Abstract
The 1997/1998 El Niño event caused mass coral bleaching and mortality in many tropical and subtropical regions, including corals on Green Island, Taiwan, in the northwestern Pacific Ocean. This study analyzed coral carbon isotope ratios (δ13C), oxygen isotope ratios (δ18O), and Sr/Ca ratios for 29 years, including the 1997/1998 El Niño period, to examine how high water temperature events are recorded in coral geochemical indicators. Sr/Ca ratios in coral skeletons from Green Island show the lowest peak, means the highest temperature during the 1997/1998 El Niño period. However, we couldn’t observe high-temperature events on δ18O. Furthermore, a negative δ13C shift was observed after El Niño events. The regime shift of δ13C might have been caused by temporal bleaching and/or a decrease in symbiotic algae due to high water temperature stress under the continuous decrease in δ13C in DIC due to the Suess effect.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74219-5.
Subject terms: Biogeochemistry, Ocean sciences
Introduction
The El Niño-Southern Oscillation (ENSO) is one of the most thermal stressors for corals and has become more frequent and severe in recent decades1. The regions that experienced the high sea surface temperature (SST) associated with the 1997/1998 El Niño event faced mass coral bleaching and mortality2. However, a few weeks of bleaching events can only be confirmed by visual inspection at various locations, making it difficult to know the occurrence of past bleaching events. Annual bands of reef-building coral skeletons have been used as paleoenvironmental archives. If a proxy of bleaching events can be found, it will be possible to reconstruct past bleaching records continuously.
Bleaching is the reduction or complete withdrawal of symbiotic algae from corals, leading to starvation, emaciation, disease, and death due to reduced nutrient availability from the symbionts3. To compensate for the lack of photosynthetic energy, reef-building corals use stored energy4–8, increased heterotrophic feeding6, decreased metabolic rates8, decreased calcification rates9,10. The long-lived massive corals are alive as their aragonite skeletons form with aragonite, and their skeletons have recorded their responses to thermal stress events. In coral skeletal growth, abrupt decreases in annual extension rates, high-density stress bands9–11, and growth hiatuses due to partial mortality11–13 have been reported. Although many factors vary coral skeletal δ13C, δ13C in seawater14, δ13C of the metabolic CO215, amount of solar radiation16, vital effects17, kinetic isotope fractionation18,19 and spawning20, the previous studies have reported the δ13C in the coral skeletons decreased after the 1997/1998 El Niño event21–24. Coral skeletal stable oxygen isotope (δ18O) is a proxy for SST and seawater δ18O18. Still, a negative shift has been reported following the 1997/1998 El Niño, associated with reduced coral extension rates13.
Coral skeletal Sr/Ca ratios are used as reliable SST proxy25, but anomalies of Sr/Ca have been reported during El Niño event13,26–28. Trace element (TE) uptake into coral skeletons might be affected by changes in skeletal microstructure29–31. For example, it causes a reduction in calcification rate due to heat stresses decreasing Ca2 + transport by the Ca-ATPase pump and causing changes in TE/Ca in the skeleton32–34. As a result, Sr/Ca is expected to be larger than that is expected from SST35.
There is still no unified view on how bleaching events are recorded in coral skeletons, and many research cases are needed. In this study, we analyzed δ13C, δ18O, and Sr/Ca ratios in Porites coral cores from Green Island, Taiwan, where coral bleaching events were reported with 1997/1998 El-Nino, to determine how high-water temperature events are recorded in coral geochemical indices.
Materials and methods
Study sites and samples
We drilled coral cores from a massive Porites colony alive at Green Island off the southeast coast of Taiwan on the 7th of April 2009. Green Island (Lutao) belongs to the tropical region in Taiwan and is located on the north of Kuroshio bifurcation, and the current speed is rapidly flowing northward36. Underwater coral drilling was performed at a depth of 12 m off the west coast of Green Island (22°39’231 N, 121°28’342E, Fig. 1) using an underwater drilling system. The coral cores are 8 cm in diameter and 2 m long. The coral tissue on the core top was soaked overnight in household bleach (HClO diluted to about 0.1% with water). Cores were sliced along the axis of maximum coral growth. After rinsing with Milli-Q water, they were dried. X-ray photographs were taken (Fig. 2) using a non-destructive transmission 2D X-ray imaging scanner TATSCAN-X137 at 37.6 or 38.6 kV with an exposure of 2.02 mA. The X-ray images were used to identify the annual density bands and determine the measurement lines of geochemical analysis.
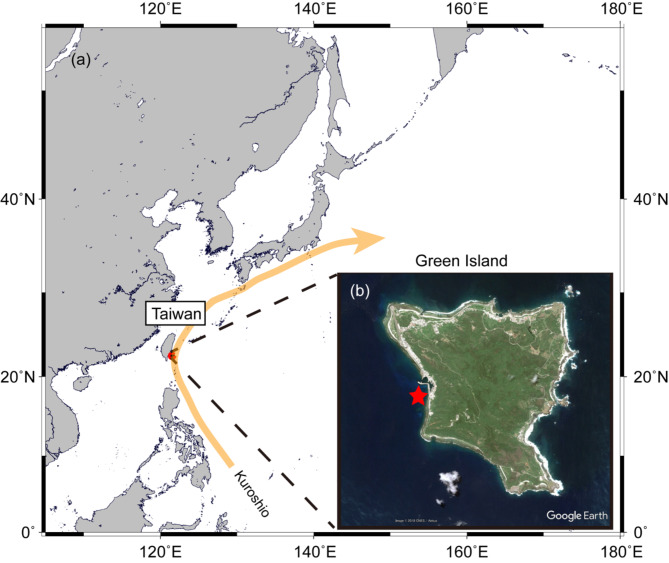
Fig. 1.
The location of Green Island in Taiwan (photo by Google earth) showing (a) north-east Pacific, (b) Green Island. A star indicates a sampling site.
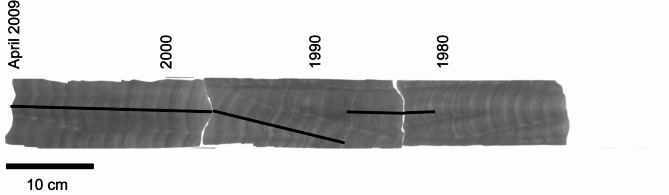
Fig. 2.
The X-ray photographs of the coral core slabs. The black lines are the tracks of geochemical analysis.
The 2 mm thick ledges were formed along each measurement line. To remove cutting dust, the ledges were ultrasonically cleaned three times for 10 min in distilled water and completely dried at 30 °C. 1365 powder samples were collected at 1 mm intervals and 2 mm widths along the maximum growth lines using the micro-sampling method with a micro drill and a digitally controlled automated stage.
Geochemical analysis
The δ18O and δ13C in coral skeletal powder were analyzed using a Finnigan MAT251 stable isotope ratio mass spectrometer system connected to an automated individual-carbonate device (Finnigan; Kiel Device II). The carbonate powder was reacted with 100% H3PO4 at 70 °C in the Kiel Device II and the resulting CO2 gas produced was induced in the MAT251. The isotopic δ-values with Vienna Pee Dee Belemnite (VPDB) scale derived from stable isotope ratios were obtained from duplicate measurement of NBS19 standard (IAEA certified values; δ18O = − 2.20‰, δ13C = + 1.95‰). Standard deviations (1σ) of the duplicate analysis of NBS19 were 0.11‰ and 0.07‰ for the coral δ18O and δ13C, respectively.
Sr/Ca ratios in coral skeletal powder were determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES, Thermo Fisher Scientific; iCAP6200) following a combined method of Schrag38, de Villiers et al.39. and Watanabe et al.40. After dissolving the sample powder in 0.5 ml of 25% HNO3, the solution was diluted with Milli-Q to Ca concentrations of 7 mg/L and analyzed by ICP-AES connected to an automatic sampler (CETAC; ASX-260). All data were calibrated against JCP-141 solution using Ca concentrations of 7 mg/L as standard. The analytical errors (RSD) were 0.181% for the coral Sr/Ca.
Age model and statistical analysis
Sr/Ca ratios compared with annual density bands were used to construct age models for all other proxies. A Sr/Ca cycle represents one year; the maximum (minimum) Sr/Ca value was associated with the minimum (maximum) SST record. Proxy data were resampled to 12 data per year using AnalySeries software ver.2.0.842. A regime shift of the proxies was tested by following Rodionov43.
SST data and NINO 3.4 index
To compare the isotopic data and Sr/Ca data, SSTs for the sample site were derived from the “Advanced Very High Resolution Radiometer (AVHRR)44” satellite dataset on the National Oceanic and Atmospheric Administration (NOAA) of Integrated Global Ocean Services System (IGOSS). The dataset is a 1°×1° grid, covering 29 years from 1980 to 2010 over 22° N − 23° N, 121° E − 122° E. The NINO3.4 index was calculated using SST data from 1981 to 2010. The NINO 3.4 index was normalized to the 1981–2010 ERSST.v5 anomaly for the NINO 3.4 region (5° N–5° S, 12° W–170° W) from Climate Explorer at the Royal Netherlands Meteorological Institute (KNMI)45. The maximum SST was 31.5 °C in August 1998, and the minimum was 21.2 °C in March 1987.
Results
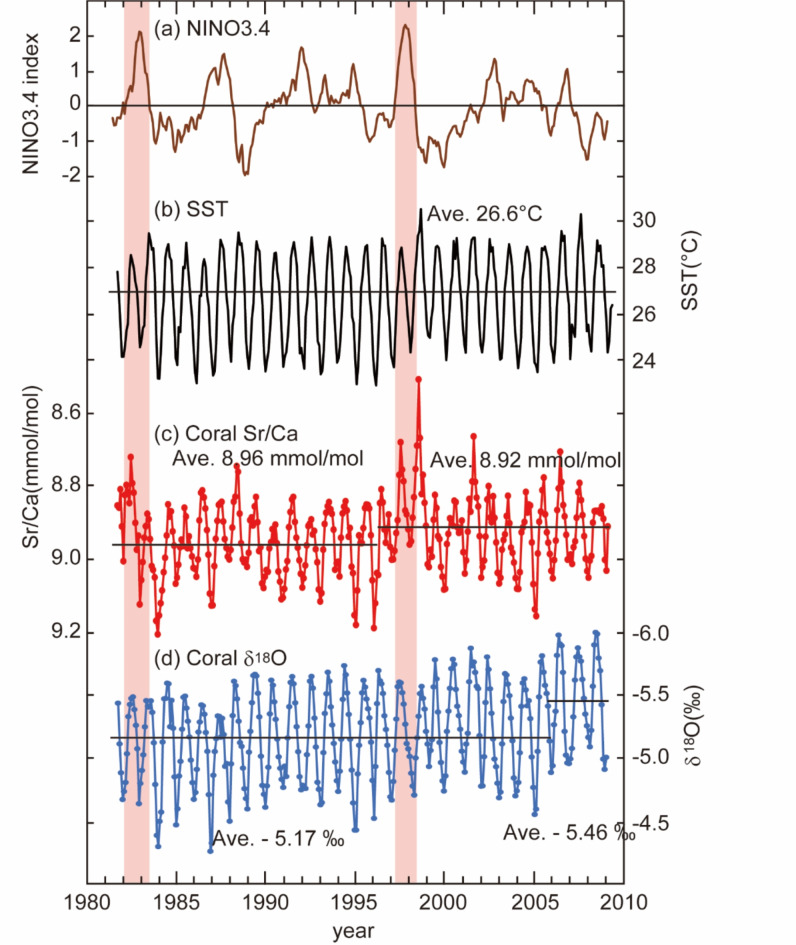
Coral Sr/Ca ratios show 27 clear annual cycles from 1983 to 2009 (Fig. 3). Sr/Ca ratios range from 8.51 mmol/mol to 9.21 mmol/mol with an average of 8.94 mmol/mol. The lowest peak of Sr/Ca ratios corresponded with the highest temperature anomaly of the SST in 1997/1998. The regime shift was observed before and after 1997 with the regime shift test, which showed the average of Sr/Ca ratios decreased from 8.96 mmol/mol to 8.92 mmol/mol. The coral Sr/Ca-SST relationship obtained from the linear regression of the monthly Sr/Ca and SST records.
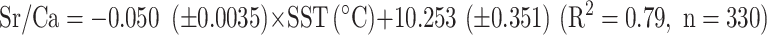 |
1 |
Fig. 3.
SST from AVHRR for 1986–2009 including 1997/1998 El Niño event (a). Records of Sr/Ca ratio (b) and δ18O (c) in coral skeletons.
Coral δ18O also shows annual cycles as Sr/Ca ratios (Fig. 3). The fluctuations of δ18O ranged from − 6.00‰ to − 4.26‰ with an average of − 5.21‰ (Fig. 3). The coral δ18O was negatively shift of − 0.29‰ before and after 2006. The coral δ18O-SST relationship between the monthly coral δ18O and SST was:
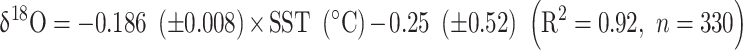 |
2 |
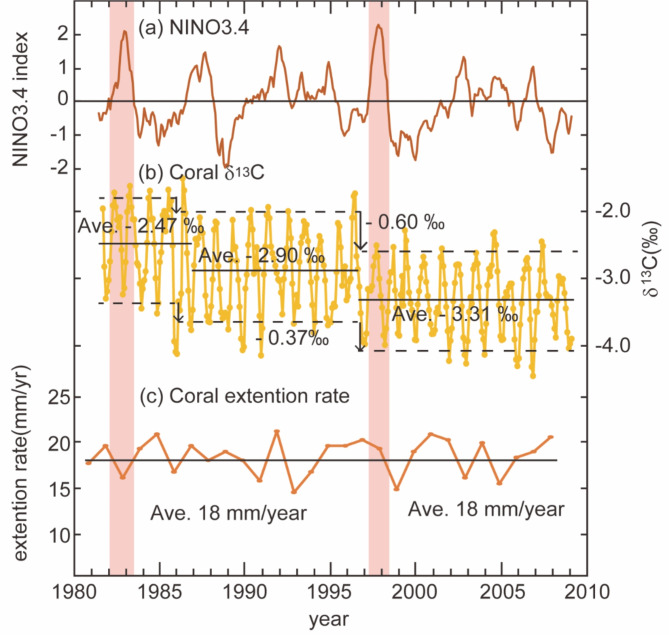
Coral δ13C shows clear annual cycles ranging from − 4.48‰ to − 1.52‰ with an average of − 3.02‰ (Fig. 4). With the regime shift test, the two shifts were observed in the 1987 and 1997/1998 El Niño years with decreases in the time series of δ13C through the 27 years. The mean δ13C values were − 2.47‰ from 1982 to 1986, − 2.90‰ from 1986 to 1996, and − 3.31‰ from 1996 to 2010. After the 1997/1998 El Niño, the mean of the maxima shifted by − 0.60‰ and the mean of the minima by − 0.37‰.
Fig. 4.
Comparison of NINO3.4 index (a) including 1997/1998 El Niño event with records of δ13C (b), and the coral extension rate (c). Black solid lines show averages in each period and broken lines show averages of maximum and minimum in each year. The solid black lines show the average of the respective periods before and after 1997/1998 El Niño, and the dashed lines show the average of the maximum and the minimum values for each year. The hatched areas represent the 1997/1998 El Niño seen from NINO3.4 index. The red hatched areas represent the particularly large 1981/1982 and 1997/1998 El Niño which NINO3.4 index indicates more than 2.
The coral annual extension rates estimated from the Sr/Ca ratios and density bands varied from 13 to 21 mm/year, with an average of 18 mm/year (Fig. 4).
Discussion
During the 1997/1998 El-Nino event, coral Sr/Ca showed apparent high-temperature anomalies consistent with the SST data, while δ18O showed no anomalies. Sr/Ca shifted negatively and maintained lower average values, and this trend is observed in other trace elements (Fig. S2; Mg/Ca). The changes in concentration of trace elements with El Niño events have been reported in previous studies13,26–28. Without the term of El Nino, Sr/Ca and δ18O maintained a linear relationship with SST. Shen et al.46 reported a slope range of − 0.051 to − 0.053 mmol/mol for the Sr/Ca-SST relationship for Porites in southern Taiwan, and the slope obtained from Eq. (1) is consistent with previous studies. The slope of the linear relationship of the coral δ18O-SST is − 0.186 from − 0.186 to − 0.209‰, which aligns with previous studies in the Pacific Ocean37,47–49.
Several factors may be responsible for decreased coral δ13C observed in 1986–1988 and 1997/1998 El Niño. While an accelerated decrease in δ13C reflecting the accumulation of anthropogenic CO2 in the surface ocean is well known (i.e., the Suess effect)50, the stepwise decrease in coral skeletons associated with the El Niño event in this study could not be only consistent with the continuous trend of the Suess effect. The negative regime shift in the coral δ13C after each El Niño event is similar to that reported by Wang et al.21 from South China Sea. They proposed three possibilities of decline for δ13C: a decrease in zooxanthellae density, an exchange to the different species of zooxanthellae with stronger thermal tolerance before and after the stepwise change of δ13C, and a kinetic effect21.
The coral physiological changes due to thermal stress with the density of zooxanthellae could be decline coral δ13C. The δ13C differences between unbleached and bleached corals, that an 85% loss of symbiotic algal density in bleached corals, were reported − 0.4~− 1‰7. The coral δ13C decline in this study, − 0.37 and − 0.60‰ for 1986–1988 and 1997/1998 El-Nino, is within the range of coral δ13C decline with bleaching events.
The stepwise decrease in coral δ13C may be due to multiple factors resulting from El Niño or decadal-scale climate change and long-term variations such as the Suess effect. The exchanging of different zooxanthellae species might maintain average coral δ13C afterward. The reconstruction of symbiosis between coral-algae with mass breaching events have been reported51. However, it has been reported that Porites corals have less diversity of symbiodinium species52. The possibility of change in coral δ13C due to symbiodinium species has room for demonstration for future studies. McConaughey18,19 suggested that slowing coral skeletal growth makes δ13C increase due to kinetic effects. However, the extension rates of the coral in this study did not change after El Niño. The coral δ13C decreasing is not explained by the kinetic effect with thermal stress event in this study.
In other previous studies, the stepwise decline of coral δ13C has been reported and observed in the coral δ13C dataset from the South China Sea53and Ogasawara54 (Fig.S1). Coral δ13C in Xisha Island coral step wisely declined − 0.26‰ for 1986–1988 and − 0.59‰ for 1997/1998. Coral δ13C in Ogasawara coral also suggests the same decline trend as − 0.21‰ in 1976–1978 of El Nino years and − 0.65‰ in 1986–1988. These results suggest the decline of coral δ13C is not only an event in southeast Taiwan but also a common regional event of the north-western Pacific.
The Kuroshio current intensified during the El Nino years; Kuroshio water intrusion increased to the northwestern Pacific55. The Kuroshio Current transports heat, tropical organisms such as reef-building corals, and fishery resources to high latitudes and is a carbon dioxide sink56,57. The Kuroshio Current and its successor basins are high carbon dioxide sinks cooled by the northward flow of water masses with low carbon dioxide partial pressures. The DIC δ13C could cause the decline of coral δ13C in El Nino years. Another possibility is that δ13C of juvenile fish and zooplankton in the Kuroshio Current is about − 20‰58, which is lower than the coral δ13C (− 3.0‰ in this study). The coral δ13C decreased after the 1997/1998 El Niño without changing the annual extension. The heterotrophic feeding also might compensate for the lack of photosynthetic energy due to the decrease in symbiotic algae59.
This study found a decreasing shift in carbon isotope ratios of coral skeletons on Green Island, Taiwan, before and after the 1997/1998 El Niño event. This is thought to be the result of a rapid decrease in carbon isotope ratios due to bleaching, a decrease in symbiotic algae, and/or a change to heterotrophy high water temperature stress and in a continuous decrease in carbon isotope ratios in the DIC due to the Suess Effect. Although similar reports have been made in some areas, it will be necessary in the future to compare the occurrence of carbon isotope ratio declines in Porites corals in areas where bleaching events have occurred. In addition, clarifying the mechanism of carbon isotope ratio fluctuation itself may make it possible to use it as a more precise indicator of bleaching or as an indicator of rates in algal symbiosis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Our thanks to CREES members in Hokkaido University and Research Center for Future Earth in National Taiwan University for their assistance in the preparation and analysis of coral cores. We would like to thank to Lü dăo Jū fu Diving service in Green Island in Taiwan for sampling coral cores specially. I would like to express my great appreciation to Dr. Samuel E. Kahung, a visiting professor at Hokkaido University, who took me time out of his busy schedule torevise my English.
Author contributions
Ikeda wrote the manuscript text and created all figures. All authors reviewed the manuscript.
Data availability
After publication, all coral data will be available on the data repository at the KIKAI Institute for Coral Reef Sciences (https://coralogy.kikaireefs.org).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: In the original version of this Article, Masataka Ikeda was incorrectly listed as the corresponding author. The correct corresponding author for this Article is Tsuyoshi Watanabe. Correspondence and request for materials should be addressed to nabe@sci.hokudai.ac.jp.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/20/2024
A Correction to this paper has been published: 10.1038/s41598-024-80208-5
References
- 1.Trenberth, K. E. & Hoar, T. J. El Ninõ and climate change. Geophys. Res. Lett. 24, 3057–3060. 10.1029/97GL03092 (1997). [Google Scholar]
- 2.Goreau, T., McClanahan, T., Hayes, R. & Strong, A. Conservation of Coral reefs after the 1998 global bleaching event. Conserv. Biol. 14, 5–15 (2000). [Google Scholar]
- 3.Brown, B. E. Coral bleaching: causes and consequences. Coral Reefs 16, S129–S138. 10.1007/s003380050249 (1997). [Google Scholar]
- 4.Fitt, W. K., Spero, H. J., Halas, J., White, M. W. & Porter, J. W. Recovery of the coral Montastrea annularis in the Florida Keys after the 1987 caribbean bleaching event. Coral Reefs 12, 57–64. 10.1007/BF00302102 (1993). [Google Scholar]
- 5.Grottoli, A. G. & Rodrigues, L. J. Bleached Porites compressa and Montipora capitata corals catabolize δ13C-enriched lipids. Coral Reefs 30, 687–692. 10.1007/s00338-011-0756-0 (2011). [Google Scholar]
- 6.Grottoli, A. G., Rodrigues, L. J. & Palardy, J. E. Heterotrophic plasticity and resilience in bleached corals. Nature 440, 1186–1189. 10.1038/nature04565 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Porter, J. W., Fitt, W. K., Spero, H. J., Rogers, C. S. & White, M. W. Bleaching in reef corals: physiological and stable isotopic responses. Proc. Natl. Acad. Sci. USA 86, 9342–9346. 10.1073/pnas.86.23.9342 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues, L. J. & Grottoli, A. G. Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol. Oceanogr. 52, 1874–1882. 10.4319/lo.2007.52.5.1874 (2007). [Google Scholar]
- 9.Barkley, H. C. & Cohen, A. L. Skeletal records of community-level bleaching in Porites corals from Palau. Coral Reefs 35, 1407–1417. 10.1007/s00338-016-1483-3 (2016). [Google Scholar]
- 10.Mallela, J., Hetzinger, S. & Halfar, J. Thermal stress markers in Colpophyllia natans provide an archive of site-specific bleaching events. Coral Reefs 35, 181–186. 10.1007/s00338-015-1350-7 (2016). [Google Scholar]
- 11.Cantin, N. E. et al. Ocean warming slows coral growth in the central Red Sea. Science 329, 322–325. 10.1126/science.1190182 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Cantin, N. E. & Lough., J. M. Surviving coral bleaching events: Porites growth anomalies on the great barrier reef. PLoS One 9, 1–12. 10.1371/journal.pone.0088720 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hetzinger, C., Pfeiffer, M., Dullo, W. C., Zinken, J. & Garbe-Schönberg, D. A change in coral extension rates and stable isotopes after El Niño induced coral bleaching and regional stress events. Sci. Rep. 6, 1–10. 10.1038/srep32879 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nozaki, Y., Rye, D. M., Turekian, K. K. & Dodge, R. E. A 200 year record of carbon-13 and carbon-14 variations in a Bermuda coral. Geophys. Res. Lett. 5, 825–828. 10.1029/GL005i010p00825 (1978). [Google Scholar]
- 15.Allison, N. & Finch, A. A. A high resolution δ13C record in a modern Porites lobata coral: insights into controls on skeletal δ13C. Geochim. Cosmochim. Acta 84, 534–542. 10.1016/j.gca.2012.02.004 (2012). [Google Scholar]
- 16.Swart, P. K., Leder, J. J., Szmant, A. M. & Dodge, R. E. The origin of variations in the isotopic record of scleractinian corals: II. Carbon. Geochim. Cosmochim. Acta 60, 2871–2885. 10.1016/0016-7037(96)00119-6 (1996). [Google Scholar]
- 17.Grottoli, A. G. & Wellington, G. M. Effect of light and zooplankton on skeletal δ13C values in the eastern Pacific corals Pavona clavus and Pavona gigantea. Coral Reefs 18, 29–41. 10.1007/s003380050150 (1999). [Google Scholar]
- 18.McCounnaughey, T. 13C and 18O isotopic disequilibrium in biological carbonatepatternstterns. Geochim. Cosmochim. Acta 53, 151–162. 10.1016/0016-7037(89)90282-2 (1989a). [Google Scholar]
- 19.McCounnaughey, T. δ13C and δ18O isotopic disequilibrium in biological carbonates: II. In vitro simulation of kinetic isotope effects. Geochim. Cosmochim. Acta 53, 163–171. 10.1016/0016-7037(89)90283-4 (1989b). [Google Scholar]
- 20.Gagan, M. K., Chivas, A. R. & Isdale, P. J. Timing coral-based climatic histories using δ13C enrichments driven by synchronized spawning. Geology 24, 1009–1012 (1996). [Google Scholar]
- 21.Wang, C. et al. Super instrumental El Niño events recorded by a Porites coral from the South China Sea. Coral Reefs 37, 295–308. 10.1007/s00338-018-1658-1 (2018). [Google Scholar]
- 22.Kajita, H. et al. Holocene sea surface temperature variations recorded in corals from Kikai Island, Japan. Geochem. J. 51, e9–e14. 10.2343/geochemj.2.0482 (2017). [Google Scholar]
- 23.Li, X. et al. Trace of the 1997 Indonesian wildfires in the marine environment from a network of coral δ13C records. Geophys. Res. Lett. 44. 10.1029/2020GL090383 (2020).
- 24.Andrews, A. H., Asami, R., Iryu, Y., Kobayashi, D. R. & Camacho, F. Bomb-produced radiocarbon in the wetern tropical Pacific Ocean: Guam coral reveals operation-specific signals from the Pacific proving grounds. J. Geoophys. Res. Oceans 121, 6351–6366. 10.1002/2016JC012043 (2016). [Google Scholar]
- 25.McCulloch, M. T., Gagan, M. K., Mortimer, G. E., Chivas, A. R. & Isdale, P. J. A high-resolution Sr/Ca and δ18O coral record from the great barrier reef, Australia, and the 1982–1983 El Niño. Geochim. Cosmochim. Acta 58, 2747–2754. 10.1016/0016-7037(94)90142-2 (1994). [Google Scholar]
- 26.Cheung, A. H. et al. Fidelity of the coral Sr/Ca paleothermometer following heat stress in the northern Garapagos. Paleoceanogr. Paleoclimatol. 36 (2021).
- 27.Mitsuguchi, T., Dang, P. D., Kitagawa, H., Uchida, T. & Shibata, Y. Coral Sr/Ca and Mg/Ca records in Con Dao Island off the Mekong Delta: assessment of their potential for monitoring ENSO and East Asian monsoon. Glob. Planet Change 63, 341–352. 10.1016/j.gloplacha.2008.08.002 (2008). [Google Scholar]
- 28.Clarke, H., D’Olivo, J. P., Conde, M., Evans, R. D. & McCulloch, M. T. Coral records of variable stress inpamcts and possible acclimatization to recent marine heat wave events on the northwest shelf of Australia. Paleoceanogr. Paleoclimatol. 34, 1672–1688. 10.1029/2018PA003509 (2019). [Google Scholar]
- 29.Meibom, M. et al. Distribution of magnesium in coral skeleton. Geophys. Res. Lett.31. 10.1029/2004GL021313 (2004).
- 30.Shirai, K. et al. Minor and trace element incorporation into branching coral Acropora nobilis skeleton. Geochem. Cosmochim. Acta 72, 5386–5400. 10.1016/j.gca.2008.07.026 (2008). [Google Scholar]
- 31.Tanaka, K. et al. Response of Acropora digitifera to ocean acidification: constraints from δ11B, Sr, mg, and Ba compositions of aragonitic skeletons cultured under variable seawater pH. Coral Reefs 34, 1139–1149. 10.1007/s00338-015-1319-6 (2015). [Google Scholar]
- 32.Clarke, H. et al. Differential response of corals to regional mass-warming events as evident from skeletal Sr/Ca and Mg/ ratios. Geochem. Geophys. Geosyst. 18, 1794–1809. 10.1002/2016GC006788 (2017). [Google Scholar]
- 33.D’Olivo, J. P. & McCulloch, M. T. Response of coral calcification and calcifying fluid composition to thermally induced bleaching stress. Sci. Rep. 7. 10.1038/s41598-017-02306 (2017). [DOI] [PMC free article] [PubMed]
- 34.D’Olivo, J. P. et al. Long-term impacts of the 1997–1998 bleaching event on the growth and resilience of massive Porites corals from the central Red Sea. Geochem. Geophys. Geosyst. 20, 2936–2954. 10.1029/2019GC008312 (2019). [Google Scholar]
- 35.Cohen, A. L. & Gaetani, G. A. Ion partitioning and the geochemistry of coral skeletons: solving the mystery of the vital effect. EMU Notes Mineral. 11, 377–397. 10.1180/EMU-notes.10.11 (2010). [Google Scholar]
- 36.Barkley, R. A. The Kuroshio Current. Sci. J. 6, 54–60 (1970). [Google Scholar]
- 37.Sowa, K., Watanabe, T., Nakamura, T., Sakai, S. & Sakamoto, T. Estimation of uncertainty for massive Porites coral skeletal density. JAMSTEC Rep. Res. Dev. 16, 31–39. 10.5918/jamstecr.16.31 (2013). [Google Scholar]
- 38.Schrag, D. P. Rapid analysis of high-precision Sr/Ca ratios in corals and other marine carbonates. Paleoceanography 14, 97–102. 10.1029/1998PA900025 (1999). [Google Scholar]
- 39.de Villiers, S., Greaves, M. & Elderfield, H. An intensity ratio calibration method for the accurate determination of Mg/Ca and Sr/Ca of marine carbonate by ICP-AES. Geochem. Geophys. Geosyst. 3. 10.1029/2001GC000169 (2002).
- 40.Watanabe, T. K., Watanabe, T., Ohmori, K. & Yamazaki, A. Improving analytical method of Sr/Ca ratios in coral skeleton for paleo-SST reconstructions using ICP-OES. Limnol. Oceanogr. Methods 18, 297–310. 10.1002/lom3.10357 (2020). [Google Scholar]
- 41.Okai, T., Suzuki, A., Kawahata, H., Terashima, S. & Imai, N. Preparation of a New Geological Survey of Japan Geochemical Reference Material; Coral JCp-1. Geostand. Geoanal. Res. 26, 95–99. 10.1111/j.1751-908X.2002.tb00627.x (2002). [Google Scholar]
- 42.Paillard, D., Labeyrie, L. & You, P. Macintosh program performs time-series analysis. Eos 77, 379. 10.1029/96EO00259 (1996). [Google Scholar]
- 43.Rodionov, S. N. A sequential algorithm for testing climate regime shifts. Geophys. Res. Lett. 31, L09204. 10.1029/2004GL019448 (2004). [Google Scholar]
- 44.Advanced Very High Resolution Radiometer. http://iridl.ldeo.columbia.edu/SOURCES/.NOAA/.NCDC/.OISST/.version2/.AVHRR/.sst/
- 45.the Climate Explorer in the Royal. Netherlands Meteorological Institute. http://climexp.knmi.nl/selectindex.cgi?id=someone@somewhere
- 46.Shen, C. C. et al. The calibration of D[Sr/Ca] versus sea surface temperature relationship for Porites corals. Geochim. Cosmochim. Acta 60, 3849–3858. 10.1016/0016-7037(96)00205-0 (1996). [Google Scholar]
- 47.Gagan, M. K. et al. Temperature and surface-ocean water balance of the Mid-holocene Tropical Western Pacific. Science 279, 1014–1018. 10.1126/science.279.5353.1014 (1998). [DOI] [PubMed] [Google Scholar]
- 48.Suzuki, A., Yukino, I. & Kawahata, H. Temperature-skeletal δ18O relationship for Porites australiensis from Ishigaki Island, the Ryukyus, Japan. Geochem. J. 33, 419–428. 10.2343/geochemj.33.419 (1999). [Google Scholar]
- 49.Abram, N. J., Webster, J. M., Davies, P. J. & Dullo, W. C. Biological response of coral reefs to sea surface temperature variation: evidence from the raised Holocene reefs of Kikai-Jima (Ryukyu Islands, Japan). Coral Reefs 20, 221–234. 10.1007/s003380100163 (2001). [Google Scholar]
- 50.Swart, P. K. et al. The 13C Suess effect in scleractinian corals mirror changes in the anthropogenic CO2 inventory of the surface oceans. Geophys. Res. Lett. 37, L05604. 10.1029/2009GL041397 (2010). [Google Scholar]
- 51.Quigley, K. M. et al. Symbioses are restructured by repeated mass coral bleaching. Sci. Adv. 8. 10.1126/sciadv.abq8349 (2022). [DOI] [PMC free article] [PubMed]
- 52.D’Angelo, C. et al. Local adaptation constrains the distribution potential of heat-tolerant Symbiodinium from Persian/Arabian Gulf. ISME J. 9, 2551–2560. 10.1038/ismej.2015.80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, X. et al. Super instrumental El Niño events recorded by a Porites coral from the South China Sea. Coral Reefs 37, 295–308. 10.1007/s00338-018-1658-1 (2018). [Google Scholar]
- 54.Fellis, T. et al. Subtropical coral reveals abrupt early-twentieth-century freshening in the western North Pacific Ocean. Geology 37, 527–530. 10.1130/G25581A.1 (2009). [Google Scholar]
- 55.Yamazaki, A., Watanabe, T., Tsunogai, U., Iwase, F. & Yamano, H. A 150-year variation of the Kuroshio transport inferred from coral nitrogen isotope signature. Paleoceanography 31. 10.1002/2015PA002880 (2016).
- 56.Takahashi, T. et al. Clobal sea-air CO2 flux based on climatological surface ocean pCO2, and seasonal biological and temperature effects. Deep-Sea Res. II 49, 1601–1622. 10.1016/S0967-0645(02)00003-6 (2002). [Google Scholar]
- 57.Midorikawa, T., Nemoto, K. & Kamiya, H. Persistently strong oceanic CO2 sink in the western subtropical North Pacific. Geophys. Res. Lett. 32. 10.1029/2004GL021952 (2005).
- 58.Kobari, T. et al. Trophic sources and pathways of mesozooplankton and fish larvae in the Kuroshio and its neighboring waters based on stable isotope ratios of carbon and nitrogen. Prog. Oceanogr. 210. 10.1016/j.pocean.2022.102952 (2023).
- 59.Grottoli, A. & Wellington, G. Effect of light and zooplankton on skeletal δ13C values in the eastern Pacific corals Pavona clavus and Pavona gigantea. Coral Reefs 18, 29–41. 10.1007/s003380050150 (1999). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
After publication, all coral data will be available on the data repository at the KIKAI Institute for Coral Reef Sciences (https://coralogy.kikaireefs.org).