G. , Shetty, A. S. , Roy, A. , D'Souza, L. , Chen, B. , Miyoshi, G. , Fishell, G. , & Tole, S. (2018). Hierarchical genetic interactions between FOXG1 and LHX2 regulate the formation of the cortical hem in the developing telencephalon. Development, 145, dev154583. 10.1242/dev.154583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, X. , Gu, X. , Zhang, Q. , Wang, Q. , Cheng, Y. , Pleasure, S. J. , & Zhao, C. (2018). FoxG1 directly represses dentate granule cell fate during forebrain development. Frontiers in Cellular Neuroscience, 12, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanashima, C. , Li, S. C. , Shen, L. , Lai, E. , & Fishell, G. (2004). Foxg1 suppresses early cortical cell fate. Science, 303, 56–59. [DOI] [PubMed] [Google Scholar]
- Hanashima, C. , Shen, L. , Li, S. C. , & Lai, E. (2002). Brain factor‐1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. The Journal of Neuroscience, 22, 6526–6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Z. H. , Li, M. , Fang, Q. J. , Liao, F. L. , Zou, S. Y. , Wu, X. , Sun, H. Y. , Zhao, X. Y. , Hu, Y. J. , Xu, X. X. , Chen, S. , Sun, Y. , Chai, R. J. , & Kong, W. J. (2021). FOXG1 promotes aging inner ear hair cell survival through activation of the autophagy pathway. Autophagy, 17, 4341–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert, J. M. , & Fishell, G. (2008). The genetics of early telencephalon patterning: Some assembly required. Nature Reviews. Neuroscience, 9, 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández‐Bejarano, M. , Gestri, G. , Spawls, L. , Nieto‐López, F. , Picker, A. , Tada, M. , Brand, M. , Bovolenta, P. , Wilson, S. W. , & Cavodeassi, F. (2015). Opposing shh and Fgf signals initiate nasotemporal patterning of the zebrafish retina. Development, 142, 3933–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettige, N. C. , & Ernst, C. (2019). FOXG1 Dose in Brain Development. Frontiers in Pediatrics, 7, 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, P. S. , Hailín, D. , Vogel, T. , & Hanashima, C. (2020). Transcription and beyond: Delineating FOXG1 function in cortical development and disorders. Frontiers in Cellular Neuroscience, 14, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, P. S. , Miyoshi, G. , & Hanashima, C. (2019). Sensory cortex wiring requires preselection of short‐ and long‐range projection neurons through an Egr‐Foxg1‐COUP‐TFI network. Nature Communications, 10, 3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta, H. , Kanai, M. , Ito, Y. , & Yamasu, K. (2003). gbx2 homeobox gene is required for the maintenance of the isthmic region in the zebrafish embryonic brain. Developmental Dynamics, 228, 433–450. [DOI] [PubMed] [Google Scholar]
- Kimmel, C. B. , Ballard, W. W. , Kimmel, S. R. , Ullmann, B. , & Schilling, T. F. (1995). Stages of embryonic development of the zebrafish. Developmental Dynamics, 203, 253–310. [DOI] [PubMed] [Google Scholar]
- Liu, B. , Zhou, K. , Wu, X. , & Zhao, C. (2018). Foxg1 deletion impairs the development of the epithalamus. Molecular Brain, 11, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Yang, M. , Su, M. , Liu, B. , Zhou, K. , Sun, C. , Ba, R. , Yu, B. , Zhang, B. , Zhang, Z. , Fan, W. , Wang, K. , Zhong, M. , Han, J. , & Zhao, C. (2022). FOXG1 sequentially orchestrates subtype specification of postmitotic cortical projection neurons. Science Advances, 8, eabh3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. X. , Zhang, D. , Xie, X. , Ouyang, G. , Liu, X. , Sun, Y. , & Xiao, W. (2013). Eaf1 and Eaf2 negatively regulate canonical Wnt/β‐catenin signaling. Development, 140, 1067–1078. [DOI] [PubMed] [Google Scholar]
- Manoli, M. , & Driever, D. (2014). nkx2.1 and nkx2.4 genes function partially redundant during development of the zebrafish hypothalamus, preoptic region, and pallidum. Frontiers in Neuroanatomy, 8, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel, M. , Martynoga, B. , Yu, T. , West, J. D. , Mason, J. O. , & Price, D. J. (2010). The transcription factor Foxg1 regulates the competence of telencephalic cells to adopt subpallial fates in mice. Development, 137, 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigo, V. , Davey, R. A. , Zuo, Y. , Cunningham, J. M. , & Tabin, C. J. (1996a). Biochemical evidence that patched is the hedgehog receptor. Nature, 384, 176–179. [DOI] [PubMed] [Google Scholar]
- Marigo, V. , Johnson, R. L. , Vortkamp, A. , & Tabin, C. J. (1996b). Sonic hedgehog differentially regulates expression of GLI and GLI3 during limb development. Developmental Biology, 180, 273–283. [DOI] [PubMed] [Google Scholar]
- Martynoga, B. , Morrison, H. , Price, D. J. , & Mason, J. O. (2005). Foxg1 is required for specification of ventral telencephalon and region‐specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Developmental Biology, 283, 113–127. [DOI] [PubMed] [Google Scholar]
- Miyake, A. , Mekata, Y. , Fujibayashi, H. , Nakanishi, K. , Konishi, M. , & Itoh, N. (2017). Brorin is required for neurogenesis, gliogenesis, and commissural axon guidance in the zebrafish forebrain. PLoS One, 12, e0176036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, T. , Dong, Z. , Berberoglu, M. A. , & Guo, S. (2011). The dorsal pallium in zebrafish, Danio rerio (Cyprinidae, Teleostei). Brain Research, 1381, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, T. , & Wullimann, M. F. (2009). An evolutionary interpretation of teleostean forebrain anatomy. Brain, Behavior and Evolution, 74, 30–42. [DOI] [PubMed] [Google Scholar]
- Mueller, T. , Wullimann, M. F. , & Guo, S. (2008). Early teleostean basal ganglia development visualized by zebrafish Dlx2a, Lhx6, Lhx7, Tbr2 (eomesa), and GAD67 gene expression. The Journal of Comparative Neurology, 507, 1245–1257. [DOI] [PubMed] [Google Scholar]
- Nadarajah, B. , & Parnavelas, J. G. (2002). Modes of neuronal migration in the developing cerebral cortex. Nature Reviews. Neuroscience, 3, 423–432. [DOI] [PubMed] [Google Scholar]
- Nery, S. , Corbin, J. G. , & Fishell, G. (2003). Dlx2 progenitor migration in wild type and Nkx2.1 mutant telencephalon. Cerebral Cortex, 13, 895–903. [DOI] [PubMed] [Google Scholar]
- Ota, S. , Hisano, Y. , Muraki, M. , Hoshijima, K. , Dahlem, T. J. , Grunwald, D. J. , Okada, Y. , & Kawahara, A. (2013). Efficient identification of TALEN‐mediated genome modifications using heteroduplex mobility assays. Genes to Cells, 18, 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley, S. , Lai, E. , & Fritzsch, B. (2006). Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Developmental Dynamics, 235, 2470–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli, A. , Montague, T. G. , Lennox, K. A. , Behlke, M. A. , & Schier, A. F. (2015). Antisense oligonucleotide‐mediated transcript knockdown in zebrafish. PLoS One, 10, e0139504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker, A. , Cavodeassi, F. , Machate, A. , Bernauer, S. , Hans, S. , Abe, G. , Kawakami, K. , Wilson, S. W. , & Brand, M. (2009). Dynamic coupling of pattern formation and morphogenesis in the developing vertebrate retina. PLoS Biology, 7, e1000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport, S. I. (1990). Integrated phylogeny of the primate brain, with special reference to humans and their diseases. Brain Research. Brain Research Reviews, 15, 267–294. [DOI] [PubMed] [Google Scholar]
- Ross, L. S. , Parrett, T. , & Easter, S. S., Jr. (1992). Axonogenesis and morphogenesis in the embryonic zebrafish brain. The Journal of Neuroscience, 12, 467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein, J. L. , Shimamura, K. , Martinez, S. , & Puelles, L. (1998). Regionalization of the prosencephalic neural plate. Annual Review of Neuroscience, 21, 445–477. [DOI] [PubMed] [Google Scholar]
- Shimizu, T. (2007). The avian brain revisited: Anatomy and evolution of the telencephalon. In Integration of comparative neuroanatomy and cognition (pp. 55–73). Keio University Publications. [Google Scholar]
- Stainier, D. Y. (2001). Zebrafish genetics and vertebrate heart formation. Nature Reviews. Genetics, 2, 39–48. [DOI] [PubMed] [Google Scholar]
- Storm, E. E. , Rubenstein, J. L. , & Martin, G. R. (2003). Dosage of Fgf8 determines whether cell survival is positively or negatively regulated in the developing forebrain. Proceedings of the National Academy of Sciences of the United States of America, 100, 1757–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyme, S. B. , Pieper, L. M. , Li, E. H. , Pandey, S. , Wang, Y. , Morris, N. S. , Sha, C. , Choi, J. W. , Herrera, K. J. , Soucy, E. R. , Zimmerman, S. , Randlett, O. , Greenwood, J. , McCarroll, S. A. , & Schier, A. F. (2019). Phenotypic landscape of schizophrenia‐associated genes defines candidates and their shared functions. Cell, 177, 478–491.e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, C. , Gong, Y. , Yang, Y. , Shen, W. , Wang, K. , Liu, J. , Xu, B. , Zhao, J. , & Zhao, C. (2012). Foxg1 has an essential role in postnatal development of the dentate gyrus. The Journal of Neuroscience, 32, 2931–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma, K. , Kumamoto, T. , & Hanashima, C. (2014). The timing of upper‐layer neurogenesis is conferred by sequential derepression and negative feedback from deep‐layer neurons. The Journal of Neuroscience, 34, 13259–13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toresson, H. , Martinez‐Barbera, J. P. , Bardsley, A. , Caubit, X. , & Krauss, S. (1998). Conservation of BF‐1 expression in amphioxus and zebrafish suggests evolutionary ancestry of anterior cell types that contribute to the vertebrate telencephalon. Development Genes and Evolution, 208, 431–439. [DOI] [PubMed] [Google Scholar]
- Viktorin, G. , Chiuchitu, C. , Rissler, M. , Varga, Z. M. , & Westerfield, M. (2009). Emx3 is required for the differentiation of dorsal telencephalic neurons. Developmental Dynamics, 238, 1984–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullimann, M. F. (2009). Secondary neurogenesis and telencephalic organization in zebrafish and mice: A brief review. Integrative Zoology, 4, 123–133. [DOI] [PubMed] [Google Scholar]
- Xuan, S. , Baptista, C. A. , Balas, G. , Tao, W. , Soares, V. C. , & Lai, E. (1995). Winged helix transcription factor BF‐1 is essential for the development of the cerebral hemispheres. Neuron, 14, 1141–1152. [DOI] [PubMed] [Google Scholar]
- Zhao, X. F. , Suh, C. S. , Prat, C. R. , Ellingsen, S. , & Fjose, A. (2009). Distinct expression of two foxg1 paralogues in zebrafish. Gene Expression Patterns, 9, 266–272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Oligonucleotides used in the current study.


