Abstract
Aim: A systematic review and network meta-analysis (NMA) was performed to evaluate the efficacy of first-line treatments for locally recurrent unresectable or metastatic triple-negative breast cancer (TNBC) patients.
Materials & methods: Databases were searched for randomized controlled trials evaluating first-line treatments for locally recurrent unresectable or metastatic TNBC patients. NMA was performed to estimate relative treatment effects on overall and progression-free survival between pembrolizumab + chemotherapy and other interventions.
Results: NMA including eight trials showed that the relative efficacy of pembrolizumab + chemotherapy was statistically superior to that of other immunotherapy- or chemotherapy-based treatment regimens.
Conclusion: Pembrolizumab + chemotherapy confers benefits in survival outcomes versus alternative interventions for the first-line treatment of locally recurrent unresectable or metastatic TNBC patients.
Keywords: : chemotherapy, immune checkpoint inhibitors, immunotherapy, network meta-analysis, PD-1, PD-L1, survival outcomes, systematic review, triple-negative breast cancer
Plain Language Summary
Clinical value of initial treatments for patients with advanced triple-negative breast cancer
What is this article about?
Around 15% of breast cancer patients have the triple-negative breast cancer (TNBC) subtype, which has the worst prognosis. Treatments targeting the immune system, such as pembrolizumab, were recently found to improve the outcomes of patients with cancer that is at an advanced stage or resistant to standard therapies. However, clinical trials evaluating the efficacy of cancer treatments typically compare only two alternative treatments. Therefore, we conducted this study to understand the relative efficacy of several commonly used initial treatments for advanced TNBC by indirectly comparing the results of all available clinical trials that were sufficiently similar. We identified trials by systematically searching the medical literature and analyzed the results of several clinical trials together to estimate the efficacy of pembrolizumab + chemotherapy compared with several other initial treatment regimens for patients with advanced TNBC.
What were the results?
We identified eight randomized controlled trials evaluating treatment regimens containing chemotherapeutic or immunotherapeutic agents in patients with previously untreated advanced TNBC. Considering all these trials together, pembrolizumab + chemotherapy was found to prolong patient survival to a greater extent than several other treatment regimens including carboplatin, docetaxel, paclitaxel, nab-paclitaxel/paclitaxel, bevacizumab + paclitaxel, ixabepilone + paclitaxel and ixabepilone + bevacizumab depending on the specific set of trials analyzed.
What do the results of the study mean?
These results indicate that pembrolizumab + chemotherapy has beneficial effects on patient survival compared with other initial treatment regimens for patients with advanced TNBC.
Tweetable Abstract
Pembrolizumab + chemotherapy confers benefits in survival outcomes versus alternative interventions for the first-line treatment of locally recurrent unresectable or metastatic TNBC patients.
Plain language summary
Summary points.
Patients with triple-negative breast cancer (TNBC) have the worst prognosis among all breast cancer patients.
Pembrolizumab + chemotherapy was recently shown to improve the survival of patients with advanced cancers or cancers refractory to standard treatments, with promising results for the treatment of breast cancer.
The purpose of this study was to compare the relative efficacy of pembrolizumab + chemotherapy to other interventions for the first-line treatment of locally recurrent unresectable or metastatic TNBC through a systematic review and network meta-analysis.
EMBASE, MEDLINE, CENTRAL, conference abstracts and clinical trial registries were searched for randomized controlled trials evaluating immunotherapy- or chemotherapy-based treatments for patients with locally recurrent unresectable or metastatic TNBC who were previously untreated for advanced disease.
Following study screening and feasibility assessment, eight unique trials were included in the network meta-analysis.
In terms of overall survival, the relative treatment efficacy of pembrolizumab + different chemotherapy agents was statistically superior to that of carboplatin, docetaxel, paclitaxel, bevacizumab + paclitaxel, ixabepilone + paclitaxel or ixabepilone + bevacizumab depending on the evidence network analyzed.
In terms of progression-free survival, the relative treatment efficacy of pembrolizumab + different chemotherapy agents was statistically superior to that of paclitaxel or nab-paclitaxel/paclitaxel combined depending on the evidence network analyzed.
These results suggest that as a first-line treatment for locally recurrent unresectable or metastatic TNBC, pembrolizumab + chemotherapy is more efficacious in improving survival outcomes compared with other interventions, with greater improvements observed for overall survival than for progression-free survival.
Breast cancer is the most common cancer in women, with 2.3 million new cases worldwide in 2020 [1]. A subtype of breast cancer, triple-negative breast cancer (TNBC) accounts for ∼15% of all breast cancer cases and is characterized by the lack of ER, PR and HER2 [2]. TNBC has the worst prognosis among all breast cancer subtypes, with a median overall survival (OS) of 13 months in patients with metastatic TNBC compared with 38 months in patients with other types of breast cancer [3]. Thus, given the complex histology and aggressive nature of TNBC, new therapeutic approaches are warranted.
In recent years, PD-1 and PD-L1 inhibitors have been shown to improve OS in patients with advanced cancers or cancers refractory to standard treatments, with promising results for the treatment of breast cancer [4]. Based on statistically and clinically meaningful improvements in OS and progression-free survival (PFS) demonstrated in the phase III randomized controlled trial (RCT) KEYNOTE-355, pembrolizumab + chemotherapy (paclitaxel, nab-paclitaxel, or gemcitabine/carboplatin) was recently granted US FDA and European Medicines Agency approval for first-line treatment of locally recurrent unresectable or metastatic TNBC in patients whose tumors express PD-L1 at the cut-off of combined positive score (CPS) ≥10 [5,6].
Network meta-analysis (NMA) is a statistical method allowing indirect comparisons among treatment regimens that have not been directly compared in head-to-head RCTs [7–9]. This approach enables a variety of stakeholders, including clinicians, developers of guidelines and health technology assessment agencies, to assess the performance of new treatments against all existing evidence on other treatments [10,11]. More specifically, NMA can combine both direct and indirect evidence on any treatments that form a connected network of trials provided that there are minimal differences among trials in terms of patient characteristics, study characteristics and outcome definitions that could modify the relative treatment effects [12]. Although clinical trial evidence suggests that pembrolizumab + chemotherapy is an effective approach to treating locally recurrent unresectable or metastatic TNBC, it has not been compared against all other immunotherapy- and chemotherapy-based regimens in head-to-head RCTs. Therefore, we aimed to estimate the relative efficacy of pembrolizumab + chemotherapy in terms of OS and PFS compared with other interventions for the first-line treatment of locally recurrent unresectable or metastatic TNBC through a systematic review (SR) and NMA.
Materials & methods
Systematic review
The SR was performed following Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [13] and employed methodology similar to that used for an SR focused on a different TNBC patient population [14]. The review was not registered in a systematic review registry. Studies were selected using pre-specified population, intervention, comparator, outcome and study design (PICOS) criteria (Supplementary Table 1). Phase II or III RCTs were eligible for inclusion if they were enrolled patients with locally recurrent unresectable or metastatic TNBC who received first-line treatment with specific chemotherapy or immunotherapy regimens. Trials were eligible for inclusion in the SR if they enrolled only TNBC patients or enrolled a broader population of breast cancer patients and reported at least one outcome of interest in a >90% TNBC patient subgroup.
Trials were identified through searches of Embase, MEDLINE and Cochrane Central Register of Controlled Trials (CENTRAL) via Ovid on 21 April 2022 (full search strategies provided in Supplementary Table 2–4). Scottish Intercollegiate Guidelines Network (SIGN) filters (www.sign.ac.uk/what-we-do/methodology/search-filters/) were used to limit Embase and MEDLINE search results to RCTs. Conference abstracts from the American Society of Clinical Oncology (2021–2022), San Antonio Breast Cancer Symposium (2021) and European Society of Medical Oncology (2021) were hand-searched to identify trials that were not yet published as journal articles. To mitigate the risk of reporting biases (i.e., missing RCTs with null or negative results not published as journal articles or conference abstracts), the US National Institute of Health Clinical Trials Registry and European Union Clinical Trial Registry were searched to identify completed trials with results available.
Title/abstract screening, full-text screening and data extraction were performed by two independent reviewers. Any discrepancies between reviewers were resolved through discussion or by involving a third reviewer. The risk of bias in included trials was assessed using the Cochrane Collaboration's Risk of Bias tool [15].
Feasibility assessment & network meta-analysis
NMA is an extension of pairwise meta-analysis that enables the indirect comparison of treatments that have not been directly compared in head-to-head trials [16]. As the validity of an NMA depends on the absence of systematic differences in treatment effects among trials in the network [16–20], a feasibility assessment was performed before commencing the NMA to determine whether the interventions of interest formed a single network of evidence for each outcome and evaluate distributions of study, patient, treatment and outcome characteristics across trials to identify potential treatment effect-modifiers [17].
After feasibility assessment, NMA was performed for the efficacy outcomes of OS and PFS. Although the assumptions of random-effects NMA models are generally preferred as they are usually more plausible than fixed-effect models, between-study heterogeneity could not be estimated in the present study because only one trial connected each intervention in the evidence networks. Therefore, NMA was performed using fixed-effects models. Proportional hazards between interventions were assumed using regression models with a contrast-based normal likelihood for the log hazard ratio (HR) and corresponding standard error for each trial [21].
Normal non-informative prior distributions for model parameters were estimated with a mean of 0 and variance of 10,000 [21]. NMA results are presented as HRs estimating of the treatment effect of each intervention relative to all other interventions in the network. The posterior distributions of relative treatment effects are summarized by the median and 95% credible interval (CrI), which reflects a 95% probability that the estimate is within the specified range. Analyses were conducted using R version 4.0.3 and OpenBugs version 3.2.3 [22].
Results
Systematic review & feasibility assessment
Study selection
The literature search yielded a total of 2930 records, of which 2332 abstracts and 154 full texts were screened by two independent reviewers (Figure 1). Six additional records were identified from conference proceedings, clinical trial registries and material provided by Merck & Co., Inc., Rahway (NJ, USA). In total, 19 records describing eight unique trials were included in the SR (Supplementary Table 5) [23–39].
Figure 1.
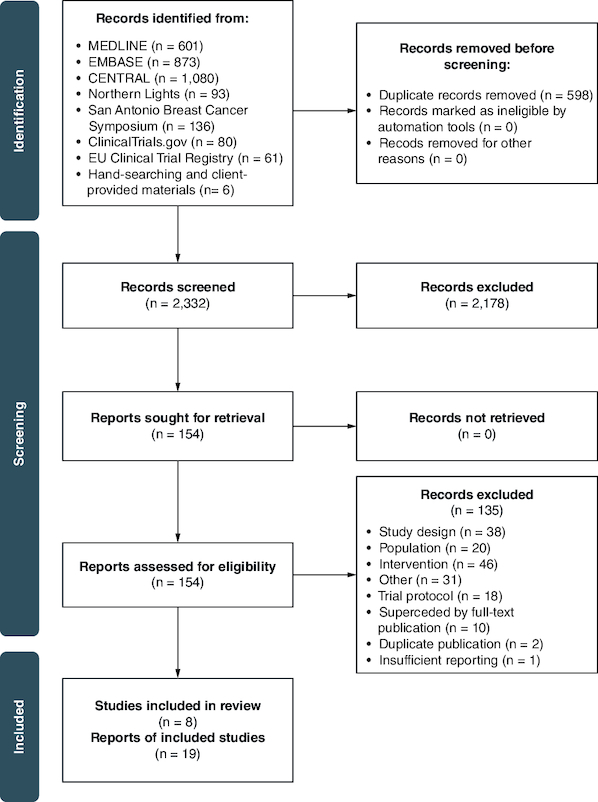
PRISMA flow diagram of the study identification and selection process.
Study characteristics
Of the eight RCTs, five were phase III, two were phase II and one was phase II/III (Table 1). Three trials were double blind, and five trials were open label. All trials were multicenter. Across trials, follow-up duration ranged from 14.8 to 43.5 months. All trials were considered to have an overall low risk of bias (Supplementary Table 6).
Table 1.
Study characteristics of included trials.
| Trial ID | Phase | Masking | Disease stage | Multicenter | Treatment | Follow-up duration, months, median (IQR) | Ref. |
|---|---|---|---|---|---|---|---|
| Alliance (CALGB 40502) | II | Open label | Stage IIIC-IV locally recurrent or metastatic | Yes | Paclitaxel ± bevacizumab | – | [19,20] |
| Nab-paclitaxel ± bevacizumab | |||||||
| Ixabepilone ± bevacizumab | |||||||
| E2100 | III | Open label | Stage IV metastatic | Yes | Paclitaxel ± bevacizumab | 41.6 (–)‡ | [21] |
| Paclitaxel | 43.5 (–)‡ | ||||||
| IMpassion130 | III | Double blind | Metastatic or unresectable locally advanced | Yes | Atezolizumab ± nab-paclitaxel | 18.8 (8.9–34.7) | [22–27] |
| Placebo ± nab-paclitaxel | |||||||
| JapicCTI-090921 | III | Open label | Metastatic or unresectable locally advanced | Yes | Nab-paclitaxel | 23 (–)‡ | [28] |
| Docetaxel | |||||||
| KEYNOTE-355 | III | Double blind | Locally recurrent or metastatic | Yes | Pembrolizumab ± chemotherapy† | 25.9 (22.8–29.9) | [29–32] |
| Chemotherapy† | 26.3 (22.7–29.7) | ||||||
| MERiDiAN | III | Double blind | Metastatic | Yes | Placebo ± paclitaxel | 14.8 (–)‡ | [33] |
| Bevacizumab ± paclitaxel | 15.0 (–)‡ | ||||||
| tnAcity | II/III | Open label | Locally advanced inoperable or metastatic | Yes | Nab-paclitaxel ± carboplatin | – | [34] |
| Nab-paclitaxel ± gemcitabine | |||||||
| Gemcitabine ± carboplatin | |||||||
| TNT | II | Open label | Metastatic | Yes | Carboplatin | – | [35] |
| Docetaxel |
Nab-paclitaxel, paclitaxel or gemcitabine/carboplatin.
Follow-up for duration for entire trial population, including non-TNBC patients.
*Intervention not of interest.
IQR: Interquartile range; TNBC: Triple-negative breast cancer.
Treatment characteristics
Of the 18 treatment arms across the eight trials, three arms evaluated paclitaxel + bevacizumab, two arms evaluated nab-paclitaxel alone, two arms evaluated paclitaxel, two arms evaluated docetaxel, one arm evaluated carboplatin alone and the remaining arms evaluated other chemotherapy combinations. The ixabepilone + bevacizumab arm in Alliance did not meet the PICOS criteria and therefore was not included in the NMA. The nab-paclitaxel + bevacizumab arm in Alliance also did not match the PICOS criteria but was included in the NMA based on this chemotherapy combination being an approved substitute for paclitaxel for the treatment of metastatic HER2-negative breast cancer [40]. Only the pembrolizumab arm of KEYNOTE-355 and atezolizumab arm of IMpassion130 evaluated PD-1/PD-L1-directed therapies. Overall, dosing and administration schedules for treatment regimens included in the NMA were comparable (Supplementary Table 7).
Patient characteristics
Median patient age ranged from 53 to 60 years (Supplementary Table 8). Most trials included only female patients, whereas two included ≤1% males. All trials that reported race/ethnicity enrolled mostly White patients. All but one trial (TNT) enrolled patients with an ECOG performance score of 0 or 1, except for one or two patients. Thus, the distributions of patient age, sex, race/ethnicity and performance status were similar across trials. Three trials (KEYNOTE-355, IMpassion130 and tnAcity) exclusively enrolled TNBC patients, whereas the remaining trials enrolled a broader population of breast cancer patients but reported at least one efficacy outcome of interest for a TNBC subgroup. E2100 reported outcomes for patients whose tumors were ER- and PR-negative, but the proportion of patients whose tumors were HER2-negative was not reported.
All trials enrolled patients irrespective of PD-L1 expression status; however, KEYNOTE-355 and IMpassion130 reported outcomes separately for PD-L1-positive and -negative subgroups. PD-L1 expression was measured using the PD-L1 IHC 22C3 pharmDx test (Dako North America, Inc, CA, USA) in KEYNOTE-355 and the PD-L1 immunohistochemical assay (Ventana Medical Systems, IN, USA) in IMpassion130. Because these two assays do not identify comparable patient populations and PD-L1 expression was expected to modify the relative treatment effect in trials comparing a PD-L1-directed therapy to chemotherapy, data from a retrospective analysis of IMpassion130 patients identified during a targeted literature search was used in the NMA. In this retrospective analysis, a model was developed to estimate HRs for OS and PFS in IMpassion130 patients with PD-L1 CPS ≥10 [41], the threshold for determining PD-L1 positivity in KEYNOTE-355. The model was implemented by testing a subset of IMpassion130 patients with both the VENTANA SP142 assay (used to determine PD-L1 IC ≥1% status in all IMpassion130 patients) and the Dako 22C3 assay (used to determine PD-L1 CPS ≥10 status in KEYNOTE-355) and evaluating the concordance between the assays to create harmonized cutoffs.
Reported outcomes
Six trials provided a definition of OS that was similar across trials: time from study entry or randomization until death from any cause (Supplementary Table 9). Two trials (E2100 and MERiDiAN) reported OS only for a broader population of breast cancer patients and not specifically for the TNBC subgroup; thus, overall population OS data from these two trials were included in the NMA unless otherwise specified.
One trial (KEYNOTE-355) evaluated PFS with both blinded independent central review (BICR) and investigator assessment (IA), three trials evaluated PFS with IA (IMpassion130, MERiDiAN and tnAcity), and four trials did not report the method of PFS assessment (Alliance, E2100, JapicCTI-090921 and TNT). Because the effect of IA versus BICR assessment on PFS is unclear, it is generally preferred to construct networks of evidence using similar outcome assessment when possible [42,43]. To facilitate the most equitable comparisons among the three trials that evaluated PFS with IA, base case networks were constructed using IA PFS from KEYNOTE-355. The results of sensitivity analysis using BICR-assessed PFS from KEYNOTE-355 were concordant with the base case findings (data not shown).
Kaplan–Meier (KM) curves for the population of interest were available in only four trials (KEYNOTE-355, TNT, Alliance and tnAcity). Thus, as it was not possible to construct connected networks for the comparisons of interest using survival data from KM curves, NMA was conducted using reported HRs (Supplementary Table 10).
A summary of safety outcomes, which were reported by four trials, is provided in Supplementary Table 11.
Network meta-analysis
Networks were constructed to compare pembrolizumab + chemotherapy to other interventions under the assumption that PD-L1 status is only a relative treatment effect modifier for PD-1/PD-L1-directed therapies. Therefore, subgroup data for patients with a PD-L1 CPS ≥10 were used for KEYNOTE-355 and IMpassion130. KEYNOTE-355 evaluated the efficacy of pembrolizumab + chemotherapy relative to chemotherapy alone by pre-assigning all patients to investigator's choice of paclitaxel, nab-paclitaxel or gemcitabine/carboplatin; patients were then randomized to receive their chemotherapy assignment alone or in combination with pembrolizumab. In scenario 1 (Figure 2), paclitaxel and nab-paclitaxel were assumed to have different efficacy, and cohorts of KEYNOTE-355 patients assigned to nab-paclitaxel, paclitaxel or gemcitabine/carboplatin were analyzed separately. In scenario 2 (Figure 3), paclitaxel and nab-paclitaxel were assumed to have the same efficacy, and cohorts of KEYNOTE-355 patients assigned to nab-paclitaxel or paclitaxel were analyzed together. Data sources and populations for each clinical end point included in the NMA are presented in Supplementary Table 12, and statistical values (i.e., HRs, logHRs and standard errors) used in the NMA are shown in Supplementary Table 13.
Figure 2.
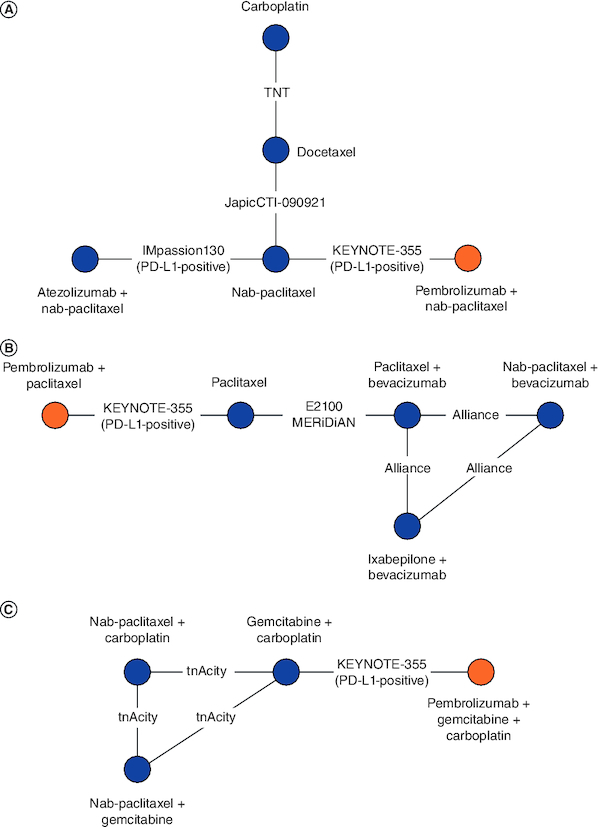
Evidence networks with KEYNOTE-355 patients assigned to nab-paclitaxel, paclitaxel and gemcitabine/carboplatin analyzed separately (scenario 1).
Cohorts of patients receiving nab-paclitaxel (A), paclitaxel (B), or gemcitabine/carboplatin (C) in KEYNOTE-355 were analyzed in separate networks, as these treatments were assumed to have different efficacies. Due to a lack of common comparators, the networks are disconnected.
Figure 3.
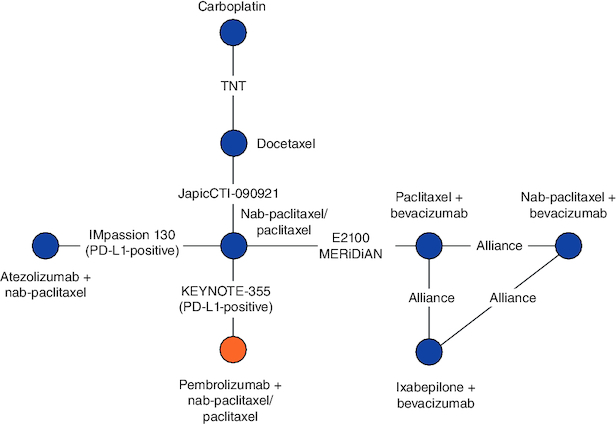
Evidence network with KEYNOTE-355 patients assigned to paclitaxel and nab-paclitaxel analyzed together (scenario 2).
Nab-paclitaxel and paclitaxel are assumed to have the same efficacy, thus cohorts of patients receiving these treatments in KEYNOTE-355 were analyzed in the same network.
KEYNOTE-355 patients assigned to nab-paclitaxel, paclitaxel & gemcitabine/carboplatin analyzed separately (scenario 1)
The networks for OS and PFS including KEYNOTE-355 patients assigned to nab-paclitaxel consisted of four trials evaluating five interventions (Figure 2A). For OS, the point estimates of the relative efficacy of pembrolizumab + nab-paclitaxel were statistically superior to that of carboplatin (HR: 0.41; 95% CrI: 0.21–0.83) and docetaxel (HR: 0.35; 95% CrI: 0.19–0.67; Table 2). Additionally, atezolizumab + nab-paclitaxel was statistically superior to carboplatin (HR: 0.51; 95% CrI: 0.28–0.91) and docetaxel (HR: 0.43; 95% CrI: 0.26–0.73). Finally, nab-paclitaxel was statistically superior to docetaxel (HR: 0.56; 95% CrI: 0.36–0.86) For PFS, the relative efficacy of pembrolizumab + nab-paclitaxel was statistically similar to that of other interventions. Atezolizumab + nab-paclitaxel was statistically superior to nab-paclitaxel (HR: 0.71; 95% CrI: 0.56–0.91).
Table 2.
Network meta-analysis including KEYNOTE-355 patients assigned to nab-paclitaxel.
| Overall survival | ||||
|---|---|---|---|---|
| Nab-paclitaxel | 1.30 (0.97, 1.75) | 0.66 (0.40, 1.09) | 0.56 (0.36, 0.86) | 1.59 (0.98, 2.57) |
| 0.77 (0.57, 1.04) | Atezolizumab + nab-paclitaxel | 0.51 (0.28, 0.91) | 0.43 (0.26, 0.73) | 1.22 (0.69, 2.16) |
| 1.52 (0.92, 2.50) | 1.97 (1.10, 3.53) | Carboplatin | 0.85 (0.66, 1.10) | 2.41 (1.20, 4.83) |
| 1.78 (1.16, 2.74) | 2.32 (1.38, 3.89) | 1.18 (0.91, 1.53) | Docetaxel | 2.84 (1.49, 5.40) |
| 0.63 (0.39, 1.02) | 0.82 (0.46, 1.44) | 0.41 (0.21, 0.83) | 0.35 (0.19, 0.67) | Pembrolizumab + nab-paclitaxel |
| Progression-free survival | ||||
|---|---|---|---|---|
| Nab-paclitaxel | 1.41 (1.10, 1.79) | 1.15 (0.73, 1.82) | 1.2 (0.81, 1.79) | 1.56 (0.99, 2.46) |
| 0.71 (0.56, 0.91) | Atezolizumab + nab-paclitaxel | 0.82 (0.49, 1.37) | 0.85 (0.54, 1.36) | 1.11 (0.66, 1.86) |
| 0.87 (0.55, 1.36) | 1.22 (0.73, 2.03) | Carboplatin | 1.04 (0.84, 1.28) | 1.35 (0.71, 2.57) |
| 0.83 (0.56, 1.24) | 1.17 (0.73, 1.87) | 0.96 (0.78, 1.19) | Docetaxel | 1.30 (0.71, 2.39) |
| 0.64 (0.41, 1.01) | 0.90 (0.54, 1.51) | 0.74 (0.39, 1.41) | 0.77 (0.42, 1.41) | Pembrolizumab + nab-paclitaxel |
Each cell represents the comparison (hazard ratio and 95% credible interval) of the row treatment vs the column treatment. All bolded values are statistically meaningful at the 0.05 level. Overall survival: deviance information criterion: 7.35; deviance: 3.35. Progression-free survival: Deviance information criterion: 7.36; deviance: 3.36.
The networks for OS and PFS including KEYNOTE-355 patients assigned to paclitaxel consisted of four trials evaluating five interventions (Figure 2B). For OS, the relative efficacy of pembrolizumab + paclitaxel was statistically superior to that of all other interventions, except nab-paclitaxel + bevacizumab (HR: 0.55; 95% CrI: 0.23–1.31; Table 3). Nab-paclitaxel + bevacizumab was statistically superior to ixabepilone + bevacizumab (HR: 0.58; 95% CrI: 0.40–0.83) and paclitaxel (HR: 0.62; 95% CrI: 0.40–0.95). For PFS, the relative efficacy of pembrolizumab + paclitaxel was statistically superior to that of paclitaxel (HR: 0.37; 95% CrI: 0.18–0.77). Nab-paclitaxel + bevacizumab was statistically superior to ixabepilone + bevacizumab (HR: 0.57; 95% CrI: 0.40–0.81) and paclitaxel (HR: 0.44; 95% CrI: 0.28–0.67). Finally, bevacizumab + paclitaxel showed statistically superior PFS versus paclitaxel (HR: 0.55; 95% CrI: 0.43–0.71).
Table 3.
Network meta-analysis including KEYNOTE-355 patients assigned to paclitaxel.
| Overall survival | ||||
|---|---|---|---|---|
| Paclitaxel | 1.20 (0.95, 1.51) | 0.94 (0.61, 1.43) | 1.62 (1.05, 2.52) | 2.95 (1.39, 6.25) |
| 0.83 (0.66, 1.05) | Bevacizumab + paclitaxel | 0.78 (0.55, 1.11) | 1.35 (0.93, 1.96) | 2.45 (1.12, 5.39) |
| 1.07 (0.70, 1.63) | 1.28 (0.90, 1.82) | Ixabepilone + bevacizumab | 1.73 (1.21, 2.48) | 3.14 (1.33, 7.47) |
| 0.62 (0.40, 0.95) | 0.74 (0.51, 1.07) | 0.58 (0.40, 0.83) | Nab-paclitaxel + bevacizumab | 1.82 (0.76, 4.34) |
| 0.34 (0.16, 0.72) | 0.41 (0.19, 0.89) | 0.32 (0.13, 0.75) | 0.55 (0.23, 1.31) | Pembrolizumab + paclitaxel |
| Progression-free survival | ||||
|---|---|---|---|---|
| Paclitaxel | 1.82 (1.41, 2.33) | 1.30 (0.85, 1.99) | 2.30 (1.48, 3.55) | 2.70 (1.29, 5.64) |
| 0.55 (0.43, 0.71) | Bevacizumab + paclitaxel | 0.72 (0.51, 1.01) | 1.27 (0.89, 1.80) | 1.49 (0.68, 3.25) |
| 0.77 (0.50, 1.17) | 1.39 (0.99, 1.96) | Ixabepilone + bevacizumab | 1.76 (1.24, 2.49) | 2.07 (0.88, 4.85) |
| 0.44 (0.28, 0.67) | 0.79 (0.55, 1.13) | 0.57 (0.40, 0.81) | Nab-paclitaxel + bevacizumab | 1.18 (0.50, 2.78) |
| 0.37 (0.18, 0.77) | 0.67 (0.31, 1.47) | 0.48 (0.21, 1.14) | 0.85 (0.36, 2.01) | Pembrolizumab + paclitaxel |
Each cell represents the comparison (HR and 95% CrI) of the row treatment vs the column treatment. All bolded values are statistically meaningful at the 0.05 level. Overall survival: deviance information criterion: 7.39; deviance: 3.38. Progression-free survival: Deviance information criterion: 7.62; deviance: 3.62.
HR: Hazard ratio; CrI: Credible interval.
The networks for OS and PFS including KEYNOTE-355 patients assigned to gemcitabine/carboplatin consisted of two trials evaluating five interventions (Figure 2C). For OS, no statistically meaningful differences between treatments were detected. For PFS, nab-paclitaxel + carboplatin was statistically superior to gemcitabine + carboplatin (HR: 0.58; 95% CrI: 0.37–0.91) and nab-paclitaxel + gemcitabine (HR: 0.59; 95% CrI: 0.41–0.86; Table 4).
Table 4.
Network meta-analysis including KEYNOTE-355 patients assigned to gemcitabine/carboplatin.
| Overall survival | |||
|---|---|---|---|
| Gemcitabine + carboplatin | 1.25 (0.82, 1.91) | 0.91 (0.66, 1.26) | 1.14 (0.79, 1.63) |
| 0.80 (0.52, 1.22) | Nab-paclitaxel + carboplatin | 0.73 (0.50, 1.07) | 0.91 (0.52, 1.58) |
| 1.10 (0.79, 1.52) | 1.37 (0.94, 2.00) | Nab-paclitaxel + gemcitabine | 1.25 (0.77, 2.02) |
| 0.88 (0.62, 1.26) | 1.10 (0.63, 1.92) | 0.80 (0.50, 1.30) | Pembrolizumab + gemcitabine/carboplatin |
| Progression-free survival | |||
|---|---|---|---|
| Gemcitabine + carboplatin | 1.72 (1.10, 2.69) | 1.02 (0.77, 1.35) | 1.21 (0.84, 1.72) |
| 0.58 (0.37, 0.91) | Nab-paclitaxel + carboplatin | 0.59 (0.41, 0.86) | 0.70 (0.40, 1.24) |
| 0.98 (0.74, 1.30) | 1.69 (1.17, 2.46) | Nab-paclitaxel + gemcitabine | 1.18 (0.75, 1.86) |
| 0.83 (0.58, 1.18) | 1.43 (0.81, 2.53) | 0.84 (0.54, 1.33) | Pembrolizumab + gemcitabine/carboplatin |
Each cell represents the comparison (HR and 95% CrI) of the row treatment vs the column treatment. All bolded values are statistically meaningful at the 0.05 level. Overall survival: deviance information criterion: 5.25; deviance: 2.25. Progression-free survival: Deviance information criterion: 5.25; deviance: 2.25.
HR: Hazard ratio; CrI: Credible interval.
KEYNOTE-355 patients assigned to nab-paclitaxel & paclitaxel analyzed together (scenario 2)
The networks for OS and PFS including KEYNOTE-355 patients assigned to nab-paclitaxel and paclitaxel combined consisted of seven trials evaluating eight interventions (Figure 3). For OS, pembrolizumab + nab-paclitaxel/paclitaxel was statistically superior to that of all other interventions, except atezolizumab + nab-paclitaxel (HR: 0.70; 95% CrI: 0.42–1.17), bevacizumab + paclitaxel (HR: 0.65; 95% CrI: 0.40–1.04) and nab-paclitaxel + bevacizumab (HR: 0.88; 95% CrI: 0.48–1.60). Atezolizumab + nab-paclitaxel was statistically superior to carboplatin (HR: 0.51; 95% CrI: 0.29–0.89) and docetaxel (HR: 0.43; 95% CrI: 0.26–0.71). Nab-paclitaxel + bevacizumab was statistically superior to ixabepilone + bevacizumab (HR: 0.58; 95% CrI: 0.40–0.83), docetaxel (HR: 0.35; 95% CrI: 0.19–0.63), carboplatin (HR: 0.41; 95% CrI: 0.21–0.78) and nab-paclitaxel/paclitaxel (HR: 0.62; 95% CrI: 0.40–0.95). Finally, bevacizumab + paclitaxel showed a statistically meaningful improvement over carboplatin (HR: 0.55; 95% CrI: 0.32–0.94) and docetaxel (HR: 0.47; 95% CrI: 0.29–0.74; Table 5). For PFS, the relative efficacy of pembrolizumab + nab-paclitaxel/paclitaxel was statistically superior to that of nab-paclitaxel/paclitaxel (HR: 0.58; 95% CrI: 0.39–0.86). Atezolizumab + nab-paclitaxel was statistically superior to nab-paclitaxel/paclitaxel (HR: 0.71; 95% CrI: 0.56–0.91). Bevacizumab + nab-paclitaxel was statistically superior to ixabepilone + bevacizumab (HR: 0.57; 95% CrI: 0.40–0.81), docetaxel (HR: 0.52; 95% CrI: 0.29–0.95), carboplatin (HR: 0.50; 95% CrI: 0.27–0.94) and nab-paclitaxel/paclitaxel (HR: 0.44; 95% CrI: 0.28–0.67). Finally, bevacizumab + paclitaxel showed a statistically meaningful improvement in PFS relative to nab-paclitaxel/paclitaxel (HR: 0.55; 95% CrI: 0.43–0.71).
Table 5.
Network meta-analysis including KEYNOTE-355 patients assigned to nab-paclitaxel or paclitaxel combined.
| Overall survival | |||||||
|---|---|---|---|---|---|---|---|
| Nab-paclitaxel/paclitaxel | 1.30 (0.97, 1.75) | 1.20 (0.95, 1.51) | 0.66 (0.41, 1.06) | 0.56 (0.37, 0.84) | 0.94 (0.61, 1.43) | 1.62 (1.05, 2.52) | 1.85 (1.23, 2.79) |
| 0.77 (0.57, 1.04) | Atezolizumab + nab-paclitaxel | 0.92 (0.63, 1.35) | 0.51 (0.29, 0.89) | 0.43 (0.26, 0.71) | 0.72 (0.43, 1.21) | 1.25 (0.74, 2.12) | 1.42 (0.86, 2.37) |
| 0.83 (0.66, 1.05) | 1.08 (0.74, 1.58) | Bevacizumab + paclitaxel | 0.55 (0.32, 0.94) | 0.47 (0.29, 0.74) | 0.78 (0.55, 1.11) | 1.35 (0.93, 1.96) | 1.54 (0.96, 2.47) |
| 1.52 (0.94, 2.45) | 1.97 (1.12, 3.47) | 1.82 (1.07, 3.10) | Carboplatin | 0.85 (0.66, 1.10) | 1.42 (0.75, 2.69) | 2.46 (1.29, 4.71) | 2.81 (1.49, 5.26) |
| 1.79 (1.19, 2.67) | 2.32 (1.40, 3.84) | 2.14 (1.34, 3.42) | 1.18 (0.91, 1.52) | Docetaxel | 1.68 (0.93, 3.00) | 2.90 (1.59, 5.25) | 3.31 (1.86, 5.87) |
| 1.07 (0.70, 1.63) | 1.38 (0.83, 2.31) | 1.28 (0.90, 1.82) | 0.70 (0.37, 1.33) | 0.60 (0.33, 1.07) | Ixabepilone + bevacizumab | 1.73 (1.20, 2.48) | 1.97 (1.09, 3.55) |
| 0.62 (0.40, 0.95) | 0.80 (0.47, 1.36) | 0.74 (0.51, 1.07) | 0.41 (0.21, 0.78) | 0.35 (0.19, 0.63) | 0.58 (0.40, 0.83) | Nab-paclitaxel + bevacizumab | 1.14 (0.62, 2.08) |
| 0.54 (0.36, 0.82) | 0.70 (0.42, 1.17) | 0.65 (0.40, 1.04) | 0.36 (0.19, 0.67) | 0.30 (0.17, 0.54) | 0.51 (0.28, 0.92) | 0.88 (0.48, 1.60) | Pembrolizumab + nab-paclitaxel/paclitaxel |
| Progression-free survival | |||||||
|---|---|---|---|---|---|---|---|
| Nab-paclitaxel/paclitaxel | 1.41 (1.10, 1.80) | 1.81 (1.41, 2.33) | 1.15 (0.73, 1.81) | 1.20 (0.80, 1.79) | 1.30 (0.86, 2.00) | 2.30 (1.48, 3.55) | 1.72 (1.17, 2.55) |
| 0.71 (0.56, 0.91) | Atezolizumab + nab-paclitaxel | 1.29 (0.91, 1.83) | 0.82 (0.49, 1.37) | 0.85 (0.53, 1.36) | 0.93 (0.57, 1.51) | 1.63 (0.99, 2.68) | 1.22 (0.77, 1.94) |
| 0.55 (0.43, 0.71) | 0.78 (0.55, 1.10) | Bevacizumab + paclitaxel | 0.64 (0.38, 1.07) | 0.66 (0.41, 1.06) | 0.72 (0.51, 1.01) | 1.27 (0.89, 1.81) | 0.95 (0.60, 1.51) |
| 0.87 (0.55, 1.36) | 1.22 (0.73, 2.04) | 1.57 (0.94, 2.63) | Carboplatin | 1.04 (0.84, 1.28) | 1.13 (0.61, 2.11) | 1.99 (1.06, 3.74) | 1.50 (0.82, 2.72) |
| 0.83 (0.56, 1.25) | 1.18 (0.73, 1.87) | 1.51 (0.94, 2.43) | 0.96 (0.78, 1.19) | Docetaxel | 1.09 (0.61, 1.95) | 1.91 (1.06, 3.46) | 1.44 (0.82, 2.51) |
| 0.77 (0.50, 1.17) | 1.08 (0.66, 1.76) | 1.39 (0.99, 1.96) | 0.88 (0.47, 1.64) | 0.92 (0.51, 1.65) | Ixabepilone + bevacizumab | 1.76 (1.24, 2.49) | 1.32 (0.74, 2.35) |
| 0.44 (0.28, 0.67) | 0.61 (0.37, 1.01) | 0.79 (0.55, 1.13) | 0.50 (0.27, 0.94) | 0.52 (0.29, 0.95) | 0.57 (0.40, 0.81) | Bevacizumab + nab-paclitaxel | 0.75 (0.42, 1.35) |
| 0.58 (0.39, 0.86) | 0.82 (0.51, 1.29) | 1.05 (0.66, 1.67) | 0.67 (0.37, 1.22) | 0.70 (0.40, 1.22) | 0.76 (0.43, 1.35) | 1.33 (0.74, 2.39) | Pembrolizumab + nab-paclitaxel/paclitaxel |
Each cell represents the comparison (HR and 95% CrI) of the row treatment vs the column treatment. All bolded values are statistically meaningful at the 0.05 level. Overall survival: deviance information criterion: 13.37; deviance: 6.38. Progression-free survival: Deviance information criterion: 13.67; deviance: 6.66.
HR: Hazard ratio; CrI: Credible interval.
Sensitivity analysis was also performed for a network for OS that did not include E2100 and MERiDiAN because these trials reported OS for the overall breast cancer population rather than the TNBC subgroup. In this analysis, the relative efficacy of pembrolizumab + nab-paclitaxel/paclitaxel in terms of OS was statistically superior to that of all other interventions, except atezolizumab + nab-paclitaxel (Supplementary Tables 14 & 15).
Discussion
A total of 19 records pertaining to eight unique RCTs that enrolled patients with locally recurrent inoperable or metastatic TNBC receiving first-line treatment were identified through a systematic literature search. These trials were assessed for their feasibility of inclusion in NMA, and networks of evidence were constructed to compare pembrolizumab + chemotherapy to other interventions. Further, separate evidence networks were constructed based on patient assignment to nab-paclitaxel, paclitaxel, nab-paclitaxel/paclitaxel combined or gemcitabine/carboplatin in KEYNOTE-355.
Considering OS, NMA showed that the relative treatment efficacy of pembrolizumab + different chemotherapy agents (nab-paclitaxel, paclitaxel, nab-paclitaxel/paclitaxel combined or gemcitabine/carboplatin) was statistically superior to that of carboplatin, docetaxel, paclitaxel, bevacizumab + paclitaxel, ixabepilone + paclitaxel or ixabepilone + bevacizumab depending on the evidence network analyzed. Considering PFS, the relative treatment efficacy of pembrolizumab + different chemotherapy agents (nab-paclitaxel, paclitaxel, nab-paclitaxel/paclitaxel combined or gemcitabine/carboplatin) was statistically superior to that of paclitaxel or nab-paclitaxel/paclitaxel combined depending on the evidence network analyzed. In the absence of clear evidence for the superiority of nab-paclitaxel over paclitaxel for TNBC patients in the first-line setting [44], we ran our analyses in two scenarios – one assuming different efficacy and one assuming the same efficacy of nab-paclitaxel and paclitaxel and found similar overall relative efficacies of pembrolizumab + chemotherapy between scenarios. Also, despite no evidence of the statistical superiority of pembrolizumab + chemotherapy over atezolizumab + chemotherapy, a numerical benefit of pembrolizumab + chemotherapy versus atezolizumab + chemotherapy was observed in all models comparing these two PD-1/PD-L1-directed therapies. Overall, these results suggest that as a first-line treatment for locally recurrent unresectable or metastatic TNBC, pembrolizumab + chemotherapy is more efficacious in improving survival outcomes compared with other interventions, with greater improvements observed for OS than for PFS.
Several factors limit the conclusions that can be drawn from this study. First, the use of subgroup data from several comparator trials precludes comparison of baseline patient characteristics for the population of interest. Five of the included trials enrolled a broader population of breast cancer patients, and although subgroup data for TNBC patients were used in the analyses when possible, baseline patient characteristics were not reported by subgroup. Therefore, this study assumed that the baseline patient characteristics for the overall study population reflect those of the TNBC subgroup. Second, the HRs in the PD-L1 CPS ≥10 population of IMpassion130 were derived in a post hoc analysis employing a model to estimate HRs for OS and PFS in all patients with CPS ≥10 as determined with IHC 22C3 pharmDx [41]. Third, because of the different chemotherapy partner options evaluated in KEYNOTE-355, networks were constructed based on chemotherapy pre-assignment. This reduced the sample size for the KEYNOTE-355 cohorts included in each analysis network and thereby decreased statistical power. Fourth, limited data availability for each comparison included in the networks restricted the analyses in several ways. Because only one trial connected each treatment in the network of evidence, between-study heterogeneity could not be estimated. Therefore, NMA was performed with a fixed-effects assumption. Fifth, most trials did not provide KM curves for the population of interest, precluding evaluation of the proportional hazards assumption. Therefore, NMA was conducted with a proportional hazard ratios model, which may not reflect variability in HRs between treatments over time. Furthermore, whereas the age and sex distributions of patients enrolled in the included trials were similar to those in the general TNBC patient population [45,46], White patients were overrepresented in most trials, thus limiting the generalizability of the results of the individual trials, as well as the NMA, to patients of other races/ethnicities.
Despite these limitations, this study also has several strengths that maximize its comprehensiveness and rigor. First, highly sensitive systematic searches in the peer-reviewed literature, recent conferences and clinical trial registries were employed to identify all published evidence from RCTs of first-line treatment for locally recurrent unresectable or metastatic TNBC. Second, the review process followed PRISMA guidelines, was guided by pre-defined eligibility criteria, and involved two independent reviewers in the study selection and data extraction processes to reduce bias in study selection and ensure data accuracy. Third, detailed feasibility assessment was conducted before proceeding with the NMA to confirm that there were minimal differences among trials included in the network that would jeopardize the validity or reliability of the analyses. Fourth, we performed scenario analyses to explore the impact of potentially different treatment efficacy between paclitaxel and nab-paclitaxel as well as sensitivity analysis to assess the impact of excluding trials that reported OS for the overall breast cancer population rather than the TNBC subgroup.
Conclusion
In conclusion, the results of this NMA suggest that among patients with locally recurrent inoperable or metastatic TNBC, first-line pembrolizumab + chemotherapy confers greater benefits in OS versus first-line carboplatin, docetaxel, paclitaxel, bevacizumab + paclitaxel, ixabepilone + paclitaxel or ixabepilone + bevacizumab and in PFS versus first-line paclitaxel or nab-paclitaxel/paclitaxel depending on the evidence network analyzed. These findings may be of value to several stakeholders including oncologists and cancer patients, health technology assessment agencies, policymakers, oncology drug developers and medical researchers. In particular, oncologists could use this information in the treatment decision-making process and to communicate with their patients, and health technology assessment agencies and policymakers could use these results as clinical benchmarks for evaluating relative efficacy of available first-line treatments for advanced TNBC patients.
Supplementary Material
Acknowledgments
We thank D Nayak (PRECISIONheor) for her contributions to this study.
Author contributions
Conception and design (all authors); acquisition and collection of data (KG Akers, AM Frederickson); statistical analyses (D Maciel); interpretation of data (all authors); draft manuscript (KG Akers, AM Frederickson); critical review of manuscript (all authors). All authors have read and approved the final version of this manuscript.
Financial disclosure
A Haiderali, M Huang and W Pan are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. KG Akers, D Maciel and AM Frederickson are employees of PRECISIONheor, a healthcare research consultancy that received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA to conduct the research described in this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Previous presentation
This work was previously presented at the 2022 National Comprehensive Cancer Network conference [46].
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Sung H, Ferlay J, Siegel RLet al. . Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clinic 71(3), 209–249 (2021). [DOI] [PubMed] [Google Scholar]; • Uses GLOBOCAN 2020 data to estimate the global burden of cancer, including the incidence and mortality rate of breast cancer.
- 2.Yao HH, Yan G, Chen Set al. . Triple-negative breast cancer: is there a treatment on the horizon? Oncotarget 8(1), 1913 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This review article summarizes the therapeutic options available to triple-negative breast cancer (TNBC) patients, including cytotoxic chemotherapies, targeted therapies and statins.
- 3.Mayer IaA, Lehmann VG, Pietenpol BD, Jennifer A. New strategies for triple-negative breast cancer-deciphering the heterogeneity. Clin. Cancer Res. 20(4), 782–790 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This review article characterizes six distinct TNBC subtypes and their potential targeting by different therapies.
- 4.Planes-Laine G, Rochigneux P, Bertucci Fet al. . PD-1/PD-L1 Targeting in Breast Cancer: The First Clinical Evidences are Emerging-A Literature Review. Cancers 11(7), 1033 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This review article summarizes the results of studies evaluating the efficacy of anti-PD-(L)1 agents against breast cancer, with a particular emphasis on TNBC.
- 5.FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple (22 February 2024).
- 6.EMA Recommends Extension of Indications for Pembrolizumab: new indication concerns the treatment of PD-L1 positive locally recurrent unresectable or metastatic TNBC. www.esmo.org/oncology-news/ema-recommends-extension-of-indications-for-pembrolizumab6 (22 February 2024).
- 7.Ades AE. A chain of evidence with mixed comparisons: models for multi-parameter synthesis and consistency of evidence. Stat. Med. 22(19), 2995–3016 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 331(7521), 897–900 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 23(20), 3105–3124 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Mills EJ, Thorlund K, Ioannidis JPA. Demystifying trial networks and network meta-analysis. BMJ 346, f2914 (2013). [DOI] [PubMed] [Google Scholar]; •• Provides an easy-to-understand explanation of network meta-analysis (NMA) methodology and how to interpret NMA results using a practical example in cardiology.
- 11.Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta-analysis. JAMA 308(12), 1246–1253 (2012). [DOI] [PubMed] [Google Scholar]; •• Provides a ‘how to’ guide for clinicians seeking to evaluate or interpret an NMA to inform medical decision-making.
- 12.Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. 11(1), 159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 62(10), 1006–1012 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thomas J, Chandler Jet al. . Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition John Wiley & Sons; Chichester (UK) (2019). [Google Scholar]
- 15.Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. 11, 159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cope S, Zhang J, Saletan S, Smiechowski B, Jansen JP, Schmid P. A process for assessing the feasibility of a network meta-analysis: a case study of everolimus in combination with hormonal therapy versus chemotherapy for advanced breast cancer. BMC Med. 12, 93 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper NJ, Sutton AJ, Morris D, Ades AE, Welton NJ. Addressing between-study heterogeneity and inconsistency in mixed treatment comparisons: application to stroke prevention treatments in individuals with non-rheumatic atrial fibrillation. Stat. Med. 28(14), 1861–1881 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med. Decis. Making 33(5), 607–617 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen JP, Fleurence R, Devine Bet al. . Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 14(4), 417–428 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Dias S, Welton NJ, Sutton AJ, Ades A. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials[Internet]. London: National Institute for Health and Care Excellence (NICE); 2014 Apr (2011). Available from: www.ncbi.nlm.nih.gov/books/NBK310366/ [PubMed]
- 21.R Development Core Team . R: a language and environment for statistical computing. (2020). www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing
- 22.Rugo HSB, Moreno-Aspitia WT, Lyss Aet al. . Randomized Phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance). J. Clin. Oncol. 33(21), 2361 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rugo HSB, Moreno-Aspitia WT, Lyss Aet al. . Long-term follow-up of CALGB 40502/NCCTG N063H (Alliance): a randomized Phase III trial of weekly paclitaxel (P) compared to weekly nanoparticle albumin bound nab-Paclitaxel (NP) or ixabepilone (Ix) +/- bevacizumab as first-line therapy for locally recurrent or metastatic breast cancer (MBC). Cancer Res. 78(4), GS3-06 (2018). https://aacrjournals.org/cancerres/article/78/4_Supplement/GS3-06/631967/Abstract-GS3-06-Long-term-follow-up-of-CALGB-40502?searchresult=1 [Google Scholar]
- 24.Miller K, Wang M, Gralow Jet al. . Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Eng. J. Med. 357(26), 2666–2676 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Schmid PA, Rugo S, Schneeweiss HSet al. . Mpassion130 trial investigators. Atezolizumab and Nab-Paclitaxel in advanced triple-negative breast cancer. N. Eng. J. Med. 379(22), 2108–2121 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Schmid P, Rugo HS, Adams Set al. . Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, Phase III trial. Lancet Oncol. 21(1), 44–59 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Adams S, Dieras V, Barrios CHet al. . Patient-reported outcomes from the Phase III IMpassion130 trial of atezolizumab plus nab-paclitaxel in metastatic triple-negative breast cancer. Ann. Oncol. 31(5), 582–589 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Emens LA, Adams S, Barrios CHet al. . IMpassion130: final OS analysis from the pivotal Phase III study of atezolizumab + nab-paclitaxel vs placebo + nab-paclitaxel in previously untreated locally advanced or metastatic triple-negative breast cancer. Ann. Oncol. 31, S1148 (1148). [Google Scholar]
- 29.Emens LA, Adams S, Barrios CHet al. . First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann. Oncol. 32(8), 983–993 (2021). [DOI] [PubMed] [Google Scholar]; • This randomized controlled trial shows that pembrolizumab + chemotherapy prolongs progression-free survival compared with placebo + chemotherapy in patients with metastatic TNBC with a PD-L1 combined positive score of ≥10.
- 30.Emens LA, Molinero L, Loi Set al. . Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. J. Natl Cancer Inst. 113(8), 1005–1016 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura K, Inoue K, Masuda Net al. . Randomized Phase II study of nab-paclitaxel as first-line chemotherapy in patients with HER2-negative metastatic breast cancer. Cancer Sci. 108(5), 987–994 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cortes J, Cescon DW, Rugo HSet al. . KEYNOTE-355: randomized, double-blind, Phase III study of pembrolizumab+chemotherapy versus placebo+chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J. Clin. Oncol. 38(15), 1000 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Cortes J, Cescon DW, Rugo HSet al. . Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, Phase III clinical trial. Lancet 396(10265), 1817–1828 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Yusof MM, Cescon DW, Rugo HSet al. . 43O Phase III KEYNOTE-355 study of pembrolizumab (pembro) vs placebo (pbo) + chemotherapy (chemo) for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (TNBC): results for patients (Pts) enrolled in Asia. Ann. Oncol. 31(6), S1257 (2020). [Google Scholar]
- 35.Rugo HS, Schmid P, Cescon DWet al. . Additional efficacy endpoints from thePhase III KEYNOTE-355 study of pembrolizumab pluschemotherapy vs placebo plus chemotherapy as first-linetherapy for locally recurrent inoperable or metastatic triple-negative breast cancer. Cancer Res. 81(Suppl. 4), GS3-01 (2021). [Google Scholar]
- 36.Miles D, Cameron D, Bondarenko Iet al. . Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): a double-blind placebo-controlled randomised Phase III trial with prospective biomarker evaluation. Eur. J. Cancer 70, 146–155 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Yardley DA, Coleman R, Conte Pet al. . nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: results from the tnAcity trial. Ann. Oncol. 29(8), 1763–1770 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tutt A, Tovey H, Cheang MCUet al. . Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat. Med. 24(5), 628–637 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breast Cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (April 5, 2022).
- 40.Rugo HS, Loi S, Adams Set al. . PD-L1 immunohistochemistry assay comparison in atezolizumab plus nab-paclitaxel-treated advanced triple-negative breast cancer. J. Natl Cancer Inst. 113(12), 1733–1743 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dello Russo C, Cappoli N, Navarra P. A comparison between the assessments of progression-free survival by local investigators versus blinded independent central reviews in Phase III oncology trials. Eur. J. Clin. Pharmacol. 76(8), 1083–1092 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Jones CF, Barrientos JFS, Monnickendam G. Investigating discrepancies in assessments of PFS by study investigators and independent review. Ann. Oncol. 28(Suppl. 5), v395–v402 (2017). [Google Scholar]
- 43.Schettini F, Giuliano M, De Placido S, Arpino G. Nab-paclitaxel for the treatment of triple-negative breast cancer: rationale, clinical data and future perspectives. Cancer Treat. Rev. 50, 129–141 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Giordano SH. Breast cancer in men. N. Eng. J. Med. 378(24), 2311–2320 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Scott LC, Mobley LR, Kuo TM, Il'yasova D. Update on triple-negative breast cancer disparities for the United States: a population-based study from the United States Cancer Statistics database, 2010 through 2014. Cancer 125(19), 3412–3417 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haiderali A, Huang M, Pan W, Fox G, Maciel D, Frederickson A. HSR22-145: Pembrolizumab plus chemotherapy for first-line treatment of advanced triple-negative breast cancer – a network meta-analysis. J. Natl Comprehen. Cancer Netw. 20(3.5), HSR22-145–HSR122-145 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


