Abstract
With advancements in antiretroviral therapy (ART), the average lifespan of people with human immunodeficiency virus (HIV) is increasing, as is the number of older adults with HIV. Accordingly, the number of patients with HIV who undergo surgery or require critical care for various reasons is increasing. Since the prognosis of people with HIV depends on the continuous and effective maintenance of ART, there is a need to consider effectively maintaining ART in people with HIV in these conditions. This case involved a 55-year-old patient with well-controlled HIV who received ART and presented to the emergency room with acute abdominal pain. He was diagnosed with extensive bowel infarction and panperitonitis and received critical care in the intensive care unit, including mechanical ventilation and continuous renal replacement therapy. The patient was administered enteral nutrition via a nasogastric tube. The patient subsequently underwent extensive small bowel resection and developed short bowel syndrome. The patient maintained ART during that period. A literature review related to the use of ART under these conditions is included in this study. This case was discussed at the [Exploring Difficult Cases in HIV Clinics] of the Korean Society for AIDS Conference held in 2023.
Keywords: HIV infection, Antiretroviral therapy, Surgical care
Introduction
The average lifespan of people with human immunodeficiency virus (HIV) has increased with the development of antiretroviral therapy (ART) [1,2]. Therefore, although newly diagnosed HIV infections are occurring less frequently than in the past, the older adult population is growing [3,4,5]. Accordingly, the prevalence of various non-communicable diseases (NCDs) among people with HIV is also rising [6,7,8,9]. Recently, HIV clinics have emphasized the management of NCDs in patients with HIV [10,11,12,13,14,15].
As various NCDs occur more frequently in individuals with HIV, the number of cases requiring surgical treatment to resolve NCDs is also increasing [16]. However, there is a lack of robust recommendations and research results regarding how ART should be administered to patients with HIV before and after surgery, particularly gastrointestinal surgery. Although these cases are not yet common in HIV clinics, the number of cases to be faced is expected to increase in the future.
It is well-known that uninterrupted ART administration is essential to maintaining a good plasma concentration of antiretrovirals (ARVs) [17,18,19]. ART inhibits the replication of HIV and suppresses the development of resistance to maintain a person's immunity [20,21]. However, in some cases of long-term surgical care, it may be challenging to use the original tablet-formulating ARVs. There is a need to report the appropriate use of ART in patients with HIV who have undergone gastrointestinal surgery requiring long-term surgical care. Therefore, we report on the experience of using ART in people with HIV who need long-term surgical care and related discussions at the Korean Society for AIDS Conference held in 2023.
Case presentation and the minutes of the conference
This case was discussed at the [Exploring Difficult Cases in HIV Clinics] of the Korean Society for AIDS Conference held in 2023. The discussion began with the presenter’s inquiries to the audience. Then, the moderator asked the panelists to participate in the discussion, and the panelists responded to the questions. The audience was also engaged in the discussions. The moderator, presenter, and panelists participating in the discussions are shown in Table 1. The following are the case presentation and minutes from the conference. Questions and panel discussions were provided during the case presentation to help readers understand.
Table 1. Participants in the [Exploring Difficult Cases in HIV Clinics] discussion.
| Moderator | |
|---|---|
| Tae Hyong Kim (Soonchunhyang University College of Medicine) | |
| Case presenter | |
| Jung Ho Kim (Yonsei University University College of Medicine) | |
| Panelists | |
| Youn Jeong Kim (Catholic University College of Medicine) | |
| Won Suk Choi (Korea University College of Medicine) | |
| Yong Pil Chong (University of Ulsan College of Medicine) |
A 55-year-old man visited the emergency room with abdominal pain that started on the morning of the day of the visit. He reported that he had consumed 600 mL of liquor with an alcohol content of 55 or higher over approximately 2 hours (from 6 PM to 8 PM) the day before visiting the hospital. He was alert but complained of abdominal pain upon review of system. On physical examination, his blood pressure was 187/86 mmHg, heart rate 91 beats/min, respiratory rate 20 breaths/min, and body temperature 37.8°C. Direct and rebound tenderness were observed throughout the abdomen.
His HIV infection was first confirmed in December 2007, and combination ART was initiated in November 2010. Lamivudine (3TC) + zidovudine + lopinavir/ritonavir was used as the initial treatment, and the virus was well controlled thereafter. No history of resistance testing for ARVs has yet been identified. He was prescribed elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine (EVG/c/TDF/FTC) from December 2014, and EVG/c/tenofovir alafenamide/FTC (EVG/c/TAF/FTC) was used from August 2017. He was maintaining EVG/c/TAF/FTC until the time of admission to the emergency room, and the last test performed before visiting the emergency room in September 2019 was HIV RNA <20 copies/mL and CD4 T cell count 747/µL. Additionally, he was taking medications for hypertension and diabetes mellitus.
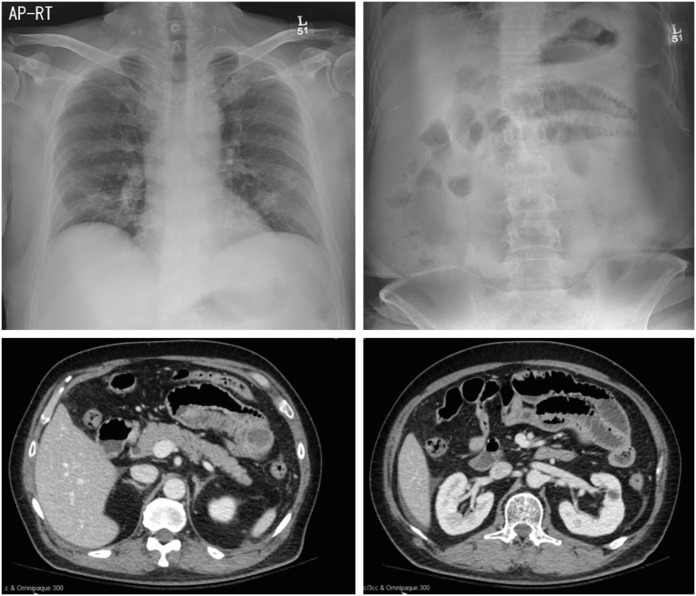
An initial blood test showed that he had a white blood cell count of 6,510/mm3, hemoglobin concentration of 13.1 g/dL, platelet count of 340,000/mm3, serum blood urea nitrogen/creatinine level of 21.0/1.20 mg/dL (estimated glomerular filtration rate 58 mL/minute 1.73 m2), and C-reactive protein level of 51.4 mg/L. Initial chest and abdominal radiography and abdominal computed tomography (CT) images are shown in Figure 1.
Figure 1. Initial chest and abdominal radiography and abdominal computed tomography images.
Abdominal CT scan revealed diffuse mild wall thickening of the small bowel loops and ascites in the perihepatic space and both paracolic gutters. Based on these findings, the patient was diagnosed with enteritis of the small bowel, acute kidney injury, pre-renal type, and well-controlled HIV infection on EVG/c/TAF/FTC. The treatment plan consisted of fasting and hydration.
1. The patient decided to fast and take only oral medications due to abdominal pain accompanying enteritis. How can ART be used?
Youn Jeong Kim: Since the patient is fasting, it may be possible to use ART, regardless of meals. I could try switching to bictegravir/TAF/FTC (BIC/TAF/FTC) or dolutegravir/abacavir/3TC (DTG/ABC/3TC), which can be taken irrespective of meals.
Won Suk Choi: I agree with Professor Youn Jeong Kim. Additionally, because the patient had mild acute kidney injury but was a pre-renal type that could recover after hydration, and the creatinine level was not very high, it appears that ART can be used without dosage adjustments to current renal function.
Jung Ho Kim: Because BIC/TAF/FTC was available at the time of admission and could be administered regardless of meals, the patient was switched to BIC/TAF/FTC.
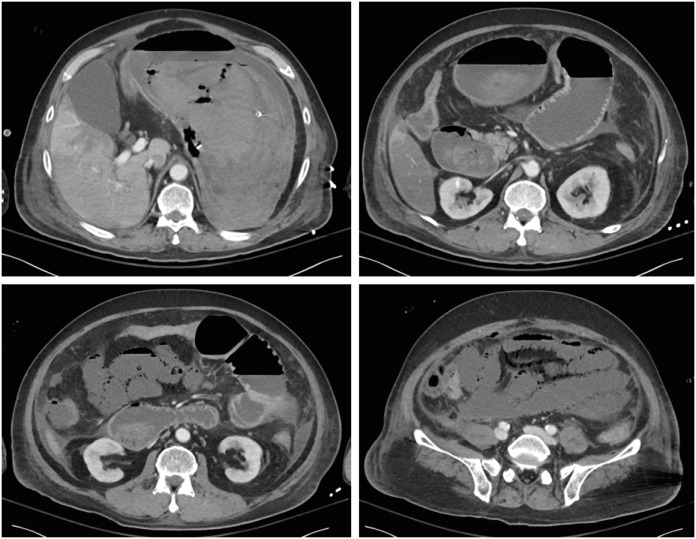
On the 5th day of admission, the patient experienced a large amount of hematemesis, resulting in a decrease in blood pressure, acute kidney injury, and respiratory failure. Endotracheal intubation was performed, continuous renal replacement therapy (CRRT) was initiated, and a nasogastric tube was inserted. A follow-up abdominal CT was performed to determine the presence of active bleeding sites (Fig. 2).
Figure 2. Follow-up computed tomography images of the abdomen.
Abdomianl CT scan shows a large hematoma in the stomach and duodenum but no definite contrast extravasation. Additionally, relatively large free air abutting a loop of the small bowel in the left lower abdomen was suspected of bowel perforation in the area, as well as non-enhancement of the small and large bowel walls from the mid jejunum to the distal ascending colon. Considering these findings, the patient had an extensive bowel infarction from the mid jejunum to the distal ascending colon and pneumoperitoneum, likely from the ischemic bowel.
2. How can ART be administered considering the status of nasogastric tube insertion due to endotracheal intubation?
Youn Jeong Kim: Because injectable ARVs are not available in Korea, the use of injectable drugs is not possible even in situations where a nasogastric tube is inserted. The original tablet formulation is also considered difficult to use, considering the state of endotracheal intubation and nasogastric tube insertion. I think I will dissolve the current ART and use it in a nasogastric tube.
Won Suk Choi: As such cases may occur in the future, it is necessary to introduce injectable ARVs in addition to oral drugs. In this patient, even if ARVs are administered via a nasogastric tube, it is questionable whether the drug would be absorbed well in such a deconditioned intestine. In cases like this patient with severe intestinal necrosis, there are concerns about absorption; therefore, it is necessary to introduce injectable ARVs.
Yong Pil Chong: I have experience in using crushed DTG/ABC/3TC. This patient had severe ileus; therefore, I am worried about whether the ARVs would be absorbed well. There are concerns regarding its absorption as an underdose and the development of resistance. There is also concern that renal function may worsen. Considering these factors, discontinuing ART may be worth considering because ARVs have recently improved, and it is not common for resistance to develop after stopping for a short period of time.
Jung Ho Kim: The patient was unable to use ART for one week due to urgent surgery for intestinal necrosis, perforation, and panperitonitis. Subsequently, the patient was administered BIC/TAF/FTC dissolved in water via a nasogastric tube.
3. How should ART be administered to the patient receiving continuous renal replacement therapy?
Youn Jeong Kim: There are limited data on the use of ART in patients receiving CRRT. However, TAF and BIC have a high protein-binding rate, and BIC/TAF/FTC is advised to be administered without dose reduction if used during hemodialysis; therefore, the same dose of BIC/TAF/FTC is likely to be administered during CRRT.
Yong Pil Chong: In general, using ART while receiving CRRT is likely to last for a short period, so there is no significant burden of overdosing on ARVs; rather, there are concerns about insufficient concentrations of ARVs in the body. In relation to the previous question, stopping ART for a short period could also be considered.
Jung Ho Kim: There are no data on the administration of ART to patients undergoing CRRT. TAF and FTC can be partially removed during CRRT, but this is insufficient to have a clinical impact. Therefore, the previously administered BIC/TAF/FTC regimen was maintained at the same dose for this patient.
As the hematemesis subsided and blood pressure stabilized, he underwent surgical treatment for peritonitis, intestinal ischemia, and intestinal perforation. Surgical findings included total necrosis of the small bowel and necrosis of the proximal ascending colon (Fig. 3).
Figure 3. Surgical specimens showing necrosis of the small bowel.
The patient underwent segmental resection of the small bowel from the proximal jejunum to the distal ileum (approximately 4 m), mobilization of the ascending colon, and an ileocecectomy. He underwent two additional surgeries, and the last surgery created a jejuno-transverse colostomy.
4. Which ART regimens can be considered for the patient with extensive small bowel resection and jejuno-transverse colostomy?
Won Suk Choi: I think there needs to be some information on where ARVs are absorbed in the gastrointestinal tract. Given the limited information, I believe the basic option is to maintain the existing ART. If injectable ARVs are possible, this may be considered a priority without concerns regarding gastrointestinal tract absorption.
Youn Jeong Kim: Considering the absorption issues in the gastrointestinal tract, ARVs with high resistance barriers are likely to be selected.
Yong Pil Chong: I prefer DTG/ABC/3TC, which has a little more data. It is important to determine which part of the gastrointestinal tract is the main part that absorbs ARVs. It seems acceptable to use NRTIs such as 3TC, TAF, and ABC, as most of them are absorbed in the duodenum. In the case of DTG/BIC/darunavir (DRV), an important factor is how well absorption occurs in the absence of the small intestine. In the case of DTG, which can be prescribed without meals, the absorption rate increases significantly by up to 50% when consumed with a meal. Therefore, it is possible to administer DTG/ABC/3TC with a meal.
Jung Ho Kim: In this patient, the primary selection points were ARVs with a high resistance barrier that were absorbed outside the jejunum or ileum, if possible. Consequently, BIC/TAF/FTC was maintained.
He gradually recovered, and ART was changed back to a tablet formulation following tracheostomy site seal-off and resumption of oral diet. He was discharged from the hospital and maintained regular parenteral nutrition and electrolyte replacement through home nursing due to short bowel syndrome.
Discussion
This case study describes the use of ART in a patient with HIV who underwent extensive gastrointestinal surgery and subsequent long-term surgical care. The effective use of ART for these patients was discussed at the 2023 Korean Society for AIDS Conference. As the number of patients with HIV undergoing surgery, especially gastrointestinal surgery, is expected to increase in the future, we have summarized how ART is administered in such cases. It was structured to review several major topics in accordance with the flow of the case presentations and the minutes of the conference.
1. How to use antiretroviral therapy when fasting is expected due to surgery, enteritis, or pancreatitis?
The discontinuation or interruption of ART can lead to viral rebound, weakening of the immune system, and potential worsening of clinical conditions [22,23,24,25,26]. Therefore, interruption of ART is not recommended. However, ART may be interrupted within a short period in certain situations. Short-term interruptions in ART, ranging from days to weeks, can occur for various reasons, such as illnesses that prevent oral intake (e.g., gastroenteritis or pancreatitis) or surgical procedures. Halting ART for a short time (less than 2 days) can generally be managed by temporarily holding all drugs in the regimen. When patients cannot take medications through any enteral route because of severe gastrointestinal disease, the Department of Health and Human Services (DHHS) guidelines recommend stopping all components of the ART regimen simultaneously, despite the differing half-lives of the drugs [17]. Once the issue is resolved, all the components should be restarted together. However, in the case of efavirenz (EFV) and rilpivirine (RPV), which are non-nucleoside reverse transcriptase inhibitors (NNRTIs) with considerably longer half-lives than those of other ARVs [27,28], caution is required because of the risk of functional monotherapy when the drug is discontinued. If ART interruption is expected, preemptively changing EFV or RPV to another drug, such as boosted protease inhibitors (PIs), or stopping EFV or RPV first and then stopping all other agents in the regimen 2–4 weeks later is possible. However, this is difficult to achieve when a sudden interruption is necessary. Long-term interruption of ART for more than 2 weeks is not recommended. Injectable ARVs such as carbotegravir and RPV may be useful when long-term fasting is required.
In clinical practice, there are cases in which a patient cannot eat food but can take medication. In particular, there may be cases where long-term fasting (two weeks or more) is necessary. In this situation, interactions between food and ARVs should be considered. Some ARVs can be taken regardless of meals, and for some ARVs, the absorption of the drug and subsequent drug plasma concentration may be affected by whether the drug is taken with a meal. Among NNRTIs, doravirine (DOR) can be administered without regard to food [29]. EFV is recommended to be consumed on an empty stomach because food increases drug absorption and central nervous system toxicities [30], whereas RPV is recommended to be consumed with a meal [31]. Additionally, RPV requires caution because it is contraindicated in combination with proton pump inhibitors [31], which are commonly used during fasting. Among PIs, the bioavailability of atazanavir (ATV) and DRV also increases when consumed with food [32,33]. Currently, widely used integrase strand transfer inhibitors (INSTIs), such as BIC, DTG, or raltegravir, do not have food requirements; however, for EVG, it is recommended that the medication be taken with meals. According to a study conducted in Japan, the administration of EVG/c/TDF/FTC under fasting conditions decreased the mean area under the curve (AUC) of EVG and TDF by 50% and 28%, respectively, relative to the administration of a standard meal [34]. The study concluded that under fasting conditions, the bioavailabilities of EVG and TDF were not equivalent to those when they were administered with food [34]. Therefore, caution should be exercised when using DRV-, ATV-, or EVG-based regimens when long-term fasting is expected. Table 2 presents ARVs that require food consideration when taking.
Table 2. Antiretrovirals that require food consideration.
| Antiretrovirals | Food consideration |
|---|---|
| EFV-based regimens | Food increases the absorption of EFV and potentially increases its central nervous system side effects |
| RPV-based regimens | Food improves absorption. RPV-based regimens should be taken with at least 390 food calories to ensure proper absorption |
| ATV/r- or ATV/c-based regimens | Food improves absorption |
| DRV/r- or DRV/c-based regimens | Food improves absorption |
| EVG/c/TDF/FTC | Food improves absorption |
| EVG/c/TAF/FTC | Food improves absorption |
EFV, efavirenz; RPV, rilpivirine; ATV/r, ritonavir-boosted atazanavir; ATV/c, cobicistat boosted atazanavir; DRV/r, ritonavir boosted darunavir; DRV/c, cobicistat boosted darunavir; EVG/c/TDF/FTC, elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine; EVG/c/TAF/FTC, elvitegravir/cobicistat/tenofovir alafenamide/emtricitabine.
2. How to use antiretroviral therapy if people with HIV have a nasogastric tube or have swallowing difficulties that make it difficult to take the original tablet form?
If people with HIV are intubated or have swallowing difficulty for one reason or another, it is difficult to take ARVs in regular tablet form. In many regions worldwide, including Korea, injectable ARVs have yet to be introduced; therefore, an alternative way of using the existing tablet formulation is needed. The European AIDS Clinical Society (EACS) guidelines version 12.0 provide a well-organized table for ARV administration in patients with swallowing difficulties [18]. Several reviews have discussed the administration of ARVs in cases of enteral feeding [35,36]. In addition to the data presented in the guidelines, the pharmacokinetics (PK) parameters of some dissolved or crushed ARVs have been reported. Here, we summarize the information about some ARVs.
For BIC/TAF/FTC, EACS guidelines version 12.0 states that tablets should be swallowed whole and should not be chewed, crushed, or split. Following this recommendation, it is difficult to use in people who are intubated or have difficulty swallowing. However, this was due to insufficient supporting data on the PK parameters when dissolving or crushing BIC/TAF/FTC. Hocqueloux et al. conducted a crossover randomized trial in healthy adults and investigated the bioavailability of dissolved and crushed BIC/TAF/FTC [37]. According to the study, BIC/TAF/FTC tablets are completely dissolved in 240 mL of water in a plastic bottle at room temperature for 6–7 minutes. For people who have nasogastric tubes, the following steps were recommended:
1) Flush the tube with 30 mL of water.
2) Administer the dissolved BIC/TAF/FTC formulation in 240 mL of water.
3) Flush the tube with 30 mL of water.
In the case of enteral feeding, separating the administration of BIC/TAF/FTC from enteral feeds by 4 hours is recommended because of the risk of interaction with polyvalent metal cations. The Cmax of the dissolved tablet was 105%/97%/96%, and the AUC was 111%/100%/99%, respectively. The Cmax of the crushed tablet was 110%/70%/66%, and the AUC was 107%/86%/84%, respectively. Dissolving BIC/TAF/FTC in water may be acceptable; however, crushing BIC/TAF/FTC tablets may lead to suboptimal TAF and FTC concentrations. According to the results of this study, if the BIC/TAF/FTC tablet cannot be swallowed whole, dissolving it in water and taking it immediately could be considered, and there were no actual problems in this case.
The DTG-containing regimen, which was released in the market before the BIC-containing regimen, was also studied for its bioavailability when crushed. Roskam-Kwint et al. investigated the effect of a crushed DTG-based fixed-dose combination of DTG/ABC/3TC and administered enteral nutrition [38]. As a result, crushing DTG/ABC/3TC leads to higher DTG exposure (AUC: +26% and Cmax: +30%) and a decrease in ABC Cmax (-17%). The 3TC concentration was not significantly affected. Higher DTG exposure did not exceed DTG intake with food or twice-daily dosing. Therefore, the authors concluded that DTG/ABC/3TC can be crushed and administered with enteral nutrition. Based on the results of this study, although not studied separately, it is expected that the single-tablet regimen (STR) of DTG/3TC can also be crushed and used for enteral nutrition.
An oral suspension form for DRV, which has the highest resistance barrier among PIs, is available in some countries. However, in countries where access to oral suspensions is unavailable, including Korea, DRV should be administered as a tablet formulation. There have been several published case reports in which ritonavir boosted darunavir (DRV/r) was crushed and used in patients with difficulty swallowing [39,40,41]. These studies reported no specific problems with serum DRV concentrations or viral suppression, even when DRV/r was crushed and used with an enteral feeding tube. Research results on DRV/c/TAF/FTC STR have been reported [42]. In this study, the DRV/c/TAF/FTC STR was split or crushed and compared with the whole swallowed STR, which is the reference method. The split showed comparable bioavailability of each DRV/c/TAF/FTC component to that of the whole tablet. In contrast, the crushed tablet showed comparable bioavailability to DRV/c, but the relative bioavailability of TAF decreased by 29% Cmax and 19% AUC compared to the whole tablet. The authors noted that the clinical significance of this change has not yet been evaluated, but it is anticipated to be minor, given the broad therapeutic range of this agent. Summarizing the results of this study, similar to the results of the BIC/TAF/FTC study above, in the case of TAF, a decrease in concentration may occur when crushed. However, at least in the case of DRV/c, it can be used by splitting or crushing. Therefore, although the manufacturer does not recommend crushing tablets, EACS guidelines 12.0 states that crushed DRV/c tablets can be used [18].
For DOR, the newest NNRTI, there are still no PK data regarding its use when crushed or dissolved. There is a case report that viral suppression was maintained in a person with HIV with the M184V mutation using crushed DOR with DTG and 3TC in a nasojejunal feeding tube [43]. However, viral suppression may have also been maintained by the co-administration of DTG. As the drug concentration for DOR could not be measured, it was impossible to compare the bioavailability of crushed DOR with that of whole tablets. Accordingly, the EACS guidelines 12.0 also recommend that the tablet be swallowed whole in the case of the DOR and DOR-based STR, DOR/TDF/3TC [18].
Table 3 shows information about representative ARVs, which can be or cannot be used by crushing or dissolving, and the references. If injectable ARTs are available, they can also be considered.
Table 3. Antiretrovirals that can be or cannot be used by crushing or dissolving.
| Antiretrovirals | Information | References | |
|---|---|---|---|
| STR | |||
| BIC/TAF/FTC | Dissolving in water and taking it immediately can be considered | [37] | |
| DTG/ABC/3TC | Can be crushed and administered with enteral nutrition | [38] | |
| DTG/3TC | Can be crushed and administered with enteral nutrition | [38] | |
| DOR/TDF/3TC | There has yet to be PK data on crushed and dissolved. The tablet must be swallowed whole | [43] | |
| NRTI | |||
| TAF/FTC | Dissolving in water and taking it immediately can be considered. Crushing tablets could reduce the TAF bioavailability but does not significantly impact TAF/FTC PK | [37,42] | |
| TDF/FTC | Dissolving in water and taking it immediately can be considered | [18] | |
| 3TC | Can be crushed and administered with enteral nutrition | [38] | |
| FTC | Dissolving in water and taking it immediately can be considered | [37] | |
| ABC | Can be crushed and administered with enteral nutrition | [38] | |
| INSTI | |||
| DTG | Can be crushed and administered with enteral nutrition. Crushing DTG leads to higher DTG exposure but does not exceed exposure intake with food or twice-daily dosing | [38] | |
| RAL | Chewing the tablets has higher drug absorption and lower drug inter-subject PK variability | [44] | |
| PI | |||
| DRV | Can be crushed and administered with enteral nutrition | [39,40,41] | |
| DRV/c | Can be crushed and administered with enteral nutrition | [42] | |
| LPV/r | Significantly lower AUC with crushed tablets | [45] | |
| NNRTI | |||
| DOR | Crushing or dissolving tablets is not recommended | [43] | |
| ETV | Dispersed in ≥5 mL water showed bioequivalence to the whole tablet | [46] | |
STR, single-tablet regimen; BIC, bictegravir; TAF, tenofovir alafenamide; FTC, emtricitabine; DTG, dolutegravir; ABC, abacavir; 3TC, lamivudine; DOR, doravirine; TDF, tenofovir disoproxil fumarate; PK, pharmacokinetics; NRTI, nucleoside reverse transcriptase inhibitors; INSTI, integrase strand transfer inhibitor; RAL, raltegravir; PI, protease inhibitor; DRV, darunavir; DRV/r, ritonavir boosted darunavir; DRV/c, cobicistat boosted darunavir; LPV/r, ritonavir boosted lopinavir; NNRTI, non-nucleoside reverse transcriptase inhibitors; ETV, etravirine.
3. How to use antiretroviral therapy if people with HIV receive continuous renal replacement therapy?
Regarding the use of ART while on CRRT, both the EACS guidelines 12.0 and the DHHS guidelines only mention chronic hemodialysis but do not provide information about CRRT [17,18]. The clinical practice guidelines for chronic kidney disease in patients infected with HIV were revised in 2014 by the Infectious Diseases Society of America [47], but they did not mention how to administer ART when receiving CRRT. This is because no research has been conducted on how CRRT affects the drug concentration of ARVs or the effectiveness of ART in patients with HIV during CRRT. The reasons for this may be that 1) there are only a small number of people with HIV who undergo CRRT, 2) it is difficult to research those who are likely to be critically ill, and 3) CRRT is generally used for a short period, so the need for research is relatively low. Moreover, in the case of CRRT, drug clearance depends on the patient's remaining renal function, effluent flow rate, filter type, and renal replacement method; therefore, it is difficult to make recommendations based on the same criteria. Nevertheless, the number of people with HIV who require CRRT may increase in the future [48,49], and in such cases, it would be helpful to describe at least a little about how to administer ART while on CRRT.
Generally, drugs with a high protein-binding are not removed by CRRT [50]. As in this presented case, in the case of BIC/TAF/FTC, although the protein binding of FTC is lower than 4%, the protein binding of BIC is over 99%, and the protein binding of TAF reaches 80%. Therefore, although many portions of FTC and some portions of TAF can be removed during CRRT, BIC/TAF/FTC can be used without dosage adjustment because the protein binding of BIC and TAF is high. Similar to BIC, DTG and DRV, which also have very high rates of protein binding, they can be used without dosage adjustments during CRRT. Although CRRT is generally conducted for a short period of time, when ART is administered during CRRT, it is crucial to monitor whether there are any adverse events and whether it is effective.
4. How to use antiretroviral therapy in people with HIV who have undergone gastrointestinal resection?
When using ART in patients who have undergone gastrointestinal resection, it is necessary to consider where the ARVs are mainly absorbed in the gastrointestinal tract and which part is removed. Depending on the site, gastrointestinal resection can be classified as gastrectomy, small bowel resection, or colon resection. Among these, most data are from gastrectomies, which are also performed for weight loss, as weight gain has recently become an issue in people with HIV [51,52].
Differences exist depending on the extent of stomach removal during gastrectomy; however, gastrectomy generally results in decreased gastric volume, absorptive surface area, motility, and gastric pH increases [53]. Therefore, it is difficult to use ATV and RPV in patients who have undergone gastrectomy because absorption may be impaired due to increased gastric pH [54,55]. Studies have reported the PK of ARVs in patients who have undergone gastrectomies. Although most studies are case reports or case series, these data can be helpful when limited data are available. Generally, PK changes are noticeable during the early postoperative period [56]. Therefore, when examining research results on the PK of ARVs after gastrectomy, it is necessary to consider the timing of sampling performed after surgery. Information on PK after gastrectomy for ARVs, commonly used in clinical practice, is summarized below.
Tempestilli et al. reported their experience with BIC/TAF/FTC in a patient who underwent Billroth II gastrectomy, followed by Roux-en-Y reconstruction for gastric cancer [57]. They investigated drug plasma concentration in patients using BIC/TAF/FTC 2 months after surgery and found a modest decrease in the PK parameters of BIC compared with population references, without consequences on antiviral efficacy. They also found an increase in TAF and FTC plasma concentrations, suggesting high bioavailability of both ARVs despite gastrectomy. Therefore, they concluded that a fixed-dose combination of BIC/TAF/FTC effectively and safely maintained high CD4 cell counts and stable plasma viral suppression without adverse events in a patient with HIV who underwent gastrectomy. However, because this study measured drug plasma concentrations 2 months after surgery, it did not address drug plasma concentrations in the early postoperative period.
A case series also reported the use of DTG in patients with HIV who underwent gastric bypass surgery [58]. According to this study, patients who underwent gastric bypass achieved long-term DTG concentrations that were comparable to historical data. In the initial postoperative period, 3 of the 4 people with HIV who participated in the study had DTG levels in the expected range for once-daily administration. In the remaining 1 person with HIV, the DTG level, measured 2 weeks after surgery, 0.5 mg/mL at 11.5 hours after once-daily administration. Due to the risk of inadequate drug concentration at the end of the dosing period, the DTG dosage was increased to 50 mg twice-daily. Two months after surgery, the DTG level on this twice-daily schedule was satisfactory. Then, the dosage was reduced to 50 mg once-daily again, consistently resulting in optimal DTG levels. To summarize this evidence, DTG could be used once-daily during the initial postoperative period. If there are concerns about absorption and concentration, DTG twice-daily can be considered for the first 2 months after surgery. This finding highlights the need to investigate PK at various time intervals after gastric bypass surgery. Mineral supplementation can be administered after bariatric surgery. When INSTIs such as BIC and DTG are used, they should be separated from the mineral supplement intake to prevent INSTIs malabsorption resulting from their chelation with minerals [56].
Studies on the use of DRV/r after gastrectomy have also been reported [59,60]. Similar to DTG, the study showed a temporary decrease in the plasma concentration of DRV/r after 3 days post-surgery; however, the concentration required to inhibit viral replication was maintained, and after 10 weeks post-surgery, the concentrations were comparable with reference. Therefore, DRV/r can also be used in patients who have undergone gastrectomy, and, similar to DTG, the use of DRV/r twice can be considered in the first 2 months after surgery.
The results of commonly used NRTIs, such as 3TC, FTC, TDF, and TAF, vary depending on the study; however, similar to the ARVs mentioned above, a transient and reversible decrease in plasma concentration is generally observed in the early postoperative period, without any consequences on CD4 cells and HIV viral load. Subsequently, the plasma concentration was maintained without significant problems [60,61,62]. Therefore, there is a high possibility that there will be no specific problems when using DTG or DRV as the core drug in combination with NRTIs in patients undergoing gastrectomy. However, when TDF/FTC was administered as pre-exposure prophylaxis (PrEP) in a patient who underwent total gastrectomy with Roux-en-Y anastomosis, the standard dose led to a reduced AUC and Cmin for both substances compared to population references, potentially compromising the effectiveness of PrEP [63]. Therefore, when using TDF/FTC for PrEP in patients who have undergone gastrectomy, it would be helpful to monitor its concentration through therapeutic drug monitoring (TDM), if available. The current evidence for ART use in patients who have undergone gastrectomy can be summarized as follows:
1) Gastrectomy generally affects the absorption of ARVs by reducing gastric volume and absorptive surface area, lowering gastric motility, and increasing gastric pH.
2) PK changes are significant in the early postoperative period, and the plasma concentrations of most ARVs decrease during this period. Twice dosing of DTG or DRV can be considered during this period (for 2 months after surgery).
3) Nevertheless, the risk of treatment failure does not increase in commonly used ART, and the plasma concentration of ARVs often recovers over time.
4) When using INSTIs, avoid simultaneous administration with mineral supplements, as this may decrease their absorption.
5) If possible, TDM can help determine the dosing.
The case covered in this paper is a case of ART for short bowel syndrome caused by extensive small bowel resection. When the small bowel is resected, the effect on absorption may vary depending on the extent of resection. The wider the resection range, the smaller the absorbable area, and as the intestinal transit time decreases, absorption may become more difficult. Additionally, several mechanisms may affect drug absorption after small bowel resection [64,65]. However, extensive small bowel resection in patients receiving ART is rare, and related studies are limited. Only one case report has been published in Japan [66]. This case report describes the use of ART in a patient with short bowel syndrome who underwent subtotal small bowel resection for non-occlusive mesenteric ischemia. In the patient, DRV, etravirine, LPV, maraviroc, RAL, and ritonavir were administered, and plasma concentrations were measured. As a result of multiple measurements, a sufficient plasma concentration was not maintained; therefore, multiple dosing was performed. Although this case was limited in that it did not include drugs such as 2nd generation INSTIs, it can be said that it is difficult to maintain the plasma concentration of ARVs in patients with extensive small bowel resection, which means that there is a high need for injectable ARVs in these patients.
The effects of colon resection on the absorption of ARVs are limited. This is because most ARVs are absorbed in the small intestine, including the duodenum, jejunum, and ileum. Therefore, ART can be administered without dosage adjustments, even in patients undergoing total colectomy. More information regarding ARV absorption after gastrointestinal surgery is well documented in ‘Prescribing Resources’ provided by the University of Liverpool [67].
In conclusion, as the average lifespan of people with HIV increases, the number of people with HIV and various comorbidities is expected to increase. Accordingly, the number of people with HIV who require critical care, such as mechanical ventilation and CRRT or who undergo gastrointestinal resection for cancer or obesity, is expected to increase. As the prognosis of people with HIV depends on the continuous and effective maintenance of ART, physicians treating HIV need to be aware of the effective maintenance of ART in this population, and this field still requires more research and data.
Footnotes
Funding: This work was supported by the National Institute of Infectious Diseases, National Institute of Health, Korea Disease Control and Prevention Agency (2022-ER1907-00).
Conflict of Interest: JYC is editorial board of Infect Chemother; however, he did not involve in the peer reviewer selection, evaluation, and decision process of this article. Otherwise, no potential conflicts of interest relevant to this article was reported.
- Conceptualization: JHK, NSK, JYC.
- Data curation: JHK, NSK.
- Formal analysis: JHK, JES, SA, YL, JAL, JYA, SJJ, NSK, J-SY, JYC.
- Methodology: JHK, JES, SA, YL, JAL, JYA, SJJ, NSK, J-SY, JYC.
- Project administration: JYC.
- Supervision: NSK, JYC.
- Writing - original draft: JHK.
- Writing - review & editing: JHK, JES, SA, YL, JAL, JYA, SJJ, NSK, J-SY, JYC.
This case was discussed at the [Exploring Difficult Cases in HIV Clinics] of the Korean Society for AIDS Conference held in 2023.
SUPPLEMENTARY MATERIAL
Korean version
References
- 1.Katz IT, Maughan-Brown B. Improved life expectancy of people living with HIV: who is left behind? Lancet HIV. 2017;4:e324–e326. doi: 10.1016/S2352-3018(17)30086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trickey A, Sabin CA, Burkholder G, Crane H, d'Arminio Monforte A, Egger M, Gill MJ, Grabar S, Guest JL, Jarrin I, Lampe FC, Obel N, Reyes JM, Stephan C, Sterling TR, Teira R, Touloumi G, Wasmuth JC, Wit F, Wittkop L, Zangerle R, Silverberg MJ, Justice A, Sterne JAC. Life expectancy after 2015 of adults with HIV on long-term antiretroviral therapy in Europe and North America: a collaborative analysis of cohort studies. Lancet HIV. 2023;10:e295–e307. doi: 10.1016/S2352-3018(23)00028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahy M, Autenrieth CS, Stanecki K, Wynd S. Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data. AIDS. 2014;28(Suppl 4):S453–S459. doi: 10.1097/QAD.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autenrieth CS, Beck EJ, Stelzle D, Mallouris C, Mahy M, Ghys P. Global and regional trends of people living with HIV aged 50 and over: Estimates and projections for 2000-2020. PLoS One. 2018;13:e0207005. doi: 10.1371/journal.pone.0207005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee M, Park WB, Kim ES, Kim Y, Park SW, Lee E, Oh MD, Kim NJ, Kim HB, Song KH, Choe PG, Kang CK, Lee CM, Choi Y, Moon SM, Choi SJ, Jeon J, Bang J. Possibility of decreasing incidence of human immunodeficiency virus infection in Korea. Infect Chemother. 2023;55:451–459. doi: 10.3947/ic.2023.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JH, Noh J, Kim W, Seong H, Kim JH, Lee WJ, Baek Y, Hyun J, Sohn Y, Cho Y, Kim MH, Ahn S, Lee Y, Ahn JY, Jeong SJ, Ku NS, Yeom JS, Kim C, Choi JY. Trends of age-related non-communicable diseases in people living with HIV and comparison with uninfected controls: a nationwide population-based study in South Korea. HIV Med. 2021;22:824–833. doi: 10.1111/hiv.13139. [DOI] [PubMed] [Google Scholar]
- 7.Jespersen NA, Axelsen F, Dollerup J, Nørgaard M, Larsen CS. The burden of non-communicable diseases and mortality in people living with HIV (PLHIV) in the pre-, early- and late-HAART era. HIV Med. 2021;22:478–490. doi: 10.1111/hiv.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bae JY, Kim SM, Choi Y, Choi JY, Kim SI, Kim SW, Park BY, Choi BY, Choi HJ. Comparison of three cardiovascular risk scores among HIV-infected patients in Korea: The Korea HIV/AIDS cohort study. Infect Chemother. 2022;54:409–418. doi: 10.3947/ic.2022.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S, Park SY, Lee E, Kim TH, Lee E. The role of age in subclinical atherosclerosis in Asian people living with human immunodeficiency virus. Infect Chemother. 2022;54:308–315. doi: 10.3947/ic.2022.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung H, Lee E, Ro JS, Lee JY, Bang J. Mortality after acute coronary syndrome in human immunodeficiency virus infection with optimal adherence: a nationwide study. Infect Chemother. 2023;55:471–478. doi: 10.3947/ic.2023.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seong H, Choi Y, Ahn KH, Choi JY, Kim SW, Kim SI, Kee MK, Choi BY, Park B, Hyun HJ, Yoon JG, Noh JY, Cheong HJ, Kim WJ, Song JY. Assessment of disease burden and immunization rates for vaccine-preventable diseases in people living with HIV: the Korea HIV/AIDS cohort study. Infect Chemother. 2023;55:441–450. doi: 10.3947/ic.2023.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy M, Ojikutu B, Andrian S, Sohng E, Minior T, Hirschhorn LR. Non-communicable diseases and HIV care and treatment: models of integrated service delivery. Trop Med Int Health. 2017;22:926–937. doi: 10.1111/tmi.12901. [DOI] [PubMed] [Google Scholar]
- 13.Lee JA, Kim Y, Lee JY, Park S, Choi JY. Identifying the unmet medical needs of HIV-positive subjects in Korea: results of a nationwide online survey. Infect Chemother. 2023;55:397–402. doi: 10.3947/ic.2023.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi JP, Lee JH, An JM, Kim J, Won N, Choi YH. The story and implications of the Korean health care facility counseling project on people living with HIV. Infect Chemother. 2023;55:167–178. doi: 10.3947/ic.2023.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JH, Kim JM, Ye M, Lee JI, Na S, Lee Y, Short D, Choi JY. Implementation of a nurse-delivered cognitive behavioral therapy for adherence and depression of people living with HIV in Korea. Infect Chemother. 2022;54:733–743. doi: 10.3947/ic.2022.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gahagan JV, Halabi WJ, Nguyen VQ, Carmichael JC, Pigazzi A, Stamos MJ, Mills SD. Colorectal surgery in patients with HIV and AIDS: trends and outcomes over a 10-year period in the USA. J Gastrointest Surg. 2016;20:1239–1246. doi: 10.1007/s11605-016-3119-x. [DOI] [PubMed] [Google Scholar]
- 17.Clinicalinfo.HIV.gov. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. [Accessed 23 April 2024]. Available at: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv.
- 18.European AIDS Clinical Society (EACS) Guidelines Version 12.0 October 2023. [Accessed 23 April 2024]. Available at: https://www.eacsociety.org/media/guidelines-12.0.pdf.
- 19.Seong H, Choi Y, Kim M, Kim JH, Song JY, Kim SW, Kim SI, Kim YJ, Park DW, Park B, Choi BY, Choi JY. Rate of and risk factors for loss to follow up in HIV-infected patients in Korea: the Korea HIV/AIDS cohort study. Infect Chemother. 2023;55:69–79. doi: 10.3947/ic.2022.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arts EJ, Wainberg MA. Mechanisms of nucleoside analog antiviral activity and resistance during human immunodeficiency virus reverse transcription. Antimicrob Agents Chemother. 1996;40:527–540. doi: 10.1128/aac.40.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh SM, Bang J, Park SW, Lee E. Resistance trends of antiretroviral agents in people with human immunodeficiency virus in Korea, 2012 - 2020. Infect Chemother. 2023;55:328–336. doi: 10.3947/ic.2022.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holkmann Olsen C, Mocroft A, Kirk O, Vella S, Blaxhult A, Clumeck N, Fisher M, Katlama C, Phillips AN, Lundgren JD EuroSIDA study group. Interruption of combination antiretroviral therapy and risk of clinical disease progression to AIDS or death. HIV Med. 2007;8:96–104. doi: 10.1111/j.1468-1293.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 23.Kousignian I, Abgrall S, Grabar S, Mahamat A, Teicher E, Rouveix E, Costagliola D Clinical Epidemiology Group of the French Hospital Database on HIV. Maintaining antiretroviral therapy reduces the risk of AIDS-defining events in patients with uncontrolled viral replication and profound immunodeficiency. Clin Infect Dis. 2008;46:296–304. doi: 10.1086/524753. [DOI] [PubMed] [Google Scholar]
- 24.Danel C, Moh R, Minga A, Anzian A, Ba-Gomis O, Kanga C, Nzunetu G, Gabillard D, Rouet F, Sorho S, Chaix ML, Eholié S, Menan H, Sauvageot D, Bissagnene E, Salamon R, Anglaret X Trivacan ANRS 1269 trial group. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367:1981–1989. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 25.DART Trial Team. Fixed duration interruptions are inferior to continuous treatment in African adults starting therapy with CD4 cell counts < 200 cells/microl. AIDS. 2008;22:237–247. doi: 10.1097/QAD.0b013e3282f2d760. [DOI] [PubMed] [Google Scholar]
- 26.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, Cohn D, Cooper D, Darbyshire J, Emery S, Fätkenheuer G, Gazzard B, Grund B, Hoy J, Klingman K, Losso M, Markowitz N, Neuhaus J, Phillips A, Rappoport C. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 27.Maggiolo F. Efavirenz: a decade of clinical experience in the treatment of HIV. J Antimicrob Chemother. 2009;64:910–928. doi: 10.1093/jac/dkp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford N, Lee J, Andrieux-Meyer I, Calmy A. Safety, efficacy, and pharmacokinetics of rilpivirine: systematic review with an emphasis on resource-limited settings. HIV AIDS (Auckl) 2011;3:35–44. doi: 10.2147/HIV.S14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colombier MA, Molina JM. Doravirine: a review. Curr Opin HIV AIDS. 2018;13:308–314. doi: 10.1097/COH.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 30.Vrouenraets SM, Wit FW, van Tongeren J, Lange JM. Efavirenz: a review. Expert Opin Pharmacother. 2007;8:851–871. doi: 10.1517/14656566.8.6.851. [DOI] [PubMed] [Google Scholar]
- 31.Garvey L, Winston A. Rilpivirine: a novel non-nucleoside reverse transcriptase inhibitor. Expert Opin Investig Drugs. 2009;18:1035–1041. doi: 10.1517/13543780903055056. [DOI] [PubMed] [Google Scholar]
- 32.Le Tiec C, Barrail A, Goujard C, Taburet AM. Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir. Clin Pharmacokinet. 2005;44:1035–1050. doi: 10.2165/00003088-200544100-00003. [DOI] [PubMed] [Google Scholar]
- 33.Rittweger M, Arastéh K. Clinical pharmacokinetics of darunavir. Clin Pharmacokinet. 2007;46:739–756. doi: 10.2165/00003088-200746090-00002. [DOI] [PubMed] [Google Scholar]
- 34.Shiomi M, Matsuki S, Ikeda A, Ishikawa T, Nishino N, Kimura M, Irie S. Effects of a protein-rich drink or a standard meal on the pharmacokinetics of elvitegravir, cobicistat, emtricitabine and tenofovir in healthy Japanese male subjects: a randomized, three-way crossover study. J Clin Pharmacol. 2014;54:640–648. doi: 10.1002/jcph.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.San C, Lê MP, Matheron S, Mourvillier B, Caseris M, Timsit JF, Wolff M, Yazdanpanah Y, Descamps D, Peytavin G. Management of oral antiretroviral administration in patients with swallowing disorders or with an enteral feeding tube. Med Mal Infect. 2020;50:537–544. doi: 10.1016/j.medmal.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Walker CK, Shaw CM, Moss Perry MV, Claborn MK. Antiretroviral therapy management in adults with HIV during ICU admission. J Pharm Pract. 2022;35:952–962. doi: 10.1177/08971900211000692. [DOI] [PubMed] [Google Scholar]
- 37.Hocqueloux L, Lefeuvre S, Bois J, Brucato S, Alix A, Valentin C, Peyro-Saint-Paul L, Got L, Fournel F, Dargere S, Prazuck T, Fournier A, Gregoire N, McNicholl I, Parienti JJ. Bioavailability of dissolved and crushed single tablets of bictegravir, emtricitabine, tenofovir alafenamide in healthy adults: the SOLUBIC randomized crossover study. J Antimicrob Chemother. 2022;78:161–168. doi: 10.1093/jac/dkac369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roskam-Kwint M, Bollen P, Colbers A, Duisenberg-van Essenberg M, Harbers V, Burger D. Crushing of dolutegravir fixed-dose combination tablets increases dolutegravir exposure. J Antimicrob Chemother. 2018;73:2430–2434. doi: 10.1093/jac/dky191. [DOI] [PubMed] [Google Scholar]
- 39.Scholten S, Mauruschat S, Hindermann S, Ranneberg B. Administration of darunavir tablets in patients with difficulties in swallowing – two case reports. J Int AIDS Soc. 2010;13(Suppl 4):P114. [Google Scholar]
- 40.Taegtmeyer AB, Müller V, Kovari H, Kullak-Ublick GA, Corti N. Effect of continuous venovenous hemodiafiltration on darunavir and raltegravir exposure after administration via a gastroduodenal tube. AIDS. 2011;25:1339–1341. doi: 10.1097/QAD.0b013e328347f40d. [DOI] [PubMed] [Google Scholar]
- 41.Kim CH, Muzevich KM, Fulco PP. Orogastric administration of crushed darunavir tablets for a critically ill patient. Can J Hosp Pharm. 2014;67:39–42. doi: 10.4212/cjhp.v67i1.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown K, Thomas D, McKenney K, Reeder M, Simonson RB, Bicer C, Nettles RE, Crauwels H. Impact of splitting or crushing on the relative bioavailability of the darunavir/cobicistat/emtricitabine/tenofovir alafenamide single-tablet regimen. Clin Pharmacol Drug Dev. 2019;8:541–548. doi: 10.1002/cpdd.632. [DOI] [PubMed] [Google Scholar]
- 43.Porter AM, Baker CR, Fulco PP. Administration of crushed doravirine via nasojejunal feeding tube in a patient with treatment-experienced human immunodeficiency virus. J Pharm Pract. 2023;36:745–746. doi: 10.1177/08971900221104258. [DOI] [PubMed] [Google Scholar]
- 44.Cattaneo D, Baldelli S, Cerea M, Landonio S, Meraviglia P, Simioni E, Cozzi V, Fucile S, Gazzaniga A, Clementi E, Galli M, Rizzardini G, Gervasoni C. Comparison of the in vivo pharmacokinetics and in vitro dissolution of raltegravir in HIV patients receiving the drug by swallowing or by chewing. Antimicrob Agents Chemother. 2012;56:6132–6136. doi: 10.1128/AAC.00942-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Best BM, Capparelli EV, Diep H, Rossi SS, Farrell MJ, Williams E, Lee G, van den Anker JN, Rakhmanina N. Pharmacokinetics of lopinavir/ritonavir crushed versus whole tablets in children. J Acquir Immune Defic Syndr. 2011;58:385–391. doi: 10.1097/QAI.0b013e318232b057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kakuda TN, Berckmans C, De Smedt G, Leemans R, Leopold L, Peeters M, Nijs S, Vyncke V, van Solingen-Ristea R, Hoetelmans RM. Single-dose pharmacokinetics of pediatric and adult formulations of etravirine and swallowability of the 200-mg tablet: results from three Phase 1 studies. Int J Clin Pharmacol Ther. 2013;51:725–737. doi: 10.5414/CP201770. [DOI] [PubMed] [Google Scholar]
- 47.Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, Atta MG, Wools-Kaloustian KK, Pham PA, Bruggeman LA, Lennox JL, Ray PE, Kalayjian RC HIV Medicine Association of the Infectious Diseases Society of America. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e96–138. doi: 10.1093/cid/ciu617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim JH, Jang H, Kim JH, Song JY, Kim SW, Kim SI, Choi BY, Choi JY. The incidence and risk factors of renal insufficiency among Korean HIV infected patients: the Korea HIV/AIDS cohort study. Infect Chemother. 2022;54:534–541. doi: 10.3947/ic.2022.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srisopa S, Kornjirakasemsan A, Treebupachatsakul P, Sonthisombat P. Incidence and risk factors of tenofovir disoproxil fumarate induced nephrotoxicity and renal function recovery, a hospital case-control study. Infect Chemother. 2023;55:226–236. doi: 10.3947/ic.2023.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Böhler J, Donauer J, Keller F. Pharmacokinetic principles during continuous renal replacement therapy: drugs and dosage. Kidney Int Suppl. 1999;(72):S24–S28. [PubMed] [Google Scholar]
- 51.Kim J, Nam HJ, Jung YJ, Lee HJ, Kim SE, Kang SJ, Park KH, Chang HH, Kim SW, Chung EK, Kim UJ, Jung SI. Weight gain and lipid profile changes in Koreans with human immunodeficiency virus undergoing integrase strand transfer inhibitor-based regimens. Infect Chemother. 2022;54:419–432. doi: 10.3947/ic.2022.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang HH. Weight gain and metabolic syndrome in human immunodeficiency virus patients. Infect Chemother. 2022;54:220–235. doi: 10.3947/ic.2022.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steenackers N, Vanuytsel T, Augustijns P, Tack J, Mertens A, Lannoo M, Van der Schueren B, Matthys C. Adaptations in gastrointestinal physiology after sleeve gastrectomy and Roux-en-Y gastric bypass. Lancet Gastroenterol Hepatol. 2021;6:225–237. doi: 10.1016/S2468-1253(20)30302-2. [DOI] [PubMed] [Google Scholar]
- 54.Tomilo DL, Smith PF, Ogundele AB, Difrancesco R, Berenson CS, Eberhardt E, Bednarczyk E, Morse GD. Inhibition of atazanavir oral absorption by lansoprazole gastric acid suppression in healthy volunteers. Pharmacotherapy. 2006;26:341–346. doi: 10.1592/phco.26.3.341. [DOI] [PubMed] [Google Scholar]
- 55.James C, Preininger L, Sweet M. Rilpivirine: a second-generation nonnucleoside reverse transcriptase inhibitor. Am J Health Syst Pharm. 2012;69:857–861. doi: 10.2146/ajhp110395. [DOI] [PubMed] [Google Scholar]
- 56.Song I, Borland J, Arya N, Wynne B, Piscitelli S. Pharmacokinetics of dolutegravir when administered with mineral supplements in healthy adult subjects. J Clin Pharmacol. 2015;55:490–496. doi: 10.1002/jcph.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tempestilli M, D'Avolio A, De Nicolò A, Agrati C, Antinori A, Cicalini S. Pharmacokinetics of bictegravir, emtricitabine and tenofovir alafenamide in a gastrectomized patient with HIV. J Antimicrob Chemother. 2021;76:3320–3322. doi: 10.1093/jac/dkab319. [DOI] [PubMed] [Google Scholar]
- 58.Piso RJ, Battegay M, Marzolini C. Dolutegravir plasma levels after gastric bypass surgery. AIDS. 2017;31:1052–1054. doi: 10.1097/QAD.0000000000001438. [DOI] [PubMed] [Google Scholar]
- 59.MacBrayne CE, Blum JD, Kiser JJ. Tenofovir, emtricitabine, and darunavir/ritonavir pharmacokinetics in an HIV-infected patient after Roux-en-Y gastric bypass surgery. Ann Pharmacother. 2014;48:816–819. doi: 10.1177/1060028014525034. [DOI] [PubMed] [Google Scholar]
- 60.Baettig V, Courlet P, Delko T, Battegay M, Marzolini C. Boosted darunavir, emtricitabine and tenofovir pharmacokinetics in sthe early and late postgastric bypass surgery periods. AIDS. 2018;32:1903–1905. doi: 10.1097/QAD.0000000000001913. [DOI] [PubMed] [Google Scholar]
- 61.Muzard L, Alvarez JC, Gbedo C, Czernichow S, Carette C. Tenofovir pharmacokinetic after sleeve-gastrectomy in four severely obese patients living with HIV. Obes Res Clin Pract. 2017;11:108–113. doi: 10.1016/j.orcp.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 62.Amouyal C, Buyse M, Lucas-Martini L, Hirt D, Genser L, Torcivia A, Bouillot JL, Oppert JM, Aron-Wisnewsky J. Sleeve gastrectomy in morbidly obese HIV patients: focus on anti-retroviral treatment absorption after surgery. Obes Surg. 2018;28:2886–2893. doi: 10.1007/s11695-018-3308-7. [DOI] [PubMed] [Google Scholar]
- 63.Roelofsen EE, Wildenbeest S, Mollema FP, Burger DM. Pharmacokinetics of tenofovir disoproxyl fumarate/emtricitabine in a client on pre-exposure prophylaxis after a total gastrectomy. AIDS. 2020;34:1989–1991. doi: 10.1097/QAD.0000000000002633. [DOI] [PubMed] [Google Scholar]
- 64.Hong WB, Tan WK, Law LS, Ong DE, Lo EA. Changes of drug pharmacokinetics in patients with short bowel syndrome: a systematic review. Eur J Drug Metab Pharmacokinet. 2021;46:465–478. doi: 10.1007/s13318-021-00696-y. [DOI] [PubMed] [Google Scholar]
- 65.Severijnen R, Bayat N, Bakker H, Tolboom J, Bongaerts G. Enteral drug absorption in patients with short small bowel : a review. Clin Pharmacokinet. 2004;43:951–962. doi: 10.2165/00003088-200443140-00001. [DOI] [PubMed] [Google Scholar]
- 66.Ikuma M, Watanabe D, Yagura H, Ashida M, Takahashi M, Shibata M, Asaoka T, Yoshino M, Uehira T, Sugiura W, Shirasaka T. Therapeutic drug monitoring of anti-human immunodeficiency virus drugs in a patient with short bowel syndrome. Intern Med. 2016;55:3059–3063. doi: 10.2169/internalmedicine.55.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.University of Liverpool. Effect of Gastrointestinal surgery on ARB absorption. [Accessed 16 July 2024]. Available at: www.hiv-druginteractions.org/prescribing_resources/hiv-guidance-gastric-surgery.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Korean version