Abstract
Residents in nursing homes face heightened COVID-19 risks. We aimed to assess the adverse events (AEs) rates and antibody responses after the first to the fifth dose of COVID-19 mRNA vaccination in a nursing home cohort. Ninety-five SARS-CoV-2 naïve participants consisted of 26 staff (median age, 51 years) and 69 residents (median age, 88 years). Life-threatening AEs were reported in neither residents nor staff. The severity of non-life-threatening AEs was graded, and severe AEs were reported only in staff. The AEs rates were considerably lower in residents, compared to those in staff. Anti-RBD IgG and the neutralizing titers (NTs) against Wuhan and Omicron BA.4/BA.5 did not differ significantly between those with ‘any AE’ and ‘no AE’ among both staff and residents two months after the second, third and fifth doses, while the anti-RBD IgG significantly differed between two groups after third dose in residents. These findings suggest that the anti-RBD IgG and the NTs increase regardless of the occurrence of AEs. Our study underscores a robust antibody response in both in staff and residents, and fewer AEs following COVID-19 vaccination in SARS-CoV-2 naïve residents than staff, supporting the recommendation for mRNA booster doses in older adults at high-risk care facilities.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73004-8.
Keywords: COVID-19, SARS-CoV-2, Omicron variants, MRNA booster vaccination, Nursing home residents, Life-threatening adverse events
Subject terms: Infectious diseases, Viral infection
Introduction
Numerous clusters of coronavirus disease 2019 (COVID-19) have been documented in nursing homes during the early phase of the pandemic1–3. Given the high case fatality rates among COVID-19 patients in these facilities3,4, residents are particularly vulnerable to SARS-CoV-2. A prior report on long-term care facilities underscored the importance of preventing COVID-19 outbreaks in such settings because both the larger number and size of clusters were linked to higher mortality5.
Vaccination against SARS-CoV-2 represents a pivotal strategy in controlling the COVID-19 pandemic. COVID-19 vaccines stimulate the production of neutralizing antibodies to SARS-CoV-2 in adults, although more recipients of COVID-19 mRNA vaccines reported adverse events (AEs) compared to placebo recipients6. Most AEs were non-life-threatening, but rare, life-threatening AEs, such as anaphylactic reaction and myocarditis, have been reported7–9.
Estimating the magnitude and durability of protection provided by COVID-19 vaccination has become challenging due to the emergence of variants that evade existing vaccine-induced immunity10. A recent study, which analyzed COVID-19 surveillance and seroprevalence data, reported that frequent COVID-19 booster vaccinations in older age groups and immunocompromised populations would effectively reduce the burden of severe COVID-1911.
Numerous studies have investigated immune responses, including immunogenicity and reactogenicity, in healthcare workers (HCW) following two doses of COVID-19 mRNA vaccination12–18. Some of these studies delineated varying degrees of correlation between humoral immune responses and AEs following COVID-19 mRNA vaccination12–17, whereas one study did not, although it was limited by a small sample size18.
We and other investigators have previously observed a substantial increase in anti-RBD IgG levels and neutralizing titers (NTs) against Omicron subvariants following booster doses of monovalent (Wuhan) COVID-19 mRNA vaccination among nursing home residents19–21. AEs following COVID-19 mRNA vaccination in general are serious concerns for the general population, particularly so for nursing home residents, who are often frail and have comorbidities. Therefore, assessing the safety profile of this vaccine in this population is crucial, as it could lead to an increased willingness to get vaccinated. While a prior study indicated that the primary vaccination series with COVID-19 mRNA elicited robust antibody responses and mostly mild AEs in nursing home residents22, the antibody responses and the reactogenicity following COVID-19 mRNA booster vaccinations in this population remain less understood. Here, we report a distinct difference in local and systemic non-life-threatening AEs between staff and residents, and robust antibody responses among the nursing home staff and residents regardless of the occurrence of AEs after booster doses of COVID-19 mRNA vaccine.
Results
Nursing home cohort of COVID-19 vaccination
The sex, age and vaccination status of 111 study participants (78 residents and 33 staff) from six nursing homes who received all five doses of COVID-19 mRNA vaccine are shown in table. The relatively small number of staff participants is due to a majority of this population not receiving the fifth dose of the mRNA vaccine. Of the 111 participants, 95 individuals were SARS-CoV-2 naïve (SARS-CoV-2 non-infected), while 16 had experienced breakthrough infections (BTIs) during the BA.5 endemic period, spanning from June 2022 to February 2023 (Fig. 1). The XBB subvariant emerged in Toyama Prefecture, from April to October 2023. The staff were significantly younger than the residents, both among SARS-CoV-2 naïve individuals (p < 0.05) and those with SARS-CoV-2 BTI (p < 0.001, Table 1).
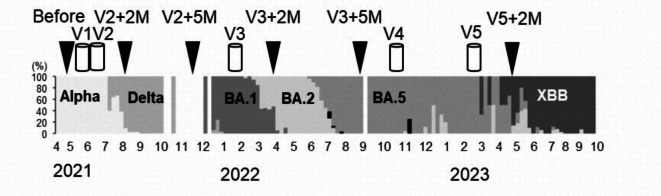
Fig. 1.
Genomic epidemiology and study design. Genomic epidemiology of SARS-CoV-2 variants or Omicron subvariants in Toyama Prefecture. Abbreviations: V2 + 2 M, V2 + 5 M; two or five months after the second dose of the vaccination (monovalent Wuhan), respectively, V3 + 2 M, V3 + 5 M; two or five months after the third dose of the vaccination (monovalent Wuhan), V5 + 2 M; two months after the fifth doses of the vaccination (bivalent Wuhan/BA.1- or Wuhan/BA.4/BA.5 adapted vaccine). Alpha; Alpha variant of SARS-CoV-2, Delta; Delta variant of SARS-CoV-2, BA1; Omicron BA.1 subvariant, BA.5; Omicron BA.5, XBB; Omicron XBB subvariant.
Table 1.
Sex, age and vaccination status of study participants.
| SARS-CoV-2 naïve | SARS-CoV-2 BTI during BA.5 endemic period | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Staff | Residents | Subtotal | p-valuea | Staff | Residents | Subtotal | p-valuea | |||
| Participants (n) | 26 | 69 | 95 | 7 | 9 | 16 | 111 | |||
| Female (%) | 14 (54) | 57 (83) | 71 (75) | p < 0.01 | 5 (71) | 8 (89) | 13 (81) | p = 0.375 | 84(76) | |
| Age, years, median (IQR) | 51 (43–59) | 88 (82–92) | 77 (66–91) | p < 0.05 | 46 (41–53) | 84 (82–86) | 68 (51–85) | p < 0.001 | 83(62–90) | |
| Vaccination history | ||||||||||
| First dose | Wuhan (BNT162b2) | 26 (100%) b | 69 (100%) | 95 (100%) | 7 (100%) | 9 (100%) | 16 (100%) | 111 (100%) | ||
| Second dose | Wuhan (BNT162b2) | 26 (100%) | 69 (100%) | 95 (100%) | 7 (100%) | 9 (100%) | 16 (100%) | 111 (100%) | ||
| Third dose | Wuhan (BNT162b2) | 8 (30.8%) | 16 (23.2%) | 24 (25.3%) | 0 (0%) | 8 (88.9%) | 8 (50.0%) | 32 (28.8%) | ||
| Wuhan (mRNA-1273) | 18 (69.2%) | 53 (76.8%) | 71 (74.7%) | 7 (100%) | 1 (11.1%) | 8 (50.0%) | 79 (71.2%) | |||
| Fourth dose | Wuhan (BNT162b2) | 5 (19.2%) | 3 (4.3%) | 8 (8.4%) | 1 (14.3%) | 2 (22.2%) | 3 (18.8%) | 11 (9.9%) | ||
| Wuhan (mRNA-1273) | 21 (80.8%) | 66 (95.7%) | 87 (91.6%) | 6 (85.7%) | 7 (77.8%) | 13 (81.3%) | 100 (90.1%) | |||
| Fifth dose | bivalent BA.1 (BNT162b2) | 1 (3.8%) | 21 (30.4%) | 22 (23.2%) | 0 (0%) | 8 (88.9%) | 8 (50.0%) | 30 (27.0%) | ||
| bivalent BA.4/BA.5 (BNT162b2) | 24 (92.3%) | 33 (47.8%) | 57 (60.0%) | 6 (85.7%) | 1 (11.1%) | 7 (43.8%) | 64 (57.7%) | |||
| bivalent BA.4/BA.5 (mRNA-1273) | 1 (3.8%) | 15 (21.7%) | 16 (16.8%) | 1 (14.3%) | 0 (0%) | 1 (6.3%) | 17 (15.3%) | |||
aThe Mann-Whitney U test or the Chi-square test was used to analyze the differences between staff and residents.
bNumbers in parentheses indicate the percentages of vaccination history at each dose.
Comparison of antibody titers between staff and residents following the fifth dose of bivalent COVID-19 vaccine
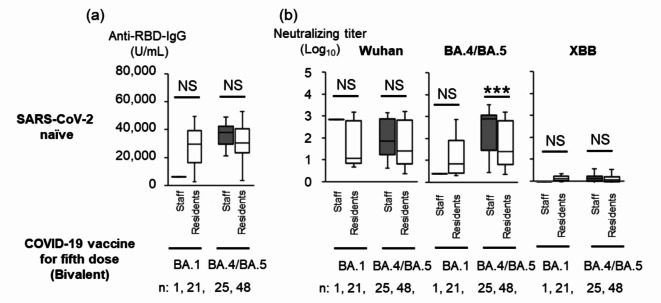
Among SARS-CoV-2 naïve participants, one staff member and 21 residents received the bivalent Wuhan/BA.1-adapted vaccine, while 25 staff members and 48 residents received the bivalent Wuhan/BA.4/BA.5-adapted vaccine as the fifth dose between November 2022 and February 2023 (Fig. 2a and b). We were unable to perform a statistical analysis of anti-RBD IgG titers and NTs against Wuhan, BA.4/BA.5 and XBB between the single staff member and 21 residents two months after the fifth dose. Although no significant difference was found in anti-RBD IgG titers and NTs against Wuhan between 25 staff members and 48 residents, we found that the NTs against BA.4/BA.5 were significantly higher in the 25 staff members than those in 48 residents (Fig. 2b, p < 0.001). NTs against XBB were negligible in 25 staff members and 48 residents two months after the fifth dose.
Fig. 2.
Anti-RBD IgG Titers and NTs against Wuhan Strain, BA.1 or BA.4/BA.5 Subvariant after the Fifth Dose of BA.1 Bivalent Vaccine or BA.4/BA.5 Bivalent Vaccine in SARS-CoV-2 Naïve Staff and Residents. Anti-RBD IgG titers (a) and NTs against SARS-CoV-2pv (b) in SARS-CoV-2 naïve staff and residents, anti-RBD IgG titers following the fifth dose. The SARS-CoV-2 naïve staff and residents received the fifth dose of BA.1 bivalent vaccine (n = 1 and 21, respectively) or BA.4/BA.5 bivalent vaccine (n = 25 and 48, respectively). Each box includes the 25th and 75th percentiles with the median as thick line; bottom and upper whiskers respectively show the smallest and largest values. Dark gray and white boxes show staff and residents, respectively. Mann-Whitney U test, ***, p < 0.001; NS, not significant. Abbreviations: RBD, receptor-binding domain; NT, neutralizing titer, SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; BTI, breakthrough infection; XBB; Omicron XBB 1.5 subvariant.
AEs following vaccination
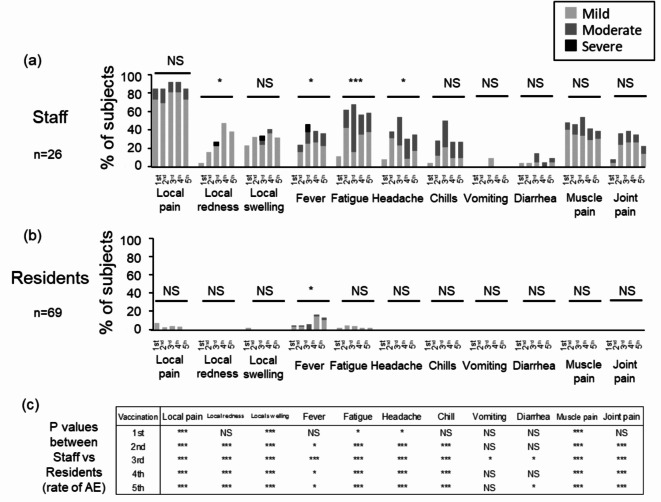
No life-threatening AEs were reported among either residents or staff. Non-life-threatening AEs were frequently observed in staff members, but rarely seen in the residents. For staff members following the third dose of mRNA vaccine, non-life-threatening AEs included local redness (22.7% mild, 0% moderate, 4.5% severe), local swelling (23.8%mild, 4.8% moderate, 4.8% severe), and fever (25.0% mild, 2.5% moderate, 8.3% severe) (Fig. 3a). Residents commonly experienced fever after the fourth (14.5% mild, 1.6% moderate, 0% severe) and fifth doses (10.3% mild, 2.9% moderate, 0% severe) of the mRNA vaccine (Fig. 3b). Severe non-life-threatening AE was not reported in the residents. The rates of local redness, fever, fatigue, and headache significantly varied across different doses in staff members. Similarly, the rate of fever differed significantly between various doses in residents. The rates of most AEs were significantly lower among residents compared to staff members after the first through fifth doses (p < 0.05) (Fig. 3a and b, and 3c). Local pain was the most frequent AE among staff (84.6–92.3%), whereas it was rarely seen among residents (0–6.8%). We also evaluated AE rates in SARS-CoV-2 naïve participants after the third to the fifth doses of the vaccines from two different manufacturers (Pfizer/BioNTech and Moderna)23–25 (Supplementary Fig. 1). Except for fatigue and headache after the fourth dose of vaccination, no significant difference was found in AE rates after the third, fourth, and fifth doses of vaccination from the manufacturers.
Fig. 3.
Comparisons of the rate of AEs between SARS-CoV-2 Naïve staff and residents after the COVID-19 vaccinations. The rate of AEs of SARS-CoV-2 naïve staff (n = 26) (a) and residents (n = 69) (b) by vaccine dose was compared using the Chi-square test. The light gray, the medium gray, or the black bars denote the case with mild, moderate, or severe AE. The p-value of the Chi-square test for comparing of the rate of AEs between staff and residents is shown in (c). ***, p < 0.001; *, p < 0.05. Abbreviations: AE, adverse event, NS, not significant.
Comparison of antibody titers between SARS-CoV-2 naïve staff and residents with and without AEs
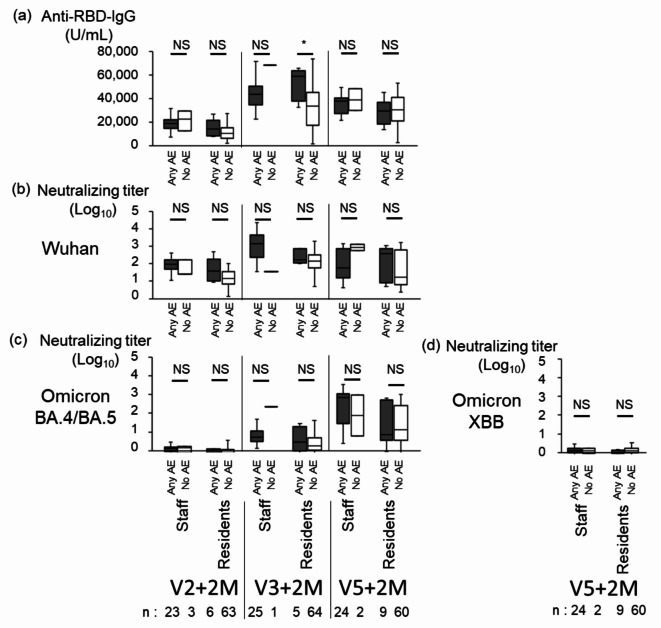
The anti-RBD IgG titer did not differ between staff with ‘any AE’ and ‘no AE’ after the second, the third and the fifth doses, and between residents with ‘any AE’ and ‘no AE’ after the second and the fifth doses, although the geometric mean anti-RBD IgG titer was significantly higher in residents with ‘any AE’ (5.2 × 104 U/mL, n = 5) than ‘no AE’ (3.4 × 104 U/mL, n = 64) after the third dose (Fig. 4a). The geometric mean NTs against Wuhan, Omicron BA.4/BA.5, and XBB did not differ between staff with ‘any AE’ and ‘no AE’, and between residents with ‘any AE’ and ‘no AE’ after the second, the third, and the fifth doses of vaccination (Fig. 4b and c, and 4d). These data indicated that the anti-RBD IgG titers and NTs similarly increased both in staff and residents regardless of the occurrence of AEs after vaccination, with one exception of anti-RBD-IgG in residents after the third dose of vaccination.
Fig. 4.
Comparison of anti-RBD IgG, and NTs between SARS-CoV-2 Naïve Staff with ‘Any AE’ and ‘no AE’, and between SARS-CoV-2 Naïve residents with ‘Any AE’ and ‘no AE’ after COVID-19 Vaccination. Anti-RBD-IgG (a), NTs against Wuhan (b), Omicron BA.4/BA.5 (c), and XBB.1.5 (d) subvariants in staff and residents with ‘any AE’ and ‘no AE’. Each box includes the 25 th and 75 th percentiles with the median as thick line; bottom and upper whiskers respectively show the smallest and largest values. Closed and open boxes show ‘any AE’ and ‘no AE’, respectively. *, p < 0.05; NS, not significant as determined by the Mann-Whitney U test. Abbreviations: RBD, receptor-binding domain; V2 + 2 M, 2 months after the second dose of the vaccine; V3 + 2 M, 2 months after the third dose of the vaccine; V5 + 2 M, 2 months after the fifth dose of the vaccine; AE, adverse event.
Discussion
In our study, data analysis across six nursing homes revealed that the rates of most AEs were significantly lower among residents compared to staff following the administration of the first through fifth doses of COVID-19 mRNA vaccination. Life-threatening AEs were reported in neither residents nor staff, and no severe non-life-threatening AEs were observed in residents following any of the first to the fifth dose of vaccination doses.
We previously reported increased levels of anti-RBD IgG and NTs against the Wuhan, Alpha, Delta, and Omicron subvariants in SARS-CoV-2-naïve participants aged ≥ 80 years, two months after the third dose of COVID-19 vaccination19. Our findings also indicate that NTs against Omicron subvariants BA.1 and BA.5 increased two months after the third dose compared to five months after the second dose in SARS-CoV-2 naïve residents aged ≥ 80 years. Taken together with these observations, our data suggest that the COVID-19 mRNA booster vaccinations are beneficial due to their low incidence of AEs and the robust antibody response among SARS-CoV-2 naïve residents.
In addition to immunogenicity, a recent study reported the effectiveness of a booster dose of COVID-19 mRNA vaccination in two distinct nursing home cohorts in the United States26. The authors demonstrated that booster vaccination reduced SARS-CoV-2 infection by 37.7–57.7%, hospital admissions by 64.1–74.4%, and SARS-CoV-2-associated deaths by 46.6–87.9%. Following the introduction of a bivalent mRNA vaccine, a retrospective, population-based cohort study in Israel indicated that the effectiveness of a bivalent BA.4/BA.5 mRNA vaccine booster dose among adults aged ≥ 65 years was 72% (95% CI; 60–81) for preventing hospitalizations due to COVID-19 and 68% (95% CI; 42–82%) for reducing COVID-19-related deaths27. Conversely, a recent study from the National Health Care Safety Network nursing homes, where most residents received a bivalent vaccine, reported vaccine effectiveness against infection at 31.2%28. Collectively, these data support the notion that booster doses of monovalent or bivalent COVID-19 mRNA vaccination are less protective against infection but effective in preventing severe diseases among nursing home residents.
In this study, we found that the NTs against BA.4/BA.5 were significantly higher in staff compared to residents following to the fifth booster dose of a bivalent BA.4/BA.5 mRNA vaccine, although no significant difference was observed for anti-RBD IgG titers and NTs against Wuhan between the two groups (Fig. 2b). A recent study reported that a bivalent Wuhan/BA.4/BA.5-adapted BNT162b2 booster similarly induced NTs against BA.4/BA.5 both in study participants aged 18–55 years and aged ≥55 years24. In our study, 25 staff members with a median age (IQR) of 48 years (43–59 years) were significantly younger than 48 residents with a median age (IQR) of 88 years (82–92 years) (p < 0.001). Considering the significant difference in NTs against BA.4/BA.5 between the two groups, it is challenging to attribute this solely to age differences. Frailty may play a role in the significant decrease observed in residents’ NTs against BA.4/BA.5 compared to staff29. The detection of negligible NTs against XBB following the bivalent Wuhan/BA.4/BA.5-adapted vaccine is in agreement with prior studies30,31.
Although the vaccination rates of the first to the third dose of COVID-19 mRNA vaccination were higher than 90% in older adults in Japan, the vaccination rate of the Omicron XBB.1.5 mRNA vaccine32 was reported to be relatively low (~ 53.7%) in older adults at the end of March 202433. Because this vaccine was reported to be associated with 76.1% reduced risk of COVID-19 hospitalization among people older than 65 years34, promotion of the variant-targeted COVID-19 vaccination is anticipated to lower the burden of severe COVID-19 in older adults.
One strength of our study is the ability to compare the rate of AEs following COVID-19 mRNA vaccinations between SARS-CoV-2 naïve staff and residents within a Japanese nursing home cohort. However, several limitations exist. Firstly, the small sample size may have limited our ability to compare anti-RBD IgG levels or NTs between ‘any AE’ and ‘no AE’ staffs and residents. Therefore, our results should not be overinterpreted. Secondly, the high dropout rate among participants in this study may have influenced the AE rate; for example, individuals experiencing severe AEs might have refused subsequent booster doses and dropped out of the study. Thirdly, observer bias may have been a confounding factor, as AE information was collected by nursing home staff from residents using a questionnaire. Fourthly, we evaluated the antibody response two months after the booster dose but did it not evaluate one month post-vaccination, when peak levels were expected. Lastly, we were unable to collect blood samples to determine antibody responses after the fourth dose of COVID-19 mRNA vaccination due to SARS-CoV-2 transmission in a few nursing homes during the study period and logistical delays in survey preparation.
In conclusion, our findings highlight fewer AEs among SARS-CoV-2 naïve nursing home residents than among the staff. Antibody responses were increased regardless of the occurrence of AEs among SARS-CoV-2 naïve nursing home residents and staff post COVID-19 mRNA vaccination. These results strongly support the recommendation of this vaccine for such high-risk populations.
Methods
Study participants
This study commenced in May 2021, involving 335 SARS-CoV-2-naive persons (194 staff and 141 residents) from six nursing homes in Toyama Prefecture, Japan19. By May 2023, two months after the fifth vaccination dose of vaccination, 111 participants (78 residents and 33 staff) had continued the study. The reasons for excluding the 224 participants were study withdrawal, retirement of staff members, not having receiving the fifth dose of vaccination, failure to complete the questionnaire, and death of participants.
Among the 111 remaining participants, 95 SARS-CoV-2 naïve participants, consisting of 26 staff (median age, 51 years) and 69 residents (median age, 88 years), were evaluated for the rate of local and systemic AEs. SARS-CoV-2 naïve case was defined as the absence of COVID-19 confirmation and a negative result of anti-nucleocapsid protein IgG. BTI cases were defined was as previously outlined19. Briefly, BTI cases were defined based on a positive result from a reverse transcription polymerase chain reaction (RT-PCR), immunochromatography tests for SARS-CoV-2, or anti-nucleocapsid protein IgG before vaccination, two months and five months after the second, the third, and the fifth doses of vaccination19.
All participants received an initial series of two doses of the COVID-19 mRNA vaccine, BNT162b2 (Pfizer/BioNTech, 30 µg per dose), targeting the Wuhan strain, administered between April and June 2021. Subsequently, for the third dose, 32 participants received the BNT162b2, while 79 received the mRNA-1273 vaccine (Moderna, 50 µg per dose), between January and March 2022. For the fourth dose, 11 participants received the BNT162b2 vaccine, and 100 received the mRNA-1273 vaccine between July 2022 and September 2022. Regarding the fifth dose, 30 participants received the bivalent Wuhan/BA.1-adapted BNT162b2 mRNA vaccine23, and 81 received either the bivalent Wuhan/BA.4/BA.5-adapted BNT162b2 mRNA vaccine24 or the bivalent mRNA-1273.222 vaccine26 between November 2022 and February 2023. The bivalent vaccines included an ancestral SARS-CoV-2 strain component along with a component from either the BA.1 subvariant or BA.4/BA.5 subvariant. The safety profiles of these bivalent vaccines were comparable to those of monovalent vaccines (BNT162b2 or mRNA-1273)23–27. In our study, we did not randomize and select the participants of the nursing homes who cooperated in the survey. The choice of vaccine type was made by the municipality and facility policy for residents, by the municipality and facility policy for staff for the first to the third vaccinations, and by the individual for the fourth and the fifth vaccines.
Blood samples were collected from May to June 2021 prior to the first COVID-19 vaccination, and then at two and five months following the primary series vaccination (August to September 2021 and November to December 2021, respectively). Subsequent blood sampling occurred two and five months after the third dose of booster vaccination (March to June 2022 and June to September 2022, respectively), and two months after the fifth dose of the vaccine (March to April 2023) (Fig. 1). Blood samples post the fourth dose could not be obtained as participants received the fifth dose before scheduled blood sampling.
This study received approval from the Ethical Review Committee of the Toyama Institute of Health (approval number #R4-14) and adhered to the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all participants or their surrogates.
Genotyping of SARS-CoV-2
Clinical samples, including nasopharyngeal swabs or saliva, which tested positive via RT-PCR or antigen tests at hospitals in Toyama Prefecture, were gathered at the Toyama Institute of Health as part of the national COVID-19 surveillance effort. In total, 2,223 samples underwent genomic analysis between March 2021 and October 2023. Whole-genome sequencing and variant classification were conducted following previously established protocols19.
AEs after vaccination
A questionnaire was distributed to study participants to assess the presence or absence of local or systemic AEs over a seven-day period following each dose of COVID-19 mRNA vaccination. Local AEs encompassed pain, redness, and swelling at the injection site, while systemic AEs included fever, fatigue, headache, chills, vomiting, diarrhea, muscle pain, joint pain, and anaphylactic shock. The severity of non-life-threatening AEs was graded according to a previously established scale35, no AE, mild AE, moderate AE, and severe AE, respectively. Pain at the injection site, fatigue, headache, chills, muscle pain, and joint pain was assessed according to the following scale: mild, does not interfere with activity; moderate, interferes with activity; and severe, prevents daily activity. Redness and swelling were measured according to the following scale: mild, 2.0 to 5.0 cm in diameter; moderate, > 5.0 to 10.0 cm in diameter; and severe, > 10.0 cm in diameter. Fever categories are designated in. Additional scales were as follows: vomiting (mild, 1 to 2 times in 24 h, moderate, > 2 times in 24 h, severe, requires intravenous hydration), and diarrhea (mild, 2 to 3 loose stools in 24 h; moderate, 4 to 5 loose stools in 24 h, or severe, 6 or more loose stools in 24 h)35. Rate of AE indicated the frequency of occurrence of each of the above AEs. Nursing staff were responsible for collecting AE information from study participants including residents with communication difficulties. Nursing home staff conducted this assessment of AEs for each sign or symptom in residents every day for the seven days after each dose of vaccination dose.
Ninety-five SARS-CoV-2 naïve participants, comprising 26 staff and 69 residents, underwent analysis of AE rates. None of the study participants required hospitalization following the vaccination.
Neutralizing activity against SARS-CoV-2 pseudotyped virus
Pseudotyped vesicular stomatitis virus (VSVs) bearing SARS-CoV-2 Spike (S) proteins were generated following established procedures36. Expression plasmids for the truncated S protein of SARS-CoV-2 variants, namely pCAGG-pm3-SARS2-SHu-d19_B.1.1.529.5 (Omicron BA.5 subvariant) and pCAGG-pm3-SARS2-SHu-d19_XBB1.5 (Omicron XBB.1.5 subvariant), were generously provided by Drs. C. Ono and Y. Matsuura of the Research Institute for Microbial Diseases, Osaka University, Japan. The SARS-CoV-2 pseudotyped VSVs (SARS-CoV-2pv) were stored at -80 °C until utilization. Neutralizing activity was assessed using a chemiluminescence reduction neutralizing test of SARS-CoV2pv bearing S proteins from the ancestral strain (Wuhan), Omicron BA.5 (B.1.1.529.5), and Omicron XBB.1.5, following previously established protocols19,36.
SARS-CoV-2 IgG ELISA
Anti- RBD IgG and anti-nucleocapsid IgG ELISA were performed as previously described19.
Statistical analysis
Post-vaccination AEs categorized by age, sex, and the mean anti-RBD IgG values and geometric mean NTs were analyzed using IBM SPSS Statistics 27.0 (IBM, Armonk, NY). The NT value underwent log transformation. Statistical significance was defined at p < 0.05. Missing data was excluded and used only data sets for which all variables were available. The Mann-Whitney U test or Friedman test was employed for comparing non-parametric variables (antibody titers and age) among groups (staff and residents, vaccination history). The Pearson’s Chi-square test was utilized to compare the rate of AEs by vaccine dose. No statistical power for the initial target sample size was calculated in this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We extend our gratitude to the residents and staff of the nursing homes and the nurses from the Toyama City Medical Association. Special thanks to Izumi Kawaguchi for their outstanding technical and secretarial support. We also appreciate the cooperation of Yukari Murotani (Alpen Rehabilitation Hospital, Toyama), Yoichi Sugimori and Mayumi Ishikuro (Nursing Home Asitanenomori, Toyama), Yosuke Hirata (Nursing Home Miyabi, Toyama), Miwa Ise (Nursing Home Harukaze, Toyama), Maki Sakai (Nursing Home Kagayaki, Toyama), Eijiro Minami, and Kazuyoshi Haba (Nursing Home Kurehaen, Toyama) in facilitating this study. Additionally, we thank Makoto Kuroda and Tsuyoshi Sekizuka at the Pathogen Genomics Center, National Institute of Infectious Diseases, Tokyo, for their technical assistance in sequence analysis, and Masanori Yagi, Moderna Japan Co., Ltd., for his critical comments on the manuscript. We would like to thank Editage (www.editage.com) for English language editing.
Abbreviations
- COVID-19
Coronavirus Disease 2019
- ELISA
Enzyme-Linked Immunosorbent Assay
- AE
Adverse event
- HCW
Healthcare worker
- NT
Neutralizing titer
- RBD
Receptor-binding domain
- S
Spike
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SARS-CoV-2pv
Severe acute respiratory syndrome coronavirus 2 pseudotyped virus
- VSVs
Vesicular stomatitis viruses
- RT-PCR
Reverse transcription polymerase chain reaction
- SD
standard deviations
- IQR
interquartile range
Author contributions
Author contribution statementM.I. and H.T. conducted conceptualization and methodology. M.I., H.T., and K.O. wrote the manuscript. S.Y., Y.S., T.S., K.T, E.M, and J.I. worked for the laboratory investigations. H.S., C.K., and K.O. supervised the study.
Funding
This work was supported in part by the [Japan Society of Public Health] under Grant [2021] (H.T.); [Daido Life Insurance Company] under Grant [2021-14] (H.T.) [2023] (M.I); and [Kurozumi Medical Foundation] under Grant [2021] (M.I.).
Data availability
Data are available within the manuscript or supplementary information. The data of this study are available on request from the corresponding author. However, part of the data is not publicly available due to restrictions e.g. their containing information that could compromise the privacy of research participants.
Declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
This study received approval from the Ethical Review Committee of the Toyama Institute of Health (approval number #R4-14) and adhered to the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all participants or their surrogates.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.White, E. M. et al. Variation in SARS-CoV-2 prevalence in U.S. skilled nursing facilities. J. Am. Geriatr. Soc.68, 2167–2173 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimizu, K. et al. Epidemiology of SARS-CoV-2 infection in nursing facilities and the impact of their clusters in a Japanese core city. J. Infect. Chemother.28, 955–961 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamura, K. et al. Impact of COVID-19 and closed transmission of SARS-CoV-2 during the first wave in Toyama Prefecture, Japan, March 30 to May 18, 2020. Jpn. J. Infect. Dis.77, 75–82 (2024). [DOI] [PubMed] [Google Scholar]
- 4.Comas-Herrera, A. et al. LT Covid International Living Report on COVID-19 and Long-Term Care (2022). https://ltccovid.org/international-living-report-covid-ltc/
- 5.Iritani, O. et al. Clusters of COVID-19 in long-term care hospitals and facilities in Japan from 16 January to 9 May 2020. Geriatr. Gerontol. Int.20, 715–719 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med.383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamprinou, M. et al. COVID-19 vaccines adverse events: Potential molecular mechanisms. Immunol. Res.71, 356–372 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allergic Reactions including Anaphylaxis after receipt of Moderna COVID-19 Vaccine-United States. December 21, 2020–January 10, 2021. MMWR Morb Mortal. Wkly. Rep.70, 125–129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mevorach, D. et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N. Eng. J. Med.385, 2140–2049 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pather, S. et al. SARS-CoV-2 omicron variants: Burden of disease, impact on vaccine effectiveness and need for variant-adapted vaccines. Front. Immunol.14, 1130539 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park, H. J. et al. Comparing frequency of booster vaccination to prevent severe COVID-19 by risk group in the United States. Nat. Commun.15, 1883 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takano, T. et al. Distinct immune cell dynamics corelate with the immunogenicity and reactogenicity of SARS-CoV-2 mRNA vaccine. Cell. Rep. Med.3, 100631 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koike, R. et al. Systemic adverse effects induced by the BNT162b2 vaccine are associated with higher antibody titers from 3 to 6 months after vaccination. Vaccines10, 451 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobashi, Y. et al. Factors associated with anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein antibody titer and neutralizing activity among healthcare workers following vaccination with the BNT162b2 vaccine. PLOS ONE17, e0269917 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy, I. et al. Correlation between adverse events and antibody titers among healthcare workers vaccinated with BNT161b mRNA COVID-19 vaccine. Vaccines10, 1220 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Held, J. et al. Reactogenicity correlates only weakly with humoral immunogenicity after COVID-19 vaccination with BNT162b2 mRNA (Comirnaty). Vaccines9, 1063 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tani, N. et al. Relation of fever intensity and antipyretic use with specific antibody response after two doses of the BNT612b2 mRNA. Vaccine40, 2062–2067 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeuchi, M., Higa, Y., Esaki, A., Nabeshima, Y. & Nakazono, A. Does reactogenicity after a second injection of the BNT162b2 vaccine predict spike IgG antibody levels in healthy Japanese subjects? PLOS ONE16, e0257668 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itamochi, M. et al. Neutralization of Omicron subvariants BA.1 and BA.5 by a booster dose of COVID-19 mRNA vaccine in a Japanese nursing home cohort. Vaccine41, 2234–2242 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canaday, D. H. et al. COVID-19 vaccine booster dose needed to achieve Omicron-specific neutralisation in nursing home residents. EBiomedicine80, 104066 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong, Y. et al. Pronounced antibody elevation after SARS-CoV-2 BNT162b2 mRNA booster vaccination in nursing home residents. Influ. Other Respir. Viruses16, 1066–1071 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oyebanji, O. A. et al. Does a lack of vaccine side effects correlate with reduced BNT162b2 mRNA vaccine response among healthcare workers and nursing home residents? Aging Clin. Exp. Res.33, 3151–3160 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winokur, P. et al. Bivalent omicron BA.1-Adapted BNT162b2 booster in adults older than 55 years. N. Engl. J. Med.388, 214–227 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usdan, L. et al. A bivalent Omicron–BA.4/BA.5-Adapted BNT162b2 booster in ≥ 12-Year-Olds in. Clin. Infect. Dis.78, 1194–1203 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalkias, S. et al. Original SARS-CoV-2 monovalent and Omicron BA.4/BA.5 bivalent COVID-19 mRNA vaccines: Phase 2/3 trial interim results. Nat. Med.29, 2325–2333 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McConeghy, K. W. et al. Infections, hospitalizations, and deaths among US nursing home residents with vs without a SARS-CoV-2 vaccine booster. JAMA Netw. Open5, e2245417 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arbel, R. et al. Effectiveness of a bivalent mRNA vaccine booster dose to prevent sever COVID-19 outcomes: A retrospective cohort study. Lancet Infect. Dis.23, 914–921 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong, E. et al. Effectiveness of up to date COVID-19 vaccination in preventing SARS-CoV-2 infection among nursing home residents–United States, November 29, 2022–. Wkly. Rep.72, 690–693 (2023). MMWR Morbid. Mortal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fulop, T., Pawelec, G., Castle, S. & Loeb, M. Immunosenescence and vaccination in nursing home residents. Clin. Infect. Dis.48, 443–448 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Kurhade, C. et al. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat. Med.29, 344–347 (2023). [DOI] [PubMed] [Google Scholar]
- 31.Amano, M. et al. Neutralization against Omicron sublineages (BA.2/BA.5/BQ.1.1/XBB/XBB.1.5) in bivalent BNT162b2-vaccinated HCWs with or without risk factors, or following BT infection with Omicron. Sci. Rep.13, 17404 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gayed, J. et al. Safety and immunogenicity of the monovalent Omicron XBB.1.5-adapted BNT162b2 COVID-19 vaccine in individuals ≥ 12 years old: A phase 2/3 trial. Vaccines12, 118 (2024). [DOI] [PMC free article] [PubMed]
- 33.The vaccination rates of COVID-19 vaccine. Cabinet Japan (2024). https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/kekkaku-kansenshou/yobou-sesshu/syukeihou_00002.html
- 34.Hansen, C. H. et al. Short-term effectiveness of the XBB.1.5 updated COVID-19 vaccine against hospitalisation in Denmark: A national cohort study. Lancet Infect. Dis.24, e73–e74 (2024). [DOI] [PubMed] [Google Scholar]
- 35.Food and Drug Administration in US. Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials (2005). https://www.fda.gov/media/73679/download [DOI] [PubMed]
- 36.Tani, H. et al. Evaluation of SARS-CoV-2 neutralizing antibodies using a vesicular stomatitis virus possessing SARS-CoV-2 spike protein. Virol. J.18, 16 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available within the manuscript or supplementary information. The data of this study are available on request from the corresponding author. However, part of the data is not publicly available due to restrictions e.g. their containing information that could compromise the privacy of research participants.