Abstract
Toxoplasma gondii is a zoonotic parasite with global distribution capable of infecting homeothermic animals. Transmission of protozoan to humans includes ingestion of water and raw food contaminated with sporulated oocysts, ingestion of raw or undercooked meat with tissue cysts, and tachyzoites' transplacental transmission. Fresh goat milk intake has already been linked to human toxoplasmosis outbreaks, but little is known about the infectious potential of this biological sample. Accordingly, the aim of the present study is to assess the survival and infectivity of T. gondii tachyzoites in fresh goat milk samples through an experimental protocol to detect this parasite via bioassay carried out with a murine model, DNA amplification, and serology. Swiss Webster mice were inoculated with fresh goat milk samples contaminated with different T. gondii RH strain tachyzoite concentrations per milliliter and stored for different refrigeration times. Animals showing clinical signs compatible to toxoplasmosis were euthanized. Milk samples contaminated with high parasitic loads and kept for a shorter refrigeration time were the most lethal ones. No significant differences were observed between mean death rates recorded for different goat milk contamination concentrations (p = 0.1888), and for the refrigeration time, contaminated milk samples were kept under (p = 0.9440). T. gondii DNA was amplified in all contaminated milk samples, but only one of the surviving mice was serologically positive. Results of the present study have shown T. gondii survival and infectivity in fresh goat milk samples, and it highlights its significant risk for public health. Therefore, molecular methods must be the tests of choice when milk samples are used to assess infection caused by protozoan in goats' dairy products.
Keywords: fresh goat milk, goats, infectivity, survival, toxoplasmosis
1. Introduction
Toxoplasmosis is a zoonosis of great importance for public health. Protozoan Toxoplasma gondii, which is its etiologic agent, has felids as its definitive hosts; birds and mammals, including humans, are its intermediate hosts [1, 2]. Protozoan transmission occurs through three main pathways: sporulated oocysts ingestion through both water and raw food contaminated by cat feces in primary infection, ingestion of tissue cysts found in raw or undercooked meat of chronically infected animals, or, yet, through transplacental transmission [3, 4]. Some other less frequent mechanisms can account for T. gondii transmission to humans, with an emphasis on fresh goat milk consumption [5].
Goats are among the domestic species mostly sensitive to toxoplasmic infection [6], since toxoplasmosis is responsible for determining important reproductive changes, such as miscarriage, neonatal death, and mummified fetuses, in them [7]. Serological surveys carried out in Brazil, in the last 3 years, have detected different antibody frequencies in goat herds; it ranged from 21% to 46% [8–13]. In addition to damage caused to animal health, T. gondii infection in goatherds can have a significant economic impact for producers [14].
According to the literature, toxoplasmosis outbreaks in humans have already been linked to fresh goat milk consumption [15–17]. However, direct protozoan detection in this biological sample has been limited to parasitic DNA detection in the analyzed samples; however, their infectivity was not assessed [18–22]. The microscopic detection of free parasites in milk samples is not carried out because the identification of tachyzoites seems to be very difficult due to the large amount of fat droplets [6].
Therefore, given the scarcity of data on T. gondii viability goat milk and on the transmission potential of this biological sample, the aim of the current study was to assess the survival and infectivity of T. gondii tachyzoites in fresh goat milk samples, based on an experimental protocol to detect this parasite through bioassay carried out with a murine model, DNA amplification, and serology.
2. Material and Methods
2.1. Ethical Considerations
The present study was approved by the Ethics Committee on Animal Use (CEUA) of Oswaldo Cruz Institute, under license number L-041/2019.
2.2. Goat Milk Sample Obtainment and T. gondii Contamination
Approximately 1 L of fresh goat milk was collected from a Saanen goat bred and maintained at the Farm School of the Veterinary Medicine School of Fluminense Federal University to carry out the present experimental protocol. This site is located in Cachoeiras de Macacu City, Rio de Janeiro State, Brazil (22°31′13.5″S 42°42′28.3″W). The milk sample was obtained through manual milking, after goat's teat cleaning (predipping) and the discharge of the first two milk jets. Then, the sample was transported to the Toxoplasmosis and Other Protozoan Diseases Laboratory (LabTOXO) of Oswaldo Cruz Institute/Fiocruz, in sterile glass vials, in isothermal box, at a temperature close to 4°C. The milk sample was aliquoted in sterile 50-mL conical tubes and stored at −70°C, until processing, at LabTOXO. One of the aliquots was previously subjected to a conventional polymerase chain reaction (PCR) to amplify the repetitive element (REP) of 529 bp of T. gondii DNA [23] to confirm the absence of this protozoan's DNA in the collected sample.
Tachyzoites of T. gondii RH strain kept in female Swiss Webster mice and obtained through peritoneal lavage were used to contaminate 30-mL aliquots of goat milk. These aliquots were contaminated at the following concentrations: 5 × 105, 5 × 104, 5 × 103, 5 × 102, and 5 × 101 parasites/milliliter. Initial concentration (5 × 105) was chosen because it is standardized in the laboratory, in the protocol to maintain the strain in the murine model. The contaminated samples, as well as an aliquot of noncontaminated milk, were stored and kept for 24, 48, 72, and 96 h under refrigeration (2°C–8°C). Milk samples were concentrated and resuspended in 2 mL of 1% PBS, supplemented with 2% antibiotic (1000 UI penicillin + 100 μg streptomycin/milliliter) for 4-day postcontamination, on a daily basis. In total, 1.5 mL of this volume was destined to assess tachyzoites' survival time and infectivity in contaminated milk; the remaining 0.5 mL was used to confirm the presence of parasite DNA in the sample.
2.3. Assessing T. gondii Tachyzoites' Survival and Infectivity in Goat Milk Samples
Concentrated 1.5-mL aliquots were intraperitoneally inoculated in groups of three female Swiss Webster mice, in the age group 25–30 days, divided based on each contamination concentration and on time interval under refrigeration, to assess T. gondii tachyzoites' survival time and infectivity in contaminated milk samples. Each animal was inoculated with 0.5 mL of the concentrated milk sample. Inoculated mice were followed up on a daily basis for 60 days, at most. Animals presenting clinical signs compatible to acute toxoplasmosis, such as prominent piloerection, dyspnea, chest breathing, and ascites, were euthanized. The sick animals were restrained manually using physical restraint. Initially, the animal was pressed lightly onto the surface of the cage grid and held by the skin of the dorsocervical region, between the index and thumb fingers, clamping its tail between the other fingers and the palm of the hand, to completely limit its movements. In this way, the right hind limb was extended to facilitate visualization and access to the biceps femoris muscle. After the immobilization and asepsis of this anatomical region, 300 μL of anesthetic overdose associating ketamine hydrochloride (300 mg/kg) and xylazine hydrochloride (30 mg/kg) was administered intramuscularly, using a 1 mL syringe and a 13 × 0.3 mm needle, to implement a humane endpoint [24]. Free tachyzoites were surveyed on slides with 10 μL of peritoneal lavage to confirm parasites' survival and infectivity.
2.4. Detecting Parasite DNA in Goat Milk Samples Contaminated With T. gondii Tachyzoites
Aliquots of 0.5 mL of milk were subjected to molecular analysis to confirm the presence of tachyzoites in samples contaminated with different concentrations and kept under different refrigeration time intervals. Initially, the concentrated samples were washed three times in 2 mL of UltraPure DNase/RNase-Free Distilled Water, Invitrogen®, to remove extraction inhibitors and to purify the DNA [25]. Then, 200 μL of concentrated milk was used for DNA extraction in commercial QIAamp DNA Blood Mini Kit, Qiagen® (250), according to the manufacturer's specifications. The extracted DNA was used to amplify the 529 bp REP of the T. gondii genome through PCR, by using primers TOX4 (CGCTGCAGGGAGGAAGACGAAAGTTG) and TOX5 (CGCTGCAGACACAGTGCATCTGGATT) [23].
Each performed reaction that used a final volume of 25 μL of PCR mix was added with 14 μL ultrapure water, 4 μL dNTP (1.25 mM), 2.5 μL buffer (10X), 0.75 μL MgCl2 (50 mM), 0.5 μL TOX4 (10 pmol/μL), 0.5 μL TOX5 (10 pmol/μL), 0.25 μL Platinum Taq DNA polymerase (Invitrogen®), and 2.5 μL extracted DNA. PCR mixes were subjected to initial denaturation at 94°C, for 7 min, and to 35 cycles at 94°C, for 1 min (denaturation), at 55°C for 1 min (annealing), at 72°C for 1 min (extension), and to final extension at 72°C for 10 min. The produced amplicons were detected through electrophoresis, in 1.5% agarose gel, stained with GelRed Nucleic Acid Gel Stain (Biotium®), and visualized under ultraviolet light in a transilluminator.
2.5. Detecting IgG Anti-T. gondii Antibodies in Surviving Mice
Surviving mice underwent cardiac puncture, followed by euthanasia, to collect approximately 1 mL of blood for IgG anti-T.gondii antibodies, 60 days after inoculation. Blood samples were centrifuged at 1000 × g for 10 min to collect the serum. Sera were subjected to an indirect fluorescent antibody test (IFAT) [26]. T. gondii tachyzoite RH strain inactivated in 2% formalin was used as antigen. Commercial anti-Mouse IgG (whole molecule)-FITC conjugate produced in goats (Sigma-Aldrich®) diluted in Evans Blue solution was used to detect anti-T. gondii IgG. Samples were considered positive when tachyzoites' total surface fluorescence was observed at a dilution ratio equal to, or higher than, 1 : 16.
2.6. Statistical Analysis
Daily inoculated mice follow-ups were recorded in Microsoft Excel® spreadsheets, from inoculation day onwards. The quantitative variable included in these spreadsheets was the number of deaths observed or euthanasias implemented per day postinoculation in the animals from the different groups inoculated with contaminated milk samples. The qualitative result regarding the presence or absence of tachyzoites in the peritoneal lavage performed was also recorded on these spreadsheets. Simple ANOVA was performed in GraphPad Prism®, version 5 to assess the difference between mean death values in relation to contamination concentration with parasites and refrigeration time. Values ≤ 0.05 were considered significant.
3. Results
In total, 45.8% (33/72) of the total number of inoculated animals died or were euthanized, because they manifested clinical signs of acute toxoplasmosis, including symptoms such as noticeable piloerection, dyspnea, antalgic position, and ascites. The highest rate of deaths/euthanasia, 90.9% (30/33), was recorded between the 6th and 9th day after inoculation (Table 1). Milk samples contaminated with 5 × 105 and 5 × 103 tachyzoites/milliliter were the most lethal ones; each of them accounted for the death of 11 animals. Contaminated milk samples kept under refrigeration for 24 h were responsible for the death of 10 animals. No significant differences were observed between mean death rates among different goat milk contamination concentrations (p = 0.1888), and for refrigeration times, contaminated milk samples were kept under (p = 0.9440). Figure 1 shows the distribution of the number of deaths caused by different contamination concentrations, and for refrigeration time, goat milk samples were kept under. Free tachyzoites were observed in the peritoneal lavage samples taken from animals found dead or euthanized.
Table 1.
Distribution of Swiss Webster mice inoculated with goat milk samples contaminated with different T. gondii tachyzoite concentrations, kept under different refrigeration times, by days of death.
| Refrigeration time | Concentration of milk contamination (parasites/milliliter) | Days (# of deaths/# of total group) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | ≥ 20 | ||
| 24 h | 5 × 105 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 3/3a | ||||||||||||||
| 5 × 104 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3a | 2/2a | ||||||||||||||
| 5 × 103 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 3/3a | ||||||||||||||
| 5 × 102 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | |
| 5 × 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3a | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | |
| Uncontaminated milk | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | |
|
| |||||||||||||||||||||
| 48 h | 5 × 105 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3 | 1/2 | 0/1 | 1/1 | |||||||||||
| 5 × 104 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | |
| 5 × 103 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3a | 2/2a | |||||||||||||
| 5 × 102 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | |
| 5 × 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | |
| Uncontaminated milk | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | |
|
| |||||||||||||||||||||
| 72 h | 5 × 105 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3a | 1/2 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 |
| 5 × 104 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | |
| 5 × 103 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3 | 0/2 | 0/2 | 1/2 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | |
| 5 × 102 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | |
| 5 × 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | |
| Uncontaminated milk | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | |
|
| |||||||||||||||||||||
| 96 h | 5 × 105 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3a | 2/2 | ||||||||||||
| 5 × 104 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 3/3a | ||||||||||||||
| 5 × 103 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 2/3 | 0/1 | 0/1 | 0/1 | 1/1 | ||||||||
| 5 × 102 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | |
| 5 × 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | |
| Uncontaminated milk | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | |
aEuthanasia implementation.
Figure 1.
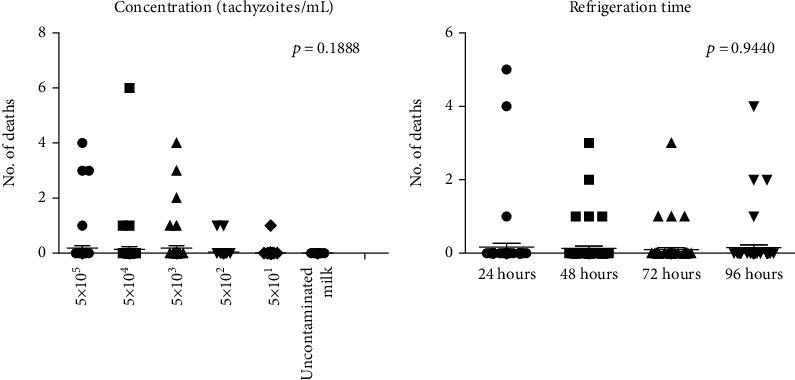
Distribution of number of deaths of Swiss Webster mice based on inoculated milk contamination concentration and refrigeration time.
The molecular analysis based on the amplification of the 529 bp fragment confirmed the presence of T. gondii DNA in all goat milk samples, regardless of the inoculated concentration and refrigeration time.
Only one of the 39 surviving mice was considered seropositive for IgG anti-T. gondii by IFAT. This surviving animal was inoculated with goat milk sample contaminated with 5 × 105 parasites/milliliter and kept under refrigeration for 72 h; it showed fluorescence of tachyzoite surface at a dilution ratio of 1 : 16.
All mice inoculated with uncontaminated milk survived for 60 days after inoculation; they showed no clinical changes at follow-up time and remained seronegative for anti-T. gondii antibodies. In addition, all uncontaminated milk samples were negative in PCR, regardless of refrigeration time.
4. Discussion
Results in the present study have shown that T. gondii tachyzoites can remain infective and viable in fresh goat milk samples under refrigeration (2°C–8°C) for 96 h (4 days), after sample collection. Walsh et al. [27] observed higher values when they assessed T. gondii tachyzoite infectivity, for 168 h (7 days), in contaminated goat milk samples kept at 4°C, in a model in vitro. Walsh et al. [27] used T. gondii RH strain to assess protozoan infectivity in experimentally contaminated goat milk samples. T. gondii RH strains belong to clonal lineage type 1; they presented high virulence, low lethal dose in a murine model, and low tachyzoite–bradyzoite interconversion [28]. Assumingly, the high intrinsic infectivity of this strain has contributed to this protozoan survival, as well as to its infectivity in milk samples kept under refrigeration for longer periods of time. This process may not be the case of clonal type 2 and 3 strains, as well as of nonclonal strains. Further studies focused on assessing the survival and infectivity of T. gondii tachyzoites of other genotypes in goat milk samples are necessary.
It is worth noticing that fresh goat milk's short perishability can be an important challenge for attempts to isolate T. gondii in samples from naturally infected animals whose samples' parasite load is unknown. Therefore, samples should be inoculated in murine models as soon as possible (few hours after collection) and kept under refrigeration (from 2°C to 8°C) to increase the possibility of T. gondii isolation from goat milk, as well as to assess its infectivity and virulence. This protocol would be the most favorable one to assess optimal tachyzoite viability [29]. This finding can be corroborated by the infectivity observed in the herein assessed goat milk samples contaminated with low tachyzoite concentrations (5 × 101 parasites/milliliter) and under refrigeration for 24 h.
T. gondii tachyzoites' infectivity in fresh goat milk samples may also be related to contamination concentration and to the protozoan strain used in the protocol. Milk samples contaminated with up to 5 × 103 tachyzoites/milliliter of T. gondii RH strain were the most lethal ones for the inoculated mice (with euthanasia implemented in 14 instances) in the current study. Dubey et al. [30] observed a correlation between goat milk samples' contamination concentration and parasite infectivity. Clinical signs of toxoplasmosis were identified in mice inoculated with goat milk samples contaminated with up to 1.6 × 10−3 tachyzoites of T. gondii GT-1 strain, clonal type 1 [30]. In addition to genotype, the parasite load found in the sample can contribute to infectivity, as well as to the manifestation of the acute disease in inoculated mice. However, little is known about the approximate amount of parasites shed in milk samples from naturally infected goats. Molecular analyses have evidenced that goats naturally or experimentally exposed to this protozoan can eliminate parasite DNA in milk; however, these analyses did not assess the infective potential of this biological sample [18, 30]. In short, studies strongly supporting the isolation of viable forms of T. gondii from goat milk remain scarce in the literature [6].
Bioassay in a murine model (outbred Swiss Webster mice) was the method of choice to assess T. gondii infectivity in goat milk. The use of outbred mice in infectivity assays carried out with T. gondii strains allows determining the degree of virulence of parasite isolates [31, 32]. Other methods, such as inoculation of milk samples in cell culture, also make it possible assessing the infective potential of goat milk in transmitting this protozoan [27]. However, T. gondii isolation in cell culture can present some disadvantages, such as the possibility of cells' contamination and greater sensitivity of cells in vitro to the components of tested biological samples, in comparison to models tested in vivo [29]. In addition, the isolation of T. gondii in cell culture does not allow us to determine the degree of virulence of the isolates, measured by mortality and morbidity values, when positive [33, 34]. The determination of virulence associated with the identification of the genotype of naturally circulating strains of T. gondii is necessary to establish the phenotypic and genotypic profiles of the isolates, as well as their potential to determine symptomatic cases of toxoplasmosis. However, alternative methods to assess T. gondii strains' infectivity and virulence are extremely necessary, since they aim at refining, reducing, or even replacing the use of murine models, from the 3R perspective.
In this study, the route of infection used in the experimental protocol was intraperitoneal. Dubey et al. [30] evaluated the survival of T. gondii tachyzoites in contaminated goat's milk by means of subcutaneous inoculations in mice. On the other hand, the infectivity of T. gondii present in artificially contaminated milk samples can also be achieved through oral administration in a murine model [35]. The choice of the intraperitoneal route in this protocol, rather than the oral route, was due to the greater sensitivity of tachyzoites to gastric juice [36]. Furthermore, this study is aimed at assessing the survival and infectivity of tachyzoites in fresh goat's milk and not the potential for parasite transmission from this biological sample, which would require a simulation of the natural transmission route. Considering that tachyzoites are the parasitic forms present in the extracellular environment and in body fluids, the choice of the intraperitoneal route of infection aimed to preserve the survival of as many parasites as possible in goat milk samples. However, Dubey [37] observed a certain degree of survival and infectivity of tachyzoites of T. gondii RH strain exposed to acid pepsin solutions in a murine model orally infected. In this sense, the protocol presented here should be tested in the future to assess the infectivity of tachyzoites present in fresh goat's milk by means of oral inoculations.
Most mice in the present study were seronegative for IgG anti-T. gondii. Dubey et al. [30] did not observe seroconversion in mice inoculated with goat milk contaminated with low T. gondii GT1 strain tachyzoite (10−4 to 10−6) concentrations. However, IgG anti-T. gondii antibodies have been detected 14 days after inoculation, onwards, in serum samples from outbred OF1 mice who were orally infected with tissue cysts of T. gondii 76 K strain [38]. Several factors can influence virulence assessment and, consequently, the kinetics of antibody production and secretion in infection caused by T. gondii in murine models, such as infecting dose, parasite strain, and evolutionary form used for infection [31]. The use of T. gondii RH strain in the current experiment can explain the absence, or low production, of antibodies against this parasite among inoculated mice. The RH strain became unable to naturally produce tissue cysts in the murine model after successive intraperitoneal passages in mice, in a laboratory environment [39]. Tissue cyst formation appears to be a key event to produce serum IgG antibodies during T. gondii infection [40]. Parasite persistence in tissues can act as a continuous stimulus for humoral immune response. Therefore, the acute nature of infection caused by T. gondii RH strain, which presents a lethal profile in mice within 3–7 days after inoculation [31], may be too accelerated to trigger antibody production.
However, a single serum sample from a mouse inoculated with contaminated milk was positive in IFAT. Despite the high inoculated parasite load (5 × 105), this animal survived the experimental protocol, as well as produced antibodies against this parasite, although at low titers (1 : 16). Intrinsic factors linked to this animal were assumingly linked to its survival; this finding points out the need of further studies to analyze the association among parasite load, seroconversion, and immunogenic susceptibility of the host. However, the inoculated parasite load seemed to be directly related to the seroconversion of the analyzed mice. Seronegativity and survival of mice herein inoculated with milk contaminated with lower amounts of tachyzoites could be explained by the control of inoculated parasite forms based on animals' immune response. Thus, lack of antigenic stimulus may have compromised antibodies' production. Therefore, the use of serological methods to indirectly detect T. gondii in serum samples from mice inoculated with fresh goat milk should be assessed with caution because parasite strains presenting virulent behavior or low cystogenic capacity and unknown concentrations of eventual parasites in the milk may not induce a humoral response in murine models.
REP 529 bp amplification through conventional PCR showed the best T. gondii detection results in the present study among all methods applied to parasite detection in contaminated goat milk samples. Molecular detection of T. gondii in milk samples of goats naturally exposed to the parasite has been widely used in Brazil and in other countries, such as Italy, Tunisia, and Poland based on the adoption of different gene targets of this parasite, as well as on conventional PCR on its variations [41–44]. Dubey et al. [30] observed the best performance of the nested PCR (nPCR) model in comparison to the conventional PCR model in the molecular detection of T. gondii in intentionally contaminated goat milk samples and to experimentally infected animals. In the current study, conventional PCR was able to amplify REP 529 bp even in samples contaminated with the lowest concentration of parasites in the milk. However, it is worth noticing that little is known about the quantification of parasite forms or of DNA excreted in milk by goats exposed to T. gondii. Therefore, despite the successful detection of DNA from this protozoan parasite in all the analyzed contaminated milk samples, future studies employing more sensitive molecular methods, such as the nPCR model, can be more effective in detecting goats naturally exposed to this protozoan parasite.
Finally, results in the present study evidenced the survival and infectivity of T. gondii RH strain tachyzoites in fresh goat milk samples at 96-h refrigeration, mainly at high concentrations of this parasite. This phenomenon, although observed in an experimental model, highlights its significant risk for public health, mainly for populations used to consume fresh goat milk. Goat milk should be inoculated in a murine model, 24 h after sample collection, and kept under refrigeration until processing time in a laboratory environment to increase the possibility of T. gondii isolation from this biological sample. However, molecular detection of T. gondii in goat milk seems to be the most effective direct method to detect the parasite in these samples, because, besides not requiring immediate processing, it can more accurately identify goats naturally exposed to this parasite. The indirect detection of T. gondii through the serology of mice inoculated with milk does not seem to be an effective method to identify the presence of this parasite, or its components, in goat milk, mainly when one is dealing with strains of unknown biological behavior that circulate naturally among goat herds.
Acknowledgments
The authors are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro for the financial support.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work is supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior ((CAPES) 88887.483483/2020-00) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro ((FAPERJ) grant E-26/201.682/2021).
References
- 1.Shapiro K., Bahia-Oliveira L., Dixon B., et al. Environmental transmission of Toxoplasma gondii: oocysts in water, soil, and food. Food and Waterborne Parasitology . 2019;15, article e00049 doi: 10.1016/j.fawpar.2019.e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barros R. A. M., Torrecilhas A. C., Marciano M. A. M., Mazuz M. L., Pereira-Chioccola V. L., Fux B. Toxoplasmosis in human and animals around the world. diagnosis and perspectives in the one health approach. Acta Tropica . 2022;231, article 106432 doi: 10.1016/j.actatropica.2022.106432. [DOI] [PubMed] [Google Scholar]
- 3.Hill D. E., Dubey J. P. Toxoplasma gondiias a parasite in food: analysis and control. Microbiology Spectrum . 2016;4(4) doi: 10.1128/microbiolspec.PFS-0011-2015. [DOI] [PubMed] [Google Scholar]
- 4.Balbino L. S., Bernardes J. C., Ladeia W. A., et al. Epidemiological study of toxoplasmosis outbreaks in Brazil. Transboundary and Emerging Diseases . 2022;69(4):2021–2028. doi: 10.1111/tbed.14214. [DOI] [PubMed] [Google Scholar]
- 5.Pinto-Ferreira F., Caldart E. T., Pasquali A. K. S., Mitsuka-Breganó R., Freire R. L., Navarro I. T. Patterns of transmission and sources of infection in outbreaks of human toxoplasmosis. Emerging Infectious Diseases . 2019;25(12):2177–2182. doi: 10.3201/eid2512.181565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubey J. P., Murata F. H. A., Cerqueira-Cézar C. K., Kwok O. C. H. Public health and economic importance of Toxoplasma gondii infections in goats: the last decade. Research in Veterinary Science . 2020;132:292–307. doi: 10.1016/j.rvsc.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Dubey J. P. Toxoplasmosis of animals and humans . 2nd. Boca Raton, Florida: CCR Press; 2010. [Google Scholar]
- 8.Pires C. R. D. S., Dias H. L. T., Costa Júnior L. M., Teixeira M. A. D. S., Prado R. S. Seroprevalence and factors associated of Toxoplasma gondii infection in goats from the Pará and Maranhão states, Brazil. Ciência Animal . 2020;30(3):48–55. [Google Scholar]
- 9.Rizzo H., Jesus T. K. S., Alcântara A. M., et al. Occurrence of anti-Toxoplasma gondii antibody and evaluation of risk infection factors in goats raised in Sergipe state, Brazil. Pesquisa Veterinária Brasileira . 2020;40(5):374–380. doi: 10.1590/1678-5150-pvb-6319. [DOI] [Google Scholar]
- 10.Romanelli P. R., Matos A. M. R. N., Pinto-Ferreira F., et al. Toxoplasma gondii and Neospora caninum infections and factors associated in goats in the Parana state, Southern Brazil. Brazilian Journal of Veterinary Parasitology . 2020;29(4, article e003620) doi: 10.1590/S1984-29612020076. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues A. A., Reis S. S., da Silva Moraes E., et al. Seroprevalence and risk factors for Neospora caninum and Toxoplasma gondii in goats of Maranhão State, Brazil. Veterinary Parasitology: Regional Studies and Reports . 2021;26, article 100634 doi: 10.1016/j.vprsr.2021.100634. [DOI] [PubMed] [Google Scholar]
- 12.Batista S. P., Silva S. D. S., Sarmento W. F., et al. Prevalence and isolation of Toxoplasma gondii in goats slaughtered for human consumption in the semi-arid of northeastern Brazil. Parasitology International . 2022;86, article 102457 doi: 10.1016/j.parint.2021.102457. [DOI] [PubMed] [Google Scholar]
- 13.Pereira Pedrini L., Santos Almeida Damiani L., Vaillant Beltrame M. A., et al. Seroprevalence and risk factors of Toxoplasma gondii in goats (Capra hircus) in southeastern Brazil. 2023. https://ssrn.com/abstract=4565157 . [DOI] [PubMed]
- 14.Millar P. R., Sobreiro L. G., Bonna I. C. F., Amendoeira M. R. R. A importância dos animais de produção na infecção por Toxoplasma gondii no Brasil. Semina: Ciências Agrárias . 2008;29(3):693–706. doi: 10.5433/1679-0359.2008v29n3p693. [DOI] [Google Scholar]
- 15.Riemann H. P., Meyer M. E., Theis J. H., Kelso T. G., Behymer D. E. Toxoplasmosis in an infant fed unpasteurized goat milk. The Journal of Pediatrics . 1975;87(4):573–576. doi: 10.1016/S0022-3476(75)80825-0. [DOI] [PubMed] [Google Scholar]
- 16.Sacks J. J., Roberto R. R., Brooks N. F. Toxoplasmosis infection associated with raw goat’s milk. JAMA . 1982;248(14):1728–1732. doi: 10.1001/jama.1982.03330140038029. [DOI] [PubMed] [Google Scholar]
- 17.Skinner L. J., Timperley A. C., Wightman D., Chatterton J. M. W., Ho-Yen D. O. Simultaneous diagnosis of toxoplasmosis in goats and goatowner's family. Scandinavian Journal of Infectious Diseases . 1990;22(3):359–361. doi: 10.3109/00365549009027060. [DOI] [PubMed] [Google Scholar]
- 18.Gazzonis A. L., Zanzani S. A., Villa L., Manfredi M. T. Toxoplasma gondii in naturally infected goats: Monitoring of specific IgG levels in serum and milk during lactation and parasitic DNA detection in milk. Preventive Veterinary Medicine . 2019;170, article 104738 doi: 10.1016/j.prevetmed.2019.104738. [DOI] [PubMed] [Google Scholar]
- 19.Khamsian E. M., Hajimohammadi B., Eslami G., Fallahzadeh M. H., Hosseini S. S. Toxoplasma gondii in milk of human and goat from the desert area in Central Iran. Iranian Journal of Parasitology . 2021;16(4):601–609. doi: 10.18502/ijpa.v16i4.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deljavan N., Moosavy M. H., Hajipour N. Molecular detection of Toxoplasma gondii DNA in goats (Capra hircus), sheep (Ovis aries), and donkey (Equus asinus) milk using PCR in East Azerbaijan Province, Iran. Research in Veterinary Science . 2022;152:58–60. doi: 10.1016/j.rvsc.2022.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Fereig R. M., Abdelbaky H. H., Mazeed A. M., et al. Prevalence of Neospora caninum and Toxoplasma gondii antibodies and DNA in raw milk of various ruminants in Egypt. Pathogens . 2022;11(11):p. 1305. doi: 10.3390/pathogens11111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Çulbasan V., Gungor C., Gundog D. A., Koskeroglu K., Onmaz N. E. Molecular surveillance of Toxoplasma gondii in raw milk and artisan cheese of sheep, goat, cow and water buffalo origin. International Journal of Dairy Technology . 2023;76(4):948–954. doi: 10.1111/1471-0307.12988. [DOI] [Google Scholar]
- 23.Homan W. L., Vercammen M., Braekeleer J. D. E., Verschueren H. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. International Journal for Parasitolology . 2000;30(1):69–75. doi: 10.1016/S0020-7519(99)00170-8. [DOI] [PubMed] [Google Scholar]
- 24.Arruda I. F., Amendoeira M. R. R., Bonifácio T. F., et al. Humane endpoints in Swiss Webster mice infected with Toxoplasma gondii RH strain. Animals . 2024;14(9):p. 1326. doi: 10.3390/ani14091326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministério da Saúde (MS) Protocolos para investigação de Toxoplasma gondii em amostras ambientais e alimentares . 1st. Brasília: MS; 2020. http://bvsms.saude.gov.br/bvs/publicacoes/protocolos_toxoplasma_amostras_ambientais_alimentares.pdf . [Google Scholar]
- 26.Camargo M. Improved technique of indirect immunofluorescence for serological diagnosis of toxoplasmosis. Revista do Instituto de Medicina Tropical de São Paulo . 1964;6(3):117–118. [PubMed] [Google Scholar]
- 27.Walsh C. P., Hammond S. E., Zajac A. M., Lindsay D. S. Survival of Toxoplasma gondii tachyzoites in goat milk: potential source of human toxoplasmosis. The Journal of Eukaryotic Microbiology . 1999;46(5):73S–74S. [PubMed] [Google Scholar]
- 28.Dardé M. L. Genetic analysis of the diversity in Toxoplasma gondii. Annali Dell'istituto Superiore Di Sanità . 2004;40(1):57–63. [PubMed] [Google Scholar]
- 29.James G. S., Sintchenko V. G., Dickeson D. J., Gilbert G. L. Comparison of cell culture, mouse inoculation, and PCR for detection of Toxoplasma gondii: effects of storage conditions on sensitivity. Journal of Clinical Microbiology . 1996;34(6):1572–1575. doi: 10.1128/jcm.34.6.1572-1575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubey J. P., Verma S. K., Ferreira L. R., et al. Detection and survival of Toxoplasma gondii in milk and cheese from experimentally infected goats. Journal of Food Protection . 2014;77(10):1747–1753. doi: 10.4315/0362-028X.JFP-14-167. [DOI] [PubMed] [Google Scholar]
- 31.Saraf P., Shwab E. K., Dubey J. P., Su C. On the determination of Toxoplasma gondii virulence in mice. Experimental Parasitology . 2017;174:25–30. doi: 10.1016/j.exppara.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q., Sibley L. D. Assays for monitoring toxoplasma gondii infectivity in the laboratory mouse. In: Tonkin C., editor. Toxoplasma gondii. Methods in Molecular Biology . 1st. Nova York: Humana; 2020. pp. 99–116. [DOI] [PubMed] [Google Scholar]
- 33.Pena H. F. J., Gennari S. M., Dubey J. P., Su C. Population structure and mouse-virulence of Toxoplasma gondii in Brazil. International Journal for Parasitology . 2008;38(5):561–569. doi: 10.1016/j.ijpara.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Fernández-Escobar M., Calero-Bernal R., Regidor-Cerrillo J., et al. Isolation, genotyping, and mouse virulence characterization of Toxoplasma gondii from free ranging Iberian pigs. Frontiers in Veterinary Science . 2020;7, article 604782 doi: 10.3389/fvets.2020.604782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiramoto R. M., Mayrbaurl-Borges M., Galisteo A. J., Jr., Meireles L. R., Macre M. S., Andrade H. F., Jr. Infectivity of cysts of the ME-49 Toxoplasma gondii strain in bovine milk and homemade cheese. Revista de Saúde Pública . 2001;35(2):113–118. doi: 10.1590/S0034-89102001000200002. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs L., Remington J. S., Melton M. L. The resistance of the encysted form of Toxoplasma gondii. The Journal of Parasitology . 1960;46(1):11–21. doi: 10.2307/3275325. [DOI] [PubMed] [Google Scholar]
- 37.Dubey J. P. Re-examination of resistance ofToxoplasma gondiitachyzoites and bradyzoites to pepsin and trypsin digestion. Parasitology . 1998;116(1):43–50. doi: 10.1017/S0031182097001935. [DOI] [PubMed] [Google Scholar]
- 38.Chardès T., Bourguin I., Mevelec M. N., Dubremetz J. F., Bou D. Antibody responses to Toxoplasma gondii in sera, intestinal secretions, and milk from orally infected mice and characterization of target antigens. Infection and Immunity . 1990;58(5):1240–1246. doi: 10.1128/iai.58.5.1240-1246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubey J. P., Shen S. K., Kwok O. C. H., Frenkel J. K. Infection and immunity with the RH strain of Toxoplasma gondii in rats and mice. The Journal of Parasitology . 1999;85(4):657–662. doi: 10.2307/3285739. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y., Ren H., Xin S., Jiang N. Comparative immunological response and pathobiology of mice inoculated with Toxoplasma gondii isolated from different hosts. The Journal of Parasitology . 2021;107(2):179–181. doi: 10.1645/20-107. [DOI] [PubMed] [Google Scholar]
- 41.Bezerra M. J. G., Kim P. C. P., Moraes E. P. B. X., et al. Detection of Toxoplasma gondii in the milk of naturally infected goats in the northeast of Brazil. Transboundary and Emerging Diseases . 2015;62(4):421–424. doi: 10.1111/tbed.12160. [DOI] [PubMed] [Google Scholar]
- 42.Mancianti F., Nardoni S., D’Ascenzi C., et al. Seroprevalence, detection of DNA in blood and milk, and genotyping of Toxoplasma gondii in a goat population in Italy. BioMed Research International . 2013;2013:6. doi: 10.1155/2013/905326.905326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amairia S., Rouatbi M., Rjeibi M. R., et al. Molecular prevalence of Toxoplasma gondii DNA in goat’s milk and seroprevalence in Northwest Tunisia. Veterinary Medicine and Science . 2016;2(3):154–160. doi: 10.1002/vms3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sroka J., Kusyk P., Bilska-Zajac E., et al. Seroprevalence of Toxoplasma gondii infection in goats from the south-west region of Poland and the detection of T. gondii DNA in goat milk. Folia Parasitologica . 2017;64(23):1–5. doi: 10.14411/fp.2017.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


