Abstract
We have examined the specific minus-strand transfer reactions that occur after the synthesis of minus strong-stop DNA and nonspecific strand switching on homopolymeric poly(rA) templates with different types of Rous sarcoma virus (RSV) reverse transcriptases. Three different types of reverse transcriptases can be isolated from virions of RSV: heterodimeric αβ and homodimeric α and β. The mechanism of minus-strand transfer was examined using a model primer-template substrate corresponding to the 5′- and 3′-terminal RNA regions of the RSV genome. The results reveal that the RNase H activity of RSV reverse transcriptases is required for minus-strand transfer. Less than 2% of strand transfer of the extended product is detectable with RNase H-deficient enzymes. We could show that the α homodimer lacking the integrase domain can perform strand transfer almost as efficiently as the αβ and αPol heterodimers. In contrast, the activities of β and Pol for minus-strand transfer are reduced. Furthermore, a two- to fivefold increase in minus-strand transfer activities was observed in the presence of human immunodeficiency virus type 1 nucleocapsid protein.
Reverse transcriptases (RTs) of retroviruses catalyze the synthesis of double-stranded DNA using the single-stranded viral genome as the template. Synthesis of the first DNA strand starts from a tRNA primer hybridized to the viral RNA at the primer-binding site complementary to the last 18 nucleotides of the tRNA. As the primer-binding site is located close to the 5′ end of the viral RNA, DNA synthesis of the minus-strand DNA can only proceed after the DNA product is transferred to the 3′ end of the RNA. This process is called minus-strand transfer. A second strand transfer event occurs during the synthesis of the plus-strand DNA (for a review, see reference 5).
These strand transfer reactions are essential for the creation of the complete long terminal repeats (LTRs). LTRs are formed when the sequences close to the ends of the RNA are duplicated. The strand transfer reactions are specific processes. In contrast, the template-switching reactions that take place between the two copies of genomic RNA during polymerization are not sequence specific.
Minus-strand transfer requires that identical direct repeats, designated R for repeated, be present at both ends of the RNA template. The length of these R regions varies in different retroviruses. R is 96 nucleotides long in human immunodeficiency virus (HIV) and includes the highly structured TAR region necessary for specific binding of the Tat transactivator protein. In contrast, R is only 21 nucleotides in length in Rous sarcoma virus (RSV) and 12 nucleotides in mouse mammary tumor virus, indicating that the R sequence is not highly conserved; rather, the major function of it appears to be to accept the transferring strand (5). In the case of HIV, it has been shown that minus-strand transfer is profoundly increased in the presence of the viral nucleocapsid (NC) protein (2, 13, 17, 20). It has been suggested that the NC protein destabilizes the stem loop in the TAR region in the donor and acceptor nucleic acid. NC functions as a nucleic acid chaperone and prevents TAR-dependent self-priming from minus strong-stop DNA (13, 17, 22).
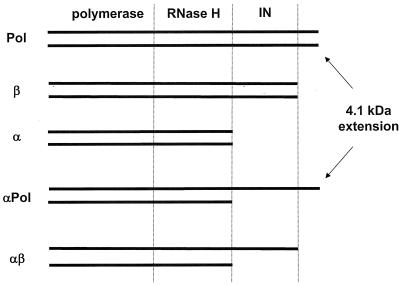
RT of RSV is a component of the Gag-Pol precursor protein. Pol is composed of the polymerase, RNase H, and integrase (IN) domains and an additional short 4.1-kDa protein located at the C terminus of the protein (Fig. 1) (1, 10–12, 16, 23, 25). Three forms of RT have been isolated from avian leukosis and sarcoma viruses: a 63-kDa protein designated α which contains the polymerase and RNase H domains; the β protein, with an apparent molecular weight of 95 kDa, which in addition to the two RT domains harbors the IN domain; and the most abundant heterodimeric αβ RT (11, 14, 15). We have shown previously that different forms of RSV RT can be expressed in and purified from insect cells using the baculovirus expression system (29, 30).
FIG. 1.
Recombinant RSV RTs.
In this study we wanted to analyze the function of the different RSV enzymes in the specific minus-strand transfer reaction. To find out more about the function of the C-terminal 4.1-kDa protein of Pol, we also included the entire Pol protein and the heterodimeric αPol RT in our studies. It has been described previously for murine leukemia virus (MLV) and HIV-1 RTs that strand transfer is severely reduced in the absence of a functional RNase H. Quantification of the transfer products yielded 3% for HIV-1 and less than 1.5% for MLV (19, 21, 26).
In order to determine the role of the RNase H activity for RSV strand transfer, two RNase H-deficient mutants which we characterized recently were also analyzed (30). In addition, we investigated the influence of the viral NC protein on the minus-strand transfer reaction. Since RSV RNA, unlike HIV RNA, does not contain a TAR region that has to be destabilized during strand transfer, we wanted to show whether the NC protein still has a positive effect on the transfer reaction of RSV.
MATERIALS AND METHODS
Buffers.
RT buffer consisted of 50 mM Tris-HCl (pH 8.0), 80 mM KCl, 6 mM MgCl2, and 5 mM dithiothreitol. STE buffer contained 20 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 10 mM EDTA. Formamide buffer contained 80% (vol/vol) formamide in 90 mM Tris-HCl (pH 8.3), 90 mM boric acid, 3 mM EDTA, 0.1% (wt/vol) xylene cyanol, and 0.1% (wt/vol) bromophenol blue.
Purification of mutated RSV RT proteins.
The methods for expressing and isolating wild-type and mutant RSV RT enzymes and the evaluation of RT enzyme activities have been described previously (29, 30).
Nonspecific template switching.
Homopolymeric poly(rA) with an average length of 357 nucleotides (Pharmacia) was hybridized to a fivefold molar excess of 5′-end 32P-labeled oligo(dT)16 in a solution containing 20 mM Tris-HCl (pH 7.5) and 50 mM NaCl by incubating the sample for 2 min at 95°C and cooling it slowly to room temperature in a heating block. Then 10 nM of the poly(rA)/oligo(dT)16 substrate was preincubated with 10 nM RT. The reaction was started by adding 250 μM dTTP. Reactions were performed in RT buffer for 10 min at 37°C in a final volume of 10 μl and stopped by the addition of an equal volume of formamide buffer. Products were analyzed on 10% denaturing polyacrylamide gels containing 7 M urea.
Substrates for minus-strand transfer.
The sequence of the donor RNA (R-U5) was 5′-GCC AUU UGA CCA UUC ACC ACA UUG GUG UGC ACC UGG GUU G-3′. The sequence of the acceptor RNA (U3-R) was 5′-GGG CUA GCU CGA UAC AAU AAA CGC CAU UUG ACC AUU CAC CAC A-3′. RNA synthesis was performed as described previously by T7 RNA polymerase runoff transcription using the corresponding DNA oligonucleotides containing the T7 promoter (27). The RNA was dephosphorylated with calf intestine phosphatase (New England Biolabs) and purified by denaturing polyacrylamide gel electrophoresis (15% acrylamide, 7 M urea). The RNA band was excised from the gel and eluted by immersing the gel slice for several hours in a buffer containing 25 mM sodium acetate (pH 4.5 to 5), 0.5 mM EDTA, and 0.1% sodium dodecyl sulfate at 37°C. The RNA was extracted from the buffer by phenol-chloroform treatment and purified over an NAP10 column (Pharmacia). The eluate was precipitated with ethanol and resuspended in H2O.
A DNA primer complementary to the last 17 nucleotides of the 3′ end of the donor R-U5 RNA was 5′-end labeled with [γ-32P]ATP (DuPont-New England Nuclear; 3,000 Ci/mmol) and T4 polynucleotide kinase (New England Biolabs) (27). After removal of the nucleotides by a NucTrap column (Stratagene), the DNA primer was hybridized to the R-U5 donor RNA (10% excess over DNA) in STE buffer by heating the sample to 90°C, followed by cooling to room temperature over several hours in a heating block.
Conversely, when the fate of the R-U5 RNA was analyzed, the RNA was 5′-end labeled and hybridized to the unlabeled DNA primer under the conditions described above.
Minus-strand transfer reactions.
Strand transfer reactions were performed in a total reaction volume of 10 μl of RT buffer. The samples contained 150 nM of the DNA/R-U5 hybrid and 150 or 220 nM of U3-R acceptor RNA. Samples were incubated for 30 min in the presence or absence of HIV-1 NC. Reactions were started by the addition of 50 nM RT and incubated at 37°C for 30 or 60 min. Reactions were stopped by the addition of an equal volume of formamide buffer and analyzed on 10% denaturing polyacrylamide gels containing 7 M urea.
Purification of HIV-1 nucleocapsid protein.
The recombinant nucleocapsid (NC) protein of HIV-1 (55 amino acid residues) was expressed and purified as described previously (33). Lyophilized NC protein was resuspended in RT buffer, frozen in small aliquots, and used only once after thawing.
RNase H assay.
RNase H activity was examined with a 45/36-mer RNA/DNA hybrid. The 45-mer RNA was synthesized chemically and possessed a fluorescent indodicarbocyanine (Cy5) label attached to its 5′ end (IBA GmbH, Göttingen, Germany). The sequence of the template RNA was 5′-Cy5-CUAAUUCCCCUUUCCCCCUCUCCUGGUGAUCCUUUCCAUCCCUGU-3′. The sequence of the DNA primer was complementary to the last 36 nucleotides of the 3′ end of the RNA. Hybridization was performed as described previously (31). RNase H cleavage was examined in RT buffer with 80 nM primer-template in the presence or absence of 3.3 μM HIV-1 NC. Samples were incubated for 15 min at 37°C. The reaction was started by the addition of RSV RT or HIV-1 RT to final concentrations of 15 or 5 nM, respectively, and stopped by the addition of formamide buffer. Reaction products were separated in the dark on denaturing 20% polyacrylamide gels containing 7 M urea. The fluorescent bands were visualized at an excitation wavelength of 635 nm using a fluoroimaging device (Fuji FLA 5000). Emission was measured via a cutoff filter (665 nm).
RESULTS
RNase H activity is not required on homopolymeric RNA templates.
During the reverse transcription process, strand displacement and template switching are important activities of RT which require efficient unwinding of nucleic acid duplexes. It has been shown previously that HIV-1 RT and avian myeloblastosis virus (AMV) RT can synthesize products that are longer than the original template. Furthermore, HIV-1 RT with a reduced RNase H activity could perform this reaction on poly(rA) templates, indicating that this reaction is RNase H independent (3).
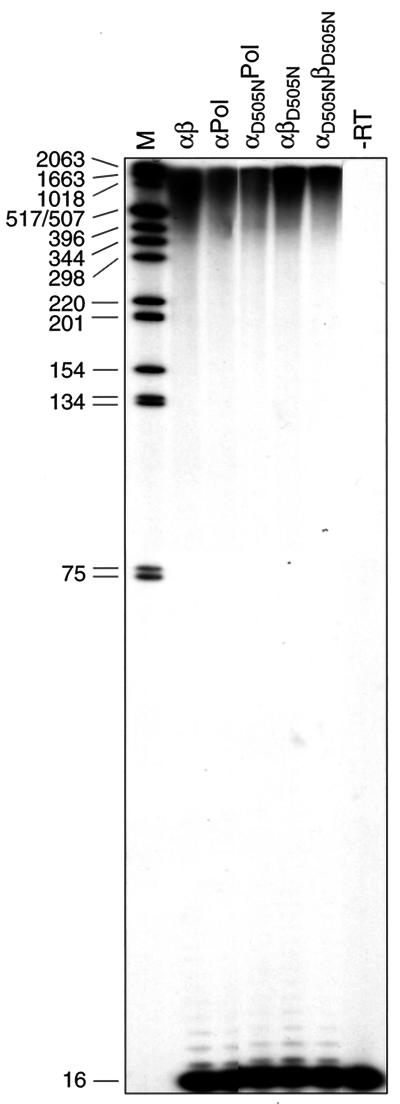
We have shown previously that the RNase H active site is located in the α subunit of heterodimeric RSV RTs. Mutating the active-site residue Asp505 to Asn leads to RNase H-deficient enzymes (30). To examine the polymerization activities of the mutants and to analyze whether the presence of an active RNase H is required to polymerize products that are longer than the original template, we used the homopolymeric poly(rA)/oligo(dT)16 as a substrate. Homopolymeric poly(rA) with an average length of 357 nucleotides was hybridized to a fivefold molar excess of radioactively labeled oligo(dT)16 (29). Figure 2 shows the polymerization products obtained with wild-type and mutant RSV RTs after addition of dTTP. The mutant RSV RTs αD505NPol and αD505NβD505N can synthesize a considerable amount of DNA product. In addition, the vast majority of the polymerization products obtained with wild-type and mutant enzymes is much longer than the average length of the poly(rA)357 template. These data indicate that the RNase H activity is not required for this mechanism and that the lack of a functional RNase H does not severely impair DNA polymerization in general.
FIG. 2.
Polymerization activities on a homopolymeric RNA template. Reactions were performed for 10 min at 37°C in RT buffer with 10 nM poly(rA)/oligo(dT)16 substrate and enzyme and 250 μM dTTP. The reactions were stopped by adding an equal volume of formamide buffer. Lane M shows DNA size markers; their sizes are indicated on the left (in nucleotides). Lane −RT, primer-template without enzyme. Samples were analyzed on denaturing 10% polyacrylamide gels with 7 M urea.
RNase H activity of RSV RTs is essential for minus-strand transfer reactions.
We wanted to establish whether RSV RTs are dependent on RNase H activity in order to perform the specific strand transfer reactions that take place during reverse transcription after synthesis of strong-stop DNA. A dependence on RNase H activity has been shown for HIV-1 RT and MLV RT (19, 21, 26).
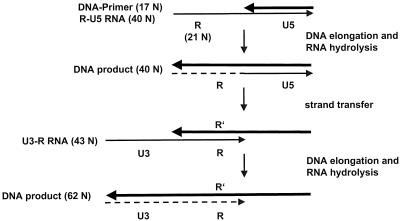
We used an oligonucleotide-based assay to analyze the strand transfer reaction (Fig. 3). A 40-mer in vitro-synthesized RNA (R-U5 RNA) that corresponded to the 5′ end of the viral RNA containing the complete R region and the 5′-terminal part of the U5 region of the LTR of viral RSV RNA was used as the donor RNA. A 5′-end-labeled DNA primer was hybridized to the RNA. The acceptor RNA U3-R comprised the 3′ terminus of the genomic viral RNA, including the 3′ terminus of the U3 region and the complete R region. In the case of RSV, the R region is only 21 nucleotides in length. Upon addition of RSV RT and deoxynucleoside triphosphates (dNTPs), strand elongation and transfer could take place. The length of the polymerization product reveals whether RT was able to extend the DNA primer only to the end of the donor RNA, yielding a 40-mer product, or whether RT could perform strand transfer and continue polymerization, so that the 62-mer end product occurs.
FIG. 3.
Schematic presentation of the in vitro assay used to analyze minus-strand transfer. The donor RNA (R-U5 RNA) and the acceptor RNA (U3-R RNA) are shown as thin lines. The bold black arrows represent the DNA primer and the DNA extension products. The broken line symbolizes the RNA degraded by the RNase H activity of RT. Extension of the DNA primer to the end of the donor RNA template yields a product of 40 nucleotides. When strand transfer occurs, a DNA of 62 nucleotides is synthesized.
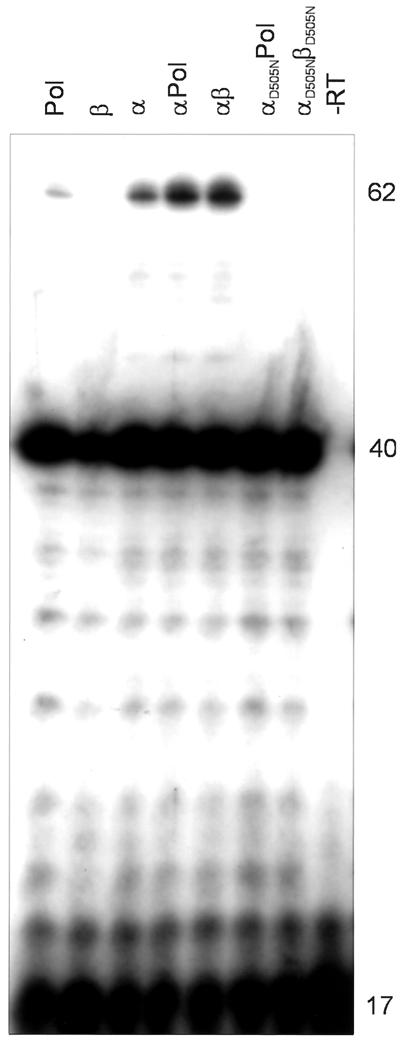
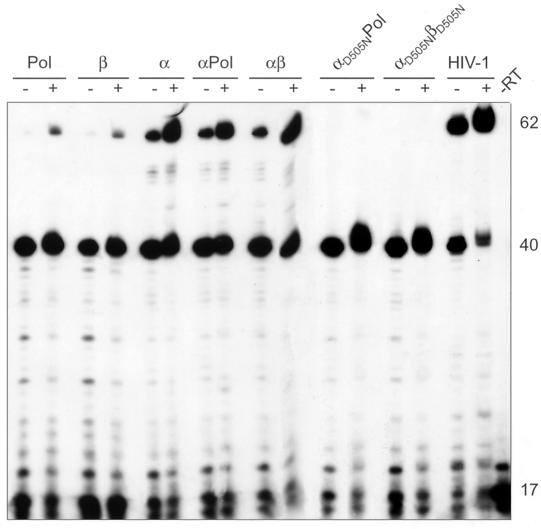
Figure 4 shows that all RSV RTs analyzed are able to extend the DNA primer to the end of the RNA donor template efficiently. Similar to the results obtained with poly(rA)/ oligo(dT)16, the heterodimeric RNase H-deficient enzymes αD505NPol and αD505NβD505N are not polymerization impaired on a heteropolymeric RNA substrate. However, we can see significant differences in the amounts of extension products that are derived from strand transfer. Strand transfer is strongly reduced with these RNase H mutants. A similar result was obtained when threefold-higher concentrations of the RNase H-deficient enzymes were used (data not shown). Quantification of the transfer products by phosphor imaging yielded values of <2% of transfer of the 40-mer extension product for αD505NPol and αD505NβD505N (Table 1). Our data indicate that the RNase H activity of RSV RT is required for efficient specific minus-strand transfer. However, the presence of the integrase domain in the β subunit which increases the affinity of integrase-containing RTs for nucleic acids (29) is not a prerequisite for efficient strand transfer, because the transfer activity of RSV RT α lacking integrase is comparable to that of αβ (Table 1).
FIG. 4.
Analysis of minus-strand transfer with different RSV RTs. Reactions were carried out for 30 min at 37°C in RT buffer. The concentrations of the donor RNA-DNA primer hybrid and the acceptor RNA were 150 nM. Each dNTP was added at 150 μM. Reactions were started by the addition of RT at a final concentration of 50 nM and analyzed as described for Fig. 1. The enzymes used are indicated above the panel. Lane −RT, substrate without enzyme added. DNA sizes are indicated on the right (in nucleotides).
TABLE 1.
Quantification of extension and strand transfer productsa
| Enzyme | HIV-1 NC protein present | % Primer extension | % Strand transfer |
|---|---|---|---|
| Pol | − | 74 | 2 |
| + | 80 | 5 | |
| β | − | 68 | 5 |
| + | 69 | 9 | |
| α | − | 91 | 10 |
| + | 93 | 48 | |
| αPol | − | 89 | 11 |
| + | 88 | 46 | |
| αβ | − | 93 | 11 |
| + | 91 | 47 | |
| αD505NPol | − | 88 | <2 |
| + | 88 | <2 | |
| αD505NβD505N | − | 90 | <2 |
| + | 90 | <2 | |
| HIV-1 RT | − | 91 | 56 |
| + | 88 | 76 |
Strand transfer experiments were performed as shown in Fig. 4 and 6. The data represent mean values for three independent experiments. The percentages of primer extended and of product transferred to the acceptor strand U3-R are shown. Quantification was performed by phosphorimaging. The 40-mer full-length product of the donor template not yet transferred and the already transferred products were regarded as substrate for strand transfer and set at 100%. The amount of transferred products was quantified as a ratio thereof. The experiments were performed in the absence or presence of HIV-1 NC protein.
Although RSV RT β and Pol are able to extend the primer to the end of the first template, they exhibit reduced transfer activities. The decreased strand transfer activity might be due to several reasons: (i) a decreased polymerase activity could lead to less extension product, and thus the strand transfer reaction would be reduced; (ii) an impaired RNase H activity could lead to a decrease in strand transfer, since we have shown above that the catalytic activity of the RNase H is required.
Fate of R-U5 donor RNA.
To analyze whether differences in the RNase H activities might be responsible for the reduced strand transfer activity of Pol and β, we analyzed the remaining RNA-oligonucleotide that is created when RT reaches the 5′ end of the donor RNA. We have shown previously (29) that the RNase H of RSV RTs does not exhibit the directed processing activity which in HIV-1 RT shortens the endonucleolytically cleaved RNA of the RNA-DNA hybrid in a 3′→5′ direction by about 10 nucleotides (9, 24, 32). This result implies that in the case of RSV RT, the remaining RNA oligonucleotide that is created when strong-stop minus-strand DNA is synthesized should be longer than that formed by HIV-1 RT. To answer these questions, we performed a strand transfer experiment with RSV RTs α, β, Pol, and αβ as well as with the RNase H-deficient mutant αD505NβD505N and HIV-1 RT. In this experiment we labeled the donor RNA R-U5 at the 5′ end (Fig. 5).
FIG. 5.
Fate of donor RNA. Reactions were performed for 30 min at 37°C in RT buffer. Reaction conditions and analysis were as described fpr Fig. 4 except the donor RNA was 5′-end labeled to visualize the products of the RNase H reaction. Lane −RT, substrate without enzyme added. DNA sizes are indicated on the right (in nucleotides).
As expected, the RNase H-deficient enzyme does not show any RNA hydrolysis. The RNA oligonucleotide that is created by the RNase H activity of RSV RTs is about 16 to 20 nucleotides long, with the major product being the 16-mer. When HIV-1 RT reaches the end of the RSV genome, the RNA is digested up to an 11-mer RNA oligonucleotide. We have shown previously that the RNase H activities of β and Pol are lower than those of the heterodimeric RSV RT αβ when using a 36/127-mer DNA/RNA primer-template substrate (29). In the experiment shown here using a different assay, we observed a similar outcome. The amount of the RNA oligonucleotide created by the β subunit appears to be less; however, because the polymerization activity of β is lower, less enzyme reaches the end of the template to create the terminal RNA fragment.
Effect of NC on minus-strand transfer.
Within retroviral particles, the viral RNA is covered with the viral nucleocapsid protein. For HIV-1 RT it has been demonstrated that minus-strand transfer is significantly improved in the presence of NC (2, 13, 17, 20).
To analyze the effect of NC protein on the transfer reaction of the different RSV RTs, recombinant HIV-1 NC protein was used (33). It has been shown that one of the major functions of NC protein during strand transfer is its nucleic acid chaperone activity, e.g., NC protein possesses the ability to catalyze an increase in the annealing rate of complementary nucleic acid strands by lowering the energy barrier for breakage and reassociation of base pairs (6, 7, 18, 22, 28, 34). This function is independent of the origin of the NC protein and justifies the use of a heterologous NC protein, in our case HIV-1 NC protein.
To determine the optimal NC protein concentration, strand transfer activity was measured at different ratios of nucleotides: NC protein with the RSV RT αβ heterodimer in comparison with HIV-1 RT. With RSV RT αβ, an improvement in strand transfer was visible at an nucleotide:NC protein ratio of 3:1 up to a ratio of 1:1. Since an increase in the NC protein concentration did not have an inhibitory effect on strand transfer, we chose to use a nucleotide:NC protein ratio of 1:1 (data not shown). Strand transfer activities of all RSV RTs and, for comparison, HIV-1 RT were tested on the RSV RNA substrate in the presence or absence of HIV-1 NC protein (Fig. 6). Our data demonstrate that strand transfer with HIV-1 RT is very efficient on the RSV RNA substrate, even in the absence of NC protein. This is probably due to a lack of extensive secondary structures like the TAR region in HIV RNA. The presence of HIV-1 NC protein improved the minus-strand transfer reaction of all RSV RTs. Depending on the RSV RT enzyme, an approximately two- to fivefold increase can be observed. The amounts of primer extended and of product transferred were determined by phosphor imaging. Table 1 shows that a four- to fivefold increase in strand transfer can be obtained with the heterodimeric RSV RTs αβ and αPol and with α. The low transfer activities of Pol and β were also increased about 2- to 2.5-fold. Furthermore, our data indicate that the residual transfer activity of the RNase H-deficient mutants cannot be stimulated significantly by the presence of the HIV-1 NC protein.
FIG. 6.
Effect of HIV-1 NC protein on the strand transfer reaction of different RSV RTs. A typical experiment is shown. Reactions were performed as described Materials and Methods at a nucleotide:NC protein ratio of 1. The absence (−) or presence (+) of HIV-1 NC protein is indicated above the panel. Products were quantitated by phosphor imaging as described in Table 1, footnote a. DNA sizes are indicated on the right (in nucleotides).
It has been shown previously that HIV-1 NC protein can interact with the RNase H domain of HIV-1 RT (4, 20). To analyze a possible stimulation of the RNase H reaction by HIV-1 NC protein, we performed RNase H activity assays (29) with the different wild-type and mutant RSV RTs in the presence or absence of HIV-1 NC as described in Materials and Methods. However, quantification of the cleaved RNA of a DNA-RNA primer-template substrate indicated neither a change in the cleavage pattern nor a significant stimulation (<1.15-fold) of the RSV RNase H activity by HIV-1 NC.
DISCUSSION
In the present study, the role of different types of RSV RT and the function of the RNase H activity for catalysis of the nonspecific template switching reaction and for the specific minus-strand transfer reaction was investigated. Our study shows that synthesis of products longer than the homopolymeric template is RNase H independent, whereas specific minus-strand transfer of RSV is strongly dependent on the presence of an active RNase H and can be improved by NC protein.
It has been shown previously that HIV-1 RT and AMV RT can synthesize products on a poly(rA)/oligo(dT) substrate that are longer than the original template. Since the reaction was dependent on the template concentration, the authors suggested that the underlying mechanism is template switching and not slippage between the extended primer and the homopolymeric template (3). However, due to the unnatural homopolymeric substrate, it cannot be excluded that slippage or other processes which do not occur under normal conditions are responsible for the synthesis of long products.
Here we demonstrate that the different RSV RTs use the homopolymeric substrate poly(rA)/oligo(dT) very efficiently. All enzymes, including the RNase H-deficient mutants αD505NPol and αD505NβD505N, synthesize DNA products that are much longer than the original poly(rA) template, with an average length of 357 bases. Our results show that an active RNase H is not necessary for efficient polymerization and for synthesizing long products on a homopolymeric RNA template.
Analysis of the specific minus-strand transfer reaction with heteropolymeric donor and acceptor RNAs that correspond to the 5′ and 3′ ends of the RSV genome shows that the RNase H function of RSV RT is required for this reaction. Less than 2% of transfer of the extended product is observed with the RNase H-deficient mutant enzymes. Similar results were obtained previously with MLV and HIV-1 RT (19, 21, 26).
The remaining RNA fragment produced by the RNase H function when RT reaches the end of the RNA template is longer with RSV RT than with HIV-1 RT (Fig. 5). Due to the lack of an RNase H 3′→5′ processing activity, the remaining RNA fragment obtained with RSV RTs is about 16 nucleotides in length, whereas HIV-1 RT creates a shorter fragment of about 11 nucleotides on the same substrate (29, 32). These results indicate differences in the mechanism of reverse transcription of different retroviruses.
Another striking result is the difference in the efficiency of strand transfer observed with the different RSV RTs. Except for RSV RT β, whose polymerization activity is somewhat reduced, all RSV RTs tested show comparable DNA primer elongation efficiencies on the donor RNA template, indicating similar polymerization activities of all enzymes tested (Table 1). In spite of this, neither RSV RT β nor Pol can perform the strand transfer reaction as efficiently. Since the RNase H activity of β is diminished (Fig. 5) (29), less substrate for strand transfer might be available. However, this does not fully explain why strand transfer efficiency is so low in the case of Pol. Figure 5 and previous results with a 36/127-mer DNA/RNA primer-template demonstrate that the RNase H activity of Pol is higher than that of β (29). We suggest that steric hindrance and/or conformational differences due to the carboxyl-terminal 4.1-kDa extension of Pol might contribute to this effect.
Our data show that the heterologous HIV-1 NC protein is capable of enhancing RSV strand transfer. Our experiments do not allow the conclusion that specific interactions of HIV-1 NC protein and RSV RT are possible. We were unable to detect a qualitative or quantitative change of RSV RNase H activity by HIV-1 NC protein (data not shown). This might be a further indication that there are no specific contacts. Specific interactions of HIV-1 RT and HIV-1 NC have been presented previously. It has been suggested that the main role of this interaction is to enhance RT processivity. In addition HIV-1 NC protein (71 amino acid residues) was shown to improve strand transfer efficiency of an RT mutant with a carboxyl-terminal deletion in the p66 subunit (4, 8, 20).
In the case of HIV-1 RT minus-strand transfer using viral RNA as a template is rather inefficient in the absence of NC protein in vitro due to formation of a stable stem-loop structure of the newly synthesized DNA strand which contains sequences complementary to the TAR region of the viral RNA. Thus, the DNA cannot anneal to the cRNA but is involved in self-priming. NC protein prevents formation of this stem-loop and thus facilitates the transfer reaction (13, 17, 22). Besides this function of NC protein, it has been shown previously that one of the major effects of NC is to promote annealing between the newly synthesized strong-stop minus-strand DNA and the complementary 3′ end of the viral RNA containing the R region (34). Since a structure corresponding to HIV-1 TAR is absent in the genome of RSV, improvement of strand transfer in the case of RSV appears to be mainly due to enhancement of the annealing reaction. However, it has also been shown that HIV-1 NC can enhance the RNase H activity of HIV-1 RT and thus improve strand transfer (20). Further experiments with HIV-1 NC in comparison with RSV NC will be necessary to elucidate the molecular basis of enhancement of strand transfer by NC in the case of RSV RT. However, since RSV RTs β and Pol exhibit rather poor strand transfer activities even in the presence of NC protein, we assume that the Pol precursor protein and β are not involved in minus-strand transfer in vivo.
ACKNOWLEDGMENTS
We thank Martina Wischnewski for assistance with enzyme purifications, Paul Rothwell for careful reading of the manuscript, and Roger Goody for support. We also thank Robert J. Gorelick, SAIC Frederick, NCI at Frederick, for providing the HIV-1 NC protein and for helpful suggestions.
This work was supported by the Max-Planck-Gesellschaft and by a grant from the Deutsche Forschungsgemeinschaft (DFG) to B.M.W.
REFERENCES
- 1.Alexander F, Leis J, Soltis D A, Crowl R M, Danho W, Poonian M S, Pan Y C, Skalka A M. Proteolytic processing of avian sarcoma and leukosis viruses Pol-Endo recombinant proteins reveals another pol gene domain. J Virol. 1987;61:534–542. doi: 10.1128/jvi.61.2.534-542.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allain B, Lapadat-Tapolsky M, Berlioz C, Darlix J L. Transactivation of the minus-strand DNA transfer by nucleocapsid protein during reverse transcription of the retroviral genome. EMBO J. 1994;13:973–981. doi: 10.1002/j.1460-2075.1994.tb06342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buiser R G, DeStefano J J, Mallaber L M, Fay P J, Bambara R A. Requirements for the catalysis of strand transfer synthesis by retroviral DNA polymerases. J Biol Chem. 1991;266:13103–13109. [PubMed] [Google Scholar]
- 4.Cameron C E, Ghosh M, Le Grice S F, Benkovic S J. Mutations in HIV reverse transcriptase which alter RNase H activity and decrease strand transfer efficiency are suppressed by HIV nucleocapsid protein. Proc Natl Acad Sci USA. 1997;94:6700–6705. doi: 10.1073/pnas.94.13.6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffin J M. Retroviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1767–1847. [Google Scholar]
- 6.Darlix J-L, Lapadat-Tapolsky M, de Rocquiny H, Roques B P. First glimpses at structure-function relationships of the nucleocapsid protein of retroviruses. J Mol Biol. 1995;254:523–537. doi: 10.1006/jmbi.1995.0635. [DOI] [PubMed] [Google Scholar]
- 7.Dib-Hajj F, Khan R, Giedroc D P. Retroviral nucleocapsid proteins possess potent nucleic acid strand renaturation activity. Protein Sci. 1993;2:231–243. doi: 10.1002/pro.5560020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Druillennec S, Caneparo A, de Rocquiny H, Roques B P. Evidence of interactions between the nucleocapsid protein NCp7 and the reverse transcriptase of HIV-1. J Biol Chem. 1999;274:11283–11288. doi: 10.1074/jbc.274.16.11283. [DOI] [PubMed] [Google Scholar]
- 9.Furfine E S, Reardon J E. Reverse transcriptase.RNase H from the human immunodeficiency virus: relationship of the DNA polymerase and RNA hydrolysis activities. J Biol Chem. 1991;266:406–412. [PubMed] [Google Scholar]
- 10.Golomb M, Grandgenett D. Endonuclease activity of purified RNA-directed DNA polymerase from avian myeloblastosis virus. J Biol Chem. 1979;254:1606–1613. [PubMed] [Google Scholar]
- 11.Grandgenett D P, Gerard G F, Green M. A single subunit from avian myeloblastosis virus with both RNA-directed DNA polymerase and ribonuclease H activity. Proc Natl Acad Sci USA. 1973;70:230–234. doi: 10.1073/pnas.70.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandgenett D P, Vora A C, Schiff R D. A 32,000-dalton nucleic acid-binding protein from avian retrovirus cores possesses DNA endonuclease activity. Virology. 1978;89:119–132. doi: 10.1016/0042-6822(78)90046-6. [DOI] [PubMed] [Google Scholar]
- 13.Guo J, Henderson L E, Bess J, Kane B, Levin J G. Human immunodeficiency virus type 1 nucleocapsid protein promotes efficient strand transfer and specific viral DNA synthesis by inhibiting TAR-dependent self-priming from minus-strand strong-stop DNA. J Virol. 1997;71:5178–5188. doi: 10.1128/jvi.71.7.5178-5188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hizi A, Joklik W K. RNA-dependent DNA polymerase of avian sarcoma virus B77. I. Isolation and partial characterization of the α, β2, and αβ forms of the enzyme. J Biol Chem. 1977;252:2281–2289. [PubMed] [Google Scholar]
- 15.Kacian D L, Watson K F, Burny A, Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971;246:365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- 16.Katz R A, Skalka A M. A C-terminal domain in the avian sarcoma-leukosis virus pol gene product is not essential for viral replication. J Virol. 1988;62:528–533. doi: 10.1128/jvi.62.2.528-533.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J K, Palaniappan C, Wu W, Fay P J, Bambara R A. Evidence for a unique mechanism of strand transfer from the transactivation response region of HIV-1. J Biol Chem. 1997;272:16769–16777. doi: 10.1074/jbc.272.27.16769. [DOI] [PubMed] [Google Scholar]
- 18.Lapadat-Tapolsky M, Pernelle C, Borie C, Darlix J L. Analysis of the nucleic acid annealing activities of nucleocapsid protein from HIV-1. Nucleic Acids Res. 1995;23:2434–2441. doi: 10.1093/nar/23.13.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo G X, Taylor J. Template switching by reverse transcriptase during DNA synthesis. J Virol. 1990;64:4321–4328. doi: 10.1128/jvi.64.9.4321-4328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peliska J A, Balasubramanian S, Giedroc D P, Benkovic S J. Recombinant HIV-1 nucleocapsid protein accelerates HIV-1 reverse transcriptase catalyzed DNA strand transfer reactions and modulates RNase H activity. Biochemistry. 1994;33:13817–13823. doi: 10.1021/bi00250a036. [DOI] [PubMed] [Google Scholar]
- 21.Peliska J A, Benkovic S J. Mechanism of DNA strand transfer reactions catalyzed by HIV-1 reverse transcriptase. Science. 1992;258:1112–1118. doi: 10.1126/science.1279806. [DOI] [PubMed] [Google Scholar]
- 22.Rein A, Henderson L E, Levin J G. Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: significance for viral replication. Trends Biochem Sci. 1998;23:297–301. doi: 10.1016/s0968-0004(98)01256-0. [DOI] [PubMed] [Google Scholar]
- 23.Rho H M, Grandgenett D P, Green M. Sequence relatedness between the subunits of avian myeloblastosis virus reverse transcriptase. J Biol Chem. 1975;250:5278–5280. [PubMed] [Google Scholar]
- 24.Schatz O, Mous J, Le Grice S F J. HIV-1 RT associated ribonuclease H displays both endonuclease and 3′ to 5′ exonuclease activities. EMBO J. 1990;9:1171–1176. doi: 10.1002/j.1460-2075.1990.tb08224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiff R D, Grandgenett D P. Virus-coded origin of a 32,000-dalton protein from avian retrovirus cores: structural relatedness of p32 and the beta polypeptide of the avian retrovirus DNA polymerase. J Virol. 1978;28:279–291. doi: 10.1128/jvi.28.1.279-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanese N, Telesnitsky A, Goff S P. Abortive reverse transcription by mutants of Moloney murine leukemia virus deficient in the reverse transcriptase-associated RNase H function. J Virol. 1991;65:4387–4397. doi: 10.1128/jvi.65.8.4387-4397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thrall S H, Krebs R, Wöhrl B M, Cellai L, Goody R S, Restle T. Pre-steady kinetic characterization of RNA-primed initiation of transcription by HIV-1 reverse transcriptase and analysis of the transition to a processive DNA-primed polymerization mode. Biochemistry. 1998;37:13349–13358. doi: 10.1021/bi981102t. [DOI] [PubMed] [Google Scholar]
- 28.Tsuchihashi Z, Brown P O. DNA strand exchange and selective DNA annealing promoted by the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1994;68:5863–5870. doi: 10.1128/jvi.68.9.5863-5870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werner S, Wöhrl B M. Soluble Rous sarcoma virus reverse transcriptases α, αβ and β purified from insect cells are processive DNA polymerases that lack an RNase H 3′ 5′-directed processing activity. J Biol Chem. 1999;274:26329–26336. doi: 10.1074/jbc.274.37.26329. [DOI] [PubMed] [Google Scholar]
- 30.Werner S, Wöhrl B M. Asymmetric subunit organization of heterodimeric Rous sarcoma virus reverse transcriptase αβ: localization of the polymerase and RNase H active sites in the α subunit. J Virol. 2000;74:3245–3252. doi: 10.1128/jvi.74.7.3245-3252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wöhrl B M, Krebs R, Goody R S, Restle T. Refined model for primer/template binding by HIV-1 reverse transcriptase: presteady-state kinetic analyses of primer/template binding and nucleotide incorporation events distinguish between different binding modes depending on the nature of the nucleic acid substrate. J Mol Biol. 1999;292:333–344. doi: 10.1006/jmbi.1999.3057. [DOI] [PubMed] [Google Scholar]
- 32.Wöhrl B M, Moelling K. Interaction of HIV-1 ribonuclease H with polypurine tract containing RNA-DNA hybrids. Biochemistry. 1990;29:10141–10147. doi: 10.1021/bi00496a001. [DOI] [PubMed] [Google Scholar]
- 33.Wu W X, Henderson L E, Copeland T D, Gorelick R J, Bosche W J, Rein A, Levin J G. Human immunodeficiency virus type 1 nucleocapsid protein reduces reverse transcriptase pausing at a secondary structure near the murine leukemia virus polypurine tract. J Virol. 1996;70:7132–7142. doi: 10.1128/jvi.70.10.7132-7142.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.You J C, McHenry C S. HIV nucleocapsid protein. Expression in Escherichia coli, purification, and characterization. J Biol Chem. 1993;268:16519–16527. [PubMed] [Google Scholar]