Abstract
Rapidly advancing evidence documents that a broad array of synthetic chemicals found ubiquitously in the environment contribute to disease and disability across the lifespan. While the early literature focused on early life exposures, endocrine-disrupting chemicals (EDCs) are now understood to contribute substantially to chronic disease in adulthood, especially metabolic, cardiovascular, and reproductive consequences as well as endocrine cancers. The contribution to mortality is substantial, with over 90,000 deaths annually and at least $39 billion/year in lost economic productivity in the United States (US) due to exposure to certain phthalates that are used as plasticizers in food packaging. Importantly, exposures are disproportionately high in low-income and minoritized populations, driving disparities in these conditions. Though non-Hispanic Blacks and Mexican Americans comprise 12.6% and 13.5% of the US population, they bear 16.5% and 14.6% of the disease burden due to EDCs, respectively. Many of these exposures can be modified through safe and simple behavioral changes supported by proactive government action to limit known hazardous exposures coupled with proactive screening of new industrial chemicals prior to their use. Routine healthcare maintenance should include guidance to reduce EDC exposures, and a recent report by the Institute of Medicine suggests that testing be conducted, particularly in populations heavily exposed to perfluoroalkyl substances—chemicals used in nonstick coatings as well as oil- and water-resistant clothing.
Keywords: endocrinology, epidemiology, environmental medicine
Introduction
Silent Spring was not a text routinely included in the medical school curriculum of the 1960s, when Rachel Carson warned about the consequences of widespread use of dichlorodiphenyltrichloroethane (DDT).[1] Except for malaria prevention in endemic regions, DDT is rarely used today; however, Carson’s prescient warnings have implications for the present-day internist. Indeed, substantial evidence suggests that pesticides used as DDT replacements contribute to myeloid leukemias and colorectal cancer.[2] While further studies are needed to better characterize the carcinogenicity of newer pesticides, one longitudinal study has associated greater organic food consumption with reduced cancer risk.[3]
Since Carson’s landmark work, roughly one to three thousand new synthetic chemicals have been approved for industrial use annually. Current estimates suggest 300,000 synthetic chemicals are used to create consumer products,[4] and many of these chemicals were introduced without testing for safety, particularly for effects on endocrine systems.[5] In 2009, the Endocrine Society published its first scientific statement describing how a broad suite of actively used chemicals impaired endocrine function with consequences for neurodevelopment, metabolism, reproduction, and carcinogenesis.6 This was soon followed by a World Health Organization joint report with the United Nations Environment Programme (UNEP) that identified EDCs as a global public health issue.7 A second Endocrine Society scientific statement followed in 2015,8 and the Strategic Alliance for International Chemicals Management—the global multi-sectoral and multi-stakeholder policy framework hosted by UNEP—“welcomed” the WHO-UNEP report, acknowledging only disagreement from the chemical and pesticide industry.9
The Endocrine Society defines EDCs as “exogenous chemical[s], or mixture[s] of chemicals, that interfere…with any aspect of hormone action.” 10 To date, the US Food and Drug Administration has identified more than 1800 chemicals that disrupt at least one of three endocrine pathways (estrogen, androgen, and thyroid).11 The actual number of EDCs is likely to be much higher, as very few chemicals have been tested for endocrine disruption, and as other receptors and the broader suite of human hormones are considered. We now appreciate that receptor binding is but one path by which hormone action can be interfered. Indeed, a recent analysis identified 10 key characteristics of EDCs, 12 with some mechanisms such as epigenetic effects on the endocrine system only recently coming under interrogation (Figure 1). 13
Figure 1.
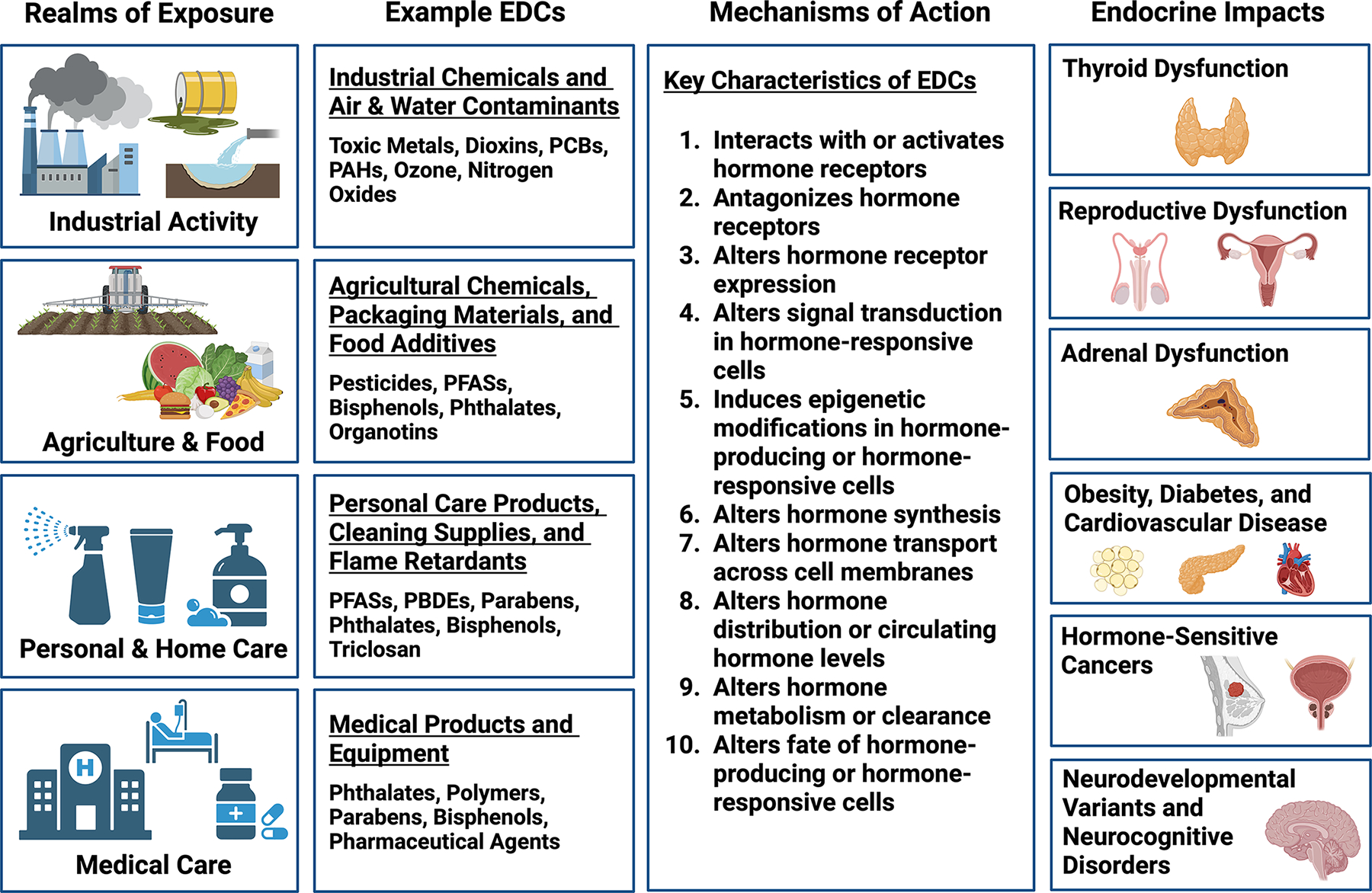
Endocrine-Disrupting Chemicals (EDCs): Sources, Mechanisms, and Impacts. This figure summarizes the realms in which EDC exposures occur, categories of EDCs and representative compounds, key characteristics by which EDCs disrupt endocrine function, and health impacts associated with EDC exposures. EDCs, endocrine-disrupting chemicals; PAHs, polycyclic aromatic hydrocarbons; PBDEs, polybrominated diphenyl ethers; PCBs, polychlorinated biphenyls; and PFASs, per/polyfluoroalkyl substances. Created with BioRender.com.
The early evidence documenting effects of endocrine-disrupting chemicals (EDCs) linked pregnancy-associated exposures with neurodevelopmental disabilities in exposed children. 14 Subsequent studies documented consequences of early life exposures that included obesity and diabetes 15 as well as alterations in male 16 and female 17 reproductive development. While these studies focused upon exposures during early life, substantial evidence has accumulated indicating that the entire lifespan is a window of EDC susceptibility. This perspective focuses on effects that have been identified as a result of adult EDC exposure. Furthermore, it describes safe and simple steps people can take to limit their exposures.
Obesity, Diabetes, and Cardiovascular Disease
There are now more than 50 chemical “obesogens.” 18 A fungicide used in marine paints and now a known contaminant of many plastic containers, tributyltin is arguably the first and prototypical obesogen with a well-characterized mechanism of action—activation of peroxisome proliferator-activated receptor-γ signaling—through which it promotes adipogenesis and increases adipose mass. 19 Furthermore, in experimental models, tributyltin’s metabolic effects have been shown to be epigenetically transmitted across multiple unexposed generations. 20
Adult exposures to synthetic chemicals are also known to increase weight gain and contribute to diabetes independent of well-known risks such as caloric excess and physical inactivity. Serum levels of per- and polyfluoroalkyl substances (PFAS) used in nonstick cooking materials and oil- and water-resistant clothing were shown to augment weight regain after a successful weight loss intervention, possibly due to reductions in resting metabolic rate. 21 In the Diabetes Prevention Program trial, total PFAS was associated with increased weight gain in the control group. 22 Studies from multiple countries have also identified increases in incident diabetes in association with antecedent measures of serum PFAS. 23–25 Bisphenols used in aluminum can linings and thermal paper receipts such as bisphenol A (BPA) and one of its emerging replacements, bisphenol S (BPS), have been associated with incident diabetes in a nested case-control study within a large representative French cohort.26
The cardiovascular consequences of these exposures are also substantial. BPA is known to reduce levels of the cardioprotective adipokine adiponectin 27 and promote oxidative stress, 28–31 a major mechanism underlying atherosclerosis and cardiovascular events. In human studies, BPA has been associated with greater carotid intimal media thickness, 32 severity of coronary artery disease on angiography,33 and reduced heart rate variability.34 Linking national representative surveys from Americans to death records, Bao et al. identified substantially increased risk for all-cause and cardiovascular, but not cancer, mortality among those with higher urinary BPA levels.35 Phthalates are EDCs known to antagonize testosterone action,36 and concerns have been raised about consequential cardiovascular risks given that low testosterone is a marker for or predictor of mortality.37 Furthermore, phthalates are potent inducers of oxidative stress,38,39 and thus may augment cardiovascular disease risk in both sexes. Indeed, linkage of urinary phthalate levels with death records revealed increases in cardiovascular mortality, independent of the increase observed with phthalates, in both sexes, suggesting approximately 90,761–107,283 preventable deaths annually in the US. 40
Male Reproduction
In animals, the distance from the anus to the genital tubercle has been identified as a sensitive biomarker of androgen action. 41,42 In males, this anogenital distance (AGD) is typically measured from the center of the anus to the anterior base of the penis (anopenile distance), or from the center of the anus to the posterior base of the scrotum (anoscrotal distance). During a “masculine programming window”43 corresponding to weeks 8–14 of gestation in humans, perineal growth and caudal migration of the tubercle in rodents relies upon androgen function, and can be disrupted by antiandrogens.44
Shortened AGD in boys due to antiandrogenic phthalate exposure is among the best described effects on reproductive development with independent observations from two birth cohorts, The Infant Development and Environment Study (TIDES)45 and the Swedish Environmental Longitudinal, Mother and Child, Asthma and Allergy (SELMA) study,46 which showed decreased anogenital distance in boys born to mothers with higher urinary levels of antiandrogenic phthalates in the first trimester of pregnancy.
Minipuberty in infancy can also be influential in shaping AGD, 47,48 and studies suggest that AGD in animals tracks thereafter to adulthood. 49 Shortened adult AGD is strongly predictive of sperm count, with each 1cm increase in AGD associated with a 4.3 million increase in sperm density per mL, strongly predicting reproductive potential. 50 A study of young adults from the general population confirmed these associations (with a doubling of risk for subfertile sperm count in the lowest 10 percentile of AGD), noting the absence of an association of AGD with contemporaneously measured sex hormones or testicular size. Together, these studies suggest a strong but intermediate link of prenatal phthalate exposure with reductions in sperm count, with AGD as an intermediate biomarker. 51 This is consistent with the concept of the testicular dysgenesis syndrome originally described by Skakkebaek, 52 in which cryptorchidism, hypospadias, and testicular cancer can arise out of decreased production of testosterone and the neohormone insulin-like factor 3 during a susceptible window of male reproductive development.53
However, multiple studies suggest that later-life exposure to EDCs can impair sperm concentration, motility, and morphology. These studies are generally cross-sectional and do not contain additional information on in utero and early life exposures. Despite these limitations, phthalates have been reported to have negative associations with one or more semen quality parameters, 15 with similar disruptions also reported for BPA, 54 BPS, 55 organophosphate pesticides, 56–58 and PFAS. 59,60 A modest number of studies have also identified increased risks for reproductive cancer. Occupational exposure to persistent pesticides has been associated with prostate cancer. 61,62 Maternal levels of brominated flame retardants have been associated with testicular cancer in their sons. 16,63 While further study is needed, PFAS exposure has been linked to kidney and testicular cancer as well as potentially prostate cancer. 64
Female Reproduction
Buck Louis et al. described an analogous ovarian dysgenesis syndrome in 2007. 65 Similar to the testicular dysgenesis syndrome, disruptions of the developing reproductive tract—including the ovary, fallopian tubes, and uterus—occur in utero; however, external phenotypes are not readily appreciated. The clinical manifestations are equally significant even if their documentation is delayed.
Epidemiologic evidence of EDC effects on female reproduction has chiefly been based on measures of adult exposure, and data have somewhat lagged behind studies of male reproductive effects. Phthalates 66 and PFAS 67 have been associated with endometriosis, and case-control studies have implicated PFAS and bisphenols with increased risk for polycystic ovarian syndrome. 68–75 The effects of BPA are perhaps not surprising in light of concordant laboratory evidence of its ovarian toxicity. 76
Multiple studies have also shown EDCs to contribute to breast cancer risk. In the Child Health and Development Studies, prenatal exposure to N-ethyl-perfluorooctane sulfonamidoacetic acid was associated with breast cancer in daughters. 77 Other studies have revealed associations of adult PFAS exposure with newly incident breast cancers. 78,79 Pesticide exposure studies have also revealed increases in cancer risk, but none are based on biological specimens; evidence for phthalates is similarly limited. 15
Disparities in EDC Exposures
Many endocrine disorders are characterized by marked disparities in disease prevalence, complications, and/or mortality—including metabolic, thyroid, and reproductive disorders. 80–84 In most instances, Black, Hispanic/Latinx, Indigenous, and low-income communities are disproportionately burdened. While recognition of the social/structural determinants of health (SDoH) has improved our understanding of the origins of these disparities, SDoH discussions often focus on educational attainment, economic opportunity, healthy food and activity ecosystems, and access to health care, 85 without substantial regard for disparities in exposures to EDCs. This is a missed opportunity as minoritized and low-income communities are known to be disproportionately exposed to a variety of toxicants linked to endocrine dysfunction, including polychlorinated biphenyls (PCBs), phthalates, bisphenols, organochlorine pesticides, air pollution, PFAS, toxic metals, and brominated flame retardants (reviewed in ref. 86). The drivers of these disparities are myriad but include neighborhood segregation, racialized labor and beauty practices, grandfathering clauses for industrial sites, suburbanization, and urban disinvestment, all of which are rooted in intersectional systems of oppression. 87,88 While the bases of these exposure disparities have deep historical roots and are nurtured by current systems of power, unlike genetics, environments are modifiable; thus, improving environmental health has the potential to mitigate endocrine health disparities.
Disease Burden and Costs due to EDCs
Expert panels organized by the Endocrine Society conservatively estimated the costs of adult diseases (Table 1) in the European Union attributable to EDCs. 89,90 Teresa Attina et al. expanded these to the US in 2016, 91 and Julia Malits et al. expanded these to Canada in 2022. 92 Trasande et al. have also estimated cardiovascular mortality due to phthalates, with over 90,000 deaths annually and at least $39 billion/year in lost economic productivity in the US alone. 40 Vladislav Obsekov et al. estimated PFAS costs in the United States. 93 Moreover, minoritized populations disproportionately account for EDC-associated healthcare costs. Though non-Hispanic Blacks and Mexican Americans comprise 12.6% and 13.5% of the US population, they bear 16.5% and 14.6% of the disease burden due to EDCs, respectively. 94 Critically, these are conservative estimates for several reasons, including: they are limited to a subset of chemicals in plastic materials that contribute to disease and disability; they are limited to a subset of diseases due to the few chemicals studied; and the cost estimates represent a subset of the entire costs due to the disease studied. Despite these limitations, EDC-associated healthcare costs are substantial.
Table 1.
Disease Burden and Costs of EDC Exposures in Adults.
| Exposure | Life stage of exposure | Outcome | US | Canada | EU | |||
|---|---|---|---|---|---|---|---|---|
| Disease Burden | Economic Cost (USD) | Disease Burden | Economic Cost (USD) | Disease Burden | Economic Cost (USD) | |||
| Phthalates | Adult | Obesity | 5900 cases | $1.7 billion | 2093 cases | $684.8 million | 53,900 cases | $20.8 billion |
| Adult | Type 2 Diabetes | 1300 cases | $91.4 million | 225 cases | $25.8 million | 20,500 cases | $807.2 million | |
| Adult Females | Endometriosis | 86,000 cases | $47.0 billion | 10,151 cases | $5.7 billion | 145,000 cases | $1.7 billion | |
| Adult Males | Male infertility | 240,100 cases | $2.5 billion | 1395 cases | $17.0 million | 618,000 cases | $6.3 billion | |
| Adults | Cardiovascular mortality | 90,800 cases | $39.9 billion | Not quantified yet. | ||||
| PFAS | Adult | Obesity | 4,294,379 Cases | $17 billion | ||||
| Adult | Kidney cancer | 142 cases | $184 million | |||||
| Adults | Couple Infertility | 593–26,160 cases | $37.6 million -$1.7 billion | |||||
| Adult Females | Hypothyroidism | 14,572 cases | $1.3–5.2 billion | |||||
| Adult Females | Type II Diabetes | 1728 cases | $140 million | |||||
| Adult Females | Endometriosis | 696–18,062 cases | $397 million - $10.2 billion | |||||
| Adult Females | Polycystic ovarian syndrome | 7209–7505 cases | $10.5–10.9 million | |||||
| Adult females | Breast cancer | 421–3095 cases | $555 million-$4.1 billion | |||||
Healthcare as a Vector of EDC Exposure
Healthcare facilities also use many products that increase the risk of EDC exposures. 95 Phthalates, for example, are abundant in polyvinylchloride-based medical devices such as blood bags, nutrition pockets, tubing, umbilical venous catheters or disposable gloves, where they can account for up to 40% of the final product. 96 They are also used to make coatings for oral medications and in flooring. Bisphenols are used in polycarbonate-based medical tubing, hemodialysis equipment, newborn incubators, syringes, and nebulizers.97 Parabens are used in medications and intravenous catheters for their antimicrobial properties. 98 Exposures are likely the greatest per unit body weight in neonatal intensive care units, where non-invasive respiratory support and feeding tubes were identified as the most significant drivers of phthalate exposure. 99 While modern healthcare has transformed human health and acknowledging that in some instances the removal of select EDCs from medical devices may augment risk, 98 the lack of knowledge of medical care as a vector of exposure undermines core principles of medical ethics. 100 Importantly, given the magnitude of plastic waste generated by modern healthcare systems, addressing EDCs in healthcare is likely to have broader benefits on health.
Unfortunately, health care providers know little about EDCs and seldom offer steps to patients to limit exposure.101–104 Given that disparities in EDC exposure are well documented,105,106 collaborative efforts are needed between scientists and healthcare organizations to develop products that improve provider knowledge about EDCs and support the use of safer alternatives in medical devices and other equipment. Healthcare providers are well positioned to communicate safe and simple steps to limit exposures; however, knowledge about environmental exposures remains limited, and health care providers still have modest self-efficacy in managing common exposures and communicating advice for prevention.101,103,107,108
Addressing Gaps in Knowledge
There are particularly large gaps in the adult epidemiologic literature that would serve to advance our understanding of EDCs and their contribution to adult chronic disease. Large national and international biobank consortia (e.g., All of Us, UK Biobank Cohort) have not measured chemical exposures in sera or urine to evaluate later life vulnerabilities to these conditions. Some notable exceptions include the Nurses’ Study 109 and the Study of Women’s Health Across the Nation, 110 but even in these samples, the researchers found limited statistical power to examine the potential effects. These cohorts have also included more women than men, which may limit their capacity to discern effects that are likely to be sexually dimorphic. Fewer still have provided mechanistic insights by examining hormone levels or leveraging multi-omic approaches to confirm or corroborate observed effects. Further work is needed to empower large-scale studies to fully interrogate the environmental drivers of disease risk in diverse populations.
Opportunities for Clinical Interventions and Prevention: PFAS and Beyond
In 2022, the National Academies of Science, Engineering, and Medicine published “Guidance on PFAS Exposure, Testing, and Clinical Follow-Up,” the first clinical guidelines on an EDC.111 These guidelines are a milestone that bring the internist into the world of environmental health. In addition to providing extensive background on PFAS contamination and potential adverse health effects, the guidelines empower clinicians with robust, actionable guidance to identify and mitigate risk. The guidelines are focused upon a subset of potential PFAS effects for which the committee found sufficient evidence for increased risk (i.e., reductions in birthweight, dyslipidemia, kidney cancer, and reduced antibody responses) while also commenting on areas with limited suggestive evidence (i.e., breast cancer, pregnancy-induced hypertension, liver enzyme elevations, testicular cancer, thyroid dysfunction, and ulcerative colitis). Key recommendations of the guidelines include:
4–1: “Clinicians advising patients on PFAS exposure reduction should begin with a conversation aimed at first determining how they might be exposed…and what exposures they are interested in reducing.”
4–2: “If patients are exposed occupationally…clinicians should consult with occupational health and safety professionals…to determine the most feasible ways to reduce that exposure.”
4–3: “Clinicians should advise patients with elevated PFAS in their drinking water that they can filter their water to reduce their exposure.”
4–4: “In areas with known PFAS contamination, clinicians should advise patients that PFAS can be present in fish, wildlife, meat, and dairy products and direct them to local consumption advisories.”
4–5: “Clinicians should direct patients interested in learning more about PFAS to authoritative sources for information on how PFAS exposure occurs and what mitigating actions they can take.”
4–6: “When clinicians are counseling parents of infants of PFAS exposure, they should discuss infant feeding and steps that can be taken to lower sources of PFAS exposure. The benefits of breastfeeding are well known; the AAP, the AAFP, and the ACOG support and recommend breastfeeding for infants, with rare exceptions. Clinicians should explain that PFAS can pass through breast milk from a mother to her baby. PFAS may also be present in other foods, such as water used to reconstitute formula and infant food, and potentially in packaged formula and baby food. It is not yet clear what types and levels of exposure to PFAS are of concern for child health and development.”
4–7: “Federal environmental health agencies should conduct research to evaluate PFAS transfer to and concentration in breast milk and formula to generate data that can help parents and clinicians make shared, informed decisions about breastfeeding.”
The guidelines identify who should be tested, how PFAS levels should be interpreted with regard to health risks, and how those risks should be monitored in adults and children. A cornerstone of all environmental health interventions, exposure reduction strategies are highlighted (included in Table 2) as are potential therapeutic approaches such as the use of bile acid sequestrants or phlebotomy, although further evidence is likely needed to advocate for broad use of these approaches. Critically, these guidelines highlight many of the key principles of modern environmental health practice: patient education, clinician engagement, shared decision-making, community engagement, patient/community/clinician advocacy, and system-wide policy development and implementation that focuses on human health.
Table 2:
Transforming Clinical Care to Reduce EDC Exposures*
| Advising Patients | |
|---|---|
| Context | Intervention |
|
Reducing EDC Exposures from Food and Beverages
Potential Exposure Reductions: pesticides, PFASs, bisphenols, phthalates, PCBs, and toxic metals |
• Choose organic foods that are preferably locally grown, and fresh or frozen foods. • Avoid canned, processed, and fast foods. • Avoid take-out containers and other food packaging. • Avoid microwave popcorn and greasy foods wrapped in paper. • Consume a diverse, fiber-rich diet. • Trim fat from meat and skin from fish. Cook meat and fish on a rack to allow the fat to drain. • Consult local guidance regarding safe sport fish consumption. • Ensure adequate intake of calcium, iron, and iodine as well as other essential vitamins and minerals. • Store food in glass, stainless steel, or porcelain containers. • Avoid heating foods in plastic containers. • Consider testing your water and filtering your drinking water using an activated carbon or reverse osmosis filtration system. • Determine whether a lead service line provides water to your home and pursue local programs to replace it. • Reduce use of plastic containers. • Avoid polyvinyl chloride (PVC), polystyrene, and polycarbonate plastics (in U.S. recycling, numbers 3, 6, and 7). |
|
Personal and Home Care Practices to Reduce EDC Exposures
Potential Exposure Reductions: flame retardants (PBDEs and OPFRs), pesticides, triclosan, fragrances, bisphenols, phthalates, particulate matter, PAHs, parabens, PFASs, and toxic metals |
• Regularly clean floors and remove dust using a damp cloth or mop. • Eliminate or drastically reduce use of household chemicals, including cleaning supplies, pesticides, and solvents. • Wash hands regularly using fragrance- and antibiotic-free soaps. • Minimize use of products packaged or stored in plastics. • Minimize handling of receipts. • Choose electrical appliances and lawncare equipment. • Use cast iron, stainless steel, glass, and enamel instead of nonstick cookware. • Increase indoor ventilation and air filtration. • Forbid smoking indoors. • Do not burn trash or yard waste. • Read product labels and avoid items containing parabens, bisphenols, and phthalates. • Avoid cosmetics with synthetic fragrances, phthalates, or toxic metals. Choose instead those labeled as “no synthetic fragrance,” “scented with essential oils,” or “phthalate-free.” • Avoid stain-resistant carpets and upholstery as well as stain-resistant treatments. • Use nylon or silk dental floss that is uncoated or coated in natural wax. |
|
Travel, Transportation, and Activity Choices to Limit EDC Exposures
Potential Exposure Reductions: particulate matter, ozone, nitrogen oxides, PAHs, and toxic metals |
• Monitor air quality and avoid outdoor activities when air pollutions levels are high. • Schedule outdoor activities, including exercise, away from busy roads and at low traffic times. • Choose efficient modes and routes of travel that limit time spent in traffic. • Choose active modes of transportation, including walking, cycling, and public transportation. |
| Educating Patients to be Environmental Health Advocates | |
|
Empowering Patient Advocacy to Improve Environmental Health
Potential Exposure Reductions: flame retardants (PBDEs and OPFRs), parabens, phthalates, bisphenols, pesticides, PFASs, particulate matter, ozone, nitrogen oxides, PAHs, toxic metals, and others |
• Encourage local school districts to reduce school bus emissions, including the introduction of “No Idling Zones.” • Demand local water utilities test for PFAS and other EDCs and encourage the establishment of more health-protective drinking water limits. • Urge local political officials to pursue sustainable development, including renewable energy, walkability, bike lanes, and expanded access to public transportation. Ensure that such policies are rooted in social equity. • Ask state legislators to set up statewide water and blood testing programs to monitor for EDCs. • Encourage local municipalities to expand green spaces and increase tree planting. • Urge park districts to eliminate synthetic turf fields and the use of pesticides. • Advocate for expanded environmental health education. • Encourage politicians to promote policies that identify and eliminate EDCs, including transparent labeling to empower individual action. • Tell retailers and manufactures you want products made without EDCs and encourage elected officials to support restrictions on EDCs in consumer products. • Join local and national organizations devoted to improving environmental health. |
| Engaging Clinicians in Environmental Health Practice and Advocacy | |
|
Practice Improvement
Potential Exposure Reductions: flame retardants (PBDEs and OPFRs), parabens, phthalates, bisphenols, pesticides, PFASs, particulate matter, ozone, nitrogen oxides, PAHs, toxic metals, and others |
• Institute clinic and hospital task forces to reduce medical waste and reduce healthcare-associated EDC exposures. • Lobby professional organizations to develop environmental health clinical practice guidelines to help providers advise patients on ways to reduce EDC exposures. |
|
Healthcare Provider Advocacy
Potential Exposure Reductions: flame retardants (PBDEs and OPFRs), parabens, phthalates, bisphenols, pesticides, PFASs, particulate matter, ozone, nitrogen oxides, PAHs, toxic metals, and others |
• Join professional organizations working to address the health threats of EDCs and become active participants in subgroups devoted to this issue. • Contact political representatives to advocate for policies that expand testing to identify EDCs, quantify human exposures in diverse populations, limit those exposures, and establish regulatory bodies to eliminate EDC exposures. • Encourage policymakers and regulators to develop and implement state and national programs to monitor EDCs in food, water, and human samples. |
Despite its sole focus on PFAS, the impact of these guidelines is difficult to overstate; however, it is also clear that expansion of clinical guidelines to address other EDCs is urgently needed. In the meantime, an array of intervention studies reveals a path forward for reducing other EDC exposures. Though large-scale intervention studies have not been conducted, small-scale interventions support the feasibility of reducing EDC exposures. Lu et al. reduced organophosphate pesticide metabolites in urine to nondetectable levels through an organic diet intervention.112 Though concerns about the additional costs associated with organic food are appropriate, a more recent dietary intervention similarly reduced pesticide metabolites in a low-income, agricultural population.113 In young girls, choosing personal care products labelled to be free of phthalates, parabens, triclosan, and benzophenones reduced personal exposure by 27%–44%.114 Another dietary intervention study that replaced diets in a small sample of families with fresh foods reduced urinary levels of phthalate metabolites and bisphenols by 53%–56%.115
Household interventions can also reduce exposure. One study performed a graded comparison of offices, common areas, and classrooms, measuring dust levels of PFAS, polybrominated diphenyl ethers (PBDEs), and organophosphorus esters (OPE), the latter having increasingly replaced PBDEs in electronics and furniture. Rooms with full “healthier” materials had 78% lower dust levels of PFAS, 65% lower OPE levels, and 45% lower PBDE levels than rooms with only partial interventions, adjusted for covariates related to insulation, electronics, and furniture.116 To be clear, not all studies have achieved expected changes in EDC levels. One study reported an increase in urinary phthalate metabolites due to substantial phthalate contamination in the coriander provided to participants.117 Use of a BPA risk score based on characteristics of food containers and packaging did not reduce urinary levels.118 Much more work is needed to formalize clinical guidance for providers to empower them to address patient questions about exposure reduction strategies; however, recognition of exposure sources and burgeoning clinical studies can assist providers in guiding their patients (Table 2).119,120
Policy Actions to Reduce Exposure
While beneficial, individual actions have limited power to address systemic threats to health; however, current policy interventions also remain inadequate. We refer the reader to a recent review that speaks to severe gaps in the regulation of chemicals in commerce and efforts to limit endocrine-disruptive effects.13 In brief, chemicals are not regulated for their endocrine-disrupting effects, and efforts are needed to improve:
Testing and identification of chemicals that disrupt endocrine systems,
Evaluating exposures,
Limiting exposures through regulations, and
Establishing an International Agency for Research on EDCs
Currently available and validated tests do not cover all endocrine modes of action. For example, US regulations require testing for estrogen agonist activity only for pesticides and drinking water contaminants. Even for estrogen and androgen signaling, the validated tests (e.g., uterotrophic assay) work best for endogenous hormones and are too insensitive for identifying EDCs. Fortunately, sensitive assays exist to test a broad number of nuclear receptors, and assays to examine receptor expression, hormone transport, hormone synthesis, and epigenetic alterations should soon be validated for inclusion in regulatory requirements. Recognizing the need to push a large suite of chemicals through a new, more scientifically up-to-date panel of tests, we suggest in vitro screening using high-throughput platforms followed by in vivo testing using zebrafish or mammalian systems to verify the absence of adverse effects using approaches that account for the integrated, multisystem nature of endocrine physiology. At the very least, such approaches should be applied prospectively to avoid the addition of new adverse exposures. Critically, we also need to regulate chemicals by class, rather than one at a time, as regrettable substitutes have emerged time and again (e.g, diisononylphthalate as a replacement for di-2-ethylhexylphthalate, and BPS for BPA).
Regulatory policies across the world use exposure and related risk to judge whether to limit chemicals of concern. However, this approach has extreme limitations, and the disease burden linked to EDCs in adults reveals the failure of a risk-based regulatory paradigm. First, current risk-based paradigms do not account for the non-linear and non-monotonic exposure-response relationships common to many EDCs.121 Indeed, the risk-based paradigm is largely based on extrapolating from no-adverse effect levels in animals to humans, which cannot be done when non-linear or non-monotonic relationships apply. There is a substantial lag from identifying new exposures to completing studies of human health effects, especially for disease outcomes with longer latencies such as diabetes or cancer. While some risk-based approaches attempt to account for age-related vulnerability, they falsely presume that the population sensitivity can be quantified a priori. Moreover, enhanced sensitivity of individuals to EDCs based on underlying disease states or their treatments remains unaddressed.122
In a hazard-based regulatory environment, chemicals identified as EDCs would simply be removed from use without regard to the exposure required to achieve an adverse effect. There are precedents for a hazard-based regulatory paradigm, especially in Europe for carcinogens; persistent, bioaccumulative and toxic chemicals; and pesticides and biocides which are EDCs. If we persist with a risk-based approach, we need broader and stronger human biomonitoring platforms, particularly to address gaps in exposure assessment in low- and middle-income countries. Such biomonitoring data will also inform educational campaigns about safe and simple steps to limit exposure.
We also suggest the establishment of a new international agency, or a broadening of the International Agency for Research on Cancer (IARC)’s scientific charge, to include endocrine disruption. Established in 1965, IARC was tasked with evaluating the evidence of carcinogenesis due to environmental hazards. Monographs describe three streams of evidence (mechanistic, animal, and epidemiological studies). An autonomous body such as the IARC can bring together diverse experts for international collaborative reports on EDCs, and further support the post-2020 process of the Strategic Alliance for International Chemicals Management.
The Plastics Treaty: An Opportunity to Address EDCs Globally
Plastics are a crucial source of many EDCs, including phthalates, bisphenols, and per- and polyfluoroalkyl substances. In February 2022, the United Nations Environment Assembly announced plans to negotiate an international, legally binding instrument to end plastic pollution. Recent public and media attention to microplastics detected in both wildlife and humans123 has coalesced the policy community around initiatives to reduce the use of single-use plastics. The arguments for this action have often focused on the visible microplastics, despite stronger evidence of human health effects induced by chemicals used as additives in plastics (e.g., phthalates and bisphenols). At the same time, there are substantial gaps in our scientific understanding of the relationships between detectable microplastics in biological specimens and the observed health effects of additive chemicals.
The dialogue around the “circular economy” and pivoting to reusable plastics as a solution to the plastic health crisis fails to address concerns about contamination of reused plastics containing EDCs. There is a need to transition to other materials (e.g., glass and stainless steel). While some suggest bioplastics as potential alternatives, early studies suggest potential endocrine activity that may pose similar risk to synthetic, fossil fuel-based plastics. There also remain gaps in information about the health effects of high-density polyethylene and other plastics that do not use phthalates or bisphenols. The global plastics treaty offers a profound opportunity; however, active engagement from the medical community is required to ensure that limiting the adverse health effects of plastics and their constituent chemicals is prioritized.
Conclusions
Patients are potentially exposed to a host of EDCs associated with cardiometabolic, reproductive, and other disorders that contribute substantially to healthcare expenditures. Moreover, differential exposure to these chemicals is a likely but underrecognized driver of health disparities. The internist can play a critical role in addressing this underlying health threat by working with patients on strategies to reduce their exposures; collaborating across the healthcare system to reduce exposures arising from clinical care; and advocating for local, national, and global policies that identify EDCs and remove them from our environment.
Funding:
This work was supported by the National Institute of Health (P30 ES027792 supporting RMS).
Footnotes
Conflicts of interest: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. RMS declares honoraria from CVS/Health and the American Medical Forum, neither of which relate to the present manuscript. All other authors declare they have no competing interests.
References
- 1.Carson R Silent spring. 2002. [Google Scholar]
- 2.Cavalier H, Trasande L, Porta M. Exposures to pesticides and risk of cancer: Evaluation of recent epidemiological evidence in humans and paths forward. International journal of cancer Journal international du cancer. 2023;152(5):879–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudry J, Assmann KE, Touvier M, et al. Association of Frequency of Organic Food Consumption With Cancer Risk: Findings From the NutriNet-Santé Prospective Cohort Study. JAMA Internal Medicine. 2018;178(12):1597–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Walker GW, Muir DCG, Nagatani-Yoshida K. Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories. Environmental Science & Technology. 2020;54(5):2575–2584. [DOI] [PubMed] [Google Scholar]
- 5.Vandenberg LN, Agerstrand M, Beronius A, et al. A proposed framework for the systematic review and integrated assessment (SYRINA) of endocrine disrupting chemicals. Environ Health. 2016;15(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, et al. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocrine Reviews. 2009;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman A, Becher G, Blumberg B, et al. Manufacturing doubt about endocrine disrupter science - A rebuttal of industry-sponsored critical comments on the UNEP/WHO report “State of the Science of Endocrine Disrupting Chemicals 2012”. Regulatory toxicology and pharmacology : RTP. 2015. [DOI] [PubMed] [Google Scholar]
- 8.Gore AC, Chappell VA, Fenton SE, et al. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015:er20151010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strategic Alliance for International Chemicals Management. Endocrine disrupting chemicals. Available at http://www.saicm.org/index.php?option=com_content&view=article&id=452&Itemid=685. 2015.
- 10.Zoeller RT, Brown TR, Doan LL, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153(9):4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding D, Xu L, Fang H, et al. The EDKB: an established knowledge base for endocrine disrupting chemicals. BMC Bioinformatics. 2010;11 Suppl 6(Suppl 6):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Merrill MA, Vandenberg LN, Smith MT, et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol. 2020;16(1):45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassotis CD, Vandenberg LN, Demeneix BA, Porta M, Slama R, Trasande L. Endocrine-disrupting chemicals: economic, regulatory, and policy implications. The lancet Diabetes & endocrinology. 2020;8(8):719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellanger M, Demeneix B, Grandjean P, Zoeller RT, Trasande L. Neurobehavioral deficits, diseases, and associated costs of exposure to endocrine-disrupting chemicals in the European union. J Clin Endocrinol Metab. 2015;100(4):1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. Endocrine-disrupting chemicals: implications for human health. The lancet Diabetes & endocrinology. 2020;8(8):703–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauser R, Skakkebaek NE, Hass U, et al. Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the European union. J Clin Endocrinol Metab. 2015;100(4):1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt PA, Sathyanarayana S, Fowler PA, Trasande L. Female Reproductive Disorders, Diseases, and Costs of Exposure to Endocrine Disrupting Chemicals in the European Union. J Clin Endocrinol Metab. 2016;101(4):1562–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heindel JJ, Alvarez JA, Atlas E, et al. Obesogens and Obesity: State-of-the-Science and Future Directions Summary from a HEEDS Workshop. Am J Clin Nutr. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamorro-Garcia R, Blumberg B. Current Research Approaches and Challenges in the Obesogen Field. Front Endocrinol (Lausanne). 2019;10:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamorro-Garcia R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect. 2013;121(3):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu G, Dhana K, Furtado JD, et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS medicine. 2018;15(2):e1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardenas A, Hauser R, Gold DR, et al. Association of Perfluoroalkyl and Polyfluoroalkyl Substances With Adiposity. JAMA network open. 2018;1(4):e181493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardenas A, Hivert MF, Gold DR, et al. Associations of Perfluoroalkyl and Polyfluoroalkyl Substances With Incident Diabetes and Microvascular Disease. Diabetes Care. 2019;42(9):1824–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P. Plasma Concentrations of Perfluoroalkyl Substances and Risk of Type 2 Diabetes: A Prospective Investigation among U.S. Women. Environmental health perspectives. 2018;126(3):037001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lind L, Zethelius B, Salihovic S, van Bavel B, Lind PM. Circulating levels of perfluoroalkyl substances and prevalent diabetes in the elderly. Diabetologia. 2014;57(3):473–479. [DOI] [PubMed] [Google Scholar]
- 26.Ranciere F, Botton J, Slama R, et al. Exposure to Bisphenol A and Bisphenol S and Incident Type 2 Diabetes: A Case-Cohort Study in the French Cohort D.E.S.I.R. Environmental health perspectives. 2019;127(10):107013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116(12):1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korkmaz A, Aydoğan M, Kolankaya D, Barlas N. Vitamin C coadministration augments bisphenol A, nonylphenol, and octylphenol induced oxidative damage on kidney of rats. Environmental Toxicology. 2011;26(4):325–337. [DOI] [PubMed] [Google Scholar]
- 29.Liu M, Jia S, Dong T, et al. The occurrence of bisphenol plasticizers in paired dust and urine samples and its association with oxidative stress. Chemosphere. 2019;216:472–478. [DOI] [PubMed] [Google Scholar]
- 30.Neier K, Marchlewicz EH, Dolinoy DC, Padmanabhan V. Assessing Human Health Risk to Endocrine Disrupting Chemicals: a Focus on Prenatal Exposures and Oxidative Stress. Endocrine disruptors (Austin, Tex). 2015;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rashid H, Ahmad F, Rahman S, et al. Iron deficiency augments bisphenol A-induced oxidative stress in rats. Toxicology. 2009;256(1–2):7–12. [DOI] [PubMed] [Google Scholar]
- 32.Lind PM, Lind L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis. 2011;218(1):207–213. [DOI] [PubMed] [Google Scholar]
- 33.Melzer D, Gates P, Osborn NJ, et al. Urinary bisphenol a concentration and angiography-defined coronary artery stenosis. PLoS One. 2012;7(8):e43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bae S, Kim JH, Lim Y-H, Park HY, Hong Y-C. Associations of Bisphenol A Exposure With Heart Rate Variability and Blood Pressure / Novelty and Significance. Hypertension. 2012;60(3):786–793. [DOI] [PubMed] [Google Scholar]
- 35.Bao W, Liu B, Rong S, Dai SY, Trasande L, Lehmler H-J. Association Between Bisphenol A Exposure and Risk of All-Cause and Cause-Specific Mortality in US Adults. JAMA network open. 2020;3(8):e2011620–e2011620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodward MJ, Obsekov V, Jacobson MH, Kahn LG, Trasande L. Phthalates and Sex Steroid Hormones Among Men From NHANES, 2013–2016. The Journal of Clinical Endocrinology & Metabolism. 2020;105(4):e1225–e1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Testosterone Endogenous and Mortality in Men: A Systematic Review and Meta-Analysis. The Journal of Clinical Endocrinology & Metabolism. 2011;96(10):3007–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan Y, Wang L, Han L, et al. Exposure to phthalates in patients with diabetes and its association with oxidative stress, adiponectin, and inflammatory cytokines. Environ Int. 2017;109:53–63. [DOI] [PubMed] [Google Scholar]
- 39.Ferguson KK, Loch-Caruso R, Meeker JD. Urinary phthalate metabolites in relation to biomarkers of inflammation and oxidative stress: NHANES 1999–2006. Environ Res. 2011;111(5):718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trasande L, Liu B, Bao W. Phthalates and attributable mortality: A population-based longitudinal cohort study and cost analysis. Environ Pollut. 2022;292(Pt A):118021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mylchreest E, Sar M, Cattley RC, Foster PMD. Disruption of Androgen-Regulated Male Reproductive Development by Di(n-Butyl) Phthalate during Late Gestation in Rats Is Different from Flutamide. Toxicology and Applied Pharmacology. 1999;156(2):81–95. [DOI] [PubMed] [Google Scholar]
- 42.McIntyre BS, Barlow NJ, Foster PM. Androgen-mediated development in male rat offspring exposed to flutamide in utero: permanence and correlation of early postnatal changes in anogenital distance and nipple retention with malformations in androgen-dependent tissues. Toxicological sciences : an official journal of the Society of Toxicology. 2001;62(2):236–249. [DOI] [PubMed] [Google Scholar]
- 43.Welsh M, Saunders PT, Sharpe RM. The critical time window for androgen-dependent development of the Wolffian duct in the rat. Endocrinology. 2007;148(7):3185–3195. [DOI] [PubMed] [Google Scholar]
- 44.Bowman CJ, Barlow NJ, Turner KJ, Wallace DG, Foster PM. Effects of in utero exposure to finasteride on androgen-dependent reproductive development in the male rat. Toxicological sciences : an official journal of the Society of Toxicology. 2003;74(2):393–406. [DOI] [PubMed] [Google Scholar]
- 45.Barrett ES, Parlett LE, Sathyanarayana S, Redmon JB, Nguyen RHN, Swan SH. Prenatal Stress as a Modifier of Associations between Phthalate Exposure and Reproductive Development: results from a Multicentre Pregnancy Cohort Study. Paediatric and Perinatal Epidemiology. 2016;30(2):105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bornehag CG, Carlstedt F, Jonsson BA, et al. Prenatal Phthalate Exposures and Anogenital Distance in Swedish Boys. Environ Health Perspect. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurtoğlu S, Baştuğ O. Mini puberty and its interpretation. Turk pediatri arsivi. 2014;49(3):186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thankamony A, Ong KK, Dunger DB, Acerini CL, Hughes IA. Anogenital distance from birth to 2 years: a population study. Environ Health Perspect. 2009;117(11):1786–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hotchkiss AK, Parks-Saldutti LG, Ostby JS, et al. A mixture of the “antiandrogens” linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biol Reprod. 2004;71(6):1852–1861. [DOI] [PubMed] [Google Scholar]
- 50.Eisenberg ML, Hsieh MH, Walters RC, Krasnow R, Lipshultz LI. The relationship between anogenital distance, fatherhood, and fertility in adult men. PLoS One. 2011;6(5):e18973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Priskorn L, Bang AK, Nordkap L, et al. Anogenital distance is associated with semen quality but not reproductive hormones in 1106 young men from the general population. Human Reproduction. 2019;34(1):12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skakkebaek NE. Endocrine disrupters and testicular dysgenesis syndrome. Hormone research. 2002;57 Suppl 2:43. [DOI] [PubMed] [Google Scholar]
- 53.Ivell R, Bathgate R. Neohormone systems as exciting targets for drug development. Trends in endocrinology and metabolism: TEM. 2006;17(4):123. [DOI] [PubMed] [Google Scholar]
- 54.Lassen TH, Frederiksen H, Jensen TK, et al. Urinary bisphenol A levels in young men: association with reproductive hormones and semen quality. Environmental health perspectives. 2014;122(5):478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghayda RA, Williams PL, Chavarro JE, et al. Urinary bisphenol S concentrations: Potential predictors of and associations with semen quality parameters among men attending a fertility center. Environ Int. 2019;131:105050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meeker JD, Ryan L, Barr DB, et al. The relationship of urinary metabolites of carbaryl/naphthalene and chlorpyrifos with human semen quality. Environmental health perspectives. 2004;112(17):1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melgarejo M, Mendiola J, Koch HM, Moñino-García M, Noguera-Velasco JA, Torres-Cantero AM. Associations between urinary organophosphate pesticide metabolite levels and reproductive parameters in men from an infertility clinic. Environmental research. 2015;137:292–298. [DOI] [PubMed] [Google Scholar]
- 58.Yucra S, Gasco M, Rubio J, Gonzales GF. Semen quality in Peruvian pesticide applicators: association between urinary organophosphate metabolites and semen parameters. Environ Health. 2008;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toft G, Jönsson BA, Lindh CH, et al. Exposure to perfluorinated compounds and human semen quality in Arctic and European populations. Hum Reprod. 2012;27(8):2532–2540. [DOI] [PubMed] [Google Scholar]
- 60.Louis GM, Chen Z, Schisterman EF, et al. Perfluorochemicals and human semen quality: the LIFE study. Environmental health perspectives. 2015;123(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lerro CC, Koutros S, Andreotti G, et al. Organophosphate insecticide use and cancer incidence among spouses of pesticide applicators in the Agricultural Health Study. Occup Environ Med. 2015;72(10):736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silva JF, Mattos IE, Luz LL, Carmo CN, Aydos RD. Exposure to pesticides and prostate cancer: systematic review of the literature. Rev Environ Health. 2016;31(3):311–327. [DOI] [PubMed] [Google Scholar]
- 63.Hardell L, Bavel B, Lindstrom G, Eriksson M, Carlberg M. In utero exposure to persistent organic pollutants in relation to testicular cancer risk. Int J Androl. 2006;29(1):228–234. [DOI] [PubMed] [Google Scholar]
- 64.Steenland K, Winquist A. PFAS and cancer, a scoping review of the epidemiologic evidence. Environ Res. 2021;194:110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buck Louis G, Cooney MA, Peterson CM. Ovarian dysgenesis syndrome. Journal of developmental origins of health and disease. 2011;2(01):25–35. [Google Scholar]
- 66.Buck Louis GM, Peterson CM, Chen Z, et al. Bisphenol A and phthalates and endometriosis: the Endometriosis: Natural History, Diagnosis and Outcomes Study. Fertility and sterility. 2013;100(1):162–169.e161–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Louis GM, Peterson CM, Chen Z, et al. Perfluorochemicals and endometriosis: the ENDO study. Epidemiology (Cambridge, Mass). 2012;23(6):799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang W, Zhou W, Wu S, et al. Perfluoroalkyl substances exposure and risk of polycystic ovarian syndrome related infertility in Chinese women. Environ Pollut. 2019;247:824–831. [DOI] [PubMed] [Google Scholar]
- 69.Vagi SJ, Azziz-Baumgartner E, Sjödin A, et al. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol A in polycystic ovary syndrome: a case-control study. BMC endocrine disorders. 2014;14:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heffernan AL, Cunningham TK, Drage DS, et al. Perfluorinated alkyl acids in the serum and follicular fluid of UK women with and without polycystic ovarian syndrome undergoing fertility treatment and associations with hormonal and metabolic parameters. Int J Hyg Environ Health. 2018;221(7):1068–1075. [DOI] [PubMed] [Google Scholar]
- 71.Akgül S, Sur Ü, Düzçeker Y, et al. Bisphenol A and phthalate levels in adolescents with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(12):1084–1087. [DOI] [PubMed] [Google Scholar]
- 72.Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocrine journal. 2004;51(2):165–169. [DOI] [PubMed] [Google Scholar]
- 73.Hossein Rashidi B, Amanlou M, Behrouzi Lak T, et al. The Association Between Bisphenol A and Polycystic Ovarian Syndrome: A Case-Control Study. Acta medica Iranica. 2017;55(12):759–764. [PubMed] [Google Scholar]
- 74.Gu J, Yuan T, Ni N, et al. Urinary concentration of personal care products and polycystic ovary syndrome: A case-control study. Environmental research. 2019;168:48–53. [DOI] [PubMed] [Google Scholar]
- 75.Jędrzejuk D, Kuliczkowska-Płaksej J, Milewicz A, Wilczewska K, Namieśnik J, Rutkowska A. Bisphenol A levels are negatively correlated with serum vitamin D-binding protein and sex hormone-binding globulin levels in women with polycystic ovary syndrome: a pilot study. Pol Arch Intern Med. 2019;129(2):133–136. [DOI] [PubMed] [Google Scholar]
- 76.Peretz J, Vrooman L, Ricke WA, et al. Bisphenol a and reproductive health: update of experimental and human evidence, 2007–2013. Environmental health perspectives. 2014;122(8):775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cohn BA, La Merrill MA, Krigbaum NY, et al. In utero exposure to poly- and perfluoroalkyl substances (PFASs) and subsequent breast cancer. Reproductive toxicology (Elmsford, NY). 2020;92:112–119. [DOI] [PubMed] [Google Scholar]
- 78.Mancini FR, Cano-Sancho G, Gambaretti J, et al. Perfluorinated alkylated substances serum concentration and breast cancer risk: Evidence from a nested case-control study in the French E3N cohort. International journal of cancer Journal international du cancer. 2020;146(4):917–928. [DOI] [PubMed] [Google Scholar]
- 79.Bonefeld-Jørgensen EC, Long M, Fredslund SO, Bossi R, Olsen J. Breast cancer risk after exposure to perfluorinated compounds in Danish women: a case-control study nested in the Danish National Birth Cohort. Cancer causes & control : CCC. 2014;25(11):1439–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mikhail N, Wali S, Brown AF. Ethnic Disparities in Diabetes. Endocrinol Metab Clin North Am. 2021;50(3):475–490. [DOI] [PubMed] [Google Scholar]
- 81.Luff MK, Kim J, Tseng CH, Livhits MJ, Yeh MW, Wu JX. Racial/ethnic disparities in thyroid cancer in California, 1999–2017. Am J Surg. 2023;225(2):298–303. [DOI] [PubMed] [Google Scholar]
- 82.Morris JR, Plowden TC, Green LJ, Edwards DRV, Jackson-Bey T. Racial and Ethnic Variation in Genetic Susceptibility: Are Disparities in Infertility Prevalence and Outcomes more than Black and White? Reprod Sci. 2022;29(7):2081–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Golden SHM R Recognizing and Addressing Health Inequitites in Endocrinology and Diabetes. Endocrinol Metab Clin North Am. 2023. [DOI] [PubMed] [Google Scholar]
- 84.Golden SH, Brown A, Cauley JA, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors--an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012;97(9):E1579–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petersen R, Pan L, Blanck HM. Racial and Ethnic Disparities in Adult Obesity in the United States: CDC’s Tracking to Inform State and Local Action. Prev Chronic Dis. 2019;16:E46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weiss MCW L; Sargis RM Hormonal Injustice: Environmental Toxicants as Drivers of Endocrine Health Disparities. Endocrinol Metab Clin North Am. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruiz D, Becerra M, Jagai JS, Ard K, Sargis RM. Disparities in Environmental Exposures to Endocrine-Disrupting Chemicals and Diabetes Risk in Vulnerable Populations. Diabetes Care. 2018;41(1):193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McDonald JA, Llanos AAM, Morton T, Zota AR. The Environmental Injustice of Beauty Products: Toward Clean and Equitable Beauty. Am J Public Health. 2022;112(1):50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trasande L, Zoeller RT, Hass U, et al. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European union. J Clin Endocrinol Metab. 2015;100(4):1245–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trasande L, Zoeller RT, Hass U, et al. Burden of disease and costs of exposure to endocrine disrupting chemicals in the European Union: an updated analysis. Andrology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Attina TM, Hauser R, Sathyanarayana S, et al. Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. The lancet Diabetes & endocrinology. 2016;4(12):996–1003. [DOI] [PubMed] [Google Scholar]
- 92.Malits J, Naidu M, Trasande L. Exposure to Endocrine Disrupting Chemicals in Canada: Population-Based Estimates of Disease Burden and Economic Costs. 2022;10(3):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Obsekov V, Kahn LG, Trasande L. Leveraging Systematic Reviews to Explore Disease Burden and Costs of Per- and Polyfluoroalkyl Substance Exposures in the United States. Exposure and Health. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Attina TM, Malits J, Naidu M, Trasande L. Racial/ethnic disparities in disease burden and costs related to exposure to endocrine-disrupting chemicals in the United States: an exploratory analysis. J Clin Epidemiol. 2019;108:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Genco M, Anderson-Shaw L, Sargis RM. Unwitting Accomplices: Endocrine Disruptors Confounding Clinical Care. The Journal of Clinical Endocrinology & Metabolism. 2020;105(10):e3822–e3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marie C, Hamlaoui S, Bernard L, et al. Exposure of hospitalised pregnant women to plasticizers contained in medical devices. BMC Womens Health. 2017;17(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bacle A, Thevenot S, Grignon C, et al. Determination of bisphenol A in water and the medical devices used in hemodialysis treatment. Int J Pharm. 2016;505(1–2):115–121. [DOI] [PubMed] [Google Scholar]
- 98.Shenep LE, Shenep MA, Cheatham W, et al. Efficacy of intravascular catheter lock solutions containing preservatives in the prevention of microbial colonization. J Hosp Infect. 2011;79(4):317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stroustrup A, Bragg JB, Busgang SA, et al. Sources of clinically significant neonatal intensive care unit phthalate exposure. J Expo Sci Environ Epidemiol. 2020;30(1):137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Genco M, Anderson-Shaw L, Sargis RM. Unwitting Accomplices: Endocrine Disruptors Confounding Clinical Care. J Clin Endocrinol Metab. 2020;105(10):e3822–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trasande L, Boscarino J, Graber N, et al. The environment in pediatric practice: a study of New York pediatricians’ attitudes, beliefs, and practices towards children’s environmental health. J Urban Health. 2006;83(4):760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trasande L, Newman N, Long L, et al. Translating Knowledge About Environmental Health to Practitioners: Are We Doing Enough? Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine. 2010;77(1):114–123. [DOI] [PubMed] [Google Scholar]
- 103.Trasande L, Schapiro ML, Falk R, et al. Pediatrician attitudes, clinical activities, and knowledge of environmental health in Wisconsin. WMJ. 2006;105(2):45–49. [PubMed] [Google Scholar]
- 104.Stotland NE, Sutton P, Trowbridge J, et al. Counseling Patients on Preventing Prenatal Environmental Exposures - A Mixed-Methods Study of Obstetricians. PLOS ONE. 2014;9(6):e98771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dodson RE, Cardona B, Zota AR, Robinson Flint J, Navarro S, Shamasunder B. Personal care product use among diverse women in California: Taking Stock Study. J Expo Sci Environ Epidemiol. 2021;31(3):487–502. [DOI] [PubMed] [Google Scholar]
- 106.Zota AR, Shamasunder B. Environmental health equity: moving toward a solution-oriented research agenda. J Expo Sci Environ Epidemiol. 2021;31(3):399–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Trasande L, Niu J, Li J, et al. The Environment and Children’s Health Care in Northwest China. BMC Pediatr. 2014;14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trasande L, Ziebold C, Schiff JS, Wallinga D, McGovern P, Oberg CN. The Environment in Pediatric Practice in Minnesota: Attitudes, Beliefs, and Practices towards Children’s Environmental Health. Minnesota Medicine, submitted. 2008. [PubMed] [Google Scholar]
- 109.Song Y, Hauser R, Hu FB, Franke AA, Liu S, Sun Q. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. International journal of obesity (2005). 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peng MQ, Karvonen-Gutierrez CA, Herman WH, Mukherjee B, Park SK. Phthalates and Incident Diabetes in Midlife Women: The Study of Women’s Health Across the Nation (SWAN). J Clin Endocrinol Metab. 2023;108(8):1947–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.National Academies of Science E, and Medicine. Guidance on PFAS Exposure, Testing, and Clinical Follow-Up. In: Guidance on PFAS Exposure, Testing, and Clinical Follow-Up. Washington (DC)2022. [PubMed] [Google Scholar]
- 112.Lu C, Toepel K, Irish R, Fenske RA, Ban DB, Bravo R. Organic Diets Significantly Lower Children’s Dietary Exposure to Organophosphorus Pesticides. Environmental Health Perspectives. 2006;114(2):260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bradman A, Quirós-Alcalá L, Castorina R, et al. Effect of Organic Diet Intervention on Pesticide Exposures in Young Children Living in Low-Income Urban and Agricultural Communities. Environ Health Perspect. 2015;123(10):1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harley KG, Kogut K, Madrigal DS, et al. Reducing Phthalate, Paraben, and Phenol Exposure from Personal Care Products in Adolescent Girls: Findings from the HERMOSA Intervention Study. Environ Health Perspect. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rudel RA, Gray JM, Engel CL, et al. Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention. Environ Health Perspect. 2011;119(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Young AS, Hauser R, James-Todd TM, et al. Impact of “healthier” materials interventions on dust concentrations of per- and polyfluoroalkyl substances, polybrominated diphenyl ethers, and organophosphate esters. Environment International. 2020:106151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sathyanarayana S, Alcedo G, Saelens BE, et al. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J Expo Sci Environ Epidemiol. 2013;23(4):378–384. [DOI] [PubMed] [Google Scholar]
- 118.Galloway TS, Baglin N, Lee BP, et al. An engaged research study to assess the effect of a ‘real-world’ dietary intervention on urinary bisphenol A (BPA) levels in teenagers. BMJ Open. 2018;8(2):e018742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sargis RM, Heindel JJ, Padmanabhan V. Interventions to Address Environmental Metabolism-Disrupting Chemicals: Changing the Narrative to Empower Action to Restore Metabolic Health. Front Endocrinol (Lausanne). 2019;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Trasande L Sicker, Fatter, Poorer: The Urgent Threat of Hormone-Disrupting Chemicals to Our Health and Future…and What We Can Do about It. New York: Houghton Mifflin Harcourt; 2019. [Google Scholar]
- 121.Duh-Leong CM M; Kassotis CD; Vandenberg C; Trasande L Regulation of endocrine disrupting chemicals to minimise their impact on health. Nat Rev Endocrinol. 2023. [DOI] [PubMed] [Google Scholar]
- 122.Sargis RM. Metabolic Disruption in Context: Clinical Avenues for Synergistic Perturbations in Energy Homeostasis by Endocrine Disrupting Chemicals. Endocrine Disruptors. 2015;3(1):e1080788–1080781–1080787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang J, Wang L, Trasande L, Kannan K. Occurrence of Polyethylene Terephthalate and Polycarbonate Microplastics in Infant and Adult Feces. Environmental Science & Technology Letters. 2021;8(11):989–994. [Google Scholar]
- 124.Schulz MC, Sargis RM. Inappropriately sweet: Environmental endocrine-disrupting chemicals and the diabetes pandemic. Adv Pharmacol. 2021;92:419–456. [DOI] [PMC free article] [PubMed] [Google Scholar]


