Abstract
PURPOSE
High densities of tumor infiltrating CD3 and CD8 T-cells are associated with superior prognosis in colorectal cancer (CRC). Their value as predictors of benefit from adjuvant chemotherapy is uncertain.
PATIENTS AND METHODS
Tumor tissue from 868 patients in the QUASAR trial (adjuvant fluorouracil/folinic acid v observation in stage II/III CRC) was analyzed by CD3 and CD8 immunohistochemistry. Pathologists, assisted by artificial intelligence, calculated CD3 and CD8 cell densities (cells/mm2) in the core tumor (CT) and invasive margin (IM). Participants were randomly partitioned into training and validation sets. The primary outcome was recurrence-free interval (RFI), 2-year RFI for assessment of biomarker-treatment interactions. Maximum-likelihood methods identified optimal high-risk/low-risk group cutpoints in the training set. Prognostic analyses were repeated in the validation set.
RESULTS
In the training set, the recurrence rate in the high-risk group was twice that in the low-risk group for all measures (CD3-CT: rate ratio [RR], 2.00, P = .0008; CD3-IM: 2.38, P < .00001; CD8-CT: 2.17, P = .0001; CD8-IM: 2.13, P = .0001). This was closely replicated in the validation set (RR, 1.96, 1.79, 1.72, 1.72, respectively). In multivariate analyses, prognostic effects were similar in colon and rectal cancers, and in stage II and III disease. Proportional reductions in recurrence with adjuvant chemotherapy were of similar magnitude in the high- and low-recurrence risk groups. Combining information from CD3-IM and CD3-CT (CD3 Score) generated high-, intermediate-, and low-risk groups with numbers needed to treat (NNTs) to prevent one disease recurrence being 11, 21, and 36, respectively.
CONCLUSION
Recurrence rates in the high-risk CD3/CD8 groups are twice those in the low-risk groups. Proportional reductions with chemotherapy are similar, allowing NNTs derived in QUASAR to be updated using contemporary, nonrandomized data sets.
INTRODUCTION
Approximately 80% of patients with stage II colorectal cancer (CRC) and 50% of those with stage III disease are cured by surgery alone.1 Adjuvant chemotherapy improves 5-year disease-free survival (DFS) by around 14 percentage points in stage III disease, whereas controversy remains regarding the more modest benefits in stage II disease.1,2
CONTEXT
Key Objective
Are CD3 and CD8 tumor infiltrating lymphocyte densities prognostic in early-stage colorectal cancer (CRC), and do they predict benefit from adjuvant chemotherapy? This is the first such predictive evaluation in a randomized data set with observation-only control.
Knowledge Generated
CD3/CD8 densities were strongly prognostic, with recurrence rates in high-risk groups twice those in low-risk groups. Both high- and low-risk groups saw proportional reductions in recurrence with adjuvant chemotherapy of similar magnitude. CD3 Score combines information from CD3 densities in the core tumor and invasive margin. Number needed to treat with high-risk CD3 Score was 11, compared with 36 for low-risk CD3 Score.
Relevance (E.M. O'Reilly)
The authors report on a prognostic tool (CD3 Score) using a CD3/CD8 tumor infiltrating lymphocytes artificial intelligence algorithm in a older large randomized trial in early stage CRC. This relatively simple tool has potential clinical application and warrants further investigation.*
*Relevance section written by JCO Associate Editor Eileen M. O'Reilly, MD.
Various clinicopathological indicators of inferior prognosis—pT4, poor tumor differentiation, lymph node yield <12, lymphovascular invasion, tumor perforation, or bowel obstruction—are used in stage II disease to improve patient selection for chemotherapy.1,3-7 However, although these factors are moderately prognostic, they do not predict sensitivity to adjuvant chemotherapy.1,3-7 Circulating tumor DNA (ctDNA) postsurgery indicates increased recurrence risk.8-10 However, again, whether chemotherapy benefits only those with detectable ctDNA has not yet been determined.11 Novel predictors of chemotherapy efficacy are therefore needed.
The immune system plays a major role in modulating cancer progression. Colorectal tumor infiltration by a variety of different immune cell types correlates with better prognosis,12 of which CD3 (universal T-cell marker) and CD8 (cytotoxic) T-cells are the best studied and have provided the most consistent results. Indeed, in a recent meta-analysis, CD3 and CD8 infiltration were associated with superior survival in both the core tumor (CT) and the invasive margin (IM) across all eligible studies and all stages of disease.12
Mounting evidence suggests that chemotherapy efficacy may in part be mediated through modulation of local and systemic immune mechanisms.13,14 In preclinical models, chemotherapy has been shown to increase activation of immune effector cells, inhibit regulatory T-cells, and increase immunogenicity.15-17 With respect to fluoropyrimidines specifically, selective depletion of immunosuppressive myeloid-derived suppressor cells has been observed in vivo,18 and after neoadjuvant treatment in rectal cancer, densities of CD3 and CD8 T-cells in the tumor stroma are known to increase.19 High baseline densities of tumor infiltrating CD3 and CD8 T-cells may therefore be beneficial for chemotherapy efficacy. However, although there is strong evidence for a prognostic effect of these markers, whether they predict sensitivity to adjuvant chemotherapy is unclear—with conflicting results from previous studies in non--randomly assigned cohorts.20-23
The QUASAR trial is the largest trial of adjuvant chemotherapy versus observation in CRC, having randomly assigned 3,239 patients, 91% of whom had stage II disease.2 It provides an ideal data set in which to test the ability of immune biomarkers to predict benefit from adjuvant chemotherapy. We have previously developed a deep learning cell classification algorithm to compute the densities of CD3 and CD8-stained T-cells within the CT and IM. Using this technology, we present here a prospectively planned, retrospective analysis of the QUASAR trial, investigating the relationship between CD3/CD8 cell densities in the CT/IM and recurrence and whether they predict benefit from adjuvant chemotherapy.
PATIENTS AND METHODS
In QUASAR, 3,239 patients with CRC (91% stage II) were randomly assigned between chemotherapy and observation across 150 sites in 19 countries between May 1994 and December 2003.2 Participants had complete primary tumor resection, no distant metastases, and uncertain need for adjuvant chemotherapy. Consenting participants were randomly assigned to 6 months of 5-fluorouracil and folinic acid chemotherapy (n = 1,622) or observation alone (n = 1,617). Radiotherapy for rectal cancers was as per the treating physician.
QUASAR was managed by the Universities of Oxford and Birmingham, United Kingdom and overseen by a trial steering committee, adhering to Good Clinical Practice guidelines and the Declaration of Helsinki. Ethical approval for this translational study was granted by the North East York (08/H0903/62) and University of Leeds research ethics committees (MREC 19-018).
Eligibility
Randomly assigned QUASAR participants with sufficient archival formalin-fixed paraffin-embedded tumor tissue for immunohistochemical (IHC) analysis were eligible. Exclusion criteria were synchronous tumors, nonadenocarcinoma/mucinous carcinoma, and assay failure (tissue loss/artifact not corrected by repeat staining).
Outcome Measures
For prognostic analyses, the primary outcome was recurrence-free interval (RFI) over the whole follow-up period. To enhance statistical power, recurrence at 2 years was the primary end point for predictive analyses comparing treatment benefit, as the effects of chemotherapy in QUASAR were seen in this period with no subsequent gain or loss of benefit.2 RFI is defined as the time in days from random assignment to first CRC recurrence or death due to CRC. Deaths from other causes without recorded recurrence were considered censoring events. Patients without events were censored at the date last known to be recurrence free.
Immunohistochemistry
Three 4-μm tissue sections were cut onto Superfrost Plus slides (VWR, Lutterworth, United Kingdom) and stained separately with anti-CD3 (2GV6; Roche Diagnostics Solutions [RDS], Tucson, AZ) and anti-CD8 (SP239; RDS) rabbit monoclonal antibodies, and hematoxylin and eosin (H&E; Mayer's hematoxylin; Scott's tap water substitute as the bluing reagent). IHC was performed using a BenchMark ULTRA instrument (RDS).
Digital Image Analysis
Digital slide images were generated using a VENTANA DP200 scanner. Pathologists (RDS), blinded to treatment allocations and outcomes, annotated the CT and IM on CD3-stained sections. CD3 annotations were transferred to adjacent CD8 images via image registration algorithms. A deep learning cell classification algorithm identified CD3+ and CD8+ cells in the CT and IM. Digital analysis reported the tissue area and detected T-cell counts, yielding cell densities (cells/mm2).
Statistical Analysis
Baseline patient characteristics were compared between treatment arms using two-tailed t-tests (continuous variables) and Mantel-Haenszel tests (categorical variables). Patient characteristics were compared with the whole trial population using the same tests. SAS version 9.2 was used for all analyses (SAS Institute, Cary, NC).
Prognostic Analyses
This study set out to analyze tissue from 850 QUASAR participants (approximately 520 with colon and 330 with rectal cancer). Before IHC review, patients were randomly partitioned into two equal-sized training and validation subsets. Optimal cutpoints to divide tumors into high- and low-recurrence risk groups by CD3-CT, CD3-IM, CD8-CT, and CD8-IM cell densities were developed in the training set using maximum-likelihood methods.24-27 Cutpoints developed in the training set were then tested in the validation set.
For both the training and validation sets, log-rank analyses of the primary end point (RFI) were used to compare the rate of recurrence in high- and low-recurrence risk groups, with a two-fold higher recurrence rate in the high- versus low-risk group considered clinically important. A two-sided P value <.05 was considered significant. With 425 patients in the training set, of whom approximately 24% would have recurred, there was more than 95% power to detect a two-fold recurrence ratio between high- and low-recurrence risk groups, assuming approximately half of the tumors would be categorized as high-risk and half as low-risk. It was decided a priori that, should the prognostic value of the cell densities be similar in both the training and validation sets, the relationship between cell density and rate of recurrence would be reported in both treatment arms combined, allowing the detection of lesser differences in prognosis between the high- and low-risk groups: with 850 patients, there would be 90% power to detect a 50% higher recurrence risk.
Log-rank methods were used to investigate whether relationships between cell densities and rate of recurrence remained significant after controlling for individual clinicopathological covariates (age, primary tumor location, nodal involvement, number of nodes examined, pathologic T stage, tumor grade, mismatch repair [MMR] status, lymphatic and/or vascular invasion), with P < .01 considered significant for tests of heterogeneity.
Predictive Analyses
Cutpoints developed in prognostic analyses were taken forward to predictive analyses. For predictive analyses, the proportional reductions in recurrence (recurrence rate ratio [RR]) were compared between high-risk and low-risk groups, with estimates of absolute chemotherapy benefit at 2 years given for each group. A log-rank test was used to assess the significance of the biomarker-treatment interaction.
Sensitivity Analyses
Since adjuvant chemotherapy is not recommended in MMR-deficient (dMMR) stage II colon cancer and, in QUASAR, chemotherapy was ineffective in those age 70 years or older, predictive analyses were repeated in the subset of patients age <70 years with MMR-proficient (pMMR) or MMR-unknown tumors.
RESULTS
Study Material
Tumor tissue from 531 (61%) colon cancers and 337 (39%) rectal cancers was selected at random from the QUASAR tissue bank (Fig 1) and analyzed by CD3 and CD8 IHC (Fig 2). In total, 2,358 of 3,239 randomly assigned patients had tissue available. Patients with tissue were slightly older (mean age 62 v 60 years) and more likely to have colon and stage III cancers. Outcomes were slightly better in those patients in the tissue bank (hazard ratio [HR] for recurrence, 0.80 [95% CI, 0.69 to 0.93]; Data Supplement, Table S1, online only).
FIG 1.
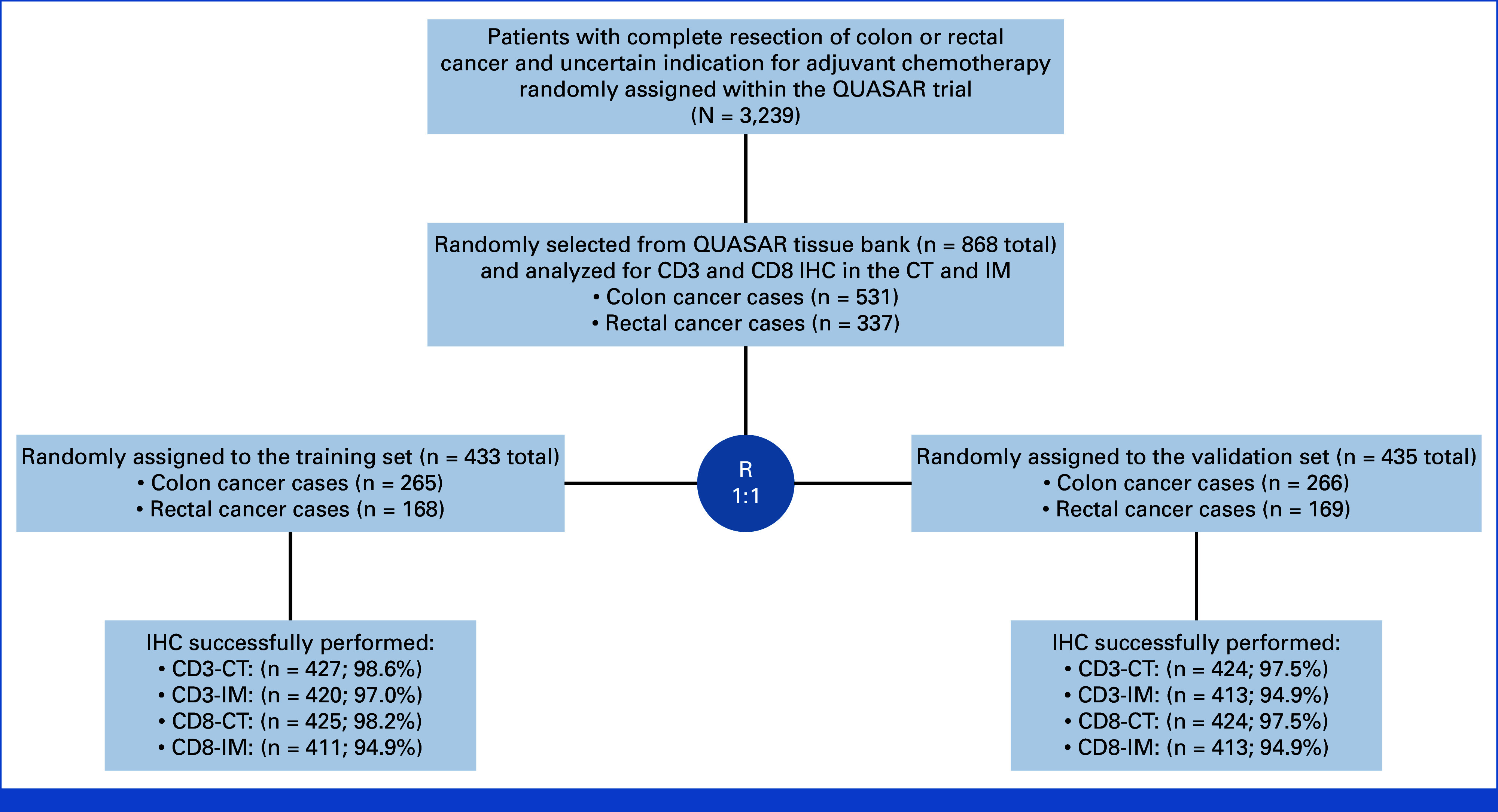
CONSORT diagram demonstrating a breakdown of the study sample. CT, core of the tumor; IHC, immunohistochemistry; IM, invasive margin.
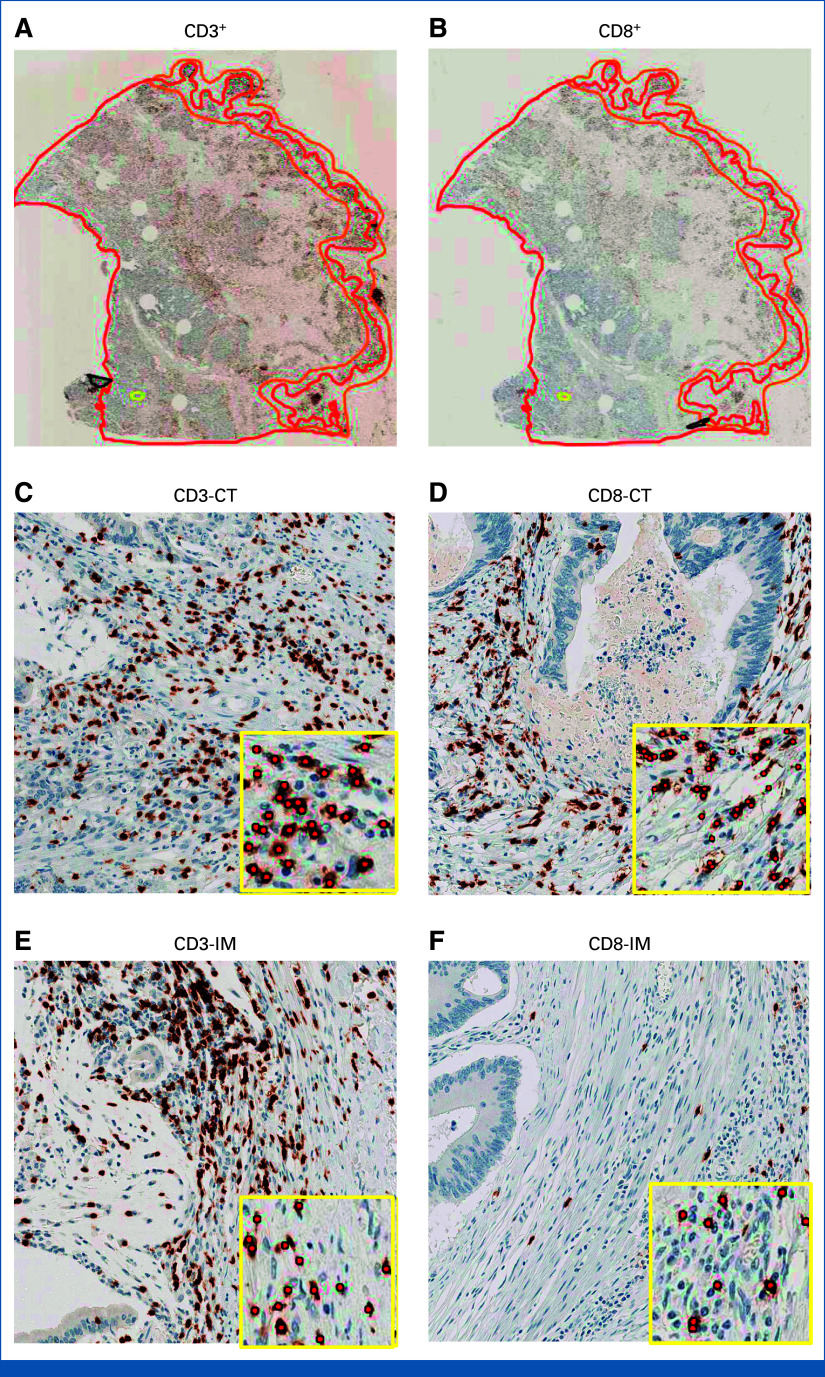
FIG 2.
Representative IHC images of CD3+ and CD8+ T-cells in the tumor microenvironment of colon cancer—(A) CD3-stained whole tissue sections were manually annotated to outline the CT (red line) and the IM (orange line); (B) CT and IM annotations were transferred onto adjacent CD8-stained sections through an image registration algorithm; (A and B) whole tissue sections and (C-F) magnified regions are shown. Insets show algorithmic cell-by-cell classification of CD3+ and CD8+ T-cells (red dots). T-cell densities were quantified (cells/mm2) for each marker in each region. CT, core tumor; IHC, immunohistochemical; IM, invasive margin.
Tissue samples selected were enriched for rectal cancer to increase the power to examine differences by tumor site. No other factors were significantly associated with selection. Outcomes were not different between the two groups (HR for relapse, 1.04 [95% CI, 0.87 to 1.23]; Data Supplement, Table S2). Samples were randomly partitioned into training and validation sets. Demographic and clinical characteristics were balanced between the two groups. CD3-CT cell density was successfully evaluated in 851 (98.0%), CD3-IM in 833 (96.0%), CD8-CT in 849 (97.0%), and CD8-IM in 820 (94.5%) patients.
Association of Cell Density Measures With Outcomes
Optimal cell density cutpoints were defined in the training set using maximum-likelihood methods. For CD3-CT, this produced a cutpoint of 318 cells/mm2, with 172 (40%) patients classified as high recurrence risk. The cutpoint was 798 cells/mm2 for CD3-IM (290 [69%] patients in high-risk group), 81 cells/mm2 for CD8-CT (246 [58%] patients in high-risk group), and 186 cells/mm2 for CD8-IM (259 [63%] patients in high-risk group; Data Supplement, Fig S1).
In the training set, the recurrence rate in the high-risk group was at least twice that of the low-risk group for all measures (CD3-CT: RR, 2.00 [95% CI, 1.33 to 2.94], P = .0008; CD3-IM: 2.38 [95% CI, 1.59 to 3.57], P < .00001; CD8-CT: 2.17 [95% CI, 1.59 to 3.57], P = .0001; CD8-IM: 2.13 [95% CI, 1.43 to 3.23], P = .0001; Figs 3A, 3C, 3E, 3G). The two-fold higher recurrence rate in the training set was closely replicated in the validation set (CD3-CT: RR, 1.96 [95% CI, 1.30 to 2.94], P = .002; CD3-IM: 1.79 [95% CI, 1.18 to 2.70], P = .005; CD8-CT: 1.72 [95% CI, 1.18 to 2.56], P = .005; CD8-IM: 1.72 [95% CI, 1.15 to 2.56], P = .008; Figs 3B, 3D, 3F, 3H; Data Supplement, Fig S2).
FIG 3.
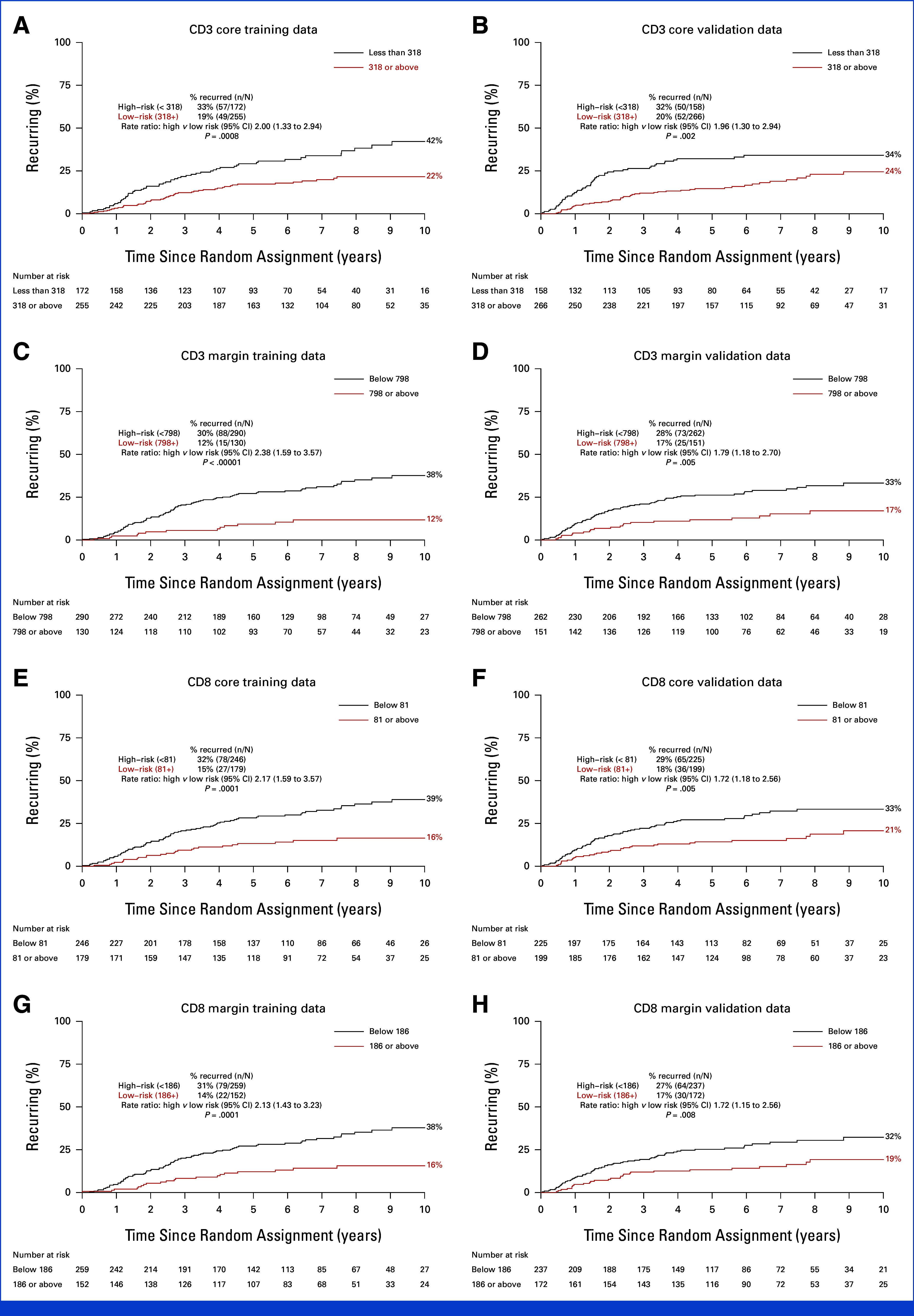
Ten-year recurrence risks for CD3 CT and IM high-risk and low-risk groups: (A and C) training and (B and D) validation sets. Ten-year recurrence risks for CD8 CT and IM high-risk and low-risk groups: (E and G) training and (F and H) validation sets. CT, core tumor; IM, invasive margin.
As the prognostic effect of each measure was similar in the training and validation sets, the two cohorts were combined to investigate whether any associations between the high-risk and low-risk groups and other patient or tumor characteristics might partly or wholly explain the association with recurrence. The proportions of tumors with high-risk CD3 and CD8 scores were similar in the training and validation sets and in those allocated chemotherapy and control. High-risk scores were, however, somewhat more likely in rectal than colon cancer, in stage III than stage II, in tumors with vascular invasion, and in pMMR than dMMR tumors (Data Supplement, Table S3). There was no significant association between CD3/CD8 densities and sex, T-stage, tumor grade, or RAS or BRAF status.
In the combined training and validation sets, the strength of the prognostic effect of each marker in each region was broadly similar, although CD3-IM had the marginally strongest prognostic effect (Chi-square 18.9, P < .0001) and then CD8-CT (18.4, P < .0001), CD3-CT (16.8, P < .0001), and CD8-IM (14.7, P < .0001). The strength of association of CD3-IM with recurrence was also least affected by adjustment for the other density measures (Data Supplement, Fig S3). Figure 4 shows recurrence RRs for high versus low CD3-IM risk groups stratified by various patient and tumor characteristics. The relationship between CD3-IM and RFI remained undiminished and highly significant after adjustment for these potential confounders. The associations of the other cell density measures with RFI were also unaffected by adjustment for other variables (Data Supplement, Figs S4-S6).
FIG 4.
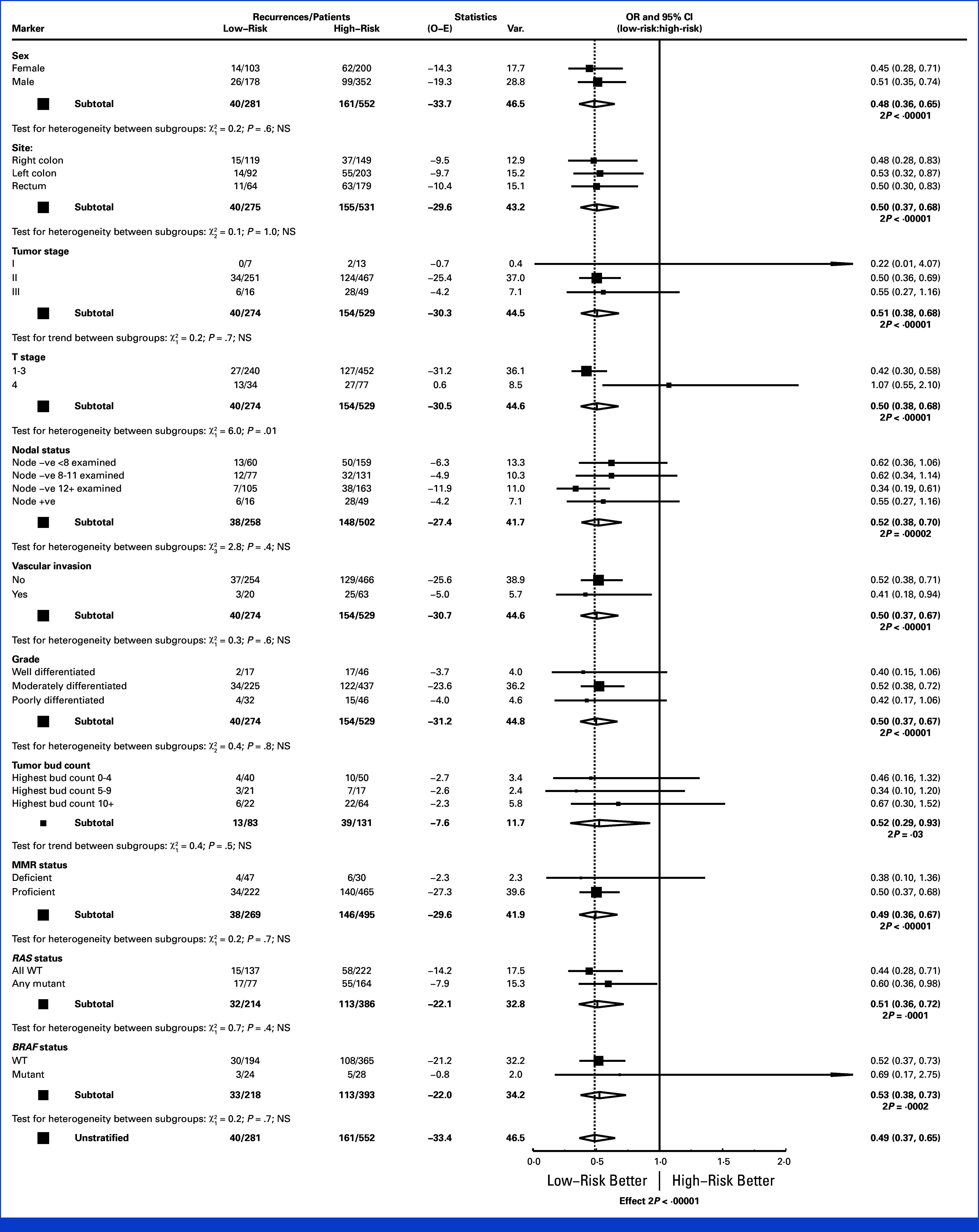
Ratio of recurrence rates in the low versus high-risk groups based on tumor margin (IM) CD3 densities, stratified by sex, site, TNM stage, T-stage, number of nodes examined, presence or absence of vascular invasion, tumor grade, tumor budding count, MMR status, and RAS and BRAF status. IM, invasive margin; MMR, mismatch repair; TNM, tumor node metastasis.
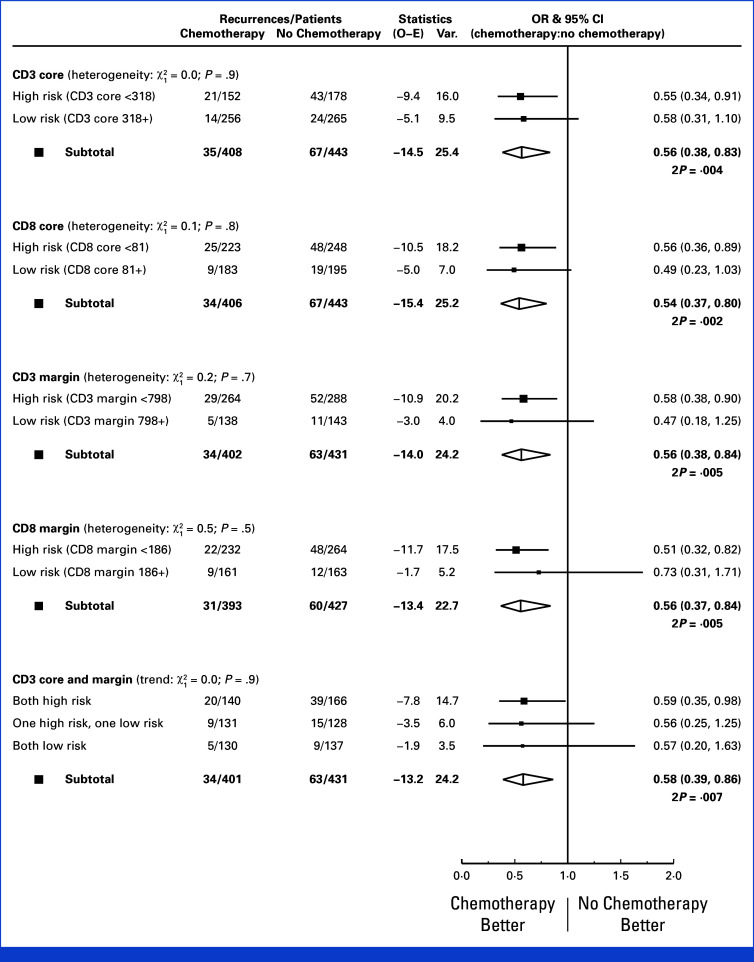
Predictive Analyses
In each of the cell density risk groups, there were similar reductions in 2-year recurrence in those allocated adjuvant chemotherapy compared with those who were not (Fig 5). Tests for interaction showed no suggestion of differences in the proportional reduction achieved with chemotherapy (CD3-CT: Pinteraction = .9; CD3-IM: Pinteraction = .7; CD8-CT: Pinteraction = .8; CD8-IM: Pinteraction = .5). There remained no predictive effect of any marker after exclusion of patients with dMMR disease and those age 70 years or older (data not shown).
FIG 5.
Rate ratios for 2-year recurrence for chemotherapy versus no chemotherapy stratified by CD3 and CD8 risk groups.
CD3 Score: Incorporating Information From Multiple Markers
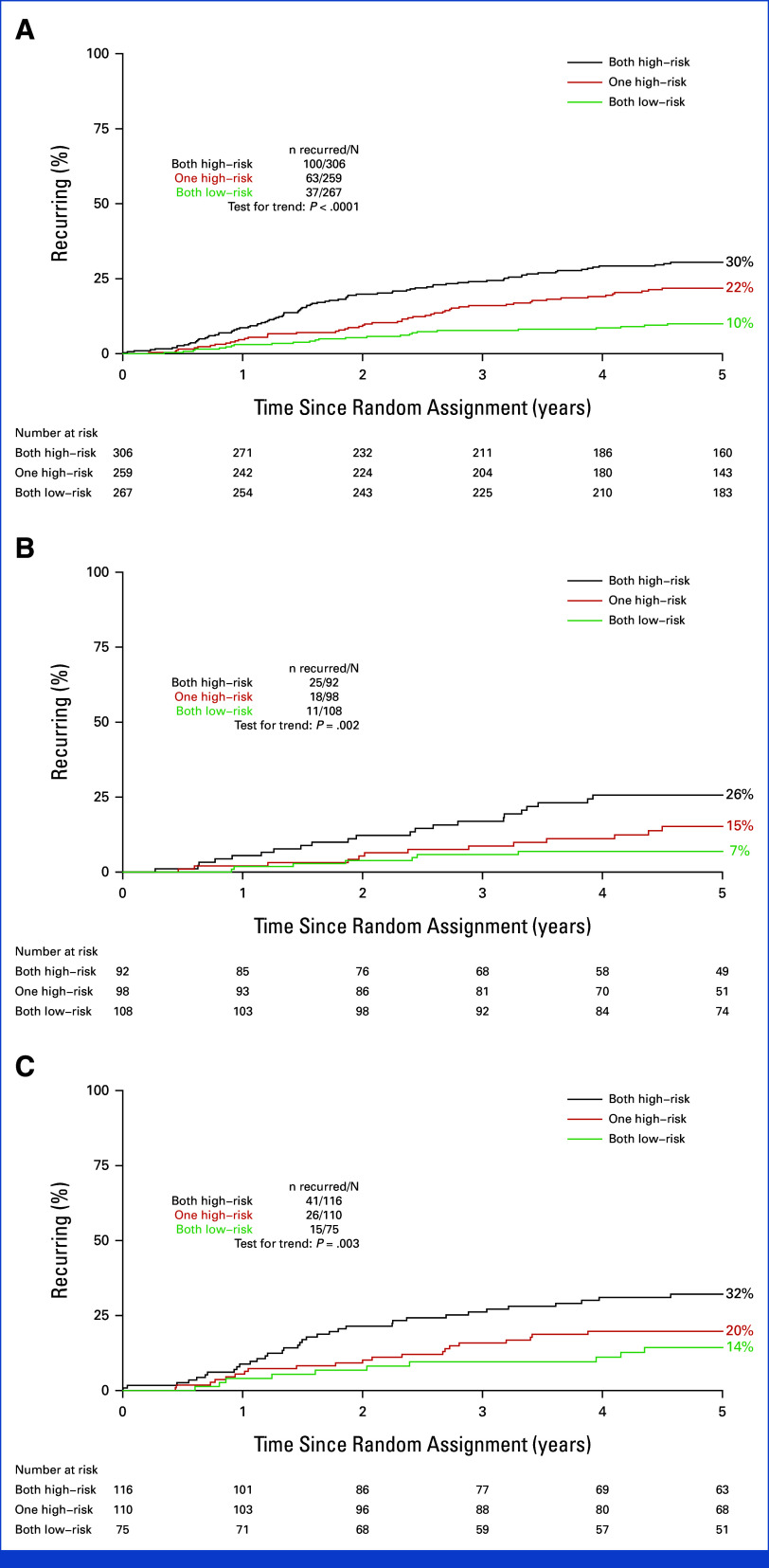
All markers were well correlated with each other but, nevertheless, a proportion of patients were classified as having high recurrence risk with one marker but low with others (Data Supplement, Fig S7). We therefore examined the utility of combining information from more than one marker to refine the prognostic effect. Given the similar prognostic strengths of each marker and to generate the most practical test for potential use in routine clinical practice, we chose to combine CD3-IM with CD3-CT (hereafter, CD3 Score), thus requiring use of a single antibody and analysis of a single IHC slide. By assessing patients with high recurrence risk by both markers, high recurrence risk by one but not both markers, and low recurrence risk by both markers, three risk groups were generated, with 306 (37%), 259 (31%), and 267 (32%) of the 832 patients with IHC results classified as high-, intermediate-, and low-risk by CD3 Score, respectively. The 5-year risk of recurrent disease was 10% for the lowest-risk group, 22% for the intermediate-risk group, and 30% for the highest-risk group (Fig 6A). Given that QUASAR chemotherapy reduced 2-year recurrence by 42%, with similar proportional reductions in the high-, intermediate-, and low-risk groups (Fig 5), the number of patients needed to treat (NNT) with adjuvant chemotherapy to prevent one recurrence of disease in the first 2 years was therefore 11 for the high-risk group, 21 for the intermediate-risk group, and 36 for the low-risk group (Table 1). The strength of the prognostic effect of the CD3 Score in comparison with other clinicopathological indicators of recurrence risk is summarized in the Data Supplement (Fig S8), showing that only the nodal status had a stronger influence on RFI.
FIG 6.
Five-year recurrence rates by CD3 Score (three risk groups defined by CD3-IM and CD3-CT): both high-risk, one high-risk, and both low-risk for (A) all with assessments; (B) just those with MMR-proficient, stage II tumors, and no risk factors (T4, poorly differentiated, vascular invasion, or <8a nodes examined); (C) MMR-proficient, stage II tumors, and one or more risk factors. aIn QUASAR, patients with 8-11 lymph nodes examined had similar recurrence risk to those with ≥12 examined and so 8-11 lymph nodes examined were not considered a high-risk characteristic. CT, core tumor; IM, invasive margin; MMR, mismatch repair. Gray et al.28
TABLE 1.
NNT With Chemotherapy to Prevent One Recurrence Within 2 Years by CD3 Score (combined CD3-IM and CD3-CT) Risk Group
| CD3 Score | Recurrences Within 2 Years, No. (%) | NNT | |
|---|---|---|---|
| Chemotherapy | Control | ||
| CD3 Score-high risk (n = 306) | 20/140 (14.3) | 39/166 (23.5) | 11 |
| CD3 Score-intermediate risk (n = 259) | 9/131 (6.9) | 15/128 (11.7) | 21 |
| CD3 Score-low risk (n = 267) | 5/130 (3.8) | 9/137 (6.6) | 36 |
| All risk groups (n = 851) | 34/401 (8.5) | 63/431 (14.6) | 16 |
Abbreviations: CT, core tumor; IM, invasive margin; NNT, number needed to treat.
The prognostic associations were equally strong when considering only the subgroups of stage II patients with low and high recurrence risk according to routinely reported clinicopathological criteria (Figs 6B and 6C). The absolute difference in 5-year recurrence risk between the high- and low-risk CD3 Score groups was 19% (26% v 7%) in stage II patients considered low recurrence risk by standard clinicopathological criteria and 18% (32% v 14%) for those considered high risk. By comparison, the difference in 5-year recurrence risk for stage II patients considered at high and low recurrence risk by standard clinicopathological criteria alone was just 8% (24% v 16%) or 9% (22% v 13%) when patients with rectal cancer were excluded (Data Supplement, Fig S9).
DISCUSSION
In this study of tissue from the QUASAR trial, high CD3 and CD8 tumor infiltrating lymphocyte (TIL) densities in both the CT and IM were strongly associated with reduced tumor recurrence risk postsurgery. However, in this first rigorous evaluation of the predictive effect of these biomarkers, CD3 and CD8 densities did not predict chemotherapy sensitivity, with the proportional reductions in recurrence with chemotherapy nearly identical in high and low recurrence risk groups. However, as the 5-year recurrence risk in high-risk tumors was twice that in low-risk tumors, the absolute reductions in recurrence with chemotherapy were also about twice as large for high-risk as compared with low-risk tumors.
The tumor node metastasis (TNM) classification has been used to guide treatment decisions in CRC for more than 50 years29 However, significant variability in recurrence risk exists within stage groups.1 The tumor immune microenvironment plays an important role in modulating CRC progression,30 suggesting that an accurate assessment of the immune response to tumors might provide useful supplementary information. To date, it has not been possible to agree a unified approach to such assessments, with the main concerns being how best to strike a balance between simplicity, value of information, and reproducibility—although common approaches have been proposed.31,32 In melanoma, quantification of TILs on an H&E-stained slide is already incorporated into the staging system,33 where a simple three-point scale of absent, nonbrisk, or brisk is used.34 However, debate continues as to whether this is the optimal method of categorization, noting issues with interobserver variability despite the simplicity of the scale35,36—an area where artificial intelligence technologies might provide a solution.
CD3/CD8 densities have been most studied as prognostic biomarkers in CRC, and results across studies show consistent associations.12 One well-established IHC-based method of CD3/CD8 TIL assessment is the Immunoscore, which is strongly associated with DFS in stage I-III colon cancer.37 This study adds to the field by testing the predictive effect of CD3/CD8 densities on benefit from adjuvant treatment in a randomized data set—a necessary step to fully evaluate the clinical applicability of the biomarkers.
A previous predictive analysis of Immunoscore in a non--randomly assigned cohort of patients who did/did not receive adjuvant chemotherapy during routine care suggested that high Immunoscore (low recurrence risk), but not low Immunoscore, might be associated with time-to-recurrence benefit from adjuvant chemotherapy.20 In our analysis, chemotherapy produced similar reductions in recurrence regardless of CD3/CD8 density, suggesting that the earlier study may have had inadequate power to detect chemotherapy benefit in the smaller Immunoscore-low subgroup.
Although there are reports to suggest chemotherapy may have either beneficial or deleterious effects on the tumor immune microenvironment,38-41 our findings are consistent with the lack of a clear biological rationale for an interaction between tumor immune cell infiltration and chemotherapy efficacy. There is a clearer rationale and evidence to support an interaction between the tumor immune contexture and immunotherapy efficacy, for which dMMR status is a well-established proxy measure.42-44 CD3/CD8 densities were higher in dMMR than pMMR tumors in this study but, as in earlier studies,37,45 the prognostic effects of CD3/CD8 densities were independent of MMR status. Interestingly, IHC-based measures of interactions between tumor and immune cells are now showing promise in predicting benefit from checkpoint inhibition in CRC,46 including in pMMR disease,47 warranting further study.
Adjuvant treatment decisions are most complex in stage II pMMR colon cancer, where risks and benefits are finely balanced. Here, the prognostic value of the CD3 Score substantially outperformed that of current standard clinicopathological prognostic factors. A high-risk CD3 Score could therefore be used to identify a subset of patients with stage II pMMR disease and no additional risk factors where adjuvant chemotherapy might have relevance. Conversely, patients with stage II disease and high-risk features or stage III disease (both higher recurrence risk groups) with a low-risk CD3 Score might wish to consider observation, particularly if elderly or frail.
Information regarding the tumor immune contexture might similarly complement that obtained from ctDNA testing. In the DYNAMIC trial, 85% of patients were ctDNA negative, and among these, standard clinicopathological risk markers remained strongly prognostic.11 CD3 Score in addition to ctDNA and clinicopathological assessments might provide optimal risk stratification. A further role for the CD3 Score might be in patient selection for neoadjuvant chemotherapy, a limitation of which is imprecision in radiological staging.48
Strengths of this study are use of tissue from QUASAR, the largest randomized trial of adjuvant chemotherapy versus observation in CRC, and internal validation using training and validation sets.
A limitation is the age of QUASAR, originally published in 2007. Current surgical and pathology standards are superior to those in place at the time of data collection. Indeed, stage II colon cancer recurrence risk was shown to have roughly halved between 2004-2008 and 2014-2019 in a recently published Danish cohort study.49 With no similar data sets to QUASAR including an observation-only control, an external validation of the findings presented here is not possible. However, the consistency of the prognostic effect of the CD3 Score across both QUASAR treatment groups means the NNT derived here can be appropriately adjusted using more recent nonrandomized data.
In summary, CD3/CD8 TIL densities provide a strong indication of recurrence risk after surgery for CRC. Proportional reductions in recurrence achieved with adjuvant chemotherapy are similar in high and low recurrence risk groups. Hence, as the 5-year recurrence risk in high-risk tumors was twice that in low-risk tumors, the number of low-risk patients needed to treat to prevent one recurrence is about twice the number of high-risk patients—information clinicians and patients may find useful in routine practice.
Christopher J.M. Williams
Honoraria: Roche Diagnostics Solutions, Merck Serono, Tactics MD, SERVIER
Research Funding: Roche Diagnostics Solutions (Inst), GlaxoSmithKline (Inst)
Liping Zhang
Employment: Roche Diagnostics Solutions
Stock and Other Ownership Interests: Roche Diagnostics Solutions, Moderna Therapeutics
Zuo Zhao
Employment: Roche Molecular Diagnostics
Stock and Other Ownership Interests: Roche Molecular Diagnostics
Tracie Gardner
Employment: Roche
Stock and Other Ownership Interests: Roche
Research Funding: Roche
Travel, Accommodations, Expenses: Roche
Nancy Sapanara
Employment: Roche
Stock and Other Ownership Interests: Roche
Xiao-Meng Xu
Employment: Roche Diagnostic Solutions
Stock and Other Ownership Interests: Roche
Isaac Bai
Employment: Roche
Dongyao Yan
Employment: Roche
Stock and Other Ownership Interests: Roche
Research Funding: Roche
Patents, Royalties, Other Intellectual Property: I am a co-inventor of IP (WO2023192946) owned by Ventana Medical Systems, Inc (Inst)
Andrea Muranyi
Employment: Roche
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: Roche
Travel, Accommodations, Expenses: Roche
Sarah Dance
Employment: Roche Diagnostics Ltd
Michael Hale
Research Funding: Roche Diagnostics Limited (Inst)
Uday Kurkure
Employment: Roche
Stock and Other Ownership Interests: Roche
Research Funding: Roche
Patents, Royalties, Other Intellectual Property: US Patent App. 18/499,208 and 18/162,458 (Inst), US Patent App. 18/499,208 and 18/162,458 (Inst)
Christoph Guetter
Employment: Roche
Stock and Other Ownership Interests: Roche Pharma AG
Patents, Royalties, Other Intellectual Property: Patents for Roche Tissue Diagnostics
Susan D. Richman
Patents, Royalties, Other Intellectual Property: IHC Patent pending. Filed by Roche, in collaboration with the University of Leeds. Technology Title: Scoring algorithm using EREG and AREG for prediction of response to EGFR directed therapies US Patent Filing: US 62/706,988
Jenny F. Seligmann
Honoraria: Pierre Fabre, Merck Serono, SERVIER, GlaxoSmithKline, Takeda Science Foundation
Consulting or Advisory Role: Elevation Oncology, GlaxoSmithKline, Sanofi, Takeda, Merck Serono, Jazz Pharmaceuticals
Research Funding: GlaxoSmithKline (Inst), Merck Serono (Inst), Pierre Fabre (Inst)
Nicholas P. West
Consulting or Advisory Role: Bristol Myers Squibb, Astellas Pharma, GlaxoSmithKline, Amgen, Pfizer
Research Funding: Roche (Inst), Pierre Fabre (Inst)
Patents, Royalties, Other Intellectual Property: UoL patent reference: 20006US1 Technology Title: Scoring algorithm using EREG and AREG for prediction of response to EGFR directed therapies US Patent Filing: US 62/706,988
Shalini Singh
Employment: Ventana Medical Systems
Stock and Other Ownership Interests: Ventana Medical Systems
Patents, Royalties, Other Intellectual Property: Method of Identifying treatment responsive Non–Small Cell Lung Cancer using ALK as a marker (Inst)
Kandavel Shanmugam
Employment: Ventana Medical Systems
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: Patents (Inst)
Travel, Accommodations, Expenses: Ventana Medical Systems
Philip Quirke
Honoraria: Roche
Consulting or Advisory Role: Roche
Speakers' Bureau: Roche
Research Funding: Roche, GeneFirst, ONI
Patents, Royalties, Other Intellectual Property: Roche have filed a patent jointly with my University I may receive income but none to date (Inst)
Travel, Accommodations, Expenses: Roche
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at ASCO Gastrointesinal Cancers Symposium, San Francisco, CA, January 24, 2023.
SUPPORT
Supported by the National Pathology Imaging Co-operative, UK (project number 104687). NPIC is supported by a £50M investment from the Data to Early Diagnosis and Precision Medicine strand of the UK government's Industrial Strategy Challenge Fund, managed and delivered by UK Research and Innovation (UKRI). Cancer Research UK fund CJM Williams (RCCCTF-Nov21/100001). Yorkshire Cancer Research (L386) funded SD Richman, prior histopathological and molecular analyses, and technical support. Roche Diagnostics Solutions provided funding for reagents, immunohistochemistry laboratory equipment, and digital pathology infrastructure at the University of Leeds. The QUASAR trial was funded by the UK Medical Research Council.
K.S. and P.Q. joint senior authors.
DATA SHARING STATEMENT
The data generated in this study are available upon request from the corresponding author. A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.23.02030.
AUTHOR CONTRIBUTIONS
Conception and design: Christopher J.M. Williams, Richard Gray, Uday Kurkure, Christoph Guetter, Gordon Hutchins, Nicholas P. West, Kandavel Shanmugam, Philip Quirke
Financial support: Kandavel Shanmugam, Philip Quirke
Administrative support: Christopher J.M. Williams, Sarah Dance
Provision of study materials or patients: Michael Shires, Gordon Hutchins, Philip Quirke
Collection and assembly of data: Christopher J.M. Williams, Richard Gray, Michael Shires, Liping Zhang, Zuo Zhao, Tracie Gardner, Nancy Sapanara, Xiao-Meng Xu, Andrea Muranyi, Sarah Dance, Gemma Hemmings, Michael Hale, Christoph Guetter, Susan D. Richman, Gordon Hutchins, Shalini Singh, Kandavel Shanmugam, Philip Quirke
Data analysis and interpretation: Christopher J.M. Williams, Richard Gray, Robert K. Hills, Zuo Zhao, Tracie Gardner, Xiao-Meng Xu, Isaac Bai, Dongyao Yan, Sarah Dance, Faranak Aghaei, Uday Kurkure, Susan D. Richman, Gordon Hutchins, Jenny F. Seligmann, Kandavel Shanmugam, Philip Quirke
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Evaluation of CD3 and CD8 T-Cell Immunohistochemistry for Prognostication and Prediction of Benefit From Adjuvant Chemotherapy in Early-Stage Colorectal Cancer Within the QUASAR Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Christopher J.M. Williams
Honoraria: Roche Diagnostics Solutions, Merck Serono, Tactics MD, SERVIER
Research Funding: Roche Diagnostics Solutions (Inst), GlaxoSmithKline (Inst)
Liping Zhang
Employment: Roche Diagnostics Solutions
Stock and Other Ownership Interests: Roche Diagnostics Solutions, Moderna Therapeutics
Zuo Zhao
Employment: Roche Molecular Diagnostics
Stock and Other Ownership Interests: Roche Molecular Diagnostics
Tracie Gardner
Employment: Roche
Stock and Other Ownership Interests: Roche
Research Funding: Roche
Travel, Accommodations, Expenses: Roche
Nancy Sapanara
Employment: Roche
Stock and Other Ownership Interests: Roche
Xiao-Meng Xu
Employment: Roche Diagnostic Solutions
Stock and Other Ownership Interests: Roche
Isaac Bai
Employment: Roche
Dongyao Yan
Employment: Roche
Stock and Other Ownership Interests: Roche
Research Funding: Roche
Patents, Royalties, Other Intellectual Property: I am a co-inventor of IP (WO2023192946) owned by Ventana Medical Systems, Inc (Inst)
Andrea Muranyi
Employment: Roche
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: Roche
Travel, Accommodations, Expenses: Roche
Sarah Dance
Employment: Roche Diagnostics Ltd
Michael Hale
Research Funding: Roche Diagnostics Limited (Inst)
Uday Kurkure
Employment: Roche
Stock and Other Ownership Interests: Roche
Research Funding: Roche
Patents, Royalties, Other Intellectual Property: US Patent App. 18/499,208 and 18/162,458 (Inst), US Patent App. 18/499,208 and 18/162,458 (Inst)
Christoph Guetter
Employment: Roche
Stock and Other Ownership Interests: Roche Pharma AG
Patents, Royalties, Other Intellectual Property: Patents for Roche Tissue Diagnostics
Susan D. Richman
Patents, Royalties, Other Intellectual Property: IHC Patent pending. Filed by Roche, in collaboration with the University of Leeds. Technology Title: Scoring algorithm using EREG and AREG for prediction of response to EGFR directed therapies US Patent Filing: US 62/706,988
Jenny F. Seligmann
Honoraria: Pierre Fabre, Merck Serono, SERVIER, GlaxoSmithKline, Takeda Science Foundation
Consulting or Advisory Role: Elevation Oncology, GlaxoSmithKline, Sanofi, Takeda, Merck Serono, Jazz Pharmaceuticals
Research Funding: GlaxoSmithKline (Inst), Merck Serono (Inst), Pierre Fabre (Inst)
Nicholas P. West
Consulting or Advisory Role: Bristol Myers Squibb, Astellas Pharma, GlaxoSmithKline, Amgen, Pfizer
Research Funding: Roche (Inst), Pierre Fabre (Inst)
Patents, Royalties, Other Intellectual Property: UoL patent reference: 20006US1 Technology Title: Scoring algorithm using EREG and AREG for prediction of response to EGFR directed therapies US Patent Filing: US 62/706,988
Shalini Singh
Employment: Ventana Medical Systems
Stock and Other Ownership Interests: Ventana Medical Systems
Patents, Royalties, Other Intellectual Property: Method of Identifying treatment responsive Non–Small Cell Lung Cancer using ALK as a marker (Inst)
Kandavel Shanmugam
Employment: Ventana Medical Systems
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: Patents (Inst)
Travel, Accommodations, Expenses: Ventana Medical Systems
Philip Quirke
Honoraria: Roche
Consulting or Advisory Role: Roche
Speakers' Bureau: Roche
Research Funding: Roche, GeneFirst, ONI
Patents, Royalties, Other Intellectual Property: Roche have filed a patent jointly with my University I may receive income but none to date (Inst)
Travel, Accommodations, Expenses: Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Böckelman C, Engelmann BE, Kaprio T, et al. : Risk of recurrence in patients with colon cancer stage II and III: A systematic review and meta-analysis of recent literature. Acta Oncol 54:5-16, 2015 [DOI] [PubMed] [Google Scholar]
- 2.QUASAR Collaborative Group : Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 370:2020-2029. 2007 [DOI] [PubMed] [Google Scholar]
- 3.Quah H-M, Chou JF, Gonen M, et al. : Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum 51:503-507, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Lee VWK, Chan KF: Tumor budding and poorly-differentiated cluster in prognostication in Stage II colon cancer. Pathol Res Pract 214:402-407, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Casadaban L, Rauscher G, Aklilu M, et al. : Adjuvant chemotherapy is associated with improved survival in patients with stage II colon cancer. Cancer 122:3277-3287. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verhoeff SR, van Erning FN, Lemmens VEPP, et al. : Adjuvant chemotherapy is not associated with improved survival for all high-risk factors in stage II colon cancer. Int J Cancer 139:187-193. 2016 [DOI] [PubMed] [Google Scholar]
- 7.Lykke J, Roikjaer O, Jess P: The relation between lymph node status and survival in stage I-III colon cancer: Results from a prospective nationwide cohort study. Colorectal Dis 15:559-565, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Tie J, Wang Y, Tomasetti C, et al. : Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med 8:346ra92, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen E, Birkenkamp-Demtröder K, Sethi H, et al. : Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J Clin Oncol 37:1547-1557, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Moding EJ, Liu Y, Nabet BY, et al. : Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small cell Lung cancer. Nat Cancer 1:176-183, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tie J, Cohen JD, Lahouel K, et al. : Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med 386:2261-2272. 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander PG, McMillan DC, Park JH: The local inflammatory response in colorectal cancer—type, location or density? A systematic review and meta-analysis. Cancer Treat Rev 83:101949, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Galluzzi L, Buqué A, Kepp O, et al. : Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28:690-714, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Zitvogel L, Galluzzi L, Smyth MJ, et al. : Mechanism of action of conventional and targeted anticancer therapies: Reinstating immunosurveillance. Immunity 39:74-88, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Maeda K, Hazama S, Tokuno K, et al. : Impact of chemotherapy for colorectal cancer on regulatory T-cells and tumor immunity. Anticancer Res 31:4569-4574, 2011 [PubMed] [Google Scholar]
- 16.Liu WM, Fowler DW, Smith P, et al. : Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer 102:115-123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohtsukasa S, Okabe S, Yamashita H, et al. : Increased expression of CEA and MHC class I in colorectal cancer cell lines exposed to chemotherapy drugs. J Cancer Res Clin Oncol 129:719-726, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Vincent J, Mignot G, Chalmin F, et al. : 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 70:3052-3061, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Teng F, Mu D, Meng X, et al. : Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am J Cancer Res 5:2064-2074, 2015 [PMC free article] [PubMed] [Google Scholar]
- 20.Mlecnik B, Bifulco C, Bindea G, et al. : Multicenter International Society for Immunotherapy of Cancer study of the consensus Immunoscore for the prediction of survival and response to chemotherapy in stage III colon cancer. J Clin Oncol 38:3638-3651, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagès F, André T, Taieb J, et al. : Prognostic and predictive value of the immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann Oncol 31:921-929, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Galon J, Hermitte F, Mlecnik B, et al. : Immunoscore as a parameter predicting time to recurrence and disease-free survival in T4N0 stage II colon cancer patients. J Clin Oncol 38, 2020. (suppl 15; abstr 4105) [Google Scholar]
- 23.Galon J, Lanzi A: Immunoscore and its introduction in clinical practice. Q J Nucl Med Mol Imaging 64:152-161, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Pace NL, Stylianou MP: Advances in and limitations of up-and-down methodology: A précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology 107:144-152, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Salanti G, Kurt U: A nonparametric changepoint model for stratifying continuous variables under order restrictions and binary outcome. Stat Methods Med Res 12:351-367, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Salanti G, Ulm K: A non-parametric framework for estimating threshold limit values. BMC Med Res Methodol 5:36, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ancukiewicz M, Finkelstein DM, Schoenfeld DA: Modelling the relationship between continuous covariates and clinical events using isotonic regression. Stat Med 22:3151-3159, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Gray RG, Quirke P, Handley K, et al. : Correlation of number of nodes examined and the 12-gene colon cancer recurrence score with recurrence in stage II colon cancer patients from QUASAR. Presented at the 2010 ASCO Gastrointestinal Cancers Symposium. January 22-24, 2010, Orlando, FL (abstr 331)
- 29.UICC : TNM Classification of Malignant Tumours (ed 1). Geneva, Switzerland, International Union Against Cancer, 1968 [Google Scholar]
- 30.Hutchins G, Southward K, Handley K, et al. : Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 29:1261-1270, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Hendry S, Salgado R, Gevaert T, et al. : Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the International Immunooncology Biomarkers Working Group: Part 1: Assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol 24:235-251, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hendry S, Salgado R, Gevaert T, et al. : Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol 24:311-335, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amin MB, Edge SB, Greene FL, et al. (eds): Melanoma skin cancer, in AJCC Cancer Staging Manual (ed 8). New York, NY, Springer, 2017 [Google Scholar]
- 34.Elder D, Murphy G: Melanocytic Tumors of the Skin: AFIP Atlas of Tumor Pathology, Series 4. Washington DC, American Registry of Pathology and Armed Forces Institute of Pathology, 2010 [Google Scholar]
- 35.Saldanha G, Flatman K, Teo KW, et al. : A novel numerical scoring system for melanoma tumor-infiltrating lymphocytes has better prognostic value than standard scoring. Am J Surg Pathol 41:906-914, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slater D, Cook M: Standards and datasets for reporting cancers: Dataset for histopathological reporting of primary cutaneous malignant melanoma and regional lymph nodes. https://www.rcpath.org/static/fb177728-072d-4b8a-97ae94319eaac5fd/Dataset-for-the-histological-reporting-of-primary-cutaneous-malignant-melanoma-and-regional-lymph-nodes.pdf [Google Scholar]
- 37.Pagès F, Mlecnik B, Marliot F, et al. : International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 391:2128-2139, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Cheema AR, Hersh EM: Patient survival after chemotherapy and its relationship to in vitro lymphocyte blastogenesis. Cancer 28:851-855, 1971 [DOI] [PubMed] [Google Scholar]
- 39.Emens LA, Machiels JP, Reilly RT, et al. : Chemotherapy: Friend or foe to cancer vaccines? Curr Opin Mol Ther 3:77-84, 2001 [PubMed] [Google Scholar]
- 40.Mathé G: Chemotherapy, a double agent in respect of immune functions. Cancer Chemother Pharmacol 1:65-68, 1978 [DOI] [PubMed] [Google Scholar]
- 41.Vacchelli E, Aranda F, Eggermont A, et al. : Trial Watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology 3:e27878, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chalabi M, Verschoor YL, Tan PB, et al. Neoadjuvant immunotherapy in locally advanced mismatch repair-deficient colon cancer. N Engl J Med 390:1949–1958, 2024 [DOI] [PubMed] [Google Scholar]
- 43.Cercek A, Lumish M, Sinopoli J, et al. : PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med 386:2363-2376, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.André T, Shiu K-K, Kim TW, et al. : Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med 383:2207-2218, 2020 [DOI] [PubMed] [Google Scholar]
- 45.Yoon HH, Shi Q, Heying EN, et al. : Intertumoral heterogeneity of CD3+ and CD8+ T-cell densities in the microenvironment of DNA mismatch-repair-deficient colon cancers: Implications for prognosis. Clin Cancer Res 25:125-133, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saberzadeh-Ardestani B, Graham RP, McMahon S, et al. : Immune marker spatial distribution and clinical outcome after PD-1 blockade in mismatch repair-deficient, advanced colorectal carcinomas. Clin Cancer Res 29:4268-4277, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antoniotti C, Rossini D, Pietrantonio F, et al. : Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol 23:876-887, 2022 [DOI] [PubMed] [Google Scholar]
- 48.Morton D, Seymour M, Magill L, et al. : Preoperative chemotherapy for operable colon cancer: Mature results of an international randomized controlled trial. J Clin Oncol 41:1541-1552, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nors J, Iversen LH, Erichsen R, et al. : Incidence of recurrence and time to recurrence in stage I to III colorectal cancer: A nationwide Danish cohort study. JAMA Oncol 10:54-62, 2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in this study are available upon request from the corresponding author. A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.23.02030.