Abstract
Adoptive transfer studies have shown that cytotoxic T lymphocytes (CTL) of high avidity, capable of recognizing low levels of peptide-MHC I molecules, are more efficient at reducing viral titers than are low-avidity CTL, thus establishing CTL avidity as a critical parameter for the ability of a CTL to clear virus in vivo. It has been well documented that CTL of high avidity are relatively CD8 independent, whereas low-avidity CTL require CD8 engagement in order to become activated. In this study we have analyzed the antiviral CTL response elicited following infection with the paramyxovirus simian virus 5 (SV5). We have identified the immunodominant and subdominant CTL responses and subsequently assessed the avidity of these responses by their CD8 dependence. This is the first study in which the relationship between immunodominance and CTL avidity has been investigated. The immunodominant response was directed against an epitope present in the viral M protein, and subdominant responses were directed against epitopes present in the P, F, and HN proteins. Similarly to other CTL responses we have analyzed, the immunodominant response and the subdominant F and HN responses were comprised of both high- and low-avidity CTL. However, the subdominant response directed against the epitope present in the P protein is novel, as it is exclusively high avidity. This high-avidity response is independent of both the route of infection and expression by recombinant SV5. A further understanding of the inherent properties of P that elicit only high-avidity CTL may allow for the design of more efficacious vaccine vectors that preferentially elicit high-avidity CTL in vivo.
The importance of cytotoxic T lymphocytes (CTL) in the clearance of viral pathogens is well established. Virus-specific CD8+ T cells clear virus by the recognition of viral peptides in the context of major histocompatibility complex (MHC) class I molecules on infected cells. Numerous studies have previously shown that CTL specific for the same peptide antigen are not always functionally equivalent. Within the T-cell population specific for a single epitope there are CTL with a broad range of avidities (2, 3, 17, 51, 53). CTL avidity can be defined by the amount of stimulation required to elicit effector function. High-avidity CTL can recognize antigen-presenting cells (APC) pulsed with 100- to 1,000-fold less peptide antigen than low-avidity CTL (3).
The in vivo importance of high-avidity CTL became evident when it was shown in a vaccinia virus clearance model that adoptively transferred high-avidity CTL were 1,000-fold more efficient at reducing viral titers than were low-avidity CTL (3, 15). High-avidity CTL have been shown to lyse virally infected cells at earlier time points, when the density of viral peptide-MHC complexes on infected cells is still low, and in addition to affect killing more quickly than do low-avidity CTL (15). Therefore, there is a strong interest in designing vaccines that can be used to preferentially elicit high-avidity CTL responses.
There has been remarkable progress in recent years on the development of reverse-genetics systems for manipulating negative-strand RNA viruses. As such, a number of nonsegmented negative-strand RNA viruses have been engineered to express a variety of foreign proteins (13, 18, 31, 38). The ability to recover paramyxoviruses and rhabdoviruses from cDNA has raised the possibility of using these viruses as therapeutic vectors to deliver antigens that will elicit long-term protective immunity against a variety of pathogens.
The paramyxovirus family of viruses includes members such as the human parainfluenza viruses, mumps virus, measles virus, and the prototypic virus simian virus 5 (SV5). A number of properties inherent in these viruses have evoked interest in using members of this family as vaccine vectors. These properties include the following: (i) the RNA genome does not integrate into host DNA, and recombination between viral genomes does not occur; (ii) the RNA genome is small, but packaging constraints are not apparent; and (iii) the technology exists to engineer these viruses to express multiple tandem-linked foreign genes. In addition to these properties, SV5 has been shown to be immunogenic in humans; however, it is not associated with any known disease (11, 19). SV5 can also replicate to high titers in many different cell types without producing apparent cytopathic effects. Finally, replication of SV5 in the respiratory tract provides an attractive route of delivering vaccines that may elicit mucosal immune responses.
The lung environment has been shown to possess a number of unique characteristics that could impact the immune responses elicited therein (7, 12, 20, 44). As part of our efforts to develop SV5 as a model for respiratory tract infections and to explore its potential use as a vaccine vector, we have analyzed the CTL response generated following immunization with this virus. Our results show that during SV5 infection of BALB/c mice the immunodominant response is directed against an epitope in the M protein, with subdominant responses elicited against epitopes in the P, F, and HN proteins. An analysis of the avidities of M, HN, and F responses showed that a mixture of high- and low-avidity CTL was present in the responding CTL populations, and this profile is typical of all antigens analyzed to date (reference 2 and data not shown). Surprisingly, the P-specific immune response consisted of almost exclusively high-avidity CTL. The finding that the SV5 P protein has an inherent property that allows for the elicitation of high-avidity CTL has important implications for the design of more potent vaccine vectors and elucidating the factors that control CTL avidity.
MATERIALS AND METHODS
Mice and cell lines.
BALB/c mice were purchased from the Frederick Cancer Research and Development Center (Frederick, Md.). P815 is a DBA/2-derived (H-2d) mastocytoma. All research performed on mice in this study has complied with federal and institutional guidelines set forth by the Wake Forest University Animal Care and Usage Committee.
Generation of CTL lines.
Responding BALB/c spleen cells (7.5 × 106) from mice previously immunized with wild-type SV5 intranasally (i.n.) were cocultured with 3.5 × 106 stimulating BALB/c splenocytes (UV inactivated) infected 20 h previously with wild-type SV5 at a multiplicity of infection (MOI) of 10. Cells were cultured together in a 24-well plate containing 2 ml of RPMI 1640 medium supplemented with 10% fetal calf serum, l-glutamine, sodium pyruvate, nonessential amino acids, HEPES, penicillin, streptomycin, 5 × 10−5 M 2-mercaptoethanol, and 5% concanavalin A supernatant. CTL lines were established from primary cultures and were maintained by weekly restimulation of 3 × 105 to 5 × 105 cells/well in the presence of 5 × 106 UV-inactivated BALB/c spleen cells infected with wild-type SV5 at an MOI of 10.
Chromium release assay.
The 51Cr release assay was carried out as described previously (1) with modifications. Target cells (106) were labeled with 200 to 300 μCi of Na251CrO4 in 200 to 300 μl of RPMI 1640 medium supplemented with 10% NCS and 10 mM HEPES for 2 h at 37°C. Where appropriate, target cells were infected 20 h previously with wild-type SV5 at an MOI of 10. Cells were then washed and added to wells along with the appropriate number of effector cells in 96-well round-bottom plates. After 4 h, supernatants were harvested and counted in an Auto-Gamma 5650 (Packard). The means of triplicate samples and percentage of 51Cr release were calculated.
Recombinant viruses.
Plasmids carrying the SV5 NP (33), P and V (46), M (41), F (35), SH (22), HN (21), and L (33) genes have been described. Vaccinia viruses expressing the HN and F proteins were kindly provided by R. Paterson and R. A. Lamb (Northwestern University) as described previously (36). Details of the cloning strategy and recovery of recombinant vaccinia virus (rVV) expressing the SV5 NP, P, M, SH, and L proteins are available upon request from the authors. Briefly, DNA fragments encoding the SV5 proteins were excised from plasmids using unique flanking restriction sites, treated with Klenow fragment of DNA polymerase to create blunt ends, and then ligated into the StuI site of pSC11ss (9). The NP gene was divided into two overlapping fragments encoding amino-terminal residues 1 to 327 (including a C-terminal 11-amino-acid hemagglutinin epitope tag for detection) and carboxy-terminal residues 182 to 509. The L gene was divided into two overlapping fragments encoding amino-terminal residues 1 to 1558 and carboxy-terminal residues 1480 to 2255. rVVs expressing the SV5 polypeptides were isolated by thymidine kinase selection using bromodeoxyuridine and beta-galactosidase screening as described previously (9). Plaque-purified virus was screened for expression by Western blotting using rabbit antisera specific for the NP, P, M, HN, and F polypeptides (28, 34), an L-specific peptide (32), or monoclonal antibodies specific for the V protein (37) or hemagglutinin epitope tag (Roche). vPE-16 used as a control virus is a recombinant vaccinia virus construct that expresses the gp160 protein from the IIIB strain of human immunodeficiency virus (16) and was a kind gift of Patricia Earl and Bernard Moss (National Institute of Allergy and Infectious Diseases, Bethesda, Md.).
Wild-type recombinant SV5 (WT rSV5) was generated using a reverse-genetics system from an infectious clone as described previously (34).
ELISPOT analyses.
Responder cells used for analysis were from mice immunized with WT rSV5, or rVVs expressing SV5 polypeptides where indicated, 6, 12, 18, or 40 days prior to enzyme-linked immunospot (ELISPOT) analysis. To enumerate high- and low-avidity cells, titrated numbers of splenocytes were cultured in the presence or absence of anti-CD8 antibody (clone 53 6.72) in the form of ascites for 15 min at 37°C. A final dilution of 1:1,000 of anti-CD8 antibody was used. Flow cytometric and functional analyses showed this concentration to be saturating. A total of 5 × 104 P815 stimulators that had been infected 18 to 24 h previously with the indicated virus and UV inactivated were then added. Cells were cocultured in plates as described previously (2). Briefly, cells were cultured in plates coated with 2 μg of anti-mouse gamma interferon (IFN-γ) monoclonal antibody R4-6A2 (PharMingen, San Diego, Calif.) per ml. After 36 h, the plates were washed and incubated for 90 min with 2 μg of biotinylated anti-mouse IFN-γ monoclonal antibody XMG1.2 (PharMingen) per ml. Following washing, the plates were incubated with 2 μg of horseradish peroxidase-conjugated avidin per ml for 90 min. Sigma Fast 3,3′-diaminobenzidine tablet sets (Sigma, St. Louis, Mo.) were subsequently added for 15 min for the detection of IFN-γ-producing cells. The number of spots was determined with the aid of a stereomicroscope. Determination of the relative avidity of cells is based on the fact that CTL of higher avidity are those that produce IFN-γ in the presence of anti-CD8 antibody following stimulation with APC infected with virus and CTL of lower avidity are those that are blocked by anti-CD8 antibody (1, 4). Nonspecific spot production was assessed by culturing the highest input number of responder cells in the presence of stimulator cells that were uninfected or were infected with viruses expressing irrelevant antigens.
Tissue sampling.
The mediastinal lymph nodes (MLN) were removed from mice immunized with SV5 i.n. and processed to give single-cell suspensions. MLN were pooled from three mice in each experiment.
Immunizations.
Mice were anesthetized by intraperitoneal (i.p.) injection with 2,2,2-tribromoethanol (Avertin). Anesthetized mice were immunized i.n. with 50 μl of phosphate-buffered saline containing the indicated amount of virus as previously described (23). Inoculations of 100 μl of viral suspensions were delivered subcutaneously (s.c.) into the scruffs of the necks of mice as previously described (6). Viral suspensions were inoculated i.p. in a volume of 100 μl.
RESULTS
SV5-specific cytolytic activity in immunized mice.
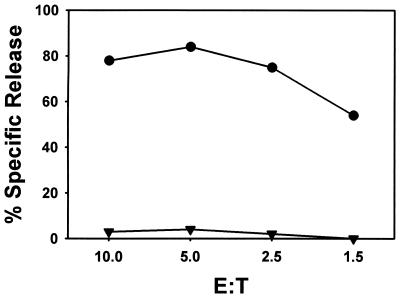
As a first step in the analysis of the immunogenicity of SV5, BALB/c mice were immunized i.n. with 106 PFU of WT SV5. Splenocytes from immunized mice were harvested 6 days postimmunization and restimulated in vitro with UV-inactivated syngeneic spleen cells that had been infected with WT SV5 (MOI = 10) 18 to 24 h previously. CTL lines were maintained in vitro by weekly restimulation with SV5-infected splenocyte stimulators. CTL activity was measured in a standard 51Cr release assay. CTL were able to lyse P815-infected targets in an antigen-specific manner, efficiently lysing infected targets but not mock-infected targets (Fig. 1). These data are in agreement with previous findings that SV5 is immunogenic in BALB/c mice and produces a potent CTL response that can be expanded in vitro (52).
FIG. 1.
CTL response of BALB/c mice immunized with SV5. CTL cultures and chromium release assays were performed as described in Materials and Methods. P815 (H-2d) cells infected with SV5 (●) or mock infected (▾) were used as targets. The data shown are representative of three independent experiments.
Mapping of the SV5 CTL response.
We have used an ELISPOT-based assay system to determine the proteins that contain epitopes that are important in the immune response to SV5. This approach has the advantage that it can be performed on cells directly ex vivo and allows for the quantitation of the number of responding CTL. The readout for the ELISPOT is the detection of secreted IFN-γ from activated CTL under specific stimulation conditions.
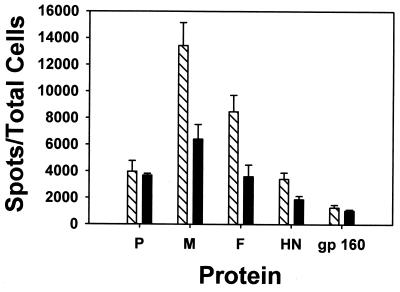
To determine the proteins that were immunogenic, mice were immunized with WT rSV5 i.n., and on day 12 postimmunization, the splenocytes were harvested and used in ELISPOT assays. The responder splenocytes were cocultured with P815 cells that had been infected with rVV expressing individual SV5 proteins or polypeptides. As shown in Fig. 2, the immunodominant response to SV5 infection in the BALB/c mice is directed against an epitope present in the M protein. Subdominant responses were detected against epitopes present in P, F, and HN proteins. CTL from SV5-infected mice recognized cells expressing the other SV5 polypeptides to the same extent as negative-control stimulator cells that were infected with a recombinant vaccinia virus expressing the human immunodeficiency virus gp160 glycoprotein (vPE-16). Since P815 cells express MHC class I antigens and not MHC class II antigens, the IFN-γ production observed was mediated through class I-restricted CTL.
FIG. 2.
Immunodominant CTL response against an epitope in the M protein and subdominant responses directed against epitopes in the P, F, and HN proteins. Mice were immunized i.n. with SV5, and 12 days postinfection splenocytes were isolated and analyzed using ELISPOT assays. P815 cells had been infected 18 h previously with recombinant vaccinia viruses as described in Materials and Methods and used as stimulators. The data shown are representative of four independent experiments.
Relative avidity of the SV5-specific responses over time.
It has previously been shown that CTL avidity can be an important determinant of the potency of antiviral CTL (3). To determine the relative avidity of the anti-SV5 CTL response, we used a modified ELISPOT assay. Our assay is based on the observation that low-avidity CTL require CD8 engagement in order to elicit effector function, whereas high-avidity CTL are relatively CD8 independent for activation (1, 4, 24, 42, 43, 49). We were able to modify the ELISPOT assay to quantify the numbers of high- and low-avidity CTL specific for SV5 polypeptides by including anti-CD8 blocking antibody in cocultures of responding splenocytes and P815-stimulating cells. Only those CTL that are of high avidity will produce IFN-γ in the presence of anti-CD8 blocking antibody (2). Therefore, the total response to each protein will be detected in the absence of antibody, and the number of low-avidity CTL (CD8 dependent) can be determined by subtracting the number of cells detected in the presence of anti-CD8 antibody from the total response.
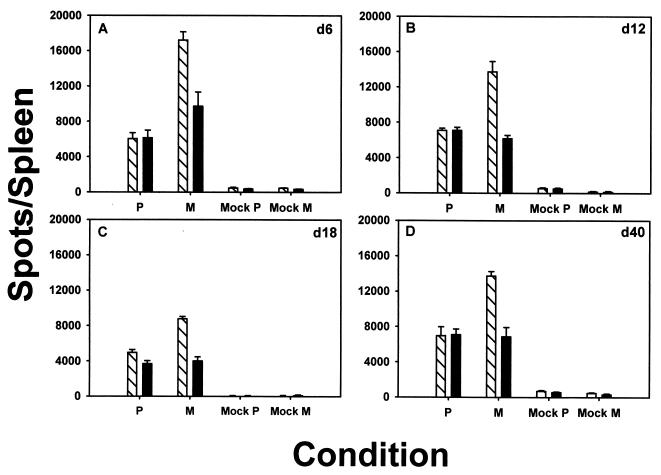
Typically, a response to a particular antigen is characterized by the presence of both high- and low-avidity CTL (2, 3, 17, 51, 53). When CTL against the M protein were analyzed on day 6 postinfection, the number of responding CTL in the presence of anti-CD8 blocking antibody (Fig. 3A, black bar) was ≈60% of the number in the absence of anti-CD8 antibody (hatched bar). Thus, the responding population specific for the immunodominant M epitope contained ≈40% CD8-dependent, or low-avidity CTL (Fig. 3A). In surprising contrast to this finding, the response to P was almost entirely CD8 independent. This suggests that 6 days after immunization, the response to P consists almost exclusively of high-avidity responders. The predominantly high-avidity P-specific CTL response detected was not exclusively due to its subdominant status, since the responses to the subdominant epitopes from HN and F were comprised of both high- and low-avidity CTL (Fig. 3A).
FIG. 3.
Predominantly high-avidity P-specific response in mice immunized with SV5. BALB/c mice were immunized i.n. with 3 × 106 PFU of SV5, and ELISPOT assays were performed 6 days (A), 12 days (B), 18 days (C), or 40 days (D) after infection as described in the legend to Fig. 2. ELISPOT assays were performed in the absence (cross-hatched bars) or presence (black bars) of anti-CD8 blocking antibody. The data shown are representative of three independent experiments.
To determine if the avidity of anti-SV5 CTL responses was maintained throughout the acute response and into the memory pool, mice were vaccinated with WT rSV5 and ELISPOT assays were performed on splenocyte responder cells isolated 12, 18, and 40 days postinfection in the presence or absence of anti-CD8 blocking antibody. Low-avidity CTL specific for the immunodominant M epitope were detectable at all time points analyzed, ranging from ≈30 to 70% of the total responding population (average, 51.9 ± 19.3% [standard deviation]). Similarly, the responses to F and HN also contained a mixture of high- and low-avidity CTL (Fig. 3). The predominantly high-avidity response to P continued through day 12 but began to decline by day 18, with the number of low-avidity cells increasing over time (Fig. 3B and C). The number of detectable P-specific CTL at day 40 postinfection was quite small (Fig. 3D). Thus, it was difficult to determine whether the numbers of high- and low-avidity CTL were different in this response; however, in two of three experiments performed, the numbers of spots in the presence and absence of anti-CD8 antibody were not statistically different. These data suggest that soon after SV5 infection, a predominantly high-avidity response against an epitope present in P is elicited, and by day 18 after infection this high-avidity response begins to decline.
To determine if the high-avidity response to P that was detected in the spleen was also present in the MLN, ELISPOT assays were performed on cells isolated from mice immunized i.n. with SV5. As seen in the previous analyses, the response to M was immunodominant, with subdominant responses apparent for P, F, and HN (Fig. 4). Also in agreement with the results shown in Fig. 3, the P-specific response at day 6 postinfection was predominantly high avidity. Interestingly, the relative proportion of CTL responding to the F-specific epitope was comparatively larger in the MLN than in the spleen at the same time point. However, the ratio of high-avidity cells to low-avidity cells was maintained. The significance of these findings is currently under investigation.
FIG. 4.
High-avidity P-specific response detected in MLN. BALB/c mice were immunized i.n. with 3 × 106 PFU of SV5, and 6 days later MLN were harvested from three mice and pooled for analysis in ELISPOT assays. ELISPOT assays were performed in the absence (cross-hatched bars) or presence (black bars) of anti-CD8 blocking antibody. The data shown are representative of three independent experiments.
Predominantly high-avidity CTL response directed against P is independent of expression by SV5.
Although the ability to selectively activate high-avidity CTL is a highly desirable attribute for vaccine purposes, the mechanism by which an antigen could display this property is unknown. As a first step in dissecting the ability of P to induce this novel high-avidity response, we determined whether the expression of P by a different virus, vaccinia virus, elicited a similar response. Mice were immunized i.n. with a recombinant vaccinia virus expressing the full-length P protein (rVV-P). A recombinant vaccinia virus expressing the full-length M protein (rVV-M) was used in parallel as a control for a protein eliciting both high- and low-avidity CTL. ELISPOT analyses with anti-CD8 blocking antibody were performed on splenocytes 6, 12, 18, and 40 days postimmunization (Fig. 5). Similarly to SV5 infection, rVV-M infection elicited CTL of both high and low avidities over the time course of the experiment (Fig. 5). Similarly to the finding for CTL activated by SV5 infection, rVV-P infection elicited a mainly high-avidity P response by day 6 after immunization (Fig. 5A). This response remained predominantly high avidity through day 12 (Fig. 5B). As seen with SV5 infection, there was a slight increase in the number of low-avidity CTL specific for P epitope(s) detected by day 18 after immunization (Fig. 5C). However, by day 40 postinfection with rVV-P the predominant response was again exclusively high avidity (Fig. 5D), suggesting that high-avidity P-specific CTL may preferentially survive into the memory population following vaccinia virus infection. These data indicate that the high-avidity response specific for the epitope(s) present in the SV5 P protein is not dependent on SV5 infection but rather is an inherent property of the polypeptide or the epitope.
FIG. 5.
The high-avidity P-specific response is independent of expression by rSV5. Mice were immunized i.n. with 106 PFU of either rVV-P or rVV-M, and ELISPOT assays were performed on splenocytes isolated 6 days (A), 12 days (B), 18 days (C), or 40 days (D) after infection. ELISPOT assays were performed in the absence (cross-hatched bars) or presence (black bars) of anti-CD8 blocking antibody. P815 cells infected with SV5 were used as stimulators. As negative controls, splenocytes from mice immunized with rVV-P (Mock P) or rVV-M (Mock M) were plated together with uninfected P815 cells. The data shown are representative of three independent experiments.
The route of immunization does not affect the avidity of the P-specific response.
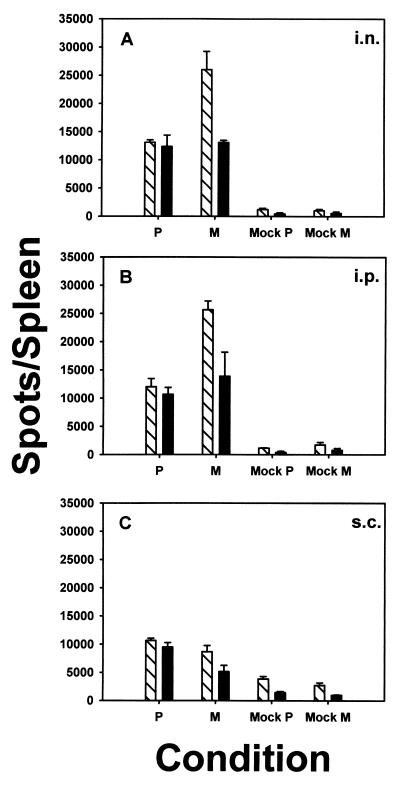
The site of antigen entry can have significant effects on the subsequent immune response. Thus, it was of interest to determine whether the observed pattern of immunodominance and the high-avidity nature of the P response were dependent on the route of immunization. As immunization with rVV-P yielded a large number of P-specific CTL while maintaining the high-avidity nature of the response, mice were immunized i.n., i.p., or s.c. with rVV-P or, as a control, with rVV-M. Intraperitoneal immunization resulted in an immune response of size and avidity similar to those obtained with i.n. immunization (compare Fig. 6A and B). Although the relative sizes of the P-specific and M-specific responses were maintained following i.n. and i.p. immunization with rVV-P or rVV-M, we observed that following s.c. infection the relative size of the M-specific response was reduced compared to that of the P-specific response (Fig. 6C). However, the high-avidity nature of the P response was maintained.
FIG. 6.
Route of immunization does not affect the avidity of the response elicited. Mice were immunized with 106 PFU of either rVV-P or rVV-M i.n. (A), i.p. (B), or s.c. (C), and ELISPOT assays were performed as described in the legend to Fig. 5 on splenocytes isolated 12 days after infection. ELISPOT assays were performed in the absence (cross-hatched bars) or presence (black bars) of anti-CD8 blocking antibody. The data shown are representative of three independent experiments.
The important result from this set of experiments is that the elicitation of the P-specific high-avidity CTL response was detected in mice immunized either i.n., i.p., or s.c. These results suggest that the elicitation of the predominantly high-avidity P-specific CTL response is independent of the route of immunization and thus the high avidity nature of the P response is unlikely to be dependent on a specialized microenvironment or APC present in the lung or draining lymph node of the lung.
DISCUSSION
A typical antigen-specific CTL response is characterized by the elicitation of both high-avidity and low-avidity cells (2, 3, 17, 51, 53). A number of studies have indicated that antiviral vaccine design should focus on increasing the number of high-avidity CTL elicited, since they are more potent than low-avidity cells in their ability to reduce viral load in vivo (3, 15). An ELISPOT assay to quantitate antigen-specific CTL with a defined avidity was developed previously (2). This assay is based on the different requirements of high- and low-avidity cells for CD8 engagement as evidenced by the ability of anti-CD8 antibody to block activation of low-avidity CTL (1, 4, 24, 42, 43, 49). As part of our efforts to utilize SV5 as a model for infection of the respiratory tract and to investigate its potential as a vaccine vector, we have applied this approach to the analysis of CTL elicited following SV5 infection in BALB/c mice. Using the ELISPOT assay we demonstrated that the immunodominant CTL response elicited during SV5 infection in BALB/c mice is directed against an epitope present in the M protein (Fig. 2). A surprising observation was made when we discovered that a subdominant response directed against an epitope present in the phosphoprotein of SV5 was almost exclusively high avidity (Fig. 3). This is the first report of an epitope with this property.
This high-avidity P-specific response is present at day 6 and continues through day 12 (Fig. 3). However, by day 18 after infection low-avidity CTL can be detected in addition to high-avidity CTL. The mechanism responsible for the increase in low-avidity CTL at day 18 following SV5 infection is unknown. However, one factor that has been shown to be important in the immune response elicited by a particular epitope is the available T-cell repertoire (14, 50). One possibility is that there is a second epitope present in the P protein that elicits low-avidity CTL but the frequency of these cells in the responding population is low, thus preventing their detection until sufficient time has passed to allow for their expansion. Work is in progress to determine if the anti-P response is directed to a single epitope or whether multiple epitopes are recognized.
As seen with WT rSV5, infection of BALB/c mice with rVV-P results in a high-avidity P-specific response that is detected through day 12 (Fig. 5A and B). By day 18 there is a slight decrease in the relative number of high-avidity CTL (Fig. 5C). However, while the low number of P-specific CTL present in the memory pool following immunization with WT rSV5 made it difficult to determine unambiguously the percentage of high-avidity CTL, a greater number of CTL were present at day 40 following rVV-P infection and here a predominantly high-avidity response was clearly detected (Fig. 5D). This result demonstrates that the high-avidity CTL response to P is independent of expression by SV5 and would suggest that during acute infection high-avidity P-specific CTL that can survive efficiently into the memory pool are elicited. It is possible that the memory responses generated as a result of immunization with WT rSV5 and rVV-P differ in the relative ratio of high-avidity to low-avidity CTL. We are currently working to increase the generation of SV5-specific memory CTL by engineering rSV5 to express cytokines. This should allow for determination of the ratio of high-avidity to low-avidity CTL elicited as a result of infection with SV5.
Our studies are the first to investigate the effect of the route of immunization on the avidity of the response elicited. Upon immunization by different routes we found that the avidity profile of the P-specific response was similar to that detected in the spleen. However, the sizes of the responses to M and P were substantially lower in mice infected s.c. (Fig. 6). This finding is in agreement with previous studies showing that the size of the immune response can vary depending on the route of infection (27, 39).
Our data suggest that the SV5 P protein or a peptide within the P protein has an inherent property that is responsible for eliciting predominantly high-avidity responses. As has been shown in previous studies, the determinant density on antigen-presenting cells is an important factor for the expansion of high-avidity CTL (1, 3, 8, 53). Stimulation with low concentrations of peptide activates high-avidity CTL, whereas high concentrations of peptide are required for the activation of low-avidity CTL. Thus, a low concentration of P peptide-MHC complexes could preferentially elicit a high-avidity response.
There are a number of factors that could impact the number of P peptide-MHC complexes displayed for recognition by CTL including the binding affinity of the peptide for the MHC and the efficiency with which the P peptide is liberated from the protein. Recent structural data have shown that the P protein of Sendai virus, a paramyxovirus related to SV5, forms highly stable homotetramers with an atypical coiled-coil structure (45). If this structure is also present in the SV5 P protein, it is possible that the determinant density of P peptide-MHC class I molecules is low on the surfaces of infected cells due to the low turnover rate of the protein. The stability of P protein during SV5 infection is currently under investigation.
The residues flanking an epitope have been shown to be important for determining the efficiency with which epitopes are presented (26, 29). Thus, a second feature that could contribute to a low level of presented P peptide is the nature of the residues flanking the peptide. If residues that prevent efficient processing of the P peptide are present, this may allow for only small amounts of P peptide to be processed and loaded into MHC class I molecules during infection, resulting in a low level of P epitope presented to CTL.
The binding affinity of peptides for MHC class I molecules requires a minimum threshold in order to be immunogenic (40, 50). If the P epitope has a binding affinity that is just above the minimum threshold for being immunogenic, it is possible that this may play a role in the population of T cells that can respond to the epitope. Further, the SV5 P protein is highly phosphorylated (25). It has been shown that phosphorylation of peptides can have negative effects on their ability to bind MHC class I molecules (5). Thus, it is possible that the epitope of interest is phosphorylated and binds MHC inefficiently. Further studies of this response should allow for the identification of the mechanism responsible for the preferential activation of high-avidity CTL by the P protein or peptide. We anticipate that the attribute(s) identified in the P protein or peptide can be engineered into other proteins or peptides in order to confer the ability to target high-avidity CTL in vivo.
Although the presence of immunodominant and subdominant epitopes has been well documented (10, 30, 47, 48), the attributes that confer these properties are not fully defined. The results from this study allow evaluation of whether there is a link between dominance and CTL avidity. The immunodominant response is by definition the response with the largest number of responding CTL. Previous studies investigating the repertoire of CTL capable of responding to the immunodominant ovalbumin epitope (SIINFEKL) have shown that the number of low-avidity precursors is greater than that of high-avidity cells (2). Thus, one could hypothesize that the increase in size of an immunodominant response could be due, at least in part, to the activation of lower-avidity precursors in addition to higher-avidity precursors. It would follow then that the smaller size of a subdominant response would be the result of the elicitation of only a subset of the CTL that possess a restricted avidity. Although the subdominant anti-P response would support this hypothesis, the presence of high- and low-avidity CTL in the anti-F and anti-HN subdominant responses at ratios similar to that found in the immunodominant response would suggest that there is not a link between avidity and immunodominance.
In summary, the study presented here examines the immune response generated following i.n. infection with SV5. The results show that in BALB/c mice an immunodominant response is elicited against an epitope present in the M protein while subdominant responses are directed against epitopes present in the P, F, and HN proteins. Upon further analysis the avidities of the responses directed against M, F, and HN were found to be similar to those of other antigens studied in the past in that both high-avidity and low-avidity CTL were elicited. Surprisingly, the subdominant response directed against the P protein was almost entirely high avidity. This high-avidity response is an inherent property of the P protein or peptide and is independent of the route of infection. The importance of this finding becomes apparent when considering the numerous studies that have shown the increased in vivo efficacy of high-avidity CTL in lowering viral load and clearing tumors (3, 15, 53). A further understanding of the factors that contribute to the preferential elicitation of high-avidity P-specific CTL and their long-term survival may allow us to confer these properties on other epitopes such that high-avidity CTL can be targeted for in vivo activation and expansion. This in turn may allow for the design of more potent vaccines.
ACKNOWLEDGMENTS
We thank Douglas Lyles for helpful comments on the manuscript. We thank Biao He and Robert Lamb for kindly supplying the SV5 infectious clone cDNA. We thank Robert Lamb and Reay Paterson for rVV expressing HN and F.
This work was supported by NIH grants AI 43592 (to M.A.M.) and AI 44282 (to G.D.P.).
REFERENCES
- 1.Alexander M A, Damico C A, Wieties K M, Hansen T H, Connolly J M. Correlation between CD8 dependency and determinant density using peptide-induced, Ld-restricted cytotoxic T lymphocytes. J Exp Med. 1991;173:849–858. doi: 10.1084/jem.173.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander-Miller M A. Differential expansion and survival of high and low avidity cytotoxic T cell populations during the immune response to a viral infection. Cell Immunol. 2000;201:58–62. doi: 10.1006/cimm.1999.1632. [DOI] [PubMed] [Google Scholar]
- 3.Alexander-Miller M A, Leggatt G R, Berzofsky J A. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander-Miller M A, Leggatt G R, Sarin A, Berzofsky J A. Role of antigen, CD8, and cytotoxic T lymphocyte (CTL) avidity in high dose antigen induction of apoptosis of effector CTL. J Exp Med. 1996;184:485–492. doi: 10.1084/jem.184.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen M H, Bonfill J E, Neisig A, Arsequell G, Sondergaard I, Valencia G, Neefjes J, Zeuthen J, Elliott T, Haurum J S. Phosphorylated peptides can be transported by TAP molecules, presented by class I MHC molecules, and recognized by phosphopeptide-specific CTL. J Immunol. 1999;163:3812–3818. [PubMed] [Google Scholar]
- 6.Bennett A M, Elvin S J, Wright A J, Jones S M, Phillpotts R J. An immunological profile of Balb/c mice protected from airborne challenge following vaccination with a live attenuated Venezuelan equine encephalitis virus vaccine. Vaccine. 2000;19:337–347. doi: 10.1016/s0264-410x(00)00123-7. [DOI] [PubMed] [Google Scholar]
- 7.Bilyk N, Holt P G. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulating factor. J Exp Med. 1993;177:1773–1777. doi: 10.1084/jem.177.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cawthon A G, Lu H, Alexander-Miller M A. Peptide requirement for CTL activation reflects the sensitivity to CD3 engagement: correlation with CD8αβ versus CD8αα expression. J Immunol. 2001;167:2577–2584. doi: 10.4049/jimmunol.167.5.2577. [DOI] [PubMed] [Google Scholar]
- 9.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Anton L C, Bennink J R, Yewdell J W. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity. 2000;12:83–93. doi: 10.1016/s1074-7613(00)80161-2. [DOI] [PubMed] [Google Scholar]
- 11.Cohn M L, Robinson E D, Thomas D, Faerber M, Carey S, Sawyer R, Goswami K K, Johnson A H, Richert J R. T cell responses to the paramyxovirus simian virus 5: studies in multiple sclerosis and normal populations. Pathobiology. 1996;64:131–135. doi: 10.1159/000164026. [DOI] [PubMed] [Google Scholar]
- 12.Constant S L, Lee K S, Bottomly K. Site of antigen delivery can influence T cell priming: pulmonary environment promotes preferential Th2-type differentiation. Eur J Immunol. 2000;30:840–847. doi: 10.1002/1521-4141(200003)30:3<840::AID-IMMU840>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Conzelmann K K. Genetic manipulation of non-segmented negative-strand RNA viruses. J Gen Virol. 1996;77:381–389. doi: 10.1099/0022-1317-77-3-381. [DOI] [PubMed] [Google Scholar]
- 14.Daly K, Nguyen P, Woodland D L, Blackman M A. Immunodominance of major histocompatibility complex class I-restricted influenza virus epitopes can be influenced by the T-cell receptor repertoire. J Virol. 1995;69:7416–7422. doi: 10.1128/jvi.69.12.7416-7422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derby M, Alexander-Miller M, Tse R, Berzofsky J. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. J Immunol. 2001;166:1690–1697. doi: 10.4049/jimmunol.166.3.1690. [DOI] [PubMed] [Google Scholar]
- 16.Earl P L, Koenig S, Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991;65:31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallimore A, Dumrese T, Hengartner H, Zinkernagel R M, Rammensee H G. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J Exp Med. 1998;187:1647–1657. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Sastre A. Negative-strand RNA viruses: applications to biotechnology. Trends Biotechnol. 1998;16:230–235. doi: 10.1016/s0167-7799(98)01192-5. [DOI] [PubMed] [Google Scholar]
- 19.Goswami K K, Lange L S, Mitchell D N, Cameron K R, Russell W C. Does simian virus 5 infect humans? J Gen Virol. 1984;65:1295–1303. doi: 10.1099/0022-1317-65-8-1295. [DOI] [PubMed] [Google Scholar]
- 20.Herscowitz H B. In defense of the lung: paradoxical role of the pulmonary alveolar macrophage. Ann Allergy. 1985;55:634–650. [PubMed] [Google Scholar]
- 21.Hiebert S W, Paterson R G, Lamb R A. Hemagglutinin-neuraminidase protein of the paramyxovirus simian virus 5: nucleotide sequence of the mRNA predicts an N-terminal membrane anchor. J Virol. 1985;54:1–6. doi: 10.1128/jvi.54.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiebert S W, Paterson R G, Lamb R A. Identification and predicted sequence of a previously unrecognized small hydrophobic protein, SH, of the paramyxovirus simian virus 5. J Virol. 1985;55:744–751. doi: 10.1128/jvi.55.3.744-751.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou S, Doherty P C, Zijlstra M, Jaenisch R, Katz J M. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992;149:1319–1325. [PubMed] [Google Scholar]
- 24.Kwan-Lim G E, Ong T, Aosai F, Stauss H, Zamoyska R. Is CD8 dependence a true reflection of TCR affinity for antigen? Int Immunol. 1993;5:1219–1228. doi: 10.1093/intimm/5.10.1219. [DOI] [PubMed] [Google Scholar]
- 25.Lamb R A, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1177–1204. [Google Scholar]
- 26.Mo A X, van Lelyveld S F, Craiu A, Rock K L. Sequences that flank subdominant and cryptic epitopes influence the proteolytic generation of MHC class I-presented peptides. J Immunol. 2000;164:4003–4010. doi: 10.4049/jimmunol.164.8.4003. [DOI] [PubMed] [Google Scholar]
- 27.Murata K, Garcia-Sastre A, Tsuji M, Rodrigues M, Rodriguez D, Rodriguez J R, Nussenzweig R S, Palese P, Esteban M, Zavala F. Characterization of in vivo primary and secondary CD8+ T cell responses induced by recombinant influenza and vaccinia viruses. Cell Immunol. 1996;173:96–107. doi: 10.1006/cimm.1996.0255. [DOI] [PubMed] [Google Scholar]
- 28.Ng D T, Randall R E, Lamb R A. Intracellular maturation and transport of the SV5 type II glycoprotein hemagglutinin-neuraminidase: specific and transient association with GRP78-BiP in the endoplasmic reticulum and extensive internalization from the cell surface. J Cell Biol. 1989;109:3273–3289. doi: 10.1083/jcb.109.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niedermann G, Butz S, Ihlenfeldt H G, Grimm R, Lucchiari M, Hoschutzky H, Jung G, Maier B, Eichmann K. Contribution of proteasome-mediated proteolysis to the hierarchy of epitopes presented by major histocompatibility complex class I molecules. Immunity. 1995;2:289–299. doi: 10.1016/1074-7613(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 30.Oukka M, Manuguerra J C, Livaditis N, Tourdot S, Riche N, Vergnon I, Cordopatis P, Kosmatopoulos K. Protection against lethal viral infection by vaccination with nonimmunodominant peptides. J Immunol. 1996;157:3039–3045. [PubMed] [Google Scholar]
- 31.Palese P. Genetic engineering of infectious negative-strand RNA viruses. Trends Microbiol. 1995;3:123–125. doi: 10.1016/s0966-842x(00)88897-6. [DOI] [PubMed] [Google Scholar]
- 32.Parks G D. Mapping of a region of the paramyxovirus L protein required for the formation of a stable complex with the viral phosphoprotein P. J Virol. 1994;68:4862–4872. doi: 10.1128/jvi.68.8.4862-4872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parks G D, Ward C D, Lamb R A. Molecular cloning of the NP and L genes of simian virus 5: identification of highly conserved domains in paramyxovirus NP and L proteins. Virus Res. 1992;22:259–279. doi: 10.1016/0168-1702(92)90057-g. [DOI] [PubMed] [Google Scholar]
- 34.Parks G D, Ward K R, Rassa J C. Increased readthrough transcription across the simian virus 5 M-F gene junction leads to growth defects and a global inhibition of viral mRNA synthesis. J Virol. 2001;75:2213–2223. doi: 10.1128/JVI.75.5.2213-2223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paterson R G, Harris T J, Lamb R A. Fusion protein of the paramyxovirus simian virus 5: nucleotide sequence of mRNA predicts a highly hydrophobic glycoprotein. Proc Natl Acad Sci USA. 1984;81:6706–6710. doi: 10.1073/pnas.81.21.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paterson R G, Lamb R A, Moss B, Murphy B R. Comparison of the relative roles of the F and HN surface glycoproteins of the paramyxovirus simian virus 5 in inducing protective immunity. J Virol. 1987;61:1972–1977. doi: 10.1128/jvi.61.6.1972-1977.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paterson R G, Leser G P, Shaughnessy M A, Lamb R A. The paramyxovirus SV5 V protein binds two atoms of zinc and is a structural component of virions. Virology. 1995;208:121–131. doi: 10.1006/viro.1995.1135. [DOI] [PubMed] [Google Scholar]
- 38.Pekosz A, He B, Lamb R A. Reverse genetics of negative-strand RNA viruses: closing the circle. Proc Natl Acad Sci USA. 1999;96:8804–8806. doi: 10.1073/pnas.96.16.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahman F, Dahmen A, Herzog-Hauff S, Bocher W O, Galle P R, Lohr H F. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology. 2000;31:521–527. doi: 10.1002/hep.510310237. [DOI] [PubMed] [Google Scholar]
- 40.Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, Kast W M, Melief C J, Oseroff C, Yuan L, Ruppert J, et al. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol. 1994;153:5586–5592. [PubMed] [Google Scholar]
- 41.Sheshberadaran H, Lamb R A. Sequence characterization of the membrane protein gene of paramyxovirus simian virus 5. Virology. 1990;176:234–243. doi: 10.1016/0042-6822(90)90248-p. [DOI] [PubMed] [Google Scholar]
- 42.Shimonkevitz R, Luescher B, Cerottini J C, MacDonald H R. Clonal analysis of cytolytic T lymphocyte-mediated lysis of target cells with inducible antigen expression: correlation between antigen density and requirement for Lyt-2/3 function. J Immunol. 1985;135:892–899. [PubMed] [Google Scholar]
- 43.Speiser D E, Kyburz D, Stubi U, Hengartner H, Zinkernagel R M. Discrepancy between in vitro measurable and in vivo virus neutralizing cytotoxic T cell reactivities. Low T cell receptor specificity and avidity sufficient for in vitro proliferation or cytotoxicity to peptide-coated target cells but not for in vivo protection. J Immunol. 1992;149:972–980. [PubMed] [Google Scholar]
- 44.Stumbles P A, Thomas J A, Pimm C L, Lee P T, Venaille T J, Proksch S, Holt P G. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J Exp Med. 1998;188:2019–2031. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarbouriech N, Curran J, Ruigrok R W, Burmeister W P. Tetrameric coiled coil domain of Sendai virus phosphoprotein. Nat Struct Biol. 2000;7:777–781. doi: 10.1038/79013. [DOI] [PubMed] [Google Scholar]
- 46.Thomas S M, Lamb R A, Paterson R G. Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell. 1988;54:891–902. doi: 10.1016/S0092-8674(88)91285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Most R G, Murali-Krishna K, Whitton J L, Oseroff C, Alexander J, Southwood S, Sidney J, Chesnut R W, Sette A, Ahmed R. Identification of Db- and Kb-restricted subdominant cytotoxic T-cell responses in lymphocytic choriomeningitis virus-infected mice. Virology. 1998;240:158–167. doi: 10.1006/viro.1997.8934. [DOI] [PubMed] [Google Scholar]
- 48.van der Most R G, Sette A, Oseroff C, Alexander J, Murali-Krishna K, Lau L L, Southwood S, Sidney J, Chesnut R W, Matloubian M, Ahmed R. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J Immunol. 1996;157:5543–5554. [PubMed] [Google Scholar]
- 49.van Emmerik N E, Daane C R, Knoop C J, Hesse C, Vaessen L M, Balk A H, Mochtar B, Claas F H, Weimar W. The avidity of allospecific cytotoxic T lymphocytes (CTL) determines their cytokine production profile. Clin Exp Immunol. 1997;110:447–453. doi: 10.1046/j.1365-2249.1997.4441458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vitiello A, Yuan L, Chesnut R W, Sidney J, Southwood S, Farness P, Jackson M R, Peterson P A, Sette A. Immunodominance analysis of CTL responses to influenza PR8 virus reveals two new dominant and subdominant Kb-restricted epitopes. J Immunol. 1996;157:5555–5562. [PubMed] [Google Scholar]
- 51.Yee C, Savage P A, Lee P P, Davis M M, Greenberg P D. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- 52.Young D F, Randall R E, Hoyle J A, Souberbielle B E. Clearance of a persistent paramyxovirus infection is mediated by cellular immune responses but not by serum-neutralizing antibody. J Virol. 1990;64:5403–5411. doi: 10.1128/jvi.64.11.5403-5411.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeh H J, III, Perry-Lalley D, Dudley M E, Rosenberg S A, Yang J C. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989–994. [PubMed] [Google Scholar]