Abstract
Four metal compounds mixed ligand of azo dye ligand (L) and metformin.(Met) were produced at aquatic ethanol for (1:1:1) (M:L:Met). The prepared compounds were identified by utilizing atomic absorption flame, FT.IR and UV–Vis spectrum manners as well as conductivity mensuration. These compounds was assayed of the gained datum the octahedral geometry was proposed into whole prepared complexes.Also in this research was studied represented examining the antibacterial and antifungal impact of the azo dye ligand (L), metformin.(Met) and (Co,Ni, Cu and Cd complexes) on four types of pathogenic, clinically isolated bacteria that are resistant to antibiotic, like Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Klebsiella pneumonia, and fungi Candida albicans from human in Iraq. The results of the sensitivity test showed the effectiveness of these compounds at a very low condensation of (10−3) in inhibiting the isolated bacteria. On the other hand, cytotoxic effects of the ligand, Met and mix ligand complexes showed anticancer activity on HepG2 cells in a serial condensation 15.6, 31, 62, 125, 250, 500 μg/ml. As the effectiveness of the compounds increases with increasing their condensation, the most effective toxicant on hepatic cancer cells is Met and cd complex and with a rate of 68.5 and 68.3 % respectively.
Keywords: Azo dye ligand, Metformin, Metal complexes, Spectral analysis, Biological activity, Toxicity study
1. Introduction
Azo dyes exemplify the largest volume for dye chemistry generated today, as well their proportional significance may raise at future. They play a critical role at managing the dye as well print market. Various methods also modulations are made to gain the desired color characteristics, yield as well particle size for dye to the improve dispersion [1]. Azo dyes are among the most widely utilized dyes, constituting more than 60 % of all dyes [2]. About 70 % of all dyes used at industry are azo dyes [3,4]. Azo dyes have been used as a very important component in the textile and printing industries [5]. Other than, used in several fields including acid-base indicators, food coloring, optical switches, plastics, cosmetics, optical data storage, nonlinear optics, liquid crystal displays, and electro-optical devices [6,7]. Further, azo dyes have been found to have biological applications, such as antioxidants, antibacterial, anticancer, anti-diabetic, and antiviral [[8], [9], [10]]. Transition metal ions have been contributed a diverse and rich field of research, its received much attention because of their applications in biology, medicine and industry [11]. Coordination compounds of azo dyes received much importance due to their ability to mimic complex molecules involved in vital biological process [12] such as oxygen transport, electron transfer, catalysis etc.,. At recent years, researchers have witnessed an interesting increase in the preparation of mixed-ligand complexes, particularly at biological domains [13]. The increase in the emergence of bacteria that are multidrug resistant and cancerous diseases has become a major global problem, and new compounds are needed to treat these diseases, so we studied the effectiveness of new substances to evaluate their inhibitory effects on bacteria and cancer cells. Metformin hydrochloride is an antihyperglycemic agent used to treat type 2 diabetes with a healthy diet, Metformin reduces blood glucose levels by decreasing glucose synthesis by the liver (gluconeogenesis).
It also increases insulin sensitivity by increasing glucose uptake and utilization by cells, and it also inhibits the activity of mitochondrial complex I and this mechanism is what gave metformin a strong effect in the treatment of diabetes and used to treat polycystic ovary syndrome. Metforamin as antimicrobial and antifungal, antiprotozoal, and antituberculosis effect either alone or in combination [14].Metformin showed a potential synergistic effect when combined with a tetracycline and fluoroquinolone group against a highly resistant strain of E.coli, S.aureus and p.aeruginosa [15,16]found metformin has anticancer activity and its mechanism of action is unknown. Azo ligand contain metoclopramide hydrochloride is antiemetic used to treat of gastroesophageal reflux disease, anticancer to expressed in a diversity of cancer tissue like lung, cervical,breast, and ovarian cancer by blocking the D2 receptor [17].Metals at low concentration within the permissible limits of global health [18],and mixed with agent lead to use to treat cancer by specifically attacking cancer cells and interacting directly with DNA. The positive charge of most metals can interact with the negative charge of the phosphate portion of DNA [19].Also, these elements and their complexes have antibacterial and antifungal effectiveness by eliminated or their growth prevented by preventing the formation of the cell wall, or through a defect in the physical or chemical structure of protein and nucleic acids,or a defect in the permeability of the cytoplasmic membranes.They can also be destroyed by a defect in the cellular enzymatic activity,as well as through Manufacturing is prohibited Proteins and nucleic acids [20].
2. Experimental
2.1. Materials
In this study, all the reagents used in this research were of the highest quality analar, which consist of Metformin.HCl, NaOH ethanol and DMSO (Merck and Aldrich chemicals). The metal chlorides, CoCl2.6H2O, NiCl2.6H2O, CuCl2.2H2O and CdCl2.H2O (Merck, BDH and Riedel) are utilized unpurified.
2.2. Physical Measurements
UV–Vis spectrum have been recorded at a Shimadzu-U.V-160.Ultraviolet spectrometer utilizing DMSO solution 10−3 at range (200–1000) nm for the (Met) as well as mixed ligand complexes. The molar conductance for mixed ligand metal complexes have been measured employing a CON 510 Conductivity at dry DMSO (10−3 M) solution on room temperature. While metal includes into complexes were determined through Atomic Absorption (A.A)Technique/Flame Emission Spectrophotometer using AA – 680. NMR spectrum (1H, 13CNMR)were acquired at DMSO-d6 solution employing NMR bruker 500mhz Germany at DMSO-d6 for the cadmium complex. Melting point have been obtained at Stuart Melting Point Apparatus for the (Met) and mixed ligand complexes. Infrared (FT.IR) spectral have been registered at 4000-400 cm−1 rang to the (Met) as well metal complexes at a Shimadzu IR-470 Spectrophotometer employing KBr. The mass spectra for the all complexes were recorded at the Agilent 6211 mass spectrometer. Sherwood Applying the Auto Magnetic Susceptibility at 25 °C allowed scientists to refine the magnetic characteristics. Elemental (C. H. N.) micro-analysis, to search using the EA 3000 single V.3 model vector device.
2.3. Preparation of the azo dye ligand
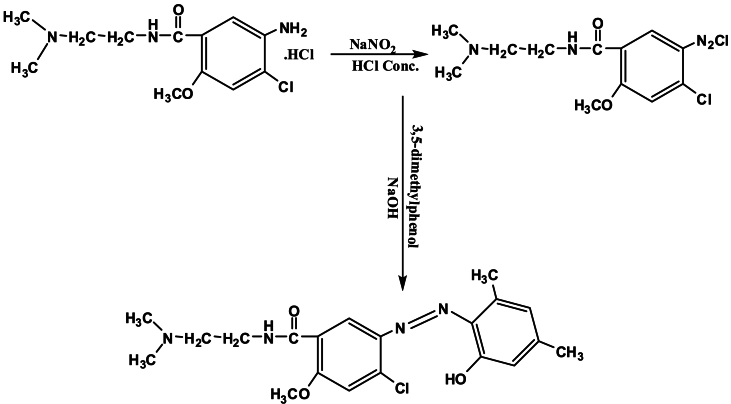
A solution was produced [21],of metoclopramide hydrochloride (0.84 gm,1 mM) in mixture (10 ml ethanol, 2 ml conc. HCl), and diazotized at 5 °C with 10 % solution of NaNO2. Diazotized solution has been added collyrium wise for stirring into a cooled ethanolic solution at (0.305 gm, 1 mM) for 3,5-dimethylphenol. Then 25 ml at 1M NaOH solution has been followed into dusky colored mix and precipitation for azo ligand has been noticed. This deposit have been filtrated, washed number ounces for (1:1) C2H5OH: H2O, mixture subsequently left into dry, the reaction is appear at Scheme 1.
Scheme 1.
Synthesis for azo ligand (L).
2.4. Preparation for mixed ligand Metal complexes
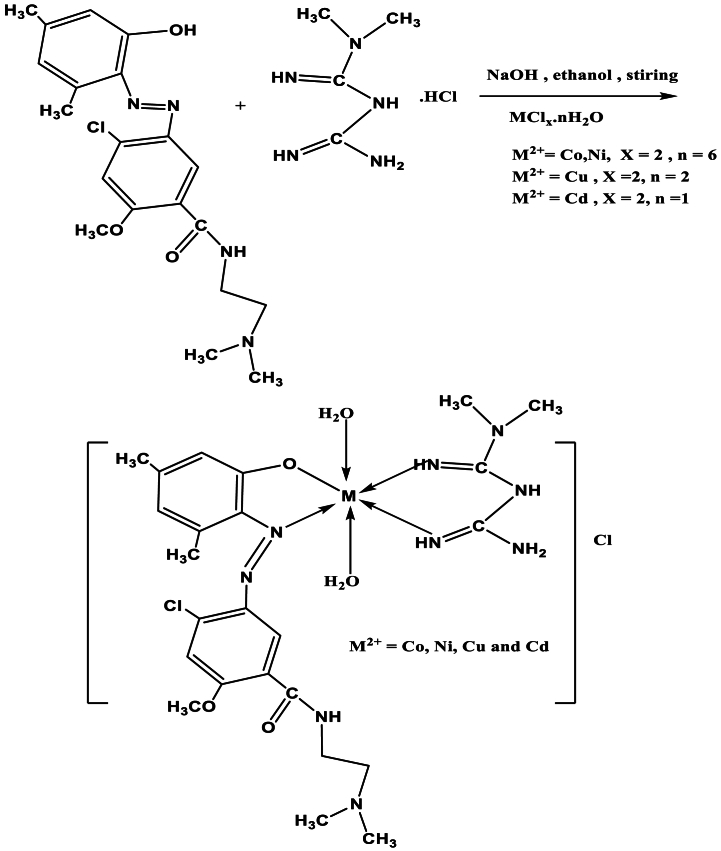
A public method has been utilized with the research for all coordinations complexes.The complexes have been prepared at a comparable method around synthesis at the molar ratio from M:L:Met (1:1:1). In the aqueous solution (10ml) for the appropriate cobalt(II) chloride hexahydrate (0.238g,1 mM), nickel(II) chloride hexahydrate (0.238g,1 mM), copper(II) chloride dihydrate (0.171g,1 mM) as well cadmium(II) chloride monohydrate (0.201g,1 mM). Has been added into a ethanolic solution(20 ml) for azo dye ligand (L), C20H25ClN4O3(0.404 gm,1 mM) that was previously prepared [1] as well added (0.04 gm,1 mM) for the NaOH was added to the previous solution as well as (0.1656 gm,1 mM) for the metformine.HCl(C4H12ClN5) in (20 ml) in an ethyl alcohol solution. The solution was refluxed about 2hr., then the precipitated solid complex has been filtrated, washed completely with (C2H5)2O as well dried at vacuo, see Scheme 2.
Scheme 2.
Preparation of mixed ligand complexes.
2.5. Antimicrobial susceptibility test
Preparation of bacterial and fungus suspension by transferring 2–3 colonies of E. coli, K.pneumoniae grew on MacConkey agar and S.epidermidis, S.aureus grew on blood agar base as for the C. albicans fungus it was grown on amedium bromocresol green agar, into 5 ml of normal saline, the turbidity of suspension was compared to 0.5 Mcfarland using Densicheck plus technique. The agar well diffusion method was used to evaluate the effect of azo dye ligand (L), Metformine.HCl (Met)and (Co,Ni, Cu and Cd) complexes on the growth of the E.coli, K.pneumoniae, S.aureus, S. epidermidis and C. albicans at concentration 10 −3. The muller hinton agar 20 ml was cultured by spreading the bacterial and fungus suspension. Well, were made by cork borer and volume 0.05 ml was added to each well in addition to DMSO as control treatment. This plates were left at room temperature for 15–20 min then incubated at 37 °C for 24 h.Three replicates from each compounds were done. measured the inhibition zone diameter by ruler [22].
2.6. Chloride content analysis (Cl %)
Chloride content of the prepared complexes was determined and analysed using the standard method [23], using Moore's method by titrating the complex against the silver nitrate present in the burette.
2.7. Cell culture
HepG2 human hepatic cell carcinoma was obtained from aliver cancer of 42 years old male and Hdfn (Human dermal fibroblast, neonatal) normal cell line the same age, were obtained from the university of malya, Malaysia. The cell sheet washed with phosphate buffer saline. 2–3 ml/trypsin/EDTA solution was added to the cell in the vessels and incubated 37 C for 2 min to detach from the vessel then [[15], [16], [17], [18], [19], [20]] ml RPMI medium added Penicillin G 103 IU, Streptomycin 0.001 g, Sodium Bicarbonate 1 %, Fetal Bovine Serum 10 % and incubated at 37 °C in CO2 incubator to grow the cells. Cell count was calculated by haematocytometer [24].
2.8. MTT cytotoxicity Protocol
The cytotoxicity effect of chemical compounds (azo dye ligand (L), Metformine.HCl (Met), (Co, Ni, Cu and Cd) complexes were performed by using MTT kit. 200 μL of medium added to the each well of microtiter plate, incubated at 37 °C, 5 % CO2 for 24 h, 200 μl of serial concentration of (Met), (L) and mix ligand complexes (15.6, 31, 62, 125, 250, 500) μg/ml. Triplicate were used for each concentration were added to the wells. The control was the cells treated with serum free medium. 10 μl of the MTT was added to well and the microplate incubated at 37 °C, 5%CO2 for 4 h. The medium was removed and 100 μl of solubilization solution was added and incubated 5 min. The absorbance was measured by Elisa at 575 nm [25,26].
3. Results and discussion
The solid compounds were generated through reaction from alcoholic solution of the azo dye ligand (L) for the aqueous solution from the metal ions and Metformin (Met) into a (M:L:Met) from (1:1:1). The metal contents of these complexes were into good correspondence for the calculated values (Table 1) contains the physical characteristics. The molar conductance from the compounds as (10−3 M) on DMSO indicating there electrolyte nature at ratio (1:1) [27], and the data recorded in (Table 2).
Table 1.
Elemental analysis and physical data for (L), (Met) and mixed ligand complexes.
| Compounds | Empirical |
Molecular weight |
Color |
M.P (°C) | Analysis Calc (Found) |
|||
|---|---|---|---|---|---|---|---|---|
| Formula | (g/mol) | (Yelid %) | M% | C% | H% | N% | ||
| Azo dye ligand (L) | C20H25ClN4O3 | 403.50 | Orange | <300 | – | 59.25 | 6.17 | 13.82 |
| (81) | (58.93) | (6.03) | (12.96) | |||||
| Metformin.HCl (Met) | C4H12ClN5 | 165.50 | white | 221 | – | – | ||
| [Co(L)(Met)(H2O)2]Cl | [CoC24H39N9O5Cl]Cl | 663 | Deep green | >350 | 8.89 | 43.43 | 5.88 | 19.00 |
| (73) | (8.37) | (42.85) | (4.91) | (18.77) | ||||
| [Ni(L)(Met)(H2O)2]Cl | [NiC24H39N9O5Cl]Cl | 662 | Orange | >350 | 8.76 | 43.50 | 5.89 | 19.33 |
| (82) | (7.32) | (42.97) | (4.87) | (18.72) | ||||
| [Cu(L)(Met) (H2O)2]Cl | [CuC24H39N9O5Cl]Cl | 668 | Light brown | >350 | 9.58 | 43.11 | 5.83 | 18.86 |
| (77) | (7.56) | (42.97) | (4.75) | (17.92) | ||||
| [Cd(L)(Met)(H2O)2]Cl | [CdC24H39N9O5Cl]Cl | 716 | Orange | >350 | 15.64 | 40.22 | 5.44 | 17.59 |
| (81) | (13.52) | (39.87) | (4.97) | (16.88) | ||||
Table 2.
UV spectra data and molar conductivity for(met) and mixed ligand complexes.
| Compounds | λ (nm) | ABS | Wave number ) (cm−1 |
Єmax (L.mol−1.cm−1) |
Assignments | Molar conductivityΛm.cm2.Mol−1 10−3M in DMSO | μeff (B.M) |
|---|---|---|---|---|---|---|---|
| Metformin. HCl (Met) | 260 | 0.539 | 38462 | 539 | π–π∗ | – | |
| 369 | 0.040 | 27100 | 40 | n–π∗ | |||
| Azo dye ligand (L) | 263 | 1.207 | 38022 | 1207 | π–π∗ | – | |
| 312 | 0.858 | 32051 | 858 | π–π∗ | |||
| 386 | 1.919 | 25907 | 1919 | n–π∗ | |||
| [Co(L)(Met)(H2O)2]Cl | 265 | 0.883 | 37736 | 883 | intra ligand | 60.2 | 4.86 |
| 386 | 0.807 | 25907 | 807 | C.T | |||
| 543 | 0.023 | 18416 | 23 | 4T1g(F)→4T1g(P) | |||
| [Ni(L)(Met)(H2O)2]Cl | 266 | 1.363 | 37594 | 1363 | intra ligand | 53.1 | 2.88 |
| 390 | 1.687 | 25641 | 1687 | C.T | |||
| 520 | 0.024 | 19230 | 24 | 3A2g→3T1g(P) | |||
| [Cu(L)(Met)(H2O)2]Cl | 263 | 0.659 | 38023 | 659 | intra ligand | 74.3 | 1.71 |
| 388 | 0.548 | 25773 | 548 | C.T | |||
| 580 | 0.011 | 10163 | 11 | 2Eg→2T2g | |||
| [Cd(L)(Met)(H2O)2]Cl | 264 | 0.645 | 37879 | 645 | intra ligand | 52.8 | Dia |
| 391 | 1.378 | 25575 | 1378 | C.T. | |||
| 527 | 0.520 | 18975 | 520 | MLCT |
3.1. Mass spectra
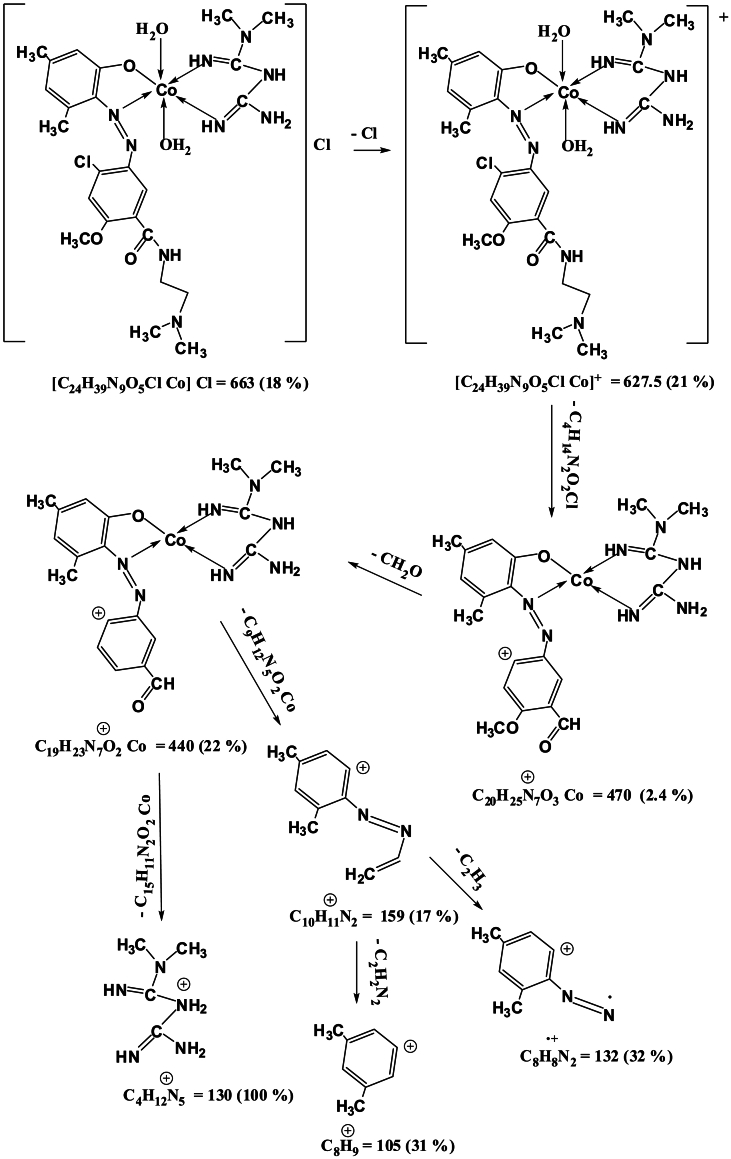
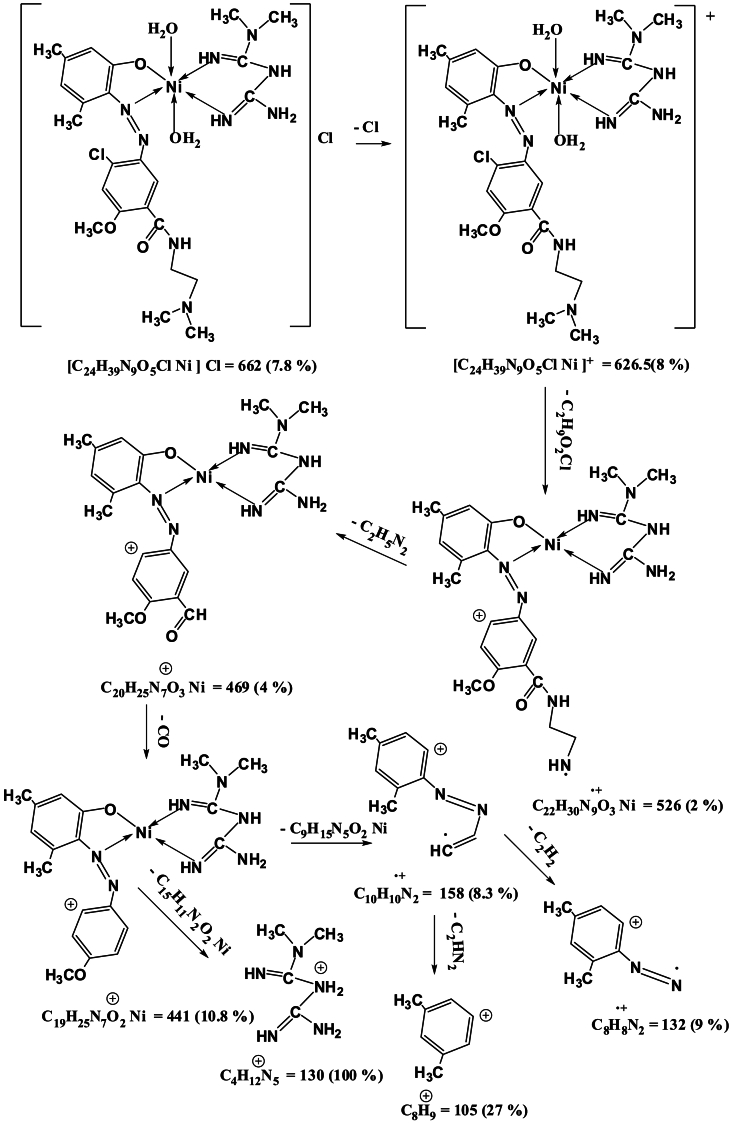
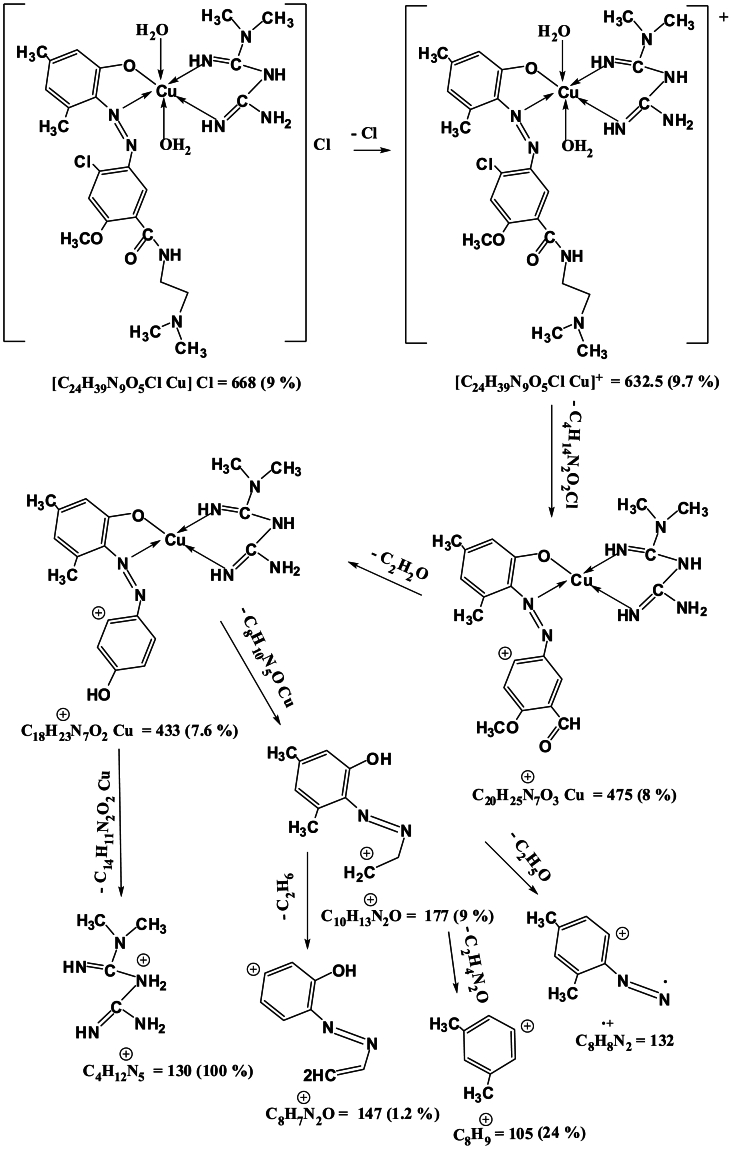
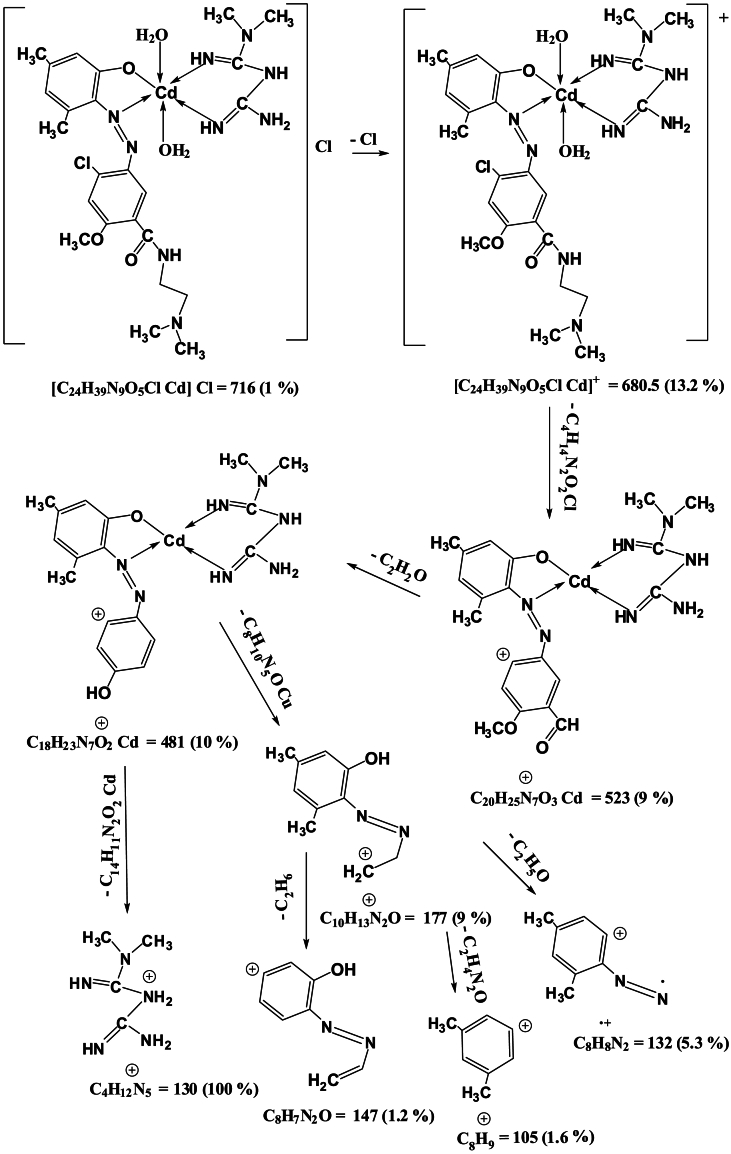
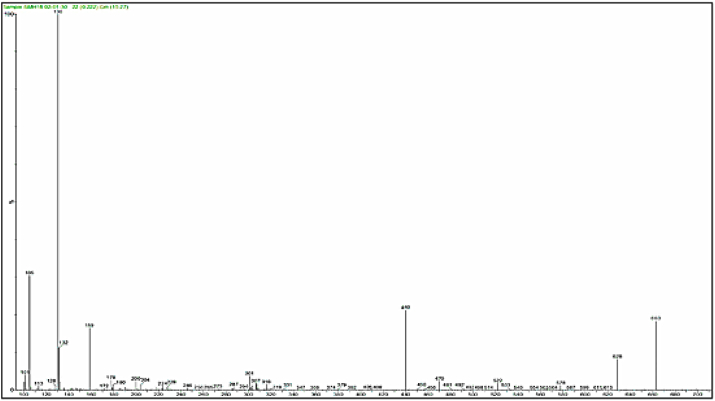
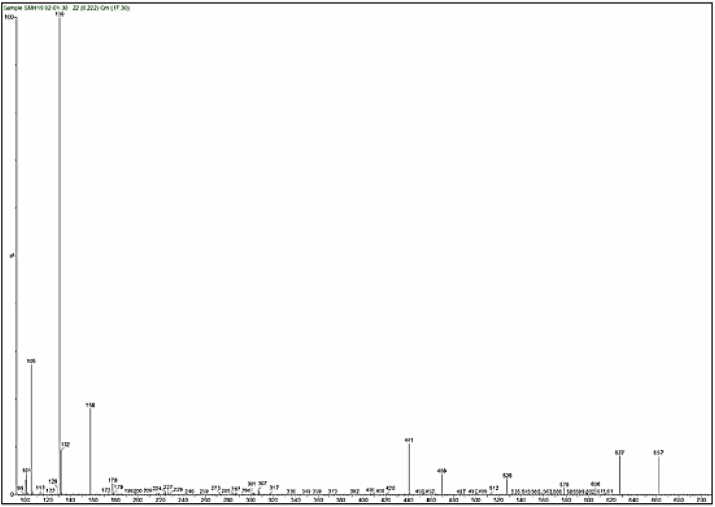
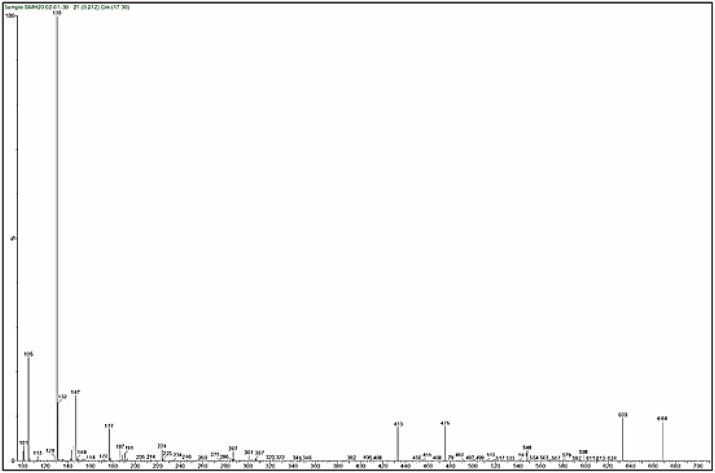
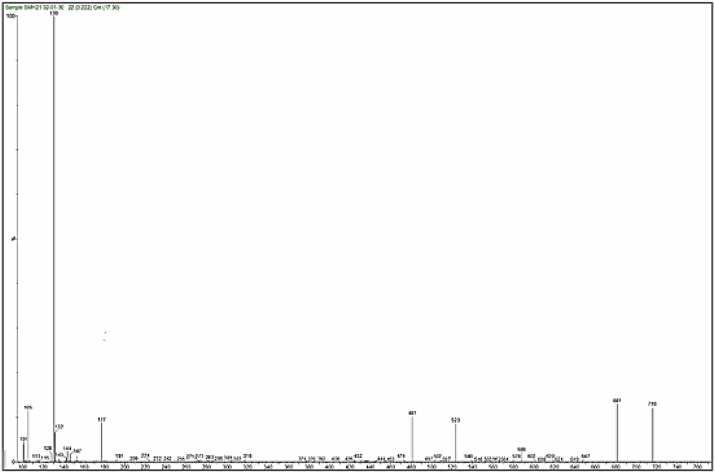
Mass spectra of the complexes displays peaks centered at m/z = 663, 662, 668 and 716 due to the formulas [C24H39N9O5ClCo]Cl, [C24H39N9O5ClNi]Cl, [C24H39N9O5ClCu]Cl and [C24H39N9O5ClCd]Cl respectively. The general pattern of fragmentation are summarized in Scheme 3, Scheme 4, Scheme 5 and 6, see Fig. 1, Fig. 2, Fig. 3, Fig. 4.
Scheme 3.
Fragmentation Pattern for [Co(L)(Met)(H2O)2]Cl Complex.
Scheme 4.
Fragmentation Pattern for [Ni(L)(Met)(H2O)2]Cl Complex.
Scheme 5.
Fragmentation Pattern for [Cu(L)(Met)(H2O)2]Cl Complex.
Scheme 6.
Fragmentation Pattern for [Cd(L)(Met)(H2O)2]Cl Complex.
Fig. 1.
Mass spectrum for [Co(L)(Met)(H2O)2]Cl complex.
Fig. 2.
Mass spectrum for [Ni(L)(Met)(H2O)2]Cl complex.
Fig. 3.
Mass spectrum for [Cu(L)(Met)(H2O)2]Cl complex.
Fig. 4.
Mass spectrum for [Cd(L)(Met)(H2O)2]Cl complex.
3.1.1. NMR spectra
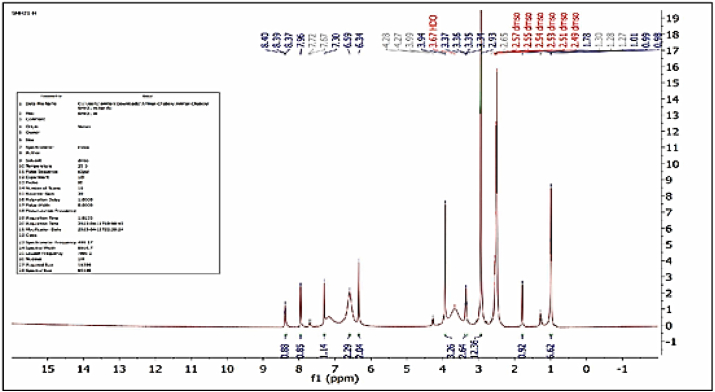
1HNMR spectrum of Cd2+ complex at dimethylsulfoxide (Fig. 5) display various signals at (δ = 7.67–7.96) ppm refers to aromatic protons [28]. Signal obtained at (δ = 8.39) ppm lead to proton of amide [29]. Finding, the signals at (δ = 1.78) and (δ = 1.27) ppm were assigned to δ(CH3) of phenol and (N-(CH3)2), signals at (δ = 2.65, 3.35) and (δ = 3.94) ppm which were assigned to (CH2) and (OCH3) groups [30]. The signals at (δ = 2.93) and (δ = 6.59) due to protons of (N-(CH3)2) and (NH,NH2)in metformin [31]. signals at (δ = 7.30) and (δ = 2.5) ppm which were assigned to(=NH) groups and DMSO- d6 [32].
Fig. 5.
1HNMR spectrum for [Cd(L)(Met)(H2O)2]Cl complex.
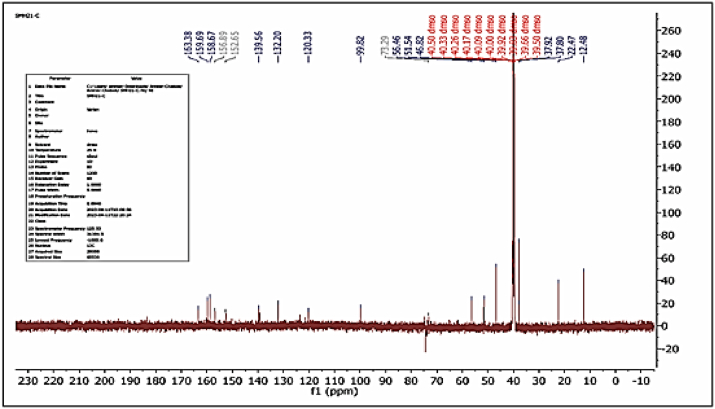
13CNMR Cd2+ complex spectrum display resonance at (δ = 12.48) and (δ = 22.47) ppm due to carbon of (CH3) in phenol ring. Signals at (δ = 37.80, 56.46) and (δ = 46.82) ppm lead to carbon of (CH2) and (N-(CH3)2) respectively. The resonance at (δ = 37.92) and (δ = 51.54) ppm indicative to carbon of (N-(CH3)2) for metformin and methoxy group (OCH3) for azo dye. The various signals at (δ = 158.67, 156.89, 152.65, 139.56, 132.20, 120.33, 99.82 and 73.29) ppm attributed to carbon atoms of aromatic rings. Signals at (δ = 163.38) ppm and (δ = 159.69) ppm due to carbon of (C=O) and (C=NH) groups and the indicative in (δ = 39.83) ppm due into DMSO-d6 [33,34], see Fig. 6.
Fig. 6.
13CNMR spectrum for [Cd(L)(Met)(H2O)2]Cl complex.
3.1.2. Electronic spectra
UV–Vis spectra to the produced compounds melted at DMSO (10−3 M/L) were gauged as well the datum formed is listed at Table 2. UV–Vis spectrum to the azo ligand shows peaks at 263, 312 and 386 nm were appointed into mild energy (π-π∗) and (n-π∗) transition, metformin spectrum shows to peaks at 260 and 369 nm due to (π-π∗) and (n-π∗) transition [35]. Co(II) spectrum appeared two peaks at 265 and 386 nm due to intra ligand and charge transfer. Peak at 543 nm assigned to electronic transition type 4T1g(F)→4T1g(P), also the value from the magnetic moment at 4.86 B M may be possessed as extra confirmation to octahedral [36]. The spectrum for Ni(II) complex display peaks in 266 and 390 nm whom were qualified into intra ligand and charge transfer. Else peak at 520 nm whom was refereed into electronic transition type 3A2g(F)→ 3T1g(P), the magnetic moment for this complex was discover in 2.88 B M whom was much close to the octahedral [37]. Cu(II) spectrum display peaks at 263 and 388 nm due to intra ligand and charge transfer. Other peak at 580 nm lead to electronic transition type 2Eg→2T2g, magnetic moment for this complex was found at 1.71 B M whom was much close to the octahedral environment [38]. The electronic spectrum of Cd(II) complex do offer the charge transferz and the magnetic susceptibility seemed the complex has diamagnetic moments, result to (d-d) transition are not likely hence electronic spectrum did not confer any productive datum, on fact this outcome are a good agreement for former work from octahedral [39].
3.1.3. FTIR spectra
FTIR spectra to the azo ligand and their metal chelates have been collated, and the data was scheduled in Table 3. The broad band in the spectrum of the azo ligand at 3371 cm−1, that was described into the stretching vibration from υ(OH) phenol, the disappearance of this band at the spectra with all produced compounds pointed out the deprotonation for phenol group to coordination with metal ion [40]. Band for azo group at 1570 cm−1 displaced into lower wave number for change during shape at spectra for all produced compounds [41]. The spectrum of metformin exhibited bands at 3371,3170 and 3294 cm−1due to υ(NH2) and υ(NH) [42]. Band at 1627 cm−1 which was assigned to υ(C=N), in complexes this band shifted to higher frequency, may be a result to the coordination [43]. extending the frequency bands for metal-nitrogen at a different rate than metal-oxygen [44,45] (according to Hock law). The structural formula of these complexes has been reached through the study of these spectra. According the result an octahedral geometry have been suggested for the metal-ligand mix complexes.
Table 3.
The main frequencies for azo ligand and metal chelate (cm−1).
| Compounds | υ (NH2) |
υ(C=N) |
υ(H2O) |
υ (M − N) |
|---|---|---|---|---|
| + |
+ |
+ |
+ |
|
| (NH)υ | υ(N=N) | υ(OH) | (M − O) υ | |
| Metformin. HCl (Met) | 3371 s. | 1627 s. – |
– | – |
| 3170 s. | ||||
| 3294 s. | ||||
| Azo dye ligand (L) | – | – | – | – |
| 3219 br. | 1585 sh. | 3371 br. | ||
| [Co(L)(Met)(H2O)2]Cl | 3470 br. | 1631 s. 1500 s. |
3561 br. | 547 w. 513 w. |
| 3394 br. | 937 sh. | |||
| 3167 sh. | 775 s. | |||
| [Ni(L)(Met)(H2O)2]Cl | 3414 br. | 1639 sh. 1535 s. |
3462 br. | 493 w. 462 w. |
| 3367 br. | 891 s. | |||
| 3181 sh. | 833 s. | |||
| [Cu(L)(Met)(H2O)2]Cl | 3482 br. | 1643 s. 1516 s. |
3491 br. | 563 w. 478 w. |
| 3340 br. | 879 sh. | |||
| 3190 sh. | 802 sh. | |||
| [Cd(L)(Met)(H2O)2]Cl | 3452 br. | 1635 sh. 1531 s. |
3549 br. | 555 w. 459 w. |
| 3382 br. | 860 sh. | |||
| 3259 sh. | 748 s. |
3.2. The effect of the ligand AZO, Met, Metal complexes on the growth of clinically isolated bacteria and fungi
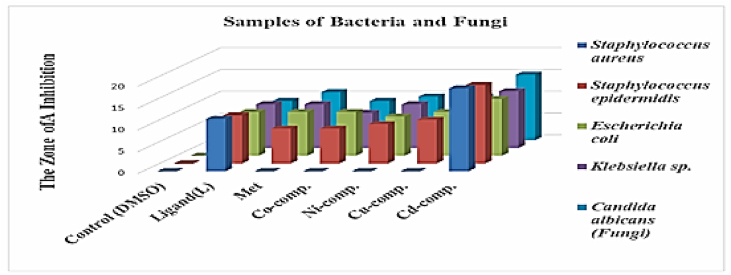
When using DMSO as a control, no inhibitory effect was observed on the growth of isolated bacteria and fungi, while the ligand azo, Met and complexes inhibit the growth of bacteria, despite the use of a very low concentration of 10⁻3 as the diameter of inhibition reached between 8 and 19 mm as shown in Fig. 7 and Table 4. If we increased the concentration, the effectiveness would have been much better. The best inhibitory activity was for the cadmium complex, and this indicates the presence of synergy between the cpmpounds included in the complex, while the rest of the complexes gave less effectiveness than the effectiveness of the ligand, due to the occurrence of antagonism between the compounds included in the complexes, which led to a reduction in the inhibitory effectiveness of the bacteria and fungi under study. This confirms what the researchers found in their study from the reaction of 2,4-dihydroxybenzaldehyde and 1,2- diaminobenzene was complexed with Cu [11], Co [11], Ni [11], Mn [11] and Uo2 [11] ions than the Schiff base ligand [46]. The principle of action of these compounds may be to inhibit the growth of isolated bacteria by their effect on the production of the bacterial cell wall that protects cells from the external environment, or to interfere in protein synthesis by linking the mechanism that builds amino acids and protein, or to affect metabolic processes such as folic acid synthesis and vitamin B is necessary for the growth of bacteria or preventing the synthesis of DNA [47]. Or it may be attributed to hydroxyl groups present in these complexes that could bind with active groups of microorganism enzymes by hydrogen bonds also precipitate proteins due to the formation of hydrogen bonds with those proteins, thus inhibiting necessary enzymes in organisms [48]. The resistance of S.aureus to most of the compounds was due to its possession of virulence factors such as gelatinase and beta-lactamase enzyme, and it was surrounded by biofilm and capsule that protects it from external influences [49,50].
Fig. 7.
Bioactivity for (L), (Met) and Mixed Ligand Complexes Against the Bacteria Species as well Fungi.
Table 4.
Effect of (L), (Met) and mixed ligand complexes for growth of bacteria and fungi clinically isolated.
| Compounds |
Staphylococcus aureus |
Staphylococcus epidermidis |
Escherichia coli |
Klebsiella pneumoniae |
Candida albicans |
|---|---|---|---|---|---|
| (G + ve) | (G + ve) | (G-ve) | (G-ve) | (Fungi) | |
| Control (DMSO) | - | - | - | - | - |
| Ligand(L) | 12 | 11 | 10 | 10 | 9 |
| Met | - | 8 | 10 | 10 | 11 |
| Co-comp. | - | 8 | 10 | 8 | 9 |
| Ni-comp. | - | 9 | 9 | 10 | 10 |
| Cu-comp. | - | 10 | 10 | 10 | 10 |
| Cd-comp. | 19 | 18 | 13 | 13 | 15 |
3.3. Cytotoxic effect of ligand, Met and Metal complexes on cell line
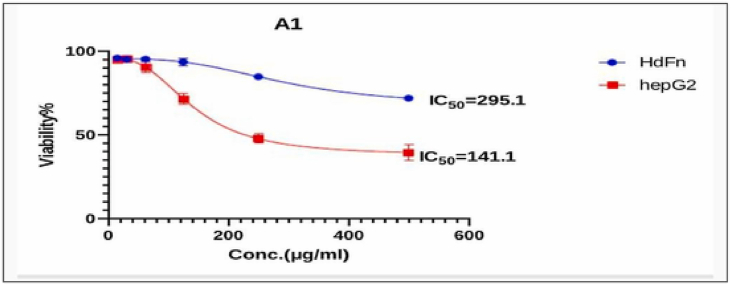
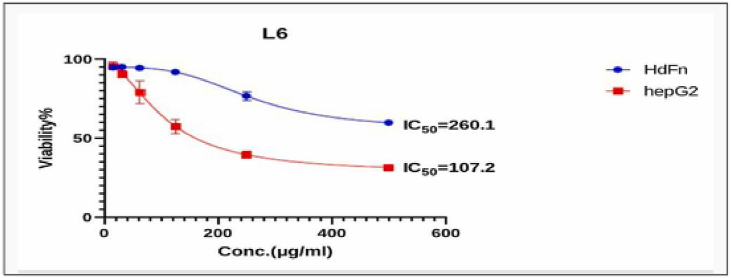
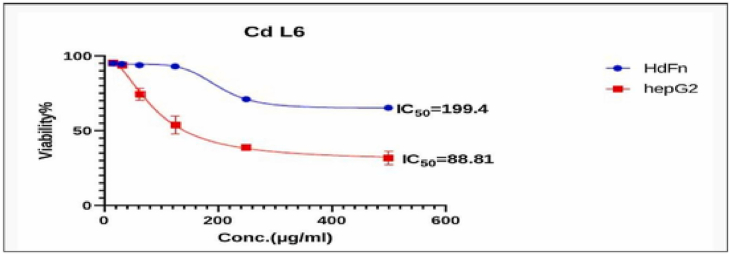
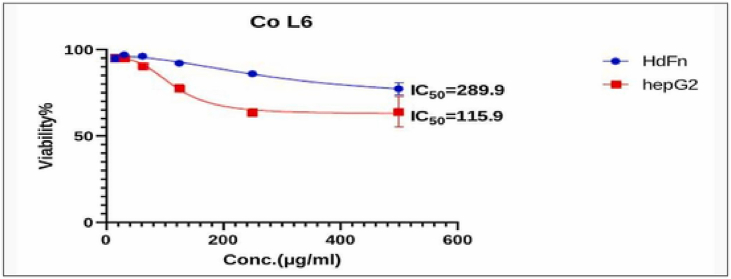
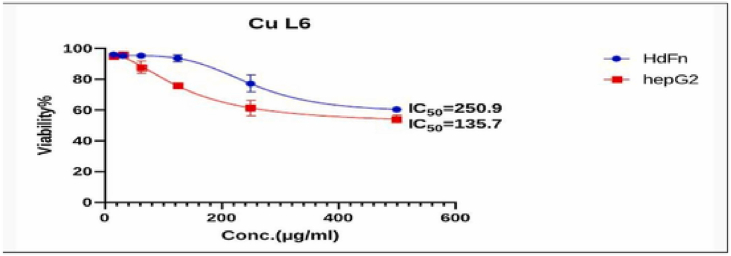
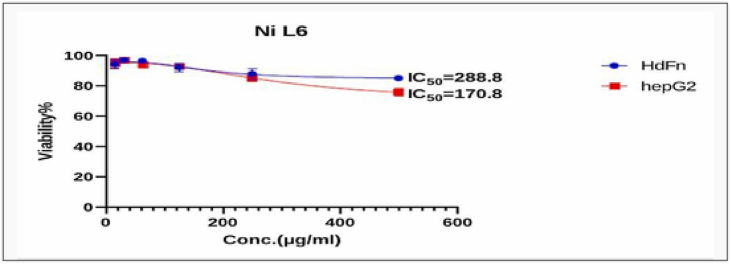
Fig. 8, Fig. 9, Fig. 10, Fig. 11, Fig. 12, Fig. 13 and Table 5, Table 6, Table 7, Table 8, Table 9, Table 10 shows the result of the effect of serial concentration of ligand,Met and metal mix ligand complexes on the normal hepatic cell Hdfn and carcinoma cell line HepG2. All complexes, ligand and Met had an effective inhibition of cancer cells. The highest inhibitory activity was met and cd complex with a percentage of (68.5 and 68.3) % respectively, then ligand azo dye 60.65 %, Cu complex 45.92 %, Co complex 35.85 and finally Ni complex 24.23 %. And compared with their effect on normal cells, their effectiveness on inhibiting cancer cells was higher than normal cells. There is no research on their inhibitory effectiveness, but it may be the effect of these complexes on DNA and change the internal structure of each cancer cell or loss of cellular communication and starvation of amino acids and interference with intracellular [51] especially when DNA binding of the copper ll compound as antioxidant [52], or inhibits the electron transport chain and ATP synthesis like metforamin agent [53].
Fig. 8.
Cytotoxicity effect of ligand azo dye on cancer HepG2 and normal HdFn cell Lne.
Fig. 9.
Cytotoxicity effect of Met on cancer HepG2 and normal HdFn cell line.
Fig. 10.
Cytotoxicity effect of Cd complex on cancer HepG2 and normal HdFn cell line.
Fig. 11.
Cytotoxicity effect Co complex on cancer HepG2 and normal HdFn cell line.
Fig. 12.
Cytotoxicity effect of Cu complex on cancer HepG2 and normal HdFn cell line.
Fig. 13.
Cytotoxicity effect of Ni complex on cancer HepG2 and normal HdFn cell line.
Table 5.
The cytotoxic effect of A1 (ligand azo dye) on HdFn and HepG2 cell line.
| Concentration μg mL−1 | Mean viability (%) ± SD |
|
|---|---|---|
| HdFn | HepG2 | |
| 500 | 71.95 ± 0.81 | 39.352 ± 4.78 |
| 250 | 84.79 ± 1.2 | 48.03 ± 2.5 |
| 125 | 93.5 ± 2.10 | 71.48 ± 3.39 |
| 62 | 95.33 ± 1.1 | 90.70 ± 3.18 |
| 31 | 95.21 ± 0.8 | 95.71 ± 0.80 |
| 15.6 | 95.949 ± 1.02 | 95.17 ± 1.28 |
Table 6.
The cytotoxic effect of L6 (Met) on HdFn and HepG2 cell line.
| Concentration μg mL−1 | Mean viability (%) ± SD |
|
|---|---|---|
| HdFn | HepG2 | |
| 500 | 59.8 ± 1.10 | 31.55 ± 1.3 |
| 250 | 76.69 ± 2.84 | 39.53 ± 2.36 |
| 125 | 91.85 ± 0.9 | 57.48 ± 4.63 |
| 62 | 94.36 ± 1.44 | 79.05 ± 7.34 |
| 31 | 95.02 ± 0.9 | 90.50 ± 2.10 |
| 15.6 | 94.83 ± 1.18 | 95.679 ± 2.65 |
Table 7.
The cytotoxic Effect of CdL6-(Cd Complex) on HdFn and HepG2 Cell Line.
| Concentration μg mL−1 | Mean viability (%) ± SD |
|
|---|---|---|
| HdFn | HepG2 | |
| 500 | 65.31 ± 1.8 | 31.7 ± 4.4 |
| 250 | 71.02 ± 1.74 | 38.92 ± 1.01 |
| 125 | 92.978 ± 1.3 | 53.89 ± 5.87 |
| 62 | 93.78 ± 0.24 | 74.34 ± 4.13 |
| 31 | 94.6 ± 1.62 | 94.05 ± 1.07 |
| 15.6 | 95.06 ± 1.6 | 95.25 ± 1.7 |
Table 8.
The cytotoxic effect of Co L6 (Co complex) on HdFn and HepG2 cell line.
| Concentration μg mL−1 | Mean viability (%) ± SD |
|
|---|---|---|
| HdFn | HepG2 | |
| 500 | 77.27 ± 3.65 | 64.15 ± 8.9 |
| 250 | 86.034 ± 0.85 | 63.69 ± 2.1 |
| 125 | 92.129 ± 1.55 | 77.62 ± 2.41 |
| 62.5 | 96.18 ± 1.2 | 90.58 ± 1.8 |
| 31.2 | 96.95 ± 1.14 | 94.79 ± 1.33 |
| 15.6 | 94.90 ± 2.19 | 95.40 ± 1.4 |
Table 9.
The Cytotoxic Effect of CuL6 (Cu complex) on HdFn and HepG2 Cell Line.
| Concentration μg mL−1 | Mean viability (%) ± SD |
|
|---|---|---|
| HdFn | HepG2 | |
| 500 | 60.378 ± 0.8 | 54.08 ± 2.56 |
| 250 | 77.08 ± 5.52 | 61.381 ± 5.20 |
| 125 | 93.59 ± 2.10 | 75.8 ± 2.05 |
| 62 | 95.33 ± 1.18 | 87.73 ± 3.9 |
| 31 | 95.216 ± 0.82 | 95.94 ± 0.90 |
| 15.6 | 95.949 ± 1.02 | 94.86 ± 0.29 |
Table 10.
The cytotoxic effect of NiL6 (Ni complex) on HdFn and HepG2 cell line.
| Concentration μg mL−1 | Mean viability (%) ± SD |
|
|---|---|---|
| HdFn | HepG2 | |
| 500 | 85.03 ± 1.03 | 75.77 ± 2.65 |
| 250 | 87.92 ± 3.57 | 85.108 ± 1.2 |
| 125 | 92.20 ± 2.77 | 92.93 ± 0.8 |
| 62 | 96.48 ± 1.29 | 94.05 ± 1.38 |
| 31 | 96.95 ± 1.14 | 96.79 ± 0.9 |
| 15.6 | 94.2 ± 2.9 | 95.02 ± 3.0 |
CRediT authorship contribution statement
Shatha M.H. Obaid: Resources, Project administration, Formal analysis, Conceptualization. Afnan E. Abd-Almonuim: Writing – original draft, Visualization, Conceptualization. Hanan Adnan Shaker Al -Naymi: Validation, Methodology, Data curation. Amer J. Jarad: Validation, Supervision, Conceptualization. Marwan Mahmood Saleh: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to express their gratitude to the Department of Chemistry, College of Education for Pure Science - Ibn-Al-Haitham, University of Baghdad for their cooperation in supplying the necessary materials for the successful completion of this research.
References
- 1.Adegoke A., Azeez G., Lawal A., Imran M. Theoretical studies of 1, 2, 3-triazole and isoxazole-linked pyrazole hybrids as antibacterial agents: an approach of docking and density functional theory. Advanced Journal of Chemistry ,Section B. 2021;3(2):148–159. [Google Scholar]
- 2.Zargar N., Zaman K.K. Mechanistic investigation of formation of some pharmacological active beta-diketo compounds and related nitrogen heterocycles. Advanced Journal of Chemistry-Section B. 2021;3:160. [Google Scholar]
- 3.Bader N., Elmajbry A., Al bork Z., Ahmida A., Geath A. Physico-chemical studies of the complexes of Hippuric acid with Cu (II), Ni (II), Zn (II), and Pb (II) ions in ethanol-water mixed solvent system. Progress in Chemical and Biochemical Research. 2020;3(1):1–6. [Google Scholar]
- 4.Mahmoodnezhad D., Taheri A. Development of a new methodology for determination of Cd, Ni, and Co at trace levels by mixed ultrasonic-assisted cloud point/solid phase extraction in micro micellar media :Optimization through response surface methodology. J. Food Compos. Anal. 2022;111 [Google Scholar]
- 5.Al-Adilee K.J., Kyhoiesh H.A.K., Taher A.M. Synthesis, characterization, biological studies, molecular docking and theoretical calculation of some transition metal complexes with new azo dye 2-[2′-(6-methoxybenzothiazolyl)azo]-3-methyl-4-nitrophenol. Results in Chemistry. 2022;4 [Google Scholar]
- 6.Khan A.A., Gul J., Naqvi S.R., Ali I., Farooq W., Liaqat R., AlMohamadi H., Stepanec L., Juchelkov′a D. Recent progress in microalgae-derived biochar for the treatment of textile industry wastewater. Chemosphere. 2022;306 doi: 10.1016/j.chemosphere.2022.135565. [DOI] [PubMed] [Google Scholar]
- 7.Shanker U., Rani M. CRC Press; 2022. Eradication of Personal Care Products by Liquid and Crystal Nanomaterials, Liquid and Crystal Nanomaterials for Water Pollutants Remediation; pp. 240–266. [Google Scholar]
- 8.Ali Y., Hamid S.A., Rashid U. Biomedical applications of aromatic azo compounds. Mini Rev. Med. Chem. 2018;18(18):1548–1558. doi: 10.2174/1389557518666180524113111. [DOI] [PubMed] [Google Scholar]
- 9.Prakash S., Somiya G., Elavarasan N., Subashini K., Kanaga S., Dhandapani R., Sivanandam M., Kumaradhas P., Thirunavukkarasu C., Sujatha V. Synthesis and characterization of novel bioactive azo compounds fused with benzothiazole and their versatile biological applications. J. Mol. Struct. 2021;1224 [Google Scholar]
- 10.Khan M.N., Parmar D.K., Das D. Recent applications of azo dyes: a paradigm shift from medicinal chemistry to biomedical sciences. Mini Rev.Med.Chem. 2021;21(9):1071–1084. doi: 10.2174/1389557520999201123210025. [DOI] [PubMed] [Google Scholar]
- 11.Ranjitha N., Krishnamurthy G., Manjunatha M.N., Bhojya Naik H.S., Malathesh P., Vasantakumarnaik N.K., Laxshmikantha J., Pradeepa K. Electrochemical determination of glucose and H2O2 using Co(II), Ni(II), Cu(II) complexes of novel 2-(1,3-benzothiazole-2ylamino)-N-(5-choro-2hydroxyphenyl) acetamide: synthesis, Structural Characterisation, antimicrobial, anticancer activity and docking studies. J. Mol. Struct. 2022;5(1274) [Google Scholar]
- 12.Vasantakumarnaik N.K., Bindu PavanV., Krishnamurthy G., Malathesh P., Venughopal N., Sunil Kumar N., Prabhakar W., Manjuraj T.3. Synthesis, spectral studies of novel 4-[(E)-2-nitrophenyl)diazenyl]-1,3-thiazol-amine (NTA/C9H7N5O2S) and its coordinated metal [CO(NTA)2.(H2O)2] and [Ni(NTA)2.(H2O)2] complexes; antioxidant activity study. Eur. Chem. Bull. 2023;12(11):449–460. Regular Issue. [Google Scholar]
- 13.Witwit I.N., Farhan H.M., Motaweq Z.Y. Preparation of mixed ligand complexes of heterocyclic azo quinoline ligand and imidazole molecule with some of divalent transition ions and their biological activity against multi drug resistance pathogenic bacteria. J. Phys. Conf. 2021;1879:1–13. [Google Scholar]
- 14.Marupuru S., Senapati P., Pathadka S., Miraj S.S., Unnikrishnan M.K., Manu M.K. Protective effect of metformin against tuberculosis infections in diabetic patients: an observational study of south Indian tertiary healthcare facility. Braz. J. Infect. Dis. 2017;21(3):312–316. doi: 10.1016/j.bjid.2017.01.001. [PMC free article] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masadeh M.M., Alzoubi K.H., Masadeh M.M., Aburashed Z.O. Metformin as a potential adjuvant antimicrobial agent against multidrug resistant bacteria. Clin. Pharmacol. 2021;13:83–90. doi: 10.2147/CPAA.S297903. PMCID: PMC8123943PMID: 34007223 Published online 2021 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aljofan M., Riethmacher D. Anticancer activity of metformin: a systematic review of the literature. Future Sci OA. 2019;5(8) doi: 10.2144/fsoa-2019-0053. Published online 2019 Aug 22. PMCID: PMC6745597PMID: 31534778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weissenrieder J.S., Neighbors J.D., Mailman R.B., Hohl R.J. Cancer and the dopamine D2 receptor: a pharmacological perspective. J Pharmacol Exp Ther. 2019;370:111–126. doi: 10.1124/jpet.119.256818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO International programme on chemical safety. A Series of Environmental Health Criterias. 1999;3 Geneva. [Google Scholar]
- 19.EL. Bindary M.M., El, Bindary A.A. Synthesis , characterization , DNA binding and biological action of dimedone arylhydrazone chelates. Appied organometallic chemistry/36/4/e6576. 2022 [Google Scholar]
- 20.Montville T.J., Matthews K.R. ASMPress; NewYork: 2005. Food Microbiology: an Introduction. [Google Scholar]
- 21.Obaid S.M.H., Abd-Almonuim A.E., Jarad A.J. Synthesis, characterization, industrial and biological application of Co(II),Ni(II),Cu(II) and Zn(II) complexes with azo ligand derived from metoclopramide hydrochloride and 3,5-dimethylphenol, Egypt. J. Chem. 2020;63(12):4719–4729. [Google Scholar]
- 22.AL-Musawi M., Khoshkalampour A., AL-Naymi H.A., Shafeeq Z.F., Doust S., Ghorbani M. Optimization and characterization of carrageenan/gelatin-based nanogel containing ginger essential oil enriched electrospun ethyl cellulose/casein nanofibers. Int,journal Biol macromol. 2023 doi: 10.1016/j.ijbiomac.2023.125969. [DOI] [PubMed] [Google Scholar]
- 23.Vogel A.I. fourth ed. Longman; London: 1978. A Textbook of Quantitative Inorganic Analysis. [Google Scholar]
- 24.Freshney R.I. A Manual of Basic Technique and Specialized Applications. 6th. John Wiley and Sons, Inc.; USA: 2010. Culture of animal cells. [Google Scholar]
- 25.Al-Sudani T.B., Mahmoudi E., Al-Naymi H.A.S., Ghorbani M., Mortazavi Moghadam F. Antibacterial and wound healing performance of a novel electrospun nanofibers based on polymethyl-methacrylate/gelatin impregnated with different content of propolis. Journal of Drug Delivery Science and TechnologyThis link is disabled. 2024;95 [Google Scholar]
- 26.Al-Naymi N.A.Sh, AL-Naymi H.A., Nashaat M.R. Toxicity stress of the durah power plant ash and its effect on the alga chlorococcum humicola (naeg) rabenhorst 1868. Arab J. Plant Protect. 2022;40(2):188–192. [Google Scholar]
- 27.Geary W.J. Characterization of coordination ompounds. Coord. Chem. Rev. 1971;7:81–122. [Google Scholar]
- 28.Patil A.R., Donde K.J., Raut S.S., Patil V.R., Lokhande R.S. Synthesis, characterization and biological activity of mixed ligand Co(II) complexes of Schiff base 2-amino-4-nitrophenol-n-salicylidine with some amino acids. J.Chem.Pharm.Res. 2012;4(2):1413–1425. [Google Scholar]
- 29.Subbaraj P., Ramu A., Raman N., Dharmaraja J. Mixed ligand complexes containing (2-hydroxy-4-methoxyphenyl)(phenol) methane and 2-aminophenol: synthesis and DNA cleavage, Inter. J.Emer.Sci.Engier. 2013;1(7):79–84. [Google Scholar]
- 30.Nair M.L.H., Sheela A. Synthesis, spectral, thermal and electrochemical studies of oxomolybdenum(V) and dioxomolybdenum(VI) complexes of an azo dye derived from 4-amino-2,3-dimethyl-1-phenyl pyrazol-5-one. Indian J. Chem. 2008;47A:1787–1792. [Google Scholar]
- 31.Olar R., Badea M., Cristurean E., Lazar V., Cernat R., Ballotescu C. Thermal behavior spectroscopic and biological characterization of Co(II),Zn(II),Pd(II) and Pt(II) complexes with N,N-dimethylbiguanide. J.Therm.Anal.Calorim. 2005;80:451–455. [Google Scholar]
- 32.Olar R., Badea M., Marinescu D., Chifiriuc M., Bleotu N,N-dimethylbiguanide complexes displaying low cytotoxicity as potential large spectrum antimicrobial agents. Eur. J. Med. Chem. 2010;45(7):3027–3034. doi: 10.1016/j.ejmech.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 33.Jarad A.J., Quiasim S.H. Synthesis and characterization of azo dyes ligands complexes with Ni(II) and Cu(II) and studies their industrial and bacterial application. Res.J.Pharm.Biol.Chem.Sci. 2018;9(2):631–642. [Google Scholar]
- 34.Al-Abdali B.I., Shakir I.M.A., Nafea H.M. Synthesis and spectroscopic studies of some transition metal complexes with mixed ligand of metformin and cysteine. Iraq.J.Sci. 2015;56(4B):3036–3047. [Google Scholar]
- 35.Jarad A.J. Synthesis, spectral and dyeing performance studies of 4-(2- aminmo-5-nitro-phenylazo)-1,5-dimethyl-2-phenyl-1,2- dihydro-pyrazol-3-one complexes with some metal ions. Baghdad Sci. J. 2016;13(4):838–845. doi: 10.21123/bsj.2016.13.4.0838. [DOI] [Google Scholar]
- 36.Hassan S.S., Kadhim N.J., Jaber Z.A. Synthesis, theoretical study, and biological evaluation of some metal ions with ligand "methyl -6-[2-(4-hydroxyphenyl) -2-((1-phenylethylidene) amino) acetamido] -2,2-dimethyl-5-oxo-1-thia-4-azabicyclo [3.2.0] heptane-3-carboxyylate. Baghdad Sci. J. 2023;20(1):102–113. doi: 10.21123/bsj.2022.6359. [DOI] [Google Scholar]
- 37.Abdulridha M.Q., Al-Hamdani A.A.S., Ahmed I. Synthesis, characterization and antioxidant activity of new azo ligand and some metal complexes of tryptamine derivatives. Baghdad Sci.J. 2024;20(3):1046–1063. doi: 10.21123/bsj.2023.8227. [DOI] [Google Scholar]
- 38.Shaalan N. Preparation, spectroscopy, biological activities and thermodynamic studies of new complexes of some metal ions with 2-[5-(2-hydroxy-phenyl)- 1,3,4-thiadiazol-2-ylimino]-methyl-naphthalen-1-ol] Baghdad Sci .J. 2022;19(4):829–837. doi: 10.21123/bsj.2022.19.4.0829. [DOI] [Google Scholar]
- 39.Salh H.M., Al-Noor T.H. Preparation, structural characterization and biological activities of curcumin-metal(II)-L-3,4-dihydroxyphenylalanin(L-dopa)complexes. Ibn Al-Haitham J.Appl.Sci. 2023;36(1):170–185. doi: 10.30526/36.1.2899. [DOI] [Google Scholar]
- 40.El-Barasil N.M., Algazalel S.F., El-Ajaily M.M., Maihub A.A., Miloud M.M., T.H, Al-Noor T.H., Mubarak H.A., Kareem M.M. Synthesis, characterization, theoretical study and biological evaluation of Schiff base and their La(III) and UO2(II) complexes. Bull. Chem. Soc. Ethiop. 2023;37(2):335–346. [Google Scholar]
- 41.Fadhil A.E., Abbas A.K. Antioxidant, antimicrobial and spectroscopic discussion of guanine azo ligandwith Cu(II) and Ag(I) complexes. Ibn Al-Haitham J.Appl.Sci. 2023;36(4):207–220. doi: 10.30526/36.4.3134. [DOI] [Google Scholar]
- 42.Jarad A.J., Dahi M.A., Taghreed H., Al-Noor T.H., El ajaily M.M., AL-Ayash S.R., Abdou A. Synthesis, spectral studies, DFT, biological evaluation, molecular docking and dyeing performance of 1-(4-((2-amino-5-methoxy)diazenyl)phenyl) ethanone complexes with some metallic ions. J. Mol. Struct. 2023;1287 doi: 10.1016/j.molstruc.2023.135703. [DOI] [Google Scholar]
- 43.Sabah A.A., Ameen A.M., Al-Daher A. Metal complexes of bis (2,6-diamine pyridine 2,5-hexanedione) macrocyclic Schiff-base ligand: preparation, characterization and thermal study. Iraqi J. Sci. 2022;63(5):1885–1893. [Google Scholar]
- 44.Abdulridha M.Q., Al-Hamdani A.A.S. Synthesis, spectral identification, thermal and antioxidant studies for Ni(II),Pd(II),Pt (IV) and Au(III) complexes with new azo ligand derivatives from tryptamine. Ibn Al-Haitham J.Appl.Sci. 2023;36(4):303–320. doi: 10.30526/36.4.3059. [DOI] [Google Scholar]
- 45.Al-Adilee K.J., Adnan S. Synthesis and spectral properties studies of novel heterocyclic mono azo dye derived from thiazole and pyridine with some transition complexes. Orien.J.Chem. 2017;33(4):1815–1827. [Google Scholar]
- 46.El-Zahed M.M., Diab M.A., El-Sonbati A.Z., Saad M.H., Eldesoky A.M., El-Bindary M.A. Synthesis , spectroscopic characterization studies of chelating complexes and their applications as antimicrobial agents, DNA binding, molecular docking, and electrochemical studies. Applied organmetallic chemistry. 2023;38(1) e 7290. [Google Scholar]
- 47.Magnúsdóttir S., Ravcheev D., de Crécy-Lagard V., Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015;6:148. doi: 10.3389/fgene.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto and Gaynor Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J.Clinic. Inves. Ret. 2006;107:135. doi: 10.1172/JCI11914. 08-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdulrahman Wisam A., Othman Iman A., Waheed Enass J. Metal complexes of ligand derived from amine compound: formation, spectral characterization, and biological evaluation. International Journal of Drug Delivery Technology. 2021;11(3):728–734. [Google Scholar]
- 50.Al-Naymi H.A.S., Mahmoudi E., Kamil M.M., Ghorbani M., Mortazavi Moghadam F. A novel designed nanofibrous mat based on hydroxypropyl methyl cellulose incorporating mango peel extract for potential use in wound care system. international Journal of Biological MacromoleculesThis link is disabled. 2024;259 doi: 10.1016/j.ijbiomac.2023.129159. [DOI] [PubMed] [Google Scholar]
- 51.Zhang U., Lou X.1, Jin L., Zhou R., Liu S., Xu N., Joshua D. Necrosis, and then stress induced necrosis-like cell death, but not apoptosis, should be the preferred cell death mode for chemotherapy: clearance of a few misconceptions. Oncoscience. 2014;1(6):407–422. doi: 10.18632/oncoscience.61. PMCID: PMC4284620PMID: 25594039 Published online 2014 Jul 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El -Bindary M.A., Fathy A.M. Azo naringenin-based copper (II) complex as DNA binder: synthesis, spectroscopic characterization, and diverse biological potentials. Appl. Organomet. Chem. 2024;38(6) e 7460. [Google Scholar]
- 53.Vancura A., Bu P., Bhagwat M., Zeng J., Vancurova I. Metformine as anticancer agent. Trends Pharmacol. Sci. 2018;39(10):867–878. doi: 10.1016/j.tips.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]