Abstract
Cattle infected with bovine spongiform encephalopathy (BSE) appear to be a reservoir for transmission of variant Creutzfeldt-Jakob disease (vCJD) to humans. Although just over 100 people have developed clinical vCJD, millions have probably been exposed to the infectivity by consumption of BSE-infected beef. It is currently not known whether some of these individuals will develop disease themselves or act as asymptomatic carriers of infectivity which might infect others in the future. We have studied agent persistence and adaptation after cross-species infection using a model of mice inoculated with hamster scrapie strain 263K. Although mice inoculated with hamster scrapie do not develop clinical disease after inoculation with 10 million hamster infectious doses, hamster scrapie infectivity persists in brain and spleen for the life span of the mice. In the present study, we were surprised to find a 1-year period postinfection with hamster scrapie where there was no evidence for replication of infectivity in mouse brain. In contrast, this period of inactive persistence was followed by a period of active replication of infectivity as well as adaptation of new strains of agent capable of causing disease in mice. In most mice, neither the early persistent phase nor the later replicative phase could be detected by immunoblot assay for protease-resistant prion protein (PrP). If similar asymptomatic carriers of infection arise after exposure of humans or animals to BSE, this could markedly increase the danger of additional spread of BSE or vCJD infection by contaminated blood, surgical instruments, or meat. If such subclinical carriers were negative for protease-resistant PrP, similar to our mice, then the recently proposed screening of brain, tonsils, or other tissues of animals and humans by present methods such as immunoblotting or immunohistochemistry might be too insensitive to identify these individuals.
Recent transmission of bovine spongiform encephalopathy (BSE) to humans in Great Britain greatly heightened concern among scientists and the public about the risk posed by transmissible spongiform encephalopathies (TSE). In addition to direct induction of clinical disease, cross-species TSE infection may in some cases result in a subclinical carrier infection. Infectious agent present in such a carrier state might eventually adapt to a more virulent form. The possibility that this scenario could occur in humans exposed to BSE has led to great concern about contamination of the blood supply and surgical instruments. The situation is complicated by the possibility that BSE has spread to sheep in Europe (12). There is no epidemiologic evidence for the transmission of sheep scrapie to humans, but nothing is known about the potential risk that sheep-derived BSE may present to humans or other species. Similarly, in the United States the risk posed by chronic wasting disease (CWD) of elk and deer to humans, wildlife, and livestock is not clear (21). In spite of the lack of evidence for natural spread of CWD to cattle or humans, the possibility that there might be infected asymptomatic carriers in animal or human populations remains a subject of increasing concern.
Cross-species transmission of TSE infectivity leading to clinical disease has been observed in a variety of animal species. Usually, the incubation period is lengthened in the first few passages but eventually stabilizes to a predictable value in the new host. Absence of cross-species transmission to host species which are not globally resistant to all TSE agents has been observed in only a few situations. These include BSE infection of hamsters (10), CWD infection of hamsters (2), transmissible mink encephalopathy infection of mice (22), and hamster scrapie strain 263K infection of mice (15). In these experiments, absence of transmission was usually based on the lack of clinical disease within the life span of the recipient, although in some cases a lack of typical central nervous system pathological changes was also documented. Only in the hamster 263K-mouse model was infectivity directly assayed by inoculation back into hamsters to search for the existence of asymptomatic carrier mice. Some carrier mice were in fact detected in the first passage but not in the second or third mouse passages, leading to the conclusion that 263K infectivity detected in the first-passage mice was merely the original inoculum and that replication had not occurred (17). In our previous experiments, we found that hamster 263K scrapie persisted in mouse brain and spleen for up to 2 years without causing clinical disease (18). This persistence required the expression of the mouse prion protein (PrP) gene and implied that the foreign scrapie agent might have replicated, since PrP was required and is a known susceptibility factor for scrapie replication. This possibility was also supported by recent experiments analyzing infectivity from mice infected with 263K hamster scrapie (11). In this earlier work, infectivity in two scrapie-inoculated mice was analyzed, and one mouse showed a significant increase in titer consistent with replication. In the present experiments, we analyzed infectivity and protease-resistant PrP (PrP-res) in 23 mice with asymptomatic carrier infections at various times following infection with 263K hamster scrapie in order to define precisely the kinetics of replication which might occur after cross-species infection. The results indicated that the original hamster scrapie agent persisted without detectable replication for over 1 year. However, during the second year significant replication of hamster agent as well as adaptation to a form virulent for mice was observed.
MATERIALS AND METHODS
Scrapie inoculations.
Primary C57BL/10 weanling mice were inoculated intracerebrally with 50 μl of a 1% hamster brain suspension containing 107 50% infective doses of hamster scrapie strain 263K. Clinical disease refers to signs of clinical scrapie including somnolence, kyphosis, tremors, stilted gait, and ataxia. Mice with these signs usually progressed to a moribund status within 2 weeks. Incubation period was the time from inoculation until development of obvious clinical disease. Secondary- and tertiary-passage mice and hamsters were inoculated intracerebrally with 50 μl of a 1% brain suspension from primary or secondary mice sacrificed at the indicated number of days postinoculation.
Immunoblot detection of PrP.
As described previously (20), hamster PrP-res was detected by immunoblotting using a hamster PrP-reactive monoclonal antibody (3F4) (14), which does not react with mouse PrP. Mouse PrP-res was detected by immunoblotting in samples negative for hamster PrP-res using a rabbit anti-PrP peptide serum (R30) (19).
RESULTS
Analysis of brain PrP-res after cross-species infection.
At several times after inoculation of mice with hamster scrapie strain 263K, mice were killed, and their brains were analyzed for the presence of PrP-res (20). First, we tested for hamster PrP-res using monoclonal antibody 3F4, which reacts with hamster PrP but not with mouse PrP (14). Hamster PrP-res was detected by immunoblotting in brains of mice at 2 h after infection but not thereafter, suggesting that this material was derived from the inoculum (Table 1). The brains that were negative for hamster PrP-res were then tested for mouse PrP-res using a polyclonal anti-PrP peptide serum (R30), capable of reacting with both mouse and hamster PrP (19). In the first passage, mouse PrP-res was not detected until 310 days postinoculation (dpi). Between 310 and 782 dpi, brain homogenates from 13 out of 36 first-passage mice were positive (Table 1). Because of its appearance so late (310 days) after scrapie inoculation, this newly generated mouse PrP-res should not have been derived from the original hamster inoculum. Instead, this result suggested that the inoculated agent had replicated in these mice. There was never evidence of clinical signs of scrapie in these first-passage mice; however, this may have been because the amount of mouse PrP-res detected in these mice was 4- to 100-fold lower than the amount usually associated with clinical disease in mice infected with Chandler/RML scrapie agent.
TABLE 1.
Detection of PrP-res and clinical disease in first-passage mice
| Day | Clinical diseasea | Hamster PrP-resb | Mouse PrP-resb |
|---|---|---|---|
| 0.1 | 0/2 | 2/2 | NT |
| 5 | 0/2 | 0/2 | 0/2 |
| 20 | 0/2 | 0/2 | 0/2 |
| 60 | 0/2 | 0/2 | 0/2 |
| 130 | 0/2 | 0/2 | 0/2 |
| 240 | 0/2 | 0/2 | 0/2 |
| 310 | 0/2 | 0/2 | 1/2 |
| 463 | 0/3 | 0/3 | 0/3 |
| 574 | 0/16 | 0/2 | 7/16 |
| 693 | 0/13 | 0/2 | 3/13 |
| 782 | 0/2 | 0/2 | 2/2 |
Values are shown as numbers of animals with clinical scrapie/total numbers of animals.
Values are shown as numbers of animals with PrP-res/total numbers of animals. NT, not tested.
Detection of scrapie infectivity for hamsters.
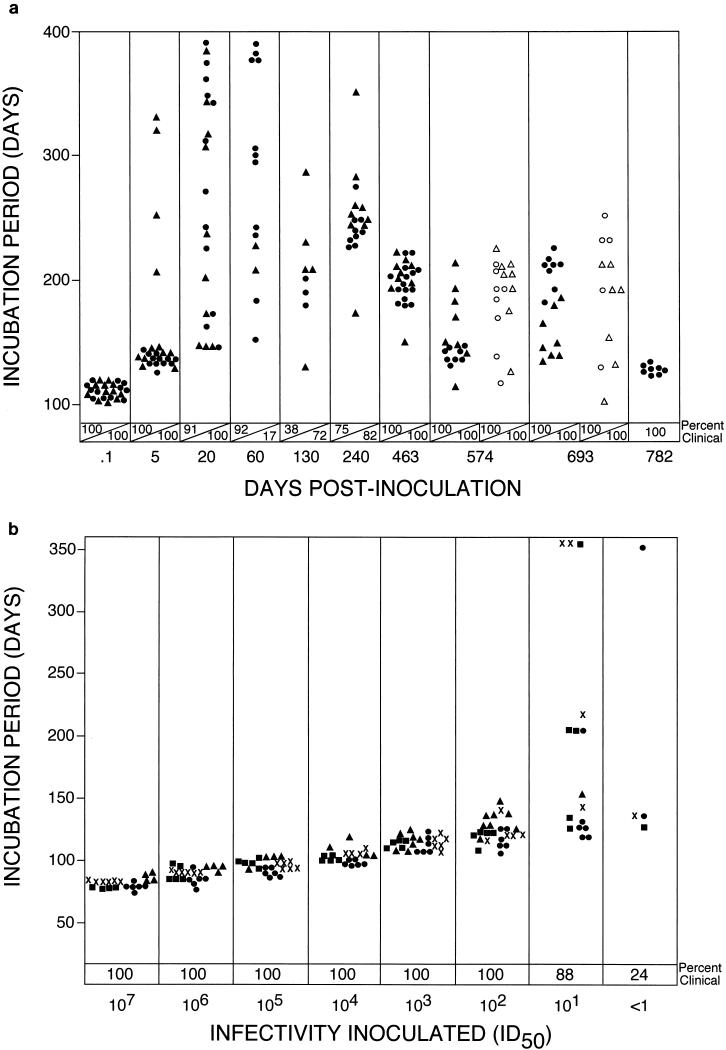
Because of the association between PrP-res and infectivity, mice expressing detectable PrP-res were likely to have high amounts of infectivity. However, we were interested to determine whether propagation of infectivity also occurred in asymptomatic PrP-res-negative mice. Such carrier mice would be detectable only by infectivity transmission experiments and would be a model for a possible situation which could develop after cross-species infection with BSE-contaminated feeds. Accordingly, brain suspensions prepared from PrP-res-negative first-passage mice sacrificed at various days postinoculation were inoculated into hamsters. All samples produced typical scrapie when inoculated into hamsters (Fig. 1a); however, the amount of infectivity varied greatly. For example, based on a 100% incidence of positive recipients and a short incubation period, high levels of infectivity were detected in mice sacrificed at 0.1 and 5 dpi. By 20 dpi, there was a marked broadening in the range of incubation periods (140 to 390 days), indicating a lower titer of infectivity. From 60 to 240 dpi, infectivity levels were very low as demonstrated by lower than 100% incidence of clinical disease in recipient hamsters. These results appear to be consistent with a partial eclipse phase seen in many viral infections. However, at subsequent times from 463 to 782 dpi all recipient hamsters became ill, and the range of incubation periods was lowered to 120 to 230 days, suggesting that infectivity titers were now higher. Furthermore, when homogenates from 574- and 693-dpi donors were diluted an additional 10-fold, there was no significant broadening of the incubation periods in hamsters, and still all recipients became ill. These results indicated that a greater-than-100-fold increase in the level of hamster-tropic infectivity occurred in the second year of this first passage into mice.
FIG. 1.
(a) Incubation period analysis in hamsters of first mouse passage of hamster scrapie strain 263K. At various times after scrapie inoculation, two mice (▴ and ●) were sacrificed at each time point except 782 dpi, at which only one mouse was sacrificed. Eight to 12 secondary hamsters were inoculated intracerebrally with 50 μl of a 1% brain suspension from each donor mouse. Homogenates from donors at 574 and 693 dpi were also diluted 10-fold before inoculation of additional hamsters (▵ and ○). (b) Incubation period analysis in hamsters of original hamster scrapie strain 263K. Hamsters were inoculated intracerebrally with 50 μl of serial 10-fold dilutions of a pooled brain suspension from hamsters inoculated with hamster scrapie strain 263K. Data from four independent titrations (●, ▴, ▪, and ✖) are shown for comparison. Note the narrow range of incubation periods observed for all dilutions until the point at which less than 100% of animals were clinically infected. ID50, 50% infective dose.
The rather broad range of incubation periods seen for both dilutions of the homogenates from 574- and 693-dpi donors contrasted markedly with what was observed in hamsters inoculated with the original strain 263K, where incubation periods remained tightly grouped except for the high dilutions which killed less than 100% of the animals (Fig. 1b). These differences suggested that the scrapie infectivity derived from some of the first-passage mice was no longer identical to that of the original hamster scrapie strain 263K.
Detection of infectivity by passage in mice.
Brain suspensions from some of the PrP-res-negative first-passage mice were also inoculated into mice to test for the presence of mouse-adapted infectivity. In contrast to the high incidence of clinical disease seen when hamsters were inoculated with these first-passage extracts (Fig. 1a), clinical disease was not induced in mice by any extract except that from the 782-dpi donor (Table 2). Interestingly, this extract also caused disease in hamsters with a brief incubation period, similar to 263K (Fig. 1a), suggesting that it might contain a mixture of 263K and mouse-adapted scrapie strains.
TABLE 2.
Detection of PrP-res and clinical disease in second-passage micea
| Day | First mouse passage
|
Second mouse passage
|
||
|---|---|---|---|---|
| Donor no. | PrP-res | Clinical scrapieb | PrP-resc | |
| 130 | 1 | − | 0/12 | 0/6 |
| 130 | 2 | − | 0/12 | 0/7 |
| 463 | 1 | − | 0/6 | 3/6 |
| 463 | 2 | − | 0/6 | 3/6 |
| 574 | 1 | − | 0/10 | 5/6 |
| 574 | 2 | − | 0/10 | 4/6 |
| 693 | 1 | − | 0/9 | 3/6 |
| 693 | 2 | − | 0/10 | 2/6 |
| 782 | 1 | + | 10/10d | 8/8 |
For the second-passage experiments, two first-passage donors at selected time points were tested, except at 782 dpi, where only one donor was used. All donors used for the second passage were negative for PrP-res except for the donor from 782 dpi. Second-passage mice which failed to develop clinical disease between 650 and 750 days of observation were sacrificed, and brains were analyzed for PrP-res.
Values are shown as numbers of mice with clinical scrapie/total numbers of mice.
Values are shown as numbers of mice with PrP-res/total numbers of mice.
Incubation period for severe clinical scrapie was 457 ± 15 days.
The second-passage mice were also monitored for the generation of PrP-res. Brain extracts from PrP-res-negative primary mice sacrificed at 130 dpi were not able to induce generation of PrP-res in the 650- to 750-day observation period (Table 2). In contrast, brains from PrP-res-negative primary mice sacrificed at later times (463 to 693 dpi) were able to induce detectable mouse PrP-res in 20 out of 36 recipients (Table 2). This indicated that a subclinical PrP-res-negative scrapie infection in these first-passage mice had been transferred to some second-passage mice. Therefore, only at these late times postinoculation were both replication of hamster scrapie infectivity and adaptation of the infectivity to mice detectable in these asymptomatic first-passage mice. Mice which received brain from the 782-dpi PrP-res-positive first-passage donor developed both brain PrP-res and clinical disease consistent with scrapie (Table 2). The recipients of this inoculum died at 457 ± 15 days, compared to hamsters receiving the same inoculum, which died between 122 and 134 days (Fig. 1a).
In order to analyze the further evolution of mouse and hamster scrapie infectivity in these experiments, at 650 to 750 dpi eight of the second-passage mice were sacrificed and brain homogenates were analyzed for infectivity by inoculation of both mice and hamsters. At present, these experiments have been monitored for over 400 days. No infectivity was detected in second-passage donors 1 and 2 derived from first-passage mice sacrificed at 130 days (Table 3). Thus, at 130 dpi in the first passage the infectivity present was capable of transmission to hamsters (Fig. 1a) but not to mice (Tables 2 and 3). Infectivity from three of the second-passage donors maintained the ability to cause disease in 100% of hamsters (donors 1 and 2 from 574 dpi and donor 1 from 782 dpi, Table 3), and for two of these donors, 574-2 and 782-1, the infectivity was also 100% lethal for mice (Table 3). In these donors, it was unclear whether there was a single new strain with dual tropism or whether separate strains infectious for mice or hamsters had coevolved. In contrast to these results, the infectivity in brain homogenate from donor 1 from 693 dpi was poorly infectious for hamsters and appeared to have adapted nearly completely to mice (incubation period of 183 ± 22 days) (Table 3). Material from two of these third-passage mice was passaged a fourth time in mice and gave incubation periods of 118 ± 3 and 120 ± 3 days and no detectable disease in hamsters (data not shown), suggesting continuing adaptation of this strain toward virulence for mice.
TABLE 3.
Analysis of third passage in hamsters and micea
| Time of first passage (days) | Second-passage donor mice
|
Third-passage recipients
|
||||
|---|---|---|---|---|---|---|
| Hamsters
|
Mice
|
|||||
| Donor no. | PrP-res | Clinical diseaseb | Incubation period (days) | Clinical diseaseb | Incubation period (days) | |
| 130 | 1 | − | 0/12 | >400 | 0/12 | >400 |
| 130 | 2 | − | 0/12 | >400 | 0/12 | >400 |
| 463 | 1 | − | 0/12 | >400 | 0/12 | >400 |
| 463 | 2 | + | 1/12c | >400 | 0/12 | >400 |
| 574 | 1 | + | 12/12 | 215 ± 11 | 0/12 | >440 |
| 574 | 2 | + | 12/12 | 180 ± 2 | 10/10 | 314 ± 18 |
| 693 | 1 | + | 1/12d | >440 | 12/12 | 183 ± 22 |
| 782 | 1 | + | 12/12 | 197 ± 12 | 12/12 | 320 ± 10 |
Fifty microliters of a 1% brain homogenate from the indicated second-passage donors was inoculated intracerebrally into groups of hamsters and mice. Donor 1 from 782-day first passage had clinical scrapie and was sacrificed at 450 dpi. Second-passage mice from other first-passage time points had no clinical scrapie and were sacrificed between 650 and 750 days. The PrP-res status of these donor mice is indicated. Incubation period was the time from inoculation until development of obvious clinical disease.
Values are shown as numbers of animals with clinical disease/total numbers of animals.
One hamster in this group developed clinical scrapie at 231 days.
One hamster in this group developed clinical scrapie at 245 days.
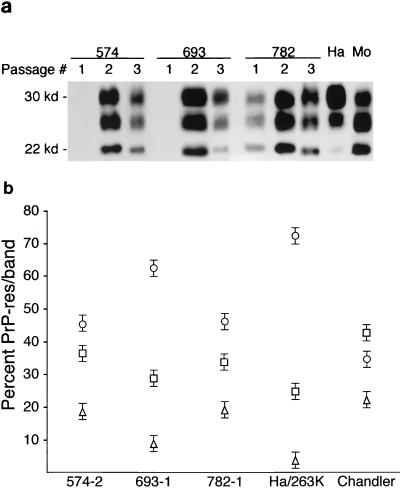
Sizing and ratios of PrP-res glycoforms on gels have previously been observed to be a possible distinguishing feature of scrapie strains (4, 9, 13). To search for possible changes in PrP glycoforms, we analyzed PrP-res from first-, second-, and third-passage mice derived from the 574-, 693-, and 782-dpi donors described in Table 3. The banding pattern of PrP-res from the mice was variable and could usually be distinguished from that seen in 263K-infected hamsters and in Chandler/RML-infected mice (Fig. 2a). When specimens from groups of third-passage mice were quantitated by PhosphorImager, the percentage of PrP-res in the bands from the 693-1 donor was significantly different from those in both the 574-2 and 782-1 donors (Fig. 2b), lending possible support to the conclusion that new strains had evolved in these passages.
FIG. 2.
(a) Immunoblot detection of PrP-res in mouse brain after one, two, or three mouse passages of hamster scrapie from the original 574-, 693-, and 782-dpi mice. First-passage lanes contain 15-mg equivalents of brain, second-passage lanes contain 5-mg equivalents, and third-passage lanes contain 1-mg equivalents. Lane 10 (Ha) contains a 1-mg equivalent of brain from a Syrian hamster with clinical scrapie induced by hamster strain 263K, and lane 11 (Mo) contains a 1-mg equivalent of brain from a mouse with clinical scrapie induced by the Chandler/RML strain. (b) Glycoform ratios of PrP-res from third-passage recipient mice. The means and standard errors of the percentages of PrP-res found in the upper (diglycosylated) (○), middle (monoglycosylated) (□), and lower (unglycosylated) (▵) bands from multiple (n = 5 or 6) third-passage mice inoculated with homogenates from second-passage donors 574-2, 693-1, and 782-1 are shown. The values for the upper and lower bands for donor 693-1 differed significantly by the Mann-Whitney U test (P < 0.001) from the corresponding values for the other two donors. Hamsters inoculated with strain 263K and mice inoculated with the Chandler/RML scrapie isolate are shown for comparison. Note that for Chandler/RML scrapie the middle band is the most abundant, and this distinguished it from all the others tested.
Histopathological analysis was also done on several of the third-passage mice to search for possible alterations in the regional distribution of pathology or PrP-res, which has previously been associated with scrapie strain differences (6). Mice studied were recipients of homogenates from the second passage of donors 574-2, 693-1, and 782-1 described in Table 3. All mice showed similar pathological changes with extensive spongiosis and astrogliosis in the brain stem, posterior colliculus, and thalamus, whereas there was less pathology in the hippocampus, cerebral cortex, and cerebellar cortex. In the recipients of the homogenate from the 693-1 donor, deposition of PrP-res was primarily in the thalamus and posterior colliculus, whereas in the other recipients heavy deposition of PrP-res was also seen in the hypothalamus, hippocampus, or brain stem (Table 4). These results provided additional support for the conclusion that different scrapie strains were evolving in these different recipients.
TABLE 4.
Distribution of PrP-res in various brain regions of third-passage mice inoculated with three different second-passage isolatesa
| Brain region | Result for isolate:
|
||
|---|---|---|---|
| 574-2 | 693-1 | 782-1 | |
| Thalamus | + | ++ | ++ |
| Brain stem | + | + | ++ |
| Posterior colliculus | ++ | ++ | ++ |
| Hypothalamus | ++ | + | ++ |
| Cerebral cortex | − | − | − |
| Cerebellum | + | − | + |
| Hippocampus | ++ | − | − |
The intensity of staining varied and was independent of the size of the positive area. Staining was most intense for isolate 782 and least intense for isolate 693. +, a few small areas of positive staining; ++, numerous areas of positive staining; −, no areas of positive staining.
DISCUSSION
In the present experiments, hamster-derived scrapie infectivity replicated in mice for two serial passages over nearly 4 years. The present results are in marked contrast to the earlier results of Kimberlin et al. (17), who concluded that 263K hamster scrapie could not replicate in mice and that only the original inoculum could persist. One likely reason for the difference with our results is that in these earlier experiments serial mouse passages were done at 319, 158, and 152 days and in our studies infectivity for second-passage mice was not detected until after 463 days (Table 2).
Our present results extended those in a recent study (11). In this earlier work, brains of two mice infected with hamster scrapie were analyzed for infectivity titer. In one of these mice, which was negative for PrP-res, the infectivity level was 1/20 of the original amount inoculated and thus might be residual inoculum. However, in the other mouse, which was positive for PrP-res, there was fivefold-more infectivity than originally inoculated, indicating that in this individual replication had probably occurred. In our analysis of infectivity in 23 mice during more than 2 years of observation, there was no evidence for replication of hamster scrapie agent for the first year postinoculation. The original inoculum was detectable several hours after infection and steadily decreased over the following months. Only after 463 days was there evidence of an increase in infectivity compared to that for the preceding days studied (Fig. 1a), and at this point there was still no detectable PrP-res. Later, there was evidence for further increases in infectivity as well as gradual adaptation toward increasing virulence for mice. Thus, in the present experiments two distinct phases were identified in asymptomatic PrP-res-negative mice, a persistent phase followed by a replicative phase, and PrP-res was not a reliable marker for either of these phases.
Apparently, adaptation for serial mouse passage is a slow process requiring extensive time in the first-passage mice. However, once in progress the adaptation that we observed was similar to that seen by Kimberlin and coworkers using this same system (15–17). At least two different strains appear to have evolved in our mice. One strain was infectious for mice but lost its virulence for hamsters (Table 3, donor 693). Another strain was virulent for hamsters but could be distinguished from the original 263K inoculum since it gave a longer incubation period in hamsters (180 to 197days) while still killing 100% of the hamsters (Table 3, donors 574-2 and 782). Infectivity for mice was also found in these same animals, giving an incubation period of 314 to 320 days. This could be either a separate strain or a manifestation of virulence for both species by a single new strain. Limiting dilution cloning will be required to distinguish between these possibilities (15).
Although adaptation of hamster scrapie to mice was noted in the second year after infection in the present experiments (Table 2), most of the infectivity present in first-passage mice maintained its virulence for hamsters. Furthermore, replication of this infectivity occurred in spite of the absence of detectable hamster PrP-res. These data would be easy to explain if the agent were known to be a conventional virus with a nucleic acid genome capable of mutation to allow adaptation to a new species. However, they are more difficult to reconcile with the protein-only hypothesis, where PrP-res might be the agent and the species tropism might be dependent on the species of the PrP used to generate the new PrP-res (8). To remain consistent with this hypothesis, one would have to speculate that the incoming hamster PrP-res would have to imprint its unique structural properties on the new PrP-res generated from mouse protease-sensitive PrP during the replication occurring over the 2-year period of these experiments (1, 3–5, 7, 9, 23). At present, there is insufficient structural information on PrP-res to be able to either validate or exclude this possibility.
The ability of TSE infectivity to both persist and adapt over such long periods may be common to many TSE agents. Furthermore, in both wildlife and agricultural settings where TSE diseases might be transferred across species barriers there could be other situations which lead to subclinical infection and unexpected adaptation or spread to additional species. For example, although sheep scrapie is thought to present no risk to humans, it may be the source of BSE in Europe (24, 25) and possibly also of CWD in the United States. If BSE were derived from sheep scrapie, then adaptation during passage in cattle may have increased its pathogenicity for humans. A similar situation could occur with CWD. CWD transmission to other cervids or livestock could change its characteristics, including its potential for transmission to people.
Although humans exposed to BSE-infected beef may be somewhat resistant to development of clinical variant Creutzfeldt-Jakob disease, as evidenced by the low number of clinically diseased people compared to the number potentially infected, there is concern that a subclinical carrier state might occur in many of these asymptomatic individuals. By analogy with the present results, this would certainly be a possibility, and if true, the danger of replication, adaptation, and further spread of the agent from these people to others might even increase at longer times postexposure. Furthermore, in the absence of precise information on the infectious dose of BSE for humans it is impossible to predict the number of possible subclinical carriers in the population. Because of the low levels of agent expressed, such a subclinical state might be detectable only by transfer of infectivity and might escape detection by present biochemical methods. Therefore, such subclinical carrier patients might pose a serious risk for contamination of surgical instruments, tissue transplants, and blood products. It will be important to be aware of these new potential risks in designing policies to prevent further spread of BSE and other TSE diseases in the coming years.
REFERENCES
- 1.Aguzzi A, Weissmann C. Spongiform encephalopathies. The prion's perplexing persistence. Nature. 1998;392:763–764. doi: 10.1038/33812. [DOI] [PubMed] [Google Scholar]
- 2.Bartz J C, Marsh R F, McKenzie D I, Aiken J M. The host range of chronic wasting disease is altered on passage in ferrets. Virology. 1998;251:297–301. doi: 10.1006/viro.1998.9427. [DOI] [PubMed] [Google Scholar]
- 3.Bessen R A, Kocisko D A, Raymond G J, Nandan S, Lansbury P T, Jr, Caughey B. Nongenetic propagation of strain-specific properties of scrapie prion protein. Nature. 1995;375:698–700. doi: 10.1038/375698a0. [DOI] [PubMed] [Google Scholar]
- 4.Bessen R A, Marsh R F. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J Virol. 1992;66:2096–2101. doi: 10.1128/jvi.66.4.2096-2101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessen R A, Marsh R F. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J Virol. 1994;68:7859–7868. doi: 10.1128/jvi.68.12.7859-7868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce M E, McConnell I, Fraser H, Dickinson A G. The disease characteristics of different strains of scrapie in Sinc congenic mouse lines: implications for the nature of the agent and host control of pathogenesis. J Gen Virol. 1991;72:595–603. doi: 10.1099/0022-1317-72-3-595. [DOI] [PubMed] [Google Scholar]
- 7.Caughey B, Raymond G J, Bessen R A. Strain-dependent differences in beta-sheet conformations of abnormal prion protein. J Biol Chem. 1998;273:32230–32235. doi: 10.1074/jbc.273.48.32230. [DOI] [PubMed] [Google Scholar]
- 8.Chesebro B. Prion protein and the transmissible spongiform encephalopathy diseases. Neuron. 1999;24:503–506. doi: 10.1016/s0896-6273(00)81105-8. [DOI] [PubMed] [Google Scholar]
- 9.Collinge J, Sidle K C, Meads J, Ironside J, Hill A F. Molecular analysis of prion strain variation and the etiology of new variant CJD. Nature. 1996;383:685–690. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- 10.Dawson M, Wells G A H, Parker B N J, Scott A C. Transmission studies of BSE in cattle, hamsters, pigs, and domestic fowl. In: Bradley R, Savey M, Marchant B, editors. Sub-acute spongiform encephalopathies. Dordrecht, The Netherlands: Commission of European Communities, Kluwer Academic Publishers; 1991. pp. 25–32. [Google Scholar]
- 11.Hill A F, Joiner S, Linehan J, Desbruslais M, Lantos P L, Collinge J. Species-barrier-independent prion replication in apparently resistant species. Proc Natl Acad Sci USA. 2000;97:10248–10253. doi: 10.1073/pnas.97.18.10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill A F, Sidle K C, Joiner S, Keyes P, Martin T C, Dawson M, Collinge J. Molecular screening of sheep for bovine spongiform encephalopathy. Neurosci Lett. 1998;255:159–162. doi: 10.1016/s0304-3940(98)00736-8. [DOI] [PubMed] [Google Scholar]
- 13.Kascsak R J, Rubenstein R, Merz P A, Carp R I, Robakis N K, Wisniewski H M, Diringer H. Immunological comparison of scrapie-associated fibrils isolated from animals infected with four different scrapie strains. J Virol. 1986;59:676–683. doi: 10.1128/jvi.59.3.676-683.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kascsak R J, Rubenstein R, Merz P A, Tonna-DeMasi M, Fersko R, Carp R I, Wisniewski H M, Diringer H. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987;61:3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimberlin R H, Walker C A. Evidence that the transmission of one source of scrapie agent to hamsters involves separation of agent strains from a mixture. J Gen Virol. 1978;39:487–496. doi: 10.1099/0022-1317-39-3-487. [DOI] [PubMed] [Google Scholar]
- 16.Kimberlin R H, Walker C A. Pathogenesis of scrapie: agent multiplication in brain at the first and second passage of hamster scrapie in mice. J Gen Virol. 1979;42:107–117. doi: 10.1099/0022-1317-42-1-107. [DOI] [PubMed] [Google Scholar]
- 17.Kimberlin R H, Walker C A, Fraser H. The genomic identity of different strains of mouse scrapie is expressed in hamsters and preserved on reisolation in mice. J Gen Virol. 1989;70:2017–2025. doi: 10.1099/0022-1317-70-8-2017. [DOI] [PubMed] [Google Scholar]
- 18.Race R, Chesebro B. Scrapie infectivity found in resistant species. Nature. 1998;392:770. doi: 10.1038/33834. [DOI] [PubMed] [Google Scholar]
- 19.Race R, Jenny A, Sutton D. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J Infect Dis. 1998;178:949–953. doi: 10.1086/515669. [DOI] [PubMed] [Google Scholar]
- 20.Race R E, Priola S A, Bessen R A, Ernst D R, Dockter J, Rall G F, Mucke L, Chesebro B, Oldstone M B A. Neuron-specific expression of a hamster prion protein minigene in transgenic mice induces susceptibility to hamster scrapie agent. Neuron. 1995;15:1183–1191. doi: 10.1016/0896-6273(95)90105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raymond G J, Bossers A, Raymond L D, O'Rourke K I, McHolland L E, Bryant III P K, Miller M W, Williams E S, Smits M, Caughey B. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J. 2000;19:4425–4430. doi: 10.1093/emboj/19.17.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor D M, Dickinson A G, Fraser H, Marsh R F. Evidence that transmissible mink encephalopathy agent is biologically inactive in mice. Neuropathol Appl Neurobiol. 1986;12:207–215. doi: 10.1111/j.1365-2990.1986.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 23.Telling G C, Parchi P, DeArmond S J, Cortelli P, Montagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P, Prusiner S B. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science. 1996;274:2079–2082. doi: 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 24.Wilesmith J W, Ryan J B, Hueston W D, Hoinville L J. Bovine spongiform encephalopathy: epidemiological features 1985 to 1990. Vet Rec. 1992;130:90–94. doi: 10.1136/vr.130.5.90. [DOI] [PubMed] [Google Scholar]
- 25.Wilesmith J W, Wells G A H, Cranwell M P, Ryan J B M. Bovine spongiform encephalopathy: epidemiological studies. Vet Rec. 1988;123:638–644. [PubMed] [Google Scholar]