Abstract
Purpose
The prevalence of Obstructive Sleep Apnea (OSA) is high, and there are many complications. Few studies have reported the relationship between OSA and kidney stones. The purpose of this study is to explore whether people at risk of OSA will increase the risk of kidney stones.
Methods
This was a cross-sectional study, and information was collected through the National Health and Nutrition Examination Survey conducted from 2015 to 2018. Multiple logistic regression analyses were employed to calculate the odds ratios (ORs) and their 95% confidence intervals (CIs) for the link between obstructive sleep apnea and the presence of kidney stones. Additionally, to assess causality and reduce observational biases, five distinct two-sample Mendelian randomization techniques were applied.
Results
Following the adjustment for relevant confounders, findings indicated a statistically significant correlation between obstructive sleep apnea (OSA) and higher prevalence of kidney stones (OR = 1.29; 95% CI: 1.00–1.66). Additionally, using the inverse-variance weighted approach in Mendelian randomization, results suggested a genetic predisposition to OSA might be causally linked to an elevated risk of developing kidney stones (OR: 1.00221, 95% CI 1.00056–1.00387).
Conclusion
OSA promotes the formation of kidney stones, and the treatment and management of OSA can improve or mitigate the occurrence of kidney stones.
Keywords: obstructive sleep apnea, kidney stones, national health and nutrition examination survey, Mendelian randomization
Introduction
Globally, the prevalence of kidney stones is alarmingly high and continues to rise annually.1–4 Research indicates that the healthcare costs associated with treating kidney stones are substantial, exerting a considerable strain on healthcare systems.5 The complications arising from kidney stones are severe and varied, including issues such as blockages in the ureter, blood in the urine, recurrent urinary tract infections, nausea, and discomfort during urination, which may lead to irreversible damage to the kidneys.6 Several factors contribute to the formation of kidney stones, including genetic predispositions, dietary habits, and metabolic abnormalities.
Obstructive sleep apnea (OSA) is a disorder marked by repeated episodes of airway obstruction during sleep, which disrupts normal sleep patterns and reduces oxygen saturation.7 It affects about 9% of women and 24% of men in the general population.8 OSA is often associated with obesity and various aspects of the metabolic syndrome. This condition fosters a metabolic environment prone to low oxygen levels and chronic inflammation, enhancing the risk of severe cardiometabolic diseases.9,10
Shahait et al found no significant variations in 24-h urine parameters related to OSA among patients with kidney stones.11 In contrast, Tallman et al identified a connection between OSA and changes in urinary analytes that increase the risk for kidney stone formation.12 Additionally, Canales et al reported that OSA is associated with declining renal function, which is intricately linked to an increased risk of kidney stone development.13
Given the escalating rates of obstructive sleep apnea (OSA) and kidney stones, their potential connection remains largely under-investigated. Studying this link and assessing the risk for kidney stone development in individuals with OSA could provide critical insights into better prevention and treatment strategies.
Mendelian randomization (MR) utilizes genetic instrumental variables (IVs) to investigate the causal relationships between an exposure and a result.14 By capitalizing on the random distribution of genetic variants at conception, this approach minimizes confounders and diminishes the potential for reverse causation.15
To explore this potential link, we analyzed data from the National Health and Nutrition Examination Survey (NHANES) covering the period from 2015 to 2018 in the United States. Subsequently, we applied a two-sample MR approach to determine whether there is a causal relationship between OSA risk and kidney stone formation.
Materials and Methods
Study Population in NHANES
We gathered data from the National Health and Nutrition Examination Survey (NHANES), overseen by the National Center for Health Statistics (NCHS), which aims to evaluate the health and nutritional status of the US population. The NHANES employs a rigorous stratified multistage probability sampling design to ensure its samples are nationally representative. All NHANES datasets are publicly available for research and can be accessed online at the NCHS website.
Our study utilized data from two NHANES cycles between 2015 and 2018, which were the only periods during which information on both kidney stones and obstructive sleep apnea (OSA) was collected. Initially, the survey included 12,488 participants. After removing minors under the age of 18 (n = 640), pregnant individuals (n = 126), those lacking OSA data (n = 7), and participants without kidney stone data (n = 584), our final sample consisted of 11,131 individuals.
The research protocols for NHANES were approved by the National Centers for Health Statistics, and informed consent was obtained from all participants involved in the survey.
Exposure and Outcome Definitions
In our study, OSA risk was treated as an exposure variable. Assessment of OSA risk was determined using answers to three yes/no questions: (1) Frequent snoring (three or more nights weekly), (2) episodes of snorting, gasping, or breath stopping (three or more nights weekly), and (3) excessive daytime sleepiness occurring 16–30 times a month despite sleeping at least 7 hours on average during weekday or work nights. Participants who reported any of these symptoms were considered to have OSA risk.
The identification of kidney stone presence was based on responses to a single question: “Have you or the sample person ever had a kidney stone?” The validity of self-reported kidney stone conditions has been supported by prior research.16 Participants who reported having kidney stones were classified as having nephrolithiasis, which was the outcome variable in our study.
Covariates
The multivariable-adjusted models encompassed a summary of potential covariates that could introduce confounding in the relationship between OSA and kidney stones. Covariates in our study included age (years), gender (male/female), poverty-to-income ratio (PIR), race, education level, smoking status (never/former/current), marital status, hypertension, diabetes, CVD, stroke, alcohol intake (never/former/mild/moderate/heavy), body mass index (BMI) and physical activity (less than moderate /moderate/ vigorous). BMI was classified into four categories: <18.5, 18.5 to <25, 25 to <30, and ≥30 kg/m2, representing underweight, normal weight, overweight, and obese populations, respectively, for all participants. Comprehensive information regarding the measurement procedures for the study variables can be accessed publicly at www.cdc.gov/nchs/nhanes/.
Assumptions and Data Sources of Two-Sample MR
In Mendelian Randomization (MR) studies, it is critical that the instrumental variables (IVs) used meet three criteria: (1) they must be strongly associated with OSA; (2) they should not relate to any confounders between the exposure and the outcome; (3) they must affect kidney stone disease only through their interaction with the exposures. We sourced genetic instruments for OSA from FinnGen’s latest release 10 (https://r10.fnngen.fi/) with data including 43,901 cases and 366,484 controls. Kidney stone disease outcomes were gathered from the UK Biobank, via the IEU’s GWAS database (https://gwas.mrcieu.ac.uk/), featuring 6536 individuals with kidney stones against 388,508 controls.
Selection of Genetic Instruments
In our research, we selected single-nucleotide polymorphisms (SNPs) that showed a robust correlation with obstructive sleep apnea (OSA) to serve as instrumental variables (IVs), applying a significance threshold of p < 5E−6. To ensure the independence of these SNPs, we conducted a linkage disequilibrium (LD) clumping process. This involved identifying SNPs with an r2 less than 0.001 and at least 10,000 kb apart, specifically focusing on those of European descent. SNPs that were palindromic were excluded, and harmonization was achieved between the alleles affecting exposure and outcome in our datasets. Additionally, we utilized the Phenoscanner database (http://www.phenoscanner.medschl.cam.ac.uk/) to remove any SNPs that exhibited pleiotropic effects, thereby addressing potential confounders in the exposure-outcome relationship.
Statistical Analysis
Cross-Sectional Study
Statistical evaluations in our study complied with the protocols established by the Centers for Disease Control and Prevention (CDC). We applied NHANES sampling weights and addressed the intricacies of the complex multistage cluster survey design. The estimation of odds ratios (ORs) and 95% confidence intervals (CIs) for the relationship between obstructive sleep apnea (OSA) and kidney stones was performed using three distinct multivariable logistic regression models. The first model did not adjust for any covariates, while the second model included adjustments for gender, age, and race. The third model incorporated additional adjustments for variables such as age, gender, race, education, income levels, marital status, smoking habits, hypertension, diabetes, cardiovascular diseases, strokes, alcohol consumption, body mass index, and physical activity levels. We also conducted a subgroup analysis through stratified multivariate regression, which considered a range of demographic and health-related factors. An interaction term was used to examine the variability in associations across different subgroups, employing the log likelihood ratio test model. Statistical significance was determined using a threshold p-value of 0.05. All statistical operations and the creation of graphical representations were performed with Stata version 17 and R version 4.3.1.
MR In the context of Mendelian randomization (MR), various methods including inverse-variance weighted (IVW), MR-Egger, weighted median, simple median, and maximum likelihood were employed to ascertain causal relationships. The MR-Egger regression intercept tested for horizontal pleiotropic effects, confirming no significant effects when the intercept was approximately zero and the p-value exceeded 0.05.17 Additionally, the MR-PRESSO test identified potential pleiotropic outliers, while heterogeneity was evaluated using the IVW method.18 Sensitivity of the results to individual SNPs was assessed with the leave-one-out approach. Visualization of the MR findings was achieved through scatter plots, funnel plots, and forest plots, utilizing the “Two-sample MR” and “MRPRESSO” packages in R.
Results
Baseline Characteristics of Participants
Table 1 shows the baseline demographic characteristics of the participants. A total of 11,131 participants were enrolled in our research, with 48.57% being male and 51.43% being female. The average age of the participants was 48.27 ± 0.41 years. The overall mean prevalence of nephrolithiasis was 11.12% and the varying rates of kidney stone formation based on whether patients have OSA. (Nephrolithiasis: Without OSA: 9.63%, With OSA: 14.27%, p < 0.001).
Table 1.
Baseline Characteristics of Participants
| Overall | Without OSAh | With OSA | p Value | |
|---|---|---|---|---|
| Age (years) | 48.27(0.41) | 47.46(0.42) | 49.98(0.54) | < 0.0001 |
| PIRa | 3.04(0.06) | 3.05(0.06) | 3.02(0.08) | 0.57 |
| Gender (%) | < 0.001 | |||
| Female | 51.43 | 53.93 | 46.14 | |
| Male | 48.57 | 46.07 | 53.86 | |
| Race (%) | 0.01 | |||
| Mexican American | 8.74 | 8.76 | 8.69 | |
| Non-Hispanic Asian | 5.84 | 6.56 | 4.32 | |
| Non-Hispanic Black | 11.30 | 11.18 | 11.56 | |
| Non-Hispanic White | 63.30 | 62.70 | 64.58 | |
| Other Hispanic | 6.64 | 6.86 | 6.18 | |
| Other Race | 4.17 | 3.94 | 4.66 | |
| Education (%) | 0.02 | |||
| Less than 9th grade | 4.83 | 5.12 | 4.23 | |
| 9–11th grade | 7.87 | 7.50 | 8.66 | |
| High school graduate | 23.97 | 23.43 | 25.15 | |
| Some college | 31.64 | 30.88 | 33.29 | |
| College or above | 31.64 | 33.07 | 28.67 | |
| Marital (%) | < 0.0001 | |||
| Divorced | 9.89 | 9.86 | 9.97 | |
| Living with partner | 9.45 | 9.65 | 9.04 | |
| Married | 53.85 | 51.37 | 59.12 | |
| Never married | 18.33 | 20.45 | 13.87 | |
| Separated | 2.58 | 2.62 | 2.51 | |
| Widowed | 5.87 | 6.06 | 5.48 | |
| Nephrolithiasis (%) | < 0.001 | |||
| No | 88.88 | 90.37 | 85.73 | |
| Yes | 11.12 | 9.63 | 14.27 | |
| Smoke (%) | < 0.0001 | |||
| Never | 56.90 | 59.86 | 50.74 | |
| Former | 25.13 | 23.92 | 27.73 | |
| Current | 17.92 | 16.22 | 21.53 | |
| Hypertension (%) | < 0.0001 | |||
| No | 60.64 | 65.24 | 50.94 | |
| Yes | 39.36 | 34.76 | 49.06 | |
| Diabetes (%) | < 0.0001 | |||
| No | 75.56 | 78.26 | 69.87 | |
| DMb | 15.88 | 13.90 | 20.07 | |
| IFGc | 7.07 | 6.46 | 8.36 | |
| IGTd | 1.49 | 1.39 | 1.70 | |
| CVDe(%) | < 0.001 | |||
| No | 91.03 | 92.14 | 88.70 | |
| Yes | 8.97 | 7.86 | 11.30 | |
| Stroke (%) | 0.03 | |||
| No | 96.95 | 97.37 | 96.39 | |
| Yes | 2.94 | 2.63 | 3.61 | |
| Alcohol (%) | 0.002 | |||
| Neverf | 9.09 | 11.84 | 8.11 | |
| Formerf | 6.32 | 6.92 | 8.41 | |
| Mildf | 34.72 | 40.34 | 41.22 | |
| Moderatef | 16.72 | 19.87 | 18.92 | |
| Heavyf | 18.62 | 21.04 | 23.35 | |
| BMIg(kg/m2) | < 0.0001 | |||
| Underweight (<18.5) | 1.50 | 1.84 | 0.78 | |
| Normal (18.5 to <25) | 25.89 | 30.99 | 15.14 | |
| Overweight (25 to <30) | 31.46 | 32.35 | 29.59 | |
| Obese (30 or greater) | 41.15 | 34.82 | 54.49 | |
| Physical activity (%) | < 0.0001 | |||
| Less than moderate | 45.12 | 42.57 | 50.49 | |
| Moderate | 26.11 | 25.86 | 26.66 | |
| Vigorous | 28.77 | 31.57 | 22.85 |
Notes: For continuous variables: survey-weighted mean (standard deviation,). For categorical variables: survey-weighted percentage, aIncome-poverty ratio, bDiabetes mellitus, cImpaired Fasting Glycaemia, dImpaired Glucose Tolerance, eCardiovascular disease, fNever corresponds to <12 drinks in lifetime; Former corresponds to ≥12 drinks in 1 year and did not drink last year, or did not drink last year but drank ≥12 drinks in lifetime; Mild corresponds to 1 drink/day for female and 2 drinks/day for male; Moderate corresponds to 2 drinks/day for female and 3 drinks/day for male; Heavy corresponds to 3 drinks/day for female and 4 drinks/day for male, gBody mass index; units are kg/m2, hObstructive Sleep Apnea.
The Risk of OSA is Positively Correlated with the Risk of Kidney Stones
In Model 1, which did not include any adjustments for variables, there was a demonstrated positive link between obstructive sleep apnea (OSA) and the development of kidney stones (OR = 1.56; 95% CI: 1.22–1.99; p < 0.001). Likewise, in Model 2, when accounting for factors such as age, gender, and race, the link between obstructive sleep apnea (OSA) and the development of kidney stones remained significant (OR = 1.46; 95% CI: 1.14–1.86; p = 0.004). In Model 3, which was fully adjusted, there was a significant positive association, indicating that people with OSA were 29% more likely to develop nephrolithiasis (OR = 1.29; 95% CI: 1.00–1.66; p = 0.05) (Table 2).
Table 2.
Association of Obstructive Sleep Apnea with Kidney Stones
| OR (95% CI), p Value | |||
|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |
| (n =11131) | (n =11131) | (n = 11,131) | |
| Kidney stones | |||
| OSAd | |||
| Without | Reference | Reference | Reference |
| With | 1.56 (1.22,1.99) <0.001 | 1.46 (1.14,1.86) 0.004 | 1.29 (1.00,1.66) 0.05 |
Notes: aModel 1: No covariates were adjusted, bModel 2: Adjusted for gender, age, and race, cModel 3: Adjusted for age, gender, race, poverty-to-income ratio, education level, marital status, smoking status, hypertension, diabetes, alcohol intake, body mass index, physical activity, stroke, cardiovascular disease, dObstructive Sleep Apnea.
Subgroup Analysis
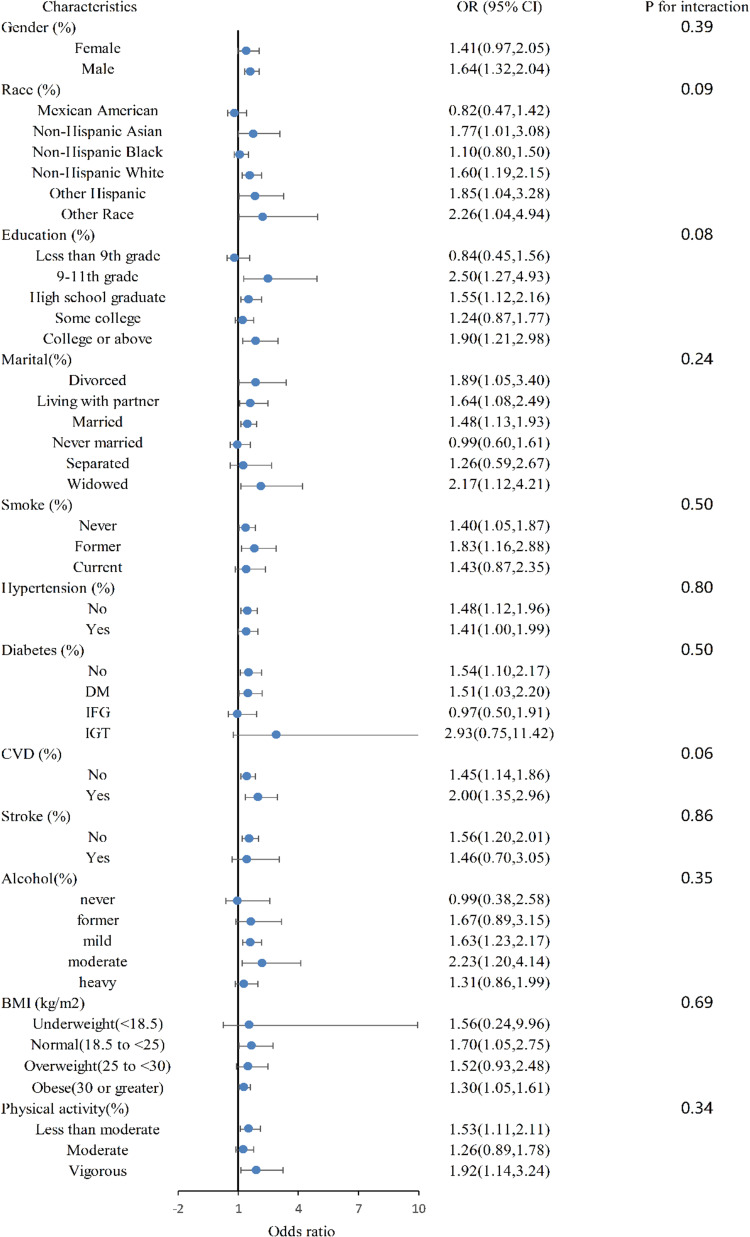
A subgroup analysis was conducted to assess the strength of the relation between OSA and kidney stones. We detected the interactions with age, gender, race, education level, marital status, smoking status, hypertension, diabetes, CVD, stroke, alcohol intake, body mass index and physical activity. However, no statistically significant correlation with the p-value for interaction was observed, suggesting that there was no dependency on factors such as age, gender, race, education level, marital status, smoking status, hypertension, diabetes, cardiovascular disease, stroke, alcohol intake, body mass index, and physical activity for this relation (overall p for interaction >0.05).The findings demonstrated that the positive relation between OSA and kidney stones was consistent across various subpopulations, including those with different age, gender, race, education level, marital status, smoking status, hypertension, diabetes, cardiovascular disease (CVD), stroke, alcohol intake, body mass index (BMI), and physical activity levels (Figure 1).
Figure 1.
Subgroup analysis (forest plots).
Results of Two-Sample MR Analysis
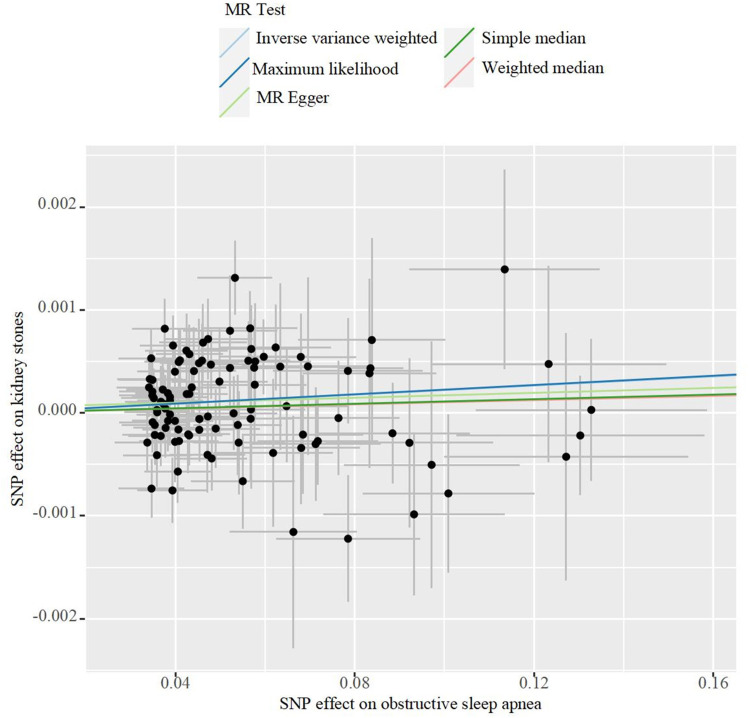
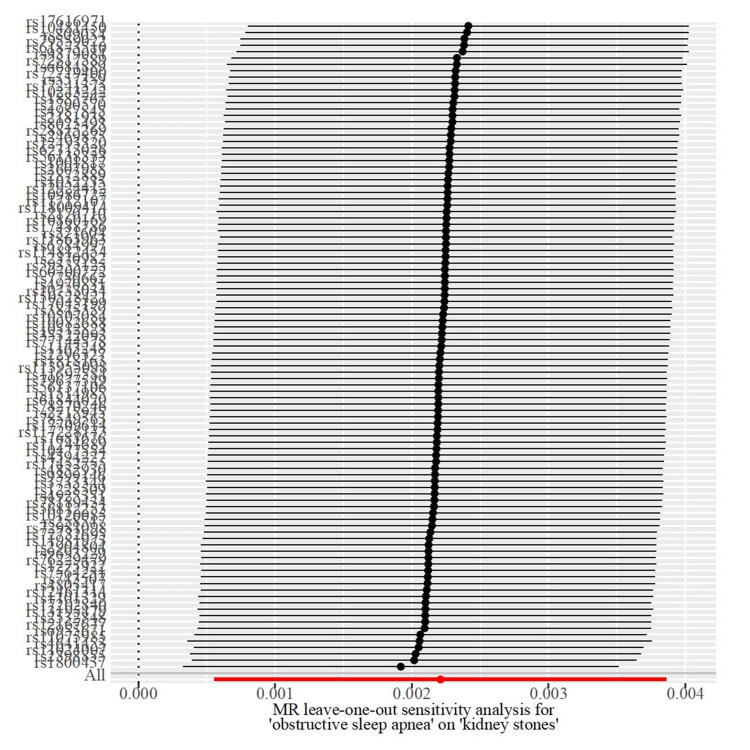
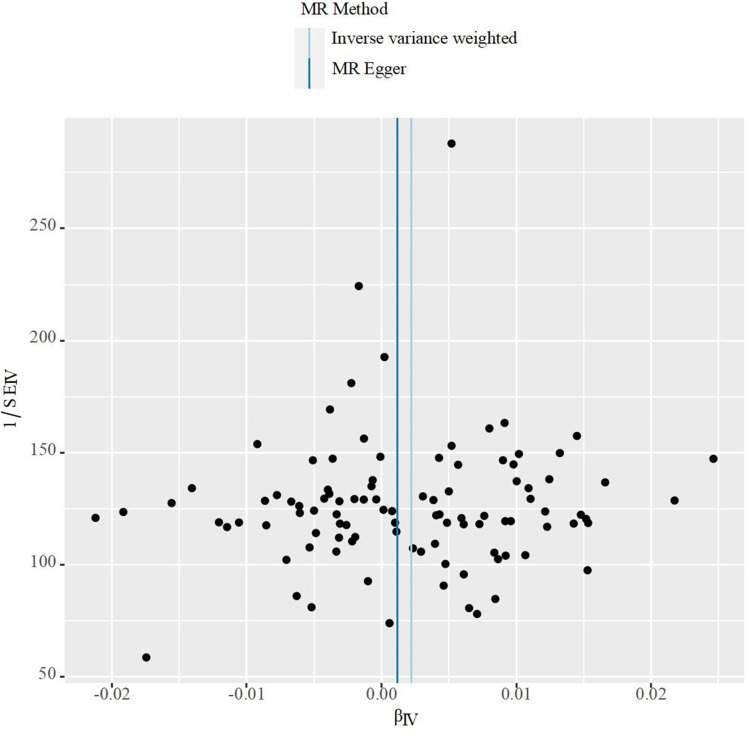
Initially, we conducted a linkage disequilibrium test and excluded SNPs with F-statistics less than 10. Subsequently, a selected set of SNPs were utilized as genetic instrumental variables in our Mendelian randomization (MR) studies, as detailed in Supplementary Table 1. The findings indicated a causal link between obstructive sleep apnea (OSA) and an increased risk of kidney stone disease (KSD), as demonstrated by the Inverse Variance Weighted (IVW) method (β: 0.00221(0.00056 0.00386), OR: 1.00221, 95% CI 1.00056–1.00387, p=0.00884), with consistent outcomes observed using the Maximum Likelihood estimation method (β: 0.00226(0.00071, 0.00380), p= 0.00420) as shown in Tables 3. Additionally, the MR-PRESSO global test did not detect any outliers. Furthermore, Cochran’s Q test confirmed the absence of significant SNP heterogeneity in the IVW approach (Q pval >0.05), as noted in Table 4. Scatter plots, leave-one-out analyses, and funnel plots pertinent to MR are available in Figures 2–4.
Table 3.
Results of Mendelian Randomization Analysis
| Method | Obstructive Sleep Apnea ~ Kidney Stones |
|---|---|
| IVW | |
| β (95 CI%) | 0.00221(0.00056, 0.00386) |
| OR (95 CI%) | 1.00221(1.00056, 1.00387) |
| p value | 0.00884 |
| MR-Egger | |
| β (95 CI%) | 0.00119(−0.00418,0.00657) |
| OR (95 CI%) | 1.00119(0.99582,1.00659) |
| p value | 0.66478 |
| Weighted median | |
| β (95 CI%) | 0.00103(0.37183,0.00329) |
| OR (95 CI%) | 1.00103(0.99877,1.00330) |
| p value | 0.37183 |
| Simple median | |
| β (95 CI%) | 0.00111(−0.00119,0.00340) |
| OR (95 CI%) | 1.00111(0.99881,1.00341) |
| p value | 0.34400 |
| Maximum likelihood | |
| β (95 CI%) | 0.00226(0.00071,0.00380) |
| OR (95 CI%) | 1.00226(1.00071,1.00381) |
| p value | 0.00420 |
| Global test p | 0.08100 |
Abbreviation: IVW, inverse variance weighted.
Table 4.
Heterogeneity and Pleiotropy Test
| Exposure | Heterogeneity Test | Pleiotropy Test | ||||
|---|---|---|---|---|---|---|
| Q | df | Q-pval | Egger-Intercept | SE | p-Value | |
| Obstructive Sleep Apnea | 120.653 | 99 | 0.069 | 5.13E-05 | 0.0001 | 0.697 |
Abbreviations: Q, Cochrane’s heterogeneity statistic; df, degrees of freedom.
Figure 2.
The scatter plot for MR analyses of causal associations between obstructive sleep apnea SNPs and kidney stones.
Figure 3.
Leave-one-out sensitivity analysis for kidney stones using SNPs associated obstructive sleep apnea.
Figure 4.
The funnel plot for MR analyses of causal associations between obstructive sleep apnea SNPs and kidney stones.
Discussion
Kidney stones typically have varied and intricate causes, along with high rates of occurrence and recurrence, and considerable differences among individuals. Current studies on the link between obstructive sleep apnea (OSA) and kidney stones are scarce. Although several studies have explored the association between OSA and kidney stones, the results remain inconsistent, and there is ongoing debate about the underlying mechanisms. For example, the study by Shahait et al11 found no significant changes in 24-hour urine parameters among OSA patients with kidney stones, suggesting a potential lack of a direct metabolic pathway link. In contrast, Tallman et al12 observed an increased risk of kidney stones in individuals with OSA, noting that changes in urinary analytes might contribute to this risk. Furthermore, the study by Canales et al13 indicated that OSA is associated with declining renal function, which is closely linked to a higher risk of kidney stone formation.
Therefore, we analyzed data from 12,488 subjects across two NHANES 2-year cycles. Upon controlling for confounding factors, our findings indicated a significant association between OSA and the prevalence of kidney stones. Additionally, a Mendelian randomization analysis of extensive genetic data suggested a potential causal connection between the two conditions.
OSA is characterized by repetitive episodes of upper airway obstruction during sleep, leading to intermittent hypoxia and hypercapnia. These episodes can trigger systemic inflammation and oxidative stress, which have been implicated in the pathogenesis of kidney stone formation. Several mechanisms may underlie the observed association between OSA and kidney calculi formation.
Periodic hypoxia, followed by oxidative stress, triggers insulin resistance in individuals with OSA.19 This resistance impairs the elimination of ammonium, leading to two known risk factors for kidney stones: increased urine acidity and lowered citrate levels in urine.20 Additionally, disruptions in blood flow, intermittent hypoxia, and heightened osmotic gradients in the renal papilla may cause microvascular damage or impairments, potentially promoting the biomineralization process and the formation of Randall’s plaques, ultimately contributing to the development of calcium-based kidney stones.21 Theoretically, hypoxia and endothelial dysfunction from OSA might adversely affect the renal papillary physiology, thereby increasing or intensifying the mineral deposition processes associated with Randall plaque formation.
Obstructive sleep apnea (OSA) has been linked to both acute and chronic systemic inflammatory states. Elevated levels of various inflammatory markers, such as C-reactive protein, adhesion molecules, interleukin 8, tumor necrosis factor α, and interleukin 6, have been observed in OSA patients.22,23 This condition is also associated with an increased production of reactive oxygen species from leukocytes.24 Recent evidence suggests that OSA induces an inflammatory response, particularly showing that OSA and intermittent hypoxemia can stimulate the NF-κB signaling pathway.25–27 Additionally, high concentrations of inflammatory proteins have been detected in both the urine and kidney stones of those affected by nephrolithiasis.28 From a mechanistic standpoint, inflammation may encourage the adherence of crystals to damaged urothelium or exposed Randall plaque surfaces, thereby elevating the likelihood of stone formation.29
Patients with OSA are observed to have elevated levels of reactive oxygen species in their bloodstream, resulting in oxidative stress.30 This leads to reduced nitric oxide bioavailability and triggers an inflammatory response, resulting in endothelial dysfunction and ultimately hypertension.31,32 Moreover, the intermittent hypoxia experienced by OSA patients stimulates baroreceptors, leading to increased sympathetic nervous activity and elevated catecholamine release, which in turn accelerates heart rate and raises blood pressure.33,34 The exact mechanism by which hypertension contributes to renal stone formation is still not well understood. Liu et al suggested that fluctuations in blood pressure directly affect the urinary microbiome, and this influence may facilitate the development of kidney stone disease.35
Sensitivity analysis plays a pivotal role in Mendelian Randomization (MR) studies. Both MR-Egger and MR-PRESSO analyses indicated no signs of directional pleiotropy, and the core results remained stable despite the removal of individual selected SNPs. This stability highlights the robustness and reliability of our MR methodology. Furthermore, the essential assumptions of instrumental variables (IVs) were upheld throughout this study, strengthening the evidence for a causal link between obstructive sleep apnea and kidney stones.
These findings suggest that while some studies have investigated the relationship between OSA and kidney stones, the mechanisms are not yet fully understood, and the conclusions are inconsistent, highlighting the need for further research to clarify the specific mechanisms and impacts of this association.
Our investigation benefits from merging a cross-sectional framework with Mendelian Randomization (MR) analysis, lending stronger credibility to our results. Extensive subgroup and sensitivity analyses were executed to refine our understanding of the associations. Despite these methodological advantages, there are notable limitations in our study. Firstly, the reliance on self-reported kidney stone data from NHANES could potentially affect the accuracy due to recall biases. Secondly, the absence of gender-specific genome-wide association study (GWAS) data restricts our exploration into more detailed interactions. Thirdly, in this study, the risk of OSA was assessed using three questions, which suggests a high risk of OSA rather than a confirmed diagnosis. Ideally, diagnosing OSA requires overnight polysomnography or polygraphy. Future research should incorporate prospective cohort studies and richer datasets to overcome these limitations and should also aim at uncovering the underlying mechanisms linking these conditions and developing preventive therapies for kidney stones in patients with OSA.
Conclusions
Our research indicates that OSA may promote the formation of kidney stones, highlighting the importance of early detection and management of OSA in individuals at risk of kidney stones.
Acknowledgments
Ying Liu, Li Wang, and Er-Hao Bao are co-first authors for this study. We express our gratitude to all individuals who contributed to and participated in the National Health and Nutrition Examination Survey, as well as to the researchers involved in the IEU open GWAS project and FinnGen database for providing genome-wide association studies data.
Funding Statement
The authors declare that there are no financial or non-financial interests that could have influenced the manuscript.
Data Sharing Statement
This study utilized publicly available datasets for analysis. The original contributions made to the research are outlined in the article/Supplementary material; for additional inquiries, please contact the corresponding author.
Ethics Approval
The Ethics Committee of the affiliated hospital of North Sichuan Medical College strictly adheres to the Declaration of Helsinki and the International Ethical Guidelines for Health-related Research Involving Humans, performing independent ethical review responsibilities. This study uses legally obtained publicly available data, meeting the conditions for exemption from review as stated in the Ethical Review Methods for Life Sciences and Medical Research Involving Humans.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no relevant financial or non-financial interests to disclose for this work.
References
- 1.Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H. Demographic and geographic variability of kidney stones in the United States. Kidney Int. 1994;46:893–899. [DOI] [PubMed] [Google Scholar]
- 2.Pozdzik A, Maalouf N, Letavernier E, et al. Meeting report of the ”Symposium on kidney stones and mineral metabolism: calcium kidney stones in 2017. J nephrol. 2019; 32:681–698. [DOI] [PubMed] [Google Scholar]
- 3.Scales CDJ, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–165. doi: 10.1016/j.eururo.2012.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croppi E, Ferraro PM, Taddei L, Gambaro G. Prevalence of renal stones in an Italian urban population: a general practice-based study. Urol Res. 2012;40:517–522. doi: 10.1007/s00240-012-0477-z [DOI] [PubMed] [Google Scholar]
- 5.Thongprayoon C, Krambeck AE, Rule AD. Determining the true burden of kidney stone disease. Nat Rev Nephrol. 2020;16:736–746. doi: 10.1038/s41581-020-0320-7 [DOI] [PubMed] [Google Scholar]
- 6.Ziemba JB, Matlaga BR. Epidemiology and economics of nephrolithiasis. Invest Clin Urol. 2017;58:299–306. doi: 10.4111/icu.2017.58.5.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rundo JV. Obstructive sleep apnea basics. Clev Clin J Med. 2019;86:2–9. doi: 10.3949/ccjm.86.s1.02 [DOI] [PubMed] [Google Scholar]
- 8.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonsignore MR, Esquinas C, Barceló A, et al. Metabolic syndrome, insulin resistance and sleepiness in real-life obstructive sleep apnoea. Europ resp J. 2012;39:1136–1143. doi: 10.1183/09031936.00151110 [DOI] [PubMed] [Google Scholar]
- 10.Mesarwi OA, Sharma EV, Jun JC, Polotsky VY. Metabolic dysfunction in obstructive sleep apnea: a critical examination of underlying mechanisms. Sleep Biol Rhythms. 2015;13:2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahait M, Nevo A, El-Asmar JM, et al. Twenty-four hour urine parameters in nephrolithiasis patients with obstructive sleep apnea syndrome. J Clin Urol. 2022;2022:1059252038. doi: 10.1177/20514158221088683 [DOI] [Google Scholar]
- 12.Tallman JE, Stone BV, Sui W, Miller NL, Hsi RS. Association between obstructive sleep apnea and 24-h urine chemistry risk factors for urinary stone disease. Urolithiasis. 2023;51:46. doi: 10.1007/s00240-023-01421-x [DOI] [PubMed] [Google Scholar]
- 13.Canales MT, Taylor BC, Ishani A, et al. Reduced renal function and sleep-disordered breathing in community-dwelling elderly men. Sleep Med. 2008;9:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephr. 2016;27:3253–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Q, Burgess S, Turman C, et al. Body mass index and breast cancer survival: a Mendelian randomization analysis. Int J Epidemiol. 2017;46:1814–1822. doi: 10.1093/ije/dyx131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. New Engl J Med. 1993;328:833–838. [DOI] [PubMed] [Google Scholar]
- 17.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985–1998. doi: 10.1093/ije/dyx102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verbanck M, Chen C, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Sign. 2005;7:1040–1052. [DOI] [PubMed] [Google Scholar]
- 20.Abate N, Chandalia M, Cabo-Chan AVJ, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392. [DOI] [PubMed] [Google Scholar]
- 21.Taylor ER, Stoller ML. Vascular theory of the formation of Randall plaques. Urolithiasis. 2015;43(1):41–45. doi: 10.1007/s00240-014-0718-4 [DOI] [PubMed] [Google Scholar]
- 22.Nadeem R, Harvey M, Singh M, et al. Patients with obstructive sleep apnea display increased carotid intima media: a meta-analysis. Int j Vasc Med. 2013;2013:839582. doi: 10.1155/2013/839582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trakada G, Chrousos G, Pejovic S, Vgontzas A. Sleep apnea and its association with the stress system, inflammation, insulin resistance and visceral obesity. Sleep Medic Clinics. 2007;2:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Resp Crit Care. 2002;165:934–939. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg H, Ye X, Wilson D, Htoo AK, Hendersen T, Liu SF. Chronic intermittent hypoxia activates nuclear factor-kappaB in cardiovascular tissues in vivo. Biochem Biophys Res Commun. 2006;343:591–596. [DOI] [PubMed] [Google Scholar]
- 26.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. [DOI] [PubMed] [Google Scholar]
- 27.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Resp Crit Care. 2006;174:824–830. [DOI] [PubMed] [Google Scholar]
- 28.Canales BK, Anderson L, Higgins L, et al. Proteome of human calcium kidney stones. Urology. 2010;76:1013–1017. doi: 10.1016/j.urology.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 29.Domingos F, Serra A. Metabolic syndrome: a multifaceted risk factor for kidney stones. Scand J Urol. 2014;48:414–419. doi: 10.3109/21681805.2014.903513 [DOI] [PubMed] [Google Scholar]
- 30.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Europ resp J. 2009;33:1467–1484. doi: 10.1183/09031936.00086608 [DOI] [PubMed] [Google Scholar]
- 31.Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--The bad ugly and good: implications to the heart and brain. Sleep Med Rev. 2015;20:27–45. doi: 10.1016/j.smrv.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 32.Victor VM, Rocha M, Solá E, Bañuls C, Garcia-Malpartida K, Hernández-Mijares A. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Design. 2009;15:2988–3002. [DOI] [PubMed] [Google Scholar]
- 33.Loh HH, Sukor N. Associations between primary aldosteronism and diabetes, poor bone health, and sleep apnea-what do we know so far? J Hum Hypertens. 2020;34:5–15. doi: 10.1038/s41371-019-0294-8 [DOI] [PubMed] [Google Scholar]
- 34.Mansukhani MP, Kara T, Caples SM, Somers VK. Chemoreflexes, sleep apnea, and sympathetic dysregulation. Curr Hypertens Rep. 2014;16:476. doi: 10.1007/s11906-014-0476-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu F, Zhang N, Jiang P, et al. Characteristics of the urinary microbiome in kidney stone patients with hypertension. J Transl Med. 2020;18:130. doi: 10.1186/s12967-020-02282-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study utilized publicly available datasets for analysis. The original contributions made to the research are outlined in the article/Supplementary material; for additional inquiries, please contact the corresponding author.