Abstract
Infection of cells by herpesviruses is initiated by the interaction of viral envelope glycoproteins with cellular receptors. In the alphaherpesvirus pseudorabies virus (PrV), the causative agent of Aujeszky's disease in pigs, the essential glycoprotein D (gD) mediates secondary attachment of virions to target cells by binding to newly identified cellular receptors (R. J. Geraghty, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear, Science 280:1618–1620, 1998). However, in the presence of compensatory mutations, infection can also occur in the absence of gD, as evidenced by the isolation in cell culture of an infectious gD-negative PrV mutant (PrV-gD− Pass) (J. Schmidt, B. G. Klupp, A. Karger, and T. C. Mettenleiter, J. Virol. 71:17–24, 1997). PrV-gD− Pass is replication competent with an only moderate reduction in specific infectivity but appears to bind to receptors different from those recognized by wild-type PrV (A. Karger, J. Schmidt, and T. C. Mettenleiter, J. Virol. 72:7341–7348, 1998). To analyze whether this alteration in receptor usage in vitro influences infection in vivo, the model host mouse and the natural host pig were intranasally infected with PrV-gD− Pass and were compared to animals infected by wild-type PrV. For mice, a comparable progress of disease was observed, and all animals infected with mutant virus died, although they exhibited a slight delay in the onset of symptoms and, correspondingly, a longer time to death. In contrast, whereas wild-type PrV-infected pigs showed clinical signs and histological and histopathological findings typical of PrV infection, no signs of disease were observed after infection with PrV-gD− Pass. Moreover, in these animals, virus-infected cells were not detectable by immunohistochemical staining of different organ samples and no virus could be isolated from nasal swabs. Mutations in glycoproteins B and H were found to correlate with, and probably contribute to, gD-independent infectivity. In conclusion, although PrV-gD− Pass is virulent in mice, it is apparently unable to infect the natural host, the pig. This altered host range in vivo correlates with a difference of receptor usage in vitro and demonstrates for the first time the importance of gD receptors in alphaherpesvirus infection of an animal host.
The alphaherpesvirus pseudorabies virus (PrV) is the causative agent of Aujeszky's disease (AD) in pigs, a serious illness which is characterized by respiratory symptoms and central nervous disorders. After oronasal uptake of the virus, primary replication occurs in the nasal and pharyngeal mucosae and the respiratory tract, leading to respiratory distress. Subsequently, after entering peripheral nerve endings, the virus ascends to the central nervous system (CNS) via trigeminal and olfactory pathways (1, 20). Replication of PrV in the CNS is characterized by a nonsuppurative meningoencephalits causing severe central nervous disorders (8, 34). PrV displays a very broad host range and is able to infect most mammals except for horses and higher primates, including humans. In most susceptible species, PrV infection is fatal, and only pigs are able to survive a productive PrV infection. They are therefore regarded as the natural host. Surviving pigs remain latently infected and productive viral replication can be reactivated in response to various stimuli (reviewed in reference 27).
The pathogenicity of PrV depends on the age of the pig, the route of infection, and the specific virulence of the infecting strain which is, at least in part, determined by viral glycoproteins (reviewed in reference 27). Up to now, 11 PrV glycoproteins have been identified. According to their relevance for viral replication in cell culture, they have been designated nonessential (gC, gE, gG, gI, gM, and gN) and essential (gB, gD, gH, gK, and gL). Glycoproteins that mediate attachment of PrV to target cells are of special interest because they may directly determine viral tropism (reviewed in reference 27). Primary attachment of PrV to target cells is mediated by binding of the nonessential gC to heparan sulfate proteoglycans (16, 24). However, this interaction is not sufficient to trigger fusion between the viral envelope and the cell membrane. As has been shown for other alphaherpesviruses (reviewed in reference 43), the essential gD mediates secondary attachment of PrV (16) to newly identified cellular gD receptors, which belong to a superfamily of immunoglobulin-like poliovirus receptor-related proteins (10, 21, 44). This gD-gD receptor interaction is thought to be necessary to initiate penetration. The wide distribution of gD receptors on different cell types and the promiscuity in receptor usage might explain at least in part the broad host range of PrV. Besides gD, gB and the gH-gL complex are required for penetration of free virions into target cells (reviewed in references 27 and 42). However, in contrast to the situations in herpes simplex virus type 1 (HSV-1) and bovine herpesvirus type 1 (BHV-1), PrV gD is dispensable for direct viral cell-to-cell spread in vitro (9, 23, 32, 37). Thus, phenotypically complemented gD-negative PrV (PrV-gD−) is able to infect primary target cells and subsequently spreads via direct cell-to-cell transmission. After intranasal infection of mice, phenotypically complemented PrV-gD− infects epithelial cells of the nasal mucosa and then invades neighboring epithelial cells and innervating peripheral sensory neurons. It then ascends to the CNS via gD-independent cell-cell transmission (1, 28). Whereas virulence of PrV-gD− is not reduced in mice (1, 13), only moderate symptoms were observed after infection of pigs with PrV-gD− (13). Since gD is essential for the entry of free wild-type PrV virions, no infectious virus was recovered from infected animals of either species (13).
Since gD is dispensable for direct cell-to-cell spread of PrV, it is possible to propagate PrV-gD− by cocultivation of infected and noninfected cells. Recently, we described the isolation of an infectious gD-negative PrV mutant (PrV-gD− Pass) after serial passaging of PrV-gD−-infected cells with noninfected cells (39). PrV-gD− Pass is replication competent with an only moderate (ca. 70-fold) reduction in specific infectivity in cell culture (17). A similar mutant has also been isolated from BHV-1 (40). This phenotype is at least partly due to compensatory mutations in gB and gH (references 40 and 41 and this paper). Infection of gD receptor-deficient Chinese hamster ovary (CHO) cells clearly demonstrated that infectivity of PrV-gD− Pass is not dependent on known gD receptors, indicating that the compensatory mutations did not induce binding of other viral proteins to gD receptors (29). This indicated that gD-independent infectivity of PrV requires hitherto unknown receptors (17). The isolation and characterization of a mutant bovine kidney cell clone (NB) which is specifically resistant to infection by PrV-gD− Pass indicated the presence of at least one alternate cellular receptor which is lacking on NB cells and is not critical for infectivity of wild-type PrV (17). Interestingly, the penetration defect of PrV-gD− Pass on NB cells could be overcome by phenotypical gD transcomplementation, indicating that this alternative receptor is used only in the absence of gD.
Since PrV offers the opportunity to analyze phenotypes not only in cell culture and animal models but also in the natural host, we were interested in testing whether the alteration in receptor usage in PrV-gD− Pass as observed in cell culture influences viral replication in animals. This should also shed light on the importance of gD receptors for alphaherpesvirus infection in vivo. To this end, mice and pigs were intranasally infected with PrV-gD− Pass and different parameters were monitored.
MATERIALS AND METHODS
Viruses and cells.
All mutants described in this study were derived from the moderately virulent PrV strain Kaplan (PrV-Ka; 15). Isolation of gG- and gD-negative infectious PrV-gD− Pass has been described (39). PrV-1112, which expresses the Escherichia coli lacZ gene from the gG locus and behaves like wild-type PrV in a multitude of tests, has also been described (25). For challenge infections, the highly virulent PrV strain NIA-3 (5) was used. Viruses were grown and titrated on porcine kidney cells (PSEK or PK-15) (39).
Isolation of complementing cell lines and viral mutants.
RK13 cell lines stably expressing wild-type or PrV-gD− Pass gB or wild-type gB and gD were isolated after PCR amplification of the corresponding open reading frames and insertion into pcDNA3 (30). RK13 cell lines expressing wild-type or PrV-gD− Pass gH under its own promoter were constructed as previously described (3). Vero cells expressing wild-type gH or the gDH hybrid protein have been published (3, 19). Construction of mutant viruses lacking gB or gH followed standard procedures (3, 12, 37). Insertion of a green fluorescent protein expression cassette facilitated the isolation of virus mutants. Mutant PrV-gD−gH− was isolated on RK13 cells expressing wild-type gD under its own promoter and gH under control of the human cytomegalovirus immediate-early promoter/enhancer. gD expression in PrV-gD− Pass was rescued after cotransfection with a genomic SphI fragment derived from the US-region of PrV-Ka encompassing the gD gene. Resulting virus progeny were titrated on Vero cells, and gD-expressing virus progeny were identified by black plaque assay (Vectastain, Camon, Germany) by using gD-specific monoclonal antibody (MAb) c14-c27 (19). One single plaque isolate, PrV-gD− Pass SPH, was used for further studies. All mutant viruses were analyzed by Southern blotting, indirect immunofluorescence, and Western blot analysis to confirm geno- and phenotypes (data not shown).
Experiments with mice.
Six-week-old female BALB/c mice were housed in isolation in plastic cages with sterile wood chips. Water and pelleted commercial food were given ad libitum. Animals were infected intranasally with 10 μl of virus suspension containing 104 PFU of PrV-Ka or PrV-gD− Pass. Animals were observed for clinical signs, and moribund animals were sacrificed. For histological analysis, animals were processed as previously described (2).
Experiments with pigs.
For all experiments, 5-week-old piglets seronegative for PrV were obtained from local farms, housed in isolation, and supplied with water and commercial food ad libitum. Animals were allowed to adjust to their housing conditions for 7 days. In the first experiment, animals were intranasally infected with 106 PFU of PrV-Ka (four animals), PrV-gD− Pass (six animals), or PrV-gD− Pass SPH (six animals) to compare virulence. Animals were observed twice a day for clinical signs, and rectal body temperature was measured daily. Virus shedding was also determined daily as described below. On day 37 postinfection (p.i.), sera of all animals were examined for the presence of complement-dependent PrV-neutralizing antibodies. The induction of a protective immunity was tested after intranasal challenge infection of surviving animals with 107 PFU of the highly virulent PrV strain NIA-3 (5). Three age-matched naive piglets were infected for control. Animals were again monitored for clinical signs, body temperature, virus excretion, and, in addition, for change in body weight.
A second experiment was performed to analyze in detail the replication of PrV in the infected animal. Six animals each were intranasally infected with 5 × 107 PFU of PrV-gD− Pass or PrV-Ka and were observed daily for clinical signs and virus shedding. Every second day p.i., one animal from each group was sacrificed and organ samples were prepared for virus isolation and immunofluorescence on frozen sections. An untreated age-matched piglet was included as control.
Determination of virus shedding.
Virus excretion was determined by titration of nasal swabs. Swabs were incubated overnight at 4°C in 1 ml of minimum essential medium supplemented with 5% fetal bovine serum (MEM–5% FBS) and were titrated on PSEK cells.
Virus neutralization.
Fifty-microliter volumes of serial twofold dilutions of sera (1:2 to 1:4,096 in MEM–5% FBS) were mixed with 50 μl of virus suspension at a titer of 4 × 103 PFU/ml. After preincubation in 96-well cell culture dishes at 37°C for 16 to 20 h, 50 μl of a PSEK cell suspension containing 2 × 105 cells per ml was added to each well, and the mixture was incubated for an additional 4 to 5 days at 37°C. Titers are given as the highest serum dilution resulting in complete virus neutralization as evidenced by inhibition of virus-specific cytopathic effect (CPE).
Tissue sampling, virus isolation, and indirect immunofluorescence.
Animals were stunned electrically and rendered unconscious and then were sacrificed and necropsied. Tissue samples from the nasal mucosa, olfactory nerve and bulb, pedunculus olfactorius, trigeminal nerve and ganglion, medulla oblongata, pons, mesencephalon, diencephalon, cerebrum, cerebellum, tonsil, and lung were prepared for virus isolation and indirect immunofluorescence. To determine virus titers, organ samples were weighed and incubated in MEM–5% FBS. Samples were homogenized in a mortar, and the final volume of tissue suspension as well as the virus titer were determined. Titers were then calculated as PFU per gram of tissue.
PrV-infected cells in organ samples were identified by indirect immunofluorescence on frozen sections using a mixture of a gB-specific MAb (A20-c26 [30, 31]) and a gC-specific MAb (B16-c8 [19]). Reactivities of wild-type and mutant virus-infected cells with the MAbs had previously been tested by indirect immunofluorescence. Tissue samples were snap frozen in n-heptane (−70°C), and cryosections were prepared and fixed with acetone at −20°C for 15 min. After equilibration to room temperature and preincubation in phosphate-buffered saline (PBS) containing 5% bovine serum albumin (BSA) for 10 min, sections were incubated with MAbs diluted in PBS–2% BSA. Subsequently, they were incubated with fluorescein isothiocyanate-labeled secondary goat F(ab)2 anti-mouse immunoglobulin G plus M (heavy and light chains) (Caltag Laboratories, Medac, Germany) diluted in PBS–2% BSA and 0.005% Evans blue. Parallel sections were incubated with a control MAb (934-H1) directed against the S protein of mouse hepatitis coronavirus (kindly provided by H. Wege, Insel Riems, Germany). Tissue from noninfected control animals was treated identically. Finally, sections were sealed with fluorescence maintenance buffer containing 2.5% DABCO (Sigma, Deisenhofen, Germany) diluted in 90% glycerol–10% PBS (pH 8.6). Fluorescence analysis and microphotography were performed in an Optiphot 2 fluorescence microscope (Nikon, Tokyo, Japan).
Virus stability in porcine nasal mucus.
Fresh nasal mucus of noninfected piglets was collected, and 100 μl of undiluted virus suspension was preincubated with 100 μl of undiluted mucus or 100 μl of medium as control for 1 h at 37°C. Assays were then titrated on PSEK cells.
Virus titration on porcine nasal mucosa explant cells.
Serial 10-fold dilutions of PrV-1112, PrV-gD− Pass, and PrV-gD− Pass SPH were plated in 24-well tissue culture dishes onto PK-15 and nasal mucosal explant cells. Two days after infection, monolayers were either stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (for PrV-1112 and PrV-gD− Pass) or crystal violet (for PrV-gD− Pass SPH) and plaques were counted.
RESULTS
PrV gD− Pass infection is fatal for mice.
To assay for virulence in mice, two 6-week-old BALB/c mice were infected intranasally with 104 PFU of PrV-Ka and six mice were infected with PrV-gD− Pass. Both groups were monitored daily for clinical signs. A typical progression of disease was observed for the PrV-Ka-infected animals (2). At 48 h p.i., loss of appetite and altered behavior like scratching of the inoculation site and hyperactivity started to appear. At 60 h p.i., severe neurological symptoms such as loss of coordination and convulsions followed by somnolence arose, indicating viral replication in the CNS. Both PrV-Ka-infected mice were dead or sacrificed in a moribund state by 72 h p.i. Mice infected with PrV-gD− Pass showed identical clinical signs invariably leading to death. However, there was a delay of 24 to 48 h in symptom development and death compared to PrV-Ka-infected mice. Immunohistological analysis showed infection of central nervous tissues in PrV-Ka and PrV-gD− Pass-infected animals at the time of death (data not shown).
Determination of virulence in pigs.
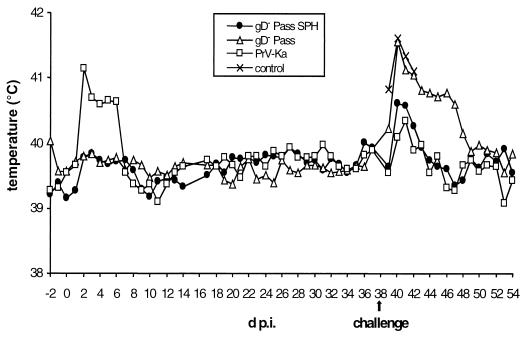
To compare virulence of PrV-gD− Pass and revertant PrV-gD− Pass SPH to PrV-Ka in the natural host, three groups of 6-week-old piglets were infected intranasally with 106 PFU of PrV-Ka (four animals), PrV-gD− Pass (six animals), and PrV-gD− Pass SPH (six animals) and were monitored for clinical signs and virus shedding. The body temperature of PrV-Ka-infected animals (Fig. 1) rose sharply at 2 days p.i. to ca. 41.5°C. At the same time, respiratory symptoms and reduction in food uptake were observed. Subsequently, the overall condition of the animals worsened. On day 4 p.i., all animals in this group displayed severe respiratory symptoms, i.e., rhinitis with purulent discharge. On day 6 p.i., two piglets showed severe CNS symptoms, including ataxia, opisthotonus, convulsions, and paralysis. One of them died on day 7 p.i. Surviving animals recovered and appeared normal on day 10 p.i. After infection with PrV-gD− Pass or PrV-gD− Pass SPH, infected animals did not show any significant rise in body temperature nor any clinical symptoms.
FIG. 1.
Body temperature after infection of pigs with PrV-Ka, PrV-gD− Pass, or PrV-gD− Pass SPH. On day 38 p.i., challenge infection with 5 × 107 PFU of PrV NIA-3 was performed. At this time point, three age-matched, naive piglets were included as controls. Mean values are shown for each group.
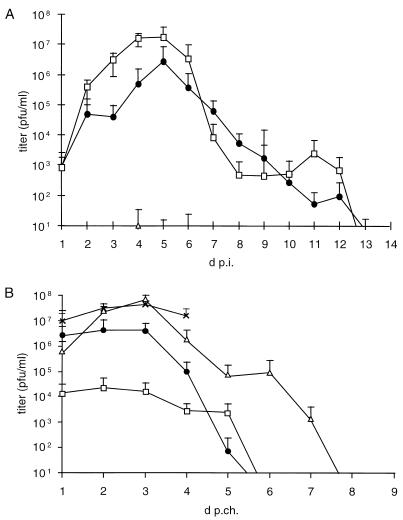
Virus shedding was quantified by titration of nasal swabs. As shown in Fig. 2A, PrV-Ka-infected animals showed the characteristic course of virus excretion, with peak titers exceeding 107 PFU/ml on day 5 p.i. Virus shedding ended on day 13 p.i. In contrast, with nasal swabs of animals infected with PrV-gD− Pass, virus was detected only occasionally in single animals at very low levels (<10 PFU/ml), which might reflect the persistence of inoculum rather than productive virus replication.
FIG. 2.
(A) Virus shedding from pigs intranasally infected with PrV-Ka, PrV-gD− Pass, or PrV gD− Pass SPH was analyzed by titration of nasal swabs during primary infection. On day 38 p.i., surviving animals and three untreated, age-matched piglets (control) were intranasally infected with 5 × 107 PFU of PrV NIA-3. (B) Virus shedding was again quantified by titration of nasal swabs. Average titers and standard deviation (error bars) are shown. □, WT; ●, gD− Pass SPH; ▵, gD− Pass; ×, control.
Interestingly, although PrV-gD− Pass SPH-infected animals did not show clinical symptoms, they excreted virus, indicative of efficient virus replication in the nasal mucosa. Compared to PrV-Ka, ca. 10-fold-lower peak titers were reached on day 5 p.i. Virus shedding also ended on day 13 p.i.
Neutralizing antibody titers.
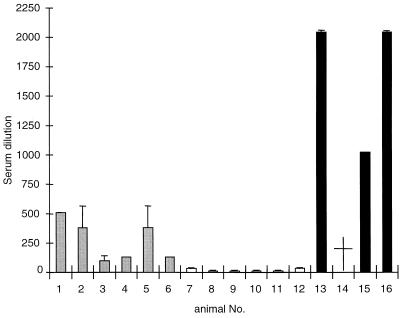
To examine if intranasal inoculation of pigs with PrV-gD− Pass resulted in an inapparent infection but induced seroconversion, titers of complement-dependent PrV-neutralizing antibodies were determined on day 37 p.i. As shown in Fig. 3, animals that had recovered from infection with PrV-Ka (animals 13 to 16) exhibited high titers of neutralizing antibodies ranging from 1:1,024 to 1:2,048. In contrast, sera from PrV-gD− Pass-infected animals (animals 7 to 12) did not contain PrV-specific neutralizing antibodies since the observed titers at between 1:2 and 1:16 were similar to those in sera from naive control animals (data not shown). Sera from PrV-gD− Pass SPH infected animals (animals 1 to 6) exhibited neutralization titers of between 1:96 and 1:512, indicating that an antibody response was induced which, however, was less pronounced than that after PrV-Ka infection.
FIG. 3.
Titers of neutralizing antibodies in sera of infected animals. On day 37 p.i., sera from PrV-Ka-infected (animals 13 to 16), PrV-gD− Pass-infected (animals 7 to 12) and PrV-gD− Pass SPH-infected (animals 1 to 6) animals were analyzed for the presence of complement-dependent neutralizing antibodies. Shown are results from two independent experiments; error bars indicate standard deviation. PrV-Ka-infected animal no. 14 (†) died during primary infection.
On day 38 p.i., surviving animals and three naive control animals were intranasally challenged with 5 × 107 PFU of the highly virulent PrV strain NIA-3. Body temperature (Fig. 1), clinical signs (Table 1, challenge infections), and virus shedding (Fig. 2B) were again monitored. In addition, body weight was determined every second day post-challenge infection (p.c.) and recorded as the change in weight compared to body weight immediately before challenge infection (Fig. 4).
TABLE 1.
Summary of clinical symptoms in pigs
| Virus | Presence of:
|
Incidence of death | ||
|---|---|---|---|---|
| Fever | Respiratory signs | Neurological signs | ||
| Primary infectiona | ||||
| PrV-Ka | +++ | +++ | ++ | 1/4 |
| PrV-gD− Pass SPH | − | − | − | 0/6 |
| PrV-gD− Pass | − | − | − | 0/6 |
| Challenge infectionb | ||||
| PrV-Ka | − | − | − | 0/3 |
| PrV-gD− Pass SPH | − | − | − | 0/6 |
| PrV-gD− Pass | +++ | +++ | ++ | 2/6 |
| Control | +++ | +++ | +++ | 3/3 |
Three groups of 6-week-old piglets were infected intranasally with 106 PFU of PrV-Ka (four animals), PrV-gD− Pass (six animals), or PrV-gD− Pass SPH (six animals). Clinical signs, such as fever, respiratory, or neurological symptoms, were recorded daily and graded as follows: +++, severe; ++, moderate; +, weak; −, none. The last column records deaths versus total number of animals per group.
Surviving animals from the primary infection as well as three age-matched naive control animals were challenge infected intranasally with 5 × 107 pfu of highly virulent PrV-NIA-3, and clinical signs were again recorded daily as described for primary infections.
FIG. 4.
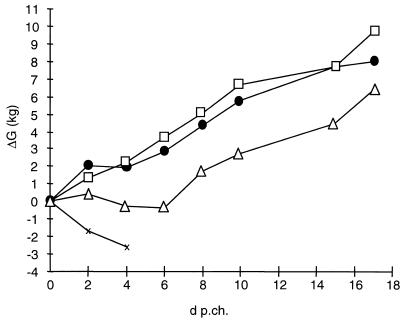
Change in body weight. Every second day after challenge, the body weight of all animals was determined and body weight change calculated in relation to the weight immediately before challenge. Indicated are average values per group. □, WT; ●, gD− Pass SPH; ▵, gD− Pass; ×, control.
Naive control animals developed high fever and displayed severe respiratory symptoms and loss of appetite as soon as 1 day p.c. On day 2 p.c., all animals suffered from severe neurological symptoms. Two piglets died on day 4 p.c., and the third was found moribund on day 5 p.c.
Animals previously infected with PrV-Ka or PrV-gD− Pass SPH showed only slightly elevated temperatures between day 1 and 5 p.c. No clinical signs or reduced food uptake was observed in previously PrV-Ka-infected animals, and all animals survived. Animals previously infected with PrV-gD− Pass SPH showed only mild respiratory symptoms on day 2 p.c. All animals fed well and survived the challenge infection without any neurological symptoms.
In contrast, animals previously infected with PrV-gD− Pass exhibited a similar sharp rise in body temperature as naive control animals, reaching over 41.5°C. Temperature normalized on day 10 p.c. Food uptake decreased from day 2 p.c. Respiratory symptoms were observed from day 3 p.c., and severe neurological symptoms started to appear on day 4 p.c. One animal died on day 4 p.c. and another one was found moribund on day 7 p.c. From day 7 p.c., surviving animals improved, and they were free of symptoms on day 10 p.c.
Shedding of challenge virus was quantified by titration of nasal swabs (Fig. 2B). Naive control animals excreted virus at titers exceeding 3 × 107 PFU/ml on day 3 p.c. Until the death of the last control animal on day 5 p.c., this high level of virus excretion was sustained. After the prior PrV-Ka infection, excretion of the challenge virus amounted to only 104 PFU/ml until day 3 p.c., and it ceased on day 6 p.c. Animals which had previously been infected with PrV-gD− Pass SPH excreted a higher amount of challenge virus than the PrV-Ka-infected animals. Titers stayed at ca. 3 × 106 PFU/ml until day 3 p.c., but excretion also ceased on day 6 p.c. Animals which had previously been infected with PrV-gD− Pass excreted virus at a level similar to that of the control animals. A peak value of ca. 4 × 107 PFU/ml was reached on day 3 p.c. Virus excretion of surviving animals ended on day 8 p.c.
The average body weight of the control group steadily declined (Fig. 4) until the death of the last animal. The animals in the PrV-gD− Pass group exhibited a stagnation in body weight until day 8 p.c., whereas animals previously infected with PrV-Ka and PrV-gD− Pass SPH showed a steady increase in average body weight.
Restriction in host range of PrV-gD− Pass.
Since the first experiment did not indicate any significant replication of PrV-gD− Pass in pigs, a second experiment was performed. Two groups of six 6-week-old piglets each were intranasally infected with 107 PFU of PrV-Ka or PrV-gD− Pass. Results for virus shedding, body temperature, and clinical signs of infection paralleled those of the first experiment (data not shown). In addition, one animal from each group was sacrificed, and organ samples were prepared for virus isolation and immunohistochemical examination at the indicated times p.i. An age-matched piglet was included as negative control. Virus titers in organ samples were determined by titration of homogenized tissue suspension on PSEK cells (Table 2). PrV-Ka-infected animals displayed a kinetic of virus replication in the different organs as is typical for AD. Highest titers were found in the different regions of the nasal mucosa. Subsequently, infection of the CNS occurred. However, no virus could be isolated from any organ at any time point in animals which had been inoculated with PrV-gD− Pass. Most strikingly, no virus could be isolated even from samples of the nasal mucosa.
TABLE 2.
Virus isolation from organs of pigs infected with PrV-Ka or PrV-gD− Pass
| Tissue examined | Virus | PFU/gram of tissuea
|
|||||
|---|---|---|---|---|---|---|---|
| 2 days p.i. | 4 days p.i. | 5 days p.i. | 6 days p.i. | 8 days p.i. | 10 days p.i. | ||
| Nasal mucosa (regio cutanea) | Ka | ++++++ | +++++ | +++++++ | ++ | ++ | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nasal mucosa (regio respiratoria) | Ka | +++ | +++++ | +++++++ | ++++ | 0 | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nasal mucosa (regio olfactoria) | Ka | +++++ | ++++ | ++++++ | + | 0 | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tonsil | Ka | ND | ++++ | +++++ | ++++ | + | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
| Lymphonodus mandibularis | Ka | + | ++ | ++ | 0 | 0 | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
| Lung | Ka | 0 | +++ | ++++ | ND | 0 | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
| Bulbus olfactorius | Ka | 0 | +++ | +++++ | 0 | 0 | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pedunculus olfactorius | Ka | + | + | ++++ | 0 | 0 | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ganglion trigeminale | Ka | ++++ | +++ | ++++ | ++++ | 0 | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
| Medulla oblongata | Ka | 0 | + | ++ | + | 0 | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pons | Ka | ++ | ++ | +++ | +++ | 0 | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
| Diencephalon | Ka | 0 | + | +++ | 0 | 0 | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cerebellum | Ka | 0 | + | + | 0 | 0 | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mesencephalon | Ka | 0 | 0 | ++ | 0 | 0 | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cerebrum | Ka | 0 | 0 | ++ | 0 | 0 | 0 |
| gD− Pass | 0 | 0 | 0 | 0 | 0 | 0 | |
+, 101 PFU; ++, 102 PFU; +++, 103 PFU; ++++, 104 PFU; +++++, 105 PFU; ++++++, 106 PFU; +++++++, 107 PFU; 0, no virus was isolated; ND, not determined.
Immunofluorescence on frozen sections.
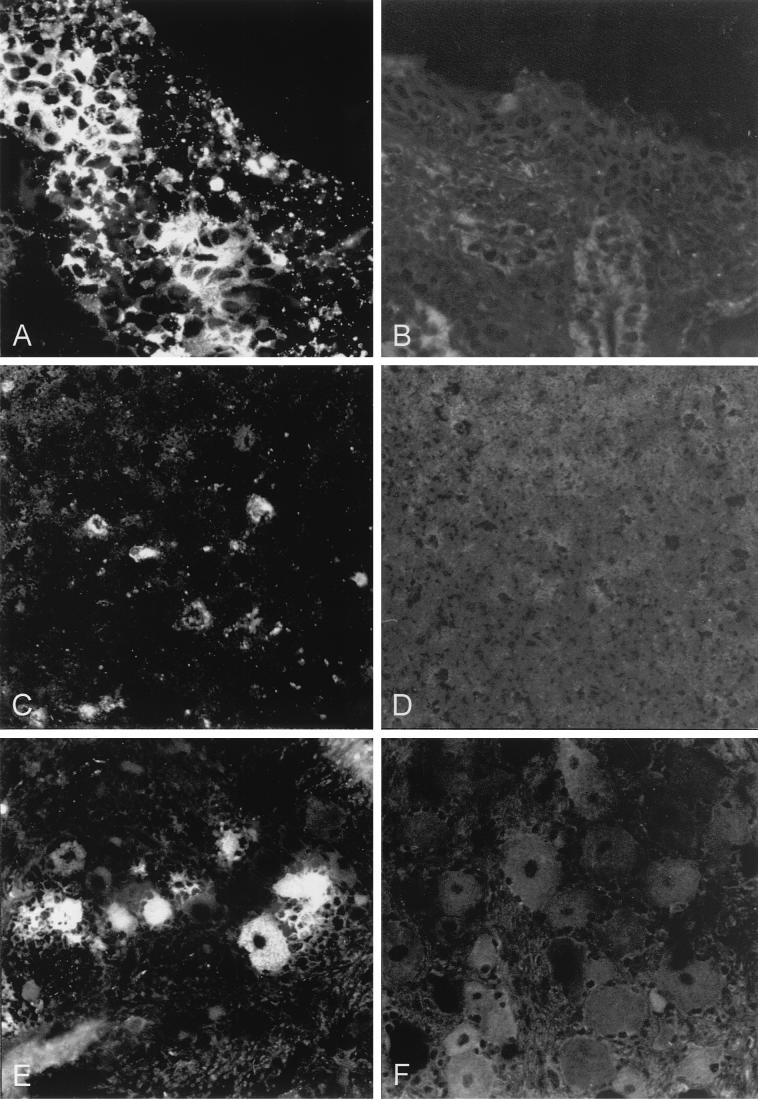
To examine whether PrV-gD− Pass infected porcine cells in vivo, indirect immunofluorescence was performed on frozen sections of PrV-Ka- or PrV-gD− Pass-infected animals (Fig. 5). There was a clear correlation between positive immunofluorescence on sections and positive virus isolation after PrV-Ka infection. Correlating with the negative results from virus isolation, we did not detect any positive signal in any organ section of PrV-gD− Pass-infected animals at any time p.i.
FIG. 5.
Immunofluorescence on frozen sections of selected organs. Frozen sections of organs prepared from PrV-Ka-infected (A, C, and E) or PrV-gD− Pass-infected (B, D, and F) animals were subjected to indirect immunofluorescence by using a MAb mixture directed against gB (A20-c26) and gC (B16-c8). (A and B) Nasal mucosa (regio cutanea); 4 days p.i. Magnification, ca. ×440. (C and D) Ganglia cells at the bulbus olfactorius; 5 days p.i. Magnification, ca. ×220. (E and F) Perikarya at the ganglion trigeminale; 4 days p.i. Magnification, ca. ×440.
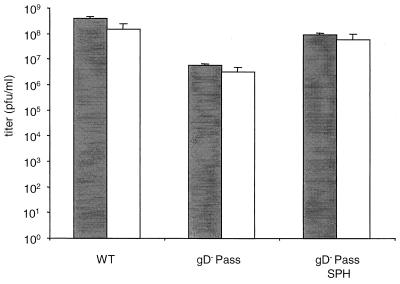
Virus inactivation by porcine nasal mucus.
Since we were not able in a multitude of tests to demonstrate infection of pigs by PrV-gD− Pass, it was necessary to rule out differences in stability of the gD-deleted PrV-gD− Pass compared to PrV-Ka which may have resulted in nonspecific inactivation of inoculated PrV-gD− Pass. Therefore, the sensitivities of PrV-Ka, PrV-gD− Pass, and PrV-gD− Pass SPH to inactivation by porcine nasal mucus were assayed (Fig. 6). Treatment of all virus suspensions resulted in similar and only moderate reductions in titer. Thus, the inability of PrV-gD− Pass to infect cells of the nasal mucosa of pigs cannot be attributed to unspecific inactivation of the PrV gD− Pass inoculum.
FIG. 6.
Virus inactivation by porcine nasal mucus. Undiluted stocks of PrV-Ka, PrV-gD− Pass, or PrV-gD− Pass SPH were preincubated with mucus (white) or cell culture medium (grey). Shown are titers from three independent experiments; error bars indicate standard deviation.
Titration on porcine nasal mucosa explant cells.
To assay whether the inability to detect virus-infected cells after in vivo infection in the nasal mucosa was reflected in a block in virus infection in explanted cells, wild-type-like PrV-1112, PrV-gD− Pass SPH, and PrV-gD− Pass were titrated on nasal mucosa explant cells and compared to PK-15 cells. Titers of PrV-1112 and PrV-gD− Pass SPH were 4 × 106 PFU/ml on PK-15 cells, and 2 × 106 and 1 × 106 PFU/ml, respectively, on porcine nasal explant cells. Titers of PrV-gD− Pass on porcine nasal explant cells amounted to 3 × 104 PFU/ml compared to 4 × 105 PFU/ml on PK-15 cells. Thus, nasal mucosal explant cells in culture are infectible by PrV-gD− Pass, albeit with a somewhat lower efficiency compared to PrV-1112 or PrV-gD− Pass SPH.
Mapping of compensatory mutations in PrV-gD− Pass.
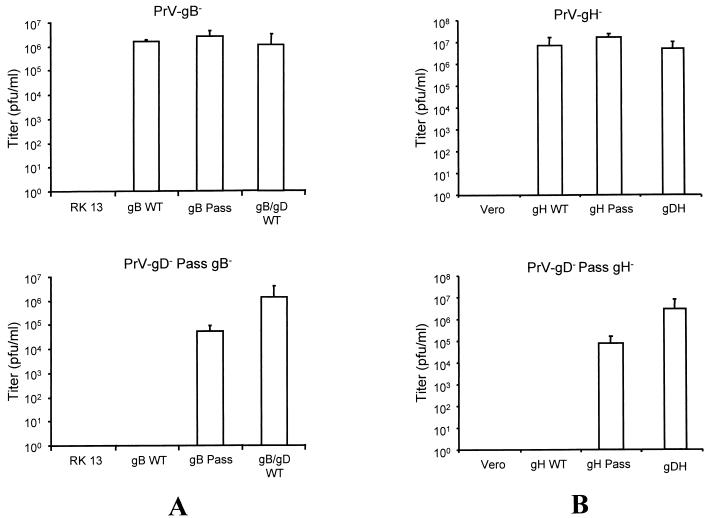
So far, the molecular basis for gD-independent infectivity in BHV-1 and PrV is unclear, although recent studies implied that mutations in gH and gB of BHV-1 could be involved (41). We initially set out to establish cell lines that express single glycoproteins of PrV-gD− Pass for the complementation of PrV-gD− and subsequent assessment of infectivity. However, in none of the assays including gB, gH, gL, and gK was any rescue of gD-independent infectivity observed. Complementation assays using transient expression of numerous (glyco)proteins either singly or in combination also did not result in any rescue of gD-independent infectivity (data not shown). Therefore, we reasoned that the wild-type form of the glycoprotein which is still expressed in PrV-gD− might interfere with the function of a mutated form in gD-independent infectivity. Consequently, to test for functional differences in gB and gH derived from either wild-type PrV or PrV-gD− Pass, mutant viruses lacking gB or gH and expressing green fluorescent protein were isolated on the basis of PrV-gD−Pass on cell lines expressing PrV-gD− Pass gB or gH. The transcomplemented virus mutants PrV-gD− Pass gB− and PrV-gD− Pass gH− were then used for infecting cells expressing wild-type gB, PrV-gD− Pass gB, or wild-type gB and gD. After complete CPE developed, supernatants were titrated on cells expressing gB of PrV-gD− Pass. As shown in Fig. 7A, the gB of PrV-gD− Pass complemented the gB deletion mutants in both viral backgrounds. In contrast, wild-type gB did not complement gB function in PrV-gD− Pass. Coexpression of wild-type gD and wild-type gB, however, restored infectivity, presumably by restoration of the gD-dependent entry pathway. Similar results were obtained with gH (Fig. 7B). Only cell lines expressing gH of PrV-gD− Pass were able to complement gH deletion mutants in both viral backgrounds, whereas wild-type gH complemented only the gH deletion mutant in a wild-type background. Again, as a positive control, the multifunctional gDH hybrid protein, which combines wild-type gD and gH function and, therefore, presumably restores gD-dependent entry, fully complemented either mutant. In summary, these data show functional differences in both gB and gH of PrV-gD− Pass compared to the respective wild-type proteins.
FIG. 7.
Transcomplementation of PrV-gD− Pass gB− (A) or PrV-gD− Pass gH− (B). (A) Either normal RK13 cells or RK13 cells expressing wild-type gB (gB WT), gB Pass, or wild-type gB and gD (gB/gD WT) were infected at an MOI of 0.1 with transcomplemented PrV-gB− (top) or PrV-gD− Pass gB− (bottom). After complete CPE was observed, supernatants were harvested and titrated on gB Pass-expressing cells. (B) Normal Vero cells or Vero cells expressing wild-type gH (gH WT), gH Pass, or the gDH hybrid protein (19) were infected with transcomplemented PrV-gH− (top) or PrV-gD− Pass gH− (bottom) at an MOI of 0.1. After complete CPE was observed, supernatants were harvested and titrated on gH Pass-expressing cells. Indicated are average titers of four independent experiments. Bars show standard deviations.
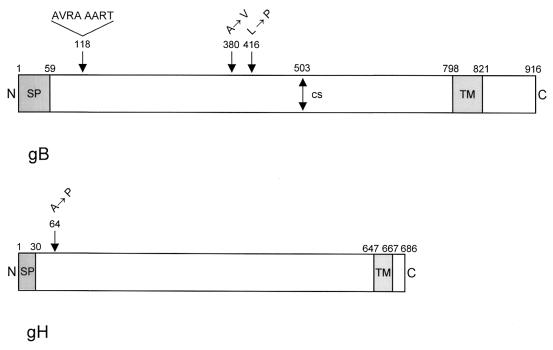
DNA sequencing of the open reading frames of gB and gH of PrV-gD− Pass revealed differences from the wild-type versions. As schematically shown in Fig. 8, there are three mutations in gB Pass compared to gB of wild-type strain Ka: an eight-amino-acid insertion at position 118, an alanine-to-valine exchange at position 380, and a leucine-to-proline substitution at position 416. The rest of the protein was identical. Interestingly, in gH, only a single amino acid exchange was observed, leading to an alanine-to-proline substitution at position 64. For control, the two other genes encoding essential glycoproteins, i.e., the UL53 (gK) and UL1 (gL) genes of PrV-gD− Pass, were also sequenced and compared to PrV-Ka. We did not detect any differences between the gK or gL gene of PrV-gD− Pass and the corresponding PrV-Ka genes. Correlating with this result, PrV-Ka gL and gK complemented the corresponding deletion mutants of PrV-gD− Pass (data not shown).
FIG. 8.
Diagram of mutations present in gB Pass and gH Pass. (Top) Schematic representation of wild-type gB (38). Mutations in gB Pass are indicated above. (Bottom) Schematic representation of wild-type gH (18). The sole mutation in gH Pass is indicated above. N, amino terminus; SP, signal peptide; TM, transmembrane domain; C, carboxy terminus; CS, furin protease cleavage site in gB.
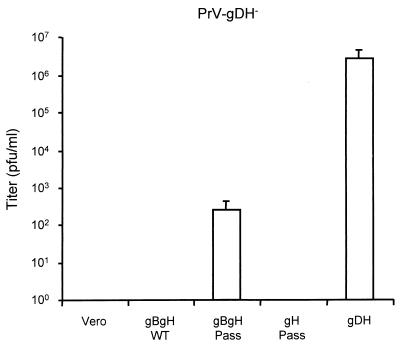
Given the interference of the wild-type versions of the glycoproteins on the function of the mutated forms, to prove that the observed mutations are indeed responsible for the gD-independent phenotype would entail the construction and complementation of a gB and gH deletion mutant of PrV-gD−, i.e., a mutant virus simultaneously lacking three essential glycoproteins. So far, we have been unable to achieve this complicated goal. However, we tried to transcomplement a double mutant, PrV-gD−gH−, by using a cell line expressing gB and gH of PrV-gD− Pass, reasoning that with only one interfering wild-type protein we might observe an effect. As shown in Fig. 9, the infectivity of PrV-gD−gH− could indeed be rescued on cells expressing gB Pass and gH Pass, although to a titer of only ca. 102 PFU/ml. However, this is significant since no infectivity at all was rescued on cells expressing the wild-type versions of gB and gH. As control, complementation with the gDH hybrid protein again resulted in rescue of more than 106 PFU per ml, presumably by restoration of the gD-dependent entry pathway.
FIG. 9.
Transcomplementation of PrV-gD−gH−. Normal Vero cells and Vero cells expressing gB and gH of wild-type PrV (gBgH WT), PrV-gD− Pass (gBgH Pass), or the gDH hybrid protein (gDH) were infected with gDH-transcomplemented PrV-gD−gH− (PrV-gDH−) at an MOI of 0.1. After the development of complete CPE, supernatants were harvested and titrated on gH Pass-expressing cells. The average titers of two independent experiments are shown. Bars show standard deviation.
In summary, our data strongly suggest that the observed mutations in gB and gH are, at least in part, responsible for mediating gD-independent entry.
DISCUSSION
Interaction between viral attachment proteins and cellular receptors is a major determinant of viral tropism in vitro and in vivo. We demonstrate here that an alphaherpesvirus mutant, PrV-gD− Pass, which had acquired the ability to infect cells via a pathway which is independent from the envelope gD and from cellular gD receptors, exhibits a drastically altered host range in vivo. PrV-gD− Pass is no longer able to infect the natural host, pigs, but is still virulent in mice.
The first interaction between PrV and target cells is mediated by the binding of gC to heparan sulfate proteoglycans (HSPG), which is similar to the situation in HSV-1 (14, 24). Since this class of primary receptors is nearly ubiquitously distributed and since the gC-HSPG interaction is not essential for infection, target cell specificity is more likely determined at least in part by other virion cell interactions. A secondary attachment step relies on binding of gD to recently identified specific cellular gD receptors. Since gD is not only involved in attachment but also essential for penetration, it is believed that binding of gD to specific receptors is the crucial link between virus attachment and initiation of membrane fusion (reviewed in references 27 and 43). In vitro studies on CHO-K1 cells demonstrated that the lack of gD receptors correlates with a relative resistance towards PrV infection (10, 29). Expression of gD receptors rendered these cells susceptible to infection, demonstrating the importance of gD receptors for in vitro host range. The use of multiple gD receptors, namely Hve B, Hve C, and Hve D, which all belong to the immunoglobulin-like protein superfamily, might explain the broad host range of PrV (10, 29, 44).
Recently, we isolated an infectious gD-negative PrV mutant, PrV-gD− Pass (39). A similar mutant could also be obtained for BHV-1 (40). For BHV-1, it has been shown that mutations in at least gB and gH contribute to gD-independent infectivity (40, 41). The data presented here for PrV correlate with these earlier findings. Moreover, for the first time, we show limited albeit significant gD-independent infectivity in a wild-type PrV-gD−gH− background by coexpression of gB and gH of PrV-gD− Pass. The only low level of gD-independent infectivity observed may be explained by the continuous expression of wild-type gB from the viral genome which could interfere with function of cellularly expressed gB Pass. With both gB and gH we indeed demonstrated functional differences between the proteins of wild-type PrV and PrV-gD− Pass so that the wild-type versions only complemented wild-type deletion mutants, whereas the glycoproteins from PrV-gD− Pass complemented wild-type and PrV-gD− Pass deletion mutants. Interestingly, the striking difference in the functional complementation of gH is associated with a single amino acid exchange at position 64 in the ectodomain resulting in an alanine-to-proline substitution, whereas in gB, three mutations also all located in the ectodomain were observed by comparison of gB from wild-type and passaged virus. Whether all three of the gB mutations are required for the observed phenotype is presently under investigation.
What is the evidence that the observed defect in PrV-gD− Pass is indeed at the level of entry and, more specifically, at receptor recognition? We recently isolated an MDBK cell clone, designated NB, which is specifically resistant toward infection with PrV-gD− Pass due to a defect in virus entry. Furthermore, NB cells do not promote direct cell-to-cell spread of PrV-gD− Pass. Therefore, initiation of infection by PrV-gD− Pass in vitro does not depend on known gD receptors but relies on the presence of a hitherto unknown receptor which is specific for PrV-gD− Pass (17, 29). Interestingly, the infectivity of PrV-gD− Pass is reduced but not eliminated by deletion of gC (17), demonstrating that tertiary receptor interactions presumably involving gB and/or gH are present. Although direct proof is lacking for a receptor binding function of gB and/or gH of PrV, elevated susceptibility of PrV toward complement-independent neutralization by a gB-specific MAb on gD-receptor deficient cells can be interpreted as indicative for receptor binding by gB (29). Since gD-independent infectivity requires the presence of compensatory mutations in gB, it is conceivable that this altered gB gained receptor binding properties unique for PrV-gD− Pass. Interestingly, an HSPG-independent cell binding function has been demonstrated for gB of BHV-1 (22). In the betaherpesvirus HCMV, binding of gH to an unidentified cellular receptor designated HCMVFR has been reported (4), and it is therefore not excluded that the observed mutation in gH of PrV-gD− Pass may also have an influence in receptor binding. An interaction of HCMV gB with cellular annexin II has also been proposed, although the biological significance of this interaction is unclear (6, 35, 36).
In this study, we analyzed PrV-gD− Pass infection in vivo. We demonstrated that PrV-gD− Pass has a virulence in mice similar to that of PrV-Ka. All characteristic symptoms of PrV infection in mice ultimately leading to death were observed, but symptoms appeared with a delay of 24 to 48 h. This delay might be linked to compensatory mutations in gB and gH required for infectivity of PrV-gD− Pass which, however, may also affect viral pathogenesis. A role for gB in determining PrV pathogenesis in pigs has recently been demonstrated by analysis of a recombinant PrV expressing the BHV-1 gB instead of it own (11). Furthermore, mutations in HSV-1 gB also influence pathogenesis in vivo (7, 45). The use of an alternative, PrV-gD− Pass-specific receptor might also contribute to the delay in symptom development, e.g., by inefficient primary replication restricted by cellular distribution or concentration of the PrV-gD− Pass receptor.
In contrast to its effect in mice, PrV-gD− Pass is not able to infect PrV's natural host. Intranasal inoculation of pigs with up to 107 PFU of PrV-gD− Pass resulted in no signs of disease at all. Inoculated pigs did not show elevated temperature or meaningful virus excretion. Furthermore, the inoculation did not induce neutralizing antibodies or a protective immunity. Since no virus could be isolated from any organ of PrV-gD− Pass-inoculated pigs, we performed detailed histochemical analyses for expression of gB or gC which could indicate an abortive infection. However, we did not observe a single antigen-positive infected cell in any organ section of PrV-gD− Pass-inoculated pigs. Since also no infection of cells in the nasal mucosa was detectable after intranasal inoculation, PrV-gD− Pass appears to be unable to infect pigs via the normal route of infection. Penetration and cell-to-cell spread of PrV-gD− Pass relies on a specific cellular receptor (17), and it is reasonable to hypothesize that cells of the porcine nasal mucosa do not express this receptor. Interestingly, cultured porcine cells, even explants from the nasal mucosa, are infectible by PrV-gD− Pass, although in the latter, infectivity is reduced ca. 10-fold. The different susceptibility of porcine cells in vitro and in vivo might be linked to alterations in cell surface molecules during growth in cell culture. Experiments to identify the receptor as well as the interacting glycoproteins responsible for the restriction in host range of PrV-gD− Pass are under way.
Although we cannot rule out the presence of additional mutations in the 150-kbp PrV-gD− Pass genome, the compensatory mutations necessary for gD-independent infectivity may suffice to explain the attenuation of PrV gD− Pass SPH in pigs compared to that of PrV-Ka and PrV-gD− Pass. We showed that mutations in gB and gH are associated with and, presumably, required for gD-independent entry of PrV and that the described mutations alter the functional properties of these glycoproteins. After intranasal infection with PrV-gD− Pass SPH, virus shedding was observed, demonstrating virus replication in the nasal mucosa. The induction of neutralizing antibodies and the establishment of a protective immunity also indicated viral replication. Since PrV-gD− Pass SPH proved to be highly attenuated, gD expression obviously did not restore virulence to PrV-gD− Pass, which correlates with experiments in cell culture. Restoration of gD expression of PrV-gD− Pass completely alleviated the entry defect on NB cells (17) but only partially restored direct cell-to-cell spread, with plaque diameters reaching only 35% of those of PrV-Ka (data not shown). If spread of PrV-gD− Pass SPH is equally compromised in vivo, primary replication would be less efficient, as indicated by the ca. 10-fold lower virus excretion. Whether PrV-gD− Pass SPH is able to invade the nervous system at all remains to be determined.
These findings are also of interest with regard to the use of nonspreading, gD-negative PrV live vaccines (13, 26, 33). The emergence in vivo of infectious gD-negative viruses, due to compensatory second-site mutations as in the case of PrV-gD− Pass, could threaten this concept. However, PrV-gD− Pass is unable to infect PrV's natural host, so this particular virus would not be selected for under natural conditions. Still, the possibility that a gD independently infectious but virulent virus might emerge cannot be ruled out.
ACKNOWLEDGMENTS
Part of this work was supported by the European Union (grant BMH4-CT97-2573) and the Deutsche Forschungsgemeinschaft (grant Me 854/4).
We thank the animal caretakers of the Friedrich-Loeffler-Institutes, Insel Riems, for their invaluable help and R. Riebe for assistance with the explant cultures.
REFERENCES
- 1.Babic N, Mettenleiter T C, Flamand A, Ugolini G. Role of essential glycoproteins gII and gp50 in transneuronal transfer of pseudorabies virus from the hypoglossal nerves of mice. J Virol. 1993;67:4421–4426. doi: 10.1128/jvi.67.7.4421-4426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babic N, Mettenleiter T C, Ugolini G, Flamand A, Coulon P. Propagation of pseudorabies virus in the nervous system of the mouse after intranasal inoculation. Virology. 1994;204:616–625. doi: 10.1006/viro.1994.1576. [DOI] [PubMed] [Google Scholar]
- 3.Babic N, Klupp B G, Makoschey B, Karger A, Flamand A, Mettenleiter T C. Glycoprotein gH of pseudorabies virus is essential for penetration and propagation in cell culture and in the nervous system of mice. J Gen Virol. 1996;77:2277–2285. doi: 10.1099/0022-1317-77-9-2277. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin B R, Zhang C O, Keay S. Cloning and epitope mapping of a functional partial fusion receptor for human cytomegalovirus gH. J Gen Virol. 2000;81:27–35. doi: 10.1099/0022-1317-81-1-27. [DOI] [PubMed] [Google Scholar]
- 5.Baskerville A, McFerran J B, Dow C. Aujeszky's disease in pigs. Vet Bull. 1973;43:465–480. [Google Scholar]
- 6.Boyle K A, Compton T. Receptor-binding properties of a soluble form of human cytomegalovirus glycoprotein B. J Virol. 1998;72:1826–1833. doi: 10.1128/jvi.72.3.1826-1833.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engel J P, Boyer E P, Goodman J L. Two novel single amino acid syncytial mutations in the carboxy terminus of glycoprotein B of herpes simplex virus type 1 confer a unique pathogenic phenotype. Virology. 1993;192:112–120. doi: 10.1006/viro.1993.1013. [DOI] [PubMed] [Google Scholar]
- 8.Enquist L W. Infection of the mammalian nervous system by pseudorabies virus (PrV) Semin Virol. 1994;5:221–231. [Google Scholar]
- 9.Fehler F, Herrmann J, Saalmüller A, Mettenleiter T C, Keil G M. Glycoprotein IV of bovine herpesvirus 1-expressing cell line complements and rescues a conditionally lethal viral mutant. J Virol. 1992;66:6372–6390. doi: 10.1128/jvi.66.2.831-839.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geraghty R J, Krummenacher C, Cohen G H, Eisenberg R J, Spear P G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 11.Gerdts V, Beyer J, Lomniczi B, Mettenleiter T C. Pseudorabies virus expressing the bovine herpesvirus 1 glycoprotein B exhibits altered neurotropism and increased neurovirulence. J Virol. 2000;74:817–827. doi: 10.1128/jvi.74.2.817-827.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham F L, Van der Eb A J. A new technique for the assay of infectivity of human adenovirus. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 13.Heffner S, Kovács F, Klupp B, Mettenleiter T C. Glycoprotein gp50-negative pseudorabies virus: a novel approach toward a nonspreading live herpesvirus vaccine. J Virol. 1993;67:1529–1537. doi: 10.1128/jvi.67.3.1529-1537.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan A S, Vatter A E. A comparison of herpes simplex and pseudorabies virus. Virology. 1959;7:394–407. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- 16.Karger A, Mettenleiter T C. Glycoproteins gIII and gp50 play dominant roles in the biphasic attachment of pseudorabies virus. Virology. 1993;194:654–664. doi: 10.1006/viro.1993.1305. [DOI] [PubMed] [Google Scholar]
- 17.Karger A, Schmidt J, Mettenleiter T C. Infectivity of a pseudorabies virus mutant lacking attachment glycoproteins C and D. J Virol. 1998;72:7341–7348. doi: 10.1128/jvi.72.9.7341-7348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klupp B G, Mettenleiter T C. Sequence and expression of the glycoprotein gH gene of pseudorabies virus. Virology. 1991;182:732–741. doi: 10.1016/0042-6822(91)90614-h. [DOI] [PubMed] [Google Scholar]
- 19.Klupp B G, Mettenleiter T C. Glycoprotein gL independent infectivity of pseudorabies virus is mediated by a gD-gH fusion protein. J Virol. 1999;73:3014–3022. doi: 10.1128/jvi.73.4.3014-3022.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kritas S K, Pensaert M B, Mettenleiter T C. Role of envelope glycoproteins gI, gp63 and gIII in the invasion and spread of Aujeszky's disease virus in the olfactory nervous pathway of the pig. J Gen Virol. 1994;75:2319–2327. doi: 10.1099/0022-1317-75-9-2319. [DOI] [PubMed] [Google Scholar]
- 21.Krummenacher C, Nicola A V, Whitbeck J C, Lou H, Hou W, Lambris J D, Geraghty R J, Spear P G, Cohen G H, Eisenberg R J. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, van Drunen Littel-van S, den Hurk, Babiuk L A, Liang X. Characterization of cell-binding properties of bovine herpesvirus 1 glycoproteins B, C, and D: identification of a dual cell-binding function of gB. J Virol. 1995;69:4758–4768. doi: 10.1128/jvi.69.8.4758-4768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ligas M W, Johnson D C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mettenleiter T C, Zsák L, Zuckermann F, Sugg N, Kern H, Ben-Porat T. Interaction of glycoprotein gIII with a cellular heparinlike substance mediates adsorption of pseudorabies virus. J Virol. 1990;64:278–286. doi: 10.1128/jvi.64.1.278-286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mettenleiter T C, Rauh I. A glycoprotein gX-βgalactosidase fusion gene as insertional marker for rapid identification of pseudorabies virus mutants. J Virol Methods. 1990;30:55–66. doi: 10.1016/0166-0934(90)90043-f. [DOI] [PubMed] [Google Scholar]
- 26.Mettenleiter T C, Klupp B G, Weiland F, Visser N. Characterization of a quadruple glycoprotein-deleted pseudorabies virus mutant for use as a biologically safe live virus vaccine. J Gen Virol. 1994;75:1723–1733. doi: 10.1099/0022-1317-75-7-1723. [DOI] [PubMed] [Google Scholar]
- 27.Mettenleiter T C. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis. Vet Res. 2000;31:99–115. doi: 10.1051/vetres:2000110. [DOI] [PubMed] [Google Scholar]
- 28.Mulder W, Pol J, Kimman T, Kok G, Priem J, Peeters B. Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI. J Virol. 1996;70:2191–2200. doi: 10.1128/jvi.70.4.2191-2200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nixdorf R, Schmidt J, Karger A, Mettenleiter T C. Infection of Chinese hamster ovary cells by pseudorabies virus. J Virol. 1999;73:8019–8026. doi: 10.1128/jvi.73.10.8019-8026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nixdorf R, Klupp B G, Karger A, Mettenleiter T C. Effect of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J Virol. 2000;74:7137–7145. doi: 10.1128/jvi.74.15.7137-7145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nixdorf R, Klupp B G, Mettenleiter T C. Role of the cytoplasmic tails of pseudorabies virus glycoproteins B, E and M in intracellular localization and virion incorporation. J Gen Virol. 2001;82:215–226. doi: 10.1099/0022-1317-82-1-215. [DOI] [PubMed] [Google Scholar]
- 32.Peeters B, de Wind N, Hooisma M, Wagenaar F, Gielkens A, Moormann R. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration but only gII is involved in membrane fusion. J Virol. 1992;66:894–905. doi: 10.1128/jvi.66.2.894-905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peeters B, Bouma A, de Bruin T, Moormann R, Gielkens A, Kimman T. Non-transmissible pseudorabies virus gp50 mutants: a new generation of safe live vaccines. Vaccine. 1994;12:375–380. doi: 10.1016/0264-410x(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 34.Pensaert M, Kluge J. Pseudorabies virus (Aujeszky's disease) In: Pensaert M, editor. Virus infections of porcines. Amsterdam, The Netherlands: Elsevier Science Publishing; 1989. [Google Scholar]
- 35.Pietropaolo R L, Compton T. Direct interaction between human cytomegalovirus glycoprotein B and cellular annexin II. J Virol. 1997;71:9803–9807. doi: 10.1128/jvi.71.12.9803-9807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pietropaolo R L, Compton T. Interference with annexin II has no effect on entry of human cytomegalovirus into fibroblast cells. J Gen Virol. 1999;80:1807–1816. doi: 10.1099/0022-1317-80-7-1807. [DOI] [PubMed] [Google Scholar]
- 37.Rauh I, Mettenleiter T C. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991;65:5348–5356. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbins A K, Dorney D J, Wathen M W, Whealy M W, Gold C, Watson R J, Holland L E, Weed S D, Levine M, Glorioso J, Enquist L W. The pseudorabies virus gII gene is closely related to the gB glycoprotein gene of herpes simplex virus. J Virol. 1987;61:2691–2701. doi: 10.1128/jvi.61.9.2691-2701.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt J, Klupp B G, Karger A, Mettenleiter T C. Adaptability in herpesviruses: glycoprotein D-independent infectivity of pseudorabies virus. J Virol. 1997;71:17–24. doi: 10.1128/jvi.71.1.17-24.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schröder C, Linde G, Fehler F, Keil G M. From essential to beneficial: glycoprotein D loses importance for replication of bovine herpesvirus 1 in cell culture. J Virol. 1997;71:25–33. doi: 10.1128/jvi.71.1.25-33.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schröder C, Keil G M. Bovine herpesvirus 1 requires glycoprotein H for infectivity and direct spreading and glycoproteins gHw450 and gB for glycoprotein D-independent cell-to-cell spread. J Gen Virol. 1999;80:57–61. doi: 10.1099/0022-1317-80-1-57. [DOI] [PubMed] [Google Scholar]
- 42.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 43.Spear P G, Eisenberg R J, Cohen G H. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 44.Warner M S, Geraghty R J, Martinez W M, Montgomery R I, Whitbeck J C, Xu R, Eisenberg R J, Cohen G H, Spear P G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 45.Yuhasz S, Stevens J G. Glycoprotein B is a specific determinant of herpes simplex virus type 1 neuroinvasiveness. J Virol. 1993;67:5948–5954. doi: 10.1128/jvi.67.10.5948-5954.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]