Drug-induced subacute cutaneous lupus erythematosus (DISCLE) accounts for about one-third of all subacute cutaneous lupus erythematosus (SCLE) cases and is clinically, histopathologically, and serologically indistinguishable from idiopathic SCLE. Its onset is temporarily and causally linked to the initiation of a culprit drug (1). Drug classes most frequently identified as DISCLE inducers include tumour necrosis factor-α inhibitors, antiepileptics, antifungals, and proton-pump inhibitors (1). Furthermore, with the advent of immune checkpoint inhibitors (ICI) for treatment of metastasized malignancies, evidence for their role as trigger of drug-induced lupus erythematosus, particularly DISCLE, is accumulating (2).
CASE REPORT
A 23-year-old female with invasive ductal breast carcinoma of no special type (cT3, cN3, G3, cM1, ER/PR negative, BRCA1 mutation) presented to our clinic with partially erosive, sharply delineated, scaling erythematous plaques on the face and scalp (Fig. 1A, B) and erosions of the lips and hard palate (Fig. 1C, D) accompanied by burning and pain. Before the onset of lesions, she received epirubicin, cyclophosphamide, paclitaxel, carboplatin and pembrolizumab with curative intent. The eruption appeared 2 weeks after the first cycle of pembrolizumab and worsened after the second infusion. A biopsy showed thin squamous epithelium with vacuolar basal cell degeneration, isolated foci of keratinocyte necrosis, scant perivascular lymphohistiocytic infiltrates, and mucin deposition in the dermis, suggestive of SCLE (Fig. 2). Direct immunofluorescence showed no specific pattern. Antibody serology tests were positive for ANA (1:640), but negative for anti-dsDNA, antibodies to extractable nuclear antigens including SSA/Ro, SSB/La, to histone, phospholipids, c-ANCA, and p-ANCA. Chest X-ray, ECG, abdomen ultrasound, and urinalysis were unremarkable. The patient did not present with cytopenia, low C3 or C4, arthritis, serositis or any other signs indicating systemic lupus erythematosus.
Fig. 1.
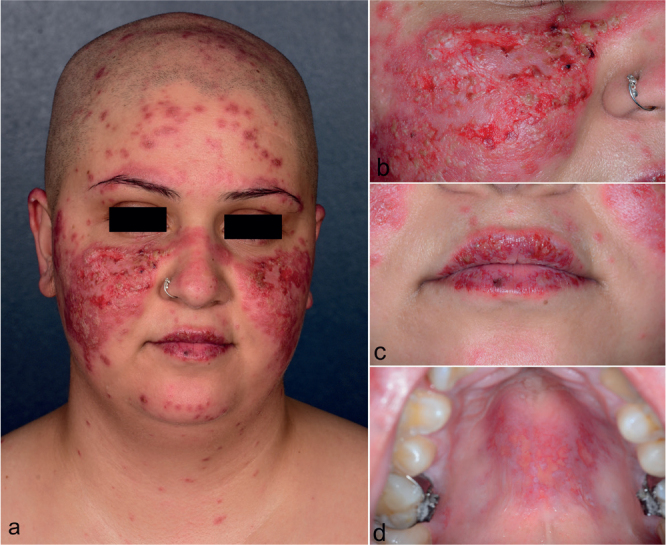
Clinical presentation of the patient after 2 cycles of pembrolizumab. (A, B) Numerous dark red plaques covered by scales and crusts on the face. Erosions on (C) the lips and (D) the hard palate.
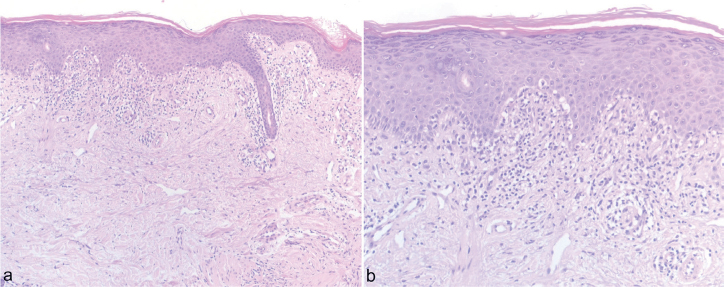
Fig. 2.
Histology from the lesion shows interface dermatitis, vacuolar basal cell degeneration, isolated foci of keratinocyte necrosis, and scant perivascular lymphohistiocytic infiltrates suggestive of subacute cutaneous lupus erythematosus. Haematoxylin eosin staining, magnification (A) x100 and (B) x200.
Due to the absence of systemic symptoms, of in vivo antibody deposition at the basement membrane zone, and of Ro-/La-antibodies, cutaneous lupus erythematosus (CLE) was diagnosed despite a histology reminiscent of SCLE.
The patient received i.v. prednisolone 100 mg for 3 days, and was then switched to oral prednisolone with dose tapering, hydroxychloroquine (HCQ) 200 mg twice daily, and topical clobetasol propionate and methylprednisolone aceponate with moderate effect. Due to the severity of the skin condition, an interdisciplinary decision was made to withdraw pembrolizumab. The patient discontinued hydroxychloroquine autonomously about 2 weeks later due to eye pain. The patient continued chemotherapy with epirubicin, cyclophosphamide, paclitaxel, and carboplatin without interruption and the cutaneous manifestations gradually improved after pembrolizumab withdrawal. Therefore, we propose pembrolizumab as culprit substance. At a 6-month follow-up, the patient presented with post-inflammatory pigmentation and milia in the midface, but showed no new lesions (Fig. 3).
Fig. 3.
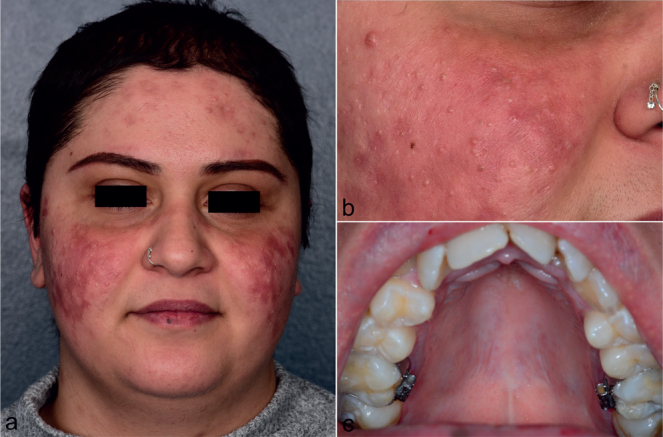
Clinical presentation of the patient at 6-month follow-up visit. Complete resolution of drug-induced subacute cutaneous lupus erythematosus with (A, B) post-inflammatory hyperpigmentation and milia in the midface, and (C) unaffected mucosa of the hard palate.
DISCUSSION
The advent of ICI therapy has revolutionized the management of metastatic cancer. However, resulting T-cell activation frequently leads to immune-related adverse events (irAEs) and exacerbation of pre-existing autoimmune conditions (3). A study by Rong et al. (4) including 8,175 patients with non-small-cell lung cancer found that 46.8% developed an irAE. The most common included pneumonitis (16.5%), hypothyroidism (10.5%), arrhythmia (11.18%), and acute kidney injury (5.8%) (4). The cutaneous irAE profile of ICI (especially PD-1/PD-L1 inhibitors) includes maculopapular rash, lichenoid reactions, eczema, vitiligo, mucositis, bullous pemphigoid, and rarely Stevens–Johnson syndrome/toxic epidermal necrolysis (5). Evidence suggests that PD-1 inhibitors including nivolumab and pembrolizumab are associated with increased rates of cutaneous lupus erythematosus relative to their global use (6).
We performed a comprehensive review of the literature and identified 21 cases of cutaneous lupus erythematosus due to PD-1 inhibitors, reported in 14 publications (Table SI) (7–20). Of these, 18 cases were diagnosed with SCLE, 2 with discoid lupus erythematosus (DLE), and in 1 case the lesions were defined as a lupus-like cutaneous reaction. In our case the patient presented with predominant facial involvement, which is not common for SCLE, but has been reported in some cases (21). Of the 18 DISCLE cases due to PD-1 inhibitors, facial skin lesions were observed in 4 cases (Table SI). However, in our patient classic annular lesions on the arms and trunk were lacking and the haemorrhagic, erosive involvement of the lips and palate was rather reminiscent of systemic lupus erythematosus. Furthermore, the patient did not present with hyperkeratotic plaques of DLE. With regard to autoantibody profiles, anti-SSA and anti-SSB antibodies were positive in about 83% (15/18) of the reported DISCLE cases, which is similar to the findings seen in classic SCLE. In summary, our case is best classified as CLE. It is conceivable that the immunological mechanisms triggered by the PD-1 inhibitors contribute to an atypical, heterogeneous clinical picture of the induced skin disease.
In 9 cases, the culprit substance needed to be withdrawn (Table SI). In 7 cases, ICI therapy continued after the skin condition resolved or was controlled. Kosche et al. (7) reported restarting nivolumab but this led to another milder DISCLE flare. Some patients developed further irAEs. Shao et al. (8) and Zitouni et al. (9) reported autoimmune hepatitis and grade 2 immune-mediated colitis preceding DISCLE development after nivolumab initiation. Liu et al. (10) reported DISCLE following tapering of prednisone for nivolumab-induced autoimmune haemolytic anaemia. One patient developed guttate psoriasis 4 months after nivolumab initiation, with DISCLE emerging 16 months later (11). In both cases reported by Bui et al. (11), anti-PD-1 therapy continued despite DISCLE flare but was later discontinued due to cancer progression. Another patient developed dermatomyositis after restarting nivolumab following DISCLE resolution (12).
The onset of CLE due to PD-1 inhibitor treatment was reported at different times after therapy initiation. In 10 of 19 cases, DISCLE appeared after 2 or 3 cycles of treatment, with a minimum of 2 weeks and a maximum of 21 months. One patient had histologically proven DLE, well controlled without treatment for over 2 years, which flared on first pembrolizumab exposure (13). The most prescribed therapy for ICI-induced DISCLE included topical and systemic steroids combined with HCQ. In one case, DISCLE resolved spontaneously within a month after pembrolizumab discontinuation (8).
Of all reported PD-1 inhibitor-induced CLE cases, 11 were triggered by nivolumab, 9 by pembrolizumab, and one by cemiplimab (Table SI). There are no reports of SCLE or DLE developing from other PD-1 inhibitors (toripalimab, dostarlimab, retifanlimab).
Although rare, PD-1/PD-L1 inhibitors may cause DISCLE. Early DISCLE diagnosis is crucial for initiating of appropriate therapy, potentially enabling continuation or reintroduction of ICI, which is often decisive for the malignancy prognosis.
Supplementary Material
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Grönhagen CM, Fored CM, Linder M, Granath F, Nyberg F. Subacute cutaneous lupus erythematosus and its association with drugs: a population-based matched case-control study of 234 patients in Sweden. Br J Dermatol 2012; 167: 296–305. 10.1111/j.1365-2133.2012.10969.x [DOI] [PubMed] [Google Scholar]
- 2.Raschi E, Antonazzo IC, Poluzzi E, De Ponti F. Drug-induced systemic lupus erythematosus: should immune checkpoint inhibitors be added to the evolving list? Ann Rheum Dis 2021; 80: e120. 10.1136/annrheumdis-2019-215819 [DOI] [PubMed] [Google Scholar]
- 3.Haanen J, Ernstoff MS, Wang Y, Menzies AM, Puzanov I, Grivas P, et al. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann Oncol 2020; 31: 724–744. 10.1016/j.annonc.2020.03.285 [DOI] [PubMed] [Google Scholar]
- 4.Rong Y, Bentley JP, Bhattacharya K, Yang Y, Chang Y, Earl S, et al. Incidence and risk factors of immune-related adverse events induced by immune checkpoint inhibitors among older adults with non-small cell lung cancer. Cancer Med 2024; 13: e6879. 10.1002/cam4.6879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhardwaj M, Chiu MN, Sah SP. Adverse cutaneous toxicities by PD-1/PD-L1 immune checkpoint inhibitors: pathogenesis, treatment, and surveillance. Cutan Ocul Toxicol 2022; 41: 73–90. 10.1080/15569527.2022.2034842 [DOI] [PubMed] [Google Scholar]
- 6.Bolton C, Chen Y, Hawthorne R, Schepel IRM, Harriss E, Hofmann SC, et al. Systematic review: monoclonal antibody-induced subacute cutaneous lupus erythematosus. Drugs R D 2020; 20: 319–330. 10.1007/s40268-020-00320-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosche C, Owen JL, Choi JN. Widespread subacute cutaneous lupus erythematosus in a patient receiving checkpoint inhibitor immunotherapy with ipilimumab and nivolumab. Dermatol Online J 2019; 25: 13030/qt4md713j8. 10.5070/D32510045821 [DOI] [PubMed] [Google Scholar]
- 8.Shao K, McGettigan S, Elenitsas R, Chu EY. Lupus-like cutaneous reaction following pembrolizumab: an immune-related adverse event associated with anti-PD-1 therapy. J Cutan Pathol 2018; 45: 74–77. 10.1111/cup.13059 [DOI] [PubMed] [Google Scholar]
- 9.Zitouni NB, Arnault JP, Dadban A, Attencourt C, Lok CC, Chaby G. Subacute cutaneous lupus erythematosus induced by nivolumab: two case reports and a literature review. Melanoma Res 2019; 29: 212–215. 10.1097/CMR.0000000000000536 [DOI] [PubMed] [Google Scholar]
- 10.Liu RC, Sebaratnam DF, Jackett L, Kao S, Lowe PM. Subacute cutaneous lupus erythematosus induced by nivolumab. Australas J Dermatol 2018; 59: e152–154. 10.1111/ajd.12681 [DOI] [PubMed] [Google Scholar]
- 11.Bui AN, Hirner J, Singer SB, Eberly-Puleo A, Larocca C, Lianet CG, et al. De novo subacute cutaneous lupus erythematosus-like eruptions in the setting of programmed death-1 or programmed death ligand-1 inhibitor therapy: clinicopathological correlation. Clin Exp Dermatol 2021; 46: 328–337. 10.1111/ced.14449 [DOI] [PubMed] [Google Scholar]
- 12.Marano AL, Clarke JM, Morse MA, Shah A, Barrow W, Selim MA, et al. Subacute cutaneous lupus erythematosus and dermatomyositis associated with anti-programmed cell death 1 therapy. Br J Dermatol 2019; 181: 580–583. 10.1111/bjd.17245 [DOI] [PubMed] [Google Scholar]
- 13.Blakeway EA, Elshimy N, Muinonen-Martin A, Marples M, Bipin Mathew B, Mitra A. Cutaneous lupus associated with pembrolizumab therapy for advanced melanoma: a report of three cases. Melanoma Res 2019; 29: 338–341. 10.1097/CMR.0000000000000587 [DOI] [PubMed] [Google Scholar]
- 14.Andersson NW, Zachariae C, Simonsen AB. Late onset of subacute cutaneous lupus erythematosus following pembrolizumab therapy. Eur J Cancer 2021; 145: 168–170. 10.1016/j.ejca.2020.12.017 [DOI] [PubMed] [Google Scholar]
- 15.Gambichler T, Doerler M, Scheel CH. Onset of subacute cutaneous lupus erythematosus after the initiation of immune checkpoint inhibitor therapy of cancer. Lupus 2021; 30: 531–533. 10.1177/0961203320983448 [DOI] [PubMed] [Google Scholar]
- 16.Ogawa-Momohara M, Muro Y, Goto K, Obuse C, Satoh M, Kono M, et al. Subacute cutaneous lupus erythematosus with melanocyte elimination induced by pembrolizumab. J Dermatol 2020;47: e217–e219. 10.1111/1346-8138.15316 [DOI] [PubMed] [Google Scholar]
- 17.Fietz S, Fröhlich A, Mauch C, de Vos-Hillebrand L, Fetter T, Landsberg J, et al. Manifestation of subacute cutaneous lupus erythematosus during treatment with anti-PD-1 antibody cemiplimab: a case report. Front Immunol 2023; 14: 1324231. 10.3389/fimmu.2023.1324231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diago A, Hueso L, Ara-Martín M, Abadías-Granado I. Subacute cutaneous lupus erythematosus induced by PD-1 Inhibitor therapy: two case reports and literature review. Australas J Dermatol 2021; 62: e347–349. 10.1111/ajd.13538 [DOI] [PubMed] [Google Scholar]
- 19.Manjunath J, Mochel M, Nutan F. Nivolumab-induced de novo discoid lupus erythematosus. Case Rep Dermatol 2022; 14: 88–92. 10.1159/000523800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khorasanchi A, Korman AM, Manne A, Meara A. Immune checkpoint inhibitor-induced subacute cutaneous lupus erythematosus: a case report and review of the literature. Front Med (Lausanne) 2024; 11: 1334718. 10.3389/fmed.2024.1334718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhn A, Specker C, Ruzicka T, Lehmann P. Methotrexate treatment for refractory subacute cutaneous lupus erythematosus. J Am Acad Dermatol 2002; 46: 600–603. 10.1067/mjd.2002.114608 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.