Abstract
Rabbit hemorrhagic disease virus 2/genotype GI.2 (RHDV2/GI.2; Caliciviridae, Lagovirus) causes a highly contagious disease with hepatic necrosis and disseminated intravascular coagulation in several Leporidae species. RHDV2 was first detected in European rabbits (Oryctolagus cuniculus) in France in 2010 and has since spread widely. We gather here data on viral detections reported in various countries and affected species, and discuss pathology, genetic differences, and novel diagnostic aspects. RHDV2 has been detected almost globally, with cases reported in Europe, Africa, Oceania, Asia, and North America as of 2023. Since 2020, large scale outbreaks have occurred in the United States and Mexico and, at the same time, cases have been reported for the first time in previously unaffected countries, such as China, Japan, Singapore, and South Africa, among others. Detections have been notified in domestic and wild European rabbits, hares and jackrabbits (Lepus spp.), several species of cottontail and brush rabbits (Sylvilagus spp.), pygmy rabbits (Brachylagus idahoensis), and red rock rabbits (Pronolagus spp.). RHDV2 has also been detected in a few non-lagomorph species. Detection of RHDV2 causing RHD in Sylvilagus spp. and Leporidae species other than those in the genera Oryctolagus and Lepus is very novel. The global spread of this fast-evolving RNA virus into previously unexploited geographic areas increases the likelihood of host range expansion as new species are exposed; animals may also be infected by nonpathogenic caliciviruses that are disseminated by almost all species, and with which genetic recombination may occur.
Keywords: cottontails, GI.2, hares, jackrabbits, Lagovirus europaeus, rabbits, rabbit hemorrhagic disease, rabbit hemorrhagic disease virus 2, Sylvilagus
The order Lagomorpha
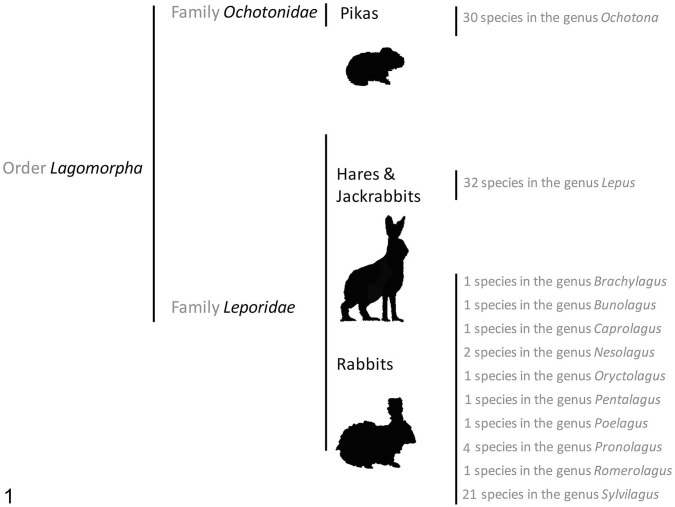
The order Lagomorpha (Fig. 1) has a long evolutionary history that can be traced back to 55 million years ago in Mongolia. 15 It comprises 2 families: Ochotonidae (pikas) and Leporidae (rabbits, hares and jackrabbits). The Leporidae family is the largest, with 66 species classified into 11 different genera.2,91 Rabbits are small lagomorphs divided into 10 genera; hares and jackrabbits are usually larger and are grouped in a single genus, Lepus. Several species within the Leporidae family face conservation challenges and, according to the International Union for Conservation of Nature, some species are categorized as vulnerable, threatened, or critically endangered.2,91
Figure 1.
The order Lagomorpha with genera in families Ochotonidae (pikas) and Leporidae (leporids). Adapted from reference 2 and updated per the International Union for Conservation of Nature–Species Survival Commission, Lagomorph Specialist Group. 91
Leporidae species can be found on all continents except Antarctica. Lepus is the most diverse genus and has a global distribution.11,62 The European rabbit (Oryctolagus cuniculus) is the single species in the genus Oryctolagus and arguably the best-known lagomorph; it is native to the Iberian Peninsula and southern France but wild European rabbits have been introduced into many other areas of the world, including the rest of Europe, certain parts of South America, most of Oceania, and numerous islands. 11 Domestic European rabbits are often kept as pets, and used for research and for meat and fur production, among other purposes. Feral populations of domestic European rabbits have proliferated in the wild and are found in various parts of the world. The genus Sylvilagus is native to the Americas and is widely distributed throughout North and South America, although some species are restricted to certain regions.110,132 There is also an established population of eastern cottontail (Sylvilagus floridanus) in Italy that was introduced for hunting purposes in the 1960s. 58
Leporids play a crucial role in many ecosystems. They are primary consumers that convert plant protein into animal protein of different weight ranges depending on the species, providing an ideal biomass intake for predators of various sizes, including genets, wild felids and canids, and eagles.56,161,167,173 The decline of native Leporidae species due to diseases such as myxomatosis or rabbit hemorrhagic disease (RHD) can lead to significant changes in the structure and function of natural ecosystems, directly impacting intermediate and top predators, and indirectly affecting other species within interconnected food webs. The wild European rabbit is a particularly well-studied Leporidae species and serves as a crucial food source for >40 terrestrial and aerial carnivore species in the Iberian Peninsula. The Iberian lynx and the Spanish imperial eagle, which include wild rabbits in ~90% of their diet, are among the carnivores that rely on this species.2,57,126
Overview of lagoviruses
The genus Lagovirus belongs to the family Caliciviridae in the order Picornavirales. Until 2023, the genus included 2 species: European brown hare syndrome virus (EBHSV; GD/FR/1989) and rabbit hemorrhagic disease virus (RHDV; GH/DE/1988; EBHSV and RHDV are antigenically and genetically similar [53–70% nucleotide homology in the vp60 gene]).107,133 The primary hosts of EBHSV and RHDV are the European brown hare (Lepus europaeus) and the European rabbit, respectively. RHD is listed by the World Organisation for Animal Health (WOAH) as a notifiable disease of terrestrial animals due to its transmissibility and significant impact on socioeconomic factors and the international trade of animals and animal products. 199
In 2017, several authors proposed a new classification based on phylogeny (Fig. 2), specifically by aligning vp60 gene sequences to determine major lineages. 107 This classification is widely used in the scientific literature about RHD (e.g.,6,160). In 2023, the International Committee on Taxonomy of Viruses (ICTV) officially adopted this classification and merged EBHSV and RHDV into Lagovirus europaeus, 90 which is divided into 2 genogroups: GI, which includes rabbit viruses (RHDV and related nonpathogenic rabbit caliciviruses [RCVs]); and GII, which includes hare viruses (EBHSV and related nonpathogenic hare caliciviruses [HaCVs]). These genogroups are further subdivided into different genotypes, each with a genetic distance of >15% from the others. The GI.1 genotype includes the first identified strains of RHDV (i.e., “classic” RHDV); the GI.2 genotype represents more recent strains classified as RHDV2, which was first detected in France in 2010, and has spread worldwide, gradually replacing previously circulating GI.1 viruses.107,151 The GI.3 and GI.4 genotypes include nonpathogenic RCVs, which are known to circulate silently in rabbit populations. Finally, within some genotypes, there are further subdivisions into different variants based on genetic distances >6%. 107 Therefore, 2 different viruses can cause RHD: RHDV (also known as Lagovirus europaeus GI.1 or simply GI.1) and RHDV2 (also known as RHDVb, Lagovirus europaeus GI.2, or simply GI.2).
Figure 2.
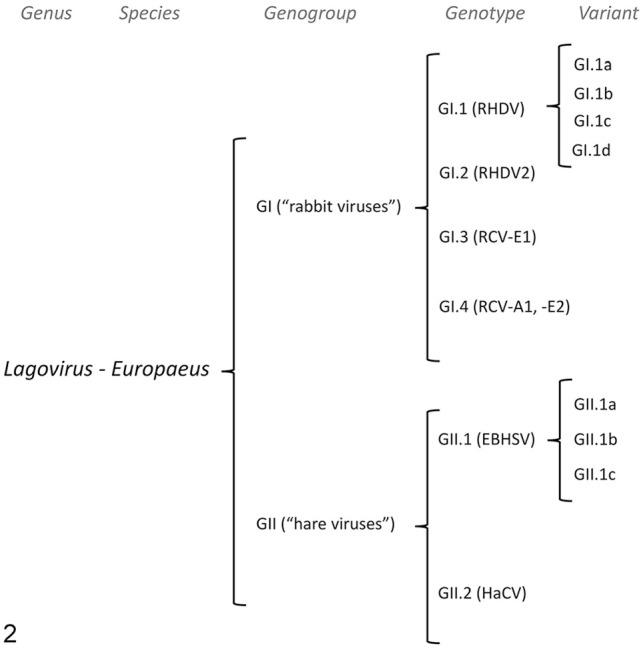
Organization of the Lagovirus genus based on vp60 sequences. 107 Genotypes are established based on genetic differences of >15%. Variants are established based on genetic differences of >6%. EBHSV = European brown hare syndrome virus; HaCV = hare calicivirus (nonpathogenic); RCV = rabbit calicivirus (nonpathogenic); RHDV = rabbit hemorrhagic disease virus.
Lagoviruses are single-stranded, positive-sense RNA viruses with an icosahedral capsid, a spherical shape, and no envelope; the viral capsid is composed of 90 arch-like dimers of the capsid protein VP60 and has 32 cup-shaped depressions reminiscent of a calyx, which gives the viral family the name Caliciviridae.46,90,136,140 The major capsid protein VP1/VP60 forms the structure of the virion; the minor structural protein VP2/VP10 provides stability following encapsidation of the viral RNA.139,140,170 The viral RNA is covalently linked to the viral genome–linked protein (VpG), which is essential for replication.115,123 The viral genome is ~7.4 kb long and contains an additional ~2.2 kb subgenomic RNA that binds to it. The genomic RNA is divided into 2 regions: ORF1, which encodes a polyprotein cleaved into nonstructural proteins (p16, p23, a 2C-like helicase, p29, the VpG, a 3C-like protease, and an RNA-dependent RNA polymerase [RdRp]) and the major structural capsid protein VP60; and ORF2, which encodes the minor structural protein VP10.136,174 The VP60 protein is the major viral antigen and has a surface loop called L1 in the P2 subdomain. This loop varies among strains and contains epitopes that induce the production of neutralizing antibodies. Cross-protective immunity among genogroups and genotypes is limited, suggesting that new viruses are likely to emerge within those levels.31,39,40,50,98,154
As with other members of the Caliciviridae family, inter-genomic recombination between different strains is a very important evolutionary feature of lagoviruses. 117 It usually occurs at the junction between the nonstructural and structural genes (RdRp-vp60), and various combinations involving both pathogenic and nonpathogenic lagoviruses have been described. 6 Recombinants are named with the genotype of the nonstructural donor with a “P” (standing for “polymerase”), followed by the structural donor. 107 For example, a GI.3P-GI.2 virus would be a RHDV2 recombinant virus between the nonstructural genes of a nonpathogenic RCV/GI.3 and the structural genes of a pathogenic RHDV2/GI.2. In addition, a less-common second recombination breakpoint at the p16-p23 boundary has been described, allowing for the occurrence of triple recombinants. 155 In this review, we will use RHDV2 generically to refer to viruses that have been identified as having GI.2 structural (capsid) protein genes, and RHDV to viruses that have been identified as having GI.1 structural (capsid) protein genes, regardless of the complete genotype; when referring to the genotype of specific recombinants, we will use the aforementioned nomenclature (i.e., GX.XP-GI.2 for RHDV2 recombinants).
Typical clinical signs of RHD are the result of severe hepatic failure and dysregulation of the coagulation system. The main differences between RHDV and RHDV2 are that the latter has a much wider host range within the Leporidae family, including several Lepus and Sylvilagus species, and most likely others, in addition to Oryctolagus, and is capable of infecting and causing disease in very young individuals.18,37,51,142 Other potential differences include a slightly longer incubation period for RHD caused by RHDV2, more variable and possibly lower apparent mortality for RHDV2, and possibly a higher proportion of rabbits with subacute or chronic presentations with RHDV2. However, highly virulent RHDV2 strains with mortality rates similar to those of RHDV have been described. 38 According to the WOAH guidelines, a method that is able to differentiate among RHDV2, RHDV, and other lagoviruses must be used to confirm a diagnosis of RHD caused by RHDV2, with RT-PCR or RT-qPCR on liver as the current method(s) of choice in most diagnostic laboratories. 198 A comprehensive review of diagnostic tests is available elsewhere. 8
Objectives and methodology
In this literature review, we gathered data on RHDV2 detections reported worldwide, focusing on reports in different lagomorph species, pathology and genetic differences, and diagnostic advances made in the context of the global spread of this virus in each geographic location. We searched the scientific literature (PubMed, Google Scholar, Web of Science, and Scopus) and the websites of official international, national, and regional animal health regulatory agencies and authorities (e.g., WOAH, U.S. Department of Agriculture–Animal and Plant Health Inspection Service [USDA-APHIS], or as indicated below).
Global detections
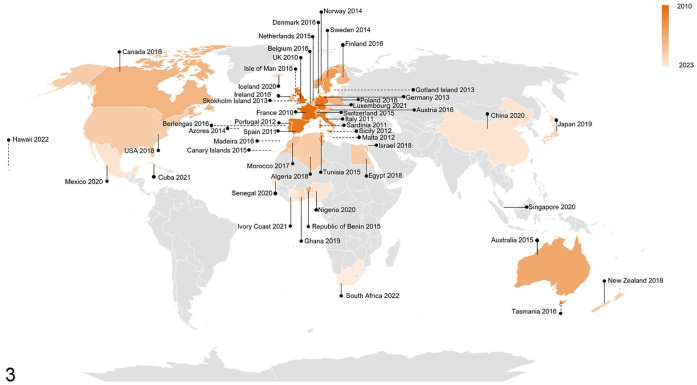
By October 2023, RHDV2 had been detected almost globally (Fig. 3). It is actively spreading to new territories and readers are encouraged to check the official reporting agencies’ websites for the latest information on reports of detection of this virus (e.g., https://wahis.woah.org/#/event-management).
Figure 3.
World map of rabbit hemorrhagic disease virus 2 detections reported between 2010 and 2023 in different countries (solid lines) and islands (dashed lines). Countries and islands with reported detections are in orange; earlier detections are darker, and the latest detections are lighter. Countries with no reported detections are in gray. References: Algeria, 152 Australia, 76 Austria, 19 Azores, 60 Belgium, 84 Benin, 176 Berlengas, 3 Canada, 13 Canary Islands, 121 China, 179 Cuba, 181 Denmark, 182 Egypt, 67 Finland, 92 France, 105 Germany, 70 Ghana, 12 Gotland, 130 Hawaii, 157 Iceland, 183 Ireland, 149 Isle of Man, 149 Israel, 184 Italy, 186 Ivory Coast (RHDV2 was most likely present at least since 2016 187 ), 188 Japan, 96 Luxembourg (RHDV2 was most likely present at least since 2015–2017 120 ), 189 Madeira, 43 Malta, 169 Mexico, 190 Morocco, 114 Netherlands, 63 New Zealand (RHDV2 was most likely present at least since 2017 124 ), 191 Nigeria, 55 Norway, 192 Poland, 69 Portugal, 7 Sardinia, 142 Senegal, 193 Sicily, 34 Singapore, 163 Skokholm, 156 South Africa, 195 Spain, 51 Sweden, 130 Switzerland, 9 Tasmania, 116 Tunisia, 144 United Kingdom, 171 United States. 196 Map developed using Bing technology and adapted.
Europe
The first detections of RHDV2 occurred almost simultaneously in different parts of Europe at the beginning of the first decade of the 2000s, and the virus is thought to have emerged on that continent. The first reports of confirmed RHDV2 detections were from northwestern and central France in April–May 2010, with rapid spread in domestic and wild European rabbit populations.103,105 At the same time as RHDV2 was being detected in several countries in continental Europe, the authors of a 2015 study reported that the virus was also present in 2 locations in England since at least May 2010, based on detections in archived samples; these represented the first confirmed detections in the entire United Kingdom. 171 Given the geographic distance between the detections and the low level of RHD surveillance in England at the time, the authors suggested that RHDV2 may have been circulating in Great Britain before that year. 171 The virus continued to circulate in Great Britain and was also detected in Scotland and Wales in following years.20,171
RHDV2 was first detected in domestic and wild rabbits in the northeast of Spain in May 2011.33,51 However, mortality events associated with a disease compatible with RHD (although not confirmed by laboratory testing) were observed earlier that year in farmed rabbits vaccinated against RHDV in eastern Spain (regions of Catalonia and Valencia), suggesting that the incursion of RHDV2 into Spain may have occurred earlier. 73 Almost simultaneously, in July 2011, RHDV2 was also detected in a rabbitry in northeastern Italy, and cases were also observed that year on the Italian island of Sardinia.103,142,186 Later, in 2012, reports of RHDV2 emerged on the Italian island of Sicily 34 and possibly on Malta (A. Lavazza and L. Capucci, unpublished results mentioned in a 2017 study 169 ).
RHDV2 was detected in wild rabbits from the north and south of mainland Portugal between November 2012 and February 2013, 7 in wild rabbits from the Swedish island of Gotland in May 2013, 130 and in domestic rabbits in Germany in 2013. 70 Cases were detected in the uninhabited island of Skokholm, near the Welsh Coast, in 2013.156,171 Further analyses of wild rabbits revealed the circulation of RHDV2 in the Portuguese Azores islands in 2014, probably due to a previous virus introduction from southern Portugal.59,60 RHDV2 was detected in domestic rabbits in Norway in 2014. 192
In 2015, RHDV2 was detected in domestic rabbits in Switzerland and the Netherlands.9,63 In 2016, detections were reported in domestic rabbits in Austria, 19 Belgium, 84 and Denmark, 182 and in domestic, wild, and/or feral domestic rabbits in Finland, 92 the Republic of Ireland, 149 Poland, 69 the Isle of Man, 149 and the Portuguese archipelagos of Berlengas and Madeira.3,43 Phylogenetic analyses of the Madeira viruses again suggested a possible introduction from the south of mainland Portugal. Detections of RHDV2 were first reported to WOAH in domestic rabbits in Iceland and Luxembourg in 2020 and 2021,183,189 respectively; however, a 2018 study mentioned detections of RHDV2 in samples of domestic rabbits from Luxembourg collected between 2015 and 2017. 120 RHDV2 was most likely detected in Croatia in 2023 180 ; the latter country has not reported the virus type yet, but per the mentioned WOAH report, the detection was in a European brown hare, suggesting that it was RHDV2 rather than RHDV.
The broader host range of RHDV2 was first recognized in Europe when the virus was detected in association with disease in hares from several countries (see also section on Lepus). During the autumn and winter of 2011, an outbreak of a disease compatible with RHD affected a significant number of Cape hares (syn. Sardinian Cape hares; Lepus capensis mediterraneus) in Sardinia, Italy, and was confirmed to be caused by RHDV2. 142 The following year, RHDV2-associated mortality was reported in Italian hares (syn. Corsican hares; Lepus corsicanus) and European brown hares, also in Italy, followed by detections in European brown hares in France in 2013 and Spain in 2014.34,104,169 Cases in mountain hares (Lepus timidus) were reported between 2016 and 2017 in Sweden and Germany.25,129 In 2017, the virus was detected in hares of unspecified species in the Netherlands. 64 In 2018–2019, the virus was detected causing mortality in Irish hares (Lepus timidus hibernicus) in Ireland, 97 European brown hares in England and Scotland,21,148 and, shortly thereafter, in Iberian hares (syn. Granada hares; Lepus granatensis) in Spain in 2020. 168
In countries in which RHDV2 was detected, there was rapid viral spread, and this new virus quickly replaced the classic RHDV strains that had been circulating. The spread of RHDV2 and the replacement of RHDV strains was faster in areas in which wild rabbit populations were abundant and the competitive advantages of RHDV2 over RHDV were enhanced,33,103 so that RHDV strains were almost completely replaced in <2 y after emergence in countries such as France, 103 Spain, 50 and Portugal. 112 However, in other countries, such as Germany and Poland, RHDV continued to co-circulate with RHDV2 for longer times.87,160
Shortly after its emergence, recombinant RHDV2 strains were detected in the Iberian Peninsula, 113 France, 104 and the Portuguese archipelagos of Azores and Madeira,10,111 infecting rabbits and also hares.104,168 Initially, most of the recombination events identified involved the junction between the genes encoding for VP60 (structural proteins) and RdRp (nonstructural proteins); RHDV2 (genotype GI.2) was the donor of the structural proteins, whereas the nonstructural proteins originated from either a pathogenic RHDV (genotype GI.1) or a nonpathogenic RCV (genotypes GI.3 or GI.4). RHDV2 recombinants encoding the nonstructural genes of EBHSV (genotype GII.1) were also reported to infect hares in Germany. 160 Furthermore, a second recombination breakpoint was detected in Portugal at the junction of the 2 genes encoding the nonstructural proteins p16 and p23, allowing the existence of triple recombinants in which RHDV2 was the donor of the structural part, a nonpathogenic lagovirus was the parental donor of p16, and RHDV genotype GI.1b was the donor of the rest of the nonstructural part. 155 The latter illustrates the high potential of lagoviruses to increase genetic diversity, leading to the emergence of new pathogenic strains. There is strong evidence that recombination contributed to the emergence of RHDV2 as a pathogenic virus in Europe, as phylogenetic and recombination analyses have shown that the first RHDV2 strains, previously considered as non-recombinants, arose from a recombination event between the GI.3 and GI.2 genotypes. 6
Detection methods evolved rapidly in response to the emergence of RHDV2 in Europe. Although it was initially reported that the virus did not agglutinate human RBCs of type O or A, 51 further studies demonstrated that RHDV2 efficiently agglutinated RBCs of type O at titers similar to those seen with most classic RHDVs, and hemagglutination (HA) was therefore often used to screen for RHDV2. 103 However, because HA cannot be used to differentiate between different lagoviruses, after the emergence of RHDV2, viral detection was mainly performed by amplification and sequencing of the partial or complete gene encoding VP60 (e.g.,50,103,105,130). Subsequently, given the different antigenic profile of RHDV2, differentiation from RHDV could also be performed using anti-RHDV monoclonal antibodies and a sandwich ELISA.103,142 Nevertheless, procedures based on RT-qPCR targeting a specific nucleotide RNA region located within the vp60 gene have been, and are by far, the most widely used assays for RHDV2 detection and viral load assessment due to their high specificity, sensitivity, and efficiency.48,61 Interestingly, RT-qPCR can detect RHDV2 RNA in commercial inactivated vaccines, but cannot detect vaccine RNA in rabbits vaccinated 15 d before, enabling the detection of circulating field strains in rabbitries following an RHD outbreak and subsequent vaccination. 42 RT-qPCR was also useful to demonstrate the likely presence of persistent infections, carriers, and unnoticed virus circulation in rabbitries after an RHD outbreak.29,41,122 Similarly, a duplex RT-qPCR designed to detect RHDV (genotype GI.1b) and RHDV2 RNA in a single analysis was developed and used to monitor RHD outbreaks and interactions between the 2 different viral types in wild rabbit populations.28,30,32
Despite the undoubted usefulness of PCR-based methods, other less-sensitive detection tests have been developed in Europe, such as a sandwich ELISA for the detection of RHDV2 antigen in rabbit liver homogenates, 53 which allowed the detection of the virus with high specificity in laboratories without PCR equipment, or a qualitative immunochromatographic test developed to be used on-site, which allowed rapid and specific detection in the field (e.g., on farms). 52 Finally, serologic analyses to detect antibodies against RHDV2 were performed with a competitive ELISA already developed for RHDV strains and later used in epidemiologic surveys of RHDV2.28,103,169
Africa
Detections of RHDV2 were first reported in 2015 in 3 locations in Africa and close-by islands. The virus was detected in domestic rabbits in Tunisia; in domestic and wild European rabbits in Tenerife, an island of the Spanish Canary Islands archipelago; and in domestic rabbits in the Republic of Benin.22,121,144,176 In Tunisia and the Canary Islands, the virus was identified during routine surveillance of annual RHD outbreaks, and the specific date of introduction was unknown. Phylogenetic analysis showed that the Tunisian viruses originated from a single introduction of European strains, most consistent with strains from Italy, which was suspected to have occurred between 2012 and 2014, 144 whereas viruses from Tenerife had the highest nucleotide identity with Portuguese strains. 121
Subsequently, reports from Ivory Coast suggested that RHDV2 was present in that country in 2016, because both rabbits and hares were affected, but the virus type was not reported to be RHDV2 until 2021.114,187,188 In 2017, RHDV2 was first detected in Morocco in samples obtained from wild rabbits, and the strains identified were GI.1bP-GI.2 recombinants, as found in the Iberian Peninsula and in the Azores and Madeira archipelagos 114 ; RHDV2 circulated widely in Morocco, at least up until 2021. 153 Subsequently, in 2018, the virus was detected in Egypt during regular surveillance for RHD outbreaks, so the date of the first introduction of the virus in that country is also unknown. 67 An August 2023 study reported that RHDV2 was circulating in the North of Algeria since at least 2018 152 ; the viruses were most similar to the GI.3P-GI.2 recombinant and the earlier detections clustered with Tunisian viruses, whereas the most recent (2020–2021) samples included in the analysis were more similar to some North American viruses. 152 RHDV2 was first reported in commercial rabbit farms in Ghana in 2019, 12 and in domestic rabbits in Senegal and Nigeria in 2020.55,193 Phylogenetic analysis of vp60 and complete sequences obtained from microbial metagenomic sequencing techniques showed that Nigerian strains had high homology with RHDV2 viruses from the Netherlands and Germany, suggesting possible virus importation.54,55,79 A subsequent study confirmed that RHDV2 had been introduced into Africa several times, with independent incursions into, at least, Tunisia, Nigeria, and Morocco, and was most likely of European origin. 22 RHDV2 was detected in South Africa in November 2022 and, per reports to WOAH, has affected domestic rabbits and native wild rabbits and hares. 195 As multiple RHDV2 detections occurred in western and northern African coastal countries, it was suggested that the virus entered each country through legally or illegally imported rabbits or related products.12,22,144
Oceania
Detections of RHDV2 in Oceania have been reported in Australia and New Zealand.175,191 The history of pathogenic lagoviruses in Australia is unique. Prior to the emergence of RHDV2, RHDV had circulated widely, mainly as a result of its use to control populations of invasive wild European rabbits. The Czech V351 (CAPM V-351) RHDV strain (a GI.1c virus) was released for this purpose in 1995 and was considered to be an ancestor of some circulating pathogenic lagoviruses.65,100,116 In 2017, the Korean K5 strain (a GI.1a virus) was also released as a biocontrol agent. 116 An incursion with active circulation of a different RHDV genotype GI.1a virus occurred in 2014. 118 Additionally, several variants of the nonpathogenic RCV (GI.4) were present in Australia prior to the release of RHDV.147,159
RHDV2 was first detected in a wild European rabbit in Australia in May 2015.76,175 Three dead wild rabbits were tested as part of surveillance of RHD outbreaks prior to the release of the K5/GI.1a in the Canberra region. While field RHDV was found in 2 carcasses, 1 animal tested positive for RHDV2. Subsequent phylogenetic analysis revealed that the virus was a GI.1bP-GI.2 recombinant.76,116 This virus was similar to some viruses previously circulating in mainland Portugal and the Azores.10,113 RHDV2 was also detected in Tasmania in 2016. 116 Retrospective serologic studies showed that RHDV2 had been circulating in Australia before its first detection, at least since spring 2014, 146 which was also supported by studies on the evolutionary history of Australian viruses and further serologic studies.116,138,158 RHDV2 had a significant impact on the wild rabbit populations between 2014 and 2018, with average population declines of up to 60% and more pronounced reductions in southern and western Australia. 146 Interestingly, only 18 months after its first confirmed detection, RHDV2 had replaced the circulating endemic RHDV viruses in Australia, and was also detected in domestic rabbits and European brown hares.78,116 Cases of RHDV2 infection continue to be detected in Australia; seroprevalence remained high until at least 2022, and it is thought to be the virus currently having the greatest impact on wild populations. 145 However, a low percentage of RHDV-seropositive animals are still being detected, suggesting co-circulation to some extent and ruling out total extinction of RHDV.145,146 In fact, the previous occurrence of multiple pathogenic and nonpathogenic lagoviruses in Australia has shaped the dynamics of RHDV2, fostering the occurrence of multiple recombinants, 117 and at least 7 different lagoviruses are believed to be circulating: RHDV genotype GI.1, RCV genotype GI.4, RHDV2 genotype GI.1bP-GI.2, RHDV-K5 genotype GI.1a, RHDV genotype GI.4eP-GI.1a, RHDV2 genotype GI.4cP-GI.2, and RHDV2 genotype GI.4eP-GI.2. 138
The use of different ELISA strategies has therefore been fundamental in shaping the Australian lagovirus landscape even before the emergence of RHDV2.145,147 However, various PCR approaches are the current method of choice and the test by which the occurrence of RHDV2 was initially confirmed in Australia. 76 A multiplex RT-PCR that was able to differentiate among all of the pathogenic lagoviruses circulating in Australia at the time was developed. 77 The assay was even able to identify mixed infections in individual animals. In addition, a SYBR green–based RT-qPCR was optimized to detect and quantify lagovirus genetic material in a single PCR reaction. 77 Monolayer cell cultures derived from European rabbit and European brown hare hepatobiliary organoids support the replication of several Australian RHDV2 (both rabbit and hare organoids) and RHDV (rabbit organoids only) 95 ; this provides a useful alternative to traditional viral culture systems, which have proven very challenging for lagoviruses.
The situation in New Zealand was somewhat similar to that in Australia. Prior to the RHDV2 incursion, the biocontrol agent CAPM V-351 RHDV was detected in 1997, possibly as a result of an illegal introduction by farmers, 135 and further releases were made in the following years. The K5 RHDV strain was also released in 2018 for a similar purpose. The nonpathogenic RCV (GI.4) was also present in New Zealand before 1997. 131 RHDV2 was first detected in a wild rabbit collected from the South Island in May 2018, but analysis of samples from rabbits collected the previous year were also positive.124,191
Asia
The first reports of RHDV2 occurrence in Asia came from Israel in 2018. 184 The same country reported cases to WOAH in 2020 and 2021. 185 The only publicly available reports from Israel referred to detections in domestic rabbits. Reports of RHDV2 occurrence in East Asia appeared in 2019–2020, including detections in Japan and China.96,179 Maritime Southeast Asia has also been affected, with detections in Singapore in 2020. 194
Since 2019, RHDV2 has been detected in several prefectures in Japan.71,96,162,165 In May 2019, all 10 rabbits in an exhibition facility from Ehime Prefecture died with gross and microscopic lesions compatible with RHD; viral particles were visualized by electron microscopy and the diagnosis was confirmed by RT-PCR followed by partial vp60 sequencing 96 ; this was the first documented case of a pathogenic lagovirus in Japan in 17 y. At least one other case of RHDV2 was detected in Ibaraki Prefecture in 2019. 162 Shortly afterward, in 2020, an outbreak with a mortality rate of 55% occurred in a colony of domestic European rabbits in a zoologic garden in Chiba Prefecture. 71 Similarly, in May 2020, 11 of 15 domestic rabbits from an exhibition facility in Tochigi Prefecture died from RHD caused by RHDV2. 165 Other prefectures were likely affected in 2020, but the viral type was not determined. 162 Whole-genome sequencing (WGS) with recombination and phylogenetic analyses of several Japanese detections in 2019–2020 revealed that at least 2 different RHDV2 recombinants, GI.3P-GI.2 and GI.1bP-GI.2, were circulating, and were more similar to viruses found in Germany and Australia in 2017, respectively, suggesting that the Japanese cases were associated with different foreign incursions. 162
In April–May 2020, large outbreaks of RHD due to RHDV2 were reported in farmed rabbits in Sichuan Province, China and later spread to other provinces.44,89,108 These were the first reports of RHDV2 detection in the country. Mortality was high, ranging from ~43% to >70%, and affected also young individuals and rabbits that had been vaccinated against RHDV. The Sichuan viruses were most closely related to detections from the Netherlands in 2016 and Germany in 2017, and were GI.1aP-GI.2 recombinants.44,108,143 Based on the phylogenetic similarities with these European strains and the reliance of the Chinese rabbit industry on imports from European countries, it was suggested that some of these introductions into China may have originated from rabbit batches or imported semen purchased in Europe.44,108,143 A 2023 study reported that a novel recombinant, distinct from the original Chinese strain, was detected between 2020 and 2021 in at least 3 different provinces (Sichuan, Jiangsu, Guangxi) separated by 1,000–1,500 km. 88 The novel recombinant had structural genes of the RHDV2 strain first detected in China and a nonstructural component of an unclassified lagovirus genotype similar to a virus that had been identified in healthy wild rabbits in the country.81,88 This recombinant induced lower mortality and had a longer replication time than the RHDV2 genotype GI.1aP-GI.2 detected in some of the first Sichuan cases. 88
In September 2020, Singapore reported their first documented cases of RHDV2. 163 All cases occurred in domestic pet rabbits and involved at least 3 households from an urban area; 8 of 11 exposed rabbits died. Rabbits from the different households had had some degree of contact with each other, and intra- and inter-household transmission, including contact at a veterinary clinic, were highlighted as important modes of dissemination in this outbreak.109,163 The source of the outbreak could not be determined, and introduction via infected rabbits or contaminated feed was initially considered unlikely. 109 No further cases were reported that year, and the outbreak was declared resolved in December 2020. 109 Subsequent WGS and recombination and phylogenetic analyses revealed that the Singapore 2020 virus was a GI.4cP-GI.2 recombinant highly homologous (~99.2%) to certain Australian viruses circulating in rabbit and hare populations since 201799,117; therefore, it was suggested that the origin of the Singapore 2020 cases might have been an introduction of a virus that originated in Australia, but the means by which this occurred were unknown. 99
The Americas
As of September 2023, RHDV2 detections in the Americas have been limited to North America, specifically to Canada, 177 the United States, 196 Mexico, 190 and Cuba. 181 Interestingly, no detections have been reported from South America. This region remains as one of the largest officially unaffected areas in the world, and continued surveillance is warranted in the current context of rapid viral spread.
RHDV2 was first detected in North America in August 2016 in a 4-mo-old rabbit from a hobby farm in Mont-Joli, Quebec, Canada; the farm had received rabbits from another farm that was also presumably affected by RHD.13,177 Mortality in the affected premises was 72–89%, and several surviving rabbits were seropositive by ELISA. Sequences from this strain of RHDV2 were most similar to a virus collected in 2011 in Navarra, Spain. 13 Following this outbreak, RHDV2 was not detected again in Canada until 2018. 178 In December 2017, a significant mortality event was reported in feral rabbits from a private property south of Nanaimo, British Columbia but was not further investigated; high mortality was then reported in a population of feral domestic rabbits from the Vancouver Island University campus in early 2018. 13 Additional cases in domestic rabbits were reported in mainland British Columbia through the summer of 2018.13,85 The strain of RHDV2 detected in British Columbia in 2018 shares only ~93% sequence homology with the strain detected in Quebec in 2016 and is thought to represent a separate incursion. 13 In 2019, additional detections were reported in the region; of note, there was one case involving pet rabbits in a downtown Vancouver apartment that shares only ~97% identity at the nucleotide level with the 2018 British Columbia index case and is thought to represent a separate incursion into the region. 13 Between 2020–2023, RHDV2 was detected several times in feral and captive domestic rabbits in other Canadian provinces in addition to British Columbia and Quebec, including Ontario and Alberta. 72
RHDV2 was first detected in the United States in 2018, affecting pet rabbits in Ohio, 196 and, shortly thereafter, in feral domestic rabbits in Washington State in 2019. 172 The sequences of the viruses from Ohio and Washington State were both similar to a strain detected in British Columbia in 2018. 166 In March 2020, RHDV2 was reported in domestic rabbits from a veterinary clinic in New York City, likely representing an introduction different from the Ohio and Washington State cases.134,166
In March 2020, RHDV2 was detected in domestic rabbits in New Mexico. 197 Since then, the virus has spread extensively throughout the southwestern United States and later to other areas of the country; RHDV2 is now considered to be endemic in most of the western United States, with sporadic detections reported in some eastern states. 197 The outbreak in the southwestern United States represents a new milestone in the global spread of RHDV2 as it is the first time that natural cases of RHD were reported in Leporidae species native to North America, including the black-tailed jackrabbit (Lepus californicus), antelope jackrabbit (Lepus alleni), desert cottontail (Sylvilagus audubonii), eastern cottontail, mountain cottontail (Sylvilagus nuttallii), western brush rabbit (Sylvilagus bachmani), riparian brush rabbit (Sylvilagus bachmani riparius), and pygmy rabbit (Brachylagus idahoensis); detections in domestic and feral domestic European rabbits occurred in parallel.17,47,101,166,197 Due to the high diversity of lagomorph species in the United States and the fact that reliable species identification is not always possible, it is likely that other species have been affected.
The first public complete RHDV2 sequences from the United States were made available in a 2020 study 134 ; 3 sequences collected in Texas, New Mexico, and Arizona between March and April 2020, at the beginning of the southwest outbreak, had high nucleotide identity (almost 99.5%) and formed a cluster distinct from the New York 2020 detections. Sequences from California collected in late 2020 and early 2021 were highly homologous (99–99.4%) to the southwestern sequences reported in the aforementioned 2020 study.18,134 No comprehensive recombination studies have yet been published, but analyses of the topology of the phylogenetic trees of the structural and nonstructural genes of the 2020–2021 California sequences suggest that they were GI.3P-GI.2 recombinants. 18 Because the virus has circulated widely and no detailed phylogenetic and recombination studies have been published yet, it is possible that other recombinants are present in the country. Of the available sequences from North America reported before 2020, the aforementioned southwestern U.S. sequences were more similar to one of the 2018 detections in British Columbia13,18; however, at this stage, it cannot be excluded that some of the cases of the southwestern outbreak and further detections in other parts of the country represent different incursions. In fact, RHDV2 detections continue to date, and as of 2023 Sep 30, the virus has been reported in 29 states, including Hawaii (USDA-APHIS RHDV2 outbreak map: https://www.aphis.usda.gov/aphis/maps/animal-health/rhd [cited Oct 2023]). Kohl and collaborators of the “RHD Awareness Team” from the University of Georgia are performing an up-to-date follow-up of the cases in the United States, compiling information from the USDA-APHIS and other sources, and the results can be viewed at https://rhdv2.org/ (cited Oct 2023).
In April 2020, almost at the same time as the first cases of the southwestern U.S. outbreak were being notified, detections were also reported in several northern states of Mexico. 190 The virus has been reported in wild and domestic Leporidae species from at least 19 states, and is considered to be endemic in Mexico as of 2021; a sequence from Chihuahua available in GenBank (MT982431.1) clusters with the 2020–2021 southwestern U.S. sequences.
Several detections of RHDV2 from Cuba have been reported to WOAH since 2021, mostly in farmed rabbits. 181 Further details of the spread and sequences are not available, but per the mentioned reports, multiple premises in different cities have been affected with up to thousands of animals being lost due to viral infection or culling.
During the recent outbreaks in the Americas, some novel detection approaches have been attempted. RT-qPCR from dried blood filter paper and ear punch samples obtained from cottontail rabbits (Sylvilagus spp.) and jackrabbits (Lepus spp.) had 100% sensitivity and specificity compared to liver at initial sampling and remained high after 6 wk, representing good alternative sample types to liver for RHDV2 surveillance in wild Leporidae species. 93 Another study compared a post-freezing and thawing magnetic-bead RNA extraction method with a single-tube rapid swab extraction method after a second freeze–thaw cycle, and demonstrated an ~1 log10 reduction in sensitivity with the latter. 86 In a California study, sensitivity and specificity of RT-qPCR from rectal swabs obtained from a pool of domestic rabbits and native North American leporids were 88% and 100%, respectively, compared with liver, suggesting that this sampling method can be used as a strategy to screen carcasses for viral RNA. 16 A pan-lagovirus immunohistochemistry (IHC) test using antibodies obtained from the WOAH RHD Reference Laboratory (Brecia, Italy; provided by Drs. A. Lavazza and L. Capucci) has been used successfully as a complementary detection tool in domestic rabbits and wild Leporidae species native to North America.16,18 In addition, RNAscope (ACDBio)-based in situ hybridization has proven very effective in localizing viral nucleic acid in different cell types. 137
Affected animal species
Oryctolagus
The first detections of RHDV2 were reported in domestic and wild European rabbits.103,105 The virus has circulated widely in this species, particularly in Europe and Oceania, and the vast majority of the experimental infections have been carried out using European rabbits. It is, therefore, the species in which the various aspects of the disease have been better characterized. As data for other species are much scarcer, disease features in European rabbits are often extrapolated to other Leporidae species, although differences are likely to occur.
Mortality associated with RHDV2 in European rabbits varies among different reports of natural disease, and is generally 10–90%, or occasionally up to 100% in small, naïve domestic populations.18,75 In wild populations, high mortality has been reported, particularly when the virus first occurs in a geographic area, and this has been one of the reasons the European rabbit has been listed as “endangered” in its natural range. 94 Case fatality in experimental settings is also variable, 0–100%,75,88,164 and 42.6–48.2% in experimental settings with the highest number of animals used (n = 94 30 and n = 110 106 ). The time from inoculation to death or euthanasia was 18 h to 9 d in the reports of experimental infections,75,88,164 with a mean time to death or euthanasia after inoculation of ~53 h in one of the experiments with the highest number of inoculated animals (n = 94 30 ). Some experimental studies did not find differences in mortality rates and clinical signs between adult rabbits and kittens.30,75 However, in a 2022 experiment, 164 shorter clinical courses and higher viral loads were observed in 4-wk-old kittens compared to adults and subadults. Significantly shorter survival time and time of onset of pyrexia were observed in 5-wk-old kittens compared with 11-wk-old rabbits in a different study. 75 Overall, differences in disease course and mortality may be due to genetic differences in the RHDV2 viruses involved, route and dose of infection, previous immunity to lagovirus infection (including other pathogenic and nonpathogenic lagoviruses, and vaccination), and other epidemiologic parameters.32,75
The clinical signs and pathology (Table 1) of RHDV2 infection in the European rabbit have been described in some detail in both natural and experimental cases.9,30,49,75,80,128,141,172 Major clinical signs include sudden death, fever, collapse, lethargy, seizures, icterus, oral bleeding, dyspnea, hypothermia, bradycardia, poor blood clotting, epistaxis, apathy, depression, anorexia, ataxia, and circling. Elevations in serum transaminases and alkaline phosphatase activities and bilirubin have been noted in pet rabbits24,141,163; however, one report mentioned significant decreases in aspartate aminotransferase and alanine aminotransferase activities in 2 pet rabbits, perhaps related to the short half-life of these enzymes in rabbits. 24 Peracute, acute, subacute, and chronic clinical presentations are described in individual animals. Sudden death without additional signs was frequently recorded in a survey of pet rabbits, 80 and it is thought to be the most common outcome in peracute disease. Subacute-to-chronic clinical courses were relatively common in the first reports of RHDV2. 103 Epistaxis or serosanguineous nasal discharge (Fig. 4) is considered a classic clinical sign of RHD; however, a large survey of commercially farmed rabbits found no clear association between serosanguineous nasal discharge and RHDV2 infection, 150 and therefore the absence of this finding should not preclude a presumptive diagnosis of RHD in cases of mass mortality.
Table 1.
Gross and microscopic lesions of rabbit hemorrhagic disease virus 2 in European rabbits (Oryctolagus cuniculus).
| Organ | Gross lesion | Microscopic lesion |
|---|---|---|
| Liver | Hepatomegaly, pallor, hemorrhages, marked acinar/lobular pattern, tan subcapsular foci | Necrosis (periportal, massive, or random); hepatocyte individualization, hypereosinophilia, pyknosis, karyorrhexis, karyolysis; vacuolar degeneration (lipid type); cholangitis, portal fibroplasia, proliferation of bile ducts (subacute-to-chronic forms); hepatocyte mineralization (subacute-to-chronic forms); multinucleated hepatocytes (subacute-to-chronic forms) |
| Lung | Congestion, hemorrhages (petechiae to more widespread distribution), foam and serosanguineous fluid in airways | Intraalveolar and perivascular hemorrhages, alveolar edema, microthrombi in septal capillaries and lobular small to medium-sized vessels |
| Spleen | Splenomegaly, friability, dark-red to black discoloration | Hyaline necrosis of red pulp, congestion and hemorrhage, lymphocytolysis in white pulp, microthrombi in central arterioles |
| Kidney | Pallor to reddening, petechiae | Interstitial, cortical, and medullary hemorrhages; microthrombi in glomerular and interstitial capillaries; tubular epithelial degeneration and necrosis; protein or hemoglobin casts |
| Heart | Petechial-to-paintbrush hemorrhages in epicardium and endocardium | Myocardial hemorrhages, cardiomyocyte degeneration and necrosis |
| Trachea | Dark-red mucosal discoloration, abundant foam | Markedly congested submucosal and lamina propria capillaries, mucosal mineralization |
| Stomach and intestines | Gastric and cecal dilation and stasis | Superficial and crypt epithelium necrosis, capillary microthrombi and hemorrhages in lamina propria capillaries |
| Thymus | Petechiae | Lymphocytolysis |
| Brain | – | Microthrombosis and rarefaction of adjacent neuropil, mild lymphoplasmacytic perivascular meningoencephalitis |
| Reproductive tract | Serosal hemorrhages in the uterus | – |
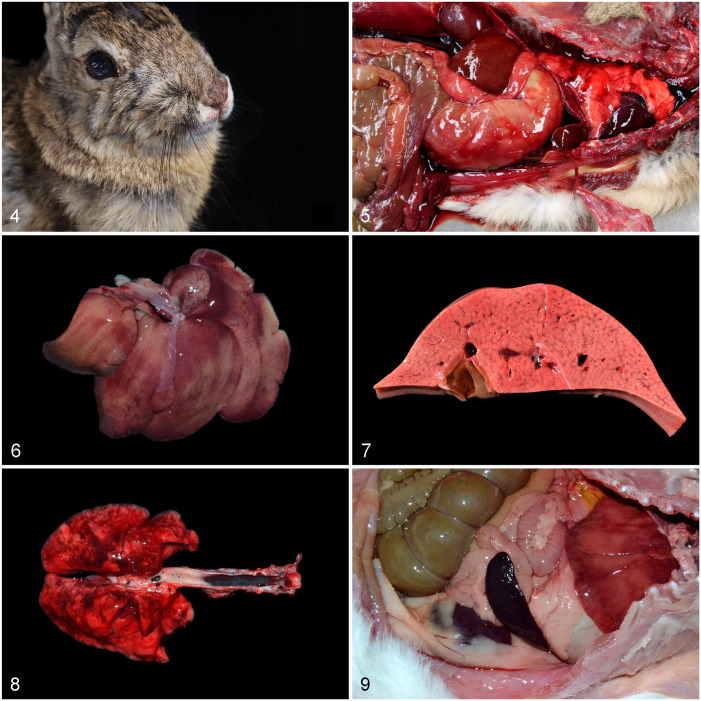
Figures 4–9.
Gross lesions of rabbit hemorrhagic disease virus 2 infection. Figure 4. Desert cottontail rabbit with blood around the nares. Figure 5. Black-tailed jackrabbit with pulmonary and serosal congestion and hemorrhages, and blood in abdominal cavity. Photo courtesy of Dr. Akinyi Nyaoke, UC Davis. Figures 6–9. Domestic rabbits. Figure 6. Diffuse hepatic pallor and reddening of the capsule with enhanced reticular pattern. Figure 7. Cut section of liver with pallor and reticular pattern. Figure 8. Pulmonary hemorrhages and congestion. Tracheal congestion and foam in the lumen. Figure 9. Marked splenomegaly and mild hepatic pallor.
On gross postmortem examination (Table 1; Figs. 4–9), a lobular or reticular pattern in the liver is frequently, albeit not always, observed, and overall enlargement, pallor, and friability usually affect this organ (Figs. 6, 7, 9). There is usually congestion and hemorrhage in several organs, being particularly severe in the liver and lungs (Figs. 5, 8). Dark-red discoloration of the tracheal mucosa has been noted as a distinctive gross change (Fig. 8). Serosanguineous fluid or blood in body cavities has also been described as a distinctive feature (Fig. 5). Less commonly, the spleen is enlarged (Fig. 9), and icterus is occasionally present. Importantly, gross lesions may be minimal to absent in some animals with RHD caused by RHDV2,9,80 and in wild and feral domestic rabbits, gross and microscopic lesions are usually obscured by postmortem decomposition or freeze–thaw artifacts. 172
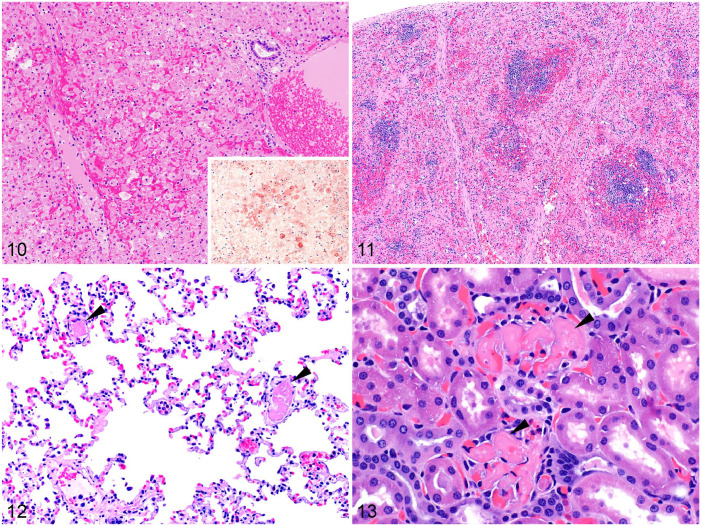
Histologically (Table 1; Figs. 10–13), hepatic necrosis with hepatocellular dissociation and apoptosis, and a periportal-to-massive distribution, is the hallmark and consistent microscopic lesion (Fig. 10). This lesion usually has diagnostic value in cases with limited gross changes. However, hepatic necrosis with random rather than periportal distribution is occasionally observed, 172 which suggests that the pattern of hepatic necrosis should not always be considered as an unequivocally distinctive feature. Preparing overnight histologic sections may be effective in establishing a presumptive diagnosis of RHD while awaiting confirmatory PCR results (J. Asin, E.E. Henderson, and F.A. Uzal, personal observations). Hepatic necrosis is sometimes associated with congestion, hemorrhage, and sinusoidal thrombosis; mild-to-marked infiltration of heterophils and macrophages is frequently seen. The spleen may have deposition of hyaline eosinophilic material in the red pulp, marked hemorrhage and congestion, and lymphocytolysis in the white pulp (Fig. 11). Pulmonary congestion, edema, and hemorrhage are observed in most cases, usually with microvascular thrombosis (Fig. 12). Microthrombi in glomerular capillaries (Fig. 13) and acute tubular injury are occasionally seen in the kidneys. Microthrombosis can be observed in virtually any organ and is the result of disseminated intravascular coagulation and perhaps viral infection of endothelial cells (Table 1).80,137
Figures 10–13.
Microscopic lesions of rabbit hemorrhagic disease virus 2 infection. H&E. Figure 10. Liver from a riparian brush rabbit, with hepatic necrosis, disorganization of hepatocyte cords, hypereosinophilia, pyknosis, karyorrhexis, and hemorrhage. Inset: positive pan-lagovirus immunohistochemistry. Case kindly donated by Dr Peter Chu, UC Davis. Figures 11–13. Domestic rabbits. Figure 11. Lymphoid depletion of follicles in the white pulp of the spleen, and red pulp necrosis with seams of hyaline eosinophilic material. Figure 12. Fibrin thrombi in 2 pulmonary vessels (arrowheads). Figure 13. Fibrin thrombi (arrowheads) in glomerular capillaries.
Lepus
RHDV2 has been detected in a number of hare and jackrabbit species (Table 2).25,26,34,104,142,166,168,195 Most cases of RHDV2-associated mortality in hares coincided with concurrent outbreaks in rabbits, suggesting the role of rabbits as the primary source of infection to initiate outbreaks in hares. However, with the exception of some outbreaks such as the one that occurred in Cape hares in Sardinia, Italy, 142 in which a significant number of hares were affected, mortality is limited in the available reports and generally lower than in European rabbits, 34 suggesting the occurrence of spillover events from rabbits to hares favored by the high infection pressure during outbreaks in the former, but causing only sporadic infections in the latter. 169 Transmission between infected hares has been postulated but not definitively demonstrated experimentally.104,129 Interestingly, viruses collected from hares can also have high genetic variability. For instance, 2 RHDV2 genotype GII.1P-GI.2 recombinants were collected from European brown hares in Germany in 2014 and 2019, 160 and a GI.1bP-GI.2 recombinant was detected in the same species in Australia in 2016.78,116
Table 2.
Detections of rabbit hemorrhagic disease virus 2 in Leporidae species other than European rabbit (Oryctolagus cuniculus).
| Common name | Scientific name | Country (island) | Reference |
|---|---|---|---|
| Antelope jackrabbit | Lepus alleni | USA | 166,197 |
| Black-tailed jackrabbit | Lepus californicus | USA, Mexico | 17,101,166,197 |
| Cape hare | Lepus capensis | South Africa | 195 |
| Desert cottontail | Sylvilagus audubonii | USA, Mexico | 17,101,166,197 |
| Eastern cottontail | Sylvilagus floridanus | USA | 125,166,197 |
| European brown hare | Lepus europaeus | Italy, Spain, France, Australia, England, Scotland, Germany | 21,68,78,104,148,169 |
| Iberian hare | Lepus granatensis | Spain | 168 |
| Irish hare | Lepus timidus hibernicus | Ireland | 26,97 |
| Italian hare | Lepus corsicanus | Italy (Sicily) | 34 |
| Mountain cottontail | Sylvilagus nuttallii | USA | 166,197 |
| Mountain hare | Lepus timidus | Sweden (Hallands Väderö), Germany | 25,129 |
| Pygmy rabbit | Brachylagus idahoensis | USA | 47 |
| Red rock rabbit | Pronolagus spp. | South Africa | 195 |
| Riparian brush rabbit | Sylvilagus bachmani riparius | USA | 27 |
| Sardinian Cape hare | Lepus capensis mediterraneus | Italy (Sardinia) | 142 |
| Scrub hare | Lepus saxatilis | South Africa | 195 |
| Western brush rabbit | Sylvilagus bachmani | USA | 16 |
Descriptions of lesions in hares and jackrabbits are limited and often based on single case reports of natural disease. Nevertheless, gross findings in hares and jackrabbits appear to be similar to those described in European rabbits with RHD and also in European brown hares with EBHS (Fig. 5). 119 Friable discolored livers with a prominent reticular pattern, congestion of viscera (particularly of the tracheal mucosa), splenomegaly, disseminated hemorrhages, and pulmonary edema have been described.17,25,26,34,97,101,129,142,168,169 Histologic findings are typically hepatic necrosis and apoptosis, usually with a massive distribution, hepatocellular fatty degeneration, and marked congestion and hemorrhage.129,142,169 Periportal-to-midzonal necrosis has been described in an Iberian hare. 168 Lesions in black-tailed jackrabbits in North America are similar to other members of the genus Lepus elsewhere, and some cases have had periportal-to-midzonal hepatocellular dissociation and necrosis or apoptosis.17,101 Hepatocyte mineralization may be a more common feature in some members of this genus compared to other Leporidae species, as it has been described in certain species such as black-tailed jackrabbits and mountain hares.101,129 Interestingly, the latter parallels a comparative description of EBHS in European brown hares and RHD caused by classic RHDV in European rabbits. 119 Some lesions typically associated with RHD in European rabbits, such as microthrombosis, heterophilic inflammation associated with the areas of hepatic necrosis, or lymphoid depletion and other splenic changes, may not be as common in black-tailed jackrabbits. 101 Viral antigen has been detected by IHC in several hare and jackrabbit species using different antibodies.18,129,168 In a study of California cases, 16 liver viral loads were significantly higher in a group of 5 black-tailed jackrabbit carcasses compared to 55 domestic European rabbits (median RT-qPCR Ct of 11.5 [Lepus] vs. 13.0 [Oryctolagus]).
Sylvilagus
The first detections of RHDV2 in rabbits of the genus Sylvilagus were reported in the southwestern United States in April–May 2020. 166 Based on publicly available information, the virus has been detected in at least 5 species of the genus Sylvilagus (Table 2).16–18,27,101,166,197 Other rabbits of this genus native to the Americas are also likely to be susceptible to the virus, and although they may have been affected, data on these species are not yet publicly available. Interestingly, the virus has been detected widely in desert cottontails in the western United States, whereas detections in eastern cottontails in other parts of the country have been rare. 197 However, according to publicly available records, researchers at the Plum Island Animal Disease Centre (NY, USA) demonstrated the susceptibility of the eastern cottontail to develop RHD after experimental inoculation with RHDV2. 125 Interestingly, the eastern cottontail is not susceptible to experimental infection with classic RHDV, 125 but develops an EBHS-like disease when inoculated with EBHSV. 102
Descriptions of RHD lesions in rabbits of the genus Sylvilagus are limited, but the changes appear to be similar to those observed in European rabbits, with hepatic necrosis or apoptosis being the main diagnostic finding (Fig. 10).18,101 Three of 5 eastern cottontails inoculated orally with an RHDV2 genotype GI.1bP-GI.2 recombinant in the Plum Island study 125 developed a disease consistent with RHD with pale friable liver, petechiae, and serosanguineous fluid in the abdomen grossly, and periportal hepatocellular degeneration and necrosis with heterophilic inflammation histologically. Interestingly, 1 of the 2 eastern cottontails that did not succumb to the disease developed a high antibody titer (1:2,560) by the end of the 21-d experimental period. A 2021 study provided a detailed description of natural lesions in 7 desert cottontails collected in the southwestern United States in April and May 2020. 101 Similar to black-tailed jackrabbits, certain histologic lesions typically associated with RHD in European rabbits were not seen in these animals, including glomerular or pulmonary fibrin microthrombi; heterophilic inflammation associated with areas of hepatic necrosis; and splenic lymphoid depletion, lymphocytolysis, macrophage hyperplasia, or red pulp necrosis. Hepatic necrosis has been observed in cases of RHD in western brush rabbits and riparian brush rabbits in California (Fig. 10). 16 Descriptions of lesions in mountain cottontails and other members of the genus Sylvilagus are not currently in the public domain, but are thought to be similar.
Viral antigen has been detected by IHC in the liver and other organs of eastern cottontails, desert cottontails, western brush rabbits, and riparian brush rabbits (Fig. 10).16,18,125 A mouse monoclonal antibody cocktail directed against several capsid epitopes (6G2, 3H6, 6D6) of pathogenic and nonpathogenic lagoviruses from the WOAH RHD Reference Laboratory (Brescia, Italy; provided by Drs. A. Lavazza and L. Capucci) was used in all cases. 40 Antigen and antibody ELISAs from the WOAH reference laboratory were used in liver homogenates and serum, respectively, from experimentally infected eastern cottontails. 125 In a study of California cases, 16 liver viral loads were significantly higher in a pool of carcasses of rabbits of the genus Sylvilagus (12 desert cottontails, 1 western brush rabbit) compared to 55 domestic European rabbits (RT-qPCR median Ct values of 11.3 [Sylvilagus] vs. 13.0 [Oryctolagus]).
Other lagomorph species
As the virus spreads around the globe, other lagomorph species restricted to specific geographic niches are being exposed. Mortality events were reported in the pygmy rabbit in Nevada (USA) in January–February 2022, and one rabbit tested positive by RT-qPCR. 47 Gross findings in affected rabbits included pulmonary congestion and hemorrhage and mild hepatic discoloration, but histopathology was not performed. Early 2023 detection reports to the WOAH include mortality in populations of red rock rabbits (syn. red rock hares; Pronolagus spp.) in South Africa. 195 To our knowledge, no further information is publicly available, but this would represent a very novel species jump within the family Leporidae (Fig. 1).
Detection in previously unaffected lagomorph species is one of the main concerns of the current rapid global spread, particularly from a conservation standpoint. Concerns focus on unique lagomorph species such as pikas (Ochotona spp.) or volcano rabbits (Romerolagus diazi), among others. There is no confirmed information about detections in these species, but the virus is present in the geographic areas where some of these unique animals live, and awareness is warranted.
Non-lagomorph species
RHDV2 RNA has been sporadically detected in non-lagomorph species from at least 4 different orders (Table 3). These specimens included 1 Mediterranean pine vole (Microtus duodecimcostatus) collected in Spain in 2014 31 ; 2 white-toothed shrews (Crocidura russula), also collected in Spain 1 y later 31 ; and several specimens of Eurasian badgers (Meles meles) collected in Portugal between 2017 and 2020. 5 In addition to the detection of viral RNA, the infectivity of the virus detected in the livers of the Mediterranean pine vole and the 2 white-toothed shrews was demonstrated by inoculation of laboratory New Zealand White rabbits (O. cuniculus) that died with RHD lesions. 31 Furthermore, RHDV2 was detected in the feces of Tasmanian devils (Sarcophilus harrisii) and red foxes (Vulpes vulpes) collected between 2017 and 2019 in Australia.36,45
Table 3.
Rabbit hemorrhagic disease virus 2 detections in non-lagomorph species.
| Common name | Scientific name | Order | Year of collection | Biological material tested | Country, region | Method used for detection | No. of positive specimens | Ref. |
|---|---|---|---|---|---|---|---|---|
| Eurasian badger | Meles meles | Carnivora | 2017–2020 | Liver, spleen, lungs, etc. | Portugal, Santarém district | RT-qPCR 61 | 10 | 5 |
| Mediterranean pine vole | Microtus duodecimcostatus | Rodentia | 2014 | Liver | Spain, Zaragoza Province | Duplex RT-qPCR 30 | 1 | 31 |
| Red fox | Vulpes vulpes | Carnivora | 2019 | Feces | Australia | Metagenomics & transcriptomics | 2 | 36 |
| Tasmanian devil | Sarcophilus harrisii | Dasyuromorphia | 2017 | Feces | Australia, Tasmania | Metagenomics & transcriptomics | 1 | 45 |
| White-toothed shrew | Crocidura russula | Eulipotyphla | 2015 | Liver | Spain, Zaragoza Province | Duplex RT-qPCR 30 | 2 | 31 |
Discussion and conclusions
Since its first reported detections in Europe in 2010, 103 the literature reviewed here suggests that RHDV2 has been more evolutionarily successful than classic RHDV strains. The detection of RHDV2 in a wider range of species is a significant improvement in its ability to survive, but nevertheless a serious concern, particularly from the standpoint of animal conservation and welfare.
RHDV2 continues its rapid global spread from its original wild and domestic rabbit host in Europe to several wild Leporidae species and previously unaffected geographic areas, mainly as a result of human intervention. 151 The current near-global presence of this emerging virus also appears to be related to its many survival strategies, including the ability to infect kittens <2-wk-old 51 ; the wider host range 18 ; genetic recombination with limited cross-protective immunity between different genotypes 127 ; apparent lower mortality, 106 possibly with an associated higher percentage of chronically infected animals, 4 which might be able to serve as virus reservoirs; prolonged disease progression 75 ; and high environmental resistance, believed to be similar to RHDV. 83
The apparent speed at which RHDV2 is evolving is likely related to 2 well-documented recombination sites at the RdRp-vp60 and p16-p23 gene boundaries. 6 Significantly, several examples of recombination involving both pathogenic and nonpathogenic lagoviruses have been documented, even including recombination events at both of these genome breakpoints simultaneously.6,155 Importantly, there is very limited knowledge of nonpathogenic rabbit or hare caliciviruses circulating in wild Leporidae species in North America; therefore, recombinants involving the structural genes of RHDV2 (genotype GI.2) and viruses of unknown genotype may potentially occur.
The phylogenetic classification of lagoviruses is based on the vp60 gene. 107 WGS has been widely used to understand the phylodynamic and molecular epidemiology of the virus, and the use of sequence analysis is expected to continue as a targeted detection and surveillance tool. Sequencing of vp60 and WGS allow for RHDV2 to be quickly identified, characterized, monitored, and potentially controlled through early detection and elimination. The 2020–2023 RHDV2 situation in North America underscores the importance of early detection and immediate intervention when dealing with a rapidly evolving RNA virus. 18 The first known North American outbreak affecting domestic rabbits in Quebec, Canada in 2016 was traced to a Spanish parent virus, 13 but this strain has not been detected since. In contrast, the strain detected in New Mexico (USA) in 2020 successfully spread to multiple states over 3 y, and has crossed the species barrier into native species in the genera Sylvilagus, Lepus, and Brachylagus.18,47,166
In this respect, the spread of the virus in North America since 2019–2020 represents an unprecedented situation. Prior to the incursion of RHDV2, there had been sporadic detections of classic RHDV and other pathogenic lagoviruses in Canada and the United States since at least 2000, but to our knowledge the virus never became endemic in those areas14,23,35,66; outbreaks of RHD caused by RHDV were also reported in domestic rabbits in Mexico in the late 1980s, but a successful control program led to viral eradication in the country. 74 However, RHDV2 has become endemic in those geographic locations in a short time, which is perhaps related to the susceptibility of some of the wild Leporidae species native to the Americas combined with human intervention and the other epidemiologic advantages of this virus. 37
Despite some subtleties, gross and microscopic lesions appear to be similar in every new Leporidae species in which the virus is detected. 101 However, information on lesions in species of genera other than Oryctolagus is scarce, and detailed descriptions and further comparative studies are warranted as the virus is detected in new species. In general, gross lesions may occasionally be discrete and nonspecific and, therefore, of limited diagnostic value, but microscopic evidence of hepatic necrosis is undoubtedly the most important parameter to be sought in diagnostic pathology. 16 Other organs that are useful to sample for histologic examination in RHD-suspect cases, at least in European rabbits, include the lungs, spleen, and kidneys. 128 However, confirmatory diagnosis is most commonly made by the detection of viral RNA in tissue samples, ideally by RT-PCR or RT-qPCR from liver. 198
As with any virus that is genetically capable of rapidly evolving and adapting to new host species, vigilance in early detection and targeted efforts to manage the spread will be critical in preventing outbreaks and minimizing further dissemination. RHDV2 models a typical RNA virus that exploits all evolutionary survival strategies. The lessons learned from this virus can be applied to other viruses and should be reviewed by scientists and policy makers before large-scale interventions are planned.
Acknowledgments
Veterinarians, biologists, and other staff at the California Animal Health and Food Safety Laboratory System, the California Department of Food and Agriculture, and the California Department of Fish and Wildlife are acknowledged for submitting and processing some of the cases from the RHD outbreaks in California that were used as examples in our review.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Javier Asin  https://orcid.org/0000-0002-6178-4801
https://orcid.org/0000-0002-6178-4801
Francisco A. Uzal  https://orcid.org/0000-0003-0681-1878
https://orcid.org/0000-0003-0681-1878
Beate M. Crossley  https://orcid.org/0000-0003-2932-7229
https://orcid.org/0000-0003-2932-7229
Eileen E. Henderson  https://orcid.org/0000-0002-5043-9308
https://orcid.org/0000-0002-5043-9308
Fábio Abade dos Santos  https://orcid.org/0000-0002-0696-7322
https://orcid.org/0000-0002-0696-7322
Contributor Information
Javier Asin, California Animal Health and Food Safety Laboratory, University of California–Davis, San Bernardino, CA, USA.
Carlos Calvete, Animal Science Department, Agri-Food Research and Technology Centre of Aragon (CITA), Agri-Food Institute of Aragón (IA2), Zaragoza, Spain.
Francisco A. Uzal, California Animal Health and Food Safety Laboratory, University of California–Davis, San Bernardino, CA, USA
Beate M. Crossley, Davis, CA, USA
Margarida Dias Duarte, National Institute for Agrarian and Veterinary Research (INIAV), Oeiras, Portugal.
Eileen E. Henderson, California Animal Health and Food Safety Laboratory, University of California–Davis, San Bernardino, CA, USA
Fábio Abade dos Santos, National Institute for Agrarian and Veterinary Research (INIAV), Oeiras, Portugal; Faculty of Veterinary Medicine, Lusofona University, Lisboa, Portugal.
References
- 1. Abade dos Santos FA. Quadro anatomo-histopatológico e diagnóstico molecular da doença hemorrágica viral em coelho-bravo [Anatomic-histopathologic picture and molecular diagnosis of viral hemorrhagic disease in wild rabbit]. Master thesis. Lisboa, Portugal: University of Lisboa, 2018. Portuguese. https://www.repository.utl.pt/handle/10400.5/15206 [Google Scholar]
- 2. Abade dos Santos FA, et al. Leporids’ emerging diseases as a threat to biodiversity. In: Saxena KS, ed. Viral Outbreaks: Global Impact and Newer Horizons. IntechOpen, 2023. doi: 10.5772/intechopen.110028. [DOI] [Google Scholar]
- 3. Abade dos Santos FA, et al. Detection of rabbit Haemorrhagic disease virus 2 during the wild rabbit (Oryctolagus cuniculus) eradication from the Berlengas archipelago, Portugal. BMC Vet Res 2017;13:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abade dos Santos FA, et al. A potential atypical case of rabbit haemorrhagic disease in a dwarf rabbit. Animals (Basel) 2020;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abade dos Santos FA, et al. Spillover events of rabbit haemorrhagic disease virus 2 (recombinant GI.4P-GI.2) from Lagomorpha to Eurasian badger. Transbound Emerg Dis 2022;69:1030–1045. [DOI] [PubMed] [Google Scholar]
- 6. Abrantes J, et al. Recombination at the emergence of the pathogenic rabbit haemorrhagic disease virus Lagovirus europaeus/GI.2. Sci Rep 2020;10:14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abrantes J, et al. New variant of rabbit hemorrhagic disease virus, Portugal, 2012–2013. Emerg Infect Dis 2013;19:1900–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abrantes J, Lopes AM. A review on the methods used for the detection and diagnosis of rabbit hemorrhagic disease virus (RHDV). Microorganisms 2021;9:972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albini S, et al. Inconspicuous post-mortem findings in rabbits from Switzerland naturally-infected with rabbit haemorrhagic disease virus 2. Schweiz Arch Tierheilkd 2022;164:375–383. [DOI] [PubMed] [Google Scholar]
- 10. Almeida T, et al. Tracking the evolution of the G1/RHDVb recombinant strains introduced from the Iberian Peninsula to the Azores islands, Portugal. Infect Genet Evol 2015;34:307–313. [DOI] [PubMed] [Google Scholar]
- 11. Alves PC, et al., eds. Lagomorph Biology: Evolution, Ecology, and Conservation. Springer, 2008. [Google Scholar]
- 12. Ambagala A, et al. Outbreak of rabbit hemorrhagic disease virus 2 infections, Ghana. Emerg Infect Dis 2021;27:1999–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ambagala A, et al. Incursions of rabbit haemorrhagic disease virus 2 in Canada—clinical, molecular and epidemiological investigation. Transbound Emerg Dis 2021;68:1711–1720. [DOI] [PubMed] [Google Scholar]
- 14. American Veterinary Medical Association. Rabbit calicivirus infection confirmed in Iowa rabbitry. J Am Vet Med Assoc 2000;216:1537. [PubMed] [Google Scholar]
- 15. Asher RJ, et al. Stem Lagomorpha and the antiquity of Glires. Science 2005;307:1091–1094. [DOI] [PubMed] [Google Scholar]
- 16. Asin J, et al. An RT-qPCR assay from rectal swabs for the detection of rabbit hemorrhagic disease virus 2 in natural cases. Transbound Emerg Dis 2023;2023:1869692. [Google Scholar]
- 17. Asin J, et al. Outbreak of rabbit hemorrhagic disease virus 2 in the southwestern United States: first detections in southern California. J Vet Diagn Invest 2021;33:728–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Asin J, et al. Early circulation of rabbit haemorrhagic disease virus type 2 in domestic and wild lagomorphs in southern California, USA (2020–2021). Transbound Emerg Dis 2022;69:e394–e405. [DOI] [PubMed] [Google Scholar]
- 19. Austrian Agency for Health and Food Safety. Rabbit hemorrhagic disease. 2023. [cited 2023 Oct]. https://www.ages.at/en/human/disease/pathogens-from-a-to-z/rabbit-hemorrhagic-disease
- 20. Baily JL, et al. RHDV variant 2 presence detected in Scotland. Vet Rec 2014;174:411. [DOI] [PubMed] [Google Scholar]
- 21. Bell DJ, et al. Rabbit haemorrhagic disease virus type 2 in hares in England. Vet Rec 2019;184:127–128. [DOI] [PubMed] [Google Scholar]
- 22. Ben Chehida F, et al. Multiple introductions of rabbit hemorrhagic disease virus Lagovirus europaeus/GI.2 in Africa. Biology (Basel) 2021;10:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bergin IL, et al. Novel calicivirus identified in rabbits, Michigan, USA. Emerg Infect Dis 2009;15:1955–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonvehí C, et al. Clinicopathologic findings of naturally occurring Rabbit Hemorrhagic Disease Virus 2 infection in pet rabbits. Vet Clin Pathol 2019;48:89–95. [DOI] [PubMed] [Google Scholar]
- 25. Buehler M, et al. Lagovirus europeus GI.2 (rabbit hemorrhagic disease virus 2) infection in captive mountain hares (Lepus timidus) in Germany. BMC Vet Res 2020;16:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Byrne AW, et al. Rabbit haemorrhagic disease virus 2 (RHDV2; GI.2) in Ireland focusing on wild Irish hares (Lepus timidus hibernicus): an overview of the first outbreaks and contextual review. Pathogens 2022;11:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. California Department of Fish and Wildlife. Rabbit hemorrhagic disease confirmed for first time in endangered riparian brush rabbits. 2022. May 27. [cited 2023 Oct]. https://wildlife.ca.gov/News/Archive/rabbit-hemorrhagic-disease-confirmed-for-first-time-in-endangered-riparian-brush-rabbits#gsc.tab=0
- 28. Calvete C, et al. Changes in European wild rabbit population dynamics and the epidemiology of rabbit haemorrhagic disease in response to artificially increased viral transmission. Transbound Emerg Dis 2022;69:2682–2696. [DOI] [PubMed] [Google Scholar]
- 29. Calvete C, et al. Infectivity of rabbit haemorrhagic disease virus excreted in rabbit faecal pellets. Vet Microbiol 2021;257:109079. [DOI] [PubMed] [Google Scholar]
- 30. Calvete C, et al. Rabbit haemorrhagic disease: cross-protection and comparative pathogenicity of GI.2/RHDV2/b and GI.1b/RHDV lagoviruses in a challenge trial. Vet Microbiol 2018;219:87–95. [DOI] [PubMed] [Google Scholar]
- 31. Calvete C, et al. Detection of rabbit hemorrhagic disease virus GI.2/RHDV2/b in the Mediterranean pine vole (Microtus duodecimcostatus) and white-toothed shrew (Crocidura russula). J Wildl Dis 2019;55:467–472. [DOI] [PubMed] [Google Scholar]
- 32. Calvete C, et al. Monitoring of rabbit hemorrhagic disease virus in European wild rabbit (Oryctolagus cuniculus) populations by PCR analysis of rabbit fecal pellets. J Wildl Dis 2022;58:394–398. [DOI] [PubMed] [Google Scholar]
- 33. Calvete C, et al. Could the new rabbit haemorrhagic disease virus variant (RHDVB) be fully replacing classical RHD strains in the Iberian Peninsula? World Rabbit Sci 2014;22:91. Letter. [Google Scholar]
- 34. Camarda A, et al. Detection of the new emerging rabbit haemorrhagic disease type 2 virus (RHDV2) in Sicily from rabbit (Oryctolagus cuniculus) and Italian hare (Lepus corsicanus). Res Vet Sci 2014;97:642–645. [DOI] [PubMed] [Google Scholar]
- 35. Campagnolo ER, et al. Outbreak of rabbit hemorrhagic disease in domestic lagomorphs. J Am Vet Med Assoc 2003;223:1151–1155, 1128. [DOI] [PubMed] [Google Scholar]
- 36. Campbell SJ, et al. Red fox viromes in urban and rural landscapes. Virus Evol 2020;6:veaa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Capucci L, et al. Viral haemorrhagic disease: RHDV type 2 ten years later. World Rabbit Sci 2022;30:1–11. [Google Scholar]
- 38. Capucci L, et al. Increased pathogenicity in rabbit haemorrhagic disease virus type 2 (RHDV2). Vet Rec 2017;180:426. [DOI] [PubMed] [Google Scholar]
- 39. Capucci L, et al. A further step in the evolution of rabbit hemorrhagic disease virus: the appearance of the first consistent antigenic variant. Virus Res 1998;58:115–126. [DOI] [PubMed] [Google Scholar]
- 40. Capucci L, et al. Antigenicity of the rabbit hemorrhagic disease virus studied by its reactivity with monoclonal antibodies. Virus Res 1995;37:221–238. [DOI] [PubMed] [Google Scholar]
- 41. Carvalho CL, et al. Progression of rabbit haemorrhagic disease virus 2 upon vaccination in an industrial rabbitry: a laboratorial approach. World Rabbit Sci 2017;25:73–85. [Google Scholar]
- 42. Carvalho CL, et al. Challenges in the rabbit haemorrhagic disease 2 (RHDV2) molecular diagnosis of vaccinated rabbits. Vet Microbiol 2017;198:43–50. [DOI] [PubMed] [Google Scholar]
- 43. Carvalho CL, et al. Emergence of rabbit haemorrhagic disease virus 2 in the archipelago of Madeira, Portugal (2016–2017). Virus Genes 2017;53:922–926. [DOI] [PubMed] [Google Scholar]
- 44. Chen W, et al. Detection of a new emerging strain of rabbit haemorrhagic disease virus 2 (GI.2) in China. J Vet Res 2022;66:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chong R, et al. Fecal viral diversity of captive and wild Tasmanian devils characterized using virion-enriched metagenomics and metatranscriptomics. J Virol 2019;93:e00205–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clarke IN, et al. The positive sense single stranded RNA viruses: Caliciviridae. In: King AMQ, et al., eds. Virus Taxonomy: Classification and Nomenclature of Viruses. Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier, 2012:977–986. [Google Scholar]
- 47. Crowell M, et al. Chapter 3.7.2. Detection of Rabbit hemorrhagic disease virus 2 in the Pygmy Rabbit (Brachylagus idahoensis) in Nevada, USA. J Wildl Dis 2023;59:342–346. [DOI] [PubMed] [Google Scholar]
- 48. Dalton KP, et al. Conventional and real time RT-PCR assays for the detection and differentiation of variant rabbit hemorrhagic disease virus (RHDVb) and its recombinants. J Virol Methods 2018;251:118–122. [DOI] [PubMed] [Google Scholar]
- 49. Dalton KP, et al. Clinical course and pathogenicity of variant rabbit haemorrhagic disease virus in experimentally infected adult and kit rabbits: significance towards control and spread. Vet Microbiol 2018;220:24–32. [DOI] [PubMed] [Google Scholar]
- 50. Dalton KP, et al. Spread of new variant RHDV in domestic rabbits on the Iberian Peninsula. Vet Microbiol 2014;169:67–73. [DOI] [PubMed] [Google Scholar]
- 51. Dalton KP, et al. Variant rabbit hemorrhagic disease virus in young rabbits, Spain. Emerg Infect Dis 2012;18:2009–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dalton KP, et al. Fast specific field detection of RHDVb. Transbound Emerg Dis 2018;65:232–234. [DOI] [PubMed] [Google Scholar]
- 53. Dalton KP, et al. ELISA for detection of variant rabbit haemorrhagic disease virus RHDV2 antigen in liver extracts. J Virol Methods 2018;251:38–42. [DOI] [PubMed] [Google Scholar]
- 54. Daodu OB, et al. Seromolecular surveillance of rabbit haemorrhagic disease virus in Nigeria. Trop Anim Health Prod 2023;55:327. [DOI] [PubMed] [Google Scholar]
- 55. Daodu OB, et al. Detection and molecular characterization of a first isolate of rabbit haemorrhagic disease virus in Nigeria. Trop Anim Health Prod 2021;53:185. [DOI] [PubMed] [Google Scholar]
- 56. Delibes-Mateos M, et al. Key role of European rabbits in the conservation of the Western Mediterranean basin hotspot. Conserv Biol 2008;22:1106–1117. [DOI] [PubMed] [Google Scholar]
- 57. Delibes-Mateos M, et al. Rabbits as a keystone species in southern Europe. Biol Conserv 2007;137:149–156. [Google Scholar]
- 58. Dori P, et al. “An American near Rome” . . . and not only! Presence of the eastern cottontail in Central Italy and potential impacts on the endemic and vulnerable Apennine hare. Mammalia 2019;83:307–312. [Google Scholar]
- 59. Duarte M, et al. Rabbit haemorrhagic disease virus 2 (RHDV2) outbreak in Azores: disclosure of common genetic markers and phylogenetic segregation within the European strains. Infect Genet Evol 2015;35:163–171. [DOI] [PubMed] [Google Scholar]
- 60. Duarte M, et al. Detection of RHDV variant 2 in the Azores. Vet Rec 2015;176:130. [DOI] [PubMed] [Google Scholar]
- 61. Duarte MD, et al. A real time Taqman RT-PCR for the detection of rabbit hemorrhagic disease virus 2 (RHDV2). J Virol Methods 2015;219:90–95. [DOI] [PubMed] [Google Scholar]
- 62. Duarte MD, et al. The health and future of the six hare species in Europe: a closer look at the Iberian hare. In: Argente M-J, et al., eds. Lagomorpha Characteristics. IntechOpen, 2020. https://www.intechopen.com/chapters/71640 [Google Scholar]
- 63. Dutch Wildlife Health Centre. Deadly rabbit disease throughout the Netherlands. 2016. [cited 2023 Oct]. https://dwhc.nl/en/deadly-rabbit-disease-throughout-the-netherlands/
- 64. Dutch Wildlife Health Centre. First case of RHDV-2 in a hare in the Netherlands. 2017 Jan 12. [cited 2023 Oct]. https://dwhc.nl/en/first-case-of-rhdv-2-in-a-hare-in-the-netherlands/
- 65. Eden J-S, et al. Comparative phylodynamics of rabbit hemorrhagic disease virus in Australia and New Zealand. J Virol 2015;89:9548–9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Embury-Hyatt C, et al. The first reported case of rabbit hemorrhagic disease in Canada. Can Vet J 2012;53:998–1002. [PMC free article] [PubMed] [Google Scholar]
- 67. Erfan AM, Shalaby AG. Genotyping of rabbit hemorrhagic disease virus detected in diseased rabbits in Egyptian Provinces by VP60 sequencing. Vet World 2020;13:1098–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Faehndrich M, et al. Status of infectious diseases in free-ranging European brown hares (Lepus europaeus) found dead between 2017 and 2020 in Schleswig-Holstein, Germany. Pathogens 2023;12:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fitzner A, Niedbalski W. Detection of rabbit haemorrhagic disease virus 2 (GI.2) in Poland. Pol J Vet Sci 2018;21:451–458. [DOI] [PubMed] [Google Scholar]
- 70. Friedrich-Loeffler-Institut. Hämorrhagische Kaninchenkrankheit: Neue Variante des RHD-Virus nun auch in Deutschland entdeckt [Rabbit haemorrhagic disease: new variant of the RHD virus now also discovered in Germany]. 2013. Oct 21. [cited 2023 Oct]. German. https://www.openagrar.de/receive/openagrar_mods_00001655
- 71. Fukui H, et al. Rabbit hemorrhagic disease virus type 2 epidemic in a rabbit colony in Japan. J Vet Med Sci 2021;83:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Government of Canada. Rabbit haemorrhagic disease (RHD) fact sheet. 2023. Apr 21. [cited 2023 Oct]. https://inspection.canada.ca/animal-health/terrestrial-animals/diseases/immediately-notifiable/rhd/fact-sheet/eng/1526322490096/1526322490704
- 73. Grasa Albajar JR, Malo Martínez M. Enfermedad vírica hemorrágica: nueva variante [Viral hemorrhagic disease: new variant]. In: XXXVII Symposium de cunicultura de Asociación Española de Cunicultura (ASESCU); Barbastro, Spain; 2012:8–35. Spanish. https://asescu.com/wp-content/uploads/2015/05/37Symposium_Barbastro2012.pdf [Google Scholar]
- 74. Gregg DA, et al. Viral haemorrhagic disease of rabbits in Mexico: epidemiology and viral characterization. Rev Sci Tech 1991;10:435–451. [DOI] [PubMed] [Google Scholar]
- 75. Hall RN, et al. Age and infectious dose significantly affect disease progression after RHDV2 infection in naïve domestic rabbits. Viruses 2021;13:1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hall RN, et al. Emerging rabbit hemorrhagic disease virus 2 (RHDVb), Australia. Emerg Infect Dis 2015;21:2276–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hall RN, et al. A strain-specific multiplex RT-PCR for Australian rabbit haemorrhagic disease viruses uncovers a new recombinant virus variant in rabbits and hares. Transbound Emerg Dis 2018;65:e444–e456. [DOI] [PubMed] [Google Scholar]
- 78. Hall RN, et al. Detection of RHDV2 in European brown hares (Lepus europaeus) in Australia. Vet Rec 2017;180:121. [DOI] [PubMed] [Google Scholar]
- 79. Happi AN, et al. Microbial metagenomic approach uncovers the first rabbit haemorrhagic disease virus genome in Sub-Saharan Africa. Sci Rep 2021;11:13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Harcourt-Brown N, et al. RHDV2 epidemic in UK pet rabbits. Part 1: clinical features, gross post mortem and histopathological findings. J Small Anim Pract 2020;61:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. He W-T, et al. Virome characterization of game animals in China reveals a spectrum of emerging pathogens. Cell 2022;185:1117–1129.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Henning J. Factors influencing the epidemiology of Rabbit hemorrhagic disease virus in New Zealand. PhD dissertation. Massey University, 2003. [cited 2023 Oct]. https://mro.massey.ac.nz/bitstream/handle/10179/1846/02_whole.pdf;jsessionid=1F3BA7F4F564EF5B367EEC4F82623573?sequence=1 [Google Scholar]
- 83. Henning J, et al. Survival of rabbit haemorrhagic disease virus (RHDV) in the environment. Epidemiol Infect 2005;133:719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hermans K, et al. Also Belgian rabbits are affected by the rabbit hemorrhagic disease type 2 virus. Vlaams Diergeneeskd Tijdschr 2016;85:309–314. [Google Scholar]
- 85. Himsworth CG, et al. An outbreak of rabbit hemorrhagic disease in British Columbia, Canada. J Wildl Dis 2021;57:983–986. [DOI] [PubMed] [Google Scholar]
- 86. Hofmeister E, et al. Comparison of magnetic bead and rapid swab RNA extraction methods for detecting rabbit hemorrhagic disease virus 2 in rabbit liver samples. Biotechniques 2023;74:156–157. [DOI] [PubMed] [Google Scholar]
- 87. Hrynkiewicz R, et al. Occurrence of Lagovirus europaeus (rabbit hemorrhagic disease virus) in domestic rabbits in southwestern Poland in 2019: case report. Microbiol Spectr 2022;10:e0229822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hu B, et al. Novel recombinant rabbit hemorrhagic disease virus 2 (RHDV2) is circulating in China within 12 months after original RHDV2 arrival. Transbound Emerg Dis 2023;2023:4787785. [Google Scholar]
- 89. Hu B, et al. Emergence of rabbit haemorrhagic disease virus 2 in China in 2020. Vet Med Sci 2021;7:236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. International Committee on Taxonomy of Viruses. History of the taxon: Lagovirus europaeus. 2023 release, MSL #39. [cited 2024 May 16]. https://ictv.global/taxonomy/taxondetails?taxnode_id=202202802&taxon_name=Rabbit%20hemorrhagic%20disease%20virus
- 91. International Union for Conservation of Nature–Species Survival Commission. Lagomorph Specialist Group. [cited 2023 Oct]. https://iucn.org/our-union/commissions/group/iucn-ssc-lagomorph-specialist-group
- 92. Isomursu M, et al. An outbreak of rabbit hemorrhagic disease in Finland. J Wildl Dis 2018;54:838–842. [DOI] [PubMed] [Google Scholar]
- 93. Jennings-Gaines JE, et al. Utilizing blood filter paper and ear punch samples for the detection of rabbit hemorrhagic disease virus 2 by RT-rtPCR. J Vet Diagn Invest 2022;34:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jiménez-Ruiz S, et al. High mortality of wild European rabbits during a natural outbreak of rabbit haemorrhagic disease GI.2 revealed by a capture-mark-recapture study. Transbound Emerg Dis 2023;2023:3451338. [Google Scholar]
- 95. Kardia E, et al. Hepatobiliary organoids derived from leporids support the replication of hepatotropic lagoviruses. J Gen Virol 2023;104:001874. [DOI] [PubMed] [Google Scholar]
- 96. Katayama A, et al. An outbreak of rabbit hemorrhagic disease (RHD) caused by Lagovirus europaeus GI.2/rabbit hemorrhagic disease virus 2 (RHDV2) in Ehime, Japan. J Vet Med Sci 2021;83:931–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kennedy A, et al. First detected case of rabbit Haemorrhagic disease virus 2 (RHDV2) in the Irish hare (Lepus timidus hibernicus). Ir Vet J 2021;74:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kinnear M, Linde CC. Capsid gene divergence in rabbit hemorrhagic disease virus. J Gen Virol 2010;91:174–181. [DOI] [PubMed] [Google Scholar]
- 99. Koh EY, et al. Rabbit haemorrhagic disease virus 2 from Singapore 2020 outbreak revealed an Australian recombinant variant. Virus Evol 2023;9:vead029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kovaliski J. Monitoring the spread of rabbit hemorrhagic disease virus as a new biological agent for control of wild European rabbits in Australia. J Wildl Dis 1998;34:421–428. [DOI] [PubMed] [Google Scholar]
- 101. Lankton JS, et al. Pathology of Lagovirus europaeus GI.2/RHDV2/b (rabbit hemorrhagic disease virus 2) in native North American lagomorphs. J Wildl Dis 2021;57:694–700. [DOI] [PubMed] [Google Scholar]
- 102. Lavazza A, et al. Field and experimental data indicate that the eastern cottontail (Sylvilagus floridanus) is susceptible to infection with European brown hare syndrome (EBHS) virus and not with rabbit haemorrhagic disease (RHD) virus. Vet Res 2015;46:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Le Gall-Reculé G, et al. Emergence of a new lagovirus related to Rabbit Haemorrhagic Disease Virus. Vet Res 2013;44:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Le Gall-Reculé G, et al. Large-scale lagovirus disease outbreaks in European brown hares (Lepus europaeus) in France caused by RHDV2 strains spatially shared with rabbits (Oryctolagus cuniculus). Vet Res 2017;48:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Le Gall-Reculé G, et al. Detection of a new variant of rabbit haemorrhagic disease virus in France. Vet Rec 2011;168:137–138. [DOI] [PubMed] [Google Scholar]
- 106. Le Minor O, et al. Evaluation de l’efficacité d’un nouveau vaccin contre le virus variant de la maladie hémorragique virale du lapin (VHD) [Evaluation of the efficacy of a new vaccine against the variant virus of rabbit hemorrhagic disease]. In: 15èmes Journées de la Recherche Cunicole; Le Mans, France; 2013:241–244. French. http://www.cuniculture.info/Docs/Magazine/Magazine2013/fichiers-pdf-JRC/P07-LeMinor.pdf [Google Scholar]
- 107. Le Pendu J, et al. Proposal for a unified classification system and nomenclature of lagoviruses. J Gen Virol 2017;98:1658–1666. [DOI] [PubMed] [Google Scholar]
- 108. Li Y, et al. First report of GI.1aP-GI.2 recombinants of rabbit hemorrhagic disease virus in domestic rabbits in China. Front Microbiol 2023;14:1188380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lim K, et al. Resolution of rabbit haemorrhagic disease virus 2 (RHDV2; Lagovirus europeus GI.2) outbreak in Singapore. Transbound Emerg Dis 2022;69:3077–3083. [DOI] [PubMed] [Google Scholar]
- 110. Long JL. Introduced Mammals of the World: Their History, Distribution and Influence. CSIRO, 2003. [Google Scholar]
- 111. Lopes AM, et al. Full genome sequences are key to disclose RHDV2 emergence in the Macaronesian islands. Virus Genes 2018;54:1–4. [DOI] [PubMed] [Google Scholar]
- 112. Lopes AM, et al. Is the new variant RHDV replacing genogroup 1 in Portuguese wild rabbit populations? Viruses 2014;7:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lopes AM, et al. Full genomic analysis of new variant rabbit hemorrhagic disease virus revealed multiple recombination events. J Gen Virol 2015;96:1309–1319. [DOI] [PubMed] [Google Scholar]
- 114. Lopes AM, et al. GI.1b/GI.1b/GI.2 recombinant rabbit hemorrhagic disease virus 2 (Lagovirus europaeus/GI.2) in Morocco, Africa. Arch Virol 2019;164:279–283. [DOI] [PubMed] [Google Scholar]
- 115. Machín Á, et al. Identification of the amino acid residue involved in rabbit hemorrhagic disease virus. J Biol Chem 2001;276:27787–27792. [DOI] [PubMed] [Google Scholar]
- 116. Mahar JE, et al. Rabbit hemorrhagic disease virus 2 (RHDV2; GI.2) is replacing endemic strains of RHDV in the Australian landscape within 18 months of its arrival. J Virol 2018;92:e01374–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mahar JE, et al. Frequent intergenotypic recombination between the non-structural and structural genes is a major driver of epidemiological fitness in caliciviruses. Virus Evol 2021;7:veab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mahar JE, et al. Detection and circulation of a novel rabbit hemorrhagic disease virus in Australia. Emerg Infect Dis 2018;24:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Marcato PS, et al. Clinical and pathological features of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev Sci Tech 1991;10:371–392. [DOI] [PubMed] [Google Scholar]
- 120. Marschang RE, et al. Rabbit hemorrhagic disease viruses detected in pet rabbits in a commercial laboratory in Europe. J Exot Pet Med 2018;27:27–30 [Google Scholar]
- 121. Martin-Alonso A, et al. Emerging rabbit haemorrhagic disease virus 2 (RHDV2) at the gates of the African continent. Infect Genet Evol 2016;44:46–50. [DOI] [PubMed] [Google Scholar]
- 122. Mendoza M, et al. Detección mediante PCR-dúplex a tiempo real y aislamiento de virus RHDV2/b durante un brote de enteropatía mucoide [Real time duplex-PCR detection and isolation of infective RHDV2/b during a mucoid enteropathy outbreak]. In: XLII Symposio de cunicultura de Asociación Española de Cunicultura (ASESCU); Murcia, Spain; 2017:95–97. Spanish. https://asescu.com/wp-content/uploads/2017/05/MEMORIAS2017.pdf [Google Scholar]
- 123. Meyers G, et al. Rabbit hemorrhagic disease virus—molecular cloning and nucleotide sequencing of a calicivirus genome. Virology 1991;184:664–676. [DOI] [PubMed] [Google Scholar]
- 124. Ministry for Primary Industries, Government of New Zealand. Protecting pet rabbits from caliciviruses. 2018. [cited 2023 Oct]. https://www.mpi.govt.nz/biosecurity/biosecurity-and-your-pets/pet-rabbits-and-caliciviruses/
- 125. Mohamed F, et al. Comparative susceptibility of eastern cottontails and New Zealand white rabbits to classical rabbit haemorrhagic disease virus (RHDV) and RHDV2. Transbound Emerg Dis 2022;69:e968–e978. [DOI] [PubMed] [Google Scholar]
- 126. Monterroso P, et al. Disease-mediated bottom-up regulation: an emergent virus affects a keystone prey, and alters the dynamics of trophic webs. Sci Rep 2016;6:36072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Müller C, et al. Immunity against Lagovirus europaeus and the impact of the immunological studies on vaccination. Vaccines (Basel) 2021;9:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Neimanis A, et al. Elucidation of the pathology and tissue distribution of Lagovirus europaeus GI.2/RHDV2 (rabbit haemorrhagic disease virus 2) in young and adult rabbits (Oryctolagus cuniculus). Vet Res 2018;49:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Neimanis AS, et al. Overcoming species barriers: an outbreak of Lagovirus europaeus GI.2/RHDV2 in an isolated population of mountain hares (Lepus timidus). BMC Vet Res 2018;14:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Neimanis AS, et al. Arrival of rabbit haemorrhagic disease virus 2 to northern Europe: emergence and outbreaks in wild and domestic rabbits (Oryctolagus cuniculus) in Sweden. Transbound Emerg Dis 2018;65:213–220. [DOI] [PubMed] [Google Scholar]
- 131. Nicholson LJ, et al. Benign rabbit calicivirus in New Zealand. Appl Environ Microbiol 2017;83:e00090–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Nielsen CK, et al. Sylvilagus floridanus, eastern cottontail. In: Smith AT, et al., eds. Lagomorphs: Pikas, Rabbits, and Hares of the World. Johns Hopkins University Press, 2018:137–140. [Google Scholar]
- 133. Nowotny N, et al. Phylogenetic analysis of rabbit haemorrhagic disease and European brown hare syndrome viruses by comparison of sequences from the capsid protein gene. Arch Virol 1997;142:657–673. [DOI] [PubMed] [Google Scholar]
- 134. O’Donnell VK, et al. Coding-complete genome sequences of emerging rabbit hemorrhagic disease virus type 2 isolates detected in 2020 in the United States. Microbiol Resour Announc 2021;10:e01064–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. O’Hara P. The illegal introduction of rabbit haemorrhagic disease virus in New Zealand. Rev Sci Tech 2006;25:119–123. [DOI] [PubMed] [Google Scholar]
- 136. Ohlinger VF, et al. Identification and characterization of the virus causing rabbit hemorrhagic disease. J Virol 1990;64:3331–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. O’Toole AD, et al. Detection of rabbit hemorrhagic disease virus 2 in formalin-fixed, paraffin-embedded tissues via in situ hybridization. J Vet Diagn Invest 2022;34:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Pacioni C, et al. Comparative epidemiology of rabbit haemorrhagic disease virus strains from viral sequence data. Viruses 2022;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Parra F, et al. The amino terminal sequence of VP60 from rabbit hemorrhagic disease virus supports its putative subgenomic origin. Virus Res 1993;27:219–228. [DOI] [PubMed] [Google Scholar]
- 140. Parra F, Prieto M. Purification and characterization of a calicivirus as the causative agent of a lethal hemorrhagic disease in rabbits. J Virol 1990;64:4013–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Pinto FF, et al. Case series: four fatal rabbit hemorrhagic disease virus infections in urban pet rabbits. Front Vet Sci 2023;10:1144227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Puggioni G, et al. The new French 2010 Rabbit Hemorrhagic Disease Virus causes an RHD-like disease in the Sardinian Cape hare (Lepus capensis mediterraneus). Vet Res 2013;44:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Qi R, et al. The outbreak of rabbit hemorrhagic virus type 2 in the interior of China may be related to imported semen. Virol Sin 2022;37:623–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Rahali N, et al. Genetic characterization and phylogenetic analysis of rabbit hemorrhagic disease virus isolated in Tunisia from 2015 to 2018. Arch Virol 2019;164:2327–2332. [DOI] [PubMed] [Google Scholar]
- 145. Ramsey DS, et al. Sustained impact of RHDV2 on wild rabbit populations across Australia eight years after its initial detection. Viruses 2023;15:1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Ramsey DSL, et al. Emerging RHDV2 suppresses the impact of endemic and novel strains of RHDV on wild rabbit populations. J Appl Ecol 2020;57:630–641. [Google Scholar]
- 147. Robinson AJ, et al. Serological evidence for the presence of a calicivirus in Australian wild rabbits, Oryctolagus cuniculus, before the introduction of rabbit haemorrhagic disease virus (RHDV): its potential influence on the specificity of a competitive ELISA for RHDV. Wildl Res 2002;29:655–662. [Google Scholar]
- 148. Rocchi M, et al. Rabbit haemorrhagic disease virus type 2 in hares in Scotland. Vet Rec 2019;185:23. [DOI] [PubMed] [Google Scholar]
- 149. Rocchi M, et al. RHDV-2 on the Isle of Man and in the Republic of Ireland. Vet Rec 2016;179:389–390. [DOI] [PubMed] [Google Scholar]
- 150. Rosell JM, et al. Myxomatosis and rabbit haemorrhagic disease: a 30-year study of the occurrence on commercial farms in Spain. Animals (Basel) 2019;9:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Rouco C, et al. Worldwide rapid spread of the novel rabbit haemorrhagic disease virus (GI.2/RHDV2/b). Transbound Emerg Dis 2019;66:1762–1764. [DOI] [PubMed] [Google Scholar]
- 152. Sahraoui L, et al. First detection and molecular characterization of rabbit hemorrhagic disease virus (RHDV) in Algeria. Front Vet Sci 2023;10:1235123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Saidi A, et al. Confirmation of the rabbit hemorrhagic disease virus type 2 (GI.2) circulation in North Africa. Acta Vet 2022;72:433–441. [Google Scholar]
- 154. Schirrmeier H, et al. Pathogenic, antigenic and molecular properties of rabbit haemorrhagic disease virus (RHDV) isolated from vaccinated rabbits: detection and characterization of antigenic variants. Arch Virol 1999;144:719–735. [DOI] [PubMed] [Google Scholar]
- 155. Silvério D, et al. Insights into the evolution of the new variant rabbit haemorrhagic disease virus (GI.2) and the identification of novel recombinant strains. Transbound Emerg Dis 2018;65:983–992. [DOI] [PubMed] [Google Scholar]
- 156. Simpson V, et al. RHDV variant 2 and Capillaria hepatica infection in rabbits. Vet Rec 2014;174:486. [DOI] [PubMed] [Google Scholar]
- 157. State of Hawai‘i Department of Agriculture. Rabbit hemorrhagic disease confirmed on Maui—quarantine order issued. 2022. Jun 21. [cited 2023 Oct]. https://hdoa.hawaii.gov/blog/main/nr-rabbitdisease/
- 158. Strive T, et al. Retrospective serological analysis reveals presence of the emerging lagovirus RHDV2 in Australia in wild rabbits at least five months prior to its first detection. Transbound Emerg Dis 2020;67:822–833. [DOI] [PubMed] [Google Scholar]
- 159. Strive T, et al. Identification and partial characterisation of a new Lagovirus in Australian wild rabbits. Virology 2009;384:97–105. [DOI] [PubMed] [Google Scholar]
- 160. Szillat KP, et al. Full-genome sequencing of German rabbit haemorrhagic disease virus uncovers recombination between RHDV (GI.2) and EBHSV (GII.1). Virus Evol 2020;6:veaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Tablado Z, et al. Breeding like rabbits: global patterns of variability and determinants of European wild rabbit reproduction. Ecography 2009;32:310–320. [Google Scholar]
- 162. Tanikawa T, et al. Genetics of the rabbit haemorrhagic disease virus strains responsible for rabbit haemorrhagic disease outbreaks in Japan between 2000 and 2020. J Gen Virol 2023;104:001846. [DOI] [PubMed] [Google Scholar]
- 163. Toh X, et al. First detection of rabbit haemorrhagic disease virus (RHDV2) in Singapore. Transbound Emerg Dis 2022;69:1521–1528. [DOI] [PubMed] [Google Scholar]
- 164. Tu T, et al. The pathogenicity comparison of Lagovirus europaeus GI.1 and GI.2 strains in China by using relative quantitative assay. Sci Rep 2022;12:20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ueda M, et al. An epidemic case of rabbit hemorrhagic disease caused by rabbit hemorrhagic disease virus 2 (Lagovirus europaeus GI.2) in Tochigi, Japan. Jpn J Zoo Wildl Med 2021;26:53–59. [Google Scholar]
- 166. U.S. Department of Agriculture–Animal and Plant Inspection Services. Emerging risk notice: rabbit hemorrhagic disease virus, type 2. 2020. Jul. [cited 2023 Oct]. https://www.aphis.usda.gov/animal_health/downloads/rhdv2.pdf
- 167. Vaughan TA, et al. Mammalogy. 5th ed. Jones & Bartlett, 2011. [Google Scholar]
- 168. Velarde R, et al. Spillover event of recombinant Lagovirus europaeus/GI.2 into the Iberian hare (Lepus granatensis) in Spain. Transbound Emerg Dis 2021;68:3187–3193. [DOI] [PubMed] [Google Scholar]
- 169. Velarde R, et al. Spillover events of infection of brown hares (Lepus europaeus) with rabbit haemorrhagic disease type 2 virus (RHDV2) caused sporadic cases of an European brown hare syndrome-like disease in Italy and Spain. Transbound Emerg Dis 2017;64:1750–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Vinjé J, et al. ICTV virus taxonomy profile: Caliciviridae. J Gen Virol 2019;100:1469–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Westcott DG, Choudhury B. Rabbit haemorrhagic disease virus 2-like variant in Great Britain. Vet Rec 2015;176:74. [DOI] [PubMed] [Google Scholar]
- 172. Williams LBA, et al. Clinical and pathologic findings in an outbreak in rabbits of natural infection by rabbit hemorrhagic disease virus 2 in the northwestern United States. J Vet Diagn Invest 2021;33:732–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Wilson DE, et al., eds. Handbook of the Mammals of the World. Vol. 6: Lagomorphs and Rodents I. Lynx Editions, 2016. [Google Scholar]
- 174. Wirblich C, et al. Genetic map of the calicivirus rabbit hemorrhagic disease virus as deduced from in vitro translation studies. J Virol 1996;70:7974–7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. World Organisation for Animal Health. Australia–Rabbit haemorrhagic disease, event 1787. WAHIS, 2015. https://wahis.woah.org/#/in-event/1787/dashboard [Google Scholar]
- 176. World Organisation for Animal Health. Benin–Rabbit haemorrhagic disease, event 1869. WAHIS, 2015. https://wahis.woah.org/#/in-event/1869/dashboard [Google Scholar]
- 177. World Organisation for Animal Health. Canada–Rabbit haemorrhagic disease, event 2008. WAHIS, 2016. https://wahis.woah.org/#/in-event/2008/dashboard [Google Scholar]
- 178. World Organisation for Animal Health. Canada–Rabbit haemorrhagic disease, event 2494. WAHIS, 2018. https://wahis.woah.org/#/in-event/2494/dashboard [Google Scholar]
- 179. World Organisation for Animal Health. China (People’s Rep. of)–Rabbit haemorrhagic disease, event 3199. WAHIS, 2020. https://wahis.woah.org/#/in-event/3199/dashboard [Google Scholar]
- 180. World Organisation for Animal Health. Croatia–Rabbit haemorrhagic disease, event 5042. WAHIS, 2023. https://wahis.woah.org/#/in-event/5042/dashboard [Google Scholar]
- 181. World Organisation for Animal Health. Cuba–Rabbit haemorrhagic disease, event 3774. WAHIS, 2021. https://wahis.woah.org/#/in-event/3774/dashboard [Google Scholar]
- 182. World Organisation for Animal Health. Denmark–Rabbit haemorrhagic disease, event 2009. WAHIS, 2016. https://wahis.woah.org/#/in-event/2009/dashboard [Google Scholar]
- 183. World Organisation for Animal Health. Iceland–Rabbit haemorrhagic disease, event 3164. WAHIS, 2020. https://wahis.woah.org/#/in-event/3164/dashboard [Google Scholar]
- 184. World Organisation for Animal Health. Israel–Rabbit haemorrhagic disease, event 2563. WAHIS, 2018. https://wahis.woah.org/#/in-event/2563/dashboard [Google Scholar]
- 185. World Organisation for Animal Health. Israel–Rabbit haemorrhagic disease, event 3745. WAHIS, 2021. https://wahis.woah.org/#/in-event/3745/dashboard [Google Scholar]
- 186. World Organisation for Animal Health. Italy–Rabbit haemorrhagic disease, event 1089. WAHIS, 2011. https://wahis.woah.org/#/in-event/1089/dashboard [Google Scholar]
- 187. World Organisation for Animal Health. Cote D’Ivoire–Rabbit haemorrhagic disease, event 2017. WAHIS, 2016. https://wahis.woah.org/#/in-event/2017/dashboard [Google Scholar]
- 188. World Organisation for Animal Health. Cote D’Ivoire–Rabbit haemorrhagic disease, event 3679. WAHIS, 2020. https://wahis.woah.org/#/in-event/3679/dashboard [Google Scholar]
- 189. World Organisation for Animal Health. Luxembourg–Rabbit haemorrhagic disease, event 3753. WAHIS, 2021. https://wahis.woah.org/#/in-event/3753/dashboard [Google Scholar]
- 190. World Organisation for Animal Health. Mexico–Rabbit haemorrhagic disease, event 3180. WAHIS, 2020. https://wahis.woah.org/#/in-event/3180/dashboard [Google Scholar]
- 191. World Organisation for Animal Health. New Zealand–Rabbit haemorrhagic disease, event 2546. WAHIS, 2018. https://wahis.woah.org/#/in-event/2546/dashboard [Google Scholar]
- 192. World Organisation for Animal Health. Norway–Rabbit haemorrhagic disease, event 1553. WAHIS, 2014. https://wahis.woah.org/#/in-event/1553/dashboard [Google Scholar]
- 193. World Organisation for Animal Health. Senegal–Rabbit haemorrhagic disease, event 3205. WAHIS, 2020. https://wahis.woah.org/#/in-event/3205/dashboard [Google Scholar]
- 194. World Organisation for Animal Health. Singapore–Rabbit haemorrhagic disease, event 3302. WAHIS, 2020. https://wahis.woah.org/#/in-event/3302/dashboard [Google Scholar]
- 195. World Organisation for Animal Health. South Africa–Rabbit haemorrhagic disease, event 4727. WAHIS, 2022. https://wahis.woah.org/#/in-event/4727/dashboard [Google Scholar]
- 196. World Organisation for Animal Health. USA–Rabbit haemorrhagic disease, event 2681. WAHIS, 2018. https://wahis.woah.org/#/in-event/2681/dashboard [Google Scholar]
- 197. World Organisation for Animal Health. USA–Rabbit haemorrhagic disease, event 2960. WAHIS, 2019. https://wahis.woah.org/#/in-event/2960/dashboard [Google Scholar]
- 198. World Organisation for Animal Health. Chapter 3.7.2. Rabbit haemorrhagic disease. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 12th ed. WOAH, 2023. https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.07.02_RHD.pdf [Google Scholar]
- 199. World Organisation for Animal Health. Chapter 13.2. Rabbit haemorrhagic disease. In: Terrestrial Animal Health Code. WOAH, 2023. https://www.woah.org/fileadmin/Home/eng/Health_standards/tahc/2023/chapitre_rabbit_haemorrhagic_disease.pdf