Abstract
Background
Solid-organ transplant recipients (SOTRs) receiving immunosuppressive therapy are expected to have worse clinical outcomes from coronavirus disease 2019 (COVID-19). However, published studies have shown mixed results, depending on adjustment for important confounders such as age, variants, and vaccination status.
Materials and Methods
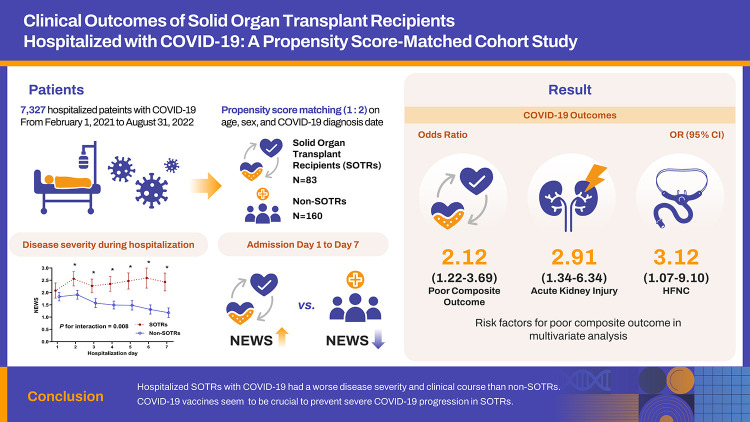
We retrospectively collected the data on 7,327 patients hospitalized with COVID-19 from two tertiary hospitals with government-designated COVID-19 regional centers. We compared clinical outcomes between SOTRs and non-SOTRs by a propensity score-matched analysis (1:2) based on age, gender, and the date of COVID-19 diagnosis. We also performed a multivariate logistic regression analysis to adjust other important confounders such as vaccination status and the Charlson comorbidity index.
Results
After matching, SOTRs (n=83) had a significantly higher risk of high-flow nasal cannula use, mechanical ventilation, acute kidney injury, and a composite of COVID-19 severity outcomes than non-SOTRs (n=160) (all P <0.05). The National Early Warning Score was significantly higher in SOTRs than in non-SOTRs from day 1 to 7 of hospitalization (P for interaction=0.008 by generalized estimating equation). In multivariate logistic regression analysis, SOTRs (odds ratio [OR], 2.14; 95% confidence interval [CI], 1.12–4.11) and male gender (OR, 2.62; 95% CI, 1.26–5.45) were associated with worse outcomes, and receiving two to three doses of COVID-19 vaccine (OR, 0.43; 95% CI, 0.24–0.79) was associated with better outcomes.
Conclusion
Hospitalized SOTRs with COVID-19 had a worse prognosis than non-SOTRs. COVID-19 vaccination should be implemented appropriately to prevent severe COVID-19 progression in this population.
Keywords: SARS-CoV-2, Immunization, Transplantation, Treatment outcome
Graphical Abstract
INTRODUCTION
The coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has reached endemic status after a prolonged pandemic around the world. The virus has undergone several mutations since its initial form, including the Delta and Omicron variants, each with varying dominant symptoms and severity. Recent mutations, such as Omicron, are milder forms that do not have a high mortality rate [1,2].
Solid organ transplant recipients (SOTRs) take life-long immunosuppressive medications to prevent rejection, so they are chronically immunocompromised. Due to the immunocompromised state of SOTRs, there has been concern since the beginning of the COVID-19 pandemic that it exacerbates the severity of infection. Indeed, there were initial reports that SOTRs were at increased risk of death from COVID-19 compared to non-SOTRs [3,4]. However, subsequent studies have been inconclusive, with some reporting no difference in mortality between SOTRs and general population [5]. The mixed results regarding the prognosis of SOTR are likely attributed to SARS-CoV-2 variants depending on the time of infection, vaccination status, patient age, and the use of therapeutic agents [6].
COVID-19 vaccination has played a crucial role in preventing infection and reducing disease severity. However, SOTRs showed diminished vaccine efficacy, relating to their immune suppression [7]. The immunogenicity rate after receiving two mRNA vaccine doses against COVID-19 is <60%, which is considerably lower than that in the general population (>90%) [8,9,10,11]. So, administering booster doses in SOTR has been confirmed to enhance the immune response and effectively alleviate the severity of COVID-19 [7], and it is currently recommended that SOTRs receive periodic boosters against COVID-19 [7,12]. However, no studies have yet analyzed the severity of the disease, clinical course, and effectiveness of vaccination in large-scale SOTRs with COVID-19 in Korea. This study compared SOTRs with matched non-transplant patients, evaluating the disease severity and in-hospital clinical course of COVID-19. Additionally, it adjusted for vaccination status to clearly assess the prognosis.
MATERIALS AND METHODS
1. Study participants
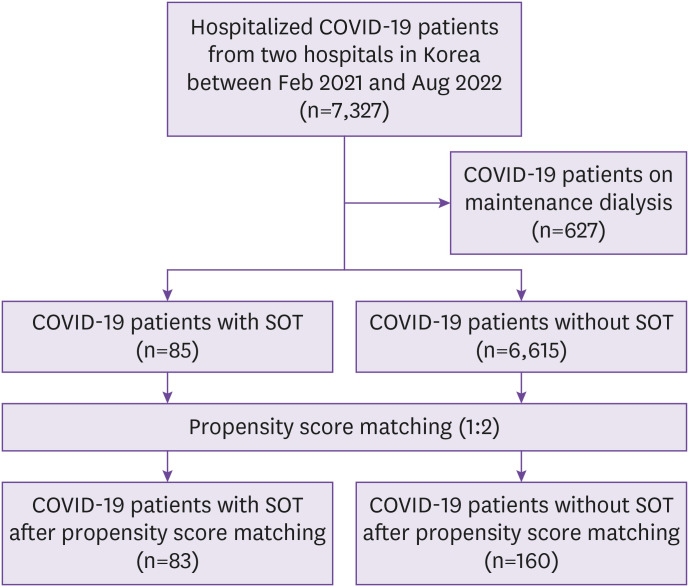
We retrospectively analyzed the data from patients with COVID-19 hospitalized in two tertiary hospitals with government-designated COVID-19 regional centers, Kyungpook National University Chilgok Hospital and Kyungpook National University Hospital, from February 1, 2021 to August 31, 2022. COVID-19 was confirmed by analyzing nasopharyngeal or oropharyngeal swab samples using real-time reverse transcriptase polymerase chain reaction for SARS-CoV-2. All hospitalized patients received symptomatic care with oxygen, antipyretics, and antitussive agents. Specialists in infectious diseases and nephrology prescribed therapeutics, such as remdesivir, antibiotics, dexamethasone, monoclonal antibodies, and tocilizumab for COVID-19, and the immunosuppressant doses were adjusted considering the patient’s clinical course. Overall, 7,327 patients with COVID-19 were hospitalized during the study period, 85 of whom were SOTRs (Fig. 1). Patients who underwent maintenance dialysis were excluded from the analysis. We compared the prognosis and clinical course during hospitalization between SOTRs and non-SOTRs.
Figure 1. Flow diagram of the study participants.
COVID-19, coronavirus disease 2019; SOT, solid organ transplant.
2. Ethics statement
The study protocol was reviewed and approved by the Daegu Joint Institutional Review Board (IRB) (DGIRB 2022-05-002-001). As this retrospective study did not infringe upon the patients’ privacy or health, informed consent was waived by the IRB. The study was conducted in accordance with the 2013 Declaration of Helsinki and the Declaration of Istanbul 2008.
3. Data collection
Patient data were retrospectively collected from electronic medical records. Baseline patient information, including demographics, comorbidities, vaccination status, severity of COVID-19, and vital signs were collected. Information on the transplant organ type and immunosuppressants was collected from SOTRs. In-hospital clinical course and outcomes included the daily National Early Warning Score (NEWS), treatment information, length of hospital stay, oxygen therapy, intensive care unit (ICU) stay, the occurrence of acute kidney injury (AKI), disease severity, survival status, and discharge information. The primary outcome was a composite of COVID-19 severity indicators. The secondary outcomes were each component of the composite outcome.
4. Definition
To consider the effectiveness of vaccination, for the first and second doses, we defined patients diagnosed with COVID-19 between 7 and 180 days after their last vaccine dose as vaccinated, and for the third dose, we defined patients diagnosed with COVID-19 more than 7 days after their last vaccine dose as vaccinated [13]. Patients diagnosed with COVID-19 at any other time were considered unvaccinated, even if they had a history of vaccination. The poor composite outcome was defined as the occurrence of at least one of the following components: supplemental oxygen use, high-flow nasal cannula (HFNC) use, mechanical ventilation, ICU transfer, occurrence of AKI, or in-hospital death. COVID-19 severity was classified into five categories according to the National Institutes of Health COVID-19 severity criteria (asymptomatic, mild, moderate, severe, and critical) [14].
The NEWS is an early warning scoring system that facilitates early detection of and response to clinical deterioration that comprises the respiratory rate, peripheral oxygen saturation, supplemental oxygen use, body temperature, systolic blood pressure, heart rate, and neurological status, all of which are assigned a score of 0–3 [15].
AKI was diagnosed according to the definition of the KDIGO guidelines: an increase in the serum creatinine level to ≥0.3 mg/dL or ≥150% from its baseline or the initiation of dialysis without a history of chronic kidney disease (CKD) [16].
5. Statistical analyses
To balance the differences in age, sex, and COVID-19 diagnosis date between SOTRs and non-SOTRs, propensity score matching (PSM) using nearest-neighbor 1:2 matching within a caliper of 0.2 was performed. PSM was performed without replacement. After matching, the absolute standardized mean differences of the matching variables are all less than 0.1, indicating a good match (Supplementary Table 1, Supplementary Fig. 1). The date of COVID-19 diagnosis was included as a matching variable to adjust for differences in severity according to the type of SARS-CoV-2 variant. Continuous variables are expressed as medians (interquartile ranges [IQRs]) and categorical variables are presented as numbers (percentages). There was no missing data for any of the continuous or categorical variables used in the analysis. The Student’s t-test or Mann–Whitney U test was used to compare differences between continuous variables. Pearson’s chi-square test or Fisher’s exact test was used to compare the differences between categorical variables. Univariate logistic regression analyses were performed to estimate the odd ratios (ORs) of composite outcomes in SOTRs, compared with those in non-SOTRs. Stepwise multivariate logistic regression analysis was performed, and the Hosmer–Lemeshow test for multivariate model showed a significance level greater than 0.05, indicating a good fit (Supplementary Table 2). The receiver operating curve showed that the model has good predictive power with an area under the curve value of 0.698 (Supplementary Fig. 2). We applied the generalized estimating equation (GEE) method to detect differences between SOTRs and non-SOTRs in the temporal changes of the NEWS. Statistical analyses were performed using R software (version 3.6.2, The R Foundation for Statistical Computing, Vienna, Austria). A P-value <0.05 indicated statistical significance.
RESULTS
1. Baseline characteristics and treatment information
Of the 85 SOTRs and 6,615 non-SOTRs, 83 SOTRs and 160 non-SOTRs were selected by PSM (Fig. 1). The median age of the patients was 60 (IQR, 51–66) years, and 73.3% were male individuals (Table 1). There were no differences in age, sex, body mass index, and disease severity in hospitalization between SOTRs and non-SOTRs. Among the SOTRs, 56 patients (67.5%) received two or three doses of the COVID-19 vaccine and 26 patients (31.3%) were unvaccinated. Among the non-SOTRs, 74patients (46.3%) received two or three doses of the COVID-19 vaccine and 80 patients (50.0%) were unvaccinated. Twenty-four patients (9.9%) had a severe disease on hospitalization, and two patients (0.8%) had a critical disease. The distribution of disease severity on hospitalization was not different between two groups (P=0.145). The Charlson Comorbidity Index and comorbidities were similar between the groups. Of the 83 SOTRs, 62 (74.7%), 17 (20.5%), 1 (1.2%), 2 (2.4%), and 1 (1.2%) were kidney, liver, heart, liver-kidney, and kidney-pancreas transplant recipient(s), respectively. Among SOTRs, 70 (84.3%) used tacrolimus, 6 (7.2%) used cyclosporine, 61 (73.4%) used mycophenolate, and 54 (65.1%) used corticosteroids as maintenance immunosuppressants. The proportion of SOTRs receiving remdesivir or dexamethasone was higher than that of non-SOTRs, and the proportion of patients receiving the regdanvimab was higher in non-SOTRs (all P <0.05). The length of hospital stay was similar between two groups.
Table 1. Baseline characteristics of study participants and treatment information.
| Variables | All (n=243) | SOTRs (n=83) | Non-SOTRs (n=160) | P-value | |
|---|---|---|---|---|---|
| Age, years | 60 (51, 66) | 60 (50, 65) | 61 (52, 67) | 0.558 | |
| <50, n (%) | 54 (22.2) | 19 (22.9) | 35 (21.9) | 0.776 | |
| 50–59, n (%) | 60 (24.7) | 21 (25.3) | 39 (24.4) | ||
| 60–69, n (%) | 90 (37.0) | 33 (39.8) | 57 (35.6) | ||
| 70–79, n (%) | 33 (13.6) | 8 (9.6) | 25 (15.6) | ||
| ≥80, n (%) | 6 (2.5) | 2 (2.5) | 4 (2.5) | ||
| Sex, male, n (%) | 178 (73.3) | 62 (74.7) | 116 (72.5) | 0.713 | |
| Body mass index, kg/m2, median (IQR) | 23.0 (20.8, 25.1) | 22.5 (20.3, 24.5) | 23.1 (20.8, 25.6) | 0.591 | |
| Body mass index, >25 kg/m2, n (%) | 61 (25.1) | 17 (20.5) | 44 (27.5) | 0.231 | |
| Vaccination status, n (%) | |||||
| Unvaccinated | 106 (43.6) | 26 (31.3) | 80 (50.0) | 0.005 | |
| One dose | 7 (2.9) | 1 (1.2) | 6 (3.7) | 0.428 | |
| Two doses | 48 (19.8) | 17 (20.5) | 31 (19.4) | 0.837 | |
| Three doses | 82 (33.7) | 39 (47.0) | 43 (26.9) | 0.002 | |
| Disease severity on hospitalization, n (%) | 0.145 | ||||
| Asymptomatic | 21 (8.6) | 3 (3.6) | 18 (11.2) | ||
| Mild | 146 (60.1) | 48 (57.8) | 98 (61.3) | ||
| Moderate | 50 (20.6) | 21 (25.3) | 29 (18.1) | ||
| Severe | 24 (9.9) | 10 (12.0) | 14 (8.7) | ||
| Critical | 2 (0.8) | 1 (1.2) | 1 (0.6) | ||
| Charlson Comorbidity Index score, median (IQR) | 2 (1, 4) | 2 (1, 4) | 2 (0, 3) | 0.528 | |
| Comorbidities, n (%) | |||||
| Congestive heart failure | 5 (2.1) | 1 (1.2) | 4 (2.5) | 0.664 | |
| Chronic obstructive pulmonary disease | 22 (9.1) | 4 (4.8) | 18 (11.2) | 0.098 | |
| Diabetes mellitus | 77 (31.7) | 37 (44.6) | 40 (25.0) | 0.002 | |
| Chronic kidney disease | 36 (14.8) | 26 (31.3) | 10 (6.2) | <0.001 | |
| Transplant organ, n (%) | |||||
| Kidney | 62 (74.7) | ||||
| Liver | 17 (20.5) | ||||
| Heart | 1 (1.2) | ||||
| Othersa | 3 (3.6) | ||||
| Treatment, n (%) | |||||
| Regdanvimab | 84 (34.6) | 21 (25.3) | 63 (39.4) | 0.029 | |
| Remdesivir | 65 (26.7) | 31 (37.3) | 34 (21.2) | 0.007 | |
| Dexamethasone | 58 (23.9) | 26 (31.3) | 32 (20.0) | 0.050 | |
| Length of hospital stay, days, median (IQR) | 8.0 (6.0, 11.0) | 8 (7.0, 12.0) | 8.0 (6.0, 10.0) | 0.166 | |
aTwo patients underwent liver-kidney transplantation, and one underwent kidney-pancreas transplantation.
SOTRs, Solid organ transplant recipients; Non-SOTRs, non-solid organ transplant recipients; IQR, interquartile range.
2. Comparison of clinical outcomes between SOTRs and non-SOTRs
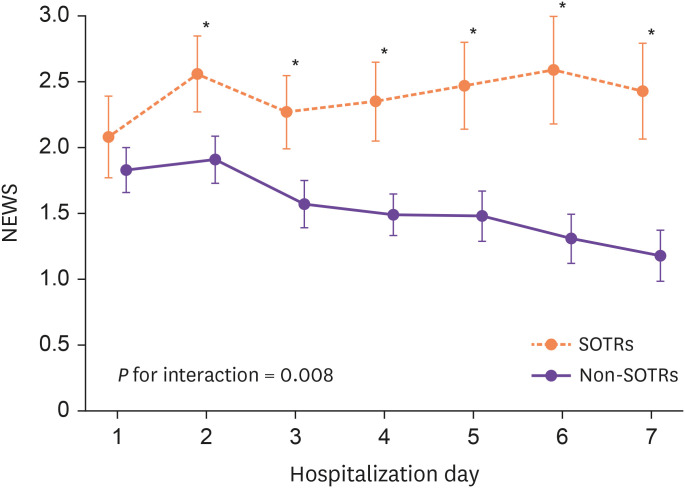
For clinical outcomes, a higher proportion of SOTRs compared with non-SOTRs required HFNC (10.8% vs. 3.8%, P=0.029), mechanical ventilation (6.0% vs. 0.6%, P=0.019), and experienced AKI (20.5% vs. 8.1%, P=0.005) and poor composite outcome (44.6% vs. 27.5%, P=0.007) (Table 2). Figure 2 shows the temporal changes in the disease severity index (NEWS) during hospitalization. The NEWS did not differ on the day of hospitalization but was significantly higher in SOTRs than in non-SOTRs from days 1 to 7 of hospitalization. The GEE model also revealed a significant interaction between the groups and time for the NEWS during hospitalization (P for interaction=0.008).
Table 2. COVID-19 severity outcomes between SOTRs and matched non-SOTRs.
| Outcomes, n (%) | All (n=243) | Transplant (n=83) | Non-transplant (n=160) | P-value | Odds ratio (95% CI) |
|---|---|---|---|---|---|
| Supplemental oxygen | 56 (23.0) | 24 (28.9) | 32 (20.0) | 0.118 | 1.63 (0.88–3.00) |
| HFNC | 15 (6.2) | 9 (10.8) | 6 (3.8) | 0.029 | 3.12 (1.07–9.10) |
| Mechanical ventilation | 6 (2.5) | 5 (6.0) | 1 (0.6) | 0.019 | 10.19 (1.17–88.74) |
| ICU transfer | 19 (7.8) | 9 (10.8) | 10 (6.2) | 0.206 | 1.82 (0.71–4.68) |
| Acute kidney injury | 30 (12.3) | 17 (20.5) | 13 (8.1) | 0.005 | 2.91 (1.34–6.34) |
| Severe or critical disease | 59 (24.3) | 24 (28.9) | 35 (21.9) | 0.225 | 1.45 (0.79–2.66) |
| In-hospital death | 11 (4.5) | 4 (4.8) | 7 (4.4) | >0.999 | 1.12 (0.32–3.95) |
| Poor composite outcome | 81 (33.3) | 37 (44.6) | 44 (27.5) | 0.007 | 2.12 (1.22–3.69) |
COVID-19, coronavirus disease 2019; SOTR, solid organ transplant recipient; HFNC, high-flow nasal cannula; ICU, intensive care unit; CI, confidence interval.
Figure 2. Changes in the NEWS during hospitalization in SOTRs and non-SOTRs.
NEWS, National Early Warning Score; SOTR, solid-organ transplant recipient.
In univariate logistic regression analysis, male gender (OR, 2.80; 95% confidence interval [CI], 1.39–5.60), one stage increase in disease severity on hospitalization (OR, 3.51; 95% CI, 2.33–5.29), pneumonia at admission (OR, 4.05; 95% CI, 2.28–7.22), diabetes mellitus (OR, 2.17; 95% CI, 1.24–3.82), chronic renal failure (OR, 2.99; 95% CI, 1.45–6.16), and SOTRs (OR, 2.12; 95% CI, 1.22–3.69) were risk factors for poor composite outcome; conversely, two or three doses vaccination (OR, 0.58; 95% CI, 0.34–0.99) was a favorable factor for good composite outcome (Table 3). In stepwise multivariate logistic regression analysis, male gender (OR, 2.62; 95% CI, 1.26–5.45) and SOTRs (OR, 2.14; 95% CI, 1.12–4.11) were risk factors for poor composite outcome; conversely, two or three doses vaccination (OR, 0.43; 95% CI, 0.24–0.79) was a favorable factor for good composite outcome.
Table 3. Risk factors for poor composite outcome.
| Outcomes | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | ||
| Age, years | |||||
| <50 | 1 | - | |||
| 50–59 | 1.88 (0.82–4.33) | 0.136 | |||
| 60–69 | 2.13 (0.98–4.59) | 0.055 | |||
| 70–79 | 1.75 (0.67–4.60) | 0.257 | |||
| ≥80 | 3.50 (0.62–19.63) | 0.154 | |||
| Male gender | 2.80 (1.39–5.60) | 0.004 | 2.62 (1.26–5.45) | 0.010 | |
| Body mass index, kg/m2 | 0.67 (0.35–1.30) | 0.238 | |||
| Body mass index, >25 kg/m2 | 0.57 (0.30–1.10) | 0.097 | |||
| Vaccination status | |||||
| Unvaccinated or one dose vaccination | 1 | - | 1 | - | |
| Two or three doses vaccination | 0.58 (0.34–0.99) | 0.046 | 0.43 (0.24–0.79) | 0.006 | |
| Disease severity on hospitalization (one stage increase) | 3.51 (2.33–5.29) | <0.001 | |||
| Modified Charlson Comorbidity Index score (one point increase) | 1.14 (0.99–1.31) | 0.059 | |||
| Congestive heart failure | 3.08 (0.50–18.79) | 0.223 | |||
| Chronic obstructive pulmonary disease | 1.43 (0.59–3.51) | 0.431 | 2.20 (0.83–5.84) | 0.114 | |
| Pneumonia at admission | 4.05 (2.28–7.22) | <0.001 | |||
| Diabetes mellitus | 2.17 (1.24–3.82) | 0.007 | 1.58 (0.85–2.95) | 0.148 | |
| Chronic kidney disease | 2.99 (1.45–6.16) | 0.003 | 2.15 (0.96–4.83) | 0.064 | |
| SOTRs | 2.12 (1.22–3.69) | 0.008 | 2.14 (1.12–4.11) | 0.021 | |
CI, confidence interval; SOTR, solid organ transplant recipient.
DISCUSSION
The study demonstrated that COVID-19 prognosis was worse for hospitalized SOTRs in Korea compared to a matched hospitalized non-SOTR cohort. COVID-19-infected SOTRs had higher oxygen requirements and more patients with AKI than non-SOTRs matched for age, sex, and time of infection. Although SOTRs and matched non-SOTRs did not differ in disease severity at the time of admission, SOTRs had a worse clinical course during hospitalization. COVID-19 vaccination was independently associated with better clinical outcomes. Based on these findings, SOTRs who are taking immunosuppressive medications should take precautions to avoid contracting COVID-19 and receive COVID-19 vaccines appropriately. Additionally, if they do contract COVID-19, they should be given aggressive therapeutic agents early to prevent severe infection.
SOTRs are susceptible to SARS-CoV-2 infection because they are immunosuppressed by continuous use of immunosuppressive agents [17]. Mortality rates among hospitalized patients in large cohorts of SOTRs with COVID-19 have ranged from 20% to 32% during the early period of the pandemic [18,19,20]. A large European study reported that kidney transplant recipients with COVID-19 had a significantly higher risk of mortality compared to patients without kidney disease [3,4]. In addition, another cohort study including 54 lung transplant recipients reported that lung transplantation was associated with an increased mortality risk compared to non-transplant patients [21]. These studies highlight that long-term maintenance immunosuppression weakens the body’s defense mechanism against COVID-19 and results in more severe disease. However, a debate exists on this matter. Some experts propose that the hyper-inflammatory reaction associated with severe COVID-19 has led to the hypothesis that immunosuppression could serve as a protective measure against severe disease [22]. The documented advantages of dexamethasone support the idea of immune modulation in disease management [22]. Interestingly, SOTRs with bacteremia and sepsis exhibited better outcomes in comparison to non-SOTRs, indicating that immunosuppression might mitigate the adverse impacts of hypercytokinemia [23]. Our findings suggest that chronic maintenance immunosuppression in SOTRs is likely to have more downsides than upsides against COVID-19, despite controversy.
As there is a difference in severity according to the SARS-CoV-2 variants, we selected patients who were hospitalized during the same period as the matched cohort for accurate comparison. The prognosis of SOTRs was worse than that of non-SOTRs; particularly, the risk of higher oxygen requirement, such as that through HFNC use and mechanical ventilation, increased. This indicated a higher incidence of severe pneumonia in SOTRs than in non-SOTRs. Because SOTRs are in an immunocompromised state due to the use of maintenance immunosuppressants, the T-cell response decreases in the early phase of COVID-19, leading to severe pneumonia [24]. Additionally, insufficient protective effects of standard vaccination and decreased response to COVID-19 therapeutics may also be associated with progression to severe pneumonia. SOTRs also have a higher risk of AKI than non-SOTRs. This may be secondary to severe infection and inflammation in SOTRs. COVID-19 can cause AKI through several mechanisms [25,26]: (1) glomerular ischemia caused by SARS-CoV-2-induced coagulopathy; (2) direct kidney invasion through SARS-CoV-2 entry in proximal tubular cells and podocytes; (3) inflammatory cytokine elevation and kidney invasion; (4) other factors that can cause kidney injury, including nephrotoxic drug use such as calcineurin inhibitors, high positive end-expiratory pressure, fluid restriction, and hemodynamic instability. In addition, the high prevalence of underlying CKD in SOTRs is also a risk factor for AKI [18,27,28].
According to the NEWS scoring system, which indicates the severity of a patient's condition, COVID-19 SOTRs had worse clinical outcomes during hospitalization compared to non-SOTRs [15]. On admission, there was no significant difference in NEWS scores between two groups. However, starting the day after admission, the SOTR group consistently had higher NEWS scores throughout the first week of hospitalization, indicating a poorer hospital outcome than in the non-SOTR group. Therefore, when treating SOTRs with COVID-19, it is crucial to consider the possibility of poor clinical course during hospitalization and to approach treatment with the potential for deterioration in mind, even if the initial severity appears favorable. Interestingly, although the severity of illness during hospitalization was significantly higher in the SOTR group, the length of hospitalization did not differ between the two groups, probably because the government quarantine period in Korea prevented patients from being discharged during the quarantine period, even if clinical outcomes improved.
Vaccination plays an important role in preventing severe cases of COVID-19. However, SOTRs showed a reduced immune response after vaccination due to maintenance immunosuppressive agents [29]. Furthermore, some researchers have reported that vaccination in SOTRs may increase the risk of rejection due to an increase in systemic immune response and some SOTRs tend to avoid vaccination due to these concerns [23]. However, the multivariate analysis confirmed that vaccination is an independent factor that reduces the risk of poor outcomes from COVID-19. In a meta-analysis of the vaccination effect on SOTRs, it was confirmed that one-time vaccination alone is insufficient to produce sufficient protective antibodies, but antibody production increases with repeated vaccinations [12]. Additionally, there was no increased risk of rejection after repeated vaccination. Therefore, clinicians should provide SOTRs who are hesitant about vaccination with sufficient information regarding the benefits and safety of vaccination and encourage them to get vaccinated.
The strength of this study is that it compared the severity and clinical course of COVID-19 in SOTRs with matched non-SOTRs in Korea. Furthermore, it shows changes in clinical course during hospitalization using the NEWS score. However, this study had some limitations. First, the number of SOTRs was relatively small because we analyzed SOTRs who required in-patient treatment. Also, the type of immunosuppressant taken is known to affect the clinical outcome of COVID-19, but due to the small number of SOTRs, we were unable to identify differences in prognosis by immunosuppressant type. Second, information on the SARS-CoV-2 variants was unavailable. In Korea, the related SARS-CoV-2 variants have not been investigated in all patients, and only a few have been randomly identified. Because the severity and prognosis of each SARS-CoV-2 variant can differ, we attempted to reduce potential bias by selecting patients diagnosed with COVID-19 during the same period when selecting a matched non-SOTR cohort.
In conclusion, hospitalized SOTRs with COVID-19 had a worse disease severity and clinical course during hospitalization than non-SOTRs. Especially, the risk of higher oxygen requirement and AKI were higher in SOTRs. COVID-19 vaccination reduced progression to severe COVID-19. Considering the risk of severe disease in SOTRs contracting COVID-19, it is recommended that COVID-19 vaccines be administered appropriately.
Footnotes
Funding: This research was supported by a grant from the project for Infectious Disease Medical Safety, funded by the Ministry of Health and Welfare, Korea (grant number: RS-2022-KH124555 (HG22C0014)).
Conflict of Interest: No conflict of interest.
- Conceptualization: JHL, KTK.
- Data curation: JHL, HYJ, JYC, JHC, SHP, CDK, YLK, EN, SB, SH, YK, HHC, SWK, JJ, KTK.
- Formal analysis: JHL, EN, YJS, JJ, KTK.
- Funding acquisition: KTK.
- Investigation: JHL, KTK.
- Methodology: JHL, KTK.
- Software: YJS, JJ.
- Supervision: KTK.
- Validation: JHL, KTK.
- Visualization: JHL, JJ.
- Writing-original draft: JHL, EN.
- Writing-review & editing: JHL, EN, KTK.
SUPPLEMENTARY MATERIALS
Standardized mean differences of matching variables before and after propensity sore matching
Results of Hosmer and Lemeshow test
Covariate balance of absolute standardized mean differences in matching variables before and after propensity score matching.
Receiver operating curve for multivariate logistic regression.
References
- 1.Gatti M, Rinaldi M, Bussini L, Bonazzetti C, Pascale R, Pasquini Z, Faní F, Pinho Guedes MN, Azzini AM, Carrara E, Palacios-Baena ZR, Caponcello G, Reyna-Villasmil E, Tacconelli E, Rodríguez-Baño J, Viale P, Giannella M ORCHESTRA study group; Infectious Diseases Unit; Department of Integrated Management of Infectious Risk; IRCCS Policlinico Sant’Orsola; Department of Medical and Surgical Sciences; University of Bologna in Bologna, Italy; Division of Infectious Diseases; Department of Diagnostics and Public Health, University of Verona in Verona, Italy; Infectious Diseases and Microbiology Unit; Hospital Universitario Virgen Macarena; Department of Medicine, University of Sevilla/Biomedicines Institute of Sevilla in Sevilla, Spain. Clinical outcome in solid organ transplant recipients affected by COVID-19 compared to general population: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28:1057–1065. doi: 10.1016/j.cmi.2022.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O'Connell AM, Simons D, Blomquist PB, Zaidi A, Nash S, Iwani Binti Abdul Aziz N, Thelwall S, Dabrera G, Myers R, Amirthalingam G, Gharbia S, Barrett JC, Elson R, Ladhani SN, Ferguson N, Zambon M, Campbell CNJ, Brown K, Hopkins S, Chand M, Ramsay M, Lopez Bernal J. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters LL, Raymer DS, Pal JD, Ambardekar AV. Association of COVID-19 vaccination with risk of COVID-19 infection, hospitalization, and death in heart transplant recipients. JAMA Cardiol. 2022;7:651–654. doi: 10.1001/jamacardio.2022.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozturk S, Turgutalp K, Arici M, Odabas AR, Altiparmak MR, Aydin Z, Cebeci E, Basturk T, Soypacaci Z, Sahin G, Elif Ozler T, Kara E, Dheir H, Eren N, Suleymanlar G, Islam M, Ogutmen MB, Sengul E, Ayar Y, Dolarslan ME, Bakirdogen S, Safak S, Gungor O, Sahin I, Mentese IB, Merhametsiz O, Oguz EG, Genek DG, Alpay N, Aktas N, Duranay M, Alagoz S, Colak H, Adibelli Z, Pembegul I, Hur E, Azak A, Taymez DG, Tatar E, Kazancioglu R, Oruc A, Yuksel E, Onan E, Turkmen K, Hasbal NB, Gurel A, Yelken B, Sahutoglu T, Gok M, Seyahi N, Sevinc M, Ozkurt S, Sipahi S, Bek SG, Bora F, Demirelli B, Oto OA, Altunoren O, Tuglular SZ, Demir ME, Ayli MD, Huddam B, Tanrisev M, Bozaci I, Gursu M, Bakar B, Tokgoz B, Tonbul HZ, Yildiz A, Sezer S, Ates K. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant. 2020;35:2083–2095. doi: 10.1093/ndt/gfaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall VG, Solera JT, Al-Alahmadi G, Marinelli T, Cardinal H, Poirier C, Huard G, Prasad GVR, De Serres SA, Isaac D, Mainra R, Lamarche C, Sapir-Pichhadze R, Gilmour S, Humar A, Kumar D. Severity of COVID-19 among solid organ transplant recipients in Canada, 2020-2021: a prospective, multicentre cohort study. CMAJ. 2022;194:E1155–E1163. doi: 10.1503/cmaj.220620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nimmo A, Gardiner D, Ushiro-Lumb I, Ravanan R, Forsythe JLR. The global impact of COVID-19 on solid organ transplantation: two years into a pandemic. Transplantation. 2022;106:1312–1329. doi: 10.1097/TP.0000000000004151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safa K, Kotton CN. COVID-19 vaccines and solid organ transplantation: more doses, more protection. Transplantation. 2023;107:21–22. doi: 10.1097/TP.0000000000004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benotmane I, Gautier-Vargas G, Cognard N, Olagne J, Heibel F, Braun-Parvez L, Martzloff J, Perrin P, Moulin B, Fafi-Kremer S, Caillard S. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99:1498–1500. doi: 10.1016/j.kint.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marion O, Del Bello A, Abravanel F, Couat C, Faguer S, Esposito L, Hebral AL, Izopet J, Kamar N. Safety and immunogenicity of anti-SARS-CoV-2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med. 2021;174:1336–1338. doi: 10.7326/M21-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang JM, Lee J, Huh KH, Joo DJ, Lee JG, Kim HR, Kim HY, Lee M, Jung I, Kim MY, Kim S, Park Y, Kim MS. Matched versus mixed COVID-19 vaccinations in Korean solid organ transplant recipients: an observational study. Transplantation. 2022;106:e392–e403. doi: 10.1097/TP.0000000000004241. [DOI] [PubMed] [Google Scholar]
- 12.Tang K, Wu X, Luo Y, Wei Z, Feng L, Wu L. Meta-analysis of immunologic response after COVID-19 mRNA vaccination in solid organ transplant recipients. J Infect. 2022;84:e73–e75. doi: 10.1016/j.jinf.2022.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson MG, Natarajan K, Irving SA, Rowley EA, Griggs EP, Gaglani M, Klein NP, Grannis SJ, DeSilva MB, Stenehjem E, Reese SE, Dickerson M, Naleway AL, Han J, Konatham D, McEvoy C, Rao S, Dixon BE, Dascomb K, Lewis N, Levy ME, Patel P, Liao IC, Kharbanda AB, Barron MA, Fadel WF, Grisel N, Goddard K, Yang DH, Wondimu MH, Murthy K, Valvi NR, Arndorfer J, Fireman B, Dunne MM, Embi P, Azziz-Baumgartner E, Zerbo O, Bozio CH, Reynolds S, Ferdinands J, Williams J, Link-Gelles R, Schrag SJ, Verani JR, Ball S, Ong TC. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance-VISION Network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:139–145. doi: 10.15585/mmwr.mm7104e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim JH, Park SD, Jeon Y, Chung YK, Kwon JW, Jeon YH, Jung HY, Park SH, Kim CD, Kim YL, Kwon KT, Choi JY, Cho JH. Clinical effectiveness and safety of remdesivir in hemodialysis patients with COVID-19. Kidney Int Rep. 2022;7:2522–2525. doi: 10.1016/j.ekir.2022.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84:465–470. doi: 10.1016/j.resuscitation.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 17.Kang JM, Kim YJ, Huh K, Kim JM, Park WB, Ahn HJ, Yang J, Lee SO, Jeong SJ, Kim MS, Kim SI. COVID-19 among solid organ transplant recipients in Korea: surveillance data of the Korean Transplantation Society, January 2020 to March 2022. Korean J Transplant. 2022;36:159–163. doi: 10.4285/kjt.22.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heldman MR, Kates OS. COVID-19 in solid organ transplant recipients: a review of the current literature. Curr Treat Options Infect Dis. 2021;13:67–82. doi: 10.1007/s40506-021-00249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravanan R, Callaghan CJ, Mumford L, Ushiro-Lumb I, Thorburn D, Casey J, Friend P, Parameshwar J, Currie I, Burnapp L, Baker R, Dudley J, Oniscu GC, Berman M, Asher J, Harvey D, Manara A, Manas D, Gardiner D, Forsythe JLR. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: A national cohort study. Am J Transplant. 2020;20:3008–3018. doi: 10.1111/ajt.16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 22.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalil AC, Syed A, Rupp ME, Chambers H, Vargas L, Maskin A, Miles CD, Langnas A, Florescu DF. Is bacteremic sepsis associated with higher mortality in transplant recipients than in nontransplant patients? A matched case-control propensity-adjusted study. Clin Infect Dis. 2015;60:216–222. doi: 10.1093/cid/ciu789. [DOI] [PubMed] [Google Scholar]
- 24.Cremoni M, Cuozzo S, Martinuzzi E, Barbosa S, Ben Hassen N, Massa F, Demonchy E, Durand M, Thaunat O, Esnault V, Le Quintrec M, Caillard S, Glaichenhaus N, Sicard A. Low T cell responsiveness in the early phase of COVID-19 associates with progression to severe pneumonia in kidney transplant recipients. Viruses. 2022;14:542. doi: 10.3390/v14030542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46:1339–1348. doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim JH, Park SH, Jeon Y, Cho JH, Jung HY, Choi JY, Kim CD, Lee YH, Seo H, Lee J, Kwon KT, Kim SW, Chang HH, Kim YL. Fatal outcomes of COVID-19 in patients with severe acute kidney injury. J Clin Med. 2020;9:1718. doi: 10.3390/jcm9061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molnar MZ, Bhalla A, Azhar A, Tsujita M, Talwar M, Balaraman V, Sodhi A, Kadaria D, Eason JD, Hayek SS, Coca SG, Shaefi S, Neyra JA, Gupta S, Leaf DE, Kovesdy CP STOP-COVID Investigators. Outcomes of critically ill solid organ transplant patients with COVID-19 in the United States. Am J Transplant. 2020;20:3061–3071. doi: 10.1111/ajt.16280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, Lundgreen KA, Reynaldi A, Khoury DS, Pattekar A, Gouma S, Kuri-Cervantes L, Hicks P, Dysinger S, Hicks A, Sharma H, Herring S, Korte S, Baxter AE, Oldridge DA, Giles JR, Weirick ME, McAllister CM, Awofolaju M, Tanenbaum N, Drapeau EM, Dougherty J, Long S, D'Andrea K, Hamilton JT, McLaughlin M, Williams JC, Adamski S, Kuthuru O;;Frank I, Betts MR, Vella LA, Grifoni A, Weiskopf D, Sette A, Hensley SE, Davenport MP, Bates P, Luning Prak ET, Greenplate AR, Wherry EJ UPenn COVID Processing Unit. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374:abm0829. doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Ayada I, Wang Y, den Hoed CM, Kamar N, Peppelenbosch MP, de Vries AC, Li P, Pan Q. Factors associated with COVID-19 vaccine response in transplant recipients: a systematic review and meta-analysis. Transplantation. 2022;106:2068–2075. doi: 10.1097/TP.0000000000004256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Standardized mean differences of matching variables before and after propensity sore matching
Results of Hosmer and Lemeshow test
Covariate balance of absolute standardized mean differences in matching variables before and after propensity score matching.
Receiver operating curve for multivariate logistic regression.