Abstract
Antimicrobial resistance (AMR) is a growing threat to human and animal health. Data are limited on the prevalence of resistant bacteria in pet rabbits. Therefore, we aimed to identify prevalent bacterial infections and AMR profiles among pet rabbits in Hong Kong (HK). Our search of the CityU Veterinary Diagnostic Laboratory (VDL) database found 301 cases of pet rabbits submitted for bacteriologic and antimicrobial susceptibility testing by veterinarians at 20 exotic veterinary clinics across HK between 2019 and 2022. The rabbits were of 8 different breeds and had a median age of 6.5 y, with 54.8% males, 40.2% females, and 5% unspecified. Of the 301 samples received, 168 (55.8%) had positive bacterial growth; 125 (74.4%) had single bacterial isolates, and 43 (25.6%) had mixed cultures. Cultures included Enterococcus faecalis (21.3%) as the most frequently isolated gram-positive bacterium, followed by Streptococcus intermedius (12.5%), and Staphylococcus aureus (11.3%). The most frequently isolated gram-negative bacteria were Pseudomonas aeruginosa (18.1%), followed by Escherichia coli (8.3%), Pasteurella multocida (6.9%), and Klebsiella pneumoniae (4.2%). Approximately 83% of the isolates had acquired resistance to at least one antimicrobial agent, and 49.4% were multidrug-resistant. The isolated bacteria had high levels of resistance to penicillin (69.8%), clindamycin (47.4%), and doxycycline (46.9%). Our findings highlight the high levels of AMR in bacteria isolated from pet rabbit clinical samples in HK; many of these bacteria are zoonotic and pose a public health threat.
Keywords: antimicrobial resistance, bacterial pathogens, clinical samples, rabbits, Hong Kong
Antimicrobial resistance (AMR) is a recognized global public health threat that occurs when microorganisms become unresponsive to antimicrobials. 26 Resistance to antimicrobials may be either intrinsic (natural resistance) or acquired (due to mutations in pre-existing or previously acquired genes or horizontal gene transfer). 2 The excessive use of antimicrobials significantly contributes to the emergence, development, and spread of AMR bacteria. The growing prevalence of AMR among various bacterial strains, including Pasteurella multocida, 13 Staphylococcus aureus, 3 Escherichia coli, 31 Enterococcus spp., 20 and others, is a serious health concern, 27 with severe consequences for bacterial infections in animals and humans. Consequently, AMR has become a growing problem that poses a threat to society, animal health, and the global environment.
Extensive research and surveillance on AMR in animals are currently conducted worldwide, with a particular focus on food-producing animals, such as chickens, cattle, and pigs. 20 These species receive significant attention from academia given their role in the epidemiology of AMR 1 ; resistant organisms can be transmitted directly from animals to foods of animal origin, farm workers, the feeding environment, 10 as well as the surrounding land and crops. 1 The widespread use of antimicrobials in veterinary medicine has contributed to an increase in AMR bacteria, which has implications for both food animals and pets. 23 These AMR bacteria, including Salmonella, Campylobacter, S. intermedius, and E. coli, 15 pose particular concerns given their potential for transmission from pets to humans. Moreover, infections caused by AMR bacteria tend to result in more severe illnesses, prolonged hospitalization, and increased healthcare costs. 34
In Hong Kong (HK), AMR bacteria are either highly prevalent or rapidly increasing, with limited, less effective, and more expensive treatment options. 5 Despite the significant concern expressed by the HK government and academic community about AMR bacteria from food and companion animals, little attention has been given to AMR in pet rabbits. Pet rabbits serve as potential sources of zoonotic AMR bacteria, highlighting the importance of practicing proper hygiene and healthcare measures when interacting with these pets to prevent disease transmission to humans. 36 In HK, rabbits constitute 2.3% of the pet population, with dogs and cats accounting for 37.7% and 18.9%, respectively. 14
To our knowledge, there is limited information regarding the prevalence of AMR bacteria in the pet rabbit population, 25 especially in HK. Therefore, we aimed to fill this knowledge gap by identifying the most prevalent bacterial infections and AMR profiles among clinical samples from pet rabbits in HK. Ultimately, a better understanding of the distribution and AMR profiles of bacterial strains in pet rabbits may lead to the development of a more effective antimicrobial stewardship program.
Materials and methods
Data collection and management
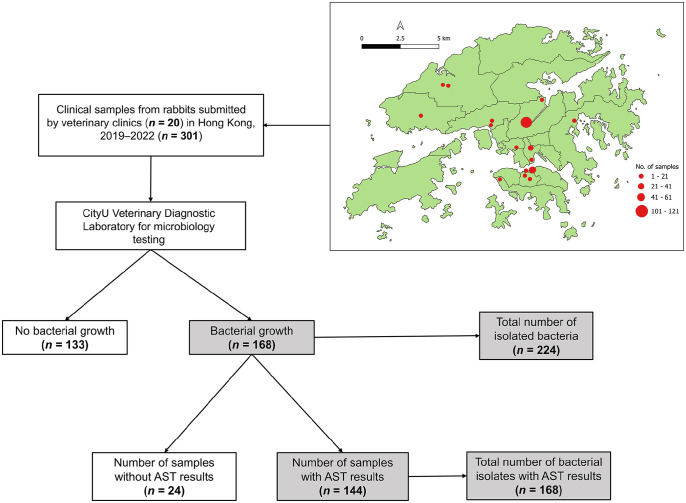
We searched the electronic medical record database of CityU Veterinary Diagnostic Laboratory (VDL; City University of Hong Kong, Hong Kong SAR, China) for rabbit clinical samples submitted by veterinarians at exotic veterinary clinics across HK (Fig. 1) from March 2019 to December 2022.
Figure 1.
A schematic diagram illustrates the distribution map of veterinary clinics, along with a flowchart describing data extraction.
We included rabbit clinical samples submitted specifically for microbiologic and/or antimicrobial susceptibility testing (AST). We downloaded 304 electronic diagnostic testing records. We excluded 3 rabbit clinical samples submitted for fungal culture. We extracted relevant data from each record, including the veterinary clinic’s name, rabbit breed, sex, date of birth, sample type, sampling site, sampling date, bacteriologic examination results, and AMR profiles.
Bacteriologic examination
Rabbit clinical samples, such as fluids (mostly urine and pus), swabs (mostly wound and abscess swabs), tissue, and fecal samples, were processed for microbiologic culture and identification following standard operating procedures. All samples were cultured on appropriate media for aerobic and anaerobic testing. Bacterial colonies were collected, and bacteria were identified using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (Bruker) and analyzed with the MALDI Biotyper (Bruker) software.
Antimicrobial susceptibility testing
A bacterial suspension was prepared from pure cultures of growing bacteria and subjected to either the disc diffusion or broth microdilution techniques for AST. The antimicrobial panel utilized varied based on the bacterial species; the panel consisted of various of the following 30 antimicrobials: penicillin (amoxicillin–clavulanic acid, ampicillin, penicillin, ticarcillin–clavulanate), aminoglycoside (amikacin, gentamicin, tobramycin, framycetin, neomycin), macrolides (azithromycin), cephalosporins (cefazolin, cefixime, cefoxitin, cefpodoxime, ceftazidime, ceftriaxone, cefuroxime axetil, cephalexin), fluoroquinolones (ciprofloxacin, enrofloxacin, ofloxacin, orbifloxacin), tetracyclines (doxycycline), florfenicol, clindamycin, marbofloxacin, cefovecin, chloramphenicol, trimethoprim–sulfamethoxazole, and fusidic acid.
Results were interpreted as sensitive or resistant based on the Clinical and Laboratory Standard Institute (CLSI) guidelines. 6 Furthermore, intrinsic resistance to specific antimicrobials was excluded from data analysis, following the latest EUCAST expert rules on intrinsic resistance and exceptional phenotypes. 11 Only acquired resistance was analyzed and presented. A variety of control strains, such as Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, and Streptococcus pneumoniae ATCC 49619, were used for routine internal quality control testing.
The multiple antibiotic resistance (MAR) index was calculated by dividing the number of antimicrobials to which bacterial isolates showed resistance by the total number of antimicrobials tested. 18 Multidrug resistance (MDR) was defined as the resistance of an isolate to ≥1 agent in ≥3 antimicrobial classes. 24
Data analysis
The extracted data were entered into Microsoft Excel and subsequently imported into R software (v.4.2.0, https://www.r-project.org/) for data analysis and visualization. Categorical variables were presented as numbers and percentages; continuous variables were reported as median and interquartile range (IQR). To compare the MAR index of isolates from different years, a one-way ANOVA was performed. A p ≤ 0.05 was considered statistically significant. QGIS software (v.3.30.3) was used to create a map of the approximate locations of veterinary clinics. 29
Results
Study population
Between March 2019 and November 2022, CityU VDL received 301 clinical samples from rabbits. Veterinarians from 20 clinics in HK (Fig. 1) collected and submitted these samples for bacteriologic testing and AST.
The rabbits were 3–9.1-y-old, with a median age of 6.5 y. Among the sampled rabbits, 54.8% were males, 40.2% females, and sex information was not available for the remaining 5% (Table 1). The breed of rabbits was not recorded for >50% of the rabbits. However, for the remaining 50%, samples originated from 8 different breeds, with the highest representation from the Lop breed (15.3%), followed by Lionhead (11.6%), and Dwarf (10.3%). Samples consisted primarily of swabs (76.7%), predominantly wound and abscess swabs, followed by fluid samples (19.3%), mainly urine samples (Table 1).
Table 1.
Characteristics of Hong Kong rabbit samples, stratified by sampling year.
| Characteristic | 2019, n (%) | 2020, n (%) | 2021, n (%) | 2022, n (%) | Total, n (%) |
|---|---|---|---|---|---|
| Sample | 26 (8.7) | 94 (31.2) | 84 (27.9) | 97 (32.2) | 301 (100.0) |
| Sample type | |||||
| Fluid (mainly urine) | 11 (19.0) | 16 (27.6) | 11 (19.0) | 20 (34.4) | 58 (19.3) |
| Swab | 15 (6.5) | 71 (30.7) | 70 (30.3) | 75 (32.5) | 231 (76.7) |
| Tissue | 0 | 5 (50.0) | 3 (30.0) | 2 (20.0) | 10 (3.3) |
| Feces | 0 | 2 (100) | 0 | 0 | 2 (0.7) |
| Sex | |||||
| Female | 11 (9.1) | 45 (37.2) | 27 (22.3) | 38 (31.4) | 121 (40.2) |
| Male | 15 (9.1) | 48 (29.1) | 46 (27.9) | 56 (33.9) | 165 (54.8) |
| Unknown | 0 | 1 (6.7) | 11 (73.3) | 3 (20.0) | 15 (5.0) |
| Age, y | |||||
| Median (IQR) | 8.7 (6.1–10.8) | 6.4 (3.1–8.7) | 5.8 (1.8–8.9) | 6.8 (2.9–9.3) | 6.5 (3.0–9.1) |
| Breed | |||||
| Angora | 4 (57.1) | 0 | 1 (14.3) | 2 (28.6) | 7 (2.3) |
| Dutch | 0 | 1 (12.5) | 2 (25.0) | 5 (62.5) | 8 (2.7) |
| Dwarf | 1 (3.2) | 2 (6.5) | 5 (16.1) | 23 (74.2) | 31 (10.3) |
| English spot | 0 | 2 (100) | 0 | 0 | 2 (0.7) |
| Lionhead | 4 (11.4) | 11 (31.4) | 7 (20.0) | 13 (37.1) | 35 (11.6) |
| Lop (Mini, French, Holland) | 4 (8.7) | 8 (17.4) | 16 (34.8) | 18 (39.1) | 46 (15.3) |
| New Zealand white | 0 | 0 | 5 (62.5) | 3 (37.5) | 8 (2.7) |
| Rex | 0 | 1 (50.0) | 1 (50.0) | 0 | 2 (0.7) |
| Unknown | 13 (8.0) | 69 (42.6) | 47 (29.0) | 33 (20.4) | 162 (53.2) |
| Bacterial infection | |||||
| Yes | 10 (6.0) | 49 (29.2) | 52 (30.9) | 57 (33.9) | 168 (55.8) |
| No | 16 (12.0) | 45 (33.8) | 32 (24.1) | 40 (30.1) | 133 (44.2) |
IQR = interquartile range.
Microbiologic results
Of the 301 samples, bacterial growth was reported in 168 (55.8%) samples; 125 (74.4%) had single bacterial growth, and 43 (25.6%) had mixed bacterial growth. A total of 35 bacterial species were isolated from the samples (Fig. 2), with gram-negative bacteria the most identified species (Table 2). Among these 35 identified bacterial species, Staphylococcus spp. was the most frequently isolated; 54% of the Staphylococcus isolates were identified as S. aureus. Within the gram-negative bacteria, the most represented were P. aeruginosa (18.1%), E. coli (8.3%), P. multocida (6.9%), and K. pneumoniae (4.2%). E. faecalis (21.3%), S. intermedius (12.5%), and S. aureus (11.3%) were the most commonly identified gram-positive bacteria (Table 2).
Figure 2.
Distribution of the bacterial isolates recovered from 168 rabbit clinical samples in Hong Kong.
Table 2.
Distribution of bacterial groups recovered from 168 rabbit clinical samples in Hong Kong, stratified by sampling year.
| Bacterial spp. | 2019, n (%) | 2020, n (%) | 2021, n (%) | 2022, n (%) | Total, n (%) |
|---|---|---|---|---|---|
| Gram-negative | 5 (3.5) | 40 (27.8) | 54 (37.5) | 45 (31.3) | 144 (64.3) |
| Gram-positive | 5 (6.3) | 21 (26.3) | 24 (30.0) | 30 (37.5) | 80 (35.7) |
| Total | 10 (4.5) | 61 (27.2) | 78 (34.8) | 75 (33.5) | 224 (100.0) |
Antimicrobial susceptibility test
The AST revealed that 82.7% of the isolates recovered from clinical rabbit samples were resistant to ≥1 antimicrobial (Fig. 3). Most of the isolates had high resistance to penicillin (69.8%), clindamycin (47.4%), and doxycycline (46.9%). However, all isolates were susceptible to fusidic acid, azithromycin, and amikacin.
Figure 3.

Frequency of antimicrobial resistance among bacterial isolates recovered from 168 rabbit clinical samples in Hong Kong.
Most (66.7%) of S. aureus isolates were resistant to 1 antimicrobial, and the remaining 33.3% were resistant to 2 antimicrobials (Table 3). Conversely, 50% of E. coli isolates were resistant to >3 antimicrobials.
Table 3.
Distribution of antimicrobial resistance in common bacterial isolates recovered from 168 rabbit clinical samples in Hong Kong.
| Bacterial isolate | Resistant to no. of antimicrobials | MDR, n (%) | Average MAR index (range) | ||||
|---|---|---|---|---|---|---|---|
| Total, n | 1, n (%) | 2, n (%) | 3, n (%) | >3, n (%) | |||
| Actinobacillus suis | 5 | 3 (60.0) | 1 (20.0) | 0 | 1 (20.0) | 1 (20.0) | 0.17 (0.07–0.40) |
| Streptococcus intermedius | 5 | 3 (60.0) | 1 (20.0) | 1 (20.0) | 0 | 1 (20.0) | 0.15 (0.09–0.30) |
| Klebsiella pneumoniae | 6 | 2 (33.3) | 2 (33.3) | 0 | 2 (33.3) | 2 (33.3) | 0.41 (0.09–1.00) |
| Staphylococcus aureus | 9 | 6 (66.7) | 3 (33.3) | 0 | 0 | 0 | 0.11 (0.06–0.18) |
| Pasteurella multocida | 9 | 1 (11.1) | 3 (33.3) | 2 (22.2) | 3 (33.3) | 5 (55.6) | 0.25 (0.09–0.50) |
| Escherichia coli | 12 | 2 (16.7) | 2 (16.7) | 2 (16.7) | 6 (50.0) | 7 (58.3) | 0.37 (0.05–0.82) |
| Enterococcus faecalis | 17 | 3 (17.7) | 3 (17.7) | 7 (41.2) | 4 (23.5) | 10 (58.8) | 0.27 (0.10–0.50) |
| Pseudomonas aeruginosa | 26 | 0 | 0 | 0 | 26 (100.0) | 26 (100.0) | 0.43 (0.31–0.60) |
MAR = multiple antibiotic resistance; MDR = multidrug resistance.
MDR was observed in 49.4% of bacterial isolates from rabbit clinical samples; E. coli, E. faecalis, P. aeruginosa, and P. multocida had the highest levels of MDR among all isolates. The average MAR index was 0.33, ranging from 0.05 to 1.00 (Fig. 4). The difference in the average MAR index between gram-positive and -negative bacterial isolates was significant (p = 0.001; Fig. 4A). However, we found no significant difference (p = 0.172) in the average MAR index among isolates from each year. In addition, differences were significant in the MAR index between bacterial species, such as Staphylococcus spp. versus Pseudomonas spp. (p = 0.002) and Staphylococcus spp. versus Haemophilus spp. (p = 0.001; Fig. 4B).
Figure 4.
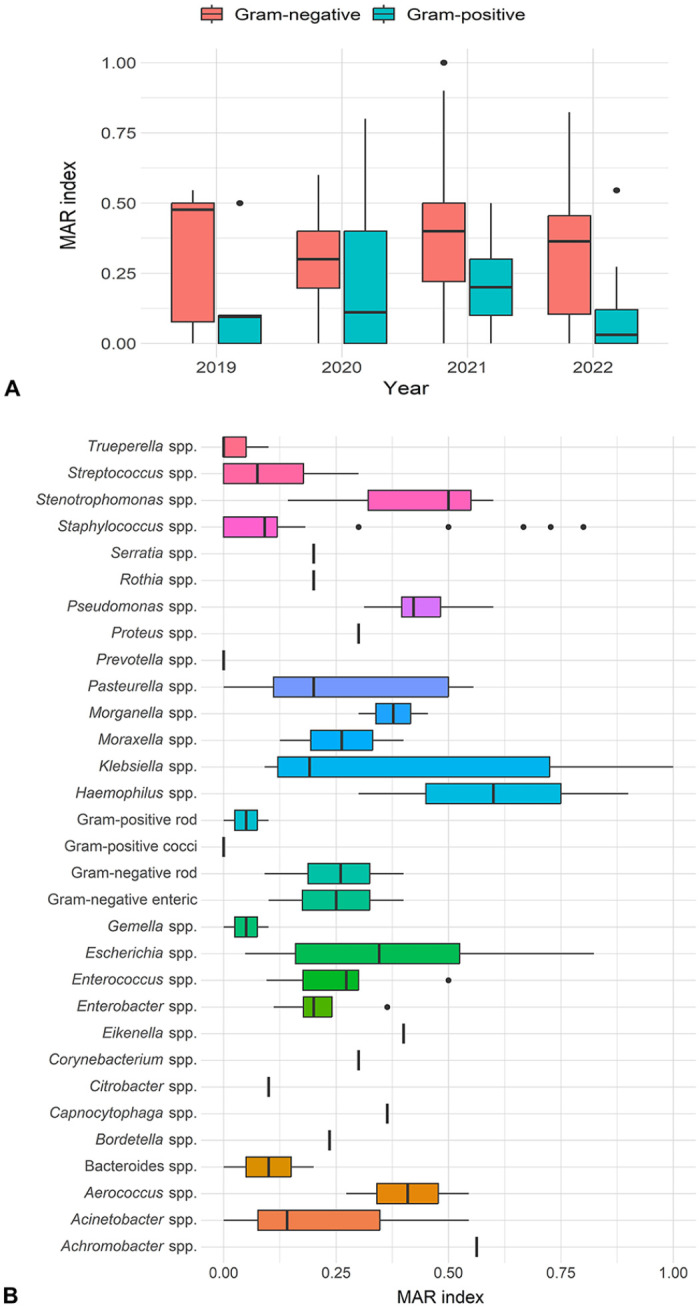
Multiple antimicrobial resistance (MAR) index for bacterial isolates recovered from 168 rabbit clinical samples in Hong Kong. A. MAR index of bacterial groups stratified by sampling year. B. MAR index of each bacterial species.
Discussion
We found that 55.8% of 301 samples from pet rabbits had bacterial growth, with Staphylococcus spp. (13.39%) and Pseudomonas spp. (12.95%) being the most frequently isolated species. The frequency of gram-negative bacterial species (64.3%) exceeded that of gram-positive species (35.7%). The predominant gram-positive species in our study were Staphylococcus spp. and Enterococcus spp., consistent with previous reports in both farmed 21 and pet 28 rabbits. A study on pet rabbits in Tunisia indicated that 69% and 73% of fecal samples contained E. coli and enterococci, respectively. 4 However, isolation rates of E. coli (57%) and enterococci (83%) differed in pet rabbits from Portugal. 31 These variations may be attributed to differences in the epidemiologic and ecologic characteristics of the study populations as well as the sample size.
E. faecalis was the most frequently identified gram-positive bacterium (21.3%) in rabbit clinical samples in HK. A Spanish study found that E. faecalis represented ~1.3% of bacterial isolates, whereas other Enterococcus spp. comprised just 1.23%. 12 This disparity in isolation rates may be explained by the substantial number of clinical samples sourced from urine in our study, especially considering that E. faecalis, a gram-positive bacterium, is commonly associated with urinary infections. 17 The small proportion of E. coli, P. multocida, and K. pneumoniae isolates found in clinical samples in our study contrasts sharply with the higher percentage of gram-positive bacteria, such as E. faecalis, S. intermedius, and S. aureus. This difference may be attributed to the increased susceptibility of gram-negative bacteria to environmental conditions compared to their gram-positive counterparts. 31
Our AST results revealed that 82.7% of bacterial isolates were resistant to ≥1 antimicrobial, with the highest resistance observed against penicillin (69.8%) and clindamycin (57.9%). Various levels of AMR have been reported for E. coli,4,22 enterococci, 31 P. multocida, 13 and S. aureus 35 in pet rabbits. For example, a UK study isolated 1 E. coli from 13 pet rabbit samples with resistance to tetracycline. 22 In contrast, a study in Portugal reported lower resistance levels among E. coli (11.4%) and enterococci (46.9%) isolates, 31 differing from our findings wherein a significant portion of E. coli isolates were resistant to multiple antimicrobials. The resistance level observed in our study, although lower, is comparable to that reported in wild (71.4% of E. coli isolates were resistant to 1 or 2 antimicrobials 8 ) and food-producing animals.30,33 Additionally, E. coli had one of the highest levels of MDR at 58.3%, aligning with data from domestic pets in Portugal (50%) 19 and sick companion animals in Singapore (57%). 16 The adaptability and effectiveness of the E. coli efflux system enables it to acquire and disseminate AMR determinants, contributing to resistance against multiple antimicrobial agents. 32
Conversely, 66.7% of S. aureus isolates in our study had resistance to ≥1 antimicrobial, with none being methicillin-resistant S. aureus (MRSA) or having MDR. Although this level of resistance was higher than reported in European farmed rabbits,3,35 the frequency of MDR isolates (92.3%) in those studies was greater, which can be explained by the more extensive use of antimicrobials in farmed rabbits. 3
P. multocida is a significant pathogen often responsible for infections in rabbits, leading to conditions such as pneumonia, otitis media, orchitis, and pyometra. 9 We found that P. multocida was one of the most frequently isolated bacteria from clinical samples of rabbits, with 11% of these isolates displaying resistance to a single antimicrobial; 55.6% exhibited MDR. Our result is similar to a report from Brazil in which 47.8% of P. multocida isolates from rabbits had resistance to ≥1 antimicrobial. 13
Our findings revealed high levels of MDR bacterial infections in clinical samples from rabbits in HK. A substantial proportion of these isolates had resistance to antimicrobial agents listed as highly important (such as clindamycin) for human medicine by the WHO. 7 Furthermore, most of the bacteria identified in our study can be transmitted from animals to their human caregivers. It is imperative to implement strategies aimed at mitigating these public health risks. This includes increasing awareness among pet owners about the prudent use of antimicrobials in treating rabbits, and promoting the utilization of antibiotic susceptibility profiles in veterinary clinics in HK.
Acknowledgments
We thank the veterinarians and veterinary clinics in Hong Kong for providing samples for analysis, and CityU VDL microbiology technologists for processing them.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by the new research initiatives at the City University of Hong Kong to Ibrahim Elsohaby (9610583).
ORCID iDs: Fraser Hill  https://orcid.org/0000-0002-0338-1895
https://orcid.org/0000-0002-0338-1895
Ibrahim Elsohaby  https://orcid.org/0000-0003-2533-988X
https://orcid.org/0000-0003-2533-988X
Contributor Information
Chen Xin, Department of Infectious Diseases and Public Health, Jockey Club College of Veterinary Medicine and Life Sciences, City University of Hong Kong, Hong Kong SAR, China.
Fraser Hill, CityU Veterinary Diagnostic Laboratory, City University of Hong Kong, Hong Kong SAR, China.
Ibrahim Elsohaby, Department of Infectious Diseases and Public Health, Jockey Club College of Veterinary Medicine and Life Sciences, City University of Hong Kong, Hong Kong SAR, China; Centre for Applied One Health Research and Policy Advice (OHRP), City University of Hong Kong, Hong Kong SAR, China.
References
- 1. Agnoletti F, et al. Longitudinal study on antimicrobial consumption and resistance in rabbit farming. Int J Antimicrob Agents 2018;51:197–205. [DOI] [PubMed] [Google Scholar]
- 2. Ali J, et al. Antimicrobial resistance mechanisms and potential synthetic treatments. Future Sci OA 2018;4:FSO290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Attili A-R, et al. Analysis of the antibiotic resistance profiles in methicillin-sensitive S. aureus pathotypes isolated on a commercial rabbit farm in Italy. Antibiotics (Basel) 2020;9:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ben Said L, et al. Antimicrobial resistance genes and virulence gene encoding intimin in Escherichia coli and Enterococcus isolated from wild rabbits (Oryctolagus cuniculus) in Tunisia. Acta Vet Hung 2019;67:477–488. [DOI] [PubMed] [Google Scholar]
- 5. Cheng VCC, et al. Strategic measures for the control of surging antimicrobial resistance in Hong Kong and mainland of China. Emerg Microbes Infect 2015;4:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 30th ed. CLSI, 2020. CLSI supplement M100. [Google Scholar]
- 7. Collignon PJ, et al. World Health Organization ranking of antimicrobials according to their importance in human medicine: a critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clin Infect Dis 2016;63:1087–1093. [DOI] [PubMed] [Google Scholar]
- 8. Costa D, et al. Mechanisms of antibiotic resistance in Escherichia coli isolates recovered from wild animals. Microb Drug Resist 2008;14:71–77. [DOI] [PubMed] [Google Scholar]
- 9. Dziva F, et al. Diagnostic and typing options for investigating diseases associated with Pasteurella multocida. Vet Microbiol 2008;128:1–22. [DOI] [PubMed] [Google Scholar]
- 10. Economou V, Gousia P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect Drug Resist 2015;8:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. European Committee on Antimicrobial Susceptibility Testing (EUCAST). Expected resistant phenotypes, v.1.2. EUCAST, 2023. Jan. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Expert_Rules/2023/Expected_Resistant_Phenotypes_v1.2_20230113.pdf
- 12. Fernández M, et al. Current situation of bacterial infections and antimicrobial resistance profiles in pet rabbits in Spain. Vet Sci 2023;10:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferreira TSP, et al. Virulence genes and antimicrobial resistance profiles of Pasteurella multocida strains isolated from rabbits in Brazil. ScientificWorldJournal 2012;2012:685028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Government of Hong Kong Special Administrative Region. Keeping of pets by households in Hong Kong SAR, 2006. https://www.censtatd.gov.hk/en/data/stat_report/product/C0000035/att/B11302262006XXXXB0100.pdf
- 15. Guardabassi L, et al. Pet animals as reservoirs of antimicrobial-resistant bacteria. J Antimicrob Chemother 2004;54:321–332. [DOI] [PubMed] [Google Scholar]
- 16. Hartantyo SHP, et al. Sick pets as potential reservoirs of antibiotic-resistant bacteria in Singapore. Antimicrob Resist Infect Control 2018;7:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kayaoglu G, Ørstavik D. Virulence factors of Enterococcus faecalis: relationship to endodontic disease. Crit Rev Oral Biol Med 2004;15:308–320. [DOI] [PubMed] [Google Scholar]
- 18. Krumperman PH. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl Environ Microbiol 1983;46:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leite-Martins LR, et al. Prevalence of antimicrobial resistance in enteric Escherichia coli from domestic pets and assessment of associated risk markers using a generalized linear mixed model. Prev Vet Med 2014;117:28–39. [DOI] [PubMed] [Google Scholar]
- 20. Lengliz S, et al. Species distribution and genes encoding antimicrobial resistance in Enterococcus spp. isolates from rabbits residing in diverse ecosystems: a new reservoir of linezolid and vancomycin resistance. J Appl Microbiol 2022;132:2760–2772. [DOI] [PubMed] [Google Scholar]
- 21. Linaje R, et al. Characterization of faecal enterococci from rabbits for the selection of probiotic strains. J Appl Microbiol 2004;96:761–771. [DOI] [PubMed] [Google Scholar]
- 22. Livermore DM, et al. Antibiotic resistance in bacteria from magpies (Pica pica) and rabbits (Oryctolagus cuniculus) from west Wales. Environ Microbiol 2001;3:658–661. [DOI] [PubMed] [Google Scholar]
- 23. Lloyd DH. Reservoirs of antimicrobial resistance in pet animals. Clin Infect Dis 2007;45(Suppl 2):S148–S152. [DOI] [PubMed] [Google Scholar]
- 24. Magiorakos A-P, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–281. [DOI] [PubMed] [Google Scholar]
- 25. Matsunaga N, et al. Analysis of fecal samples from Amami rabbits (Pentalagus furnessi) indicates low levels of antimicrobial resistance in Escherichia coli. Eur J Wildl Res 2020;66:84. [Google Scholar]
- 26. Palma E, et al. Antimicrobial resistance in veterinary medicine: an overview. Int J Mol Sci 2020;21:1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pinto L, et al. Genomic and proteomic evaluation of antibiotic resistance in Salmonella strains. J Proteomics 2010;73:1535–1541. [DOI] [PubMed] [Google Scholar]
- 28. Poeta P, et al. Characterization of antibiotic resistance genes and virulence factors in faecal enterococci of wild animals in Portugal. J Vet Med B Infect Dis Vet Public Health 2005;52:396–402. [DOI] [PubMed] [Google Scholar]
- 29. QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. https://qgis.org/en/site/ [Google Scholar]
- 30. Sáenz Y, et al. Antibiotic resistance in Escherichia coli isolates obtained from animals, foods and humans in Spain. Int J Antimicrob Agents 2001;18:353–358. [DOI] [PubMed] [Google Scholar]
- 31. Silva N, et al. Molecular characterization of antimicrobial resistance in enterococci and Escherichia coli isolates from European wild rabbit (Oryctolagus cuniculus). Sci Total Environ 2010;408:4871–4876. [DOI] [PubMed] [Google Scholar]
- 32. Szmolka A, Nagy B. Multidrug resistant commensal Escherichia coli in animals and its impact for public health. Front Microbiol 2013;4:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teshager T, et al. Surveillance of antimicrobial resistance in Escherichia coli strains isolated from pigs at Spanish slaughterhouses. Int J Antimicrob Agents 2000;15:137–142. [DOI] [PubMed] [Google Scholar]
- 34. Umber JK, Bender JB. Pets and antimicrobial resistance. Vet Clin North Am Small Anim Pract 2009;39:279–292. [DOI] [PubMed] [Google Scholar]
- 35. Vancraeynest D, et al. Antimicrobial resistance and resistance genes in Staphylococcus aureus strains from rabbits. Vet Microbiol 2004;101:245–251. [DOI] [PubMed] [Google Scholar]
- 36. Varela K, et al. A review of zoonotic disease threats to pet owners: a compendium of measures to prevent zoonotic diseases associated with non-traditional pets: rodents and other small mammals, reptiles, amphibians, backyard poultry, and other selected animals. Vector Borne Zoonotic Dis 2022;22:303–360. [DOI] [PMC free article] [PubMed] [Google Scholar]