Abstract
Cardiovascular disease (CVD) is a major chronic disease worldwide and its risk factors have long been investigating in epidemiological studies. Our study aims to develop a body composition-based risk score and integrate it into the Framingham Risk Score (FRS) to improve CVD prediction among well-functioning older adults. We included 1882 older adults from the Health, Aging and Body Composition (Health ABC) study to screen body composition variables obtained from the Dual-energy X-ray absorptiometry (DXA). Three models were developed and compared: the 4-DXA model, the refit FRS, and the refit FRS plus 4-DXA model. C-statistics were 0.62 (95% CI: 0.59, 0.65) for the refit FRS, 0.58 (95% CI: 0.55, 0.61) for the 4-DXA model, and 0.63 (95% CI: 0.60, 0.66) for the refit FRS plus 4-DXA model. Compared to the refit FRS, the refit FRS plus 4-DXA model slightly improved CVD outcome prediction as the discrimination slope, net reclassification index, and the integrated discrimination index were 0.053 (95% CI: 0.041, 0.066), 0.098 (95% CI = – 0.0033, 0.20) and 0.013 (95% CI: 0.0069–0.019). This study provides a model for more accurate risk stratification and draws more attention on DXA-based indices in the clinical setting. It also encourages further research in validating the developed risk score in more diverse population and in investigating a broader range of CVD risk factors.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74185-y.
Keywords: Cardiovascular disease, Body composition indices, Dual-energy X-ray absorptiometry, Framingham risk score, Older adults
Subject terms: Risk factors, Predictive markers, Cardiovascular diseases, Geriatrics, Cardiology
Introduction
Cardiovascular disease (CVD) is a major health concern worldwide, affecting over half a billion people and accounting for 20.5 million deaths in 20211. In an effort to lower the prevalence of cardiovascular disease and its complications, significant emphasis has been placed on its early risk prediction and risk mitigation2. The Framingham Risk Score (FRS), first developed using data from the Framingham Heart Study to predict the risk of incident coronary heart disease over ten years, is one of the earliest as well as the most widely used algorithms to evaluate cardiovascular risk nowadays3. However, several studies revealed that the FRS had inconsistent performance in different groups and has either overestimated or underestimated the risk among older adults4–7.
A broader range of CVD risk factors could be incorporated to refine the risk assessment framework. And, certain body composition indices were proven to be associated with the risk of CVD occurance. Body composition is a multi-dimensional concept that refers to the amount of fat, muscle, bone, and water in the body. DXA (Dual-energy X-ray absorptiometry) was developed 30 years ago as a method for body composition measurements, including both body bone mass and soft tissue composition8. Prior studies have both identified the statistical correlation and pathophysiological mechanism between body composition measurements and CVD. For instance, obesity and elevated body mass index (BMI) are statistically associated with higher hazards of CVD in various cohort studies9–13. The accumulation of visceral fat, as a result of obesity, leads to dysregulation of various adipocyte-derived bioactive molecules (adipocytokines), which may cause chronic systemic low-grade inflammation and CVD14. Moreover, the excessive adipose tissue in the myocardium can cause alterations in its structure and functionality, which directly increases the risk of CVD15. In addition, many other body composition measures, such as reduced bone mineral density (BMD) at the lumbar spine, femur neck, thoracic, total hip, and for all total were proven to be associated with an increased risk of CVD in varied cohorts16–18. This inverse relationship could be attributed to the pathophysiological mechansims such as alterations in signaling pathways shared by normal bone remodeling and arterial calcification, low-grade inflammation, and vascular calcification19,20. However, these studies only incorporated a certain subset of body composition indices. There has not yet been a study that investigates the association between a comprehensive pool of body composition measurements and the occurance of CVD. Little is known about whether and how multiple body composition indices jointly contributed to the prediction of CVD. As a result, in this study, we included a pool of 87 body composition indices and tried to find the association.
The objectives of the present study were as follows: (1) to investigate a broader range of body composition variables and their association with CVD; (2) to create a body composition variable based model of biomarkers for prognostic stratification; (3) to determine whether incorporating the body composition variables in the traditional FRS improves risk prediction.
Materials and methods
Data and study participants
The Health, Aging and Body Composition (Health ABC) Study is a longitudinal cohort designed to examine risk factors for aging-related changes in body composition and physical function among initially well-functioning older adults. From March 1997 to July 1998, 3075 black and white individuals aged 68–80 years were recruited from a list of Medicare beneficiaries provided by the Health Care Financing Administration at two study sites across the United States (Pittsburgh, Pennsylvania, and Memphis, Tennessee). The inclusion criteria of the Health ABC study were (i) free of life-threatening illness, (ii) self-reported ability to walk a quarter of a mile, climb ten steps without resting, and perform basic activities of daily living without assistance, and (iii) no intention to move out of the current geographic area for at least three years. Details about the Health ABC study have been previously published elsewhere21,22. The study was approved by the Institutional Review Board of the University of California, San Francisco (H5254-12688-14), the University of Tennessee (95-05531-FB), and the University of Pittsburgh (#960212). All participants provided written informed consent, and all methods in the study were performed in accordance with the principles of the Declaration of Helsinki.
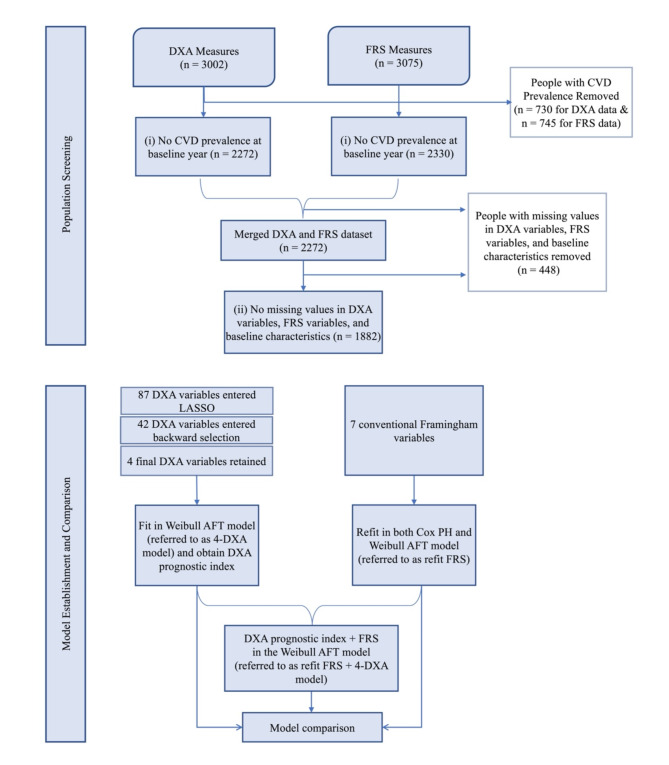
Among the total number of 3002 participants who completed DXA measures and 3075 participants who had FRS at the baseline year, we excluded 730 and 745 participants respectively due to prevalent CVD or prior CVD. We then removed 58 participants who had FRS but did not complete DXA measure and ended up with 2272 participants. In addition, we excluded another 390 participants with missing values in either FRS or DXA measures. A total of 1882 participants were included. The details of data processing, statistical modeling, and assessments of our established models are summarized in Fig. 1.
Fig. 1.
Flowchart of the current study on the Health ABC population.
Body composition
Body composition indices (lean mass, fat mass, bone mineral content, and bone mineral density) were measured by DXA using the fan-beam technology (model QDR 4500 A; Hologic Inc, Waltham, MA). After completing the scans, the body composition results for the total body were provided by the system’s most recent software release, version 8.21. The calculation of total body fat free mass (FFM), total leg muscle mass, and other body composition indices followed the standard procedures23. The validity and reproducibility of the body composition data in the Health ABC Study were reported elsewhere24. In our study, we first manually removed 16 irrelevant variables (e.g. scan_id, scan_date) from the original DXA dataframe. These 16 variables contain the parameters and the meta-data of the DXA scan and therefore were deemed irrelevant in body composition variables (Supplementary Table S1). Then, since the same measures from the left and right sides of the body were highly correlated (e.g., left arm fat free mass and right arm fat free mass), we took measures only from the side of non-dominant hand and disregarded the variable from the other side for each individual in our study. We believed that measures from the non-dominant side were less influenced by non-aging related reasons such as exercise and trauma and therefore, their relationship with CVD could be more unperturbed and stable. Finally, a total of 87 DXA indices were selected and analyzed in the present study (Supplementary Table S2).
Framingham risk score
The FRS included seven components: age, gender, smoking status, diabetes, SBP, total cholesterol, and HDL-C (High-density Lipoprotein). Age in years, gender (male or female), and smoking status (current smoker or not) were self-reported. SBP was calculated as the average of two measurements by a conventional mercury sphygmomanometer with an appropriately sized cuff, taken in the seated position after five minutes of quiet rest. Baseline blood samples were obtained at the clinic in the morning (mean time 9:25 A.M.; 50% of specimens were collected between 8:55 and 9:58 A.M.) after overnight fasting of at least eight hours. The specimens were then aliquoted into cryovials, frozen at − 70 °C, and shipped to the core laboratory at the University of Vermont. Total cholesterol (mg/dL) and HDL-C (mg/dL) were measured by a colorimetric technique (Johnson & Johnson Vitros 950 analyzer, New Brunswick, New Jersey). Diabetes was assessed by self-report, medication use, or a positive diagnosis by fasting blood glucose level or oral glucose tolerance test.
Outcomes
Outcomes of interest included incident CVD and CVD mortality (N = 613). Incident CVD included the following events: acute myocardial infarction (death of part of the myocardium due to occlusion of a coronary artery from any cause, including spasm, embolus, thrombosis, or the rupture of a plaque), angina pectoris (symptoms, such as chest pain, chest tightness, or shortness of breath, produced by myocardial ischemia that do not result in infarction), or congestive heart failure (a constellation of symptoms and physical signs that occur in a participant whose cardiac output cannot match metabolic need despite adequate filling pressures). The event must result in at least one overnight hospitalization. CVD mortality refers to inpatient death due to CVD.
Follow-up for outcomes occurred every six months, either by telephone or annual visits to clinical centers; participants were asked about hospitalizations and major outpatient procedures. CVD events were adjudicated based on interviews, reviews of all hospital records, death certificates, and other documents by a panel of experts. Deaths were ascertained by the review of local obituaries, by the report to the clinical centers by family members, or by semiannual telephone contacts. Diagnoses and causes of death were adjudicated based on interviews, reviews of all hospital records, death certificates, and other support documents by a panel of physicians. The follow-up time was calculated as the difference between the time from the baseline visit and the first CVD event date or date of death due to CVD, whichever came first. Participants were censored at the date of the last contact or by the end of the follow-up period (30 April 2010 for Memphis and 30 June 2010 for Pittsburgh), whichever came first.
Confounders
Confounders included study site (Pittsburgh or Memphis), race (Black or White), education (less than high school, high school or equivalent, or more than high school), physical activities, alcohol consumption, and body mass index (BMI) calculated as body weight in kilograms divided by the square of standing height in meters.
Statistical analysis
We described the baseline characteristics by participants who were included and excluded in our study; median and interquartile range were used for continuous variables and count and proportion were used for categorical variables. Mood’s median test and chi-square test for continuous and categorical variables were conducted to compare the statistics between the included and excluded population.
We applied the Cox proportional hazards model with the least absolute shrinkage and selection operator25 (LASSO) to select body composition variables. Each body composition variable was standardized. The LASSO method adds an L-1 norm term to the ordinary least square loss function and minimizes it, leading to a shrinkage of some coefficients to zero. The LASSO was chosen over the Ridge method based on the sparse nature of feature selection. It also led to fewer variables compared to the Elastic Net method, which achieved very slight improvement in the C-statistics (0.579 vs. 0.577) but selected 46 features (42 for LASSO). Therefore, we adopted the full LASSO setting. The penalization coefficient was selected using a 5-fold cross-validation and grid search technique. We selected a penalization coefficient of 0.0046, at which the model has achieved maximum prediction accuracy. Subsequently, we used the stepwise backward selection technique to further reduce the number of variables retained in the Cox model using the Bayesian information criterion stopping criteria. The final subset of body composition variables was then included in a fully parametric Accelerated Failure Time (AFT) model based on the Weibull distribution for a prediction. We estimated the 4000-day risk of CVD and CVD mortality based on this model.
Sample size calculations were performed assuming that our developed models could achieve moderate performance (0.6–0.7) in C-statistics. On the basis of a two-sided 0.05 significance level t-test, a total sample size of 313 participants (ncases = 86, ncontrols = 227) provides 80% power for a C-statistics that equals 0.6. And a total sample size of 79 (ncases = 22, ncontrols = 56) provides 80% power for a C-statistics that equals 0.7. Therefore, our sample size of 1882 was adequeate for the statistical analysis performed.
As a comparative reference for the body composition risk model, the variables from the FRS model26 were refitted to the Health ABC cohort (referred to as refit FRS). The refit FRS combined with the variables from the body composition risk model was also fit (referred to as refit FRS plus 4-DXA model).
Model performance was assessed by discrimination and calibration. For discrimination, both the C-statistics and the discrimination slope were reported. The category-free net reclassification index (NRI > 0) and integrated discrimination index (IDI) were used to assess the performance of reclassification and the improvement in discrimination over the refit FRS. Calibration performance was assessed with a calibration plot and summarized the risk scores using the Hosmer-Lemeshow statistic. Calibration in the large was also reported as the difference between the observed 4000-day event frequency and the mean predicted risk score. Distribution-free (nonparametric) 95% CIs were reported for median values and bootstrapped intervals for point estimates of performance metrics when asymptotic intervals were unavailable.
All analyses were performed using R and Python 3.9.
Results
Baseline characteristics
Among the 1,882 participants, the median age was 73.0 years; 45.4% were men (Table 1). The median follow-up time was 13.4 years (range: 0.02–15.9 years). A total of 613 incident CVD or cardiovascular mortality occurred (28.04 per 1000 person-years).
Table 1.
Baseline characteristics of the study cohort.
| Median (Interquartile range) | |||
|---|---|---|---|
| FRS and DXA baseline (N = 1882) |
Excluded due to missing values (N = 448) | p-value | |
| Age, years | 73.0 (71.0, 76.0) | 74.0 (71.0, 76.0) | 0.14 |
| Men (%) | 854 (45.4%) | 203 (45.3%) | 1.00 |
| Maximum sagittal diameter (mm) | 234.0 (210.0, 257.0) | 236.0 (210.0, 260.2) | 0.18 |
| Pelvic bone mineral density (g/cm2) | 1.1 (1.0, 1.3) | 1.2 (1.0, 1.3) | 0.09 |
| Lumb spine bone mineral content (g) | 48.6 (38.0, 61.8) | 48.8 (37.9, 65.2) | 0.95 |
| Thigh intramuscular fat density sectional density (HU) | 25.5 (24.0, 27.1) | 24.9 (23.2, 26.9) | < 0.001 |
| Incidence (%) | 613 (32.6%) | 114 (25.4%) | < 0.005 |
| Body mass index (kg/m2) | 26.8 (23.9, 29.9) | 27.2 (24.2, 31.1) | 0.16 |
| Total cholesterol (mg/dL) | 203.0 (179.0, 228.0) | 201.0 (177.0, 226.8) | 0.39 |
| High-density lipoprotein cholesterol (mg/dL) | 52.0 (43.0, 64.0) | 52.0 (43.0, 63.0) | 0.55 |
| Diabetes (%) | 233 (12.4%) | 79 (17.6%) | < 0.005 |
| Systolic blood pressure (mm Hg) | 134.0 (122.0, 148.0) | 133.0 (121.0, 149.0) | 0.80 |
| Current smoker (%) | 187 (9.9%) | 48 (10.7%) | 0.69 |
Median and interquartile range were used for continuous variables and count and proportion were used for categorical variables. P-values were reported from the Mood’s median test for continuous variables and chi-sqaured test for categorical variables. FRS Framingham risk score; DXA dual-energy X-ray absorptiometry.
Variable selection process
From the 87 variables included in the LASSO model, 42 were retained after selection. Further refinement was achieved through the stepwise backward elimination technique based on the Bayesian information criterion. Four variables, maximum sagittal diameter (mm), pelvic Bone Mineral Density (BMD) (g/cm2), lumbar spine Bone Mineral Content (BMC) (g), and thigh intermuscular fat density SD (Sectional Density) (HU), were left and included in the final risk score prediction. The two consecutive variable selection procedures have identified the most statistically significant variables, which ensured that only those with the strongest associations with CVD risk were included in the final model. The preserved four DXA variables also have better simplicity, interpretability, and practicality in clinical settings compared to the 42 LASSO-preserved variables.
The median and interquartile range of the demographics, the selected 4 DXA variables, and the variables included in the refit FRS were summarized in Table 1. The Mood’s median test and the chi-square test were performed between the included and excluded samples. The median of thigh intramuscular fat sectional density, incidence rate, and the proportion of diabetes between these two populations were significantly different (P < 0.05). All other baseline demographics, FRS variables, and DXA measures did not differ significantly between the included and excluded population.
Risk score
The 4-DXA risk score reflected the probability of a cardiovascular event occurring within 4000-days and was given by:
where the prognostic index combined the measurements of four DXA variables as follows:
where absag_d represents maximum sagittal diameter (mm), lspibmc represents lumbar spine bone mineral content, pelvbmd represents pelvic bone mineral density, and thimfsd represents thigh intermuscular fat density SD (Sectional Density) (HU). Table 2 provided the Cox proportional hazards model coefficients of the refit FRS model, with and without the addition of the prognostic index from the 4-DXA model. Total cholesterol was not a significant risk predictor with (P = 0.14) or without (P = 0.12) the prognostic index from the 4-DXA model in our study cohort. Gender was statistically significant in the refit FRS (P < 0.005) but became statistically insignificant (P = 0.076) when adding the prognostic index to the refit FRS. All other refit FRS variables were statistically significant, both with and without the prognostic index.
Table 2.
Risk prediction models for the primary endpoint of cardiovascular diseases.
| Refit FRS | Refit FRS plus 4-DXA model | |||
|---|---|---|---|---|
| Coefficients (95% CI) | p-value | Coefficients (95% CI) | p-value | |
| Age, years | 0.045 (0.017, 0.074) | < 0.005 | 0.042 (0.014, 0.071) | < 0.005 |
| Men | 0.27 (0.093, 0.44) | < 0.005 | 0.16 (– 0.017, 0.34) | 0.076 |
| Total cholesterol (mg/dL) | 0.062 (– 0.020, 0.14) | 0.14 | 0.065 (– 0.017, 0.15) | 0.12 |
| High-density lipoprotein cholesterol (mg/dL) | – 0.19 (– 0.29, – 0.095) | < 0.001 | – 0.17 (– 0.26, – 0.071) | < 0.001 |
| Diabetes | 0.49 (0.28, 0.70) | < 0.001 | 0.43 (0.22, 0.64) | < 0.001 |
| Systolic blood pressure (mm Hg) | 0.17 (0.090, 0.25) | < 0.001 | 0.16 (0.080, 0.24) | < 0.001 |
| Current smoker | 0.26 (0.088, 0.60) | < 0.01 | 0.38 (0.13, 0.64) | < 0.005 |
| 4-DXA prognostic index | – | – 0.21 (– 0.30, – 0.13) | < 0.001 | |
All continuous variables were standardized. FRS variables were refitted using a Cox proportional hazards model with and without the 4-DXA prognostic index. FRS Framingham risk score; DXA Dual-energy X-ray absorptiometry; CI confidence interval.
Model performance
Table 3 showed the performance metrics for the refit FRS, the 4-DXA model, and the combination of both models. The C-statistic was 0.62 (95% Confidence Interval (CI): 0.59, 0.65) for the refit FRS model and 0.58 (95% CI: 0.55, 0.61,
Table 3.
Comparative performance metrics for the FRS model, the 4-DXA model, and the refit FRS plus 4-DXA model.
| Refit FRS | 4-DXA model | Refit FRS plus 4-DXA model | |
|---|---|---|---|
| C-statistics |
0.62 (0.59, 0.65) Reference |
0.58 (0.55, 0.61) P = 0.031 |
0.63 (0.60, 0.66) P = 0.046 |
| Discrimination slope | 0.040 (0.029, 0.050) | 0.025 (0.017, 0.033) | 0.053 (0.041, 0.066) |
| Quintile | 2.53 (1.81, 3.26) | 2.02 (1.69, 2.34) | 2.84 (2.14, 3.54) |
| Hosmer-Lemeshow |
17.07 P = 0.029 |
17.28 P = 0.027 |
11.38 P = 0.18 |
| Integrated discrimination index | 1 [Reference] |
– 0.015 (– 0.026, – 0.0034) P = 0.011 |
0.013 (0.0069, 0.019) P < 0.001 |
| Net reclassifcation index | 1 [Reference] |
– 0.12 (– 0.22, – 0.021); P = 0.018 |
0.098 (– 0.0033, 0.20) P = 0.058 |
| Event net reclassification index | 1 [Reference] | – 0.11 (– 0.19, – 0.020) | – 0.0097 (– 0.093, 0.075) |
| No-event net reclassification index | 1 [Reference] | – 0.015 (– 0.069, 0.037) | 0.11 (0.054, 0.16) |
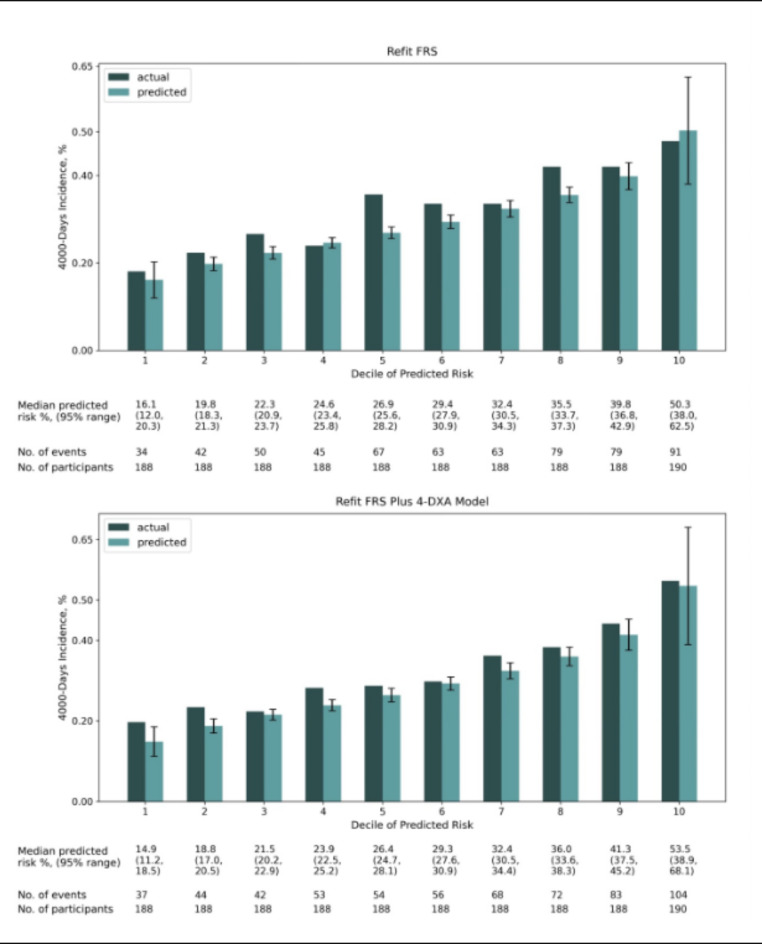
Fig. 2.
Agreement between observed vs. predicted 4000-day incidence of cardiovascular disease with the refit FRS model, and the refit FRS plus 4-DXA model. Predicted 4000-day incidence for each decile is the median predicted risk in percentage. Error bars indicates the 95% prediction intervals. FRS Framingham risk score; DXA Dual-energy X-ray absorptiometry.
Discussion
The present study aimed to select body composition variables predictive for CVD among a broader range of variables obtained through DXA. We also examined whether incorporating the selected features could improve the predictability of traditional FRS among older adults. We selected four features among 87 body composition variables and established a 4-DXA risk prediction model for CVD. The 4-DXA model alone did not outperform the refit FRS but combining the prognostic index obtained by the selected four DXA variables can provide additional predictive value for CVD risk assessment. However, the improvement was modest. The potential reasons for the non-significant findings could be attributed to several factors. First, the Health ABC study recruited relatively healthy older adults that are capable of walking a quarter of a mile, climbing ten steps without resting, and performing basic activities of daily living without assistance. These conditions may lead to healthy body composition indices that might not manifest the risk of CVD. Second, aiming for simplicity and practicality in clinical settings, the developed 4-DXA prognostic index only consisted of linear terms and ignored the interaction between individual variable with time, the interaction between variables, and the higher order terms such as quadratic terms. This might sacrifice certain predictive accuracy compared to a more complex model that includes other interaction terms.
The results also elucidated some clinical implications. The improvement in predicting the risk within each decile group in Fig. 2 suggests that body composition measures could be used to fine-tune risk stratification. If the DXA scans are already being conducted in clinical settings, this additional data could be leveraged to refine CVD risk predictions without significant cost of time. However, due to the limited accuracy achieved from both the refit FRS and the refit FRS plus 4-DXA model, one should use these models with more caution in the healthy older adults in the clinical setting. Further research should validate the efficacy of both FRS and DXA variables in healthy older adults population. A more diverse cohort is also needed for more reliability and more accurate identification of CVD risk prediction variables.
Four body composition indices, maximum sagittal diameter, pelvic bone mineral density, lumbar spine bone mineral content, and thigh intermuscular fat density SD (Sectional Density), were retained after variable selction in the DXA-based prognostic model. We found that the older adults with higher lumber spine bone mineral content, higher max abdominal sagittal diameter, lower pelvic bone mineral density, and lower thigh intramuscular fat sectional density had a higher risk of CVD outcomes. The relationship between each individual variable and CVD is as follow:
Abdominal sagittal diameter measures the distance from the back to the upper abdomen and can be used to reflect visceral obesity. Sagittal abdominal diameter was suggested to be positively related to the risk of coronary heart disease in large prospective studies from the National Health Nutrition and Examination Survey 2011–201627, Kaiser Permanente of Northern California subscribers28, and Risérus et al.’s survey in Sweden29. There are also several underlying biological mechanisms between the elevated abdominal sagittal diameter and the increased risk of CVD. For instance, abdominal sagittal diameter estimates of the accumulation of visceral adipose tissue and can serve as a strong measure of visceral obesity30. The excessive accumulation of adipose tissue can cause instability in body weight homeostasis, insulin resistance, and alterations in lipids, blood pressure, coagulation, fibrinolysis and inflammation, which leads to endothelial dysfunction and atherosclerosis31.
The negative association between pelvic BMD and CVD found in the present study can also be verified in other research32–36. For instance, Trivedi and Khaw35 found that BMD measured at the hip is inversely associated with all-cause mortality and cardiovascular disease mortality from the population of over one thousand older men in the Cambridge General Practice Health Study. This association may be explained by shared pathophysiological mechanisms between osteoporosis and atherosclerosis. Both conditions involve the calcification process, where cells resembling osteoblasts, osteoclasts, and chondrocytes also contribute to the formation of calcium hydroxyapatite crystals. Genetic factors like OPG and MGP mutations also increase the risk of both osteoporosis and arterial calcification37. These mechanisms highlight the biological link between low bone mineral density and cardiovascular disease.
There have also been new findings regarding the other two selected variables, lumbar spine BMC and thigh intramuscular fat sectional density. The present study showed a positive association between lumbar spine BMC with CVD incidence. Although Farhat and Cauley38, using the same study cohort, concluded a negative association between lumbar spine BMD with CVD outcomes, this relationship only presented in the white men and black women group, but was missing in the black men and white women group. Therefore, it is likely that the relationship becomes insubstantial when tested in the general population regardless of race and gender. Furthermore, in the systematic review by Khandkar et al.39, there was no significant association between lumbar spine BMD and CVD. As for intramuscular thigh muscle fat, its association with CVD outcomes was also conflicting. Some study shows that the thigh intramuscular fat density is positively correlated with CVD risk40,41. A study, which focuses on the same population as our study, shows intramuscular thigh muscle fat is independently associated with CVD risk42.
Our study has several strengths. First, our study considered a broader range of body composition variables and investigated their potential association with CVD. We also conducted multiple comparisons between the 4-DXA model, the refit FRS, and the refit FRS plus 4-DXA model. Second, the present study proposed a novel method that combines the LASSO-penalized Cox PH model and backward elimination for variable selection. We addressed collinearity between body composition variables in the LASSO and preserved statistical significance when conducting stepwise backward elimination. Third, this study supplemented the traditional FRS by adding body composition variables, which led to a more accurate prediction of the risk of CVD.
Nevertheless, the limitations of the present study also warrant mentioning. First, the Health ABC cohort population was sampled from well-functioning older adults who are free of life-threatening illness and possess good mobility. The sample might have better indices in the examination compared with the older population that has limitation in functioning and mobility and therefore, might not be fully representative of the general older population. In addition, our results may not be generalizable to a younger population that are less than 70 years old. Furthermore, additional biases might be introduced by the significant differences between the included and excluded population in thigh intramuscular fat sectional density, the rate of diabetes, and the rate of incidence. Third, we were unable to determine whether the selected body composition variables were causally associated with CVD or whether there might be other confounders of CVD. A larger and more comprehensive cohort is needed to validate the developed models. The FRS, 4-DXA model, and the FRS plus 4-DXA model should also be refitted and the improvement of incorporating 4-DXA prognostic index into the FRS will be assessed. Better strategies for excluding the population due to missing values might reduce potential biases. Final causal relationship should also be investigated to address the importance of each selected variable and to contribute to better prevention and therapy of CVD.
Conclusion
In conclusion, among the older adults, the combination of four selected DXA variables slightly improved the performance of FRS in predicting cardiovascular endpoints, but the accuracy is still modest. Further study is needed to validate the effect of FRS and each individual body composition variable in both the healthy older adults population and the general population on CVD risk prediction. More effective measures to improve the predictability of FRS should also be investigated and included.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
C.W. designed the study. L.C., X.W., and T.L. carried out the study and conducted statistical analysis. H.X., H.L., S.X., and J.Y. interpreted the acquired results. L.C. and X.W. drafted the manuscript. All authors provided critical feedback for important intellectual content.
Funding
The research was supported by the Kunshan Government Research Funding.
Data availability
The dataset utilized in our research, known as the Health, Aging and Body Composition (Health ABC) Study, is a longitudinal cohort dataset designed to investigate the risk factors associated with aging-related changes in body composition and physical function in initially well-functioning older adults. This dataset was assembled through extensive field work and follow-ups by the National Institute on Aging (NIA) and the National Institute of Health (NIH). The datasets analysed during the current study are available in the National Institute on Aging’s website https://www.nia.nih.gov/healthabc-study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lihui Chen and Xinran Wang.
References
- 1.Martin, S. S. et al. 2024 Heart Disease and Stroke statistics: a report of US and Global Data from the American Heart Association. Circulation149, e347–e913 (2024). [DOI] [PubMed] [Google Scholar]
- 2.O’Sullivan, J. W. et al. Polygenic risk scores for cardiovascular disease: A scientific statement from the American Heart Association. Circulation2022, 146 (2022). [DOI] [PMC free article] [PubMed]
- 3.Wilson, P. W. F. et al. Prediction of coronary heart disease using risk factor categories. Circulation97, 1837–1847 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Rodondi, N. et al. Framingham risk score and alternatives for prediction of coronary heart disease in older adults. PLoS ONE7, e34287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung, J. Y., Lin, S. L., Lee, R. S., Lam, T. H. & Schooling, C. M. Framingham risk score for predicting cardiovascular disease in older adults in Hong Kong. Hong Kong Med. J. Xianggang Yi Xue Za Zhi24(Suppl 4), 8–11 (2018). [PubMed] [Google Scholar]
- 6.May, M., Lawlor, D. A., Brindle, P., Patel, R. & Ebrahim, S. Cardiovascular disease risk assessment in older women: can we improve on Framingham? British women’s heart and health prospective cohort study. Heart92, 1396 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Ruijter, W. et al. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ338, a3083–a3083 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepherd, J. A., Ng, B. K., Sommer, M. J. & Heymsfield, S. B. Body composition by DXA. Bone104, 101–105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan, S. S. et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol.3, 280–287 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Després, J. P. Body fat distribution and risk of cardiovascular disease: an update. Circulation126, 1301–1313 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Flint, A. J. et al. Body mass index, waist circumference, and risk of coronary heart disease: a prospective study among men and women. Obes. Res. Clin. Pract.4, e171–e181 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su, T. T. et al. Body composition indices and predicted cardiovascular disease risk profile among urban dwellers in Malaysia. BioMed Res. Int.2015, 1–7 (2015). [DOI] [PMC free article] [PubMed]
- 13.Ortega, F. B., Lavie, C. J. & Blair, S. N. Obesity and cardiovascular disease. Circ. Res.118, 1752–1770 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Kihara, S. & Matsuzawa, Y. Fat distribution and cardiovascular disease risk. Curr. Cardiovasc. Risk Rep.9, 8 (2015). [Google Scholar]
- 15.Ashraf, M. J. & Baweja, P. Obesity: the ‘huge’ problem in cardiovascular diseases. Mo Med.110, 499–504 (2013). [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmadi, N. et al. The relation of low levels of bone mineral density with coronary artery calcium and mortality. Osteoporos. Int. J. Establ Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA29, 1609–1616 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Park, J. et al. Prognostic value of lower bone mineral density in predicting adverse cardiovascular disease in Asian women. Heart Br. Card Soc.107, 1040–1046 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Xiao, S. et al. Prevalence of cardiovascular diseases in relation to total bone mineral density and prevalent fractures: a population-based cross-sectional study. Nutr. Metab. Cardiovasc. Dis.32, 134–141 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Kim, H. et al. Low bone mineral density is associated with coronary arterial calcification progression and incident cardiovascular events in patients with chronic kidney disease. Clin. Kidney J.15, 119–127 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veronese, N. et al. Relationship between low bone mineral density and fractures with incident cardiovascular disease: a systematic review and meta-analysis. J. Bone Min. Res. Off J. Am. Soc. Bone Min. Res.32, 1126–1135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman, A. B. et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc.51, 323–330 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Rooks, R. N. et al. The Association of Race and Socioeconomic Status with Cardiovascular Disease indicators among older adults in the Health, Aging, and body composition study. J. Gerontol. B Psychol. Sci. Soc. Sci.57, S247–S256 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Visser, M. et al. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. J. Appl. Physiol.87, 1513–1520 (1999). [DOI] [PubMed] [Google Scholar]
- 24.Tylavsky, F. et al. QDR 4500A DXA overestimates fat-free mass compared with criterion methods. J. Appl. Physiol.94, 959–965 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Tibshirani, R. Regression shrinkage and selection via the Lasso. J. R Stat. Soc. Ser. B Methodol.58, 267–288 (1996). [Google Scholar]
- 26.D’Agostino, R. B. et al. Primary and subsequent coronary risk appraisal: new results from the Framingham Study. Am. Heart J.139, 0272–0281 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Li, C. et al. Sagittal abdominal diameter and its socioeconomic correlates: perspective of sex differences. BMC Public. Health21, 486 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iribarren, C., Darbinian, J. A., Lo, J. C., Fireman, B. H. & Go, A. S. Value of the sagittal abdominal diameter in coronary heart disease risk assessment: cohort study in a large, multiethnic population. Am. J. Epidemiol.164, 1150–1159 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Risérus, U., De Faire, U., Berglund, L. & Hellénius, M. L. Sagittal abdominal diameter as a screening tool in clinical research: cutoffs for cardiometabolic risk. J. Obes.2010, 1–7 (2010). [DOI] [PMC free article] [PubMed]
- 30.Saad, M. A. N. et al. Sagittal abdominal diameter as a marker of visceral obesity in older primary care patients. J. Geriatr. Cardiol. JGC17, 279–283 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Gaal, L. F. & Mertens, I. L. De Block, C. E. Mechanisms linking obesity with cardiovascular disease. Nature444, 875–880 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Budoff, M. J. et al. Measurement of phantomless thoracic bone mineral density on coronary artery calcium CT scans acquired with various CT scanner models. Radiology267, 830–836 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Chen, Z. et al. Bone mineral density of extremities is associated with coronary calcification and biopsy-verified vascular calcification in living-donor renal transplant recipients. J. Bone Min. Metab.35, 536–543 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Iseri, K. et al. Bone mineral density at different sites and 5 years mortality in end-stage renal disease patients: a cohort study. Bone130, 115075 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Trivedi, D. P. & Khaw, K. T. Bone Mineral density at the hip predicts mortality in elderly men. Osteoporos. Int.12, 259–265 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Wallén, E. F. et al. High prevalence of cardio-metabolic risk factors among adolescents with intellectual disability. Acta Paediatr.98, 853–859 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Shen, C. et al. Relation between bone mineral density, bone loss and the risk of cardiovascular disease in a Chinese cohort. Am. J. Cardiol.110, 1138–1142 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Farhat, G. N. & Cauley, J. A. The link between osteoporosis and cardiovascular disease. Clin. Cases Min. Bone Metab. Off J. Ital. Soc. Osteoporos. Min. Metab. Skelet. Dis.5, 19–34 (2008). [PMC free article] [PubMed] [Google Scholar]
- 39.Khandkar, C., Vaidya, K., Karimi Galougahi, K. & Patel, S. Low bone mineral density and coronary artery disease: a systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc37, 100891 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassler, E. M. et al. Distribution of subcutaneous and intermuscular fatty tissue of the mid-thigh measured by MRI—A putative indicator of serum adiponectin level and individual factors of cardio-metabolic risk. PLOS ONE16, e0259952 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Pelt, R. E., Evans, E. M., Schechtman, K. B., Ehsani, A. A. & Kohrt, W. M. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am. J. Physiol. -Endocrinol Metab.282, E1023–E1028 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Huynh, K. et al. Association between Thigh muscle Fat Infiltration and Incident Heart failure. JACC Heart Fail.10, 485–493 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset utilized in our research, known as the Health, Aging and Body Composition (Health ABC) Study, is a longitudinal cohort dataset designed to investigate the risk factors associated with aging-related changes in body composition and physical function in initially well-functioning older adults. This dataset was assembled through extensive field work and follow-ups by the National Institute on Aging (NIA) and the National Institute of Health (NIH). The datasets analysed during the current study are available in the National Institute on Aging’s website https://www.nia.nih.gov/healthabc-study.