Supplemental Digital Content is Available in the Text.
Key Words: daily oral antiretroviral therapy, HIV-DAQ, HIV-PP-R, HIV-PP-RC, patient-reported outcome measures, people with HIV
Abstract
Background:
Patient-reported outcome measures (PROMs) can provide data on the barriers and facilitators of adherence to daily oral antiretroviral therapy (OART) regimens. We aimed to develop PROMs to understand the perspectives of people with HIV (PWH) on (1) facilitators/barriers to daily OART regimen adherence and (2) a hypothetical switch to a long-acting (LA)-OART regimen.
Methods:
Following the US food and drug administration patient-reported outcome guidance, targeted literature reviews and concept elicitation interviews with clinicians (n = 7) and PWH (n = 28) were conducted to develop conceptual models (CMs) of facilitators/barriers to OART regimen adherence. Three de novo PROMs were developed after an item-generation meeting. Three waves of cognitive debriefing interviews were conducted among PWH (n = 30) to demonstrate content validity and refine the PROMs.
Results:
The targeted literature review identified 25 facilitators/barriers; an additional 16 facilitators/barriers were added by clinicians and PWH and represented in 2 CMs. During the item-generation meeting, the CMs were used to develop 3 de novo PROMs: (1) HIV Patient Perspective of Regimen, (2) HIV Patient Perspective of Regimen Change, and (3) HIV Drivers of Adherence Questionnaire. In the cognitive debriefing interviews, PWH corroborated the relevancy of items in the PROMs, and minor adjustments were made for clarity.
Conclusion:
Three content-valid PROMs were developed to understand the treatment experience of PWH taking daily OART and how that experience may be altered upon a switch to weekly LA-OART. Data from future LA-OART clinical trials will help define a scoring guide and evaluate the structure and measurement properties of the PROMs.
INTRODUCTION
Modern daily oral antiretroviral therapy (OART) has transformed survival outcomes in people living with HIV, bringing life expectancy closer to that in the general population.1,2 The treatment is lifelong, and high adherence to OART is required to suppress viral replication, slow progression, reduce transmission, and improve health-related quality of life.3–5 However, adherence to daily OART among people with HIV (PWH) is suboptimal, estimated to be between 27% and 80%,4 in part because of treatment fatigue and sociobehavioral stigma.6 Consequently, a growing body of research has been conducted over the past decade to develop long-acting oral antiretroviral therapies (LA-OARTs) to simplify dosing and address challenges, such as stigma, fear of disclosing HIV status, and daily treatment fatigue, and, as a result, improve adherence to OART.6
It is essential to get a full appreciation of PWH perspectives of daily OART treatment attributes that affect adherence and attributes of LA-OART that may facilitate OART adherence or may create new barriers. Small-scale qualitative research acts as a starting point to elicit such insights and can be used as the basis from which patient-reported outcome measures (PROMs) can be developed. These PROMs can then be applied on a larger scale to provide a comprehensive understanding of the facilitators of and barriers to adherence and to compare treatment options. Although multiple PROMs are available to measure treatment satisfaction, adherence, and/or quality of life among PWH, none directly assess barriers and facilitators or provide PWH perspectives when considering a switch from daily OART to LA-OART.7
This study was conducted to identify, adapt, or construct PROMs to understand PWH perspectives on (1) the facilitators of and barriers to daily OART adherence and (2) the hypothetical switch to LA-OART. This work informed the development of 3 new draft PROMs for use in LA-OART clinical trials to evaluate adherence attributes of and considerations in switching to LA-OART among PWH.
METHODS
Study Design
This study was conducted in 5 steps, comprising a targeted literature review (TLR), clinician concept elicitation (CE), PWH CE, item generation, and cognitive debriefing (CD), as shown in Figure 1.
FIGURE 1.
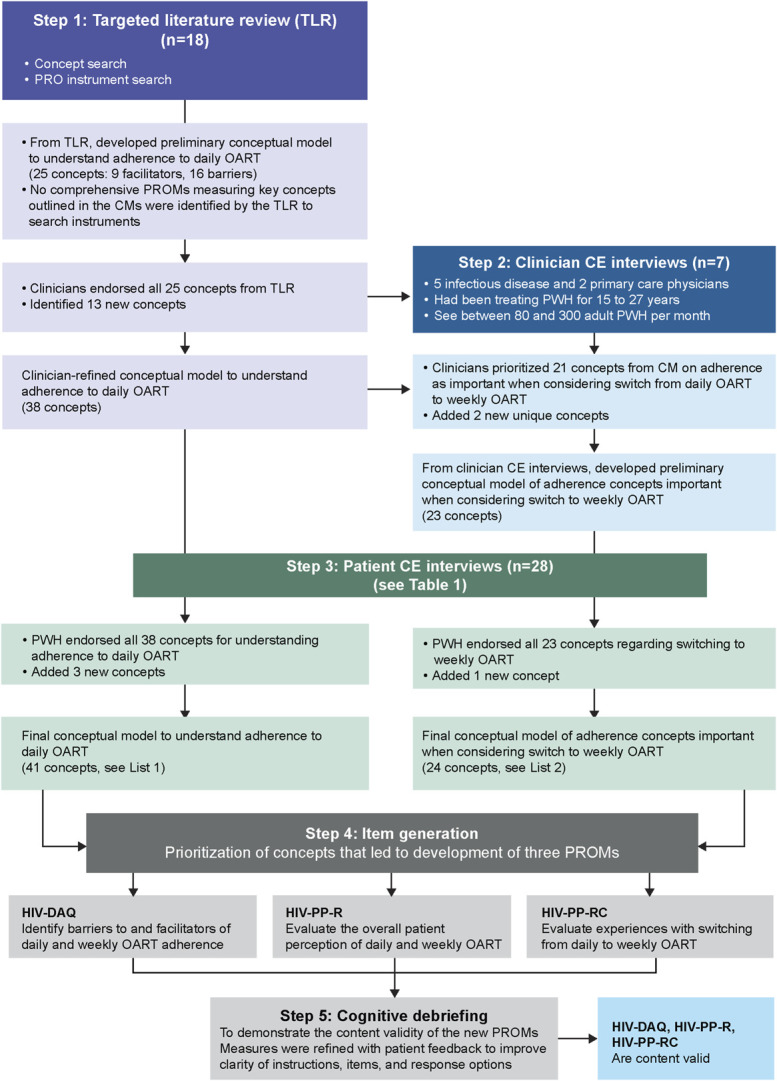
Model development steps.
Steps 1–3: Literature Review, Clinician CE, and PWH CE
An initial TLR was conducted to identify facilitators and barriers to adherence to OART in PWH to create preliminary conceptual models (CMs) capturing the most relevant concepts of the PWH treatment experience. The aim of the TLR was also to identify treatment-related concepts associated with long-acting therapies in analog indications (eg, diabetes, contraceptives, and antipsychotics) that are relevant for PWH.
After the TLR, a noninterventional, cross-sectional, qualitative CE interview study in the United States with clinicians and PWH (n = 7 and 28, respectively, in accordance with US Food and Drug Administration [FDA] guidance8) was conducted to understand perspectives on the treatment experiences of PWH, including facilitators and barriers to both daily OART treatment and a hypothetical switch to weekly OART, and determine the relevance of concepts.8 The PWH CE interviews were conducted in 6 chronological waves (n = 5 PWH for waves 1–5, n = 3 PWH for wave 6) to assess concept saturation. The PWH sample size was expected to be sufficient to achieve concept saturation, the point at which no new data are being derived from the interviews. This approach is in line with the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Good Research Practices Task Force recommendations for CE interviews. Weekly OART was framed as hypothetical because this dosing option does not currently exist. Recruited physicians were required to be actively engaged in patient care (seeing >30 patients/month for initiation or maintenance of antiretroviral therapy), predominantly treating adult patients, having >5 years of clinical practice experience treating patients, and able/willing to speak in English about patient treatment experience. PWH were between 18 and 70 years of age, living in the United States, English-speaking, willing to share treatment experiences, diagnosed with HIV confirmed by their physician or medical record, and currently taking daily OART or on a drug holiday, and had not been in a clinical trial in the prior year. Key aspirational targets of 4 subgroups of PWH considered to be at high risk of HIV infection were identified: men who have sex with men, heterosexual men or women, people who inject drugs, and transgender persons.9–11 Other targets by subgroup, age group, race/ethnicity, and geographic location were broadly selected based on the epidemiological data of HIV incidence and prevalence in the United States. A specialist recruitment vendor applied an inclusive sampling strategy based on FDA guidance on representativeness and generalizability. One-on-one CE interviews were conducted over the telephone by trained qualitative interviewers (length: 60 minutes for clinicians and 75–90 minutes for PWH), following a semistructured discussion guide. All participants were provided gift-card incentives for their time. Audio recordings of interviews were transcribed verbatim. Researchers coded each interview and produced a framework based on the number of PWH who mentioned or prioritized each concept. Key PWH quotations were assembled to provide insight into the language and concepts that PWH used. The preliminary CMs were updated based on the CE feedback.
A second TLR was then conducted to identify PROM(s) that may be appropriate to measure key concepts outlined in the CMs. Detailed methods for the TLR and CE interviews have been included in Supplemental Digital Content 2 (see Supplementary Methods, http://links.lww.com/QAI/C322) and have previously been described.12
Step 4: Item Generation
An item-generation meeting (IGM) was conducted among researchers, including experts in clinical outcomes assessment and PROM development, to develop PROMs that would evaluate the overall perception of daily/weekly OART regimens, capture the experiences of PWH who switch from daily to weekly OART, and measure barriers to and facilitators of taking daily and weekly OART. Item generation (creation of questions and response scales) was performed in accordance with the FDA's patient-focused drug development guidance and item-generation principles, outlined in the ISPOR Patient-Reported Outcome Good Research Practices Task Force report.13–15 The IGM led to the drafting of 3 de novo questionnaires, in paper and electronic formats. The first PROM was developed to evaluate the overall PWH perception of daily and weekly OART (HIV Patient Perspective of Regimen [HIV-PP-R]); the second PROM was developed to evaluate experiences with switching from daily to weekly OART (HIV Patient Perspective of Regimen Change [HIV-PP-RC]); and the third PROM was developed to identify barriers to and facilitators of daily and weekly OART adherence (HIV Drivers of Adherence Questionnaire [HIV-DAQ]).
Step 5: CD
Cross-sectional qualitative CD interviews (n = 30) were performed through 1:1 audio conference to ensure that the PROMs developed after the IGM were relevant and understandable to PWH.16,17 Specifically, CD sought to evaluate the comprehensibility and comprehensiveness of instructions, recall period, formats, and response scales and appropriateness of the items to their treatment experience. Eligible participants met the following inclusion criteria: aged 18–70 years; willing/able to provide informed consent; resided within any state in the United States and could complete the interview in English; had a diagnosis of HIV, which was confirmed by their physician or medical record; and those who were using daily OART or were on a drug holiday. Regarding the CE interviews, a specialist recruitment vendor applied an inclusive sampling strategy based on FDA guidance on representativeness and generalizability. Targets were identified as previously described in the CE section. An aspirational target for new-to-therapy PWH, defined for this study as PWH who have ≤6 months on their first HIV regimen of daily OART, was created to represent subpopulations with less OART treatment experience. Efforts were made to recruit PWH by subgroup, age group, and race/ethnicity from the Centers for Disease Control and Prevention (CDC)–prioritized areas for HIV.17
A standardized PWH cognitive-debriefing interview discussion guide was developed to include open-ended questions to avoid bias and allow for a free-flowing discussion. It included sample probing questions to guide more in-depth discussion on topics and to ascertain PWH understanding. Telephone interviews lasting 60–120 minutes were conducted through audio conference by trained qualitative researchers. Aligned with ISPOR good practices, PWH were asked to complete the PROMs, verbalizing their thought process when providing a response to each question (the “think aloud” technique).15 Afterward, PWH were asked about, and invited to give feedback on, the meaning and relevance of individual items, fit and adequacy of response scales to reflect their experience, and clarity of the items, instructions, and sentence structures. PWH were also provided an opportunity to suggest changes to the draft PROMs. Interviews were conducted across 3 waves (n = 10 in each wave) to allow for updates to the PROMs and/or interview discussion guide between waves. Half of PWH in each wave responded to the paper format, and the other half responded to the electronic format.
Audio files from completed interviews were transcribed verbatim and deidentified. All quantitative (categorical and continuous variables) data were analyzed to generate tables of descriptive statistics (count). Responses to each PROM item were reviewed. PWH feedback on the comprehensiveness of PROM(s) and the relevance and comprehension of each item were analyzed. PWH quotations for items and the overall PROMs were assembled to provide additional details on the language PWH used to describe their experiences and to inform updates to the measures. Where updates were deemed relevant, items were updated at the conclusion of each wave, and the updated measures were tested in the next wave. An item-tracking matrix was developed to track changes to the measures as the waves progressed.
Ethics Approval
PWH interviews for both CE and CD were conducted in accordance with the Declaration of Helsinki, Good Clinical Practice, the regulations of the US FDA as described in 21 Code of Federal Regulations 50 and 56, applicable laws, and the Western Institutional Review Board Copernicus Group, Inc (WCG IRB), requirements. Informed consent was obtained from PWH before performing any study-related procedures. WCG IRB approval of the materials was received on April 9, 2021 (WCG IRB study tracking number 20211189).
RESULTS
TLR and CE Interviews
The concept-focused TLR initially identified 1211 studies, of which 18 studies were deemed relevant and selected for data abstraction (see Figure, Supplemental Digital Content 1, http://links.lww.com/QAI/C321). The concepts identified from the TLR supported the development of 2 preliminary CMs: one for identifying barriers to and facilitators of adherence to daily OART and the other for identifying barriers to and facilitators of adherence after a hypothetical switch from daily to LA-OART.
CE interviews with 7 clinicians supported the inclusion of all 25 concepts from the TLR and identified 13 new concepts relevant to PWH experiences.
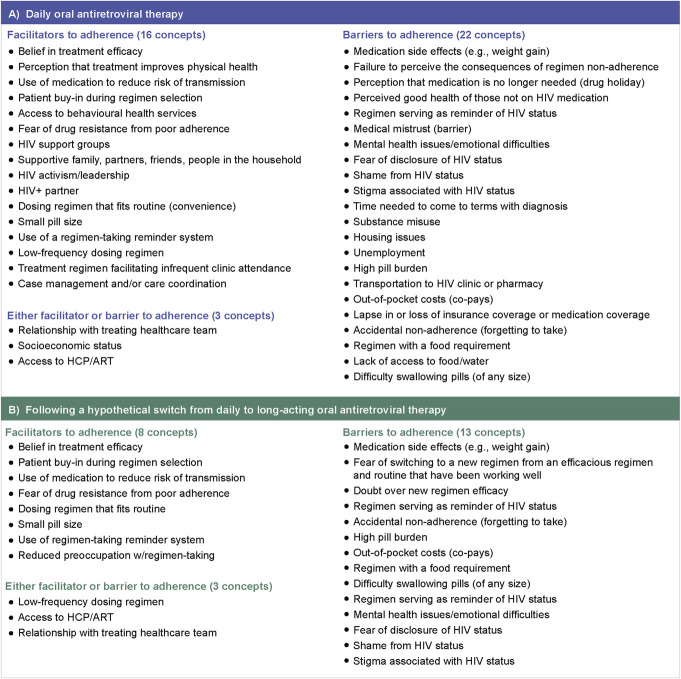
A total of 28 PWH were included in CE interviews, and most were men who have sex with men, African Americans, on ≥ third daily OART, and with >10 years of treatment experience (Table 1). During CE interviews, PWH affirmed all 38 concepts from the TLR, and clinician CE identified 3 new concepts. Final CMs were developed based on the outcomes from the CE interviews (Fig. 2). For the adherence-related concepts, saturation was achieved after wave 3, and for the switch-related concepts, saturation was achieved after wave 4.
TABLE 1.
Characteristics of Patients Who Participated in Concept Elicitation and Cognitive-Debriefing Interviews
| Patient Demographics | Concept Elicitation (N = 28) | Cognitive Debriefing (N = 30) |
| Age (yr), mean ± SD | NC | 47.2 ± 11.7 |
| Age groups (yr), n (%)* | ||
| 18–29 | 5 (18) | 4 (13) |
| 30–49 | 9 (32) | 11 (37) |
| 50–70 | 14 (50) | 15 (50) |
| Subgroups, n (%)* | ||
| Men who have sex with men | 18 (64) | 19 (63) |
| Heterosexual men | 1 (4) | 2 (7) |
| Heterosexual women | 7 (25) | 8 (27) |
| People who inject drugs | 3 (11) | 2 (7) |
| Transgender persons | 1 (4) | 2 (7) |
| Race/ethnicity, n (%)* | ||
| Caucasian or White | 6 (21) | 6 (20) |
| African American, African, or Black | 15 (54) | 14 (47) |
| Hispanic/Latinx/Central, or Spanish origin | 3 (11) | 6 (20) |
| Asian | — | 2 (7) |
| Other (mixed racial background, prefer not to say) | 4 (14) | 2 (7) |
| Geography, n (%)* | ||
| CDC-prioritized area for HIV | 19 (68) | 18 (60) |
| Current living situation, n (%) | ||
| Lives alone | 13 (46) | 14 (47) |
| Lives with someone | 14 (50) | 16 (53) |
| Other (nursing facility) | 1 (4) | — |
| Treatment history with daily OART, n (%)† | ||
| On 1st daily OART | 3 (11) | 4 (13) |
| On 2nd daily OART | 4 (14) | 2 (7) |
| On ≥3rd daily OART | 20 (71) | 24 (80) |
| Treatment experience, n (%) | ||
| <5 yrs | 2 (7) | 3 (10) |
| 5–10 yrs | 10 (36) | 4 (13) |
| >10 yrs | 16 (57) | 23 (77) |
| Highest level of education completed, n (%) | ||
| High school graduate, diploma, or the equivalent | 6 (21) | 4 (13) |
| Some college credit, no degree | 9 (32) | 11 (37) |
| Trade/technical/vocational training | 1 (4) | 2 (7) |
| Associate/bachelor’s degree | 5 (18) | 9 (30) |
| Some postgraduate work or postgraduate degree | 7 (25) | 4 (13) |
| Employment status, n (%) | ||
| Full-time employment | 11 (39) | 12 (40) |
| Part-time employment | 3 (11) | 5 (17) |
| Out of work and looking for work or not looking for work | 4 (14) | 5 (17) |
| Retired | — | 3 (10) |
| Other (unable to work, disabled, military) | 10 (36) | 5 (17) |
| Comorbidities and/or health conditions in addition to living with HIV, n (%) | — | 24 (80) |
Aspirational target groups; a single patient may identify as one or more subgroups.
Treatment history was not captured for 1 patient.
NC, not collected.
FIGURE 2.
Final CM after clinician and PWH CE for PWH treatment experience on (A) daily OART and (B) following a hypothetical switch from daily to LA-OART.
The TLR for PROM instrument search initially identified 140 instruments, of which 56 measured concepts of interest (ie, PWH perception of OART, considerations in switching to LA-OART, and the facilitators of and barriers to adherence) and so they were mapped to the CM. Of these, 15 instruments were assessed for content validity and psychometric strength. None of these 15 instruments covered more than 12.5% of concepts of primary CM. Moreover, no single PROM or combination of existing PROMs captured all the concepts in the preliminary CMs to understand adherence to daily OART or to understand adherence considerations in switching to an LA-OART.
Item Generation
The IGM led to the development of 3 de novo questionnaires in paper and electronic formats: (1) HIV-PP-R, (2) HIV-PP-RC, and (3) HIV-DAQ. The initial HIV-PP-R measured 8 concepts from the CMs, each of which is a facilitator or barrier to both daily OART and LA-OART using 1 item each for each concept: belief in treatment efficacy, use of medication to reduce risk of transmission, fear of resistance, convenience, preoccupation with regimen-taking, fear of disclosure of HIV status, medication side effects, and regimen serving as a reminder of HIV status. Three additional global items were included: overall burden, overall satisfaction, and willingness to continue the trial regimen. The HIV-PP-RC questionnaire (10 items) shared the same concepts and in the same order as are present in the HIV-PP-R, except that the “willingness to continue trial regimen” item is excluded. The HIV-PP-RC differed from the HIV-PP-R in that the HIV-PP-RC explores PWH perspectives of the current (trial) regimen compared with their immediate pretrial regimen, thereby allowing PWH to provide a relative assessment of their experiences. The HIV-DAQ questionnaire comprised 4 parts: overall adherence (Part 1; 4 items), factors or beliefs that make adherence either easy or difficult (Parts 2 and 3; 9 items), and reasons for nonadherence (Part 4; 9 items in addition to 1 free-response option). The concepts and the item language for each concept in Part 4 are the same as in Parts 2 and 3, although the stem and response scales are different.
Cognitive Debriefing
The mean age of PWH was approximately 47.2 years, 19 (63%) PWH were men who have sex with men, 14 (47%) PWH were African American, 23 (77%) PWH had >10 years' treatment experience, 24 (80%) PWH had other comorbidities, and 18 (60%) PWH resided in CDC-prioritized areas for HIV (Table 1).
HIV-PP-R
PWH considered a total of 10 items of the HIV-PP-R relevant in wave 1. Item 11, “willingness to continue trial regimen,” was not considered relevant because PWH found it to be misleading, less relevant, and/or difficult to answer. This item was removed from the PROM after receiving similar feedback in wave 2 (see Table, Supplemental Digital Content 2, http://links.lww.com/QAI/C322). One or more PWH in each wave reported reliance on laboratory results to inform their response to 3 medical items (“belief in treatment efficacy,” “use of medication to reduce risk of transmission,” and “fear of resistance from poor adherence”). As a result, the instructions of the questionnaire were refined to emphasize that the PWH perspective was being sought, rather than their understanding of their laboratory results (see Table, Supplemental Digital Content 2, http://links.lww.com/QAI/C322). Minor refinements to 3 items (“reduced preoccupation with regimen-taking,” “medication side effects,” and “overall burden”) were made to improve clarity following feedback from wave 2. All PWH demonstrated an ability to choose an appropriate response to all items, although response options for the “overall satisfaction” item were refined to improve clarity, and an option was added to the “medication side effects” for PWH who had not experienced any side effects.
Two recall periods were tested, 2 and 4 weeks. PWH reported the ability to recall events for the past 2 and 4 weeks and generally showed no strong preference for either recall period. The decision to proceed with the 4-week recall period was due to PWH reporting a slight preference for the longer recall period in consideration of a weekly hypothetical product. The characteristics of the final HIV-PP-R are included in Table 2.
2. HIV-PP-RC
TABLE 2.
Characteristics of PROMs Developed After CD Interviews
| Characteristics | PROMs | ||
| HIV-PP-R | HIV-PP-RC | HIV-DAQ | |
| Description | Intended to explore patient perceptions (positive, negative, overall) of current HIV regimen | Intended to explore patient perceptions (positive, negative, overall) of the current (trial) HIV regimen compared with the pretrial HIV regimen | Intended to explore regimen-taking behavior during the course of the trial |
| Concepts covered | 1. “Belief in treatment efficacy” 2. “Use of medication to reduce risk of transmission” 3. “Fear of resistance from poor adherence” 4. “Dosing regimen that fits routine (convenience)” 5. “Reduced preoccupation with regimen-taking” 6. “Fear of disclosure of HIV status” 7. “Medication side effects” 8. “Regimen serving as reminder of HIV status” 9. “Overall burden” 10. “Overall satisfaction” |
1. “Belief in treatment efficacy” 2. “Use of medication to reduce risk of transmission” 3. “Fear of resistance from poor adherence” 4. “Dosing regimen that fits routine (convenience)” 5. “Reduced preoccupation with regimen-taking” 6. “Fear of disclosure of HIV status” 7. “Medication side effects” 8. “Regimen serving as reminder of HIV status” 9. “Overall burden” 10. “Overall satisfaction” |
Part 1: Overall adherence “Overall adherence” “Ease of adherence” “Accidental non-adherence” “Use of a regimen-taking reminder system” Part 2: Factors that made adherence either easy or difficult “Small pill size” “Low-frequency dosing regimen” “Dosing regimen that fits routine (convenience)” “Medication side effects” “Regimen serving as reminder of HIV status” Part 3: Beliefs or feelings that make adherence either easy or difficult “Treatment efficacy,” “Use of medication to reduce risk of transmission” “Fear of resistance from poor adherence” “Fear of disclosure of HIV status” Part 4: Reasons for non-adherence—similar concepts to Parts 2 and 3 |
| Number of items | 10 | 10 | 22 |
| Recall period | 2 wk/4 wk | None | 2 wk/4 wk |
| Sample item | In the past X weeks…has your HIV regimen fit conveniently into your lifestyle? | Which HIV regimen serves as more of a reminder of your HIV status? | In the past X weeks…have you forgotten to take your HIV pills at the day(s) and time(s) that were advised by your health care provider? |
| Sample response options | Not at all; a little bit; somewhat; quite a bit; very much | Current regimen more of a reminder; current and prior regimen similar reminders; prior regimen more of a reminder | Not at all; some of the time; most of the time; all of the time |
The HIV-PP-RC shared the same concepts as the HIV-PP-R, and thus, PWH considered items relevant. Minor revisions were made to the items, response options, and instructions to improve clarity (see Table, Supplemental Digital Content 2, http://links.lww.com/QAI/C322). No items were removed but, as with the HIV-PP-R, the instructions of the questionnaire were refined to emphasize the PWH perspective rather than their understanding of their laboratory results. The number of response options was reduced from 5 to 3 for items 1–9, whereas 5 response options were retained for item 10, satisfaction. The HIV-PP-RC has no recall period and instead focuses on the comparison of the PWH current (trial) regimen to their experience with the regimen immediately before entering the trial/switching to the current regimen. The characteristics of the final HIV-PP-RC are included in Table 2.
3. HIV-DAQ
Consistent with changes to the HIV-PP-R and HIV-PP-RC, HIV-DAQ instructions were refined to emphasize that the PWH perspective was being sought. After waves 1 and 2, the language of multiple items was refined, and instructions were slightly modified to improve clarity (see Table, Supplemental Digital Content 2, http://links.lww.com/QAI/C322). After wave 2, 4 items in the section testing facilitators of and barriers to adherence (eg, “belief in treatment efficacy” and “fear of disclosure of HIV status”) were separated into a new section with a unique set of instructions to reaffirm that PWH perspectives were being sought versus understanding of clinical data. To improve distinguishability between options, the response scale was reduced from a 5-point scale to a 4-point scale after wave 2.
Two recall periods were tested, 2 and 4 weeks. Regarding the HIV-PP-R, PWH reported no strong preference for either recall period. The decision to proceed with the 4-week recall period was due to the PWH report of slight preference for the longer recall period in consideration of a weekly hypothetical product. The characteristics of the final HIV-DAQ are included in Table 2.
DISCUSSION
There is a lack of PROMs that measure PWH experience of switching from daily OART to LA-OART. To the best of our knowledge, this study is the first to develop a PROM to understand the treatment experience of taking daily OART and the experience after switching from a daily to a weekly OART. CD interviews with PWH confirmed the relevancy of the items in the 3 PROMs. The overall findings suggest that the 3 new PROMs are content-valid for PWH who have switched from daily to weekly OART, regardless of the duration they had been on daily OART.
The European Medicines Agency and the FDA recommend using PROMs for the evaluation and optimization of new treatments.14,18–21 In the case of equal efficacy and safety of drugs or treatment regimens, PROMs-directed satisfaction, compliance, and comfort with treatment serve as a guide for determining the most appropriate regimen.22 For a PROM to be useful in Health Technology Assessment of HIV therapies, it should be relevant to HIV, demonstrate added benefit over current therapies, be validated, show consistency in assessment, and be codified.23 As opposed to generic PROMs, HIV-specific PROMs have better sensitivity and high specificity for certain HIV-specific domains with no significant ceiling and floor effect.24 There are no PROMs that aim to measure facilitators of and barriers to medication adherence in HIV. Quality-of-life questionnaires, such as the World Health Organization Quality of Life-HIV abbreviated version, measure the impact of disease on the lives of PWH, rather than their ability to take their HIV treatment. Therefore, most concepts in these 3 de novo PROMs are not covered by the World Health Organization Quality of Life-HIV abbreviated version and similar instruments. The HIV Treatment Satisfaction Questionnaire status version and the HIV Treatment Satisfaction Questionnaire change version measure satisfaction with the current regimen and satisfaction with the current regimen compared with the prestudy regimen, respectively,7 but they do not adequately capture the concepts identified in the CMs of daily/weekly OART treatment experience. Moreover, existing PROMs do not measure key concepts that were prioritized by IGM stakeholders, such as “reduced preoccupation with regimen-taking” and “regimen serving as a reminder of HIV status.” The HIV-PP-R and HIV-PP-RC developed in this study address this gap. The evidence generated from the HIV-PP-R and HIV-PP-RC may help identify unmet needs that have been mitigated by the switch to weekly OART and provide support in the everyday clinical practice setting.
Because switching from daily to weekly OART may affect adherence, it is crucial to assess the facilitators of and barriers to weekly OART while retaining specificity to the HIV condition. Adherence Barriers Questionnaire for HIV Patients25 and the Chronic Treatment Acceptance Questionnaire (ACCEPT)26 are the 2 PROMs evaluating adherence in PWH that had the highest representation of concepts outlined in the CMs and IGM; however, they contained some irrelevant items and do not measure 2 key concepts (ie, “use of medication to reduce risk of transmission” and “regimen serving as reminder of HIV status”). Based on our CE interviews with PWH, some of the language used in items in those questionnaires are not aligned with the language of today's PWH. The HIV-DAQ was created to fill this gap; it will allow a better understanding of support and other needs for PWH to successfully adhere, particularly for the difficulties unique to weekly OART, if any. This information can aid PWH support programs/materials in everyday clinical practice and real-world studies, aiming to mitigate some of these challenges.
The patient-centered data generated through the PROMs in clinical trials can guide clinicians and PWH in shared decision-making, support pharmaceutical labeling and reimbursement claims, and inform health care policy.27,28 Regulatory authorities advise using PROMs (eg, release of the FDA patient-focused drug development guidance in 2018) in drug research and development.16,29–31 Patient advocacy groups encourage the use of PROMs, which may result in increased implementation of patient-reported outcome endpoints in clinical trials.31,32 The Health Resources & Services Administration Ryan White HIV/AIDS Program also endorses using PROMs as a way to elevate patient care.33 According to the Luckett and King34 guiding principles toward selecting a PROM, the PROM items (individual questions) should be appropriate to the study and the items should be aggregated into summary scales. Data from future weekly OART clinical trials will allow scalability (designing the measurement properties and structure: scoring guide, definitions of meaningful change in scores, and endpoints using these scores) for these 3 de novo PROMs. The patient data generated through these PROMs, if collected in a scientifically rigorous way, would help identify the PWH experiences of switching from daily to weekly OART and how they affect adherence, aiding overall treatment optimization.
Limitations and Strengths
This study had some limitations, common among such research, such as insufficient representation of harder-to-reach PWH subgroups, because not all aspirational targets were met due to recruiting challenges. Furthermore, targets were not set for sex workers and persons in prison (subgroups of high risk) because of complications in recruitment. This could potentially limit the generalizability of findings to these populations. Conduct of telephone versus face-to-face interviews may create potential bias. Although the discussion guide was developed with the intention to build rapport, it may not develop between some participants and moderators, especially over the telephone, potentially resulting in reduced disclosure of sensitive information about living with HIV. Telephone conversations may also be more prone to distractions. However, it is important to note that some of the interviews were conducted during the height of the COVID-19 pandemic, when conducting face-to-face interviews was challenging. The development of PROMs was limited to US PWH; hence, the PROMs need to be validated for translations to other languages for use outside the United States.
This study also has several strengths. The steps taken in the development and content validation of these 3 PROMs followed the US FDA patient-reported outcome development guidance. Aspirational targets were created, and attempts were made to fulfill those targets. Further work is needed to assess the psychometric performance of these 3 PROMs and to complete a translation and cultural validation process for use outside of the United States. To date, the measures have been translated and culturally validated in over 40 languages across North and South America, Europe, Africa, and Asia.
To our knowledge, this study is the first to develop PROMs for use in clinical trials for PWH to understand the treatment experience of taking daily OART and how that experience may be altered upon switching to LA-OART. PWH who participated in CD interviews corroborated the relevancy of the items in the 3 PROMs. Measures were refined with PWH feedback to improve the clarity of instructions, items, and response options. The overall findings suggest that the 3 new PROMs are content-valid. Data from future weekly OART clinical trials will guide the measurement properties and structure of these PROMs.
Supplementary Material
ACKNOWLEDGMENTS
Leena Patel, Olga Klibanov, Daria Renshaw, Nathaniel Grubbs, and Suzanne Morgen of IQVIA, Inc., provided medical writing and editorial support, which was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ.
Footnotes
Supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ.
A poster presenting part of this work, with the title “Development of Conceptual Models to Understand Patient Experiences with and Attributes of Adherence to HIV Oral Antiretroviral Therapy (OART) and Considerations in Switching to Long-acting OART,” was presented at the HIV Drug Therapy Glasgow 2022; October 23–26, 2022; Glasgow, Scotland. Another poster presenting part of this work, with the title “Establishing Content Validity of 3 new patient-reported outcome (PRO) measures for use in HIV long-acting oral antiretroviral therapy (LA-OART) clinical trials,” was presented at the ISPOR Europe 2022; November 6–9, 2022; Vienna, Austria.
E.F. and T.L.S. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, and shareholders of Merck & Co., Inc., Rahway, NJ. J.R.B., A.B., E.H., S.I.G., S.K., and M.R. are current employees and A.M. was an employee of IQVIA at the time of study conduct, an organization that received funding for the conduct of this study from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
All authors have substantially contributed to the study’s conception, design, or implementation. All authors were involved in data analysis and interpretation. All authors had access to the study results, reviewed and revised the manuscript, and approved the decision to submit for publication.
Contributor Information
Julie R. Bailey, Email: julie.bailey@iqvia.com.
Eileen Fonseca, Email: eileen.fonseca@merck.com.
Alexander Borsa, Email: alexanderborsa@gmail.com.
Emily Hawryluk, Email: emily.hawryluk@iqvia.com.
Steven I. Gubernick, Email: steve.gubernick@iqvia.com.
Anna de la Motte, Email: ajdelamotte@gmail.com.
Stella Karantzoulis, Email: stella.karantzoulis@iqvia.com.
Matthew Reaney, Email: matthew.reaney@iqvia.com.
REFERENCES
- 1.Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society–USA panel. JAMA. 2016;316:191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altice F, Evuarherhe O, Shina S, et al. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. Patient Prefer Adherence. 2019;13:475–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iacob SA, Iacob DG, Jugulete G. Improving the adherence to antiretroviral therapy, a difficult but essential task for a successful HIV treatment—clinical points of view and practical considerations. Front Pharmacol. 2017;8:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angel JB, Freilich J, Arthurs E, et al. Adherence to oral antiretroviral therapy in Canada, 2010–2020. AIDS. 2023;37:2031–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ndung’u T. Will long-acting antiretroviral therapy be a game changer globally? Med. 2021;2:115–117. [DOI] [PubMed] [Google Scholar]
- 7.Woodcock A, Bradley C. Validation of the revised 10-item HIV Treatment Satisfaction Questionnaire status version and new change version. Value Health. 2006;9:320–333. [DOI] [PubMed] [Google Scholar]
- 8.US Department of Health and Human Services, Food and Drug Administration. Patient-Focused Drug Development: Selecting, Developing, or Modifying Fit-for-Purpose Clinical Outcome Assessments. Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. Draft Guidance (FDA-2022-D-1385). 2022. Available at: https://www.fda.gov/media/159500/download. Accessed February 1, 2024. [Google Scholar]
- 9.Centers for Disease Control and Prevention Prevention. National HIV Behavioral Surveillance (NHBS). Last reviewed: June 27, 2023. Available at: https://www.cdc.gov/hiv/statistics/systems/nhbs/index.html. Accessed February 1, 2024. [Google Scholar]
- 10.World Health Organization. Policy Brief: Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations, 2016 Update. World Health Organization; 2017. Available at: https:/apps.who.int/iris/handle/10665/258967. Accessed February 1, 2024. [Google Scholar]
- 11.Center for Disease Control and Prevention. HIV Incidence: Estimated Annual Infections in the US, 2014─2018. Available at: https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed February 1, 2024. [Google Scholar]
- 12.Bailey J, Javidnia P, Mao J, et al. P032. Development of conceptual models to understand patient experiences with and attributes of adherence to HIV oral antiretroviral therapy and considerations in switching to long-acting oral antiretroviral therapy. J Int AIDS Soc. 2022;22:e26009. [Google Scholar]
- 13.U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH). Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims; 2009. Available at: https://www.fda.gov/media/77832/download. Accessed February 1, 2024. [Google Scholar]
- 14.Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2—assessing respondent understanding. Value Health. 2011;14:978–988. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Patient-Focused Drug Development: Collecting Comprehensive and Representative Input. Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders (2020). 2020. Available at: https://www.fda.gov/media/139088/download. Accessed February 1, 2024. [Google Scholar]
- 16.Bailey JR, Javidnia P, Fonseca E, et al. PCR253 Establishing content validity of three new patient reported outcome (PRO) measures for use in HIV long-acting oral antiretroviral therapy (LA-OART) clinical trials. Value Health. 2022;25:S439. [Google Scholar]
- 17.Centers for Disease Control and Prevention. Ending the HIV Epidemic in the U.S. (EHE). 2023. Available at: https://www.cdc.gov/endhiv/about-ehe/index.html#:∼:text=Launched%20in%202019%20and%20led,States%20by%2090%25%20by%202030. Accessed February 1, 2024. [Google Scholar]
- 18.U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH). Patient-Focused Drug Development: Incorporating Clinical Outcome Assessments into Endpoints for Regulatory Decision-Making. Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. 2023. Available at: https://www.fda.gov/media/166830/download. Accessed February 1, 2024. [Google Scholar]
- 19.European Medicines Agency (EMA). EMA Regulatory Science to 2025 Strategic Reflection. 2020. Available at: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/ema-regulatory-science-2025-strategic-reflection_en.pdf. Accessed February 1, 2024. [Google Scholar]
- 20.European Medicines Agency (EMA). EMA's Regulatory Science to 2025 – Mid-point Achievements to End 2022. 2023. Available at: https://www.ema.europa.eu/en/documents/report/emas-regulatory-science-strategy-2025-mid-point-achievements-end-2022_en.pdf. Accessed February 1, 2024. [Google Scholar]
- 21.European Medicines Agency (EMA). Appendix 2 to the Guideline on the Evaluation of Anticancer Medicinal Products in Man - the Use of Patient-Reported Outcome (PRO) Measures in Oncology Studies. Available at: https://www.ema.europa.eu/en/documents/other/appendix-2-guideline-evaluation-anticancer-medicinal-products-man_en.pdf. Accessed February 1, 2024. [Google Scholar]
- 22.Antela A, Bernardino JI, de Quirós JCL-B, et al. Patient-reported outcomes (PROs) in HIV infection: points to consider and challenges. Infect Dis Ther. 2022;11:2017–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kall M, Marcellin F, Harding R, et al. Patient-reported outcomes to enhance person-centred HIV care. Lancet HIV. 2020;7:e59–e68. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Zhu Y, Duan X, et al. HIV-specific reported outcome measures: systematic review of psychometric properties. JMIR Public Health Surveill. 2022;8:e39015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller S, Wilke T, Gorasso V, et al. Adaption and validation of the adherence barriers questionnaire for HIV patients on antiretroviral therapy (ABQ-HIV). BMC Infect Dis. 2018;18:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnould B, Gilet H, Patrick DL, et al. Item reduction, scoring, and first validation of the ACCEPTance by the Patients of their Treatment (ACCEPT©) Questionnaire. Patient. 2017;10:81–92. [DOI] [PubMed] [Google Scholar]
- 27.Calvert M, Kyte D, Mercieca-Bebber R, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA. 2018;319:483–494. [DOI] [PubMed] [Google Scholar]
- 28.Cruz Rivera S, McMullan C, Jones L, et al. The impact of patient-reported outcome data from clinical trials: perspectives from international stakeholders. J Patient Rep Outcomes. 2020;4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kluetz PG, O'Connor DJ, Soltys K. Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada. Lancet Oncol. 2018;19:e267–e274. [DOI] [PubMed] [Google Scholar]
- 30.Organisation for Economic Co-operation and Development (OECD). Recommendations to OECD Ministers of Health from the High Level Reflection Group on the Future of Health Statistics. Strengthening the International Comparison of Health System Performance through Patient-Reported Indicators; 2017. Available at: https://www.oecd.org/health/Recommendations-from-high-level-reflection-group-on-the-future-of-health-statistics.pdf. Accessed February 1, 2024. [Google Scholar]
- 31.Mercieca-Bebber R, King MT, Calvert MJ, et al. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.European AIDS Treatment Group (EATG). PROMs in HIV Research and Development: Analysis of Community Needs and Engagement. 2021. Available at: https://www.eatg.org/publications/proms-in-hiv-research-and-development-analysis-of-community-needs-and-engagement/. Accessed February 1, 2024. [Google Scholar]
- 33.Pearson D. Elevating Patient Voices to Improve HIV Care: Patient-Reported Outcome Measures (PROMS) and Patient-Reported Experience Measures (PREMS). 2022. Available at: https://targethiv.org/sites/default/files/media/documents/2022-09/PROMS_PREMS_Literature_Overview_FINAL_0.pdf. Accessed February 1, 2024. [Google Scholar]
- 34.Luckett T, King M. Choosing patient-reported outcome measures for cancer clinical research–practical principles and an algorithm to assist non-specialist researchers. Eur J Cancer. 2010;46:3149–3157. [DOI] [PubMed] [Google Scholar]