Abstract
Background
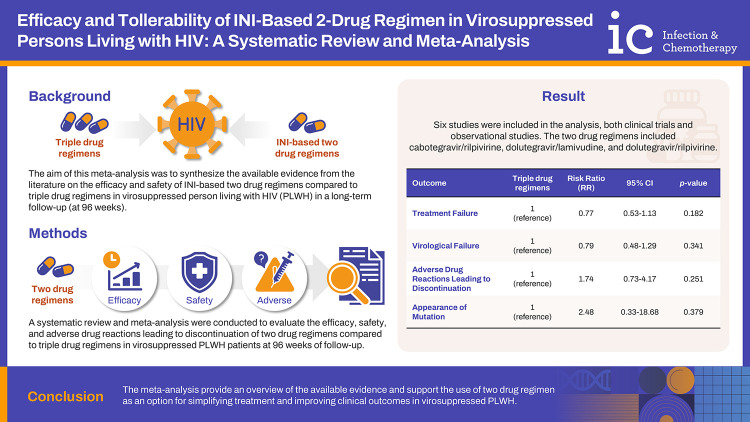
The aim of this meta-analysis was to synthesize the available evidence from the literature on the efficacy and safety of integrase inhibitor-based two drug regimens compared to triple drug regimens in virosuppressed people living with HIV (PLWH) in a long-term follow-up (at 96 weeks).
Materials and Methods
A systematic review and meta-analysis were conducted to evaluate the efficacy, safety, and adverse drug reactions leading to discontinuation of two drug regimens compared to triple drug regimens in virosuppressed PLWH patients at 96 weeks of follow-up. We searched MEDLINE, Google Scholar, and the Cochrane Library up to March 15, 2024, and studies were selected for eligibility based on predefined criteria. Data were extracted independently by two reviewers, and risk ratios (RRs) were calculated as the measure of association between therapy and incidence of events.
Results
Six studies were included in the analysis, both clinical trials and observational studies. The two drug regimens included cabotegravir/rilpivirine, dolutegravir/lamivudine, and dolutegravir/rilpivirine. No significant differences were observed in treatment failure (RR, 0.77; 95% confidence interval [CI], 0.53–1.13; P=0.182), virological failure (RR, 0.79; 95% CI, 0.48–1.29; P=0.341), adverse drug reactions leading to discontinuation (RR, 1.74; 95% CI, 0.73–4.17; P=0.215), or appearance of mutation (RR, 2.48; 95% CI, 0.33–18.68; P=0.379) between two drug regimen and triple drug regimen groups at 96 weeks of follow up.
Conclusion
The meta-analysis provide an overview of the available evidence and supports the use of two drug regimens as an option for simplifying treatment and improving clinical outcomes in virosuppressed PLWH.
Keywords: HIV, Virosuppressed, Two drug regimens, Efficacy, Meta-analysis
Graphical Abstract
INTRODUCTION
Combination antiretroviral therapy (ART) has changed HIV infection from a fatal disease to a manageable chronic condition, significantly improving the life expectancy and quality of life of people living with HIV (PLWH) worldwide [1].
Because of the effectiveness of traditional triple drug regimens (3-DR), the attention today is focused on the comorbidities, linked to increased age and systemic inflammation, which have become the main cause of mortality and morbidity among PLWH [2]. Thus, the clinical challenges of ART in this population are today, tolerability, adherence, long-term toxicity, polypharmacy, and drug interactions. A growing interest has developed in exploring alternative treatment strategies, for example, two drug regimens (2-DR), to reduce drug toxicity. The 2-DR provides some advantages: reducing pill burden, minimizing drug interactions, preserving future treatment options, and potentially mitigating long-term toxicities associated with prolonged exposure to multiple ART drugs [3].
Several studies including meta-analyses investigated the efficacy of 2-DR compared to 3-DR in both naïve and virosuppressed PLWH [4,5]. In virosuppressed PLWH the latest meta-analyses were published in 2016, including different drugs combinations [5], and in the majority of cases drugs not in line with recent guidelines [6,7]. Today the 2-DR suggested by the international guidelines are dolutegravir/lamivudine (DTG/3TC) or emtricitabine (FTC), DTG/rilpivirine (RPV), cabotegravir/rilpivirine (CAB/RPV), darunavir/cobicistat plus 3TC or FTC in the European AIDS Clinical Society guidelines [6]; DTG/3TC or emtricitabine, DTG/RPV, boosted protease inhibitor plus 3TC, DTG plus darunavir/cobicistat, CAB/RPV in Department of Health and Human Service guidelines [7]. Another point of discussion is that the analysis of the efficacy and tolerability of dual therapy in virosuppressed PLWH was limited to the first 48 weeks of treatment. In more recent years several clinical trials and observational studies have investigated the efficacy and safety of 2-DR compared to 3-DR in particular with integrase inhibitor (INI)-based therapy [8,9,10,11,12,13].
Thus, questions remain regarding the optimal selection of agents for 2-DR and the potential impact on long-term outcomes such as drug resistance and treatment durability. This meta-analysis aimed at synthesizing the available evidence from the literature on the long-term efficacy, development of drug resistance, and adverse drug reactions leading to discontinuation (ADRLD) of 2-DR, including only INI-based two drug regimens, actually in use, compared to 3-DR in virosuppressed PLWH.
MATERIALS AND METHODS
1. Search strategy and selection criteria
A systematic review and meta-analysis of randomized controlled trials (RCTs) and observational studies comparing the efficacy and tolerability of CAB/RPV, DTG/3TC, or DTG/RPV versus 3-DR in virosuppressed-HIV patients at 96 weeks of follow-up was performed. The study was conducted in accordance with the PRISMA guidelines [14].
We screened original reports using MEDLINE, Google Scholar, and the Cochrane Library from January 1, 2010, to March 15, 2024, involving both medical subject heading terminology and relevant keywords to identify articles that evaluate the efficacy and tolerability of CAB/RPV, DTG/3TC or DTG/RPV versus 3-DR in virosuppressed-HIV PLWH at 96 weeks of follow-up. We chose January 1, 2010 considering the first study published on DTG in MEDLINE.
The following items were used to search the studies: “cabotegravir” or “dolutegravir” and “switch” or “suppressed” or “suppression”. In addition, the reference lists of all studies retrieved as full papers were manually searched to identify any other study that might be eligible for inclusion.
All studies included had to fulfil the following characteristics and inclusion criteria: (1) to show original data from RCTs or observational studies; (2) to investigate the efficacy and tolerability of CAB/RPV, DTG/3TC or DTG/RPV versus 3-DR in virosuppressed PLWH patients at 96 weeks of follow-up; (3) report at least one of the outcomes clearly defined as treatment failure (TF), i.e. stopping or modification of ART at 96 weeks; virological failure (VF), i.e. HIV RNA more than 50 copies/mL at 96 weeks of follow up; development of mutations conferring resistance to ART at 96 weeks of follow up; and tolerability response, i.e. the (4) to be published in the English language as a full paper. The exclusion criteria of the meta-analysis were: (1) meta-analyses, letters, reviews, meeting abstracts, or editorial comments; (2) duplicate publications or studies reporting duplicate data and studies not published in English.
If required, the authors of studies not reporting clearly defined outcomes were contacted to retrieve the information.
Six researchers (AR, SM, MP, MaPa, MR, VZ) independently screened the title, abstract and key words of all citations to identify potentially eligible articles. Reasons for the exclusion of any study were recorded independently. After that, studies selected during the first screening were retrieved as full texts to be assessed for inclusion. In the case of disagreement, the reviewers re-evaluated the article together; if a consensus was not reached, the corresponding author (NC) was consulted.
2. Data analysis
Three authors (AR, SM, MP) working independently extracted the data using a data-collection form previously established. The following relevant information was collected from every article included in the analysis: the last name of the first author, year of publication, country where the population was enrolled, calendar period of enrolment, study design, sample size, baseline patient characteristics, and occurrence of the endpoint evaluated in each treatment group. The corresponding author was contacted if additional data were needed to identify patients enrolled in the study. If more than one study enrolled the same patient population, only the most complete article was included in the analysis.
Two reviewers (AR, PG) independently performed the quality appraisal of each study. Risk of bias assessment of RCTs was conducted using the Cochrane Risk of Bias Tool [15]. The Newcastle-Ottawa Scale (NOS) was used to assess the quality of observational studies [16]. The articles based on the NOS score were divided into three groups: 0–3 (fair), 4–6 (moderate), and 7–9 (good). In the case of discrepancies between the researchers, the quality assessment was jointly re-evaluated. If a consensus was not reached, a third reviewer (NC) decided.
TF, VF, ADRLD, development of mutations conferring resistance at 96 weeks of treatment were the outcomes of this meta-analysis. Risk ratios (RRs) were used as the meta-analytic measure of association between therapy and the incidence of events. For each study, a proportion of patients with an event for the two therapeutic approaches were used to calculate RR using a 2×2 table.
Heterogeneity between the studies was assessed using the Q statistic and I2 . I2 values between 25% and 49% indicated low heterogeneity, between 50% and 75% indicated moderate heterogeneity and a I2 value of 75% or above indicated high heterogeneity; a P-value of Q statistic less than 0.10 was considered significant [17]. Considering the different population sizes of the studies we chose to perform only random-effect size. If both-armed zero-event (BA0E) was present we included it when treatment effects were unlikely but excluded it when there was a decisive treatment effect [18]. In the latter case, a sensitivity analysis including BA0E was performed.
Where not specified, tests were two-sided, and P-values <0.05 were considered significant. All statistical analyses were performed using Stata/IC (version 16, Stata Corporation, College Station, TX, USA) [19].
RESULTS
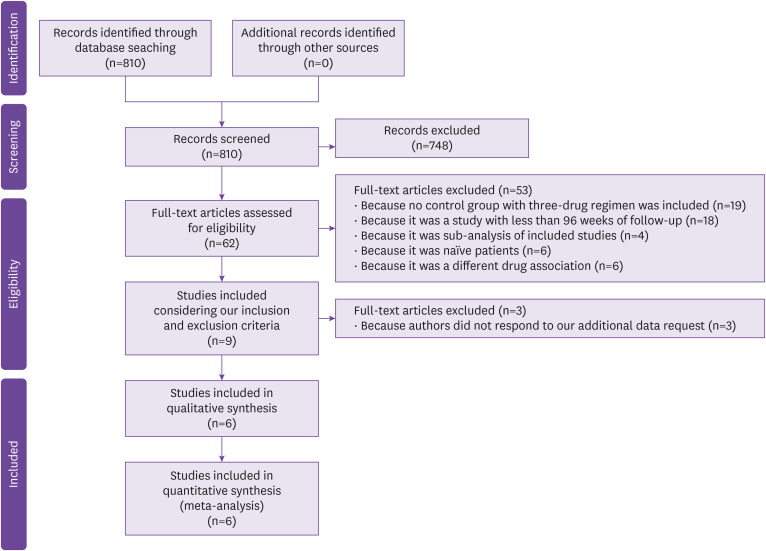
Figure 1 shows the PRISMA flow diagram for article selection. A total of 810 citations from the electronic search were identified; among them, 748 were excluded on the basis of the title and abstract, and 53 for other causes (Fig. 1). Of the nine full articles selected, three had all the data needed for our meta-analysis while for the other six, we needed further data, so we made a data request to the corresponding authors. Only three responded to our request, the others were excluded. In conclusion, six studies were included in our analysis (Fig. 1).
Figure 1. PRISMA Flow chart of studies included.
The characteristics of the 6 studies included are described in detail in Table 1. Three studies were observational [12,20,21] and three studies were clinical trials [10,11,13], two phase 3 trials [10,11], and one open label, single center randomized control trial [13]. Five studies reported outcomes at 96 weeks of follow-up [11,12,13,20,21] and one at 144 weeks of follow-up [10]. Only two studies included the data considering a snapshot at 48 weeks of follow-up [11,13]. Precisely, 3 corresponding authors responded to our data request [12,20,21]: to all were asked to include data at 96 weeks of follow up after switch. Fabbiani M, et al. included data of TF and VF [12]; De Socio GV, et al. include data of TF, VF and ADRLD at 96 weeks [20]; Borghetti A, et al. included data of VF at 96 weeks [21].
Table 1. Characteristics of studies included in the meta-analysis.
| First author [ref] | Year | Country | Study design | Enrolment period | Dual drug regimen group | Triple drug regimen group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regimen | No. pts | Median age (IQR), years | Male sex (%) | CD4 cells per μL, median (IQR) | Regimen | No. pts | Median age (IQR), years | Male sex (%) | CD4 cells, mean | |||||
| De Socio G. V. [20] | 2023 | Italy | Observational cohort | 2018–2023 | Dolutegravir/lamivudine | 110 | 56 (44–63) | 82 (74.5) | 297 (172–473) | Bictegravir/emtricitabine /tenofovir alafenamide | 214 | 56.5 (48–61.5) | 172 (80.4) | 214 (59–365) |
| Orkin [11] | 2022 | Canada, France, Germany, Italy, Japan, Netherlands, Russia, South Africa, Spain, UK, USA | Randomised, multicenter, parallel-group, open label, phase 3 study | 2016–2017 | Cabotegravir/rilpivirine | 283 (ITT) | 34 (29–42) | 220 (78.0) | 624 (473–839) | Dolutegravir/abacavir/lamivudine | 283 | 34 (29–43) | 219 (77.0) | 625 (472–799) |
| Trujillo-Rodriguez [13] | 2022 | Spain | Open label, single center, non-inferiority, randomized clinical trial | 2017–2018 | Dolutegravir/lamivudine | 50 (ITT) | 44 (26–37) | 44 (88.0) | 750 (590–917) | Elvitegravir/cobicistat/tenofovir alafenamide/emtricitabine or dolutegravir/abacavir/lamivudine | 53 | 33 (28–44) | 51 (96.2) | 794 (596–1,123) |
| Osiyemei [10] | 2022 | USA*, Australia, Belgium, Canada, France, Germany, Japan, Netherland, Spain, United Kingdom | Phase 3, randomized, open-label, non-inferiority study, ongoing | 2018–ongoing | Dolutegravir/lamivudine | 369 (ITT) | 40 (20–74) | 290 (79.0) | 682 (133–1,904) | TAF-based triple drug regimens | 372 (ITT) | 39 (18–73) | 280 (75.0) | 720 (119–1,810) |
| Borghetti [21] | 2022 | Italy | Observational cohort | 2014–2020 | Dolutegravir/lamivudine | 204 | 49 (40–56) | 160 (78.4) | 262 (156–359) | Dolutegravir/tenofovir/emtricitabine or dolutegravir | 118 | 50 (45–54) | 85 (72.0) | 190 (82–313) |
| Dolutegravir/abacavir/lamivudine | 306 | 50 (42–56) | 214 (69.9) | 200 (82–313) | ||||||||||
| Fabbiani [12] | 2021 | Italy | Observational cohort | 2016–2019 | Dolutegravir/lamivudine or dolutegravir/rilpivirine | 332 (ITT) | 52.4 (44.8–57.0) | 324 (73.8) | 676 (488–896) | Elvitegravir- dolutegravir- raltegravir-based triple drug regimens | 1,334 | 50.4 (42.8–55.3) | 980 (73.5) | 674 (475–892) |
IQR, interquartile range; USA, United States of America; ITT, Intention to treat.
The patients enrolled in the studies ranged from 103 to 1,666, with a total of 1,348 patients treated with 2-DR and 2,680 with 3-DR. Precisely, of the 1,348 patients treated with 2-DR, 283 were treated with CAB/RPV, 733 with DTG/3TC and 332 with DTG/3TC or DTG/RPV (Table 1). The most used 3-DR were elvitegravir-DTG-raltegravir-based triple drug regimens, TAF-based triple drug regimen, and DTG/abacavir/3TC.
Quality assessments were reported in Supplementary Table 1 and Table 2. Both observational and clinical studies had low risk of bias, except one clinical trials that showed some concerns [11].
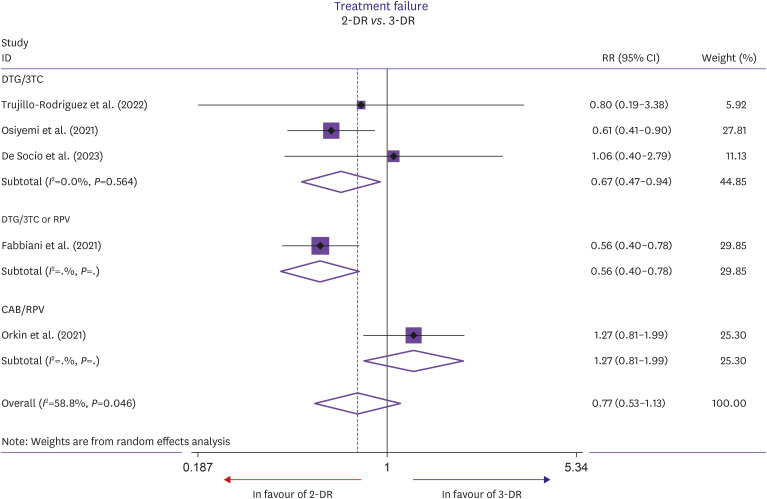
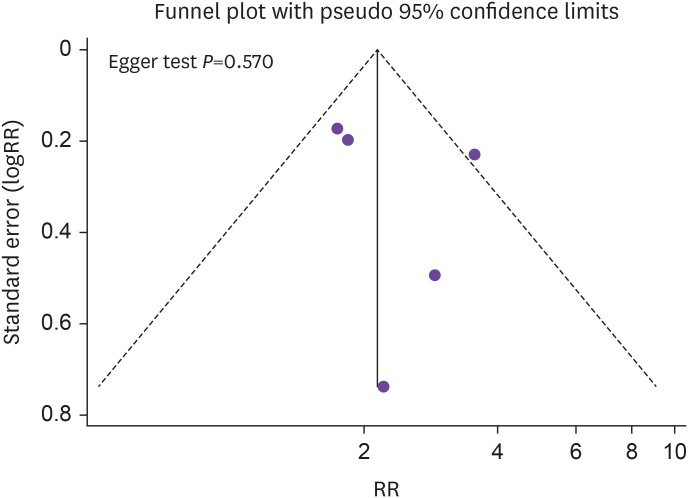
The outcomes evaluated at week 96 of follow up are shown in Figure 2 and Table 2. Precisely, the RR of TF showed no difference in the 2-DR group compared to the 3-DR group (RR, 0.77; 95% CI, 0.53–1.13; P=0.182; Fig. 2), as well as VF (RR, 0.78; 95% CI, 0.48–1.29; P=0.341, Table 2), ADRLD (RR, 1.74; 95% CI, 0.73–4.17; P=0.215; Table 2) and the appearance of mutations conferring resistance (RR, 2.48; 95% CI, 0.33–18.68; P=0.379; Table 2). Intermediate heterogeneity was observed analyzing TF (I2=58.8%, P=0.046) and ADRLD (I2=66.6%, P=0.018), while low heterogeneity was observed analyzing VF (I2=0%, P=0.919) and appearance of mutations conferring resistance (I2=0%, P=0.631). Also considering only the 3 clinical trials there were no differences in TF, VF (Table 3), but we founded a higher RR in ADRLD in patients underwent to 2-DR (RR, 3.38; 95% CI, 1.58–7.24; P=0.002). No small-study effect was observed (Egger test, P=0.570) (Fig. 3).
Figure 2. Forest plot of risk ratios of treatment failure in patients receiving two drug regimens or triple drug regimens.
Table 2. Summary of meta-analysis results in the achievemnt of the outcomes including only 96 weeks of follow up.
| Outcome | No. of studies [ref] | No. of patients experimental/control group | No. (%) of events experimental/control group | RR (efficacy) | 95% Confidence interval (efficacy) | P-value | Heterogeneity test (I2, %; P) |
|---|---|---|---|---|---|---|---|
| Treatment failure | 5 [10,11,12,13,20] | 1,128/2,237 | 117/350 | 0.77 | 0.53–1.13 | 0.182 | 58.8%, 0.046 |
| Virological failure | 6 [10,11,12,13,20,21] | 1,223/2,491 | 22/65 | 0.79 | 0.48–1.29 | 0.341 | 0.0%, 0.919 |
| Adverse drugs leading to discontinuation | 5 [10,11,12,13,20] | 1,128/2,228 | 53/117 | 1.74 | 0.73–4.17 | 0.215 | 66.6%, 0.018 |
| Appearance of mutation | 3 [10,11,13] | 686/680 | 3/0 | 2.48 | 0.33–18.68 | 0.379 | 0.0%, 0.631 |
RR, risk ratio.
Table 3. Summary of meta-analysis results in the achievemnt of the outcomes including only clinical trials.
| Outcome | No. of studies [ref] | No. of patients experimental/control group | No. (%) of events experimental/control group | RR (efficacy) | 95% Confidence interval (efficacy) | P-value | Heterogeneity test (I2, %; P) |
|---|---|---|---|---|---|---|---|
| Treatment failure | 3 [10,11,13] | 686/689 | 77/93 | 0.86 | 0.48–1.54 | 0.608 | 65.7%, 0.054 |
| Virological failure | 3 [10,11,13] | 686/680 | 10/13 | 0.82 | 0.36–1.86 | 0.635 | 0.0%, 0.498 |
| Adverse drugs leading to discontinuation | 3 [10,11,13] | 686/680 | 29/8 | 3.38 | 1.58–7.24 | 0.002 | 0.0%, 0.758 |
| Appearance of mutation | 3 [10,11,13] | 686/680 | 3/0 | 2.48 | 0.33–18.68 | 0.379 | 0.0%, 0.631 |
RR, risk ratio.
Figure 3. Funnel plot of treatment failure at 96 weeks.
RR, risk ratio.
DISCUSSION
Today, 2-DR is an option that clinicians increasingly use in their antiretroviral treatment strategy. These regimens have been extensively studied in trials compared with 3-DR, both in naïve and experienced PLWH who have achieved viral suppression. In some cases, 2-DR affords a metabolic improvement or, in other cases, a reduction in pharmacokinetic interactions or a better tolerability. In some cases, the adherence can also be improved using the dual long-acting regimen, which takes advantage of bimonthly administration in vials rather than daily oral administration. The assumption is naturally that 2-DR guarantee similar long-term efficacy and tolerability of 3-DR.
Although the data on the tolerability and efficacy at 48 weeks of the switch to dual were robust, the optimal selection of agents for 2-DR and the long-term efficacy and tolerability remain open questions. The aim of our meta-analysis was to evaluate if 2-DR and 3-DR show similar long term (96 weeks) efficacy and tolerability, by analyzing studies comparing 3-DR with INI-based 2-DR, currently recommended by guidelines. The references considered in the discussion and not in the analysis included studies that didn’t fit our inclusion criteria or included duplicated data. In every case, considering their scientific impact, we decided to include them in the discussion.
In a long-term follow-up, when compared to 3-DR in virosuppressed PLWH, the switch to dual therapy showed the same rate of efficacy, expressed as treatment and virological failures and development of viral mutants. Moreover, the two strategies showed also a similar tolerability, expressed as a rate of adverse drug reactions leading to discontinuation.
Analyzing data from the literature, it is clear that in the case of switch in stable experienced PLWH with achieved viral suppression, there is no difference between a 2-DR or 3-DR, but the majority of the studies evaluated the data at week 48 of treatment. In a recent 2020 review, Cento and Perno evaluated the role of DTG/3TC 2 vs. 3-DR in a series of studies [22]. They concluded that 2-DR was an excellent alternative to the classic 3-DR in already virosuppressed PLWH, both in terms of efficacy and tolerability.
In a more recent work Libre et al. analyzed the results of the randomized SALSA trial, which compared virosuppressed patients who switched to DTG/3TC to those who remained on 3-DR [23]. The authors concluded that switching to DTG/3TC was non-inferior to continuing current antiretroviral regimen (CAR) for maintaining virologic suppression at week 48. No resistance supporting the efficacy, good safety, and high genetic barrier of DTG/3TC was observed. In a recent real-life study, Lagi et al. compared DTG/RPV and DTG/3TC regimens in PLWH, who switched from a standard three-drug regimen [24]. This study showed that, in clinical practice, a two-drug regimen with DTG/3TC or DTG/RPV is characterized by a low discontinuation rate and VF in virologically suppressed PLWHs switching from a non-nucleoside reverse transcriptase inhibitor-based three antiretroviral drug regimen. Another recent Spanish work analyzed the comparison between the DTG/3TC regimen and the 3-DR based on TAF/FTC/bictegravir (BIC) in a retrospective real-life study that enrolled both naive and experienced patients [25]. The authors concluded that 2-DR showed non-inferiority in terms of virological effectiveness versus 3-DR. Additionally, the durability and safety of 2-DR were confirmed to be similar to 3-DR. Moreover, Van Wick et al. in the Tango study analyzed also the metabolic impact of the switch to 2-DR: switching from 3- or 4-drug TAF-based regimens to DTG/3TC could improve metabolic parameters, particularly when switching from boosted regimens [26]. This is also due to the greater efficacy of integrase inhibitors, like DTG, which ensure a high genetic barrier and therefore a good durability in different combinations of antiretroviral regimens [27].
Regarding DTG/RPV, the Sword studies demonstrated good efficacy and tolerability of this regimen versus the 3-DR [28]. DTG/RPV was non-inferior to CAR over 48 weeks in participants with HIV suppression and showed a safety profile consistent with its components. The results support the use of this 2-DR to maintain HIV suppression. Another Spanish multicenter study evaluated the efficacy and tolerability of this regimen in real life in the switch strategy [29]. The authors highlighted that this study confirms the effectiveness, tolerability and safety of DTG/RPV in real-world clinical practice in a different population from clinical trials, with many years of HIV infection, low CD4+ nadir, several previous treatment lines, more than half with VFs, and one-third diagnosed with AIDS. The switch to DTG/RPV was safe, with few discontinuations due to adverse effects. Modifications of the lipid and liver profiles were favorable. There were no relevant changes in kidney function. Regarding the CAB/RPV regimen, it is a new type of administration with long-latency intramuscular injections which represent a revolution from a posological point of view. The Solar study compared CAB/RPV with the 3-DR gold standard (TAF/FTC/BIC) [30]. These data support the use of long-acting CAB/RPV, dosed every 2 months, as a complete antiretroviral regimen that has similar efficacy to a commonly used integrase strand transfer inhibitor-based first-line regimen, while addressing unmet psychosocial issues associated with daily oral treatment. The data were also confirmed at 96 weeks with the atlas-2 study, in which the authors concluded that long-acting CAB/RPV, dosed every 8 weeks, had non-inferior efficacy compared with a posology of every 4 weeks through the 96-week analysis, with both regimens maintaining a high levels of virological suppression [31]. All these results show the durable safety, efficacy and acceptability of dosing long-acting CAB/RPV monthly and every 2 months as maintenance therapy for people living with HIV-1.
Considering the data from the literature, dual therapies have shown good efficacy and tolerability both in trials and in real-life experiences. The present systematic review and meta-analysis confirmed these data also considering the drugs available today and a long-term follow-up
Although no significant differences were observed in the present study between the dual therapy and triple drug regimen groups, considering only the 3 clinical trials [10,11,13], the patients in 2-DR showed a tendency to have higher rate of adverse drug reactions leading to discontinuation (RR, 3.38; 95% CI, 1.58-7.24; P=0.002, Supplementary Fig. 1). For the Orkin trial that used CAB/RPV as two drug regimen, the use of an injectable form could have determined an increase in ADRLD [11]. The other two studies, Osiyemi et al. [10] and Trujillo-Rodriguez et al. [13], used DTG/3TC, but only in the first ADRLD appear higher in two drug regimens compared to three drug regimens. However, we underline that in the present meta-anaysis we defined as ADRLD all adverse reactions that resulted in discontinuation, whether or not they were attributed to the drug. In the Osiyemi study, at 96 weeks there were 14 total ADRLD in the DTG/3TC arm, but only 4 were attributed to the drug, and 4 ADRLD in the TAF-based regimens, but only 1 was attributed to the drug [10]. Moreover, it is important to note that those included in the DTG/3TC arm were transitioning from a three drug regimen, whereas those included in the three-drug regimen arm continued a TAF-based regimen: it probably resulted in an increase in ADRLD in patients with two drug regimen.
The principle of tailoring always applies to optimize treatment choices for the individual patient. Overall, from the literature data, dual therapy appears to be comparable to the 3-drug regimens in virosuppressed PLWH on switch. This may give clinicians greater guidance in the possibility of simplifying ongoing 3-drug regimens if necessary. At the same time the long-term management of PLWH is more complex, and to optimize it, clinicians have to evaluate more insidious parameters that escape normal clinical practice. In the long term, it will be interesting to evaluate not only the efficacy and tolerability, but the ability of 2-DR to allow pharmacological saving that has clinical significance and not just merely the economic advantage. On the other hand, it is necessary to understand whether the adoption of one fewer drug in the regimen guarantees similar effectiveness of 3-DR on the reservoir in the long-term follow-up. Otherwise, an eventual low-level viral replication could be associated with higher inflammatory potential with a greater risk of a comorbidity and a negative impact on the long-term prognosis of patients.
In conclusion, our meta-analysis was on 2-DR compared to 3-DR in experienced patients with a follow-up of at least 96 weeks. Overall, no differences in efficacy and tolerability were highlighted between the two regimens. This supports the use of 2-DR in simplification schemes for virosuppressed PLWH.
ACKNOWLEDGEMENT
We thank Vittorio Simeon, Section of Medical Statistics, Department of Mental Health and Public Medicine, University of Campania Luigi Vanvitelli, Naples.
Footnotes
Funding: None.
Conflict of Interest: No conflict of interest.
- Conceptualization: AR, SM, MP, NC.
- Data curation: AR, SM, MP, AB, GVS, MF, MGP, RP, VZ, MTR.
- Formal analysis: AR, PG.
- Investigation: AR, PG, NC, SM.
- Methodology: AR, PG, NC, MP.
- Supervision: NC, MP, AR.
- Writing - original draft: AR, SM, MP, NC, AB, GVS, MF.
- Writing - review & editing: AR, SM, MP, NC, AB, GVS, MF.
SUPPLEMENTARY MATERIALS
Newcastle - Ottawa quality assessment scale cohort studies
Revised Cochrane risk-of-bias tool for randomized trials
Forest plot of risk ratios of adverse drug reactions leading discontinuation in patients receiving two drug regimens or triple drug regimens including only clinical trials.
References
- 1.Mazzitelli M, Sasset L, Gardin S, Leoni D, Trunfio M, Scaglione V, Mengato D, Agostini E, Vania E, Putaggio C, Cattelan A. Real-life experience on dolutegravir and lamivudine as initial or switch therapy in a silver population living with HIV. Viruses. 2023;15:1740. doi: 10.3390/v15081740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno S, Perno CF, Mallon PW, Behrens G, Corbeau P, Routy JP, Darcis G. Two-drug vs. three-drug combinations for HIV-1: Do we have enough data to make the switch? HIV Med. 2019;20(Suppl 4):2–12. doi: 10.1111/hiv.12716. [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo-Tenorio C, Pasquau J, Vinuesa D, Ferra S, Terrón A, SanJoaquín I, Payeras A, Martínez OJ, López-Ruz MÁ, Omar M, de la Torre-Lima J, López-Lirola A, Palomares J, Blanco JR, Montero M, García-Vallecillos C. DOLAVI real-life study of dolutegravir plus lamivudine in naive HIV-1 patients (48 weeks) Viruses. 2022;14:524. doi: 10.3390/v14030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisaturo M, Onorato L, Russo A, Martini S, Chiodini P, Signoriello S, Maggi P, Coppola N. Risk of failure in dual therapy versus triple therapy in naïve HIV patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:28–35. doi: 10.1016/j.cmi.2020.09.048. [DOI] [PubMed] [Google Scholar]
- 5.Achhra AC, Mwasakifwa G, Amin J, Boyd MA. Efficacy and safety of contemporary dual-drug antiretroviral regimens as first-line treatment or as a simplification strategy: a systematic review and meta-analysis. Lancet HIV. 2016;3:e351–e360. doi: 10.1016/S2352-3018(16)30015-7. [DOI] [PubMed] [Google Scholar]
- 6.European AIDS Clinical Society (EACS) EACS Guidelines. [Accessed 26 February 2024]. Available at: https://www.eacsociety.org/guidelines/eacs-guidelines/
- 7.Department of Health and Human Services (HHS) Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. [Accessed 17 March 2024]. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf.
- 8.Sequera-Arquelladas S, Hidalgo-Tenorio C, López-Cortés L, Gutiérrez A, Santos J, Téllez F, Omar M, Ferra-Murcia S, Fernández E, Javier R, García-Vallecillos C, Pasquau J. DOLAMA 200: effectiveness and safety of a dual therapy with dolutegravir plus lamivudine in treatment-experienced HIV-1 infected real world participants in Spain. Viruses. 2024;16:259. doi: 10.3390/v16020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aboud M, Orkin C, Podzamczer D, Bogner JR, Baker D, Khuong-Josses MA, Parks D, Angelis K, Kahl LP, Blair EA, Adkison K, Underwood M, Matthews JE, Wynne B, Vandermeulen K, Gartland M, Smith K. Efficacy and safety of dolutegravir-rilpivirine for maintenance of virological suppression in adults with HIV-1: 100-week data from the randomised, open-label, phase 3 SWORD-1 and SWORD-2 studies. Lancet HIV. 2019;6:e576–e587. doi: 10.1016/S2352-3018(19)30149-3. [DOI] [PubMed] [Google Scholar]
- 10.Osiyemi O, De Wit S, Ajana F, Bisshop F, Portilla J, Routy JP, Wyen C, Ait-Khaled M, Leone P, Pappa KA, Wang R, Wright J, George N, Wynne B, Aboud M, van Wyk J, Smith KY. Efficacy and safety of switching to dolutegravir/lamivudine versus continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: results through week 144 from the phase 3, noninferiority TANGO randomized trial. Clin Infect Dis. 2022;75:975–986. doi: 10.1093/cid/ciac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orkin C, Oka S, Philibert P, Brinson C, Bassa A, Gusev D, Degen O, García JG, Morell EB, Tan DHS, D'Amico R, Dorey D, Griffith S, Thiagarajah S, St Clair M, Van Solingen-Ristea R, Crauwels H, Ford SL, Patel P, Chounta V, Vanveggel S, Cutrell A, Van Eygen V, Vandermeulen K, Margolis DA, Smith KY, Spreen WR. Long-acting cabotegravir plus rilpivirine for treatment in adults with HIV-1 infection: 96-week results of the randomised, open-label, phase 3 FLAIR study. Lancet HIV. 2021;8:e185–e196. doi: 10.1016/S2352-3018(20)30340-4. [DOI] [PubMed] [Google Scholar]
- 12.Fabbiani M, Rossetti B, Ciccullo A, Oreni L, Lagi F, Celani L, Colafigli M, De Vito A, Mazzitelli M, Dusina A, Durante M, Montagnani F, Rusconi S, Capetti A, Sterrantino G, D'Ettorre G, Di Giambenedetto S ODOACRE Study Group. Efficacy and durability of two- vs. three-drug integrase inhibitor-based regimens in virologically suppressed HIV-infected patients: Data from real-life ODOACRE cohort. HIV Med. 2021;22:843–853. doi: 10.1111/hiv.13146. [DOI] [PubMed] [Google Scholar]
- 13.Trujillo-Rodríguez M, Muñoz-Muela E, Serna-Gallego A, Milanés-Guisado Y, Praena-Fernández JM, Álvarez-Ríos AI, Herrera-Hidalgo L, Domínguez M, Lozano C, Romero-Vazquez G, Roca C, Espinosa N, Gutiérrez-Valencia A, López-Cortés LF. Immunological and inflammatory changes after simplifying to dual therapy in virologically suppressed HIV-infected patients through week 96 in a randomized trial. Clin Microbiol Infect. 2022;28:1151.e9–1151.e16. doi: 10.1016/j.cmi.2022.02.041. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 16.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Cheng J, Pullenayegum E, Marshall JK, Iorio A, Thabane L. Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: a simulation study. BMJ Open. 2016;6:e010983. doi: 10.1136/bmjopen-2015-010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.StataCorp. Stata statistical software: Release 16; StataCorp LLC. Texas: College Station; 2019. [Google Scholar]
- 20.De Socio GV, Tordi S, Altobelli D, Gidari A, Zoffoli A, Francisci D. Dolutegravir/lamivudine versus tenofovir alafenamide/emtricitabine/bictegravir as a switch strategy in a real-life cohort of virogically suppressed people living with HIV. J Clin Med. 2023;12:7759. doi: 10.3390/jcm12247759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borghetti A, Alkhatib M, Dusina A, Duca L, Borghi V, Zazzi M, Di Giambenedetto S. Virological outcomes with dolutegravir plus either lamivudine or two NRTIs as switch strategies: a multi-cohort study. J Antimicrob Chemother. 2022;77:740–746. doi: 10.1093/jac/dkab429. [DOI] [PubMed] [Google Scholar]
- 22.Cento V, Perno CF. Two-drug regimens with dolutegravir plus rilpivirine or lamivudine in HIV-1 treatment-naïve, virologically-suppressed patients: latest evidence from the literature on their efficacy and safety. J Glob Antimicrob Resist. 2020;20:228–237. doi: 10.1016/j.jgar.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Llibre JM, Brites C, Cheng CY, Osiyemi O, Galera C, Hocqueloux L, Maggiolo F, Degen O, Taylor S, Blair E, Man C, Wynne B, Oyee J, Underwood M, Curtis L, Bontempo G, van Wyk J. Efficacy and safety of switching to the 2-drug regimen dolutegravir/lamivudine versus continuing a 3- or 4-drug regimen for maintaining virologic suppression in adults living with human immunodeficiency virus 1 (HIV-1): week 48 results from the phase 3, noninferiority SALSA randomized trial. Clin Infect Dis. 2023;76:720–729. doi: 10.1093/cid/ciac130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagi F, Giacomelli A, Borghi V, Ciccullo A, Taramasso L, Madeddu G, D'Ettorre G, Giacometti A, Ducci F, De Vito A, Pincino R, Di Giambenedetto S, Mussini C, Antinori S, Sterrantino G. Efficacy and tolerability of dolutegravir/lamivudine versus dolutegravir/rilpivirine in switching from a three-drug regimen based on nonnucleoside reverse transcriptase inhibitors: A retrospective cohort study. J Med Virol. 2023;95:e29149. doi: 10.1002/jmv.29149. [DOI] [PubMed] [Google Scholar]
- 25.Mendoza I, Lázaro A, Espinosa A, Sánchez L, Horta AM, Torralba M. Effectiveness, durability and safety of dolutegravir and lamivudine versus bictegravir, emtricitabine and tenofovir alafenamide in a real-world cohort of HIV-infected adults. PLoS One. 2023;18:e0291480. doi: 10.1371/journal.pone.0291480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Wyk J, Ait-Khaled M, Santos J, Scholten S, Wohlfeiler M, Ajana F, Jones B, Nascimento MC, Tenorio AR, Smith DE, Wright J, Wynne B. Brief report: improvement in metabolic health parameters at week 48 after switching from a tenofovir alafenamide-based 3- or 4-drug regimen to the 2-drug regimen of dolutegravir/lamivudine: the TANGO study. J Acquir Immune Defic Syndr. 2021;87:794–800. doi: 10.1097/QAI.0000000000002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taramasso L, De Vito A, Ricci ED, Orofino G, Squillace N, Menzaghi B, Molteni C, Gulminetti R, De Socio GV, Pellicanò GF, Sarchi E, Celesia BM, Calza L, Rusconi S, Valsecchi L, Martinelli CV, Cascio A, Maggi P, Vichi F, Angioni G, Guadagnino G, Cenderello G, Dentone C, Bandera A, Falasca K, Bonfanti P, Di Biagio A, Madeddu G Behalf of the CISAI Study Group. Durability of dolutegravir-based regimens: a 5-year prospective observational study. AIDS Patient Care STDS. 2021;35:342–353. doi: 10.1089/apc.2021.0089. [DOI] [PubMed] [Google Scholar]
- 28.Llibre JM, Hung CC, Brinson C, Castelli F, Girard PM, Kahl LP, Blair EA, Angelis K, Wynne B, Vandermeulen K, Underwood M, Smith K, Gartland M, Aboud M. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391:839–849. doi: 10.1016/S0140-6736(17)33095-7. [DOI] [PubMed] [Google Scholar]
- 29.Palacios R, Gómez-Ayerbe C, Casado JL, Tejerina F, Montes ML, Castaño M, Ocampo A, Rial D, Ribera E, Galindo MJ, Hidalgo C, Fariñas C, Montero M, Payeras T, Fanjul F, de la Torre J, Santos J. Efficacy and safety of dolutegravir/rilpivirine in real-world clinical practice. GeSIDA study 1119. HIV Med. 2023;24:933–937. doi: 10.1111/hiv.13489. [DOI] [PubMed] [Google Scholar]
- 30.Ramgopal MN, Castagna A, Cazanave C, Diaz-Brito V, Dretler R, Oka S, Osiyemi O, Walmsley S, Sims J, Di Perri G, Sutton K, Sutherland-Phillips D, Berni A, Latham CL, Zhang F, D'Amico R, Pascual Bernáldez M, Van Solingen-Ristea R, Van Eygen V, Patel P, Chounta V, Spreen WR, Garges HP, Smith K, van Wyk J. Efficacy, safety, and tolerability of switching to long-acting cabotegravir plus rilpivirine versus continuing fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with HIV, 12-month results (SOLAR): a randomised, open-label, phase 3b, non-inferiority trial. Lancet HIV. 2023;10:e566–e577. doi: 10.1016/S2352-3018(23)00136-4. [DOI] [PubMed] [Google Scholar]
- 31.Jaeger H, Overton ET, Richmond G, Rizzardini G, Andrade-Villanueva JF, Mngqibisa R, Hermida AO, Thalme A, Belonosova E, Ajana F, Benn PD, Wang Y, Hudson KJ, Español CM, Ford SL, Crauwels H, Margolis DA, Talarico CL, Smith KY, van Eygen V, Van Solingen-Ristea R, Vanveggel S, Spreen WR. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 96-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet HIV. 2021;8:e679–e689. doi: 10.1016/S2352-3018(21)00185-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Newcastle - Ottawa quality assessment scale cohort studies
Revised Cochrane risk-of-bias tool for randomized trials
Forest plot of risk ratios of adverse drug reactions leading discontinuation in patients receiving two drug regimens or triple drug regimens including only clinical trials.