Abstract
Background
While polystyrene microplastics (PS-MPs) are emerging as potentially significant health threats, linked to cancer and reproductive dysfunction, their precise effects on human health remain largely unknown. We aimed to investigate the underlying mechanisms promoting microplastic-induced damage in the reproductive system.
Methods
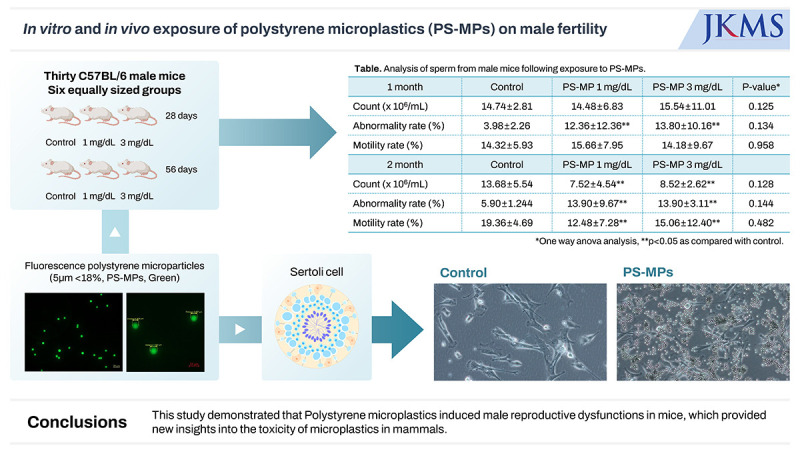
Thirty C57BL/6 male mice were randomly allocated into six equal-sized groups. Mice were exposed to fluorescent PS-MPs (5 µm, < 18%, green) at a dose of 1 and 3 mg/dL via oral gavage for 28 and 56 days, respectively (control, 0 mg/dL). The presence of antibodies and inflammatory and oxidative stress markers were evaluated using western blotting. Sperm analysis was also performed. Mouse testis Sertoli TM4 cells were divided into two groups: control (medium only) and PS-MPs (medium containing, 1,000 μg/mL) groups and cultured in vitro for 1, 24, 48, or 72 hours. The cells were cultured in a Ham’s F12: Dulbecco's Modified Eagle Medium medium with 0.25% fetal bovine serum at 37°C with humidified atmosphere of 5% carbon dioxide in the air. Protein analyses for interleukin (IL)-6, IL-10, NADPH-oxidase (NOX)-2, NOX-4, hypoxia-inducible transcription factor (HIF)-2α, monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor (TNF)-α, and transforming growth factor (TGF)-β were performed using western blotting.
Results
The testes were evaluated after 28 and 56 days of exposure. Varying sizes of PS-MPs were detected in the testes (ranging from 5.870 to 7.768 µm). Significant differences in sperm concentration, motility, and the proportion of normal sperm were observed between the two groups. An increase in TGF-β, HIF-2α, and NOX-4 levels was observed using western blot analysis. However, no dose-dependent correlations were observed between the two groups. In vitro evaluation of the PS-MPs group displayed PS-MP penetration of the lumen of Sertoli cells after 1 hour. Further PS-MP aggregation within Sertoli cells was observed at 24, 48, and 72 hours. A significant increase in inflammatory protein expressions (IL-10, TGF-β, MCP-1, IL-6, TNF-α, and HIF-2α) was observed through western blotting, although oxidative agents did not show a significant increase.
Conclusion
PS-MPs induced reproductive dysfunction in male mice provide new insights into PS-MPs-associated toxicity in mammals.
Keywords: Infertility, Microplastics, Sertoli Cells, Male, Mice
Graphical Abstract
INTRODUCTION
Microplastics (MPs), typically sized < 5 mm, are derived from the degradation of plastic objects in the environment or commercial production.1,2 Owing to their increasing production and extremely low natural biodegradation in ecosystems, MPs have recently been considered as environmental pollutants.3 During the coronavirus disease 2019 pandemic, the use of synthetic fiber-based face masks and disposable food containers was believed to have accelerated MP exposure.4
MPs are emerging as a serious threat to human health worldwide. Recent studies have revealed that humans constantly inhale and ingest MPs.5,6 Although numerous studies have investigated the effects of MPs on human health, our understanding of whether MPs pose a substantial risk to human health remains limited.7,8 Furthermore, some studies have suggested a potential link between the components of MPs and the occurrence of ovarian, breast, and prostate cancers within the human body.9,10 These research findings imply a potential risk of MP-associated adverse effects on human health.
Recent studies have suggested a potential effect of MPs on the reproductive system. One study demonstrated that the internal components of MPs can mimic hormones and consequently affect reproductive function.11 In women, MPs can interact with the endocrine hormone, estrogen, potentially increasing the risk of infertility.12 Furthermore, environmental MPs interacting with other environmental contaminants may impair reproductive function. For instance, certain studies have indicated that MPs can adsorb harmful substances such as saturated fatty acids, which may influence the reproductive system.13
While experimental studies in animals have investigated the association between MPs and reproductive dysfunction, the potential health risks of MPs on the reproductive system, particularly in male mammals, have yet to be determined.14,15 Therefore, additional data on the effects of MPs on the male reproductive system using mammalian models is needed for human health risk assessments of MPs. This study examined the toxicity of polystyrene MPs (PS-MPs) on the male reproductive system of mice using both in vivo and in vitro experiments.
METHODS
PS-MPs are continuously detected in the environment. In this study, pure MPs were used for model construction, while modified MPs with green fluorescence were used to assess the penetration and related toxicity in the testes of mice. We used fluorescent PS-MPs (Fluoro-Max green, 468/508 nm; red, 542/612 nm) with a diameter of 6 μm in this study, purchased from ThermoFisher Scientific Ltd. (Waltham, MA, USA).
In vitro study, cell culture, and experimental design
TM4 mouse Sertoli cells were divided into two groups for in vitro culturing: a control group cultured in medium only, and a PS-MPs group cultured in medium supplemented with 1,000 μg/mL PS-MPs. The cells were incubated for 1, 24, 48, or 72 hours. The culture conditions for the TM4 mouse Sertoli cells were as follows: a controlled environment of Ham’s F12 Dulbecco’s Modified Eagle Medium medium supplemented with 0.25% fetal bovine serum, a constant temperature of 37°C, and a humidified incubator maintained at 5% CO2. This setup ensured optimal growth conditions for Sertoli cells, allowing for an accurate assessment of the effect of PS-MPs.
In vivo study, animal model, and experimental design
Thirty-six six-week-old C57BL/6 male mice were randomly allocated to six equal-sized groups. Mice were exposed to fluorescent PS-MPs (5 µm, < 18%, green) at a dose of 0 (control), 1 mg/dL, and 3 mg/dL via oral gavage for 28 and 56 days. An oral gavage was administered once daily in the morning (between 9:00 a.m. and 10:00 a.m.). On days 28 and 56, blood samples were collected from the right atrium under general anesthesia for biochemical analysis, and the extracted serum was kept at −20°C until further analysis. The testes, gastrointestinal tract, liver, lungs, and kidneys were removed from each mouse for fluorescent PS-MPs detection. Frozen sections were prepared at a thickness of 7 μm.
Sperm count, abnormality, and motility analysis
The sperm parameters were evaluated as follows: the fresh testes and epididymis of mice were immediately transferred to a sterilized bench, where the external adipose tissue was pre-detached, fully minced, and squeezed into individual 2 mL centrifuge tubes. Subsequently, 1 mL normal saline was added to release the sperm. The tubes were then incubated in a 37°C water bath for 15 minutes to obtain a fresh sperm suspension. For the sperm count and motility assessment, the initial sperm suspension of each mouse was diluted to 10 mL with 37°C saline, and a 2 μL sample was pipetted into a hemocytometer to count the dead sperm (X1). The remaining sperm suspension was inactivated in a 90°C water bath for 5 minutes, and a 2 μL sample was pipetted into a hemocytometer to obtain the total sperm count (X2). Sperm motility, with 7 replicates for each group, was defined as:
| A (%) = (X2 − X1)/X2 |
Western blot analysis
For preparation of tissue lysate, frozen tissues were homogenized at 4°C using a 200 μL protein extraction solution (Cat. No. EBA-1049; ELPis, Daejeon, Korea) supplemented with 1% protease (P8340; Sigma, St. Louis, MO, USA) and phosphatase inhibitor cocktail 2 (P5726; Sigma), followed by centrifugation at 12,000 rpm for 20 minutes at 4°C. The supernatants were collected as total tissue lysates. Equal amounts of protein lysates (30 μg/lane) were resolved on an sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel, and the separated bands were transferred onto a 0.45 μm polyvinylidene difluoride membrane (Cat. No. IPVH00010; Merck, Cork, Ireland). Subsequently, unspecific sites were blocked on the membranes using 5% skimmed milk in phosphate buffered saline with tween® at room temperature for 1 hour. The membranes were incubated with the primary antibodies at 4°C overnight. Membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. After washing with tris-buffered saline, the protein bands were visualized using a ChemiDoc™ Imaging system (Bio-Rad, Hercules, CA, USA). Protein expression was quantified using ImageJ software, standardized to the expression of a housekeeping gene, and expressed as a fold change compared to the control samples.
Statistical analysis
Significant differences observed between the two groups were evaluated using a two-tailed Student’s t-test, and one-way analysis of variance was applied for multiple treatment comparisons. All statistical analyses were conducted using SPSS software (version 20.0; IBM Corp., Armonk, NY, USA). Differences were considered statistically significant at P < 0.05.
Ethics statement
This study was approved by the Institutional Review Board of Korea University and conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (KOREA-2021-0177).
RESULTS
In vitro study
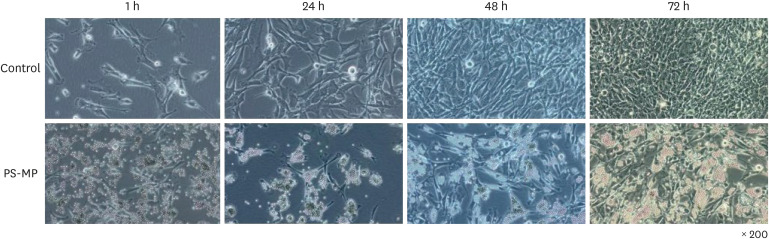
In primary cultures and colony formation assays with Sertoli cells exposed to PS-MPs, an accumulation of MPs was observed within the cells of the treated group compared with the control group, at 200× magnification (Fig. 1). This accumulation was evident at 24, 48, and 72 hours and was associated with the enlargement of interstitial spaces and cell vacuolization. These morphological changes resulted in inhibited cell division within Sertoli cells specifically in the PS-MPs group, indicating a disruptive effect of PS-MPs on normal cellular processes in the testicular environment. This reduction in cell division was consistently observed across all samples in multiple experiments.
Fig. 1. Morphology and characterization of primary cell cultures and colony formation of Sertoli cells exposed to PS-MPs.
PS-MPs = polystyrene microplastics.
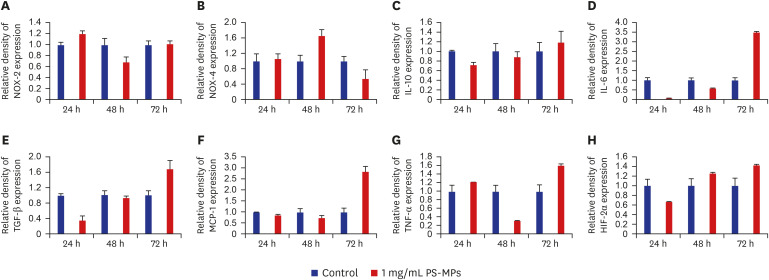
Regarding inflammation-associated factors, a significant increase in interleukin (IL)-10, transforming growth factor (TGF)-β, monocyte chemoattractant protein-1 (MCP-1), IL-6, and tumor necrosis factor (TNF)-α levels was detected in the PS-MPs group, as observed by Western blot analysis (Fig. 2, Supplementary Fig. 1). At 72 hours, a significant increase in the expression of TGF-β (1:1.6), IL-10 (1:1.2), MCP-1 (1:2.9), IL-6 (1:3.5), TNF-α (1:1.6), and hypoxia-inducible transcription factor (HIF)-2α (1:1.4) was observed in the PS-MPs-treated group. Except for TNF-α and MCP-1, an increase in inflammatory-related factors was observed over time; however, no significant increase in the levels of the oxidative agents, NADPH-oxidase (NOX)-2 and NOX-4, was observed.
Fig. 2. Western blot analysis of antibody expression in Sertoli cells. The protein level of (A) NOX-2, (B) NOX-4, (C) IL-10, (D) IL-6, (E) TGF-β, (F) MCP-1, (G) TNF-α, and (H) HIF-2α.
NOX = NADPH-oxidase, PS-MPs = polystyrene microplastics, IL = interleukin, TGF-β = transforming growth factor-β, MCP-1 = monocyte chemoattractant protein-1, TNF-α = tumor necrosis factor-α, HIF-2α = hypoxia-inducible transcription factor-2α.
In vivo study
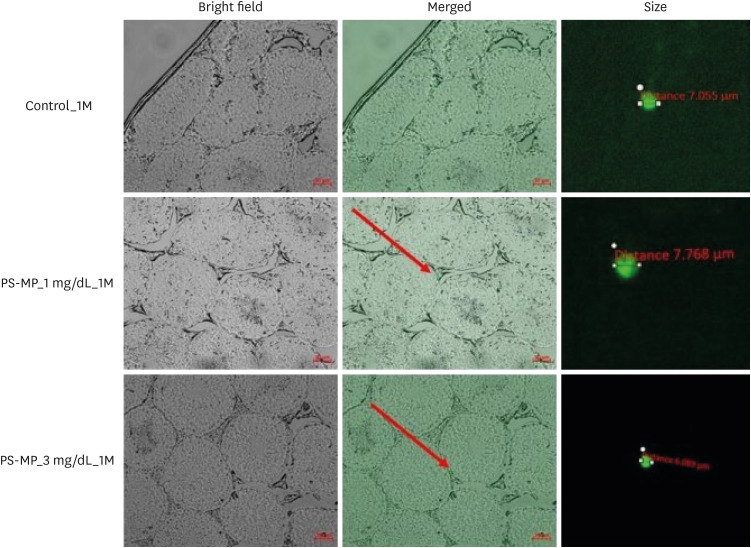
Our findings indicated that exposure of PS-MPs for 28 and 56 days resulted their detection in the mouse testes. The smallest and largest detected PS-MPs measured 5.870 µm and 7.768 µm, respectively (Fig. 3). However, accumulation of PS-MPs in the testes and destruction of the blood-testis barrier (BTB) were not observed.
Fig. 3. Fluorescent PS-MPs in the testes of mice after exposure for 28 days.
PS-MPs = polystyrene microplastics, 1M = 1 month.
A significant difference was observed between the control and PS-MPs exposure groups (1 mg/dL, 3 mg/dL; 1 and 2 months) in terms of the proportion of normal sperms (P < 0.05), as observed in sperm analysis (Table 1, Supplementary Fig. 2). In the 28-day group, no significant differences were observed in sperm count and motility between the control and PS-MPs groups (1 and 3 mg/dL). However, following 2 months of exposure, the PS-MPs exposed groups showed a statistically significant difference in cell count and motility when compared with the control group. However, no differences were observed between the tested concentrations of PS-MPs. In addition, no significant differences were observed among the three groups in sperm count, motility, or proportion of normal sperm shape (P > 0.05).
Table 1. Analysis of sperm from male mice following exposure to PS-MPs.
| Groups | Control | PS-MP 1 mg/dL | PS-MP 3 mg/dL | P valuea | |
|---|---|---|---|---|---|
| 1 month | |||||
| Count, × 106/mL | 14.74 ± 2.81 | 14.48 ± 6.83 | 15.54 ± 11.01 | 0.125 | |
| Abnormality rate, % | 3.98 ± 2.26 | 12.36 ± 12.36* | 13.80 ± 10.16* | 0.134 | |
| Motility rate, % | 14.32 ± 5.93 | 15.66 ± 7.95 | 14.18 ± 9.67 | 0.958 | |
| 2 months | |||||
| Count, × 106/mL | 13.68 ± 5.54 | 7.52 ± 4.54* | 8.52 ± 2.62* | 0.128 | |
| Abnormality rate, % | 5.90 ± 1.24 | 13.90 ± 9.67* | 13.90 ± 3.11* | 0.144 | |
| Motility rate, % | 19.36 ± 4.69 | 12.48 ± 7.28* | 15.06 ± 12.40* | 0.482 | |
Values are presented as mean ± standard deviation.
Sperm were observed with double tail deformity, double head deformity, folded body deformity, folded neck deformity, and unstable head deformity.
PS-MPs = polystyrene microplastics.
aOne-way analysis of variance analysis.
*P < 0.05 as compared with the control group.
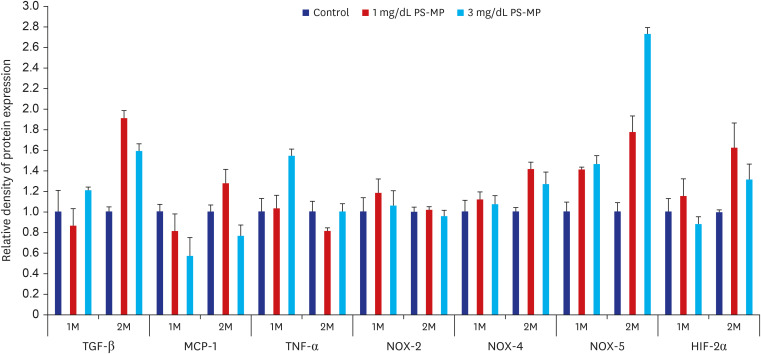
Western blot analysis revealed a significant increase in TGF-β and HIF-2α expression levels in the PS-MP groups when compared to the control group. Additionally, a notable increase in the expression levels of NOX-4 and NOX-5, factors related to oxidative stress, was observed. However, this finding could not be corroborated in the in vitro study. The increase was substantiated with longer treatment durations but no correlation was observed with the administered dose of PS-MPs (Fig. 4, Supplementary Fig. 3).
Fig. 4. Western blot analysis of antibody expression in mice testes (TGF-β, MCP-1, TNF-α, NOX-2, NOX-4, NOX-5, and HIF-2α).
PS-MPs = polystyrene microplastics, 1M = 1 month, 2M = 2 months, TGF-β = transforming growth factor-β, MCP-1 = monocyte chemoattractant protein-1, TNF-α = tumor necrosis factor-α, NOX = NADPH-oxidase, HIF-2α = hypoxia-inducible transcription factor-2α.
DISCUSSION
Male infertility accounts for approximately 20% of all infertility cases and 30–40% of contributing cases.16 The testes and epididymis, crucial male reproductive organs, are responsible for spermatogenesis and are highly sensitive to environmental contaminants such as heavy metals, endocrine disruptors, and ingested MP particles.17,18,19 Sertoli cells, the structural component of the seminiferous epithelium, form the BTB as well as provide physical support and a microenvironment for spermatogenesis. The BTB contributes partially to the immune-privileged status of the testis by preventing autoantigens present in the late spermatocytes and post-meiotic germ cells from being recognized by host immune cells, thus suppressing immune responses in the testis.20 It is virtually impossible for extrinsic toxicants to pass through the BTB, a protective barrier that preserves reproductive function by preventing toxicants from reaching germ cells. Previous studies have suggested that micron-sized particles have difficulty entering Sertoli cells in mice owing to the protection provided by the BTB.21 However, Li et al.22 reported a reduction in the expression of BTB-related proteins such as occludin, connexin-43, N-cadherin, and claudin-11, indicating that exposure to PS-MPs could induce disruption of the BTB in mice. Our study assumed that PS-MPs could pass through the BTB and conducted experiments to determine whether PS-MPs are associated with infertility. Ingested MPs could pass through the BTB in the testes, leading to enlarged interstitial spaces and cell vacuolation.
The effects of PS-MPs on reproductive dysfunction have been previously described. Although the exact mechanism has not been determined, possible mechanisms may include MP-induced developmental toxicity, oxidative stress, mitogen-activated protein kinase (MAPK), Akt and mammalian target of rapamycin signaling, NLRP3 inflammasome and fibrotic signaling, steroidogenic and endocrine signaling, cell death, and apoptosis.23 Several studies on the effect of MPs on male fertility have suggested that PS-MPs aggravate oxidative stress.12,23 However, our in vitro study demonstrated that the interaction with PS-MPs did not contribute to changes in expression levels of oxygen-activating enzymes, specifically NOX-2 and NOX-4. This suggests that MPs do not directly induce oxidative stress under cell culture conditions. In contrast, the in vivo studies revealed an increase in NOX4 and NOX5 levels, which was distinctive compared to the cell culture environment. This suggests that PS-MPs may not directly interact with oxygen-activating enzymes or may indirectly influence their regulation via divergent signaling pathways. The in vivo environment is inherently more complex than in vitro cell culture systems. In in vivo conditions, multiple cell types, tissues, and systemic factors interact, creating conditions where indirect mechanisms can contribute to oxidative stress. Inflammatory cytokines and immune cells can be recruited to the testes in vivo, indirectly promoting oxidative stress. This is supported by our findings of the increased expression levels of inflammatory markers such as IL-6, TNF-α, and MCP-1 in vivo. These inflammatory responses can enhance the activity of oxidative stress pathways, including NOX enzymes, thus contributing to the observed oxidative stress.12 Additionally, in vivo, Sertoli cells interact with other testicular cell types, including germ cells and Leydig cells. These interactions are critical for maintaining testicular homeostasis and responding to stress. MPs may induce oxidative stress in other cell types, which in turn could influence Sertoli cell function through paracrine signaling. The absence of these interactions in vitro might explain the lack of significant changes in oxidative stress markers in isolated Sertoli cells.22 It is plausible that PS-MPs might alter systemic metabolic processes, leading to increased production of reactive oxygen species from other organs that circulate and affect the testes, thereby inducing oxidative stress through indirect mechanisms present only in vivo.23
The present study confirmed that PS-MPs are able to induce inflammatory responses by directly passing through the BTB and infiltrating Sertoli cells. An increase in inflammatory-related factors such as IL-10, TGF-β, MCP-1, IL-6, and TNF-α was observed after treatment with PS-MPs, suggesting the activation of immune responses and cellular stress pathways by MPs. The above-mentioned factors are involved in inflammation and immunity regulation and are generally modulated by signaling pathways such as nuclear factor κB, MAPK, or JAK-STAT.24,25,26 Consequently, the present study concluded that MAPK, inflammation, and toxicity have a role to play. An increase in inflammatory responses can potentially affect cellular physiology and function and cause reproductive dysfunction such as infertility. Further research is needed to gain a more precise understanding of the underlying mechanisms and interactions, thereby facilitating a comprehensive assessment of the effects of PS-MPs and their potential impacts.
Research on the relationships between size, duration of exposure, and dosage of PS-MPs and reproductive dysfunction is currently limited. In terms of the duration of exposure, longer exposure has been shown to correlate with decreased reproductive capabilities. However, no size-dependent relationship has been established, similar to nanoplastics.15 Studies investigating exposure dosage have indicated significant increases in oxidative stress and inflammatory responses in the testes in groups exposed to higher doses of PS-MPs (0.15 mg/day and 1.5 mg/day).22 The present study established a correlation between toxicity in Sertoli cells and the duration of exposure to PS-MPs, but no significant difference was observed between the groups treated with 1 and 3 mg/dL PS-MPs. The lack of a clear dose-response effect between the 1 mg/dL and 3 mg/dL MP exposure groups may be attributed to a saturation effect, where the biological response reaches a maximum at lower exposure levels, making additional increases in dose less impactful. This phenomenon has been observed in other studies, suggesting that even low doses of MPs can elicit significant biological effects. Li et al.22 reported similar findings, where lower doses of PS-MPs induced maximal oxidative stress responses in the testes of rats, with no significant differences observed at higher doses.2 Similarly, Dubey et al.23 noted that the reproductive toxicity of MPs did not increase proportionally with an increase in dosage, indicating a potential threshold effect.3 These comparisons suggest that the reproductive system may be highly sensitive to MPs, and exposure beyond a certain threshold may not further exacerbate existing adverse effects. Understanding this threshold is crucial for assessing the risks associated with MPs exposure and determining their safe levels. More evidence is needed to comprehensively understand dose-dependency in PS-MP exposure.
This study had some limitations. Comparing between the effects of MP doses and exposure durations in mice and human exposure levels is challenging. Current estimates suggest that humans ingest thousands of MP particles annually through food, water, and air.6,27 Our study doses (1 and 3 mg/dL) and durations (28 and 56 days) are significantly higher than typical environmental exposures, which were necessary to observe pronounced effects within a shorter timeframe. However, experimental studies often use MP concentrations far exceeding environmental levels to identify potential health effects.28 These elevated experimental conditions help to understand the possible effects at the upper limits of human intake, but they do not accurately represent everyday exposure.29 Ethical constraints prevent the exact replication of human exposure levels in experimental settings, posing challenges to direct applicability. Therefore, further studies are needed to bridge the gap between experimental and environmental conditions to comprehensively evaluate the human health implications of MP exposure.
In conclusion, PS-MPs induced male reproductive dysfunction in mice. Although a clear dose-response relationship was not established, increased PS-MPs duration of exposure correlated with a decline in sperm count, normal morphology, and motility, ultimately resulting in reduced reproductive capabilities. These findings provide new insights into the toxicity of MPs in mammalian systems and highlight their potential risks in terms of male reproductive dysfunction.
Footnotes
Funding: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, Information, and Communications Technology (ICT) and Future Planning (NRF-2021R1l1A2060109), and a Korea University Grant. None of the funding agencies had any role in the study concept and design, experiments, data analysis, manuscript writing, or decision to publish.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Tae BS.
- Data curation: Ko YJ.
- Formal analysis: Ko YJ, Tae BS.
- Funding acquisition: Cha JJ, Kim C, Seo MY, Lee SH, Bae JH, Tae BS.
- Investigation: Jeon BJ, Tae BS.
- Methodology: Tae BS.
- Validation: Tae BS.
- Visualization: Jeon BJ, Ko YJ, Tae BS.
- Writing - original draft: Jeon BJ.
- Writing - review & editing: Park JY, Bae JH, Tae BS.
SUPPLEMENTARY MATERIALS
Western blot analysis of various inflammatory and oxidative stress markers in Sertoli cells at different time points (24 hours, 48 hours, and 72 hours) following exposure to 1 mg/dL PS-MPs. Markers include NOX-2, NOX-4, IL-10, IL-6, TGF-β, MCP-1, TNF-α, and HIF-2α.
Serum testosterone levels in mice exposed to PS-MPs. The levels are compared between control, 1 mg/dL, and 3 mg/dL PS-MP exposure groups for 1M and 2M.
Western blot analysis of various inflammatory and oxidative stress markers in Sertoli cells and mice testes exposed to PS-MPs. Markers include TGF-β, MCP-1, TNF-α, NOX-2, NOX-4, NOX-5, and HIF-2α. Protein expression levels are shown for control, 1 mg/dL PS-MPs, and 3 mg/dL PS-MPs groups at 1 month and 2 months.
References
- 1.Hartmann NB, Hüffer T, Thompson RC, Hassellöv M, Verschoor A, Daugaard AE, et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ Sci Technol. 2019;53(3):1039–1047. doi: 10.1021/acs.est.8b05297. [DOI] [PubMed] [Google Scholar]
- 2.de Souza Machado AA, Kloas W, Zarfl C, Hempel S, Rillig MC. Microplastics as an emerging threat to terrestrial ecosystems. Glob Change Biol. 2018;24(4):1405–1416. doi: 10.1111/gcb.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prata JC. Airborne microplastics: consequences to human health? Environ Pollut. 2018;234:115–126. doi: 10.1016/j.envpol.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 4.Kannan K, Vimalkumar K. A review of human exposure to microplastics and insights into microplastics as obesogens. Front Endocrinol (Lausanne) 2021;12:724989. doi: 10.3389/fendo.2021.724989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitrano DM, Wick P, Nowack B. Placing nanoplastics in the context of global plastic pollution. Nat Nanotechnol. 2021;16(5):491–500. doi: 10.1038/s41565-021-00888-2. [DOI] [PubMed] [Google Scholar]
- 6.Wright SL, Kelly FJ. Plastic and human health: a micro issue? Environ Sci Technol. 2017;51(12):6634–6647. doi: 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- 7.Lu K, Zhan D, Fang Y, Li L, Chen G, Chen S, et al. Microplastics, potential threat to patients with lung diseases. Front Toxicol. 2022;4:958414. doi: 10.3389/ftox.2022.958414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamura T, Hamaguchi M, Hasegawa Y, Hashimoto Y, Majima S, Senmaru T, et al. Oral exposure to polystyrene microplastics of mice on a normal or high-fat diet and intestinal and metabolic outcomes. Environ Health Perspect. 2023;131(2):27006. doi: 10.1289/EHP11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JH, Hong S, Kim OH, Kim CH, Kim J, Kim JW, et al. Polypropylene microplastics promote metastatic features in human breast cancer. Sci Rep. 2023;13(1):6252. doi: 10.1038/s41598-023-33393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edaes FS, de Souza CB. BPS and BPF are as carcinogenic as BPA and are not viable alternatives for its replacement. Endocr Metab Immune Disord Drug Targets. 2022;22(9):927–934. doi: 10.2174/1871530322666220316141032. [DOI] [PubMed] [Google Scholar]
- 11.Amran NH, Zaid SSM, Meng GY, Salleh A, Mokhtar MH. Protective role of kelulut honey against toxicity effects of polystyrene microplastics on morphology, hormones, and sex steroid receptor expression in the uterus of rats. Toxics. 2023;11(4):324. doi: 10.3390/toxics11040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei Z, Wang Y, Wang S, Xie J, Han Q, Chen M. Comparing the effects of polystyrene microplastics exposure on reproduction and fertility in male and female mice. Toxicology. 2022;465:153059. doi: 10.1016/j.tox.2021.153059. [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Wei F, Qiu S, Xing B, Hou J. Polystyrene microplastics trigger adiposity in mice by remodeling gut microbiota and boosting fatty acid synthesis. Sci Total Environ. 2023;890:164297. doi: 10.1016/j.scitotenv.2023.164297. [DOI] [PubMed] [Google Scholar]
- 14.Zhao T, Shen L, Ye X, Bai G, Liao C, Chen Z, et al. Prenatal and postnatal exposure to polystyrene microplastics induces testis developmental disorder and affects male fertility in mice. J Hazard Mater. 2023;445:130544. doi: 10.1016/j.jhazmat.2022.130544. [DOI] [PubMed] [Google Scholar]
- 15.Xu W, Yuan Y, Tian Y, Cheng C, Chen Y, Zeng L, et al. Oral exposure to polystyrene nanoplastics reduced male fertility and even caused male infertility by inducing testicular and sperm toxicities in mice. J Hazard Mater. 2023;454:131470. doi: 10.1016/j.jhazmat.2023.131470. [DOI] [PubMed] [Google Scholar]
- 16.Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, et al. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed) 1985;291(6510):1693–1697. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao HT, Xu R, Cao WX, Qian LL, Wang M, Lu L, et al. Effects of six priority controlled phthalate esters with long-term low-dose integrated exposure on male reproductive toxicity in rats. Food Chem Toxicol. 2017;101:94–104. doi: 10.1016/j.fct.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Liu TM, Lee EH, Lim B, Shyh-Chang N. Concise review: balancing stem cell self-renewal and differentiation with PLZF. Stem Cells. 2016;34(2):277–287. doi: 10.1002/stem.2270. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Tang Y, Chen B, Zhao Y, Aguilar ZP, Tao X, et al. Inhibition of testosterone synthesis induced by oral TiO2 NPs is associated with ROS-MAPK(ERK1/2)-StAR signaling pathway in SD rat. Toxicol Res (Camb) 2021;10(4):937–946. doi: 10.1093/toxres/tfab077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mruk DD, Cheng CY. The mammalian blood-testis barrier: its biology and regulation. Endocr Rev. 2015;36(5):564–591. doi: 10.1210/er.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan Z, Yang WX. Nanoparticles and spermatogenesis: how do nanoparticles affect spermatogenesis and penetrate the blood-testis barrier. Nanomedicine (Lond) 2012;7(4):579–596. doi: 10.2217/nnm.12.20. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Wang Q, Yu H, Yang L, Sun Y, Xu N, et al. Polystyrene microplastics induce blood-testis barrier disruption regulated by the MAPK-Nrf2 signaling pathway in rats. Environ Sci Pollut Res Int. 2021;28(35):47921–47931. doi: 10.1007/s11356-021-13911-9. [DOI] [PubMed] [Google Scholar]
- 23.Dubey I, Khan S, Kushwaha S. Developmental and reproductive toxic effects of exposure to microplastics: a review of associated signaling pathways. Front Toxicol. 2022;4:901798. doi: 10.3389/ftox.2022.901798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13(9):679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 26.O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66(1):311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE. Human consumption of microplastics. Environ Sci Technol. 2019;53(12):7068–7074. doi: 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- 28.Lenz R, Enders K, Nielsen TG. Microplastic exposure studies should be environmentally realistic. Proc Natl Acad Sci U S A. 2016;113(29):E4121–E4122. doi: 10.1073/pnas.1606615113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T. Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ. 2020;702:134455. doi: 10.1016/j.scitotenv.2019.134455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blot analysis of various inflammatory and oxidative stress markers in Sertoli cells at different time points (24 hours, 48 hours, and 72 hours) following exposure to 1 mg/dL PS-MPs. Markers include NOX-2, NOX-4, IL-10, IL-6, TGF-β, MCP-1, TNF-α, and HIF-2α.
Serum testosterone levels in mice exposed to PS-MPs. The levels are compared between control, 1 mg/dL, and 3 mg/dL PS-MP exposure groups for 1M and 2M.
Western blot analysis of various inflammatory and oxidative stress markers in Sertoli cells and mice testes exposed to PS-MPs. Markers include TGF-β, MCP-1, TNF-α, NOX-2, NOX-4, NOX-5, and HIF-2α. Protein expression levels are shown for control, 1 mg/dL PS-MPs, and 3 mg/dL PS-MPs groups at 1 month and 2 months.