Abstract
Background
Nicotinamide has demonstrated efficacy in the treatment of melasma. Topical antioxidants and humectants may enhance its performance. Currently, there is no controlled trial on the combination of 10% nicotinamide, 5% magnesium ascorbyl phosphate, and 5% hyaluronic acid, a dermo-cosmetic compound, in comparison to 4% hydroquinone for the treatment of melasma. This study aimed to explore the tolerability and efficacy of the association of the combined product versus hydroquinone.
Methods
A randomized, double-blind trial involving women with facial melasma was conducted. Participants were instructed to apply the combined product (NIC group) twice daily or 4% hydroquinone for 60 days (HQ group) at night and placebo in the morning. Evaluations were performed at inclusion, after 14 and 60 days of treatment, measuring the modified Melasma Area and Severity Index (mMASI), Melasma Quality of Life Scale (MELASQoL), and colorimetric luminosity. The Global Aesthetic Improvement Scale (GAIS) was assessed by a blinded evaluator.
Results
Both interventions led to a progressive improvement in mMASI, MELASQoL, and GAIS, without a difference between them on D14 and D60 (p>0.2). For NIC, the mean reduction (95% CI) in mMASI was 16% (8–24%) on D14 and 32% (23–41%) on D60, while for HQ, it was 10% (7–24%) on D14 and 43% (34–52%) on D60. Reduction in colorimetric luminosity was greater in the HQ group at D60 (p=0.01). No serious side effects were identified. Of the initially included 50 patients, one was lost to follow-up in the HQ group on D60, and one withdrew consent from the NIC group, both unrelated to treatment.
Conclusion
The association of 10% nicotinamide, 5% magnesium ascorbyl phosphate, and 5% hyaluronic acid was safe and well-tolerated, although its overall clinical efficacy was numerically inferior to 4% hydroquinone. This regimen can be considered for patients with poor tolerability to hydroquinone.
Clinical Trial Registration
#RBR-4mkfmr8.
Keywords: melasma, hyperpigmentation, nicotinamide, hydroquinone, antioxidants
Introduction
Melasma is a chronic acquired hyperpigmentation of the skin, characterized by the presence of brownish macules in sun-exposed areas, particularly on the face. While it can affect both genders and various ethnicities, it is more prevalent in women during childbearing age, especially those with intermediate Fitzpatrick skin types (III to V).1,2 The development of melasma is influenced by hormonal and external factors such as sun exposure, cosmetics, and photosensitizing medications, alongside genetic susceptibility.3,4
The standard treatment for melasma typically involves a combination of sun protection with depigmenting agents, with hydroquinone (HQ) being the most widely used and studied topical bleaching. HQ is a potent tyrosinase inhibitor, however, it also exhibits melanocytotoxic activity. While it yields satisfactory results, it may sensitize the skin of some patients and lead to conditions such as hypochromia in confetti and ochronosis, especially after prolonged use, and in higher concentrations.5
Sunlight exposure and the release of inflammatory cytokines contribute to disrupting the balance between oxidant and antioxidant agents, leading to increased oxidative stress, which contributes to sustained melanogenesis in melasma.6–8 Numerous studies have underscored the effectiveness of both topical and oral antioxidants in mitigating hyperpigmentation.5,9–13
Nicotinamide (NIC), or niacinamide, aside from playing a role in energy metabolism and DNA synthesis regulation, exhibits notable biological effects, including depigmenting and photoprotective actions. NIC can prevent photocarcinogenesis and protect against UV-induced immunosuppression, leading to improvement in epidermal homeostasis and cellular bioenergetics in oxidative stress-exposed skin. In addition, it delays the transfer of melanosomes from melanocytes to keratinocytes.14–18 Also restores the skin barrier by increasing protein and ceramide synthesis and keratinocyte differentiation.
Vitamin C (VC) is a potent antioxidant and is the most abundant in human skin. In the cytoplasm of keratinocytes, melanocytes, and fibroblasts, enzymes related to VC participate in the neutralization of free radicals and reduction of solar erythema and melanogenesis.13,19 Topical VC also prevents oxidative damage from UVA radiation and particulate hydrocarbon pollutants, reversing epithelial damage, collagen and lipid synthesis in the skin barrier.20,21
L-ascorbic acid is the most biologically active form of VC. However, the hydrophobic structure from the stratum corneum reduces its penetration, and pH above 3.5 reduces the L-ascorbic acid stability. In this context, the industry challenge is to develop stable formulations and VC derivatives that have efficient transepidermal delivery to maximize the concentration of active vitamin C in the skin, like magnesium ascorbyl phosphate (VCPMG).21
Melasma is characterized by a local defective skin barrier function.22,23 Topical hyaluronic acid (HA) enhances epidermal hydration, and binds to extracellular matrix molecules and cell surface receptors, thereby regulating cellular behavior by controlling the tissue’s macro and microenvironments. Humectants, such as HA, contribute to the restoration of the skin barrier and facilitate the penetration of active substances through the skin.24
It is important to explore safe and effective alternatives to HQ. To date, no randomized controlled study has evaluated the response to the combination of NIC + VCPMG + HA in the treatment of facial melasma Therefore, we propose a study that compares this combination with HQ.
Materials and Methods
A multicenter, randomized, double-blinded clinical trial involving 50 women with facial melasma was conducted at UNIFESP (São Paulo - SP), FMB-Unesp (Botucatu - SP), and Corium Clinic (Presidente Prudente - SP). Participants were recruited from dermatological patients between May and August 2023. The diagnosis of facial melasma was established through clinical and dermoscopic examination by an experienced dermatologist. The study protocol received approval from the Institutional Review Board, all participants signed informed consent, and the protocol was registered in the Brazilian Registry of Clinical Trials (https://ensaiosclinicos.gov.br/rg/RBR-4mkfmr8).
Inclusion criteria comprised women aged between 18 and 60 years old, presenting moderate to severe facial melasma (Melasma Area and Severity Index - mMASI≥4), without treatment for at least 45 days, except for sunscreen use during the washout period. Pregnant and lactating women and individuals with hypersensitivity or previous side effects to the studied medications were not included.
Participants were instructed to apply, on the facial spots, a gel cream containing 10% NIC + 5% VCPMG + 5% HA (NIC group) twice daily or to use only 4% HQ gel cream at night and an identical placebo in the morning (HQ group) for 60 days.
The treatment protocols were randomized in blocks through computer simulation, and the participants were consecutively allocated. The researchers had no access to the randomization list (central randomization). At the inclusion (D0), each participant received a brown envelope containing a numbered metal tube, with numbers ranging from 1 to 50, and they were randomized into two groups:
- NIC Group: received broad-spectrum tinted sunscreen (Episol color, SPF 70) and two tubes (morning and night) containing 10% NIC + 5% VCPMG + 5% HA gel cream, applied twice daily for 60 days.
- HQ Group: received the broad-spectrum tinted sunscreen, a tube with 4% HQ gel cream for nightly use, and another identical tube containing a topical placebo for morning use for 60 days.
Clinical and demographic data were evaluated at the inclusion. At D0, D14, and D60, standardized photographs, colorimetric measurements, assessments of clinical severity using mMASI, and evaluations of the quality of life through the Melasma Quality of Life Scale (MELASQoL) were performed.1,25
Colorimetry was assessed by calculating the difference in colorimetric luminosity (Dif*L) between the skin affected by melasma and the adjacent unaffected skin (<2 cm distance), measured using the CR-400 Chroma Meter (Konica Minolta). The site was selected as the area of maximum melasma pigmentation on D0. To maintain consistency in the location for future evaluations, the assessed area was marked in a standardized clinical photograph. The assessment of luminosity differences between affected and unaffected skin is a highly accurate measure. Evaluating the contrast between these areas effectively reflects the treatment’s impact on the patient’s daily life. A cell cycle from the basal layer to the stratum corneum takes approximately 28 days. Therefore, 60 days (final assessment) ensures at least 2 cell cycles, making it possible to evaluate the response to therapies instituted for melasma.
The primary outcome was the change in mMASI from baseline at each visit. Secondary outcomes included differences in MELASQoL, Dif*L, and GAIS (Global Aesthetic Improvement Scale). GAIS is a 5-point scale used to evaluate the overall improvement in a patient’s appearance after a specific treatment or procedure, ranging from “worse” to “very much improved”.26 These assessments were conducted by a blinded investigator not involved in the initial evaluations or in the follow-up assessments.
Additionally, participants were questioned during follow-up assessments on day 14 and day 60 regarding product tolerability, compliance, adverse effects, and the daily frequency of sunscreen applications. They also provided a subjective improvement evaluation at D14 and D60, categorizing the evolution of melasma as worsening, neutral, mild improvement, moderate improvement, significant improvement, and exceptional improvement.
The sample size was calculated to detect a minimum of a 10% difference in mMASI reduction between the groups, assuming an equivalent standard deviation. We set the power at 90% and alpha at 5%, accounting for up to a 10% potential dropout rate.27 The dropout criteria were loss to follow-up (failure to attend assessments on Day 14 and/or Day 60) or withdrawal of consent.
All outcomes were analyzed on an intention-to-treat basis.28 The absolute reduction in scores (D14-D0 and D60-D0) and the difference in the categorical scores (eg, GAIS and subjective perception of improvement) between groups were assessed using the Mann–Whitney test. The frequency of irritative adverse effects was compared between the groups using Fisher’s exact test.29,30 Significance was set at a p-value <0.05.31
Results
The main clinical and demographic variables of the groups at inclusion are presented in Table 1, and no differences were observed between the groups (p >0.1). Out of the initially enrolled 50 patients, there was one loss to follow-up on day 60 in the HQ group, and one withdrawal of consent in the NIC group, both of which were unrelated to the treatment. The patient in the HQ group did not attend the follow-up appointment due to difficulties with transportation logistics to the research center. The patient in the NIC group withdrew consent due to personal reasons. The study flowchart according to CONSORT 2010 is presented in Figure 1.
Table 1.
Clinical and Demographic Data of the Participants (n = 49)
| Variables | NIC | HQ |
|---|---|---|
| n | 24 | 25 |
| Age (years), mean (SD) | 44.0 (5.7) | 44.8 (7.1) |
| Skin phototype, n (%) | ||
| II | 2 (8%) | 3 (12%) |
| III | 10 (42%) | 9 (36%) |
| IV | 11 (46%) | 8 (32%) |
| V | 1 (4%) | 5 (20%) |
| Age of onset, mean (SD) | 31.4 (6.1) | 28.3 (8.9) |
| Family occurrence, n (%) | 17 (71%) | 20 (80%) |
| mMASI, mean (SD) | 6.2 (3.1) | 7.2 (4.4) |
| MELASQoL, mean (SD) | 44.7 (17.9) | 50.7 (15.3) |
| Dif*L, mean (SD) | 5.0 (2.1) | 4.7 (2.7) |
Note: Dif*L: difference between colorimetric luminosity (*L) from the melasma to the adjacent unaffected skin.
Abbreviations: mMASI, modified Melasma Area Severity Index; MELASQoL, Melasma Quality of Life Scale.
Figure 1.
Study flowchart according to CONSORT 2010.
The main outcomes are summarized in Figure 2. Both groups showed progressive improvement in clinical outcomes (Figure 3) and quality of life, with no significant difference between the groups (p >0.2). Within the NIC group, the mean reduction (95% CI) in mMASI was 16% (8-24%) on D14 and 32% (23–41%) on D60, while for the HQ group, it was 10% (7-24%) on D14 and 43% (34–52%) on D60, respectively.
Figure 2.
Main outcomes for D14 and D60. (A) Percentage reduction in mMASI score; (B) Percentage reductions in colorimetric difference between healthy and affected skin; (C) percentage reduction in MELASQoL; (D) and (E) GAIS scores at D14 and D60. GAIS is a 5-point scale used to evaluate the overall improvement in a patient’s appearance after a specific treatment or procedure, ranging from “worse” to “very much improved”. These assessments were conducted by a blinded investigator.
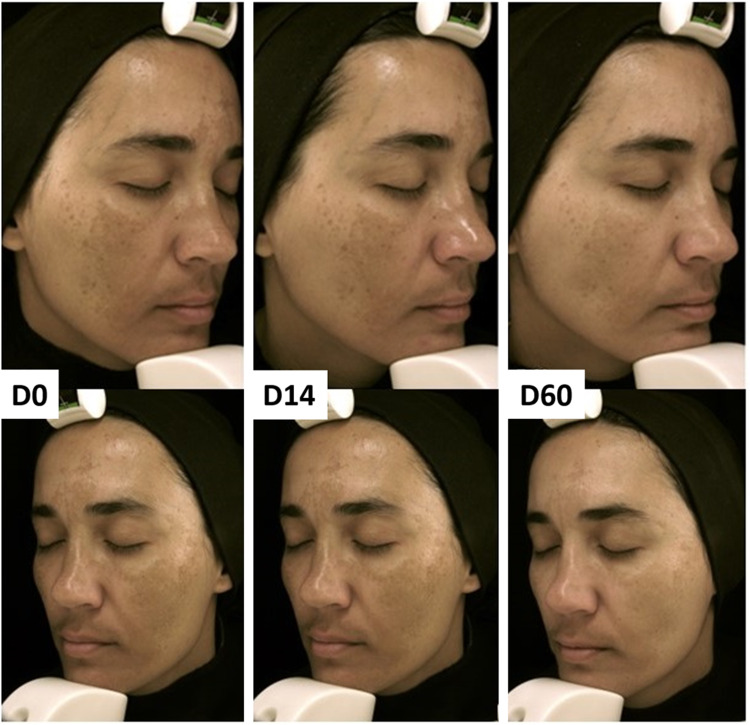
Figure 3.
Facial melasma treated with topical 10% nicotinamide + 5% magnesium ascorbyl phosphate + 5% hyaluronic acid. Standardized photography from the inclusion, after 14 and 60 days.
Additionally, both groups demonstrated reductions in MELASQoL (95% CI) on D14 and D60, with a mean reduction of 18% (12–23%) on D14 and 30% (22–39%) on D60 in the NIC group. The HQ group showed reductions of 20% (10–31%) on D14 and 33% (22–46%) on D60.
Reductions in luminosity difference (colorimetry) were observed in the NIC group, from 7% (3–22%) on D14 to 17% (0–34%) on D60. In the HQ group, the reductions were from 18% (6–43%) on day 14 to 27% (13–43%) on D60.
The GAIS score did not differ between the groups on both D14 and D60. Any improvement was evidenced in 5 (21%) and 11 (46%) participants in the NIC group on D14 and D60, respectively, and 11 (44%) and 18 (75%) in the HQ group.
There were no serious adverse effects related to the treatments. In the NIC group, seven cases of mild itching (29%) were reported on day 14, characterized by itching lasting a few minutes after application, without redness. In comparison, the HQ group noted nine cases (36%, p = 0.85) of irritation or dryness. By day 60, the NIC group observed three cases (13%, p = 0.11) of mild acne (fewer than 5 inflammatory lesions on the cheek) and three additional cases (13%) of mild itching, again lasting a few minutes post-application and without redness. In contrast, the HQ group recorded nine cases (38%, p = 0.10) of irritation or dryness. Importantly, all reported cases resolved spontaneously, with no need for intervention or treatment interruption (Figure 4).
Figure 4.
Frequency of the main adverse effects of the study according to the group at D14 and D60.
All participants who experienced no adverse effects used the treatment for at least six days per week, and all applied sunscreen at least twice a day.
According to participants’ subjective evaluations, on D14, perceived improvement was reported by 71% of NIC group participants and 76% of HQ group participants (p = 0.15); on D60, both groups showed improvement in 92% of cases (p = 0.78).
Discussion
A consistent improvement was observed in clinical measures, quality of life scores, and colorimetric measurements in women with melasma treated with 4% HQ gel cream for 60 days. Similarly, those treated with 10% NIC + 5% VCPMG + 5% HA gel cream up to day 60 also showed progressive favorable results. The participants of the study represented a usual sample of Brazilian adult women with melasma, regarding clinical and epidemiological data from previous studies.10,11,32–38
In this trial, the topical combination of NIC + VCPMG + HA reached numerically inferior clinical results compared to HQ on D14 or D60. In a prior study comparing the 4% NIC and 4% HQ, where participants applied one product to each side of the face, a significant improvement was observed in 44% of patients using NIC and 55% using HQ after two months. However, side effects were more prevalent with HQ (29%) compared to NIC (18%), indicating the better tolerability of NIC and a higher safety profile.15 This study also assessed histopathological characteristics of melasma-affected skin before and after the use of 4% NIC, revealing a significant reduction in melanin content in the epidermis, as well as decreased inflammatory infiltrate and solar elastosis.15
The efficacy of topical NIC in skin depigmentation was evaluated through a randomized clinical trial involving 18 Japanese women. NIC 5% was applied to one side of the face, while a moisturizer was applied to the contralateral side. The results after eight weeks demonstrated a reduction in pigmentation in the intervention side compared to the control side.39 In our sample, the topical combination of NIC + VCPMG + HA promoted 32% mMASI reduction in eight weeks, with excellent tolerability. This may be particularly relevant for melasma patients from sunny regions or with sensitive skin, who may have reduced tolerability to treatments with retinoids and hydroquinone.
After 16 weeks of twice-daily application, 10% NIC resulted in a mean 36% reduction in mMASI in 14 Chinese patients with facial melasma, and no adverse effects were observed. However, the comparator group, which combined this regimen with biweekly salicylic acid peels, performed better.40
Another study aimed to investigate the role of interleukin 6 (IL-6) and endothelin-1 (ET-1) receptors in the increased dendricity of melanocytes in hyperpigmented facial lesions and the action of NIC in suppressing these ligands. The study observed that IL-6 and ET-1 increased melanocyte dendricity, consequently enhancing the transfer of melanosomes from melanocytes to keratinocytes. Therefore, this study demonstrated that NIC was effective in reducing the release of IL-6 and ET-1 from keratinocytes, inhibiting melanocyte dendricity, confirming its role in skin depigmenting.41
VCPMG is a stable, lipophilic, and esterified form of VC that remains stable at neutral pH.21 An in vitro study has shown its potential to protect keratinocytes against UVA irradiation, possibly by increasing glutathione levels.42 Additionally, other in vitro results demonstrated that it induces collagen synthesis to a similar extent as ascorbic acid, and contributes to skin depigmentation.43,44 Further histologic studies on the topical combination of NIC + VCPMG + HA are warranted.
The time taken for a basal keratinocyte to reach the stratum corneum is approximately 28 days, and most trials for topic treatments for melasma assess clinical outcomes at 60-90 days. The evaluation conducted on Day 14 primarily aimed to identify adverse effects rather than assess potential early depigmenting effects of the treatments. The assessment of the isolated effects of the 5% VCPMG, or the 10% NIC in melasma demands specific designs.
Possible limitations of the study include the short duration of the intervention, modest sample size, lack of histopathologic analysis, as well as the assessment of relapses after stopping the treatments. The use of this combination in longer treatment regimens for melasma is warranted.
Conclusion
The topical combination of NIC + VCPMG + AH, was found to be safe and well-tolerated, although its overall clinical efficacy was numerically inferior to 4% hydroquinone. It can be considered as an alternative treatment for facial melasma, especially for patients with poor tolerability to HQ.
Acknowledgments
To the patients in this study who gave written informed consent to the publication of their case details and images.
Funding Statement
There is no funding to report.
Data Sharing Statement
The raw data of this study is available under contact with the corresponding author.
Ethics Statement
The study was approved by the Research Ethics Committee of the UNIFESP (#6.142.230). All procedures in studies involving human participants were performed in accordance with the Declaration of Helsinki (as revised in 2013).
Informed-Consent Statement
Written informed consent was obtained from patients to publish their data and images in this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89(5):771–782. doi: 10.1590/abd1806-4841.20143063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dias MO, Minagawa FH, Teixeira de Abreu AF, et al. Prevalence of facial melasma among adult women in a multiracial population. Int J Dermatol. 2024;63(4):e89–e91. doi: 10.1111/ijd.17078 [DOI] [PubMed] [Google Scholar]
- 3.Esposito ACC, Cassiano DP, da Silva CN, et al. Update on melasma-part i: pathogenesis. Dermatol Ther. 2022;12(9):1967–1988. doi: 10.1007/s13555-022-00779-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4..de Abreu AFT, Dias MO, Barbosa MMC, de Amorim RP, Miot HA, Esposito ACC. Factors associated with facial melasma severity in Brazilian women: an internet-based survey. An Bras Dermatol. 2024;99. doi: 10.1016/j.abd.2023.12.008 [DOI] [PubMed] [Google Scholar]
- 5.Cassiano DP, Esposito ACC, da Silva CN, et al. Update on melasma-part II: treatment. Dermatol Ther. 2022;12(9):1989–2012. doi: 10.1007/s13555-022-00780-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seckin HY, Kalkan G, Bas Y, et al. Oxidative stress status in patients with melasma. Cutan Ocul Toxicol. 2014;33(3):212–217. doi: 10.3109/15569527.2013.834496 [DOI] [PubMed] [Google Scholar]
- 7.Katiyar S, Yadav D. Correlation of oxidative stress with melasma: an overview. Curr Pharm Des. 2022;28(3):225–231. doi: 10.2174/1381612827666211104154928 [DOI] [PubMed] [Google Scholar]
- 8.Choubey V, Sarkar R, Garg V, Kaushik S, Ghunawat S, Sonthalia S. Role of oxidative stress in melasma: a prospective study on serum and blood markers of oxidative stress in melasma patients. Int J Dermatol. 2017;56(9):939–943. doi: 10.1111/ijd.13695 [DOI] [PubMed] [Google Scholar]
- 9.Babbush KM, Babbush RA, Khachemoune A. Treatment of melasma: a review of less commonly used antioxidants. Int J Dermatol. 2021;60(2):166–173. doi: 10.1111/ijd.15133 [DOI] [PubMed] [Google Scholar]
- 10.Lima PB, Dias JAF, Esposito ACC, Miot LDB, Miot HA. French maritime pine bark extract (pycnogenol) in association with triple combination cream for the treatment of facial melasma in women: a double-blind, randomized, placebo-controlled trial. J Eur Acad Dermatol Venereol. 2021;35(2):502–508. doi: 10.1111/jdv.16896 [DOI] [PubMed] [Google Scholar]
- 11.Lima PB, Dias JAF, Cassiano D, et al. A comparative study of topical 5% cysteamine versus 4% hydroquinone in the treatment of facial melasma in women. Int J Dermatol. 2020;59(12):1531–1536. doi: 10.1111/ijd.15146 [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Kim J, Lee YI, Almurayshid A, Jung JY, Lee JH. Effect of a topical antioxidant serum containing vitamin C, vitamin E, and ferulic acid after Q-switched 1064-nm Nd: YAG laser for treatment of environment-induced skin pigmentation. J Cosmet Dermatol. 2020;19(10):2576–2582. doi: 10.1111/jocd.13323 [DOI] [PubMed] [Google Scholar]
- 13.Correia G, Magina S. Efficacy of topical vitamin C in melasma and photoaging: a systematic review. J Cosmet Dermatol. 2023;22(7):1938–1945. doi: 10.1111/jocd.15748 [DOI] [PubMed] [Google Scholar]
- 14.Zhen AX, Piao MJ, Kang KA, et al. Niacinamide protects skin cells from oxidative stress induced by particulate matter. Biomol Ther. 2019;27(6):562–569. doi: 10.4062/biomolther.2019.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarrete-Solis J, Castanedo-Cazares JP, Torres-Alvarez B, et al. A double-blind, randomized clinical trial of niacinamide 4% versus hydroquinone 4% in the treatment of melasma. Dermatol Res Pract. 2011;2011:379173. doi: 10.1155/2011/379173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76(2):135–141. [PubMed] [Google Scholar]
- 17.Berardesca E, Ardigo M, Cameli N, Mariano M, Agozzino M, Matts PJ. Randomized, double-blinded, vehicle-controlled, split-face study to evaluate the effects of topical application of a Gold Silk Sericin/Niacinamide/Signaline complex on biophysical parameters related to skin ageing. Int J Cosmet Sci. 2015;37(6):606–612. doi: 10.1111/ics.12237 [DOI] [PubMed] [Google Scholar]
- 18.Madaan P, Sikka P, Malik DS. Cosmeceutical aptitudes of niacinamide: a review. Recent Adv Antiinfect Drug Discov. 2021;16(3):196–208. doi: 10.2174/2772434416666211129105629 [DOI] [PubMed] [Google Scholar]
- 19.Huh CH, Seo KI, Park JY, Lim JG, Eun HC, Park KC. A randomized, double-blind, placebo-controlled trial of vitamin C iontophoresis in melasma. Dermatology. 2003;206(4):316–320. doi: 10.1159/000069943 [DOI] [PubMed] [Google Scholar]
- 20.Dimitrov A, Zanini M, Zucchi H, et al. Vitamin C prevents epidermal damage induced by PM-associated pollutants and UVA1 combined exposure. Exp Dermatol. 2021;30(11):1693–1698. doi: 10.1111/exd.14315 [DOI] [PubMed] [Google Scholar]
- 21.Al-Niaimi F, Chiang NYZ. Topical vitamin c and the skin: mechanisms of action and clinical applications. J Clin Aesthet Dermatol. 2017;10(7):14–17. [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DJ, Lee J, Ha J, Park KC, Ortonne JP, Kang HY. Defective barrier function in melasma skin. J Eur Acad Dermatol Venereol. 2012;26(12):1533–1537. doi: 10.1111/j.1468-3083.2011.04337.x [DOI] [PubMed] [Google Scholar]
- 23.Esposito ACC, Brianezi G, de Souza NP, Miot LDB, Miot HA. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32(2):101–108. doi: 10.5021/ad.2020.32.2.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Disphanurat W, Srisantithum B. Efficacy and safety of 0.15% isobutylamido thiazolyl resorcinol combined with hyaluronic acid vs 0.15% isobutylamido thiazolyl resorcinol or hyaluronic acid alone in melasma treatment: a randomized evaluator-blind trial. J Cosmet Dermatol. 2021;20(11):3563–3572. doi: 10.1111/jocd.14031 [DOI] [PubMed] [Google Scholar]
- 25.Maranzatto CF, Miot HA, Miot LD, Meneguin S. Psychometrican analysis and dimensional structure of the Brazilian version of melasma quality of life scale (MELASQoL-BP). An Bras Dermatol. 2016;91(4):422–428. doi: 10.1590/abd1806-4841.20165014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, Smith S. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of restylane versus zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29(6):588–595. doi: 10.1046/j.1524-4725.2003.29150.x [DOI] [PubMed] [Google Scholar]
- 27.Miot HA. Sample size in clinical and experimental studies. J Vasc Bras. 2011;10(4):275–278. doi: 10.1590/s1677-54492011000400001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagatin E, Miot HA. How to design and write a clinical research protocol in Cosmetic Dermatology. An Bras Dermatol. 2013;88(1):69–75. doi: 10.1590/s0365-05962013000100008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miola AC, Miot HA. Comparing categorical variables in clinical and experimental studies. J Vasc Bras. 2022;21:e20210225. doi: 10.1590/1677-5449.20210225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miot HA. Analysis of data with dependent measures in clinical and experimental studies. J Vasc Bras. 2023;22:e20220150. doi: 10.1590/1677-5449.202201502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miola AC, Miot HA. P-value and effect-size in clinical and experimental studies. J Vasc Bras. 2021;20:e20210038. doi: 10.1590/1677-5449.210038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Silva CN, Miot HA, Grassi TF, Dias-Melicio LA, Santos L, Esposito ACC. Expression of endothelin-1, endothelin receptor-a, and endothelin receptor-B in facial melasma compared to adjacent skin. Clin Cosmet Invest Dermatol. 2023;16:2847–2853. doi: 10.2147/CCID.S402168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dias JAF, Lima PB, Cassiano DP, et al. Oral ketotifen associated with famotidine for the treatment of facial melasma: a randomized, double-blind, placebo-controlled trial. J Eur Acad Dermatol Venereol. 2022;36(2):e123–e125. doi: 10.1111/jdv.17692 [DOI] [PubMed] [Google Scholar]
- 34.Tamega Ade A, Miot LD, Bonfietti C, Gige TC, Marques ME, Miot HA. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27(2):151–156. doi: 10.1111/j.1468-3083.2011.04430.x [DOI] [PubMed] [Google Scholar]
- 35.Cassiano D, Esposito ACC, Hassun K, et al. Efficacy and safety of microneedling and oral tranexamic acid in the treatment of facial melasma in women: an open, evaluator-blinded, randomized clinical trial. J Am Acad Dermatol. 2020;83(4):1176–1178. doi: 10.1016/j.jaad.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 36.Holanda IRM, de Almeida Correa Alfredo M, Cassiano DP, et al. Efficacy of oral 5 mg melatonin in the treatment of facial melasma in women: a double-blind, randomized, placebo-controlled clinical trial. J Eur Acad Dermatol Venereol. 2024;38(7). doi: 10.1111/jdv.19784 [DOI] [PubMed] [Google Scholar]
- 37.de Amorim RP, Barbosa MMC, Cassiano DP, et al. Sequential therapy with topical clobetasol for 14 days followed by hydroquinone versus hydroquinone alone in facial melasma treatment: a randomized, double-blind, controlled clinical trial. Int J Dermatol. 2024;63(9):1221–1226. doi: 10.1111/ijd.17094 [DOI] [PubMed] [Google Scholar]
- 38.Alfredo MAC, Holanda IRM, Cassiano DP, Esposito ACC, Lima PB, Miot HA. Lack of efficacy of oral N-acetylcysteine in the treatment of facial melasma in women: a randomized, double-blind, placebo-controlled clinical trial. An Bras Dermatol. 2024. doi: 10.1016/j.abd.2023.10.005 [DOI] [PubMed] [Google Scholar]
- 39.Hakozaki T, Minwalla L, Zhuang J, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol. 2002;147(1):20–31. doi: 10.1046/j.1365-2133.2002.04834.x [DOI] [PubMed] [Google Scholar]
- 40.Cheng Y, Zhang L, You Y. The effects of supramolecular nicotinamide combined with supramolecular salicylic acid on chloasma. J Cosmet Dermatol. 2024;23(2):681–686. doi: 10.1111/jocd.16010 [DOI] [PubMed] [Google Scholar]
- 41.Hakozaki T, Wang J, Laughlin T, Jarrold B, Zhao W, Furue M. Role of interleukin-6 and endothelin-1 receptors in enhanced melanocyte dendricity of facial spots and suppression of their ligands by niacinamide and tranexamic acid. J Eur Acad Dermatol Venereol. 2024;38(Suppl 2):3–10. doi: 10.1111/jdv.19719 [DOI] [PubMed] [Google Scholar]
- 42.Hwang TL, Tsai CJ, Chen JL, Changchien TT, Wang CC, Wu CM. Magnesium ascorbyl phosphate and coenzyme Q10 protect keratinocytes against UVA irradiation by suppressing glutathione depletion. Mol Med Rep. 2012;6(2):375–378. doi: 10.3892/mmr.2012.933 [DOI] [PubMed] [Google Scholar]
- 43.Geesin JC, Gordon JS, Berg RA. Regulation of collagen synthesis in human dermal fibroblasts by the sodium and magnesium salts of ascorbyl-2-phosphate. Skin Pharmacol. 1993;6(1):65–71. doi: 10.1159/000211089 [DOI] [PubMed] [Google Scholar]
- 44.Kameyama K, Sakai C, Kondoh S, et al. Inhibitory effect of magnesium L-ascorbyl-2-phosphate (VC-PMG) on melanogenesis in vitro and in vivo. J Am Acad Dermatol. 1996;34(1):29–33. doi: 10.1016/s0190-9622(96)90830-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data of this study is available under contact with the corresponding author.