Abstract
Exposure to corticosterone attenuates hippocampal CA1 long-term potentiation (LTP) via intracellular Zn2+ dysregulation. Here we report that effusol, a phenanthrene isolated from Chinese medicine Juncus effusus, rescues CA1 LTP attenuated by corticosterone. In vivo microdialysis experiment indicated that both increases in extracellular glutamate induced under perfusion with corticosterone and high K+ are suppressed in the hippocampus by co-perfusion with effusol. Because corticosterone and high K+ also increase extracellular Zn2+ level, followed by intracellular Zn2+ dysregulation, the effect of effusol on both the increases was examined in brain slice experiments. Effusol did not suppress increase in extracellular Zn2+ in the hippocampal CA1 of brain slices bathed in corticosterone, but suppressed increase in intracellular Zn2+, which may be linked with suppressing the increase in extracellular glutamate in vivo. In vivo CA1 LTP was attenuated under perfusion with corticosterone prior to LTP induction, while the attenuation was rescued by co-perfusion with effusol, suggesting that the rescuing effect of effusol is due to suppressing the increase in intracellular Zn2+ in CA1 pyramidal cells. The present study indicates that CA1 LTP attenuated by corticosterone is canceled by effusol, which rescues intracellular Zn2+ dysregulation via suppressing extracellular glutamate accumulation. It is likely that effusol defends the hippocampal function against stress-induced cognitive decline.
Keywords: Effusol, Juncus effusus, Zn2+, Corticosterone, Hippocampus, Stress
Introduction
Exposure to stress activates the hypothalamo-pituitary-adrenocortical axis, followed by increasing glucocorticoid (GC) secretion from the adrenal cortex. When blood glucocorticoid concentration is increased, glucocorticoid transport to the brain extracellular fluid is increased through the blood–brain barrier. This increase modifies cognitive activity bidirectionally (McEwen and Sapolsky 1995; Kim and Yoon 1998; Kim and Diamond 2002). When glucocorticoids (corticosterone in rodents) are abnormally secreted after exposure to stressful circumstances, the secretion causes cognitive decline via affected synaptic plasticity (McEwen et al. 2015; Joëls and de Kloet 2017).
The hippocampus plays a key role for cognitive activity and is vulnerable to exposure to stress. The hippocampus expresses mineralocorticoid (MC) and GC receptors. In the hippocampus, corticosterone accelerates glutamate release from the neuron terminals, which is induced by the rapid (non-genomic) action of membrane MC receptors (Karst et al. 2005; Joëls et al. 2008). Zincergic neuron, which is a subclass of glutamatergic neurons, concentrates glutamate and zinc in the presynaptic vesicles and activity-dependently releases glutamate and Zn2+. In the hippocampal CA1, therefore, corticosterone accelerates release of glutamate and Zn2+ from zincergic Schafffer collaterals after exposure to stress, resulting in accumulation of glutamate and Zn2+ in the extracellular compartment (Takeda et al. 2018a). Extracellular Zn2+ accumulation plays a crucial role for cognitive decline in cooperation with extracellular glutamate signaling (Sandi 2011; Suzuki et al. 2018); extracellular glutamate-mediated α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor activation induces intracellular Zn2+ dysregulation via influx of extracellular Zn2+ (Takeda et al. 2011), resulting in cognitive decline (Lynch 2004).
The influx of extracellular Zn2+ preferentially occurs through Ca2+- and Zn2+-permeable GluR2-lacking AMPA receptors after accumulation of extracellular glutamate in the CA1 (Frederickson et al. 2005; Kwak and Weiss 2006; Weiss 2011). Extracellular glutamate-induced cognitive decline, which is induced after exposure to high K+ in addition to corticosterone, is due to intracellular Zn2+ dysregulation. Although intracellular Ca2+ dysregulation is also induced, its involvement in extracellular glutamate-induced cognitive decline is much less (Takeda et al. 2018a, b).
In the hippocampus, on the other hand, loss of Zn2+ in zincergic neuron terminals, which is induced by adrenalectomy, reduces seizure-induced neuronal death. The data suggest that elevated corticosterone level accelerates neuronal death in the hippocampus caused by epilepsy (Suh et al. 2001), probably via synaptic Zn2+ dysregulation, which is associated with extracellular glutamate-mediated influx of extracellular Zn2+.
Juncus effusus, which is a traditional Chinese medicinal perennial herb, has been used in China to treat insomnia and akathisia (Liao et al. 2011; Wang et al. 2012). The traditional Chinese medicine Juncus effusus contains considerable amount of phenanthrenes and is also used for treatments, e.g., anxiety and insomnia (Shima et al. 1991; Greca et al. 1993, 1994). Effusol and dehydroeffusol are phenanthrenes isolated from Juncus effusus and concentration-dependently enhance γ-aminobutyric acidA (GABAA) receptor-mediated current, suggesting that this activity on the GABAA receptor is involved in the sedative and anxiolytic effects in the traditional use of Juncus effusus (Singhuber et al. 2012). However, there is no report on the effect of effusol and dehydroeffusol on stress. Here we report that effusol (Fig. 1) isolated from Juncus effusus rescues CA1 long-term potentiation (LTP) attenuated with corticosterone.
Fig. 1.
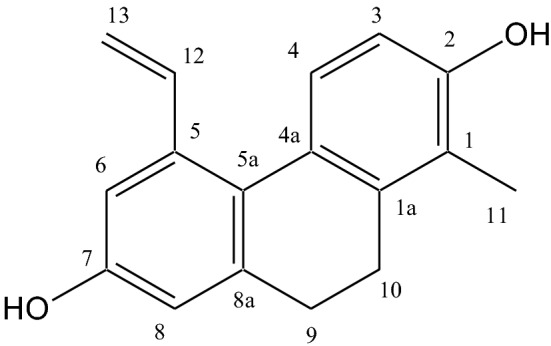
Structure of effusol
Materials and Methods
Animals
Wistar rats (male, 6–9 weeks) were obtained from Japan SLC (Hamamatsu, Japan). They had access to water and feed ad libitum under the standard laboratory conditions (23 ± 1 °C, 55 ± 5% humidity). The present experiments were performed according to the Guidelines for the Care and Use of Laboratory Animals of the University of Shizuoka, which refer to American Association for Laboratory Animals Science and the guidelines laid down by the NIH (NIH Guide for the Care and Use of Laboratory Animals) in the USA.
Chemicals
ZnAF-2DA and ZnAF-2 (Kd = 2.7 × 10−9 M for Zn2+), which were provided from Sekisui Medical Co., LTD (Hachimantai, Japan), are membrane-permeable (intracellular) and membrane-impermeable (extracellular) Zn2+ indicators, respectively. When ZnAF-2DA is taken up into neurons, it is hydrolyzed by esterase in the cytosol to produce ZnAF-2, which does not pass through the plasma membrane. ZnAF-2 is specifically bound to Zn2+ and is not bound to other divalent cations, e.g., Ca2+, Mg2+, and Cu2+ (Hirano et al. 2002). Calcium orange AM, an intracellular Ca2+ indicator, was obtained from Molecular Probes, Inc. (Eugene, OR). The indicators, which were dissolved in dimethyl sulfoxide, were diluted with Ringer solution containing 119 mM NaCl, 2.5 mM KCl, 1.3 mM MgSO4, 1.0 mM NaH2PO4, 2.5 mM CaCl2, 26.2 mM NaHCO3, and 11 mM d-glucose (pH 7.3).
Extraction and Isolation of Effusol
Rush (Igusa, Juncus effusus) was kindly supplied from HAGIHARA & CO., LTD. (Kurashiki, Japan). The aerial part of dried Rush (Igusa) (cut into 2–3 cm, 400 g) were extracted three times in MeOH (8 l) under reflux conditions for 2 h and filtered. The filtrate was concentrated under reduced pressure and the residue was suspended in H2O (1 l). This suspension was successively extracted with n-hexane (1 l × 3), EtOAc (1 l × 3), successively. The EtOAc extract (6.56 g) was passed through a silica gel (Mitsubishi Chemical Co., Japan) column (i.d. 32 × 300 mm), and the adsorbed materials were eluted successively with 5–40% MeOH in CHCl3 to obtain 17 fractions (fraction A-Q). The fraction C (361.8 mg) and D (362.6 mg) were purified by preparative HPLC [COSMOSIL Cholester, i.d. 20 × 250 mm (NACALAI TESQUE, INC., Japan); mobile phase, H2O–CH3CN (60:40)] to give effusol (Fig. 1) (77 mg) (Greca et al. 1996).
1H and 13C NMR spectra were recorded on a Jeol lambda-500 spectrometer (Jeol, Tokyo, Japan) (500 MHz for 1H and 126 MHz for 13C) in CD3OD. The chemical shifts are given in δ (ppm) values relative to that of the solvent [CD3OD (δH 3.31; δC 49.15)] on a tetramethylsilane scale. ESI–MS was performed on a JMS-T100LC mass spectrometer (Jeol, Tokyo, Japan). The structure of effusol was identified by spectroscopic methods including 1D and 2D NMR (1H, 13C, COSY, HMQC, and HMBC) and mass spectrometry (ESI–MS). 1H-NMR (CD3OD) δ: 2.16 (3H, s, H-11), 2.57 (2H, m, H-9), 2.62 (2H, m, H-10), 5.15 (1H, dd, J = 11, 1.5 Hz, H-13b), 5.598 (1H, dd, J = 17.5, 1.5 Hz, H-13a), 6.606 (1H, br s, H-8), 6.614 (1H, d, J = 8 Hz, H-3), 6.81 (1H, d, J = 3 Hz, H-6), 6.89 (1H, dd, J = 17, 11 Hz, H-12), 7.15 (1H, d, J = 8.5 Hz, H-4). 13C-NMR (CD3OD) δ: 11.7 (C-11), 26.5 (C-10), 31.4 (C-9), 112.3 (C-3), 113.47 (C-13), 113.49 (C-6), 114.9 (C-8), 121.9 (C-1), 127.0 (C-4a), 127.7 (C-5a), 128.2 (C-4), 137.2 (C-5), 140.1 (C-1a), 140.3 (C-12), 141.6 (C-8a), 154.9 (C-2), 156.2 (C-7). ESI–MS m/z: 251.05 [M−H]−, 253.12 [M+H]+.
In Vivo Intracellular Zn2+ Dynamics
Rats anesthetized with chloral hydrate (400 mg/kg) were individually placed in a stereotaxic apparatus. Injection cannulae (internal diameter, 0.15 mm; outer diameter, 0.35 mm) were slowly and carefully inserted into the hippocampal CA1 (3.3 mm posterior to the bregma, 2.2 mm lateral, and 2.2 mm inferior to the dura) to reduce cellular excitation and damages. Thirty minutes later, ZnAF-2DA (100 μM) in artificial cerebrospinal fluid (ACSF), which was composed of 127 mM NaCl, 2.5 mM KCl, 0.9 mM MgCl2, 1.2 mM Na2HPO4, 1.3 mM CaCl2, 21 mM NaHCO3 and 3.4 mM d-glucose (pH 7.3), and corticosterone (500 ng/ml) in ACSF containing 100 μM ZnAF-2DA were bilaterally injected into the CA1 pyramidal cell layer through the cannulae at the rate of 0.25 μl/min for 16 min. Ten minutes later, the rats were decapitated after the injection cannulae were slowly and carefully pulled out the brain. The brain was quickly excised from the rats and placed in ice-cold choline-Ringer solution bubbled with 95% O2 and 5% CO2, which was composed of 124 mM choline chloride, 2.5 mM KCl, 2.5 mM MgCl2, 1.25 mM NaH2PO4, 0.5 mM CaCl2, 26 mM NaHCO3, and 10 mM glucose (pH 7.3) to block neuronal excitation. Brain slices (400 μm thickness) were coronally prepared by a vibratome ZERO-1 (Dosaka, Kyoto, Japan) in the same solution, followed by maintaining brain slices in the same solution for 30 min. The brain slices were moved to a chamber containing choline-Ringer solution. Intracellular ZnAF-2 fluorescence (laser, 488.4 nm; emission, 500–550 nm) was determined in the stratum radiatum of the CA1 with a confocal laser-scanning microscopic system (Nikon A1 confocal microscopes, Nikon Corp.).
In Vivo Microdialysis
Anesthetized rats were placed in a stereotaxic apparatus in the same manner. A guide tube was surgically inserted into the hippocampus (5.6 mm posterior to the bregma, 5.2 mm lateral, and 3.6 mm inferior to the dura) of an anesthetized rat and fixed. Seventy-two hours after fixation, a microdialysis probe (3-mm membrane, Eicom Co., Kyoto) was inserted through the guide tube. The hippocampus was pre-perfused with ACSF at 4.0 µl/min for 2 h and perfused with ACSF at the same flow rate for 25 min to determine the basal levels of extracellular glutamate, followed by perfusion with 1000 ng/ml corticosterone in ACSF or 150 mM KCl in ACSF for 20 min. In another experiment, the hippocampus was perfused with ACSF at 4.0 µl/min for 25 min to determine the basal levels of extracellular glutamate in the same manner, perfused with ACSF containing 100 μM effusol for 25 min, and perfused with 1000 ng/ml corticosterone in ACSF containing 100 μM effusol or 150 mM KCl in ACSF containing 100 μM effusol for 20 min.
Glutamate concentration in the perfusate samples collected for 5 min was determined by high-performance liquid chromatography [column, CAPCELL PAK C18 UG120A (1 mm × 150 mm) (Shiseido Co Ltd, Tokyo, Japan)] using the pre-column derivatization technique with o-phthaldialdehyde and a fluorescence detector (NANOSPACE SI-2, Shiseido Co Ltd). Mobile phase (pH 6.0) was composed of 0.1 M potassium dihydrogen phosphate, 0.1 M di-sodium hydrogen phosphate, 10% acetonitrile, 0.5 mM EDTA-2Na, and 3% tetrahydrofuran. Glutamate concentrations were calculated based on the peak area of the standard solution that was measured before and after analysis of samples.
In Vitro Extracellular and Intracellular Zn2+ Imaging
Coronal brain slices (400 µm) prepared in the same manner were bathed in Ringer solution bubbled with 95% O2 and 5% CO2. To determine intracellular Zn2+ levels, brain slices were loaded for 30 min in 10 µM ZnAF-2DA in Ringer solution and then moved to a chamber containing Ringer solution to rinse extracellular ZnAF-2DA in brain slices for at least 15 min. The brain slices were moved to a chamber containing 100 μM effusol in Ringer solution. ZnAF-2 fluorescence was measured for 60 s in the CA1 stratum radiatum and then measured for 300 s in the same manner after exposing the brain slices to corticosterone (final concentration, 1000 ng/ml). To determine extracellular Zn2+ levels, in another experiment, brain slices were moved to a chamber containing 10 µM ZnAF-2 in Ringer solution. Extracellular ZnAF-2 fluorescence was measured in the same manner.
In Vivo CA1 LTP
LTP was recorded according to the previous method (Suzuki et al. 2018).
Data Analysis
Comparison of the means of unpaired data was done by Student’s t test. Differences between treated groups were also evaluated by one-way ANOVA followed by post hoc test using the Tukey’s test for multiple comparisons. A value of p < 0.05 was considered significant (the statistical software, GraphPad Prism 5). Data were shown as mean ± standard error.
Results
To check Zn2+ dynamics after expose to glucocorticoid in vivo, 500 ng/ml corticosterone was locally injected into the hippocampal CA1. Intracellular Zn2+ level determined with ZnAF-2 was increased in the CA1 (Fig. 2), The data suggest that this increase is linked with accumulation of extracellular glutamate induced with corticosterone (Suzuki et al. 2018).
Fig. 2.
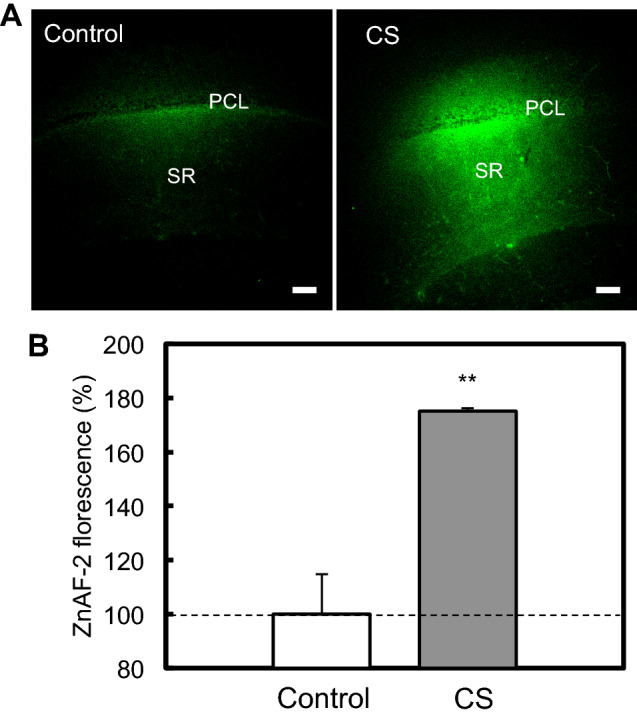
Corticosterone increases the concentration of intracellular Zn2+ in the hippocampal CA1. a ACSF (control, n = 5) and Corticosterone (500 ng/ml) in ACSF (n = 5) containing 100 μM ZnAF-2DA were bilaterally injected into the CA1 pyramidal cell layer (PCL) via cannulae at the rate of 0.25 μl/min for 16 min. Intracellular ZnAF-2 fluorescence was captured in the CA1 10 min after injection. PCL pyramidal cell layer; Bar, 100 μm. b Each bar and line shows the rate (%) of ZnAF-2 fluorescence in the stratum radiatum (SR) to the control ZnAF-2 fluorescence in the SR. **p < 0.01, vs. control (t test)
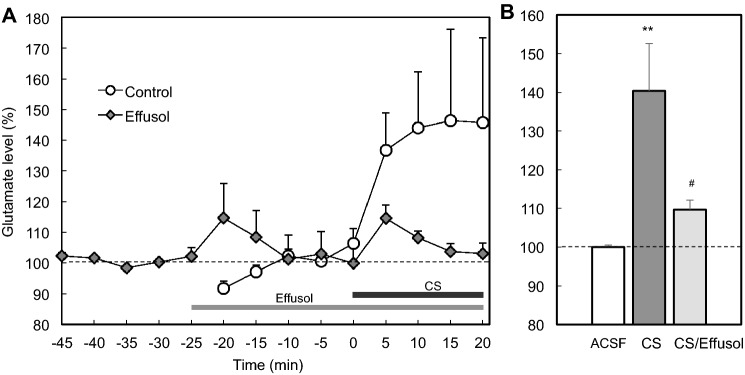
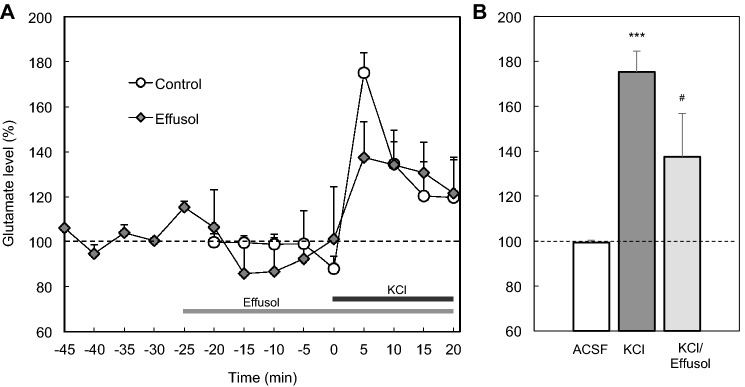
When accumulation of extracellular glutamate was assessed under perfusion with corticosterone, glutamate concentration in the perfusates was increased and this increase was suppressed under co-perfusion with effusol (Fig. 3). Increased glutamate concentration in the perfusates, which was induced under perfusion with high K+, was also suppressed under co-perfusion with effusol (Fig. 4). Because glutamate is released with Zn2+ from zincergic Schafffer collaterals, it is estimated that extracellular Zn2+ also accumulates in the CA1 after exposure to corticosterone and high K+.
Fig. 3.
Extracellular glutamate increased with corticosterone is suppressed in the presence of effusol. a The CA1 was perfused with ACSF for 25 min (time, −25 to 0 min, n = 6) and 1000 ng/ml corticosterone (CS) in ACSF for 20 min (time, 0–20 min) as indicated by a black bar (control group). The CA1 was also perfused with ACSF for 25 min (time, −50 to −25 min, n = 6), 100 μM effusol in ACSF for 25 min (time, −25 to 0 min), and 100 μM effusol + 1000 ng/ml corticosterone (CS) in ACSF for 20 min (time, 0–20 min, n = 6) as indicated by a gray and black bar, respectively (effusol group). b Each bar and line shows concentration of glutamate in the perfusate collected for 20 min (time, 0–20 min) to each basal concentration of glutamate in perfusate under the perfusion with ACSF for 25 min, which is shown as 100%. **p < 0.01, vs. ACSF; #p < 0.05, vs. cs (Tukey’s test)
Fig. 4.
Extracellular glutamate increased with high K+ is suppressed in the presence of effusol. a The CA1 was perfused with ACSF for 25 min (time, −25 to 0 min, n = 9) and 150 mM KCl in ACSF for 20 min (time, 0–20 min) as indicated by a black bar (control group). The CA1 was also perfused with ACSF for 25 min (time, −50 to −25 min, n = 6), 100 μM effusol for 25 min (time, −25 to 0 min), and 100 μM effusol + 150 mM KCl for 20 min (time, 0–20 min) as indicated by a gray and black bar, respectively. b Each bar and line shows concentration of glutamate in the perfusate collected for 5 min (time, 0–5 min) to each basal concentration of glutamate in perfusate under the perfusion with ACSF for 25 min, which is shown as 100%. ***p < 0.001, vs. ACSF; #p < 0.05, vs. cs (Tukey’s test)
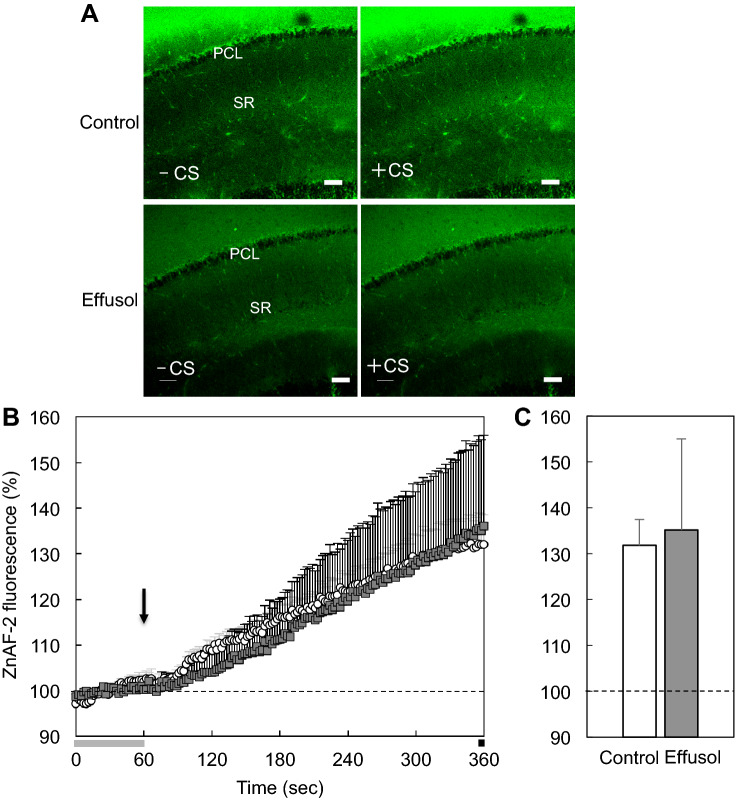
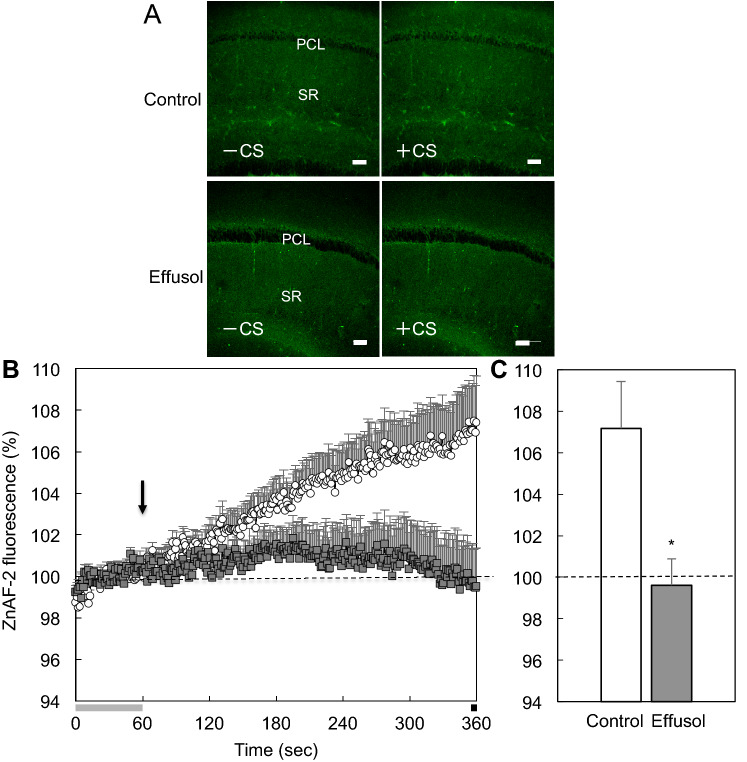
Thus, we tested whether accumulation of extracellular Zn2+ induced with corticosterone is suppressed in the presence of effusol. Extracellular levels of Zn2+ were rapidly increased in the CA1 after exposing brain slices to corticosterone, while this increase was not suppressed in the presence of effusol (Fig. 5). In contrast, intracellular levels of Zn2+ were also rapidly increased in the CA1 after exposing brain slices to corticosterone, and this increase was almost completely blocked in the presence of effusol (Fig. 6).
Fig. 5.
Extracellular Zn2+ increased with corticosterone is not suppressed in the presence of effusol. a Extracellular ZnAF-2 fluorescence in the CA1 of brain slices bathed in Ringer solution containing 10 µM ZnAF-2 and 100 µM effusol in Ringer solution containing 10 µM ZnAF-2 was imaged 0 s (base, − CS) and 300 s after exposure to corticosterone (the final concentration, 1000 ng/ml) (+ CS). Bar, 100 μm. b Corticosterone was added to brain slices as indicated by the arrow 60 s after measuring the basal fluorescence of ZnAF-2 and the changes in ZnAF-2 fluorescence were measured for 300 s (Control, n = 4; effusol, n = 3). c Each point and line shows the rate of ZnAF-2 fluorescence measured for the last 5 s shown by the black bar in B to the basal fluorescence ZnAF-2 shown by the gray bar in (b)
Fig. 6.
Intracellular Zn2+ increased with corticosterone is suppressed in the presence of effusol. a Intracellular ZnAF-2 fluorescence in the CA1 of brain slices, which were loaded with ZnAF-2DA, bathed in Ringer solution and 100 µM effusol in Ringer solution was imaged 0 s (base, − CS) and 300 s after exposure to corticosterone (the final concentration, 1000 ng/ml) (+ CS). Bar, 100 μm. b Corticosterone was added to brain slices as indicated by the arrow 60 s after measuring the basal fluorescence of ZnAF-2 and the changes in ZnAF-2 fluorescence were measured for 300 s (Control, n = 13; effusol, n = 10). c Each point and line shows the rate of ZnAF-2 fluorescence measured for the last 5 s shown by the black bar in (b) to the basal fluorescence ZnAF-2 shown by the gray bar in (b). *p < 0.01, vs. control (t test)
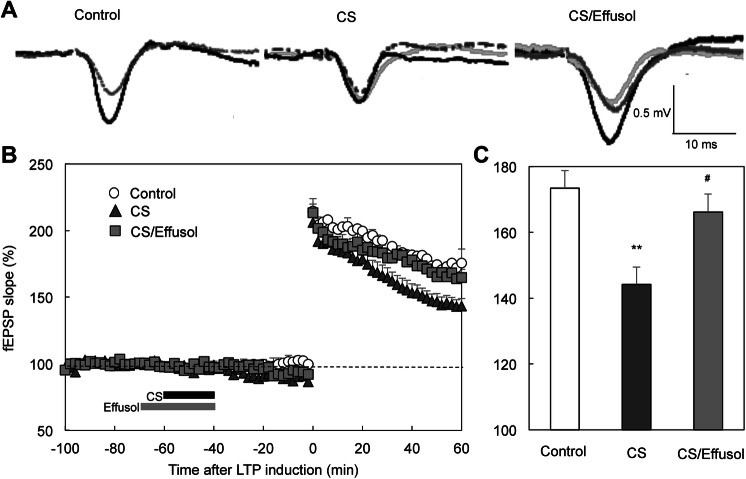
It is possible that effusol-mediated block of increase in intracellular Zn2+ leads to rescuing corticosterone toxicity in the CA1. CA1 LTP was attenuated by the pre-perfusion with corticosterone for 20 min, but not by the co-perfusion with effusol (Fig. 7).
Fig. 7.
Effusol rescues CA1 LTP attenuated by corticosterone. a Representative fEPSP recordings at the time -80 min (gray dotted line), -50 min (light gray line), and 50 min (black line) are indicated. b LTP was induced under pre-perfusion with 500 ng/ml corticosterone as indicated by a black bar (CS, time −60 to 40 min) (n = 15) or 500 ng/ml corticosterone + 100 µM effusol as indicated by a gray bar (time −70 to 40 min) (n = 10). c The magnitude of LTP. **p < 0.01 vs. control (n = 15), #p < 0.05 vs. CS (Tukey’s test)
Discussion
Glucocorticoid concentration is readily increased in the hippocampal extracellular fluid after exposure to acute stress and the increase activates membrane MC and GC receptors, followed by cognitive decline via aberrant synaptic plasticity (Sajadi et al. 2006; Khaksari et al. 2007; Dorey et al. 2011). The activation of presynaptic and postsynaptic membrane MC receptors modifies CA1 pyramidal cell function. Corticosterone pre-synaptically elevates glutamate release probability and post-synaptically reduces potassium current, followed by enhanced excitability of CA1 pyramidal cells (Olijslagers et al. 2008). In the CA1, approximately half of the Schaffer collaterals release Zn2+ with glutamate (Sindreu et al. 2003). The Schaffer collaterals increases release of glutamate and Zn2+ via membrane MC receptor activation after exposure to corticosterone and the influx of extracellular Zn2+ into CA1 pyramidal cells occurs, probably through Ca2+- and Zn2+-permeable AMPA receptors, resulting in intracellular Zn2+ dysregulation. On the other hand, intracellular Zn2+ dysregulation is also caused by membrane GC receptor activation in the CA1 (Suzuki et al. 2018). The activation of membrane MC and GC receptors, which is poorly understood, may occur via differential mechanisms after exposure to corticosterone, which attenuates CA1 LTP. In the present study, in vivo Zn2+ imaging with ZnAF-2 indicated that a rapid increase in intracellular Zn2+ is induced in the CA1 after local exposure to corticosterone in the CA1, probably via activating membrane MC receptors. Hippocampal LTP attenuated with corticosterone is closely linked with neuronal Zn2+ dysregulation after exposure to stress (Takeda et al. 2009, 2012). Therefore, natural products, which rescue intracellular Zn2+ dysregulation, might lead to defend hippocampal function against stress-related cognitive decline.
The present study examined the effect of effusol, a phenanthrene isolated from Chinese medicine Juncus effusus on corticosterone toxicity, which induces intracellular Zn2+ dysregulation in the CA1 via accumulation of extracellular glutamate. In vivo microdialysis experiment indicated that both increases in extracellular glutamate induced under perfusion with corticosterone and high K+ in the CA1 are suppressed by co-perfusion with effusol. Because corticosterone and high K+ also induce increase in extracellular Zn2+, followed by intracellular Zn2+ dysregulation (Takeda et al. 2011, 2018a; Suzuki et al. 2018), the effect of effusol on both the increases was examined in brain slice experiments. Effusol did not suppress increase in extracellular Zn2+ in the CA1 of brain slices bathed in corticosterone. In contrast, effusol suppressed the increase in intracellular Zn2+, which may be linked with suppressing increase in extracellular glutamate in vivo. CA1 LTP was attenuated under pre-perfusion with corticosterone, while the attenuation was rescued by co-perfusion with effusol. The attenuation is observed under co-perfusion with CaEDTA, an extracellular Zn2+ chelator, that blocks increase in intracellular Zn2+ induced with corticosterone (Takeda et al. 2012), suggesting that the rescuing effect of effusol is due to blocking increase in intracellular Zn2+ in CA1 pyramidal cells. The data that effusol had no effect on the basal fEPSP slope recording suggest the substantial action of effusol on the attenuated CA1 LTP.
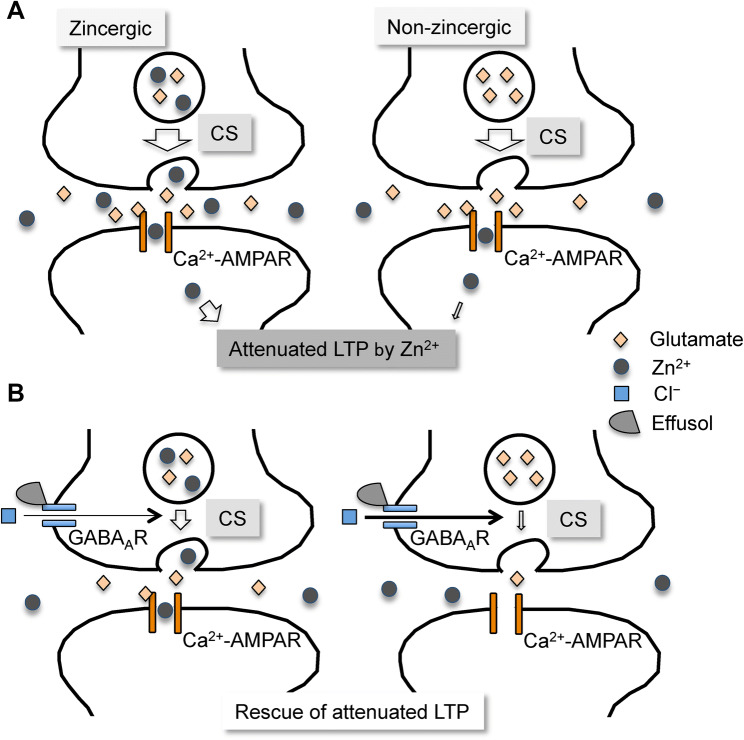
Glutamatergic Schaffer collaterals are composed of zincergic and non-zincergic terminals (Sindreu et al. 2003). In conclusion, it is estimated that effusol-mediated activation of GABAA receptors (Singhuber et al. 2012) suppresses glutamate release from Schaffer collaterals, followed by blocking intracellular Zn2+ dysregulation in CA1 pyramidal cells that rescues CA1 LTP attenuated by corticosterone (Fig. 8). Because effusol did not suppress increase in extracellular Zn2+ in the CA1 of brain slices bathed in corticosterone, it is likely that effusol-mediated activation of GABAA receptors preferential occurs in non-zincergic Schaffer collaterals in comparison with zincergic Schaffer collaterals, resulting in no significant suppression of increasing extracellular Zn2+ (Fig. 8). Zn2+ released with glutamate serves as a negative feedback factor against glutamate release from zincergic Schaffer collaterals (Takeda et al. 2007). Thus, it is possible that presynaptic GABAA receptors, which are activated by effusol, are more functional for non-zincergic Schaffer collaterals than zincergic Schaffer collaterals. Effusol may defend the hippocampal function against stress-induced cognitive decline. It might be also effective in neurological disorders associated with glutamate excitotoxicity.
Fig. 8.
Proposed mechanism on rescuing action of effusol in attenuated LTP. a After exposure to corticosterone, Zn2+ is co-released with glutamate at zincergic Schaffer collateral-CA1 pyramidal cell synapses and taken up into CA1 pyramidal cells, probably through Ca2+- and Zn2+-permeable AMPA receptors (Ca2+-AMPAR), followed by excess intracellular Zn2+ that attenuates LTP (left). Extracellular Zn2+ is also taken up into CA1 pyramidal cells in the same manner by glutamate release from non-zincergic Schaffer collaterals (right). b Effusol reduces extracellular glutamate accumulation and blocks increase in intracellular Zn2+ in CA1 pyramidal cells, probably via less activation of Ca2+-AMPAR, resulting in rescuing the attenuated LTP by Zn2+. Presynaptic inhibitory action via GABAA receptors might be more functional for non-zincergic Schaffer collaterals than zincergic Schaffer collaterals
Abbreviations
- LTP
Long-term potentiation
- MC
Mineralocorticoid
- GC
Glucocorticoid
- CS
Corticosterone
- AMPA
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- GABA
γ-Aminobutyric acid
- ACSF
Artificial cerebrospinal fluid
- PCL
Pyramidal cell layer
- SR
Stratum radiatum
Author Contributions
Planned experiments: H.T. and A.T. Performed the Experiments: Y.S., T.M, and T.F. Analyzed data: H.K. and M.S. Wrote the paper: H.T. and A.T. All authors reviewed the manuscript.
Compliance with Ethical Standards
Conflict of interest
All authors have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Dorey R, Piérard C, Shinkaruk S, Tronche C, Chauveau F, Baudonnat M, Béracochéa D (2011) Membrane mineralocorticoid but not glucocorticoid receptors of the dorsal hippocampus mediate the rapid effects of corticosterone on memory retrieval. Neuropsychopharmacology 36:2639–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson CJ, Koh JY, Bush AI (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci 6:449–462 [DOI] [PubMed] [Google Scholar]
- Greca MD, Fiorentino A, Molinaro A, Monaco P, Previtera L (1993) A bioactive dihydrodibenzoxepin from Juncus effusus. Phytochemistry 34:1182–1184 [Google Scholar]
- Greca MD, Fiorention A, Monaco P, Previtera L (1994) Cycloartane triterpenes from Juncus effusus. Phytochemistry 35:1017–1022 [DOI] [PubMed] [Google Scholar]
- Greca MD, Fiorentino A, Monaco P, Pinto G, Pollio A, Previtera L (1996) Action of antialgal compounds from Juncus effusus L. on Selenastrum capricornutum. J Chem Ecol 22:587–603 [DOI] [PubMed] [Google Scholar]
- Hirano T, Kikuchi K, Urano Y, Nagano T (2002) Improvement and biological applications of fluorescent probes for zinc, ZnAFs. J Am Chem Soc 124:6555–6562 [DOI] [PubMed] [Google Scholar]
- Joëls M, de Kloet ER (2017) The brain mineralocorticoid receptor: a saga in three episodes. J Endocrinol 234:T49–T66 [DOI] [PubMed] [Google Scholar]
- Joëls M, Karst H, DeRijk R, de Kloet ER (2008) The coming out of the brain mineralocorticoid receptor. Trends Neurosci 31:1–7 [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M (2005) Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 102:19204–19207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaksari M, Rashidy-Pour A, Vafaei AA (2007) Central mineralocorticoid receptors are indispensable for corticosterone-induced impairment of memory retrieval in rats. Neuroscience 149:729–738 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM (2002) The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci 3:453–462 [DOI] [PubMed] [Google Scholar]
- Kim J, Yoon KS (1998) Stress: metaplastic effects in the hippocampus. Trends Neurosci 21:505–509 [DOI] [PubMed] [Google Scholar]
- Kwak S, Weiss JH (2006) Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr Opin Neurobiol 16:281–287 [DOI] [PubMed] [Google Scholar]
- Liao YJ, Zhai HF, Zhang B, Duan TX, Huang JM (2011) Anxiolytic and sedative effects of dehydroeffusol from Juncus effusus in mice. Planta Med 77:416–420 [DOI] [PubMed] [Google Scholar]
- Lynch MA (2004) Long-term potentiation and memory. Physiol Rev 84:87–136 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM (1995) Stress and cognitive function. Curr Opin Neurobiol 5:205–216 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C (2015) Mechanisms of stress in the brain. Nat Neurosci 18:1353–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olijslagers JE, De Kloet ER, Elgersma Y, Van Woerden GM, Joëls M, Karst K (2008) Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors. Eur J Neurosci 27:2542–2550 [DOI] [PubMed] [Google Scholar]
- Sajadi AA, Samaei SA, Rashidy-Pour A (2006) Intra-hippocampal microinjections of anisomycin did not block glucocorticoid-induced impairment of memory retrieval in rats: an evidence for non-genomic effects of glucocorticoids. Behav Brain Res 173:158–162 [DOI] [PubMed] [Google Scholar]
- Sandi C (2011) Glucocorticoids act on glutamatergic pathways to affect memory processes. Trends Neurosci 34:165–176 [DOI] [PubMed] [Google Scholar]
- Shima K, Toyota M, Asakawa Y (1991) Phenanthrene derivatives from the medullae of Juncus effusus. Phytochemistry 30:3149–3151 [Google Scholar]
- Sindreu CB, Varoqui H, Erickson JD, Perez-Clausell J (2003) Boutons containing vesicular zinc define a subpopulation of synapses with low AMPAR content in rat hippocampus. Cereb Cortex 13:823–829 [DOI] [PubMed] [Google Scholar]
- Singhuber J, Baburin I, Khom S, Zehl M, Urban E, Hering S, Kopp B (2012) GABA(A) receptor modulators from the Chinese herbal drug Junci Medulla—the pith of Juncus effusus. Planta Med 78:455–458 [DOI] [PubMed] [Google Scholar]
- Suh SW, Jo SM, Vajda Z, Danscher G (2001) Adrenalectomy causes loss of zinc ions in zinc-enriched (ZEN) terminals and decreases seizure-induced neuronal death. Brain Res 895:25–32 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Sato Y, Tamura K, Tamano H, Takeda A (2018) Rapid intracellular Zn2+ dysregulation via membrane corticosteroid receptor activation affects in vivo CA1 LTP. Mol Neurobiol. 10.1007/s12035-018-1159-9 [DOI] [PubMed] [Google Scholar]
- Takeda A, Fuke S, Tsutsumi W, Oku N (2007) Negative modulation of presynaptic activity by zinc released from Schaffer collaterals. J Neurosci Res 85:3666–3672 [DOI] [PubMed] [Google Scholar]
- Takeda A, Ando M, Kanno S, Oku N (2009) Unique response of zinc in the hippocampus to behavioral stress and attenuation of subsequent mossy fiber long-term potentiation. NeuroToxicology 30:712–717 [DOI] [PubMed] [Google Scholar]
- Takeda A, Takada S, Nakamura M, Suzuki M, Tamano H, Ando M, Oku N (2011) Transient increase in Zn2+ in hippocampal CA1 pyramidal neurons causes reversible memory deficit. PLoS ONE 6:e28615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Suzuki M, Tamano H, Takada S, Ide K, Oku K (2012) Involvement of glucocorticoid-mediated Zn2+ signaling in attenuation of hippocampal CA1 LTP by acute stress. Neurochem Int 60:394–399 [DOI] [PubMed] [Google Scholar]
- Takeda A, Koike Y, Osawa M, Tamano H (2018a) Characteristic of extracellular Zn2+ influx in the middle-aged dentate gyrus and its involvement in attenuation of LTP. Mol Neurobiol 55:2185–2195 [DOI] [PubMed] [Google Scholar]
- Takeda A, Tamano H, Hisatsune M, Murakami T, Nakada H, Fujii H (2018b) Maintained LTP and memory are lost by Zn2+ influx into dentate granule cells, but not Ca2+ influx. Mol Neurobiol 55:1498–1508 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang Y, Zhai H, Liao Y, Zhang B, Huang J (2012) Phenanthrenes from Juncus effusus with anxiolytic and sedative activities. Nat Prod Res 26:1234–1239 [DOI] [PubMed] [Google Scholar]
- Weiss JH (2011) Ca permeable AMPA channels in diseases of the nervous system. Front Mol Neurosci 4:42 [DOI] [PMC free article] [PubMed] [Google Scholar]