Abstract
Head and neck cancer (HNC) is the sixth most diagnosed cancer, and treatment typically consists of surgical removal of the tumor followed by ionizing radiation (IR). While excellent at controlling tumor growth, IR often damages salivary glands due to their proximity to common tumor sites. Radiation damage to salivary glands results in loss of secretory function, causing severe and chronic reductions in salivary flow. This leads to the patient-reported sensation of dry mouth, termed xerostomia, which significantly reduces quality of life for HNC patients and survivors. The mechanisms underlying salivary gland damage remain elusive, and therefore, treatment options are scarce. Available therapies provide temporary symptom relief, but there is no standard of care for permanent restoration of function. There is a significant gap in understanding the chronic mechanistic responses to radiation as well as treatments that can be given in the months to years following cessation of treatment. HNC cases are steadily rising; particularly, the number of young patients diagnosed with nonfatal human papillomavirus + HNC continues to increase. The growing number of HNC diagnoses and improved prognoses results in more people living with xerostomia, which highlights the mounting need for restorative treatments. Mechanisms underlying chronic damage include decreases in acinar differentiation markers, increases in acinar cell proliferation, immune and inflammatory dysregulation, and metabolic changes including increases in amino acids and reductions in glycolysis and oxidative phosphorylation, fibrosis, and dysregulated neuronal responses. Currently, promising treatment options include adenoviral gene transfers and stem cell therapy. Thus, this review describes in depth known mechanisms contributing to chronic damage and discusses therapeutic advances in treating chronically damaged glands. Understanding the chronic response to radiation offers potential in development of new therapeutics to reverse salivary gland damage and improve the quality of life of HNC survivors.
Keywords: salivary glands, xerostomia, quality of life, head and neck cancer, submandibular glands, parotid glands
Clinical Relevance
More than 890,000 new cases of head and neck cancer (HNC) are diagnosed globally every year, and more than 54,000 cases of HNC were diagnosed in 2022 in the United States per Surveillance, Epidemiology, and End Results Program estimates (Barsouk et al. 2023). Risk factors for HNC include age, sex, diet, oral infection, history of infection with human papilloma virus or Epstein-Barr virus, and tobacco and alcohol use. Treatment for HNC typically involves surgical resection of the tumor followed by ionizing radiation (IR) therapy or chemoradiotherapy (Kaidar-Person et al. 2018); however, treatment options are dependent on tumor stage and location. An adverse side effect of targeted head and neck radiation therapy is damage to healthy surrounding tissues, including the salivary glands. The salivary glands are highly radiosensitive, with the parotid glands (PGs) being the most radiosensitive, and radiation damage to salivary tissue leads to hyposalivation and xerostomia, the patient-reported sensation of severe dry mouth (Grundmann et al. 2009). Reduced salivary output results in significantly diminished quality of life, with severe symptoms such as oral infections, increased cavities and canker sores, dysphagia, and malnutrition. Eighty percent of patients report some degree of xerostomia while undergoing treatment; stimulated and unstimulated saliva output are reduced to approximately 20% to 30% of pretreatment levels (Dirix et al. 2006; Jensen et al. 2019). While some patients do regain partial salivary function after the termination of IR, most patients report hyposalivation for months to years following treatment; unfortunately, saliva output does not return to levels comparable to before radiation, and saliva composition remains altered (Eisbruch et al. 1999; Pinna et al. 2015; Jensen et al. 2019; Winter et al. 2021; Rades et al. 2022). Various prevention and treatment options for xerostomia exist, such as saliva stimulants and topical analgesics; however, they often are accompanied by severe side effects, provide only symptom control, and do not permanently restore the production of saliva endogenously (Chibly et al. 2014; Bockel et al. 2018; Jensen et al. 2019). New advances in technology, such as intensity-modulated radiation therapy and volumetric modulated arc therapy, allow for radiation targeting to the shape and location of the tumor (Jasmer et al. 2020). Several studies indicate that these therapies result in a significant reduction in radiation dose to nonmalignant tissues, but patients treated with these therapies still report xerostomia (Jasmer et al. 2020). A cumulative radiation dose of <26 Gy to the PGs and <39 Gy to the submandibular glands (SMGs) is recommended for recovery of function. In the present review, we aim to evaluate what is currently known on the chronic phenotypes (defined as 30+ d after IR) of radiation-induced salivary gland damage. More extensive phenotypic comparisons between rodents, larger animals, and humans have been reviewed by Grundmann et al. (2009). Further, we discuss experimental therapies that alter the chronic phenotype and therapies that can be administered at chronic time points to treat xerostomia. Understanding the mechanisms that underlie salivary gland damage is critical to identify therapeutic targets to permanently restore function.
Chronic Phenotypes Underlying Salivary Gland Dysfunction
Acinar Cell Proliferation, Polarity, and Differentiation
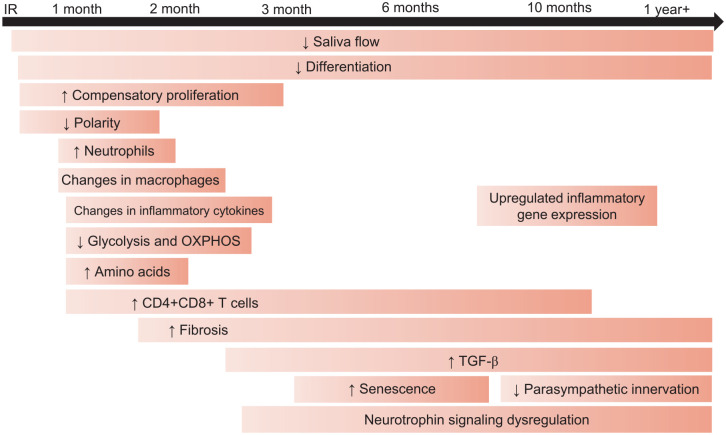
Compensatory proliferation is a conserved response to tissue injury that serves to replace cells that underwent cell death during the aforementioned injury. In radiation-damaged murine salivary glands, compensatory proliferation, as measured by PCNA or Ki-67, begins as acutely at 48 h after IR in the intercalated duct and is detected in acinar and granular convoluted tubule cells in the parotid and SMGs at day 5 after IR. Compensatory proliferation in the acinar compartment persists with elevated levels present through day 90 in mouse models (Fig.) (Wong et al. 2019). In addition, another study reported significant increases in proliferating acinar cells as evidenced by increased Ki-67+NKCC1+ dual staining at all time points (Luitje et al. 2021).
Figure.
Timeline of phenotypes influencing the chronic radiation damage response. Following ionizing radiation (IR) therapy, animal models show a reduction in salivary flow beginning at day 3 that persists through chronic time points (Jasmer et al. 2020). Increases in proliferation markers and reductions in differentiation are observed through day 90, while a loss of apical basolateral polarity is observed through day 30 (Chibly et al. 2018; Emmerson et al. 2018; Wong et al. 2019; Lombaert et al. 2020). Changes in the immune and inflammatory response, including increases in double positive T cells and neutrophils and changes in proinflammatory cytokines, are observed at day 30, and the increase in double-positive T cells is seen through 10 mo, as measured by scRNAseq (Hu et al. 2021; Rheinheimer et al. 2023; Gunning et al. 2024). Both increases and decreases in macrophages have been reported around 1 mo following IR (Hu et al. 2021; Gunning et al. 2024). Reductions in glycolytic flux and oxidative phosphorylation (OXPHOS) have been reported at 30 and 60 d after IR, and increases in amino acids have been observed at 30 d after IR (Meeks et al. 2021; Buss et al. 2024). Fibrosis is generally reported 4 to 6 mo following IR but has been observed as soon as 8 wk post-IR and is often associated with increases in transforming growth factor–β (Lombaert et al. 2020). Increases in cellular senescence markers and reductions in anti-senescence cytokines have been observed at 5 wk post-IR in animal models and after 4 mo in clinical studies (Marmary et al. 2016; Hu et al. 2021). Data from clinical studies illustrate neuronal changes, including reduced parasympathetic innervation and dysregulation of neurotrophin signaling, begin around 4 mo and persist for years following IR (Emmerson et al. 2018; Chibly et al. 2023).
Establishment of apical-basolateral polarity, mediated through atypical-protein kinase C zeta (aPKCζ), is necessary for epithelial wound healing, and irradiated salivary glands show loss of activated aPKCζ at day 5, which continues through day 30 (Fig.) (Wong et al. 2019). Inactivation of aPKCζ is significantly correlated with the induction of compensatory proliferation, and Prkcz−/− mice show increased baseline proliferation that does not increase with 5 Gy IR (Wong et al. 2019). Another study reported Prkcz−/− mice treated with therapeutic insulin-like growth factor (IGF)—1 injections do not experience an improvement in saliva flow compared with Prkcz−/− or wild-type (WT) IR-only controls, indicating aPKCζ is necessary for IGF-1–mediated restoration of function (Chibly et al. 2018).
Despite the increase in proliferation following radiation-induced apoptosis, animal studies demonstrate these cells do not undergo differentiation, as evidenced by reductions in amylase and aquaporins (AQP; Fig.) (Jasmer et al. 2020). The elevated proliferative response coalesced with reductions in differentiation markers is highly correlated with loss of salivary output (Grundmann et al. 2010; Hill et al. 2014; Morgan-Bathke et al. 2014; Meyer et al. 2023). In mice treated with 5 × 6 Gy fractionated IR, researchers identified significant decreases in AMY2 and AQP5 expression 300 d post-IR (Lombaert et al. 2020). One study reported significant decreases in the expression of acinar cell markers AQP3 and AMY1 in human SMGs collected approximately 2 y after IR (Emmerson et al. 2018). Another clinical study assessing acinar cell changes in parotid and SMGs following radiation found significant loss of acinar cells and MIST1+ cells in samples collected from ~10 wk to 1 y following IR (Luitje et al. 2021). After 1 y, acinar cells and distinct acini were present in human submandibular and PGs, and MIST1+ cells were increased but remained significantly lower than in control glands. Further, this study reported that samples collected less than 1 y following IR had loss of functional markers NKCC1 and AQP5, but these markers were restored in samples collected at longer time points (Luitje et al. 2021). Therapies that alter the proliferative and redifferentiation response are a promising option to improve the chronic damage phenotype.
Stem and Progenitor Cell Phenotypes
Salivary gland stem and progenitor cells (SPCs) are multipotent cells that can differentiate into functional acinar cells. Reactivation of the SPC population in radiation-damaged salivary glands is a potential therapeutic option to replace damaged cells. One study reported increased senescence-associated β-galactosidase–positive staining in ducts, which are thought to contain SPCs, in murine SMGs 8 wk after 15 Gy IR (Peng et al. 2020). This study also reported increased p16-positive staining in the SPC population of human SMGs (Peng et al. 2020). Label-retaining cells (LRCs) express markers of salivary progenitors, including keratin-5, keratin-14, and kit, and have the capacity to proliferate following damage (Chibly et al. 2018). One study reported a significant increase in nuclear translocation of Yes-associated protein (Yap) in murine acinar label-retaining cells (LRCs) at 30 d post-IR (Chibly et al. 2018). Inactivation of aPKCζ is significantly correlated with induction of compensatory proliferation, specifically in progenitor cells, or LRCs, in the acinar compartment of PGs (Chibly et al. 2018; Wong et al. 2019). Prkcz−/− mice have a genetic deletion of aPKCζ; these mice have higher baseline levels of LRC proliferation compared with WT mice as shown by increased Ki-67–positive cells, and 5 Gy IR does not significantly increase LRC proliferation (Chibly et al. 2018). Targeting SPCs represents a potential therapeutic avenue.
Inflammation and Senescence
Acute inflammation is necessary for complete wound healing in many models, but chronic inflammation can inhibit tissue regeneration. Inflammation is often associated with cellular senescence, with radiation-induced senescence reported in multiple tissues such as the lung, brain, and skin (Kim et al. 2023). One study described significant increases in inflammatory and immune related genes Clec12a, Pld4, and Lyz2 in murine SMGs 300 d post-IR (Lombaert et al. 2020). The same study identified increased CD45 staining and significantly upregulated mRNA expression of LYZ in minipigs at day 300 post–15 Gy IR (Lombaert et al. 2020). A study conducted by Hu and colleagues reported significant reductions in mRNA expression (ARG1, AIF1, ADGRE, ITGAX, HGF, and C1Qa) and protein levels (AIF1, F4/80) of macrophage markers at 5 wk following radiation in miniature pig PGs (Hu et al. 2021). Interestingly, because ARG1, an M2 pro-resolving macrophage marker, is significantly reduced, this may indicate that macrophages in radiation-damaged PGs may have an M1 proinflammatory phenotype. The role of macrophages in chronically radiation-induced salivary gland damage is not well understood; however, we posit that macrophages are major contributors to perpetuating a chronic inflammatory milieu. Likewise, this study found significant increases in mRNA expression and protein levels of proinflammatory cytokines in irradiated parotid tissue, including interleukin (IL)–6, interferon gamma, and tumor necrosis factor. In addition, another study using RNAseq reported significant upregulation of genes related to inflammation in parotid and SMG clinical biopsies at chronic time points (Chibly et al. 2023). Finally, a clinical study reported a significant increase in γH2AX-positive senescent ductal cells in SMGs resected from patients 4 to 8 mo following IR (Marmary et al. 2016). Further, vascular endothelial growth factor (VEGF), an anti-senescence cytokine, is significantly reduced in minipig PGs at 5 wk after 20 Gy (Hu et al. 2021).
Single-cell RNA sequencing (scRNAseq) identified a notable increase in T cells in mouse PGs at 10 mo after IR; specifically, CD4+, CD4+CD8+, and FoxP3+ T cells increased (Rheinheimer et al. 2023). Surprisingly, double-positive T cells had the highest number of radiation-induced differentially expressed genes (DEGs), with DEGs enriched within lymphocyte differentiation, apoptosis, V(D)J recombination, axonogenesis, and ERK signaling pathways (Rheinheimer et al. 2023). In addition, this study reported notable communication between acinar cells and T cells, as evidenced by multiple ligands on acinar cells corresponding to receptors on T cells. A recent study reported significant increases in F4/80+CD11b+ macrophages, Ly-6G+CD11b+ neutrophils, and CD3+/CD4+CD8+ T cells measured by flow cytometry at day 30 in mice treated with 5 Gy IR (Gunning et al. 2024). Interestingly, they observed significant decreases in certain pro- and anti-inflammatory cytokines at day 30 post-IR (Gunning et al. 2024). Together, these studies depict chronic inflammation in radiation-damaged salivary glands, with T cells and cytokines being intricately involved. The chronic inflammatory milieu coupled with increased cellular senescence likely perpetuates prolonged loss of function.
Neuronal Responses
Parasympathetic activity is vital for salivary gland function. Neurturin (NRTN) is a neurotrophic factor necessary for parasympathetic development; data indicate that parasympathetic neurons are involved in salivary gland regeneration (Ferreira et al. 2018). In human SMGs, a significant decrease in parasympathetic innervation measured by GFRA2, CHRM1, and CHRM3 expression was observed in samples collected approximately 2 y after IR (Fig.) (Emmerson et al. 2018). A study using bulk RNAseq reported neurotrophin signaling is a key dysregulated pathway in radiation-damaged salivary glands at chronic time points. RNAseq analysis and quantitative polymerase chain reaction validation showed significant upregulation of NGFR and NTRK2 expression in human parotid and SMGs, and scRNAseq of murine SMG revealed that these genes are differentially expressed in myoepithelial cells (Chibly et al. 2023). Immunofluorescent staining confirmed upregulation of NGFR and TRKA in irradiated human SMG myoepithelial cells (Chibly et al. 2023). These findings demonstrate neuronal responses are chronically dysregulated in radiation-damaged salivary glands.
Fibrosis
Development of glandular fibrosis contributes to chronic hyposalivation and can develop as early as 8 wk after IR (Fig.) (Grundmann et al. 2009; Brook 2020). The mechanisms underlying radiation-induced fibrosis in salivary glands are not fully elucidated, but generation of reactive oxygen species and chronic inflammation are hypothesized to be involved (Straub et al. 2015). A study treating mice with 6 × 5 Gy IR reported increases in fibrosis at 300 d post-IR as measured by Masson’s trichome staining (Lombaert et al. 2020). Further, this study identified increased expression in genes related to matrix deposition, extracellular matrix remodeling, and fibrosis (Lombaert et al. 2020). In addition, this study reported increased fibrosis in porcine PGs, as measured by Masson’s trichrome staining and significant upregulation of genes related to extracellular matrix (ECM) remodeling and fibrosis, at day 300 post–15 Gy IR (Lombaert et al. 2020). A study using RNAseq reported significant upregulation of genes related to fibrosis in both parotid and SMG biopsies taken from HNC patients 4 mo to 7 y following IR (Chibly et al. 2023). Another clinical study reported a significant positive correlation between fibrotic area and increased levels of proinflammatory cytokines in saliva at days 35, 80, and 105 after ~66 Gy fractionated radiation (Zlygosteva et al. 2023). Furthermore, this study also depicted a significant negative correlation between fibrotic area and saliva volume at chronic time points. Conversely, transforming growth factor (TGF)–β is an immunosuppressive cytokine thought to be involved in the development of fibrosis (Zhang et al. 2020). In addition to radiation, other models of salivary gland damage, including ductal ligation and Sjögren’s disease, have illustrated the involvement of TGF-β in glandular fibrosis (Woods et al. 2015; Sisto et al. 2018). Fibrosis is a contributing factor to chronic salivary gland damage and hypofunction following radiation.
Metabolic Changes
Changes in energy metabolism are observed in wound-healing models to provide fuel for tissue regeneration. One study using liquid chromatography–mass spectrometry in irradiated murine PGs reported the lowest percentage of upregulated metabolites at a chronic time point (day 30) when compared with earlier time points (Buss et al. 2023). Pathway analysis using GSEA KEGG IDs did not detect any significant pathway enrichments at day 30; however, MetaMapp network analysis identified tryptophan, tyrosine, and diabetic cardiomyopathy as significantly altered metabolite communities at day 30 post-IR. Further, researchers used weighted gene correlation network analysis and reported significant enrichment of arginine biosynthesis. In addition, they identified significant increases in tryptophan, serine, lysine, arginine, glutamine, histidine, proline, methionine, threonine, phenylalanine, tyrosine, and asparagine in PGs 30 d after IR (Fig.). These data depict increases in amino acid metabolism in radiation-damaged murine PGs that may indicate mechanistic attempts to heal itself. Another study conducted by the same group reported decreases in glycolytic flux and oxidative phosphorylation as evidenced by significant reductions in extracellular acidification rate and oxygen consumption rate in murine PGs at chronic time points (Fig.) (Buss et al. 2024). Further, the adenosine triphosphate (ATP) production rate significantly decreased at days 30 and 60 post-IR, and the mitochondrial DNA copy number significantly increased at day 30 post-IR (Buss et al. 2024). These researchers concluded that irradiated salivary glands exhibit a mitochondrial dysfunction phenotype at chronic time points that may hinder the ability to repair. Aberrant metabolic signaling may contribute to chronic loss of function in radiation-damaged glands.
Therapies That Alter the Chronic Phenotype
Palliative care aims to alleviate symptoms of xerostomia and improve quality of life for HNC survivors but fails to do so. Consequently, therapeutics that alter the chronic phenotype and offer long-term functional restoration are needed. Table 1 summarizes studies that administer therapies at acute time points (<14 d), or prior to IR, that affect the chronic phenotype.
Table 1.
Summary of Studies That Administer Therapies at Acute Time Points (<14 d), or prior to IR, That Alter the Chronic Phenotype.
| Species | Study | Intervention | Timing of Intervention | Clinically Relevant Outcome | Proposed Therapeutic Mechanism |
|---|---|---|---|---|---|
| Mouse | Marmary et al. (2016) | Exogenous IL-6 administration or IL-6 genetic knockout | IL-6 administration 3.5 to 4 h prior to IR | Improved salivary flow | Reduced γH2AX-positive senescent ductal cells |
| Mouse | Hai et al. (2018) | Shh adenoviral vector delivery | Day 3 after IR | N/A | Reduced senescence, DNA damage, oxidative stress, and IL-6 |
| Mouse | Ferreira et al. (2018) | Human NRTN gene transfer | 24 h prior to IR | Preservation of salivary flow | Preservation of parasympathetic markers |
| Mouse | Gilman et al. (2019) | P2X7R antagonist A438079 | 1 h before IR | Improved salivary flow | Reduction in prostaglandin production |
| Mouse | Lombaert et al. (2020) | NRTN (CERE-120) gene transfer via AAV2 [20] | 10 d prior to IR | Improved salivary flow | Attenuated fibrosis, reduced inflammatory gene expression, increased proacinar gene expression |
| Mouse | Zhao et al. (2020) | Shh gene transfer | 3 d after IR | Improved salivary flow | Increased AQP5 protein levels |
| Mouse | Blitzer et al. (2021) | Human IFN-γ treated BM-MSC transfer | 1 d after IR | N/A | Increased amylase, decreased fibrosis |
| Mouse | Gilman et al. (2021) | Indomethacin | Days 3, 5, and 7 post-IR | Improved salivary flow | Increased amylase area |
| Mouse | Li et al. (2022) | Cevimeline-alginate hydrogel | Day 14 post-IR | Improved salivary flow | Increased AQP5, increased nerve activity, regeneration of innervated acini, increased macrophages, reduced T cells, increased pHH3+SOX2+, and pHH3+SOX2- |
| Mouse | Meyer et al. (2023) | AICAR or metformin | Days 4 to 6 post-IR | Improved salivary flow | Attenuation of compensatory proliferation, increased redifferentiation, increased phosphorylated aPKCζ |
| Mouse | Gunning et al. (2024) Jasmer et al. 2020 |
IGF-1 | Days 4 to 6 post-IR | Improved salivary flow [12] | Attenuation of compensatory proliferation [12], reduction in macrophages, neutrophils, and double-positive T cells [28] |
| Minipig | Lombaert et al. (2020) | NRTN (CERE-120) gene transfer via AAV2 | 1 wk prior to IR | Improved salivary flow | Prevented loss of gland weight, increased NRTN protein levels, reduction in expression of immune-related genes |
| Human | Steenbakkers et al. (2022) | Parotid sparing versus stem cell–sparing IR | During IR treatment | Reduced xerostomia scores in stem cell–sparing group | Increased stem cell sparing |
AAV2, adeno-associated serotype 2; aPKCζ, atypical-protein kinase C zeta; BM-MSC, bone marrow mesenchymal stem cells; IFN, interferon; IGF, insulin-like growth factor; IL, interleukin; IR, ionizing radiation; N/A, not applicable; NRTN, neurturin.
Compensatory Proliferation and Differentiation
In animal studies, increased compensatory proliferation is correlated to reduced saliva output. A variety of therapeutics with different mechanisms can alter the proliferative phenotype. Mice treated with IGF-1 injections at days 4 to 7 following IR have significantly improved saliva output, reduced proliferation, and increased amylase area through day 90 post-IR (Jasmer et al. 2020). Rapamycin inhibits mTOR signaling; treating mice with the rapamycin analog CCI-779 restores salivary function, reduces proliferation, and increases differentiation as measured by positive amylase area (Jasmer et al. 2020).
To understand prostaglandin involvement in radiation-induced salivary gland dysfunction, researchers treated mice with indomethacin, a cyclooxygenase inhibitor, prior to and after 5 Gy IR. Interestingly, no difference in salivary flow was observed at day 30 when indomethacin was given prior to radiation compared with controls (Gilman et al. 2021). However, mice receiving indomethacin at days 3, 5, and 7 post-IR had significantly higher saliva flow rates and improved amylase area at day 30 post-IR. Activation of AMPK via AICAR or metformin treatment at days 4 to 6 following 5 Gy IR significantly attenuated compensatory proliferation, increased redifferentiation and apical-basolateral polarity, and improved saliva flow at day 30 (Meyer et al. 2023).
Senescence and Inflammation
It is well established that acute inflammation is necessary for complete wound healing, but aberrant, chronic inflammation contributes to many pathological conditions. A study employing IL-6 genetic knockout mice found a significant reduction in γH2AX-positive senescent ductal cells in SMGs at 8 wk post-IR (Marmary et al. 2016). Interestingly, treatment with endogenous IL-6 or HIL6 significantly reduced γH2AX-positive senescent ductal cells. Further, both IL-6 genetic knockout and endogenous IL-6 supplementation significantly improved saliva flow in mice when compared with IR-only controls. One study delivered Shh adenoviral vectors into mouse salivary glands at 3 d after IR and reported a significant reduction in markers of senescence, DNA damage, oxidative stress, and IL-6 at day 90 following IR (Hai et al. 2018). The P2X7 receptor is activated by extracellular ATP and is involved in multiple inflammatory conditions; P2X7R−/− knockout mice or mice treated with a P2X7R antagonist have significantly improved saliva flow at 30 d post-IR when compared with WT IR-only controls (Gilman et al. 2019). Interestingly, a study using a cevimeline-alginate hydrogel to mimic cholinergic muscarinic stimulation in mice found a significant increase in CD206+CD68+ macrophages and a significant decrease in CD3+ T cells at day 70 when compared with control mice (Li et al. 2022). Finally, a study treating mice with therapeutic IGF-1 injections at days 4 to 6 following 5 Gy IR reported attenuation of the increase observed in F4/80+CD11b+ macrophages, Ly-6G+CD11b+ neutrophils, and CD3+/CD4+CD8+ double-positive T cells (Gunning et al. 2024). An acute inflammatory response is necessary for tissue regeneration; however, these data illustrate that chronic inflammation in the salivary gland contributes to long-term dysfunction, and therapies that dampen the inflammatory response may be beneficial.
Neuronal Changes
To increase the understanding of NRTN involvement in salivary gland dysfunction, researchers employed adenoviral gene transfer of human NRTN to salivary glands in mice 24 h prior to 5 × 6 Gy IR (Lombaert et al. 2020). Saliva flow rates significantly improved at day 90 and 120 post-IR in mice with NRTN transfer compared with radiation-only controls. mRNA expression of Nrtn increased significantly, and the expression of parasympathetic and sympathetic genes Vacht and Th was significantly restored with NRTN transfer. Another study reported that NRTN adenoviral transfer 24 h after IR preserved saliva flow and parasympathetic markers in mice (Ferreira et al. 2018).
In mice treated with 1 dose of a cevimeline-alginate hydrogel that acts as a muscarinic-cholinergic receptor agonist, researchers reported glandular repair at day 70 as measured by increased AQP5, pHH3+SOX2+, and pHH3+SOX2− cells and significant improvements in saliva flow at days 49 and 56 but not day 63 post-IR (Li et al. 2022). Further, they report increased nerve activity and regeneration of innervated acini as evidenced by a significant increase in GFRa2+ filaments (Li et al. 2022). Interestingly, mice treated weekly with this hydrogel had significantly improved saliva output at days 49, 63, 77, and 91 post-IR (Li et al. 2022). Another study used NRTN transfer via adeno-associated serotype 2 (AAV2) vector 10 d prior to 5 × 6 Gy radiation to further understand NRTN involvement in glandular damage (Lombaert et al. 2020). NRTN transfer administered 10 d prior to IR in mice improved salivary gland function at 90, 120, and 300 d in minipigs at 16 wk (Lombaert et al. 2020).
Stem Cell Therapy
Transplantation of salivary SPCs leads to restoration of glandular function in animal models; therefore, one research group posited that radiation techniques that spare compartments containing SPCs may preserve salivary gland function following IR (van Luijk et al. 2015). To test this clinically, HNC patients were treated with radiation using either a standard parotid-sparing or a stem cell–sparing technique (Steenbakkers et al. 2022). Interestingly, no significant differences were found in stimulated saliva production, but patients treated with stem cell–sparing IR reported lower xerostomia scores than those treated with standard parotid-sparing IR (Steenbakkers et al. 2022). A pilot study harvested bone marrow mesenchymal stem cells (BM-MSCs) from HNC patients with xerostomia and transplanted them into mice 1 d after 15 Gy IR (Blitzer et al. 2021). When compared to IR-only controls, mice receiving BM-MSCs experienced less salivary gland damage as measured by increased amylase and decreased fibrosis at 3 mo post-IR (Blitzer et al. 2021). Another study aimed to elucidate the role of aPKCζ in LRCs; Prkcz−/− mice were treated with IGF-1 at days 4 to 7 post-IR (Chibly et al. 2018). WT mice treated with IR+IGF-1 experienced a significant reduction in nuclear Yap in LRCs compared with IR-only WT mice, while the Prkcz−/− mice treated with IR+IGF-1 had no significant difference in nuclear Yap in LRCs when compared with IR-only controls (Chibly et al. 2018).
Adenoviral Gene Transfer
3re-IR treatment with NRTN AAV2 gene transfer reduced fibrosis as measured by Masson’s trichrome staining and reduced expression of genes related to stromal and ECM remodeling at day 300 post-IR when compared with IR mice receiving GFP gene transfer (Lombaert et al. 2020). Further, pre-IR NRTN gene transfer reduced the expression of inflammation and immune-related genes including Cma1, Pld4, and Lyz2. Further, restoration of proacinar gene expression SMGc was observed in mice treated with NRTN. In minipigs, NRTN gene transfer 1 wk prior to 15 Gy IR resulted in an increase in acinar markers AQP5 and AMY2 and a significant downregulation of immune-related genes LYZ, SERPING3, and MMP2 compared with IR-only contralateral PGs (Lombaert et al. 2020). In addition, saliva flow significantly improved at 16 wk post-IR from PGs receiving a lower dose of NRTN (Lombaert et al. 2020). In animal studies, Sonic Hedgehog (Shh) gene transfer yields promising results. Interestingly, one study reported that Shh gene transfer into mouse SMGs 3 d after 15 Gy IR significantly increased saliva flow and mRNA and protein levels of AQP5 at day 90 post-IR; lineage tracing revealed that Aif1/Iba1 macrophages, basal/myoepithelial, and endothelial cells were responsive to Shh gene transfer (Zhao et al. 2020). Further, this study revealed that F4/80+ macrophages are necessary for Shh-mediated restoration of function; depletion of macrophages via clodronate liposomes prior to Shh gene transfer resulted in no significant improvement in saliva flow at day 90 post IR.
Potential Therapies for Chronic Damage
A significant gap, particularly in mechanistic work and animal models, is the lack of treatments that can be administered in the weeks to months following radiotherapy. There is a substantial body of work outlining acute mechanisms and treatments given in the days prior to and in the week after radiation, yet there remains a critical need to understand long-term injury mechanisms and to develop appropriate therapeutics to repair chronically damaged glands. This is essential to improving quality of life in HNC survivors. Table 2 summarizes studies that administer treatments at chronic time points (>1 mo) following IR.
Table 2.
Summary of Therapies That Can Be Administered at Chronic Time Points (>1 mo) after IR.
| Species | Study | Intervention | Timing of Intervention | Clinically Relevant Outcome | Proposed Therapeutic Mechanism |
|---|---|---|---|---|---|
| Mouse | Teos et al. (2016) | hAQP1 AAV2 gene transfer | 2- or 8-mo post-IR | Improved salivary flow | Increased water permeability of acinar cells |
| Minipig | Hu et al. (2018) | Shh gene transfer | 4 wk after IR | Improved salivary flow | Improved blood supply, parasympathetic innervation, prevented fibrosis, attenuation of reduction in macrophages and increase in cytokines, improved VEGF |
| Human, phase I | Alevizos et al. (2017) | hAQP1 AAV2 gene transfer | At least 5 y after IR | Improved salivary flow, gene transfer well tolerated | N/A |
| Human, phase I | Lynggaard, Gronhoj, et al. (2022), Lynggaard, Jersie-Christensen, et al. (2022) | Adipose tissue–derived mesenchymal stem/stromal cells | At least 2 y after IR | Improved stimulated and unstimulated salivary flow | Significant alterations in salivary proteome |
AAV2, adeno-associated serotype 2; IR, ionizing radiation; N/A, not applicable; VEGF, vascular endothelial growth factor.
Adenoviral Gene Transfer
AAV2 gene transfer may be a promising option for chronic xerostomia and has shown efficacy in select animal and human trials. Several animal studies have reported significant improvements in saliva flow when human aquaporin-1 (hAQP1) AAV2 transfer was performed at least 3 mo following IR (D’Agostino et al. 2020). One study reported increased water permeability and improved saliva flow after hAQP1 transfer to murine SMGs at 2 or 8 mo post–15 Gy IR (Teos et al. 2016). In a phase I clinical trial, hAQP1 gene transfer was performed on human subjects at least 5 y after IR (Baum et al. 2012), which was well tolerated and significantly improved parotid saliva flow after 42 d. The subjects in this trial had increased saliva flow at their final study visit 3 to 4.7 y following hAQP1 transfer (Alevizos et al. 2017).
One study delivered Shh to minipig PGs at 4 wk post-IR and observed increased saliva output, improved blood supply, parasympathetic innervation, and reduced fibrogenesis in glandular tissue at 20 wk post-IR (Hu et al. 2018). This study also reported radiation-induced upregulation of proinflammatory cytokines that was reversed with Shh gene transfer. Finally, IR significantly reduced VEGF protein levels, an anti-senescence cytokine, and Shh gene transfer at 4 wk post-IR restored VEGF levels at 5 wk post-IR.
Stem Cell Therapy
MSCs are stem cells found in vascularized tissues and derived primarily from bone marrow or adipose tissue (Brown et al. 2019). Radiation causes dysfunction of SPC populations in salivary glands, and SPCs have been shown to be necessary for tissue regeneration (Aure et al. 2015; Chibly et al. 2018). Resident salivary gland SPCs can differentiate into acinar cells to compensate for acinar cell apoptosis following radiation (Ninche et al. 2020). Recently, animal and clinical studies have shown potential for MSCs to improve glandular function. The mechanism of action is not fully elucidated; in vivo studies depict paracrine effects, release of cytokines and growth factors, enhanced vascularization, and reduced inflammation as possible mechanisms in various tissues following radiation injury (Guan et al. 2023).
Preclinical studies show potential for stem cell therapy in treating xerostomia. To test this clinically, a phase I trial in HNC patients with evidence of salivary gland hypofunction transferred adipose tissue–derived mesenchymal stem/stromal cells (AT-MSCs) to submandibular and PGs at least 2 y following the end of radiation therapy (Lynggaard, Gronhoj, et al. 2022). This study reported intraglandular treatment with AT-MSC significantly increased unstimulated and stimulated saliva flow at 4 mo after treatment (Lynggaard, Gronhoj, et al. 2022). Further analysis of these data illustrates a significant effect on the salivary proteome (Lynggaard, Jersie-Christensen, et al. 2022). At 120 d following AT-MSC treatment, 99 proteins in saliva were significantly different when compared with baseline values. It is important to note that while improvements were observed, AT-MSC did not restore healthy conditions, as 212 proteins in saliva from the AT-MSC group remain significantly different from healthy controls (Lynggaard, Jersie-Christensen, et al. 2022). The proteins most downregulated after AT-MSC versus baseline were the high mobility group protein B2, hornerin, and tight junction protein ZO-1, and the most upregulated included 60 S ribosomal protein L19, endophilin-B2, and statherin. Currently, there is 1 ongoing clinical trial (NCT06012604) transplanting umbilical cord MSCs into parotid and SMGs of HNC patients 2 y after IR (Strojan et al. 2023). This phase I study will assess toxicity and efficacy via saliva flow. In addition, there is another ongoing phase I clinical trial (NCT04489732) transplanting IFN-γ–stimulated BM-MSCs into SMGs 2 y after IR in HNC survivors (Blitzer et al. 2023).
Summary and Future Directions
Salivary glands are highly radiosensitive, and many HNC patients and survivors experience chronic xerostomia, which affects quality of life. The chronic response to radiation is not fully understood, but current data illustrate increased inflammation, senescence, loss of stem and progenitor cells, fibrosis, and decreased parasympathetic innervation as contributing phenotypes. Further, therapies that can influence the chronic response as well as be administered at chronic time points following radiation therapy are limited. Future research should aim to identify precise mechanisms that prevent salivary glands from regenerating and how these mechanisms perpetuate long-term loss of function. Further, developing therapies that restore salivary gland function when administered months to years after radiation therapy will improve quality of life for HNC survivors living with xerostomia.
Author Contributions
J. Gunning, contributed to design, data analysis and interpretation, drafted the manuscript; K.H. Limesand, contributed to conception, design, data analysis and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The salary support for authors was funded by National Institute of Dental and Craniofacial Research NIDCR R01DE029166 to K.H.L. The funders had no role in preparation or decision to publish the manuscript.
ORCID iD: K.H. Limesand  https://orcid.org/0000-0003-4295-4665
https://orcid.org/0000-0003-4295-4665
References
- Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, Billings ME, Goldsmith CM, Tandon M, Helmerhorst EJ, Catalan MA, et al. 2017. Late responses to adenoviral-mediated transfer of the aquaporin-1 gene for radiation-induced salivary hypofunction. Gene Ther. 24(3):176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aure MH, Konieczny SF, Ovitt CE. 2015. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev Cell. 33(2):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsouk A, Aluru JS, Rawla P, Saginala K, Barsouk A. 2023. Epidemiology, risk factors, and prevention of head and neck squamous cell carcinoma. Med Sci (Basel). 11(2):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, Goldsmith CM, Burbelo PD, Citrin DE, Mitchell JB, et al. 2012. Early responses to adenoviral-mediated transfer of the aquaporin-1 cdna for radiation-induced salivary hypofunction. Proc Natl Acad Sci U S A. 109(47):19403–19407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer GC, Glazer T, Burr A, Gustafson S, Ganz O, Meyers R, McDowell KA, Nickel KP, Mattison RJ, Weiss M, et al. 2023. Marrow-derived autologous stromal cells for the restoration of salivary hypofunction (marsh): a pilot, first-in-human study of interferon gamma-stimulated marrow mesenchymal stromal cells for treatment of radiation-induced xerostomia. Cytotherapy. 25(11):1139–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer GC, Paz C, Glassey A, Ganz O, Giri J, Pennati A, Meyers R, Lunga T, Robbins D, Thibeault S, et al. 2021. A pilot study to assess the salivary gland regenerative potential of bone marrow mesenchymal stromal cells from treated head and neck cancer patients. Res Sq. [epub ahead of print 18 Oct 2021] in press. doi: 10.21203/rs.3.rs-965122/v1 [DOI] [Google Scholar]
- Bockel S, Vallard A, Levy A, Francois S, Bourdis M, Le Gallic C, Riccobono D, Annede P, Drouet M, Tao Y, et al. 2018. Pharmacological modulation of radiation-induced oral mucosal complications. Cancer Radiother. 22(5):429–437. [DOI] [PubMed] [Google Scholar]
- Brook I. 2020. Late side effects of radiation treatment for head and neck cancer. Radiat Oncol J. 38(2):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR. 2019. Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med. 13(9):1738–1755. [DOI] [PubMed] [Google Scholar]
- Buss LG, De Oliveira Pessoa D, Snider JM, Padi M, Martinez JA, Limesand KH. 2023. Metabolomics analysis of pathways underlying radiation-induced salivary gland dysfunction stages. PLoS One. 18(11):e0294355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss LG, Rheinheimer BA, Limesand KH. 2024. Radiation-induced changes in energy metabolism result in mitochondrial dysfunction in salivary glands. Sci Rep. 14(1):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibly AM, Nguyen T, Limesand KH. 2014. Palliative care for salivary gland dysfunction highlights the need for regenerative therapies: a review on radiation and salivary gland stem cells. J Palliat Care Med. 4(4):1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibly AM, Patel VN, Aure MH, Pasquale MC, Genomics NN, Computational Biology C, Martin GE, Ghannam M, Andrade J, Denegre NG, et al. 2023. Neurotrophin signaling is a central mechanism of salivary dysfunction after irradiation that disrupts myoepithelial cells. NPJ Regen Med. 8(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibly AM, Wong WY, Pier M, Cheng H, Mu Y, Chen J, Ghosh S, Limesand KH. 2018. aPKCzeta-dependent repression of yap is necessary for functional restoration of irradiated salivary glands with IGF-1. Sci Rep. 8(1):6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino C, Elkashty OA, Chivasso C, Perret J, Tran SD, Delporte C. 2020. Insight into salivary gland aquaporins. Cells. 9(6):1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirix P, Nuyts S, Van den Bogaert W. 2006. Radiation-induced xerostomia in patients with head and neck cancer: a literature review. Cancer. 107(11):2525–2534. [DOI] [PubMed] [Google Scholar]
- Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. 1999. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 45(3):577–587. [DOI] [PubMed] [Google Scholar]
- Emmerson E, May AJ, Berthoin L, Cruz-Pacheco N, Nathan S, Mattingly AJ, Chang JL, Ryan WR, Tward AD, Knox SM. 2018. Salivary glands regenerate after radiation injury through SOX2-mediated secretory cell replacement. EMBO Mol Med. 10(3):e8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JNA, Zheng C, Lombaert IMA, Goldsmith CM, Cotrim AP, Symonds JM, Patel VN, Hoffman MP. 2018. Neurturin gene therapy protects parasympathetic function to prevent irradiation-induced murine salivary gland hypofunction. Mol Ther Methods Clin Dev. 9:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman K, Camden JM, Woods L, Weisman G, Limesand K. 2021. Indomethacin treatment post-irradiation improves mouse parotid salivary gland function via modulation of prostaglandin e2 signaling. Front Bioeng Biotechnol. 9:697671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman KE, Camden JM, Klein RR, Zhang Q, Weisman GA, Limesand KH. 2019. P2x7 receptor deletion suppresses gamma-radiation-induced hyposalivation. Am J Physiol Regul Integr Comp Physiol. 316(5):R687–R696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann O, Fillinger JL, Victory KR, Burd R, Limesand KH. 2010. Restoration of radiation therapy-induced salivary gland dysfunction in mice by post therapy IGF-1 administration. BMC Cancer. 10:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann O, Mitchell GC, Limesand KH. 2009. Sensitivity of salivary glands to radiation: from animal models to therapies. J Dent Res. 88(10):894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Zhang J, Jiang N, Tian M, Wang H, Liang B. 2023. Efficacy of mesenchymal stem cell therapy in rodent models of radiation-induced xerostomia and oral mucositis: a systematic review. Stem Cell Res Ther. 14(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning JA, Gilman KE, Zuniga TM, Simpson RJ, Limesand KH. 2024. Parotid glands have a dysregulated immune response following radiation therapy. PLoS One. 19(3):e0297387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai B, Zhao Q, Deveau MA, Liu F. 2018. Delivery of sonic hedgehog gene repressed irradiation-induced cellular senescence in salivary glands by promoting DNA repair and reducing oxidative stress. Theranostics. 8(4):1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill G, Headon D, Harris ZI, Huttner K, Limesand KH. 2014. Pharmacological activation of the EDA/EDAR signaling pathway restores salivary gland function following radiation-induced damage. PLoS One. 9(11):e112840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Du C, Yang Z, Yang Y, Zhu Z, Shan Z, Zhang C, Wang S, Liu F. 2021. Transient activation of hedgehog signaling inhibits cellular senescence and inflammation in radiated swine salivary glands through preserving resident macrophages. Int J Mol Sci. 22(24):13493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Zhu Z, Hai B, Chang S, Ma L, Xu Y, Li X, Feng X, Wu X, Zhao Q, et al. 2018. Intragland Shh gene delivery mitigated irradiation-induced hyposalivation in a miniature pig model. Theranostics. 8(16):4321–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmer KJ, Gilman KE, Munoz Forti K, Weisman GA, Limesand KH. 2020. Radiation-induced salivary gland dysfunction: mechanisms, therapeutics and future directions. J Clin Med. 9(12):4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SB, Vissink A, Limesand KH, Reyland ME. 2019. Salivary gland hypofunction and xerostomia in head and neck radiation patients. J Natl Cancer Inst Monogr. 2019(53):lgz016. [DOI] [PubMed] [Google Scholar]
- Kaidar-Person O, Gil Z, Billan S. 2018. Precision medicine in head and neck cancer. Drug Resist Updat. 40:13–16. [DOI] [PubMed] [Google Scholar]
- Kim JH, Brown SL, Gordon MN. 2023. Radiation-induced senescence: therapeutic opportunities. Radiat Oncol. 18(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Sudiwala S, Berthoin L, Mohabbat S, Gaylord EA, Sinada H, Cruz Pacheco N, Chang JC, Jeon O, Lombaert IMA, et al. 2022. Long-term functional regeneration of radiation-damaged salivary glands through delivery of a neurogenic hydrogel. Sci Adv. 8(51):eadc8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IMA, Patel VN, Jones CE, Villier DC, Canada AE, Moore MR, Berenstein E, Zheng C, Goldsmith CM, Chorini JA, et al. 2020. CERE-120 prevents irradiation-induced hypofunction and restores immune homeostasis in porcine salivary glands. Mol Ther Methods Clin Dev. 18:839–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luitje ME, Israel AK, Cummings MA, Giampoli EJ, Allen PD, Newlands SD, Ovitt CE. 2021. Long-term maintenance of acinar cells in human submandibular glands after radiation therapy. Int J Radiat Oncol Biol Phys. 109(4):1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynggaard CD, Gronhoj C, Christensen R, Fischer-Nielsen A, Melchiors J, Specht L, Andersen E, Mortensen J, Oturai P, Barfod GH, et al. 2022. Intraglandular off-the-shelf allogeneic mesenchymal stem cell treatment in patients with radiation-induced xerostomia: a safety study (MESRIX-II). Stem Cells Transl Med. 11(5):478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynggaard CD, Jersie-Christensen R, Juhl M, Jensen SB, Gronhoj C, Melchiors J, Jacobsen S, Moller-Hansen M, Herly M, Ekblond A, et al. 2022. Intraglandular mesenchymal stem cell treatment induces changes in the salivary proteome of irradiated patients. Commun Med (Lond). 2(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmary Y, Adar R, Gaska S, Wygoda A, Maly A, Cohen J, Eliashar R, Mizrachi L, Orfaig-Geva C, Baum BJ, et al. 2016. Radiation-induced loss of salivary gland function is driven by cellular senescence and prevented by IL6 modulation. Cancer Res. 76(5):1170–1180. [DOI] [PubMed] [Google Scholar]
- Meeks L, De Oliveira Pessoa D, Martinez JA, Limesand KH, Padi M. 2021. Integration of metabolomics and transcriptomics reveals convergent pathways driving radiation-induced salivary gland dysfunction. Physiol Genomics. 53(3):85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RK, Gilman KE, Rheinheimer BA, Meeks L, Limesand KH. 2023. AMPK activation restores salivary function following radiation treatment. J Dent Res. 102(5):546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Bathke M, Hill GA, Harris ZI, Lin HH, Chibly AM, Klein RR, Burd R, Ann DK, Limesand KH. 2014. Autophagy correlates with maintenance of salivary gland function following radiation. Sci Rep. 4:5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninche N, Kwak M, Ghazizadeh S. 2020. Diverse epithelial cell populations contribute to the regeneration of secretory units in injured salivary glands. Development. 147(19):dev192807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Wu Y, Brouwer U, van Vliet T, Wang B, Demaria M, Barazzuol L, Coppes RP. 2020. Cellular senescence contributes to radiation-induced hyposalivation by affecting the stem/progenitor cell niche. Cell Death Dis. 11(10):854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna R, Campus G, Cumbo E, Mura I, Milia E. 2015. Xerostomia induced by radiotherapy: an overview of the physiopathology, clinical evidence, and management of the oral damage. Ther Clin Risk Manag. 11:171–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rades D, Warwas B, Gerull K, Pries R, Leichtle A, Bruchhage KL, Hakim SG, Schild SE, Cremers F. 2022. Prognostic factors for complete recovery from xerostomia after radiotherapy of head-and-neck cancers. In Vivo. 36(4):1795–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinheimer BA, Pasquale MC, Limesand K, Hoffman MP, Chibly AM. 2023. Evaluating the transcriptional landscape and cell-cell communication networks in chronically irradiated parotid glands. iScience. 26(5):106660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisto M, Lorusso L, Ingravallo G, Tamma R, Ribatti D, Lisi S. 2018. The tGF-beta1 signaling pathway as an attractive target in the fibrosis pathogenesis of Sjogren’s syndrome. Mediators Inflamm. 2018:1965935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbakkers R, van Rijn-Dekker MI, Stokman MA, Kierkels RGJ, van der Schaaf A, van den Hoek JGM, Bijl HP, Kramer MCA, Coppes RP, Langendijk JA, et al. 2022. Parotid gland stem cell sparing radiation therapy for patients with head and neck cancer: a double-blind randomized controlled trial. Int J Radiat Oncol Biol Phys. 112(2):306–316. [DOI] [PubMed] [Google Scholar]
- Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. 2015. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 141(11):1985–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strojan P, Plavc G, Kokalj M, Mitrovic G, Blatnik O, Lezaic L, Socan A, Bavec A, Tesic N, Hartman K, et al. 2023. Post-radiation xerostomia therapy with allogeneic mesenchymal stromal stem cells in patients with head and neck cancer: study protocol for phase I clinical trial. Radiol Oncol. 57(4):538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teos LY, Zheng CY, Liu X, Swaim WD, Goldsmith CM, Cotrim AP, Baum BJ, Ambudkar IS. 2016. Adenovirus-mediated hAQP1 expression in irradiated mouse salivary glands causes recovery of saliva secretion by enhancing acinar cell volume decrease. Gene Ther. 23(7):572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Luijk P, Pringle S, Deasy JO, Moiseenko VV, Faber H, Hovan A, Baanstra M, van der Laan HP, Kierkels RG, van der Schaaf A, et al. 2015. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci Transl Med. 7(305):305ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C, Keimel R, Gugatschka M, Kolb D, Leitinger G, Roblegg E. 2021. Investigation of changes in saliva in radiotherapy-induced head neck cancer patients. Int J Environ Res Public Health. 18(4):1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WY, Allie S, Limesand KH. 2019. PKCzeta and JNK signaling regulate radiation-induced compensatory proliferation in parotid salivary glands. PLoS One. 14(7):e0219572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods LT, Camden JM, El-Sayed FG, Khalafalla MG, Petris MJ, Erb L, Weisman GA. 2015. Increased expression of TGF-beta signaling components in a mouse model of fibrosis induced by submandibular gland duct ligation. PLoS One. 10(5):e0123641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yun JS, Han D, Yook JI, Kim HS, Cho ES. 2020. TGF-beta pathway in salivary gland fibrosis. Int J Mol Sci. 21(23):9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Zhang L, Hai B, Wang J, Baetge CL, Deveau MA, Kapler GM, Feng JQ, Liu F. 2020. Transient activation of the hedgehog-gli pathway rescues radiotherapy-induced dry mouth via recovering salivary gland resident macrophages. Cancer Res. 80(24):5531–5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlygosteva O, Juvkam IS, Aass HCD, Galtung HK, Soland TM, Malinen E, Edin NFJ. 2023. Cytokine levels in saliva are associated with salivary gland fibrosis and hyposalivation in mice after fractionated radiotherapy of the head and neck. Int J Mol Sci. 24(20):15218. [DOI] [PMC free article] [PubMed] [Google Scholar]