Abstract
Peripheral nerve surgeries are a complex undertaking that require a multifaceted team approach. For optimal outcomes, early involvement of therapy, planning with the surgical team, and communication with the patient are crucial. This facilitates compliance and is an integral component of the recovery process after these large procedures.
Key words: Nerve repair, Prehabilitation, Rehabilitation, Therapy
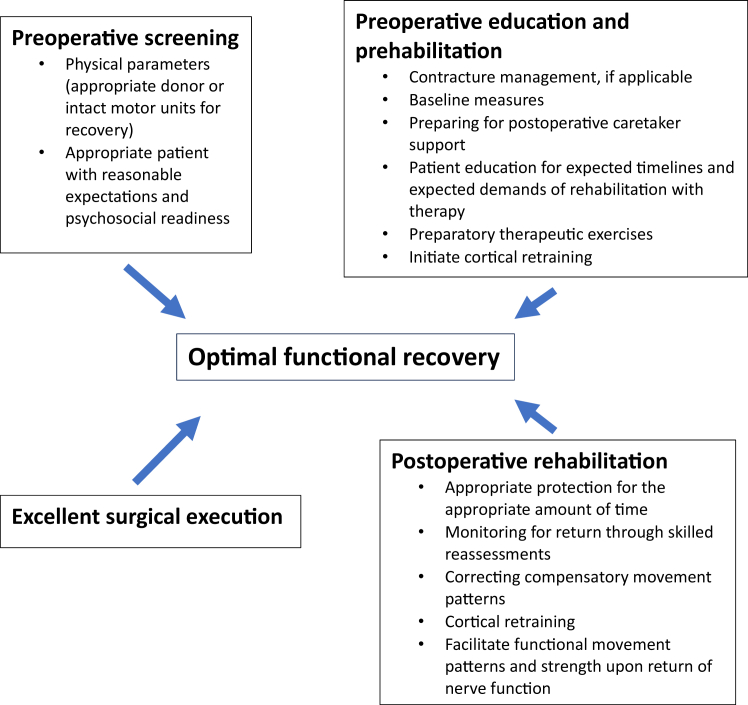
Nerve transfers and peripheral nerve surgeries are a means of restoring movement and, when performed with sensory nerve transfers, sensation with the ultimate purpose of enhancing meaningful function. In the setting of peripheral nerve surgery specifically, attentive care coordination by the surgical and hand therapy personnel (knowledgeable occupational or physical therapists) before and after the surgery is imperative. Even after excellent technical execution of an indicated procedure on a motivated patient, these complex surgeries are still heavily reliant upon rehabilitation to achieve their optimal outcomes (Figure 1). Traditionally, these procedures have gravitated toward tertiary referral centers where large teams of professionals can aid patients in recovery. Centers of excellence for peripheral nerve surgery have been designed to facilitate communication between the surgical and rehabilitative teams to help adapt recovery strategies to protect technically challenging operations while tailoring progression to the individual patient after surgery.
Figure 1.
Factors that are imperative for achieving optimal functional recovery.
Preoperative planning with a multidisciplinary approach can illuminate issues that would otherwise risk the operative procedure’s success. Having the patient participate in preoperative rehabilitation can enhance the surgical outcomes postoperatively.1 The appropriate rehabilitation postoperatively maximizes recovery. Appropriate rehabilitation involves well-timed assessments with modifications to therapeutic exercises by knowledgeable therapists to facilitate progress dynamically as structures heal. Success of the postoperative rehabilitation depends on both the treating occupational or physical therapist and the patient understanding how to initially protect repaired soft tissue structures and how to appropriately progress movement and limb use to optimize function. Efforts at each phase of the process can be optimized. The purpose of this article is to discuss the phases of prehabilitation and rehabilitation and strategies to optimize both to improve outcomes of peripheral nerve surgery.
The specific rehabilitation protocol is tailored to the individual structures repaired; however, there are overlapping concepts that can be applied. The shared aspects include the following: evaluation by both the surgical team and therapist of the patient to determine whether they are an appropriate candidate, preoperative patient education about the procedure and expected rehabilitation timelines for recovery, and finally participation in prehabilitation and rehabilitation therapy. Prehabilitation therapy includes addressing contractures, correcting compensatory movement patterns, correcting muscle imbalances from injury, neuromuscular retraining to facilitate active muscle contractions for the donor muscle of the nerve, and strengthening for functional movement patterns.2 Preoperative education can help ensure safety during a postoperative protective phase and facilitate the initiation of cortical remapping prior to surgery, which some prior literature has suggested is critical for successful outcomes.3
Preoperative Patient Selection
Patient selection involves not only meeting the physical parameters, including appropriate donor nerve or intact muscle motor units for recovery and contractures that have been managed to allow movement for the repair to work, but also demonstrating psychological and emotional readiness for the surgery and the rehabilitation process. It is of primary importance is to ascertain the patient’s goals for the surgery. There should be mindful inquiry about what the patient specifically expects to gain. General goals such as “I need to use my hand like normal…” or “I want to be able to use my hand like before [my injury]…” are not appropriate and indicate a need for further education about expectations from the surgical procedure and likelihood of functional recovery. Specific patient goals guide the health care team in efforts to help the patient achieve meaningful functional gains and provide the patient the opportunity to operationally define and visualize a successful and realistic outcome. An example of a specific goal may be “I want to pinch my thumb to the tips of my fingers to pick up small objects, like pills, or to eat finger foods at meals.” The Patient-Specific Functional Scale is a patient-rated outcome measure, in which the patient defines five important activities they now find difficult and rate the level of difficulty of performing the task from 0 (unable to perform) to 10 (able to perform as before injury); pre- and postintervention use of this metric has been found to be reliable, valid, and responsive for patients with upper extremity dysfunctions.4
Body diagrams are also a necessary tool to gather information about the location of pain and sensory disturbances. If pain is widespread, particularly in a nonneuroanatomic distribution, further investigation about patient expectations and level of understanding are a requisite to clarify whether the expected outcomes of surgery are indicated. A Visual Analog Scale or Numerical Pain Scale should be used preoperatively to gather the status of pain prior to surgery.5 If pain is a chronic issue that causes limited participation in functional activities, tools, such as the Fear-Avoidance Beliefs Questionnaire, can help further explore of the impact pain may have in the postoperative rehabilitation process.6 Preoperative kinesiophobia, or fear related to movement of the injured part, may affect participation in postoperative rehabilitation, negatively impacting the outcomes of surgery. It is recommended that preoperative subjective evaluations include questions that include measures of pain, descriptions of sensory disturbances, and measures of psychosocial components, such sadness, frustration, ability to cope and hope.7 If the patient psychologically is struggling with their injury, psychological support from the multidisciplinary team is recommended. Techniques, such as Acceptance and Commitment Therapy, cognitive behavioral therapy, and mindfulness, may be considered.8
Preoperative Education and Prehabilitation
Prehabilitation includes taking baseline measurements, addressing contractures, providing education about postoperative timelines and the rehabilitation involved, and describing any therapeutic exercises to be performed preoperatively to prepare for the procedure.
Prior to the surgical procedure, baseline measurements should be taken. Baseline measurements include passive and active range of motion for affected joints (°), grip and pinch strength (kg), dexterity, sensation, and patient-rated pain and function assessments. Consistency in measurement tools among surgeons and therapists also provides meaningful information, i.e., will sensory outcomes be defined using the Medical Research Council grade, “Ten Test,” static or moving two-point discrimination, or Semmes-Weinstein monofilament testing?
Addressing contractures prepares for the active movement that the motor nerve repair or transfer is expected to provide. This may include a period of therapy that involves manual therapy techniques and positioning and progressive range of motion orthoses to optimize range of motion.
The preoperative period is the optimal time to manage patient expectations regarding the outcome of surgery. Ensuring that the patient and the family or caregivers have realistic expectations can enhance patient satisfaction. If a patient has an unrealistic understanding of outcomes, then disappointment and dissatisfaction may result. Having the patient and/or caregivers repeat back their understanding helps ensure that appropriate expectations have been communicated. In a systematic review by Yen et al,9 the “teach back method” was found to be effective for confirming patient understanding with resultant improved patient satisfaction scores.
The initial therapy sessions after surgery are not the optimal time for learning complex concepts. Patients typically are in pain and are fearful of doing something that will ruin their surgery. Psychosocially, they are often distracted by the burden of their postoperative demands on their caretakers and overwhelmed by the timeline to recovery. Education prior to surgery can allow patients to plan for accommodations with anticipated limited functionality. Having assistance at home preplanned promotes compliance with precautions during the protection phase. Planning for postoperative accommodations may include assistance from a social worker. It is also recommended that postoperative rehabilitation be scheduled prior to surgery.10 Performance of preoperative therapeutic exercises improves postoperative outcomes. Practicing the therapeutic exercises preoperatively will establish a habit, making this part of the rehabilitation less mentally demanding postoperatively.11 Specifically, for motor nerve transfers, the donor activation focus rehabilitation approach has been proven to be effective. In this approach, the donor muscle is activated preoperatively throughout the day with the verbal cue to “flood the donor.” Preoperative resistive strengthening of the donor will help preserve muscle function postoperatively when the innervation of the muscle has changed.12 This requires communication between the surgeon and treating occupational or physical therapist about the planned procedure, including the specific muscle donating innervation and which muscle is the recipient.
Injury and immobilization result in changes in the somatosensory cortex of the brain. This cortical reorganization often means that there is a disconnect between the brain and the injured body region.13 Merzenich and Jenkins14 showed evidence of the capacity for neuroplasticity and the potential for reorganization of the somatosensory cortex of the brain with proper stimulus in simians. Cortical retraining is a crucial aspect of the prehabilitation and rehabilitation processes for nerve repairs and transfers. As stated previously, initial postoperative therapy sessions are not ideal for learning complex therapeutic concepts; therefore, activities for cortical retraining are best initiated preoperatively. Recovery of function after nerve transfers specifically requires both the successful reinnervation of the target muscle and cortical retraining of the new innervation pathway to achieve motor function. Graded motor imagery is a rehabilitation approach that uses techniques of laterality, imagery, and mirror therapy for cortical remapping with clinically documented clinical success.15, 16, 17
Addressing compensatory movement patterns and muscle imbalances should also begin preoperatively. Bringing the patient’s awareness to these problems can lay the foundation for postoperative work. The patient should be encouraged to use the operative arm as much as possible to prevent disuse weakness and cortical changes.
Postoperative Protective Phase
Nerve repairs and nerve transfers require initial protection postoperatively. A well-positioned sling and well-fitting positioning orthotics ensure that repaired structures are not stressed. Slings and positioning orthoses can be used past the protection phase to position muscles at an optimal length to generate force. An overly stretched or shortened position places the sarcomeres of the muscle at a disadvantage to generate force for movement. Ideal positioning may help the muscle regain strength and control more rapidly.18 Additionally, the support of a sling will limit traction from the weight of the arm from gravity on the brachial plexus.12
Facilitating Active Movement of the Recipient
For motor nerve transfers, movement may be restricted at the discretion of the surgeon for up to 6 weeks to protect the coaptation site. Orthoses and slings are used after the protection phase to place muscles in an advantageous position for active movement. The timeline between protective phase and detecting motor activity at the recipient muscle varies and is referred to as the “silent phase.”18,19 Once an appropriately individualized home management program has been established, the frequency of therapy sessions is scheduled to allow for monitoring of motor function return and to ensure patient compliance with therapeutic activities, but also to preserve the limited visits allowed by insurance carriers. Patients may be seen every 2 weeks to monthly at this stage of recovery.
During the silent phase of recovery from a motor nerve transfer, Kahn and Moore12 recommend continuing to “flood the donor” with active contractions of the donor muscle every hour for 10–20 repetitions and to perform passive range of motion in the desired movement pattern 2–3 times a day. Larocerie-Salgado et al19 advocate using mental imagery and mirror therapy for cortical reorganization. Performing movements with the contralateral limb coupled with mental imagery may also be effective.
If applicable, strengthening any proximal weakness from disuse and addressing compensatory movement patterns will also need to be addressed. The use of the protracted position of the scapula to position the arm in a sling or to guard against pain results in scapular dyskinesia and poor proximal stabilization. Attention should be directed to evaluating and treating muscle imbalances of weakness in the middle and lower trapezius and serratus anterior and excessive activity of the upper trapezius and levator scapulae.20,21
Strengthening and Functional Movement
The strengthening phase is gratifying. At this point, restrictions for protecting repairs are expired, and the patient is seeing the intended active movement. Strengthening is enhanced by immediate feedback and modifications to correct movement patterns. Patient should be cued to focus on the correct movement rather than compensatory use of stronger muscles to compensate. Bimanual tasks enhance the feedback to both hemispheres of the brain and solidify motor learning patterns. Therapist techniques, such as gravity-eliminated positions, place and hold in midrange, and active-assisted motions, are useful tools for enhancing recovery.20 Initially, strengthening sessions should be short in duration to avoid excessive fatigue and effort.22
For motor nerve transfers, neuromuscular retraining to separate active movement of the recipient muscle from the donor can begin when strength of the recipient muscle is graded as a 3/5. If using neuromuscular electrical stimulation, a low frequency of 20 Hz is recommended to avoid excessively fatiguing the recipient muscle.12,23
Conclusions
In conclusion, the intricacies of peripheral nerve reconstruction combined with pre- and postrehabilitation strategies underscore the paramount importance of a collaborative and comprehensive strategy for achieving optimal outcomes. Hand and peripheral nerve surgeons at the forefront of this intricate field must recognize that success extends beyond the operating room, necessitating a seamless integration of operative and rehabilitation efforts. The synergy between these two crucial teams not only fosters patient engagement but also serves as the linchpin for procedural success. For optimal results, initiating rehabilitation prior to surgery is recommended, if not imperative, using a personalized, patient-centric methodology. This approach, which is characterized by nuanced feedback delivery, not only respects the mechanical integrity of the repair, but also accounts for the intricate neuroadaptation required for subsequent functional gains. To realize optimal outcomes for our patients we must demonstrate a unified commitment to a tailored, collaborative, and patient-focused continuum of care.
Footnotes
Declaration of interests: No benefits in any form have been received or will be received related directly to this article.
References
- 1.Novak C.B., von der Heyde R.L. Evidence and techniques in rehabilitation following nerve injuries. Hand Clin. 2013;29(3):383–392. doi: 10.1016/j.hcl.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Doherty C, Brown E, Berger M, et al. Contemporary approaches to peripheral nerve surgery. Plast Surg (Oakv). Published online September 9, 2022:22925503221120571. [DOI] [PMC free article] [PubMed]
- 3.Baldassarre B.M., Lavorato A., Titolo P., et al. Principles of cortical plasticity in peripheral nerve surgery. Surg Technol Int. 2020;36:444–452. [PubMed] [Google Scholar]
- 4.Hefford C., Abbott J.H., Arnold R., Baxter G.D. The patient-specific functional scale: validity, reliability, and responsiveness in patients with upper extremity musculoskeletal problems. J Orthop Sports Phys Ther. 2012;42(2):56–65. doi: 10.2519/jospt.2012.3953. [DOI] [PubMed] [Google Scholar]
- 5.Haefeli M., Elfering A. Pain assessment. Eur Spine J. 2006;15(1):S17–S24. doi: 10.1007/s00586-005-1044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gatchel R.J., Neblett R., Kishino N., Ray C.T. Fear-avoidance beliefs and chronic pain. J Orthop Sports Phys Ther. 2016;46(2):38–43. doi: 10.2519/jospt.2016.0601. [DOI] [PubMed] [Google Scholar]
- 7.Novak C.B. Evaluation of the patient with nerve injury or nerve compression. Nerve Surg. 2014;1 [Google Scholar]
- 8.Forman E.M., Herbert J.D., Moitra E., Yeomans P.D., Geller P.A. A randomized controlled effectiveness trial of acceptance and commitment therapy and cognitive therapy for anxiety and depression. Behav Modif. 2007;31(6):772–799. doi: 10.1177/0145445507302202. [DOI] [PubMed] [Google Scholar]
- 9.Yen P.H., Leasure A.R. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed Pract. 2019;36(6):284–289. [PMC free article] [PubMed] [Google Scholar]
- 10.McLaughlin MT, Moura SP, Edalatpour A, Seitz AJ, Michelotti BF. Insurance status predicts hand therapy adherence following flexor tendon repair: a retrospective cohort study. Plast Reconstr Surg. Published online May 16, 2023:10.1097/PRS.0000000000010702. [DOI] [PubMed]
- 11.Mendelsohn A.I. Creatures of habit: the neuroscience of habit and purposeful behavior. BiolPsychiatry. 2019;85(11):e49–e51. doi: 10.1016/j.biopsych.2019.03.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn L.C., Moore A.M. Donor activation focused rehabilitation approach: maximizing outcomes after nerve transfers. Hand Clin. 2016;32(2):263–277. doi: 10.1016/j.hcl.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Delcour M., Russier M., Castets F., et al. Early movement restriction leads to maladaptive plasticity in the sensorimotor cortex and to movement disorders. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-34312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merzenich M.M., Jenkins W.M. Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. J Hand Ther. 1993;6(2):89–104. doi: 10.1016/s0894-1130(12)80290-0. [DOI] [PubMed] [Google Scholar]
- 15.McCabe C. Mirror visual feedback therapy. A practical approach. J Hand Ther. 2011;24(2):170–179. doi: 10.1016/j.jht.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 16.McCabe C.S., Haigh R.C., Blake D.R. Mirror visual feedback for the treatment of complex regional pain syndrome (type 1) Curr Pain Headache Rep. 2008;12(2):103–107. doi: 10.1007/s11916-008-0020-7. [DOI] [PubMed] [Google Scholar]
- 17.Priganc V.W., Stralka S.W. Graded Motor Imagery. J Hand Ther. 2011;24(2):164–169. doi: 10.1016/j.jht.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Hill J.L., Turner L.C., Jones R.D., Jimulia D.T., Miller C., Power D.M. The stages of rehabilitation following motor nerve transfer surgery. J Musculoskelet Surg Res. 2019;3:60. [Google Scholar]
- 19.Larocerie-Salgado J., Chinchalkar S., Ross D.C., Gillis J., Doherty C.D., Miller T.A. Rehabilitation following nerve transfer surgery. Tech Hand Up Extrem Surg. 2022;26(2):71–77. doi: 10.1097/BTH.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 20.Moore A.M., Novak C.B. Advances in nerve transfer surgery. J Hand Ther. 2014;27(2):96–104. doi: 10.1016/j.jht.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Cricchio M., Frazer C. Scapulothoracic and scapulohumeral exercises: a narrative review of electromyographic studies. J Hand Ther. 2011;24(4):322–333. doi: 10.1016/j.jht.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Skirven T.M., editor. Rehabilitation of the Hand and Upper Extremity. 7th edition. Elsevier Mosby; 2021. [Google Scholar]
- 23.Chan K.M., Curran M.W.T., Gordon T. The use of brief post-surgical low frequency electrical stimulation to enhance nerve regeneration in clinical practice. J Physiol. 2016;594(13):3553–3559. doi: 10.1113/JP270892. [DOI] [PMC free article] [PubMed] [Google Scholar]