Abstract
Objectives
DEPDC5-related epilepsy carries an increased risk of sudden unexpected death in epilepsy. We evaluated the occurrence and features of ictal central apnea (ICA) in patients with pathogenic sequence variant in DEPDC5.
Methods
We reviewed data of 108 patients collected in 2 independent cohorts of patients with focal epilepsy who prospectively underwent long-term video-EEG monitoring (LTVM) with cardiorespiratory polygraphy. All patients underwent (1) at least an overnight polysomnography, (2) a high-field (3T) brain MRI study, and (3) CSF analysis when clinically indicated. Genetic testing (next-generation sequencing [NGS]) was offered for diagnostic purposes to patients with focal epilepsy of unknown etiology.
Results
In this cohort, NGS was finally performed in 29 patients, resulting in DEPDC5 pathogenic mutations in 5 patients. According to the presence of ictal apnea events, 5 of 14 patients with ICA showed pathogenic DEPDC5 variants (35%) while none of the 15 patients without ICA showed pathogenic mutation. Notably, DEPDC5 patients showed ICA in all recorded seizures (n = 15) with apnea duration ranging from 20 seconds to more than 1 minute. All seizures were characterized by motor arrest without overt automatic behaviors during ictal apnea. Scalp EEG showed the involvement of temporal lobe leads in all events. Severe oxygen desaturation was observed in 2 cases.
Discussion
In our cohort, ictal central apnea was a common finding in DEPDC5. These results support (1) the need for respiratory polygraphy during LTVM in DEPDC5-related epilepsy and (2) the potential relevance of genetic testing in patients with focal epilepsy of unknown etiology and ictal apnea.
Introduction
Sudden unexpected death in epilepsy (SUDEP) has a global incidence ranging from 0.22 to 1.2 per 1,000 individuals per year,1 accounting for up to 17% of death in patients with epilepsy, making it the second leading cause of potential life-years lost due to neurologic diseases, after stroke.2 The etiology of SUDEP is heterogeneous, encompassing cardiac and respiratory dysregulations across various epilepsy syndromes.2
DEPDC5 is a common causative gene in familial focal epilepsy with or without malformations of cortical development.3,4 Its pathogenic variants also confer a significantly higher risk of SUDEP,5-8 providing opportunities to investigate the pathophysiology intersecting neurodevelopment, epilepsy, and cardiorespiratory functions.9 Recently, a mouse model of DEPDC5 demonstrated interictal respiratory dysregulations and ictal apnea long before the terminal cardiac asystole.9
For these reasons the investigation of ictal central apnea (ICA) and respiratory alterations during seizures in patients harboring DEPDC5 pathogenic variants is of clinical relevance.
Considering the importance to gain a mechanistic understanding of DEPDC5-related epilepsy and SUDEP, here we report that ictal central respiratory alterations are a common finding in DEPDC5-related seizures.
Methods
Patients
We reviewed video-polygraphic data from patients with pathogenic DEPDC5 sequence variant who were prospectively recruited in a previous study aimed at characterizing the incidence and features of ictal respiratory alterations in patients with epilepsy.10,11 In brief, a group of 108 adolescent and adult patients with focal epilepsy was evaluated in 2 epilepsy centers (Modena Academic Hospital and IRCCS E. Medea-Conegliano, Italy) from April 2020 to December 2022. All the patients included in this study underwent (1) prolonged video-EEG monitoring, including at least an overnight polysomnography; (2) a high-field (3T) brain MRI study with a dedicated epilepsy protocol12; (3) CSF analysis when clinically indicated (suspect of autoimmune encephalitis, infectious, inflammatory causes).
Study Measures and Definitions
Each patient underwent long-term video-EEG monitoring (LTVM) with a 10–20 EEG system integrated with a standard precordial single-channel ECG, pulse oximetry for SpO2 measurement, and thoracoabdominal belt for respiratory inductance plethysmography. According to published criteria,10,11,13-15 ICA was defined as a respiratory arrest lasting 5 or more seconds, visible on the pneumographic channel, and preceded and followed by stable breathing for at least 5 seconds. Postictal apnea was identified as a respiratory arrest starting within 5 seconds after the termination of ictal discharge. Data collected included apnea duration, hypoxemia (duration, nadir, and degree of oxygen desaturation), apnea awareness, and heart rate changes. Hypoxemia was defined as a drop of SpO2 value below 95% and classified as mild 90%–94%, moderate 75%–89%, or severe <75%.10-15 Tachycardia and bradycardia were defined as heart rate >100 beats per minute and <60 beats per minute, or a >20% deviation from baseline, respectively.14
In this cohort of patients with focal epilepsy, a genetic analysis for clinical-diagnostic purposes was offered to patients who resulted, at the end of the diagnostic workup, without a definite etiology (epilepsy of unknown etiology). In both centers, DNA, extracted from blood samples, was analyzed by NGS with custom panels covering 196 to 216 genes associated with epilepsy (eAppendix 1), including genes known to cause epilepsies related to alterations in the mTOR pathway.3,4
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the local ethical committees (Area Vasta Emilia Nord,N.322/15 and 679/2022/SPER/UNIMO; CET Area Nord Veneto N.22494).
Patients gave written informed consent for the use of their clinical records in this study. The study was conducted in accordance with the World Medical Association Declaration of Helsinki.
Data Availability
Data will be made available on reasonable request.
Results
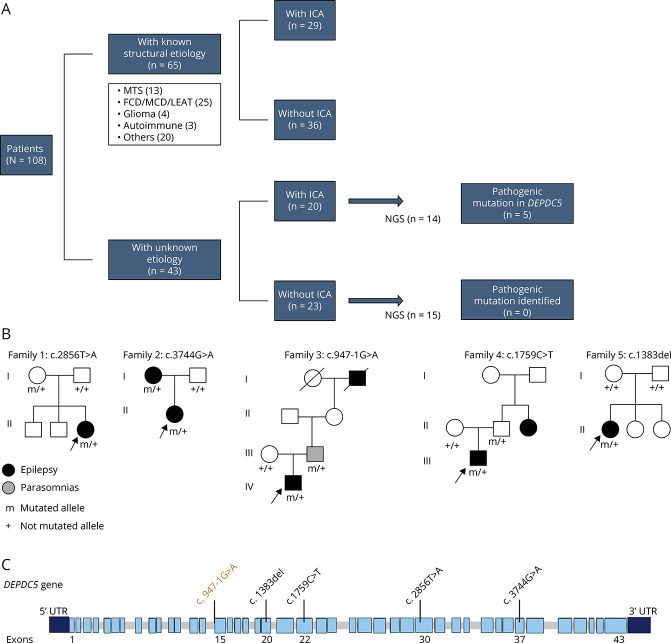
Among the 108 patients (mean age 35.07 ± 14.54 years; 46 female), 49 patients (45%) showed seizures with ICA. According to etiology, 65 of 108 patients had known/structural etiologies (29 with ICA) while 43 patients were MRI-negative or had unknown etiology (20 with ICA) (Figure 1A).
Figure 1. DEPDC5 Families and Genetic Findings.
(A) Flowchart of the investigated patients according to etiology, ictal respiratory findings (ICA), and genetic testing (NGS). (B) Pedigrees of families with the proband indicated by an arrow. Patients with a mutation are indicated by m/+, and patients negative for the mutation are indicated by +/+. (C) Schematic structure of the DEPDC5 gene (NM_001242896) with the position of sequence variants found in our patients. Variants indicated in black are nonsense or frameshift mutations while the variant in orange is predicted to alter mRNA splicing. FCD = focal cortical dysplasia; ICA = ictal central apnea; LEAT = low-grade epilepsy-related tumors; MCD = other malformations of cortical development; NGS = next-generation sequencing.
Genetic testing was performed in 29 patients with unknown etiology, resulting in 5 patients with pathogenic DEPDC5 variants (Table; Figure 1, B and C). No other pathogenic mutations in epilepsy-related genes were observed. According to polygraphic respiratory findings, NGS analysis returned no pathogenic mutations in the 15 patients without ICA while of 14 patients with ICA, 5 showed pathogenic DEPDC5 variants (35.7%) (Figure 1A). 100% of the patients with DEPDC5 mutations showed seizure-related respiratory alteration with different degrees and severity (Table; Figure 2). None of the patients had a history of snoring or sleep apnea, and overnight sleep polygraphy did not show obstructive apnea in any patient.
Table.
Clinical Features and Ictal Central Apnea of DEPDC5 Patients
| Patient # 1 | Patient # 2 | Patient # 3 | Patient # 4 | Patient # 5 | |
| Age, y/sex | 24/f | 21/f | 24/m | 58/m | 55/f |
| Epilepsy onset, yrs | 3 | 8 | 11 | 3 | 10 |
| Prevalent seizure type | FIAS | FIAS | FIAS | FIAS | FIAS |
| ASMs at last follow-up | BRV, VPA | CBZ, LEV | CBZ | VPA, LTG, CNB | LCM |
| Drug resistance | Yes | Yes | No | Yes | Yes |
| Interictal EEG | Left temporal slow waves and spikes | Bilateral frontotemporal slow spike and waves | Bilateral frontocentral slow spike and waves | Right temporal slow waves and spikes | Left temporal slow waves and spikes |
| Epilepsy family history | No | Yes, proband mother | Yes, proband paternal grandfather | Yes, proband paternal uncle | No |
| SUDEP family history | No | No | No | No | No |
| Brain MRI | Suspected left insular/mesial temporal FCD | Negative | Negative | Negative | Negative |
| FDG-PET | n.a. | Left fronto-opercular hypometabolism | n.a. | n.a. | Left temporal hypometabolism |
| DEPDC5 pathogenic variant | c.2856T>A, p.Cys952Ter | c.3244G>A, p.Thr1248 | c.947-1G>A, | c.1759C>T, p.Arg587Ter | c.1383del, p.Tyr462Metfs*55 |
| Ictal central apnea | |||||
| No. of seizures with apnea/total recorded seizures | 4/4 | 1/1 | 3/3 | 6/6 | 1/1 |
| Scalp EEG ictal onset (lobe/side) | T/left | FT/bilateral | F/left | T/right | T/left |
| Seizure duration (mean, sec.) | 89 | 70 | 43 | 34.5 | 30 |
| Apnea duration (mean, sec.) | 77 | 60 | 35 | 26 | 25 |
| Latency of ICA from ictal onset (mean, s) | 4 s | 2 s | 0 s | 3 s | 4 s |
| Oxygen desaturation nadir | 71% | 75% | n.a. | n.a. | 92% |
| Heart rate changes | Tachycardia | — | — | Tachycardia | Tachycardia |
| Vigilance at seizure onset | Sleep | Awake | Sleep | Sleep | Sleep |
| Awareness of respiratory distress | No | No | No | No | No |
Abbreviations: BRV = brivaracetam; CBZ = carbamazepine; CNB = cenobamate; F = frontal; FIAS = focal impaired awareness seizures; FT = frontotemporal; LCM = lacosamide; LEV = levetiracetam; LTG = lamotrigine; SUDEP = sudden unexplained death in epilepsy; T = temporal; VPA = valproate.
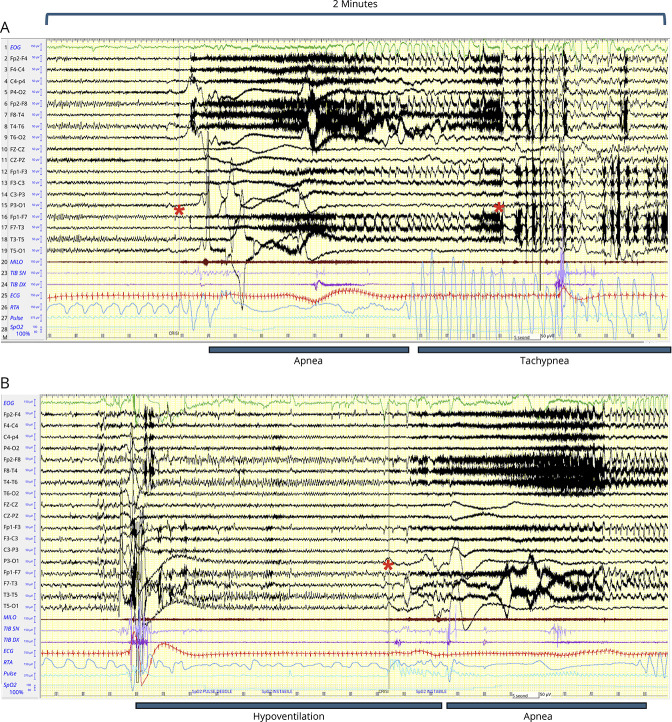
Figure 2. Polygraphic Features of Ictal Apnea in Patient 1.
In both A and B, 2 minutes of long-term EEG monitoring are shown for 2 seizures. (A) Regular breathing (RTA channel) is interrupted with the appearance of a prolonged apnea (>30 s). Red asterisks mark the start and termination of the ictal discharge on scalp EEG. The apnea was followed by a marked tachypnea with gasping that started during the seizure then prolonging into the postictal period for several seconds. ECG shows tachycardia during the apnea persisting in the postictal period. The EEG channels show EMG artifacts during the first part of the seizure; then, a generalized EEG suppression with anterior slow waves is evident in the second part of the seizure and in the postictal period. Oxygen saturation is 100% at seizure onset; then, a decrease is observed until 74%. (B) A second seizure with a similar sequence is reported. However, in this ictal event, the apnea period is preceded by a prolonged period of hypoventilation that precedes the EEG seizure onset (red asterisk). This event was more prolonged and associated with an oxygen desaturation until 71%. The end of the seizure and the postictal period are not shown. EEG channels show bipolar recordings according to the 10–20 international system. Filters: high-pass 0.1 Hz; low-pass 35 Hz. Amplitude 10 uV/mm. Polygraphic channels are as follows: channel 1: ocular movement (green color); channel 20 (milo): EMG activity from mylohyoid muscle (brown color); channels 23–24 (tib dx, tib sx): EMG activity from right and left tibialis anterior muscles (purple color); channel 25 (ECG): cardiac activity (red color); channel 26 (RTA): thoracoabdominal respiration (blue). The last 2 channels: pulse and oxygen saturation (light blue).
A total of 15 seizures were recorded in DEPDC5 patients, with 100% showing ICA (Table). During LTVM, only focal seizures were recorded, emerging from sleep in 4 of 5 patients. ICA was associated with an electrographic arousal and in the longest events also with a brief clinical arousal followed by body turning and sleep. All seizures were characterized by motor arrest without overt automatic behaviors during ictal apnea. Scalp EEG showed the involvement of the temporal lobe in all events. No focal to bilateral tonic-clonic (FBTC) seizures were recorded. Seizure duration ranged from 30 to 130 seconds with ICA lasting from 20 seconds to more than 1 minute (in patient #1). Severe oxygen desaturation (≤75%) was recorded in 2 patients who showed ICAs with the longest duration. In patient #1, the apnea was followed by a marked tachypnea with gasping that started during the seizure then prolonging into the postictal period for several seconds (Figure 2). Notably, no patient was aware of the apnea. In addition, the video of the events did not show behavioral reports of overt physical distress/discomfort. Patients were interrogated actively in some events by the EEG technician. We asked every patient whether they were in some way aware of stop breathing during the seizures, and none reported awareness of apnea or distress.
Short case descriptions of the 5 patients are reported in eAppendix 1.
Discussion
Ictal/postictal respiratory alterations represent a potential electroclinical biomarker of SUDEP because terminal respiratory disruption has been demonstrated to be a relevant mechanism leading to fatal outcomes after focal to bilateral tonic-clonic seizures.14,16 Furthermore, central apnea during focal seizures may represent a biomarker of seizure-related fragility of respiratory centers.17,18 Ictal central apnea can be an underreported clinical correlate of focal seizures, being observed in 30%–45% of patients with focal epilepsy admitted to epilepsy monitoring units.10,13-15
In this study, we documented that patients with pathogenic mutations of DEPDC5 exhibit ictal apnea during focal seizures. Specifically, we observed ICA occurring in 100% of the recorded seizures of patients with DEPDC5 pathogenic variants. These results certainly need to be evaluated with caution, given the relatively small sample size, but strongly suggest that ictal respiratory changes are frequent in DEPDC5. Moreover, in 2 of 5 patients, ICA was prolonged and associated with severe oxygen desaturation. Notably, none of the patients was aware of the apnea or reported subjective breathing distress or choking.10,13-15
Overall, our findings highlight the necessity for active monitoring of respiratory parameters during seizures to detect ictal apnea. Cardiorespiratory monitoring is clinically relevant, given that DEPDC5-related epilepsy is associated with an increased risk of SUDEP. Breathing alterations here reported show similarities with a recently developed mouse model of DEPDC5-related epilepsy, characterized by ictal apnea followed by terminal apnea in fatal seizures.8 Moreover, the absence of structural or functional cardiac abnormalities documented in patients with DEPDC5-related epilepsy further supports the potential role of respiratory abnormalities underlying SUDEP risk in this population.5,6
In NGS-tested patients with ICA, we observed DEPDC5 pathogenic variants in 5 of 14 (35.71%) while none of the patients undergoing NGS testing without ICA presented mutations of DEPDC5 or other epilepsy-related genes. These data suggest that it might be of significant value to think about a broader application of genetic testing in patients with focal seizures and ICA who do not have an acquired structural etiology.
This study has limitations. First, this was not a prospective study aiming at estimating the incidence of pathogenic mutations in patients with epilepsy and ictal respiratory alterations. Instead, we capitalized on previous studies conducted to investigate the incidence and characteristics of ICA in epilepsy monitoring units to inquire about DEPDC5 and ictal respiratory alterations. Therefore, our findings should be interpreted with caution and as hypothesis generating results for replication in wider patient cohorts. Nonetheless, our results could open new research lines to increase our understanding of epilepsy related to GATOR1 variants and the role of ictal respiratory dysfunction and its relevance to SUDEP in genetic epilepsies.
Acknowledgment
The authors thank all the patients for their participation in this study. The authors also thank all the EEG technicians of the Epilepsy Monitoring Units.
Appendix. Authors
| Name | Location | Contribution |
| Stefano Meletti, MD, PhD | Department of Biomedical Metabolic Sciences and Neurosciences, University of Modena and Reggio Emilia; Neurophysiology Unit and Epilepsy Centre, Neuroscience Department, Modena AOU, Italy | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Gian Marco Duma, PhD | Epilepsy Unit, IRCCS E. Medea Scientific Institute, Conegliano, Italy | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Margherita Burani, MD | Department of Biomedical Metabolic Sciences and Neurosciences, University of Modena and Reggio Emilia; Neurophysiology Unit and Epilepsy Centre, Neuroscience Department, Modena AOU, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Alberto Danieli, MD | Epilepsy Unit, IRCCS E. Medea Scientific Institute, Conegliano, Italy | Major role in the acquisition of data; analysis or interpretation of data |
| Giada Giovannini, MD, PhD | Neurophysiology Unit and Epilepsy Centre, Neuroscience Department, Modena AOU, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Elisa Osanni, MD | Epilepsy Unit, IRCCS E. Medea Scientific Institute, Conegliano, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Elisa Micalizzi, MD | Department of Biomedical Metabolic Sciences and Neurosciences, University of Modena and Reggio Emilia; Neurophysiology Unit and Epilepsy Centre, IRCCS Ospedale Policlinico San Martino, Genoa, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Fabiana Mambretti, MSc | Laboratory of Molecular Genetics, IRCCS E. Medea Scientific Institute, Bosisio Parini, Lecco, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Matteo Pugnaghi, MD | Neurophysiology Unit and Epilepsy Centre, Neuroscience Department, Modena AOU, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Anna E. Vaudano, MD, PhD | Department of Biomedical Metabolic Sciences and Neurosciences, University of Modena and Reggio Emilia; Neurophysiology Unit and Epilepsy Centre, Neuroscience Department, Modena AOU, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Paolo Bonanni, MD | Epilepsy Unit, IRCCS E. Medea Scientific Institute, Conegliano, Italy | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Study Funding
The study was supported by Ricerca Corrente 2024 funds for biomedical research of the Italian Ministry of Health.
Disclosure
S. Meletti received research grant support from the Ministry of Health (MOH); has received personal compensation as scientific advisory board member for UCB, Jazz pharmaceuticals, and EISAI. A.E. Vaudano has received speaker's or consultancy fees from Angelini. M. Burani, G. Giovannini, M. Pugnaghi, E. Micalizzi report no disclosures. P. Bonanni has received speaker's or consultancy fees from Angelini, EISAI, Livanova. G.M. Duma, A. Danieli, E. Osanni, F. Mambretti report no disclosures. Go to Neurology.org/NG for full disclosures.
References
- 1.Harden C, Tomson T, Gloss D, et al. . Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of neurology and the American Epilepsy Society. Neurology. 2017;88(17):1674-1680. doi: 10.1212/WNL.0000000000003685 [DOI] [PubMed] [Google Scholar]
- 2.Li R, Buchanan GF. Scurrying to understand sudden expected death in epilepsy: insights from animal models. Epilepsy Curr. 2019;19(6):390-396. doi: 10.1177/1535759719874787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baulac S, Baldassari S. DEPDC5-related epilepsy. 2016. [updated 2023 Mar 9]. In: Adam MP, Feldman J, Mirzaa GM, et al., eds. GeneReviews® [Internet]. : University of Washington, Seattle; 1993-2024. [Google Scholar]
- 4.Baldassari S, Picard F, Verbeek NE, et al. . The landscape of epilepsy-related GATOR1 variants. Genet Med. 2019;21(2):398-408. doi: 10.1038/s41436-018-0060-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacq A, Roussel D, Bonduelle T, et al. . Cardiac investigations in sudden unexpected death in DEPDC5-related epilepsy. Ann Neurol. 2022;91(1):101-116. doi: 10.1002/ana.26256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagnall RD, Crompton DE, Petrovski S, et al. . Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann Neurol. 2016;79(4):522-534. doi: 10.1002/ana.24596 [DOI] [PubMed] [Google Scholar]
- 7.Weckhuysen S, Marsan E, Lambrecq V, et al. . Involvement of GATOR complex genes in familial focal epilepsies and focal cortical dysplasia. Epilepsia. 2016;57(6):994-1003. doi: 10.1111/epi.13391 [DOI] [PubMed] [Google Scholar]
- 8.Nascimento FA, Borlot F, Cossette P, Minassian BA, Andrade DM. Two definite cases of sudden unexpected death in epilepsy in a family with a DEPDC5 mutation. Neurol Genet. 2015;1(4):e28. doi: 10.1212/NXG.0000000000000028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kao HY, Yao Y, Yang T, et al. . Sudden unexpected death in epilepsy and respiratory defects in a mouse model of DEPDC5-related epilepsy. Ann Neurol. 2023;94(5):812-824. doi: 10.1002/ana.26773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Micalizzi E, Vaudano AE, Ballerini A, et al. . Ictal apnea: a prospective monocentric study in patients with epilepsy. Eur J Neurol. 2022;29(12):3701-3710. doi: 10.1111/ene.15547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micalizzi E, Ballerini A, Giovannini G, et al. . The role of the amygdala in ictal central apnea: insights from brain MRI morphometry. Ann Clin Transl Neurol. 2024;11(1):121-132. doi: 10.1002/acn3.51938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaudano AE, Ballerini A, Zucchini F, et al. . Impact of an optimized epilepsy surgery imaging protocol for focal epilepsy: a monocentric prospective study. Epileptic Disord. 2023;25(1):45-56. doi: 10.1002/epd2.20050 [DOI] [PubMed] [Google Scholar]
- 13.Lacuey N, Zonjy B, Hampson JP, et al. . The incidence and significance of periictal apnea in epileptic seizures. Epilepsia. 2018;59(3):573-582. doi: 10.1111/epi.14006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilella L, Lacuey N, Hampson JP, et al. . Incidence, recurrence, and risk factors for peri-ictal central apnea and sudden unexpected death in epilepsy. Front Neurol. 2019;10:166. doi: 10.3389/fneur.2019.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131(Pt 12):3239-3245. doi: 10.1093/brain/awn277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryvlin P, Nashef L, Lhatoo SD, et al. . Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12(10):966-977. doi: 10.1016/S1474-4422(13)70214-X [DOI] [PubMed] [Google Scholar]
- 17.Nashef L, Walker F, Allen P, Sander JW, Shorvon SD, Fish DR. Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry. 1996;60(3):297-300. doi: 10.1136/jnnp.60.3.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuele SU, Afshari M, Afshari ZS, et al. . Ictal central apnea as a predictor for sudden unexpected death in epilepsy. Epilepsy Behav. 2011;22(2):401-403. doi: 10.1016/j.yebeh.2011.06.036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on reasonable request.