Abstract
Introduction
Obesity has previously been correlated with an elevated risk of reproductive system diseases in women. The waist-hip ratio (WHR) has been shown to be correlated with visceral fat, making it one of the most commonly used indicators of abdominal obesity. However, little is known about the relationship between WHR and infertility. Therefore, the aim of this study was to evaluate the effect of the WHR on infertility in women of childbearing age.
Methods
The study used cross-sectional data from women aged 20–45 who participated in the National Health and Nutrition Examination Survey (NHANES), which was conducted between 2017 and 2020. We collected details of their waist circumference, hip circumference, fertility status, and several other essential variables. We used multivariate logistic regression analysis and subgroup analyses to assess the association between WHR and infertility.
Results
There were 976 participants, with 12.0% (117/976) who experienced infertility. After adjusting for potential confounding factors, our multivariate logistic regression analysis revealed that every 0.1 unit increase in WHR resulted in a more than 35% higher risk of infertility (odds ratio [OR; 95% confidence interval [CI]: 1.35 [1.01∼1.81], p = 0.043). Compared to the group with WHR <0.85, the risk of infertility increased in the group with WHR ≥0.85, with an adjusted OR of 1.74 (95% CI: 1.06∼2.85). When WHR was treated as a continuous variable, it was observed that each 0.1 unit increase in WHR was associated with a relatively high risk in the secondary infertility population after adjusting all covariates, with an OR of 1.66 (95% CI: 1.14∼2.40, p = 0.01). When WHR was analyzed as a categorical variable, the group with WHR ≥0.85 exhibited a significantly higher risk of secondary infertility than the group with WHR <0.85, with the OR of 2.75 (95% CI: 1.35–5.59, p = 0.01) after adjusting for all covariates. Furthermore, the interaction analysis indicated that there was a significant interaction between age status on WHR and the risk of infertility.
Conclusion
WHR showed a positive correlation with the risk of infertility. This study highlights the importance of effectively managing abdominal fat and promoting the maintenance of optimal WHR levels to mitigate the progression of infertility, particularly for younger women.
Keywords: Obesity, Infertility, Waist-hip ratio, NHANES, Cross-sectional analysis
Introduction
Infertility is the inability to conceive despite regular unprotected intercourse without the use of contraception for 1 year or more. It is classified into two groups included primary infertility and secondary infertility [1, 2]. Primary infertility is defined as a woman who has never been diagnosed with a clinical pregnancy and meets the criteria of being classified as being infertile, while secondary infertility is defined as a woman unable to establish a clinical pregnancy who has previously been diagnosed with a clinical pregnancy [1, 2]. It has a significant global impact, with approximately one in seven couples in developed countries and one in four couples in developing countries being affected by this issue [3–5]. In the USA, the estimated prevalence of infertility among women of reproductive age ranges from about 6.7–14.2% [6, 7]. As a result, the Centers for Disease Control and Prevention (CDC) has focused on prioritizing both the diagnosis and treatment of infertility [8]. However, the determinants of infertility remain incompletely understood. It can stem from various sources including some medical factors (such as abnormalities of the ovaries, uterus, fallopian tubes, or endocrine system), occupational factors (such as shift work, stress, exposure to physical radiation, and toxic chemicals), and lifestyle factors (such as age, nutrition, exercise, obesity, psychological stress, smoking, and alcohol consumption), as well as environmental pollution [9–11].
Previous studies have shown that abdominal obesity is one of the risk factors for insulin resistance, metabolic syndrome, diabetes mellitus, hypertension, coronary heart disease, and heart failure [12–19]. Pasquali suggested in both sexes, obesity, particularly the abdominal obesity phenotype, may impair fertility [20]. This adverse effect appears to be primarily associated with disorders of sex hormone secretion and/or metabolism, resulting in a state of relative hyperandrogenism in women with obesity [20].
Several studies have investigated the impact of abdominal obesity and body fat distribution on female fertility. In a study by Shoujing Liang et al. [1], it was found that a WHR above 0.85 may act as a protective factor against secondary infertility. The risk of secondary infertility decreased for the group with WHR >0.85 compared to the group with WHR ≤0.85. These findings contradict the prevailing view that abdominal obesity may have a detrimental effect on fertility due to its association with metabolic disorders [3]. In contrast, another study found no association between abdominal obesity and fertility [21]. One possible explanation is that WHR is just one of several parameters used to assess abdominal obesity. It should also be noted that most participants in this study had a normal body mass index (BMI) [1]. However, the available evidence of the association between WHR and the risk of infertility is limited and inconsistent.
The present study used data from the National Health and Nutrition Examination Survey (NHANES) to evaluate waist-to-hip ratio (WHR) in US female adults with and without infertility. We hypothesized that females with infertility would have a higher WHR compared to healthy individuals in this population. Additionally, we hypothesized that females with a WHR of ≥0.85 would have a higher prevalence of infertility compared to those with a WHR of <0.85. Furthermore, we identified the WHR associated with the prevalence of primary and secondary infertility. The subgroup analysis examined age, ethnicity, marital status, family poverty income ratio (PIR), physical activity, smoking status, alcohol status, menstrual periods, and trouble sleeping as they have been shown in previous studies to affect the prevalence of infertility [3, 4, 22, 23]. Understanding these associations can assist health authorities in making decisions about health promotion programs and interventions to prevent infertility in women of reproductive age.
Materials and Methods
Study Design
This cross-sectional study utilized publicly available data from the National Health and Nutrition Survey (NHANES) conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) [4, 22, 23]. NHANES is a health-related program of studies designed to assess the health and nutritional status of noninstitutionalized US citizens. Survey participants were selected using a multistage, stratified probability design as a representative sample [4, 22, 23]. Moreover, NHANES collects various health-related data, including demographic characteristics, physical examination results, laboratory findings, and dietary habits through home visits, screening, and laboratory testing conducted by a mobile examination center. The survey’s design, methods, and data are available to the public. The data of the present study were obtained from women aged 20–45 years during the March 2017–2020 cycle and can be accessed on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm; accessed on July 10, 2023). The study protocol received approval from the NCHS Research Ethics Review Board, and informed consent was obtained from all participants. As the study relied on deidentified data, ethical approval and consent were not required [18]. In accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline, this study is reported.
Inclusion and Exclusion Criteria
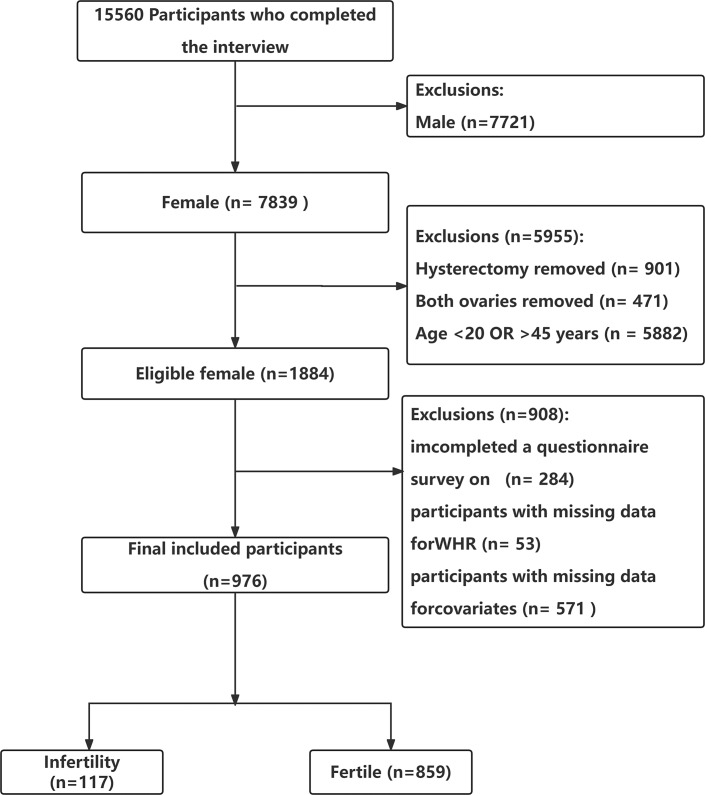
Women who participated in the NHANES during the March 2017–2020 cycle provided the data for this cross-sectional research because only this cycle included a reproductive health questionnaire with questions on infertility and the value of hip circumference (HC). The inclusion criteria were (1) female participants aged 20–45 years [22–25]. (2) Female participants who positively responded to the question: “Have you ever tried to get pregnant for at least 1 year without getting pregnant?” [22–25]. The exclusion criteria were (1) female participants with a history of hysterectomy, or bilateral oophorectomy, as defined in previous NHANES studies [22–25]. History of hysterectomy used in previous studies using NHANES data. History of hysterectomy was defined as a dichotomous variable based on the question: “Have you had a hysterectomy, that is, surgery to remove your uterus or womb?” (Variable Name in NHANES: RHD280) from the reproductive health questionnaire. Participants who answered “yes” were identified as having a history of hysterectomy [23–25]. And the definition of “bilateral oophorectomy” was determined by the question: “Have you had both of your ovaries removed (either when you had your uterus removed or at another time)” (Variable Name in NHANES: RHQ305) from the reproductive health questionnaire. Participants who answered “yes” were identified as having undergone bilateral oophorectomy [23–25]. (2) Female participants with missing values for waist circumference (WC) and HC. (3) Female participants with missing data for covariates, such as the ratio of family income to poverty (PIR), pelvic infection (PID), BMI, and hypertension. After applying the inclusion and exclusion criteria, a total of 976 participants were selected for the study. Figure 1 illustrates the sample selection process.
Fig. 1.
Flowchart of the study population enrollment. WHR, waist-hip ratio.
Outcomes and Covariates
Anthropometric measures, including BMI, WC, waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR), provide a simple, cost-effective, and repeatable approach to assessing abdominal obesity [26–29]. Among these indices, WHR is considered to be a more accurate indicator of abdominal obesity, particularly in individuals with larger body sizes [30]. However, it is important to note that individuals with larger body sizes but without abdominal obesity may exhibit elevated WC measurements, potentially leading to misdiagnosis. Similarly, individuals with higher body size but with abdominal obesity may display lower WHtR measurements, increasing the risk of missed diagnoses [31]. Therefore, we chose to use WHR as an alternative to assess abdominal obesity in this study.
The WHR was calculated as the division of WC (in centimeters) by HC (in centimeters) [29, 32]. The anthropometric measurements, such as height, weight, WC, and HC, were obtained through physical examinations. BMI was calculated by dividing weight in kilograms by the square of height in meters and expressed as kg/m2. The following cutoffs of the different measures were used for categorical variable definitions. In accordance with previous literature, we classified BMI into four groups: underweight (<18.5), normal weight (18.5–24.9), overweight (25.0–29.9), obesity (≥30) [4]. For WHR, the cutoff was placed ≥0.85 for females for an obesity definition [32].
The primary outcome of the study was the incidence of infertility. Infertility was determined based on responses to the question “Have you ever attempted to conceive for a minimum period of 1 year without achieving pregnancy?” (Variable Name in NHANES: RHQ074) from the reproductive health questionnaire. Participants who answered affirmatively were categorized as infertility (1 = infertility, 0 = fertile) [33, 34]. And according to a previous study [23], infertility was further divided into primary infertility group and secondary infertility group based on the answer from a question “Ever been pregnant?” (Variable Name in NHANES: RHQ131) from the reproductive health questionnaire. Furthermore, if a women with infertility answering “yes” denoted a secondary infertility case, whereas if a women with infertility answering “no” denoted a primary infertility case.
To identify potential covariates associated with infertility and WHR, a comprehensive selection was made based on relevant literature [4, 9, 29, 34]. These covariates encompassed demographic factors such as age, ethnicity, educational level, marital status, family PIR, smoking status, physical activity, hypertension, diabetes, dietary supplements, and trouble sleeping. Additionally, reproductive factors including regular menstrual periods and treatment for pelvic infection/pelvic inflammatory disease (PID) were considered in the analysis.
According to preceding literature definitions [35], ethnicity was classified into four categories: non-Hispanic white, non-Hispanic black, Mexican-American, and other races. Marital status was categorized as married, living with a partner, or living alone. Educational attainment was grouped into three categories: less than 9 years, 9–12 years, and more than 12 years. According to a US government report [35], family income was stratified into two groups using the PIR: low (PIR ≤1.3) and high (PIR >1.3) [35]. Smoking status was categorized as never smokers (smoked less than 100 cigarettes), current smokers, and former smokers (quit smoking after consuming more than 100 cigarettes), as per literature definitions [35]. Physical activity was classified as sedentary, moderate (at least 10 min of movement within the last 30 days, resulting in only light sweating or a mild to moderate increase in breathing or heart rate), and vigorous (at least 10 min of activity within the last 30 days, resulting in profuse sweating or an increase in breathing or heart rate) [35]. Previous disease history (hypertension, diabetes) was determined based on the inquiry in the questionnaire of whether the doctor had informed of the condition in the past. Trouble sleeping was defined a dichotomous variable based on the question: “Have you ever told a doctor or other health professional that you have trouble sleeping?”. Participants who answered “yes” were identified as having trouble sleeping [36]. And the definition of dietary supplements was determined by the question: “Have you use of dietary supplements and medications during the past month. Have you used or taken any vitamins, minerals or other dietary supplements in the past month? Include those products prescribed by a health professional such as a doctor or dentist, and those that do not require a prescription.” Participants who answered “yes” were identified as having taken dietary supplements [35].
Statistical Analysis
In this study, publicly accessible datasets were subjected to a secondary examination. Means and standard deviations and frequencies (percentages) were used to describe demographic and clinical data. The t test was used to analyze the normal distribution and the Kruskal-Wallis test to analyze the skewed distribution in continuous variables, and χ2 tests for categorical variables.
Participants were divided into two groups based on their WHR values, using a cutoff of ≥0.85 as defined for obesity in females [37]. Group 1 comprised individuals with WHR <0.85 (n = 320), while group 2 consisted of those with WHR ≥0.85 (n = 656).
Univariate logistic regression was used to identify factors that were associated with the prevalence of infertility. Multivariable analyses were performed to identify WHR associated with the prevalence of infertility, as well as primary and secondary infertility. In multivariate logistic regression, WHR was analyzed as a continuous and categorical variable. Both nonadjusted and multivariate-adjusted models were employed. Adjustment variables were selected if they resulted in at least a 10% change in the matched odds ratio (OR). The results were reported as OR and 95% confidence interval (CI).
In model 1, adjustments were made for sociodemographic factors including age, ethnicity, educational level, marital status, and family poverty income ratio. Model 2 included additional adjustments for hypertension, diabetes, regular menstrual periods, and trouble sleeping. Model 3 further accounted for smoking status.
Furthermore, logistic regression models were used to investigate potential interactions and perform subgroup analyses based on various factors, including age, ethnicity, marital status, poverty income ratio, physical activity level, smoking status, alcohol consumption, regular menstrual periods, and trouble sleeping. These variables were selected based on clinical interest and previous scientific literature [3, 4, 38, 39]. The likelihood ratio test was used to examine interactions among subgroups and WHR.
The sample size was determined based on available data, and no priori estimate of statistical power was made. All analyses were conducted using R 4.2.2 and Free Statistics software version 1.9.1 [40]. A descriptive study was performed on the full participant sample, and statistical significance was set at a two-tailed p value of <0.05.
Results
Characteristics of the Study Participants
A total of 976 participants with available data on waist-to-hip ratio (WHR) and infertility were included in this analysis. Among these individuals, 117 (12.0%) reported experiencing infertility. Table 1 presents the clinical characteristics of the study population based on different WHR levels. The mean age of the participants was 32.1 ± 7.5 years, with the majority identifying as non-Hispanic black (32.2%, n = 314). Of the participants, 500 (51.2%) participants were classified as obesity (≥30 kg/m2) and 202 (20.7%) participants were classified as overweight (25.0–29.9 kg/m2). In addition, it was found that participants with WHR ≥0.85 had a higher mean age of 33.3 ± 7.4 years compared to those with WHR <0.85 who had a mean age of 29.8 ± 7.1 years (p < 0.001). Furthermore, the former group exhibited a higher prevalence of obesity (≥30 kg/m2: 66.5% vs. 20.0%, p < 0.001), were more prone to suffering from hypertension (14.2% vs. 3.1%, p < 0.001) and diabetes (6.4% vs. 1.2%, p < 0.001), and were more likely to engage in less physical activity (sedentary: 47.7% vs. 36.9%, p < 0.001). Notably, participants in the group of WHR ≥0.85 had a significantly higher prevalence of infertility compared to those in the group of WHR <0.85 (14.2% vs. 7.5%, p = 0.003).
Table 1.
Characteristics of the study participants by categories of WHR (n = 976)
| Variables | WHR | |||
|---|---|---|---|---|
| total (n = 976) | <0.85 (n = 320) | ≥0.85 (n = 656) | p value | |
| Age, mean(SD), years | 32.1±7.5 | 29.8±7.1 | 33.3±7.4 | <0.001 |
| Ethnicity, n (%) | 0.008 | |||
| Non-Hispanic white | 271 (27.8) | 97 (30.3) | 174 (26.5) | |
| Non-Hispanic black | 314 (32.2) | 105 (32.8) | 209 (31.9) | |
| Mexican American | 152 (15.6) | 32 (10) | 120 (18.3) | |
| Others | 239 (24.5) | 86 (26.9) | 153 (23.3) | |
| Education level, n (%) | 0.002 | |||
| <9 years | 46 (4.7) | 13 (4.1) | 33 (5.0) | |
| 9–12 years | 359 (36.8) | 94 (29.4) | 265 (40.4) | |
| >12 years | 571 (58.5) | 213 (66.6) | 358 (54.6) | |
| Marital status, n (%) | <0.001 | |||
| Married or living with a partner | 504 (51.6) | 136 (42.5) | 368 (56.1) | |
| Living alone | 472 (48.4) | 184 (57.5) | 288 (43.9) | |
| PIR, n (%) | 0.386 | |||
| ≤1.3 | 483 (49.5) | 152 (47.5) | 331 (50.5) | |
| >1.3 | 493 (50.5) | 168 (52.5) | 325 (49.5) | |
| Dietary supplements taken, n (%) | 0.118 | |||
| No | 538 (55.1) | 165 (51.6) | 373 (56.9) | |
| Yes | 438 (44.9) | 155 (48.4) | 283 (43.1) | |
| BMI, kg/m2 | 31.2±9.0 | 25.5±7.0 | 34.0±8.5 | <0.001 |
| BMI, n (%) | <0.001 | |||
| Underweight (<18.5) | 25 (2.6) | 22 (6.9) | 3 (0.5) | |
| Normal weight (18.5–24.9) | 249 (25.5) | 165 (51.6) | 84 (12.8) | |
| Overweight (25.0–29.9) | 202 (20.7) | 69 (21.6) | 133 (20.3) | |
| Obesity (≥30) | 500 (51.2) | 64 (20.0) | 436 (66.5) | |
| Hypertension, n (%) | <0.001 | |||
| No | 873 (89.4) | 310 (96.9) | 563 (85.8) | |
| Yes | 103 (10.6) | 10 (3.1) | 93 (14.2) | |
| Diabetes, n (%) | <0.001 | |||
| No | 930 (95.3) | 316 (98.8) | 614 (93.6) | |
| Yes | 46 (4.7) | 4 (1.2) | 42 (6.4) | |
| Physical activity, n (%) | <0.001 | |||
| Sedentary | 431 (44.2) | 118 (36.9) | 313 (47.7) | |
| Moderate | 279 (28.6) | 123 (38.4) | 156 (23.8) | |
| Vigorous | 266 (27.3) | 79 (24.7) | 187 (28.5) | |
| Smoking status, n (%) | 0.018 | |||
| Never | 638 (65.4) | 229 (71.6) | 409 (62.3) | |
| Former | 129 (13.2) | 35 (10.9) | 94 (14.3) | |
| Current | 209 (21.4) | 56 (17.5) | 153 (23.3) | |
| Alcohol drinker, n (%) | 0.636 | |||
| No | 101 (10.3) | 31 (9.7) | 70 (10.7) | |
| Yes | 875 (89.7) | 289 (90.3) | 586 (89.3) | |
| Infertility, n (%) | 0.003 | |||
| No | 859 (88.0) | 296 (92.5) | 563 (85.8) | |
| Yes | 117 (12.0) | 24 (7.5) | 93 (14.2) | |
| PID, n (%) | 0.55 | |||
| No | 905 (92.7) | 299 (93.4) | 606 (92.4) | |
| Yes | 71 (7.3) | 21 (6.6) | 50 (7.6) | |
| Regular menstrual periods, n (%) | 0.862 | |||
| No | 66 (6.8) | 21 (6.6) | 45 (6.9) | |
| Yes | 910 (93.2) | 299 (93.4) | 611 (93.1) | |
| Trouble sleeping, n (%) | 0.005 | |||
| No | 738 (75.7) | 260 (81.2) | 478 (73.0) | |
| Yes | 237 (24.3) | 60 (18.8) | 177 (27.0) | |
Data presented are mean ± SD or N (%).
SD, standard deviation; WHR, waist-hip ratio; BMl, body mass index; PIR, family poverty income ratio; PID, pelvic inflammatory disease.
Previous literature showed that they did not use sampling weights in their analyses. This is because including variables used in the calculation of sampling weights in statistical models can lead to lower accuracy of effect estimates [39, 41, 42]. Therefore, unweighted analyses were deemed more suitable.
However, to assess the robustness of our findings, we also assess the relationship between WHR and infertility with weighted samples. We used full sample MEC exam weight for the weighted analysis. For the analyses of NHANES 2017-March 2020 pre-pandemic data, a 3.2-year MEC weight (WTMECPRP) set was used. A descriptive analysis was performed for all participants. In the descriptive analysis, categorical data were expressed as unweighted numbers (weighted percentages), whereas continuous data were expressed as means (standard error). The characteristics of participants of the 976 included participants are shown in online supplementary Table S1 (for all online suppl. material, see https://doi.org/10.1159/000538974). The study found that participants with WHR ≥0.85 had a significantly higher prevalence of infertility compared to those with WHR <0.85 (15.27% vs. 7.11%, p = 0.0072).
Associations between WHR and the Risk of Infertility
The univariate analysis demonstrated that ethnicity, marital status, family income, BMI, diabetes, smoking status, trouble sleeping and WHR were associated with infertility (Table 2). Table 3 summarizes the OR and 95% CIs for infertility risk based on WHR categories: WHR ≥0.85 and WHR <0.85. The group with WHR ≥0.85 exhibited a significantly higher risk of infertility, with an unadjusted OR of 2.04 (95% CI: 1.27–3.26). After adjusting for all covariates, the OR remained significant at 1.74 (95% CI: 1.06–2.85). These results were consistent across all models, indicating their robustness (Table 3). Additionally, treating WHR as a continuous variable, each 0.1 unit increase in WHR was associated with a more than 35% increase in the odds of infertility, even after accounting for potential confounders (model 3, OR (95% CI): 1.35 (1.02–1.80), p = 0.038).
Table 2.
Univariate logistic regression between infertility and WHR and other risk factors
| Variables | OR (95% CI) | p value |
|---|---|---|
| Age, years | 1.02 (1.00–1.05) | 0.069 |
| Race and ethnicity | ||
| Non-Hispanic white | 1.00 (ref) | |
| Non-Hispanic black | 0.55 (0.33–0.92) | 0.022 |
| Mexican American | 0.75 (0.42–1.36) | 0.350 |
| Others | 0.81 (0.49–1.34) | 0.402 |
| Education level | ||
| <9 years | 1.00 (ref) | |
| 9–12 years | 1.15 (0.43–3.05) | 0.786 |
| >12 years | 1.11 (0.42–2.90) | 0.834 |
| Marital status | ||
| Married or living with a partner | 1.00 (ref) | |
| Living alone | 0.49 (0.33–0.74) | 0.001 |
| PIR | ||
| ≤1.3 | 1.00 (ref) | |
| >1.3 | 1.54 (1.04–2.27) | 0.033 |
| Dietary supplements taken | 0.91 (0.61–1.34) | 0.620 |
| BMI, kg/m2 | 1.03 (1.00–1.05) | 0.016 |
| Hypertension | 0.96 (0.51–1.82) | 0.911 |
| Diabetes | 2.77 (1.39–5.52) | 0.004 |
| Physical activity | ||
| Sedentary | 1.00 (ref) | |
| Moderate | 0.64 (0.39–1.03) | 0.068 |
| Vigorous | 0.82 (0.51–1.30) | 0.389 |
| Smoking status | ||
| Never | 1.00 (ref) | |
| Former | 1.48 (0.84–2.60) | 0.171 |
| Current | 1.9 (1.22–2.96) | 0.005 |
| Alcohol drinker | 1.44 (0.71–2.94) | 0.317 |
| PID | ||
| No | 1.00 (ref) | |
| Yes | 1.38 (0.70–2.71) | 0.347 |
| Regular menstrual periods | ||
| No | 1.00 (ref) | |
| Yes | 0.85 (0.41–1.77) | 0.670 |
| Trouble sleeping | ||
| No | 1.00 (ref) | |
| Yes | 1.74 (1.15–2.64) | 0.008 |
| WHRa | 1.51 (1.17–1.97) | 0.002 |
Data presented are OR and 95% CI.
WHR, waist-hip ratio; BMl, body mass index; PIR, family poverty income ratio; PID, pelvic inflammatory disease; OR, odds ratio; 95% CI, 95% confidence interval.
aThe OR was examined by per 0.1-unit increase of WHR.
Table 3.
Multivariate logistic regression was used to determine the relationship between WHR and infertility
| Variable | Non-adjusted model | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| WHRa (n = 976) | 1.51 (1.17–1.97) | 0.002 | 1.47 (1.12–1.94) | 0.005 | 1.40 (1.05–1.85) | 0.021 | 1.35 (1.02–1.80) | 0.038 |
| Binary variable | ||||||||
| WHR <0.85 (n = 320) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| WHR ≥0.85 (n = 656) | 2.04 (1.27–3.26) | 0.003 | 1.89 (1.16–3.07) | 0.010 | 1.80 (1.10–2.94) | 0.019 | 1.74 (1.06–2.85) | 0.028 |
Data presented are OR and 95% CI.
WHR, waist-hip ratio; OR, odds ratio; 95% CI, 95% confidence interval.
aThe OR was examined by per 0.1-unit increase of WHR.
Model 1: adjusted for sociodemographic variables (age, race and ethnicity, educational level, marital status, and family poverty income ratio).
Model 2: adjusted for model 1 + hypertension, diabetes, regular menstrual periods, and trouble sleeping.
Model 3: adjusted for model 2+ smoking status.
Furthermore multivariate logistic regression was used to determine the weighted ORs and 95% CIs for the relationship between WHR and infertility. The results are displayed in online suppl. Table S2. When WHR was used as a continuous variable, we found that each 0.1 unit increase in WHR (OR = 1.76, 95% CI: 1.25–2.48, p = 0.002) was associated with a relatively high risk in infertility population in the crude model. The OR for each 0.1 unit increase in WHR was consistently significant in model 1 and model 2. And when WHR was analyzed as a categorical variable, the group with WHR ≥0.85 exhibited a significantly higher risk of infertility than the group with WHR <0.85, with an unadjusted OR of 2.35 (95% CI: 1.27–4.37, p = 0.01).
Associations between WHR and the Risk of Primary and Secondary Infertility
Considering the possible differences in factors between primary and secondary infertility, we further conducted a separate analysis in these two groups. Multivariable analyses were performed to identify WHR associated with the prevalence of primary and secondary infertility. The results are displayed in Table 4 and Table 5. When treating WHR as a continuous variable, we found that each 0.1 unit increase in WHR (OR = 1.99, 95% CI 1.41–2.80, p < 0.001) was associated with a relatively high risk in the secondary infertility population in the crude model (Table 4). And WHR was associated with a 66% increase in the odds of secondary infertility, even after accounting for potential confounders, each 0.1 unit increase in WHR (model 3, OR [95% CI]: 1.66 [1.14–2.40], p = 0.01) (Table 5). However, no significant association was observed in women with primary infertility.
Table 4.
Multivariate logistic regression was used to determine the associations between WHR and the risk of primary and secondary infertility for US women aged 20–45 years, weighted (crude model)
| Variable | Primary population (n = 859) | Secondary population (n = 959) | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| WHRa | 1.05 (0.87–1.26) | 0.60 | 1.99 (1.41–2.80) | <0.001 |
| Binary variable | ||||
| WHR <0.85 | 1.00 (reference) | 1.00 (reference) | ||
| WHR ≥0.85 | 1.08 (0.79–1.47) | 0.61 | 3.44 (1.86–6.35) | <0.001 |
Data presented are OR and 95% CI.
WHR, waist-hip ratio; OR, odds ratio; 95% CI, 95% confidence interval.
Crude model: no covariate was adjusted.
aThe OR was examined by per 0.1-unit increase of WHR.
Table 5.
Multivariate logistic regression was used to determine the associations between WHR and the risk of primary and secondary infertility for US women aged 20–45 years, weighted (adjusted model)
| Variable | Primary population (n = 859) | Secondary population (n = 959) | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| WHRa | 1.04 (0.86–1.24) | 0.68 | 1.66 (1.14–2.40) | 0.01 |
| Binary variable | ||||
| WHR <0.85 | 1.00(reference) | 1.00 (reference) | ||
| WHR ≥0.85 | 1.05 (0.78–1.41) | 0.68 | 2.75 (1.35–5.59) | 0.01 |
Data presented are OR and 95% CI.
Adjusted model: age, race and ethnicity, educational level, marital status, PIR, hypertension, diabetes, regular menstrual periods, trouble sleeping, and smoking status.
WHR, waist-hip ratio; OR, odds ratio; 95% CI, 95% confidence interval.
aThe OR was examined by per 0.1-unit increase of WHR.
When WHR was analyzed as a categorical variable, the group with WHR ≥0.85 exhibited a significantly higher risk of secondary infertility than the group with WHR <0.85, with an unadjusted OR of 3.44 (95% CI: 1.86–6.35, p < 0.001) (Table 4). After adjusting for all covariates, the OR remained significant at 2.75 (95% CI: 1.35–5.59, p = 0.01) (Table 5). There was no significant difference detected between the group with a WHR of ≥0.85 and the group with a WHR of <0.85 in the primary infertility population, both in the crude model and in model 3 after adjusting for all covariates.
Subgroup Analyses
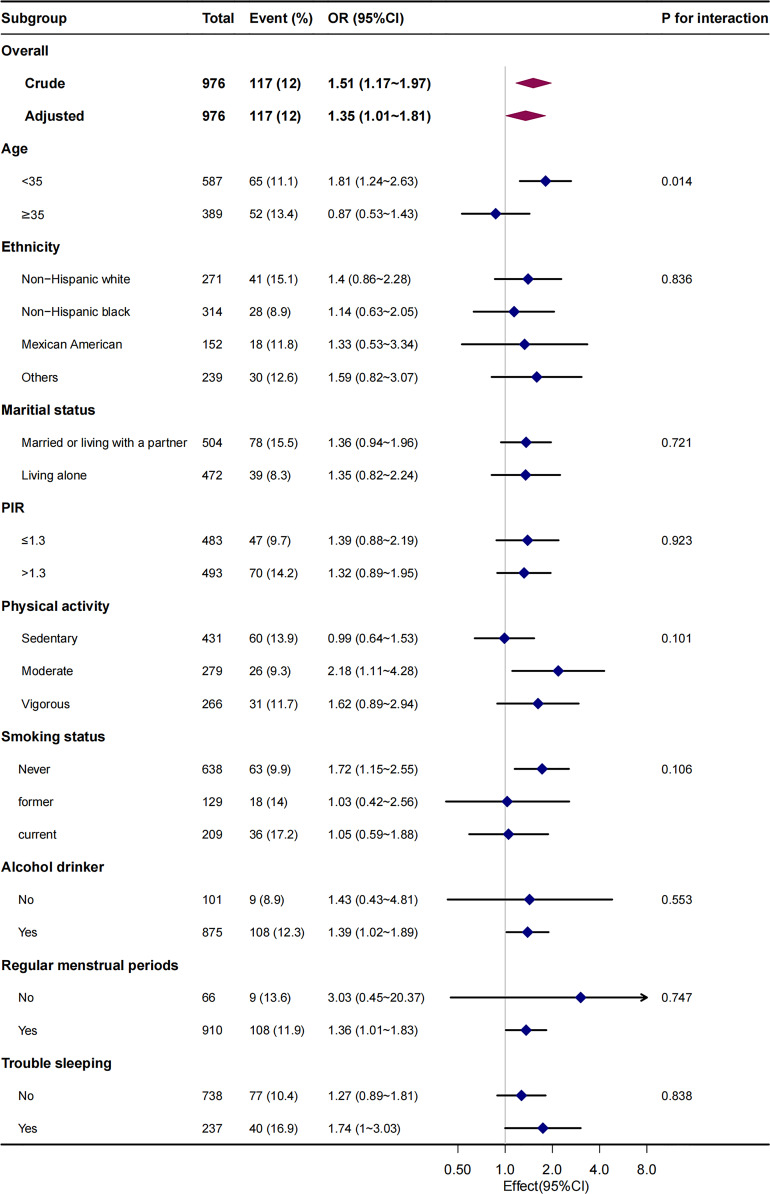
We also performed a stratified analysis according to age, ethnicity, marital status, family income, physical activity, smoking status, alcohol consumption, regular menstrual periods, and trouble sleeping (Fig. 2). For incident infertility, we observed significant differences in age (the p value for interaction likelihood ratio test was p = 0.014) across strata. After adjusting for age, race, and ethnicity, educational level, marital status, PIR, hypertension, diabetes, regular menstrual periods, trouble sleeping, and smoking status, when WHR was used as a continuous variable, as each 0.1 unit increase in WHR, the risk of infertility was significantly increased in the group of age <35 (OR, 1.81; 95% CI: 1.24–2.63), but not in the group of age ≥35 (OR, 0.87; 95% CI: 0.53–1.43). The interaction remained significant when WHR was transformed into a categorical variable (the p value for the interaction was 0.015).
Fig. 2.
Stratified multivariable analysis of the association between WHR and infertility according to baseline characteristics. Each stratification adjusts for all factors (age, ethnicity, educational level, marital status, family poverty income ratio, physical activity, dietary supplements taken, smoking status, alcohol drinking status, hypertension, diabetes, regular menstrual periods, and trouble sleeping) except for the stratification factor itself. The likelihood ratio test was used to examine interactions among subgroups and WHR and statistical significance was set at a two-tailed p value of <0.05. PIR, family poverty income ratio; WHR, waist-hip ratio.
To assess the robustness of our findings, we also assess the relationship between WHR and infertility with weighted samples. After adjusting for age, race, and ethnicity, educational level, marital status, PIR, hypertension, diabetes, regular menstrual periods, trouble sleeping, and smoking status, when WHR was used as a continuous variable, as each 0.1 unit increase in WHR, the risk of infertility was significantly increased in the group of age <35 (the weighted OR, 1.96; 95% CI: 1.35–2.86), meanwhile, it was not significant in the group of age ≥35 (the weighted OR: 0.80, 95% Cl: 0.46–1.38). The interaction between age status on WHR and infertility prevalence was significant (the p value for interaction likelihood ratio test was p = 0.014). The interaction remained significant when WHR was transformed into a categorical variable (the p value for the interaction was 0.015) (online suppl. Table S3).
Discussion
This cross-sectional study analyzed NHANES (2017–2020) data to investigate the association between WHR and infertility in American women of childbearing age. The findings indicate a significant positive correlation between WHR and infertility, even after accounting for potential confounding factors such as age, ethnicity, educational level, marital status, family poverty income ratio, hypertension, diabetes, regular menstrual periods, trouble sleeping, and smoking status. The results are consistent with those of previous studies. In a study, researchers analyzed a prospective cohort of 500 women aged between 20 and 42 who were undergoing artificial insemination. The objective was to examine the effect of body fat distribution on conception rates, while controlling for weight and other variables. The findings indicated that a higher WHR had a detrimental effect on the female reproductive process. Following adjustment for variables including age, body fatness, reasons for artificial insemination, cycle length and regularity, smoking, and parity, each 0.1 unit increase in WHR was found to correlate with a 30% decrease in the likelihood of conception per cycle (hazard ratio 0.706; 95% CI 0.562–0.887) [43]. In contrast, Venkatesh et al. [44] included in their analyses 257,193 individuals who self-identifying as females of white ancestry aged 40–69 years in UKBB. They conducted a Mendelian randomization study and concluded that obesity was associated with a range of female reproductive disorders, including uterine fibroids, polycystic ovary syndrome, heavy menstrual bleeding, and pre-eclampsia. And WHR at baseline assessment was positively associated with the prevalence of most female reproductive disorders in UKBB, with the association observed between WHR and PCOS (OR [95% CI] per 1-SD higher WHR = 1.48 [1.41–1.55], p = 3.32 × 10−26), associations with WHR (ORs for endometriosis, 1.07; heavy menstrual bleeding, 1.15; miscarriage (sporadic), 1.04; pre-eclampsia, 1.13; uterine fibroids, 1.08). However, infertility was the only disorder for which WHR was inversely associated with disease (OR [95% CI] = 0.927 [0.884–0.969], p = 4.08 × 10−4). And the study conducted by Liang et al. [1] indicated that WHR above 0.85 may act as a protective factor for secondary infertility. The risk for secondary infertility decreased for the group with WHR >0.85, with an unadjusted OR of 0.637 (95% CI: 0.428∼0.950), compared with the group with WHR ≤0.85. After adjusting all covariates, the OR and 95% CI was 0.650 (95% CI: 0.428–0.987). The results presented contradict the prevailing view that abdominal obesity may have a detrimental effect on fertility due to its association with metabolic disorders [20]. Another study demonstrated that abdominal obesity was not associated with fertility [21]. One possible explanation was that WHR is just one of several parameters used to assess abdominal obesity, and it should be noted that most participants in this study had a normal BMI [43].
Our studies have confirmed a relationship between WHR and infertility, although the mechanisms remain unclear. After reviewing the literature, it appears that there may be several underlying mechanisms that mediate the correlation. First, a higher WHR tends to reflect more abdominal fat and less hip fat. Previous studies have shown that adipose factors, primarily from adipose tissue, are associated with a range of metabolic-related diseases. Abdominal fat mainly produces harmful adipokines that lead to worse clinical outcomes, while hip fat has the opposite effect. In addition, excessive abdominal fat can directly or indirectly cause sympathetic hyperactivity and abnormal secretion of adipose factors including adiponectin and leptin, which in turn leads to the occurrence and development of insulin resistance and chronic inflammation, which are the several established independent risk factors of infertility. Therefore, a higher WHR increases the risk of infertility, which may be caused by abnormal fat distribution and dysfunction of adipokines. Second, studies have found that adipose tissue can have an impact on female health. In particular, women with excess abdominal adiposity may be at risk of health problems. This is because adipose tissue can disrupt the normal functioning of the hypothalamic-pituitary-gonadal axis, which is a part of the endocrine system that controls hormone levels and regulates reproduction. This disruption leads to reduced levels of gonadotropins, resulting in symptoms such as irregular menstrual cycles and anovulation, which ultimately contribute to infertility [1]. Third, Ricardo et al. [45] reported that the effect of testosterone on WHR appears to be opposite to that of estradiol. High WHRs are associated with elevated testosterone levels in pre- and perimenopausal women, as well as in medical conditions where this hormone is naturally increased, such as polycystic syndrome or morbid obesity. It is worth noting that estrogens promote fat accumulation in hips, buttocks, thighs, and bosom in women, while androgens promote fat accumulation in the abdomen, leading to weight gain. The study found that women with the smallest WHRs during their fertile phase had the highest concentrations of testosterone and estradiol in their saliva. This suggests that these women may have a higher turnover of estradiol, which in turn promotes fat deposition in the hips and buttocks. Additionally, the study found that women with the lowest estradiol levels and the highest testosterone levels during their fertile days had the highest WHRs. Furthermore, in women, reproductive disorders such as PCOS are frequently associated with obesity, and women with obesity often display more severe symptoms such as anovulation and hyperandrogenism than do normal-weight women with PCOS. Adipocytes from women with PCOS are characterized by cell size enlargement, insulin resistance, and the abnormal secretion of adipokines [46]. To sum up, higher WHR may directly or indirectly affect the prevalence of infertility by the above mechanisms.
Furthermore, stratified analyses suggest that younger women in the age group of <35 with a WHR of ≥0.85 may experience a greater risk of infertility compared to those with a WHR of <0.85. Further research is required to fully understand the underlying mechanisms. Based on previous literature [2, 23], similar results suggest that excessive visceral adipose tissue in younger women may have more adverse effects on reproductive endocrinology, and the specific mechanism needs to be further studied. Age has been proven in prior research to have an impact on the prevalence of infertility. Fecundity reportedly decreased for females in their late thirties and early forties. The likelihood of infertility rose from 10% to 20% after age 35–45% in the early forties among women with previously confirmed fertility. In women aged >35 years, the effects were weakened, which may be mainly caused by the decline of ovarian function or any other possible decline in the reproductive system. Women aged >35 years always face the challenge of declining ovarian reserve, oocyte number, and quality, all of which are the main causes of infertility, and the effects of WHR may be weakened.
However, the present study has some limitations that need to be taken into account. First, we cannot establish causality based on the results obtained due to the cross-sectional nature of our analysis [47]. Second, we do not know whether female infertility accounts for pregnancy failure, male infertility, or a combination of both because no information was available from NHANES. Finally, our secondary analysis was limited by the unavailability of new data, which may result in residual confounding due to unmeasured factors. For example, we were unable to control for potential confounders such as family history of infertility and frequency of intercourse, as we did not have access to these data. Future studies should address these factors. However, it is important to exercise caution should be exercised when interpreting these findings due to the limited sample size in our study. Therefore, it is imperative that additional well-designed prospective studies are conducted to further explore this area.
The NHANES provides us with a valuable opportunity to investigate the potential association between WHR and infertility. It also allows us to investigate the dose-response relationship between these variables while taking into account numerous covariates and conducting a variety of stratified analyses.
Conclusions
In conclusion, our study found that WHR is an independent risk factor for infertility. The group with WHR ≥0.85 had a significantly higher risk of secondary infertility than the group with WHR <0.85. However, no significant association was observed in women with primary infertility. The study found a significant interaction between age status on WHR and the risk of infertility. Specifically, younger women in the age group of <35 with a WHR of ≥0.85 may experience a greater risk of infertility compared to those with a WHR of <0.85. The study highlights the importance of managing abdominal fat in the progression of infertility, particularly for younger women. These results draw attention to the association between WHR and infertility.
Acknowledgments
We sincerely thank Dr. Jie Liu, PhD, Department of Vascular and Endovascular Surgery, PLA General Hospital, China, for his valuable feedback and suggestions on the manuscript.
Statement of Ethics
The US National Center for Health Statistics Research Ethics Review Board granted ethical approval for NHANES (Continuation of Protocol No. 2011-17, Protocol No. 2018-01) (available at: https://www.cdc.gov/nchs/nhanes/irba98.htm). NHANES is a publicly available dataset. The study’s analysis is a secondary analysis of NHANES data. Therefore, ethical approval is exempt under the US Health and Human Services (HHS) regulations at 45 CFR 46.104. The regulations are available at https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/common-rule-subpart-a-46104/index.html. Written informed consent was obtained prior to conducting household interviews and health examinations. Participants were assured that the data collected would only be used for the stated purposes and would not be disclosed or released to others without their consent. The consent form is available at https://www.cdc.gov/nchs/nhanes/genetics/genetic_participants.htm.
Conflict of Interest Statement
The remaining authors declare that they have no conflict of interest.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Jun Lai: conceptualization, formal analysis, writing – original draft. Zongyan Liu and Yufeng Wei: data collection. Xinqing Li, Yuanyue Liao, and Zuomiao Xiao: writing and review. Yongxiao Cao: editing, project administration, and conceptualization. All authors contributed to and approved the final manuscript.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
NHANES data used in this work is publicly available. All raw data are available on the NHANES website (https://www.cdc.gov/nchs/nhanes/). Further inquiries can be directed to the corresponding author.
Supplementary Material.
References
- 1. Liang S, Chen Y, Wang Q, Chen H, Cui C, Xu X, et al. Prevalence and associated factors of infertility among 20-49 year old women in Henan Province, China. Reprod Health. 2021;18(1):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang X, Zhu R, Han H, Jin J. Body fat distribution and female infertility: a cross-sectional analysis among US women. Reprod Sci. 2023;30(11):3243–52. [DOI] [PubMed] [Google Scholar]
- 3. Carson SA, Kallen AN. Diagnosis and management of infertility: a review. JAMA. 2021;326(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu L, Zhou B, Zhu X, Cheng F, Pan Y, ZY, et al. Association between body mass index and female infertility in the United States: data from National Health and Nutrition Examination Survey 2013-2018. Int J Gen Med. 2022;15:1821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MasCarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Key Statistics from the national survey of family growth. Available from: https://www.cdc.gov/nchs/nsfg/key_statistics/i.htm#infertility. Accessed August 27, 2023.
- 7. Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324–31.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Macaluso M, Wright-Schnapp TJ, Chandra A, Johnson R, Satterwhite CL, Pulver A, et al. A public health focus on infertility prevention, detection, and management. Fertil Steril. 2010;93(1):16.e1–10. [DOI] [PubMed] [Google Scholar]
- 9. Zhu F, Chen C, Zhang Y, Chen S, Huang X, Li J, et al. Elevated blood mercury level has a non-linear association with infertility in U.S. women: data from the NHANES 2013-2016. Reprod Toxicol. 2020;91:53–8. [DOI] [PubMed] [Google Scholar]
- 10. Lee S, Min JY, Min KB. Female infertility associated with blood lead and cadmium levels. Int J Environ Res Public Health. 2020;17(5):1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma R, Biedenharn KR, Fedor JM, Agarwal A. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol. 2013;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ge Q, Qi Z, Xu Z, Li M, Zheng H, Duan X, et al. Comparison of different obesity indices related with hypertension among different sex and age groups in China. Nutr Metab Cardiovasc Dis. 2021;31(3):793–801. [DOI] [PubMed] [Google Scholar]
- 13. Zhang F, Ren J, Zhang P, Jin H, Qu Y, Yu Y, et al. Strong association of Waist Circumference (WC), Body Mass Index (BMI), Waist-to-Height ratio (WHtR), and Waist-to-Hip ratio (WHR) with diabetes: a population-based cross-sectional study in Jilin province, China. J Diabetes Res. 2021;2021:8812431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhattacharya K, Sengupta P, Dutta S, Chaudhuri P, Das Mukhopadhyay L, Syamal AK. Waist-to-height ratio and BMI as predictive markers for insulin resistance in women with PCOS in Kolkata, India. Endocrine. 2021;72(1):86–95. [DOI] [PubMed] [Google Scholar]
- 15. Hassan S, Oladele C, Galusha D, Adams OP, Maharaj RG, Nazario CM, et al. Anthropometric measures of obesity and associated cardiovascular disease risk in the Eastern Caribbean Health Outcomes Research Network (ECHORN) cohort study. BMC Public Health. 2021;21(1):399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xue R, Li Q, Geng Y, Wang H, Wang F, Zhang S. Abdominal obesity and risk of CVD: a dose–response meta-analysis of thirty-one prospective studies. Br J Nutr. 2021;126(9):1420–30. [DOI] [PubMed] [Google Scholar]
- 17. Rico-Martín S, Calderón-García JF, Sánchez-Rey P, Franco Antonio C, Martínez Alvarez M, Sánchez Muñoz Torrero JF. Effectiveness of body roundness index in predicting metabolic syndrome: a systematic review and meta-analysis. Obes Rev. 2020;21(7):e13023. [DOI] [PubMed] [Google Scholar]
- 18. Yalcin G, Ozsoy E, Karabag T. The relationship of body composition indices with the significance, extension and severity of coronary artery disease. Nutr Metab Cardiovasc Dis. 2020;30(12):2279–85. [DOI] [PubMed] [Google Scholar]
- 19. Emdin CA, Khera AV, Natarajan P, Klarin D, Zekavat SM, Hsiao AJ, et al. Genetic association of waist-to-Hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA. 2017;317(6):626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pasquali R. Obesity, fat distribution and infertility. Maturitas. 2006;54(4):363–71. [DOI] [PubMed] [Google Scholar]
- 21. Loy SL, Cheung YB, Soh SE, Ng S, Tint MT, Aris IM, et al. Female adiposity and time-to-pregnancy: a multiethnic prospective cohort. Hum Reprod. 2018;33(11):2141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang J, Xu Y, Wang Z, Ji X, Qiu Q, Mai Z, et al. Association between metabolic healthy obesity and female infertility: the national health and nutrition examination survey, 2013-2020. BMC Public Health. 2023;23(1):1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang R, Feng Y, Chen J, Chen Y, Ma F. Association between polyunsaturated fatty acid intake and infertility among American women aged 20-44 years. Front Public Health. 2022;10:938343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin J, Lin X, Qiu J, You X, Xu J. Association between heavy metals exposure and infertility among American women aged 20–44 years: a cross-sectional analysis from 2013 to 2018 NHANES data. Front Public Health. 2023;11:1122183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yin YH, Zhou SY, Lu DF, Chen XP, Liu B, Lu S, et al. Higher waist circumference is associated with increased likelihood of female infertility: NHANES 2017-2020 results. Front Endocrinol. 2023;14:1216413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seidell JC, Oosterlee A, Thijssen MA, Burema J, Deurenberg P, Hautvast JG, et al. Assessment of intra-abdominal and subcutaneous abdominal fat: relation between anthropometry and computed tomography. Am J Clin Nutr. 1987;45(1):7–13. [DOI] [PubMed] [Google Scholar]
- 27. Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35(1):83–92. [DOI] [PubMed] [Google Scholar]
- 28. Andreacchi AT, Griffith LE, Guindon GE, Mayhew A, Bassim C, Pigeyre M, et al. Body mass index, waist circumference, waist-to-hip ratio, and body fat in relation to health care use in the Canadian longitudinal study on aging. Int J Obes. 2021;45(3):666–76. [DOI] [PubMed] [Google Scholar]
- 29. Wang Z, Huang X, Li J, Liu N, Wei Q. Association between waist-hip ratio and subclinical myocardial injury in the general population: insights from the NHANES. Front Endocrinol. 2022;13:975327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carmienke S, Freitag MH, Pischon T, Schlattmann P, Fankhaenel T, Goebel H, et al. General and abdominal obesity parameters and their combination in relation to mortality: a systematic review and meta-regression analysis. Eur J Clin Nutr. 2013;67(6):573–85. [DOI] [PubMed] [Google Scholar]
- 31. Streng KW, Voors AA, Hillege HL, Anker SD, Cleland JG, Dickstein K, et al. Waist-to-hip ratio and mortality in heart failure. Eur J Heart Fail. 2018;20(9):1269–77. [DOI] [PubMed] [Google Scholar]
- 32. Anusruti A, Jansen EHJM, Gào X, Xuan Y, Brenner H, Schöttker B. Longitudinal associations of body mass index, waist circumference, and waist-to-hip ratio with biomarkers of oxidative stress in older adults: results of a large cohort study. Obes Facts. 2020;13(1):66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arya S, Dwivedi AK, Alvarado L, Kupesic-Plavsic S. Exposure of U.S. population to endocrine disruptive chemicals (Parabens, Benzophenone-3, Bisphenol-A and Triclosan) and their associations with female infertility. Environ Pollut. 2020;265(Pt A):114763. [DOI] [PubMed] [Google Scholar]
- 34. Anyalechi GE, Hong J, Kreisel K, Torrone E, Boulet S, Gorwitz R, et al. Self-reported infertility and associated pelvic inflammatory disease among women of reproductive age-national health and nutrition examination survey, United States, 2013-2016. Sex Transm Dis. 2019;46(7):446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu H, Wang L, Chen C, Dong Z, Yu S. Association between dietary niacin intake and migraine among American adults: national health and nutrition examination survey. Nutrients. 2022;14(15):3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kang X, Luo J, Zhang D, Li J. Associations between organophosphate esters metabolites and sleep disorder and trouble sleeping in adults: a machine-learning approach. Environ Sci Pollut Res Int. 2022;29(44):67287–300. [DOI] [PubMed] [Google Scholar]
- 37. World Health Organization . Waist circumference and waist-hip ratio: report of a WHO expert consultation. Geneva: WHO; 2008. [Google Scholar]
- 38. He S, Wan L. Associations between smoking status and infertility: a cross-sectional analysis among USA women aged 18-45 years. Front Endocrinol. 2023;14:1140739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liang Z, Liu J. Sleep behavior and self-reported infertility: a cross-sectional analysis among U.S. Women. Front Endocrinol. 2022;13:818567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ruan Z, Lu T, Chen Y, Yuan M, Yu H, Liu R, et al. Association between psoriasis and nonalcoholic fatty liver disease among outpatient US adults. JAMA Dermatol. 2022;158(7):745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lewis RC, Meeker JD. Biomarkers of exposure to molybdenum and other metals in relation to testosterone among men from the United States national health and nutrition examination survey 2011-2012. Fertil Steril. 2015;103(1):172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Monica K, Silver BL, Meeker JD. Blood cadmium is elevated in iron deficient U.S. Children: a cross-sectional study. Environ Health. 2013;12(117):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zaadstra BM, Seidell JC, Van Noord PA, te Velde ER, Habbema JD, Vrieswijk B, et al. Fat and female fecundity: prospective study of effect of body fat distribution on conception rates. BMJ. 1993;306(6876):484–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Venkatesh SS, Ferreira T, Benonisdottir S, Rahmioglu N, Becker CM, Granne I, et al. Obesity and risk of female reproductive conditions: a Mendelian randomisation study. PLoS Med. 2022;19(2):e1003679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mondragón-Ceballos R, Granados MDG, Cerda-Molina AL, Chavira-Ramírez R, Estela Hernández-López L. Waist-to-Hip ratio, but not body mass index, is associated with testosterone and estradiol concentrations in young women. Int J Endocrinol. 2015;2015:654046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li J, Papadopoulos V, Vihma V. Steroid biosynthesis in adipose tissue. Steroids. 2015;103:89–104. [DOI] [PubMed] [Google Scholar]
- 47. Xiao Y, Xiao Z. Association between serum klotho and kidney stones in US middle-aged and older individuals with diabetes mellitus: results from 2007 to 2016 national health and nutrition survey. Am J Nephrol. 2023;54(5–6):224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
NHANES data used in this work is publicly available. All raw data are available on the NHANES website (https://www.cdc.gov/nchs/nhanes/). Further inquiries can be directed to the corresponding author.