Highlights
-
•
Combining a halo frame with radiotherapy allows for safe reossification of unstable cervical metastases.
-
•
After removal of the halo frame, range of motion of the neck is mostly preserved.
-
•
The treatment combination could be an acceptable alternative to cervical spondylodesis.
Keywords: Cervical spinal metastases, Spinal instability, Halo fixation, Radiotherapy, Reossification, Spondylodesis, Range of motion, Palliation
Abstract
Background and purpose
Currently no minimally invasive surgical treatment exists to provide immediate stability for unstable cervical/cervicothoracic metastases. Long-construct spondylodesis carries a high complication risk and has severe impact on residual range of motion. This study explores temporary halo fixation and radiotherapy as an alternative to long-construct cervical spondylodesis.
Materials and methods
This retrospective cohort study included twenty patients with multiple unstable cervical metastases treated between 2013–2023. All patients underwent halo fixation for an intended duration of three months to allow for safe reossification of lytic lesions following radiotherapy, with a dose fractionation scheme best suited to the histological origin of the tumor.
Results
Immobilization with halo fixation lasted a median 83 days (range, 41–132 days). Radiotherapy started on average 7 days after halo fixation (range, −35–118 days). The median pain score at baseline was 8, and was 0 at halo removal and at last follow-up. All patients had no or minor neurological deficits at baseline and did not develop new neurological deficits. At halo removal, 17/18 patients showed radiographic evidence of reossification. The majority of patients experienced minor limitations or had full range of motion of the neck at last follow-up.
Conclusion
Patients with multiple unstable cervical metastases treated with halo fixation and radiotherapy showed complete pain response or substantial pain reduction, reossification of the vertebrae and a, mostly, preserved range of motion. In selected neurologically intact patients, this treatment might be a patient-friendly alternative to fixation. Prospective evaluation of this treatment combination is needed.
Introduction
With improving survival rates due to advancements in detection methods and systemic treatments, more cancer patients are at risk for developing bone metastases, with the spinal column most commonly affected [1]. Quality of life of patients with symptomatic spinal metastases can be severely impaired due to local tumor pain, pain due to mechanical instability, pathological fractures, and the occurrence of neurological deficits [2], [3].
Of all spinal metastases, 8–20 % are located in the cervical spine [4]. Anatomically, this is a challenging location and the optimal treatment for patients with multiple unstable metastases in the cervical/cervicothoracic spine is unknown. Many relevant factors need to be carefully balanced when selecting a treatment, such as preserving range of motion, providing stability and alignment, restoring or retaining neurological function, obtaining local tumor control, and preventing complications.
Radiotherapy is effective for palliation and local tumor control, but cannot provide immediate mechanical stability. The standard surgical approach for multiple unstable cervical metastases is long-construct cervical spondylodesis, applying long bridging constructs with screws and rods. This type of surgery provides immediate stability but severely impairs range of motion and carries a high risk of complications [5], [6], [7]. Currently, there is no minimally invasive treatment option for unstable cervical spinal metastases that provides immediate stability and reduces pain.
Previous studies have reported on reossification and increased bone density after radiotherapy for bone metastases [8], [9]. The phenomenon of reossification after radiotherapy allows for a treatment combination with immediate stabilization offered by the application of a halo frame combined with fractionated radiotherapy to provide long-term stability. The halo frame can be removed when the vertebrae have reossified sufficiently.
In the current study, we present outcomes of patients with (impending) multiple unstable cervical metastases treated with temporary halo fixation and radiotherapy aiming for immediate cervical stabilization, local tumor control, and long-term preservation of mobility. The study explored this treatment as a potentially more patient-friendly alternative to long-construct cervical spondylodesis.
Materials and methods
We retrospectively identified all patients with unstable cervical metastases, including lesions at the cervicothoracic junction, who were treated with halo fixation and radiotherapy at our tertiary referral hospital in the Netherlands between September 2013 and July 2023. All histological origins of the metastases were allowed. Some patients were participant of a prospective cohort of patients with bone metastases (PRospective Evaluation of interventional StudiEs on boNe meTastases (PRESENT) (NL49273.041.14) [10] and we used cohort data for the current study. Patients who were not included in the PRESENT cohort and were alive at the time of data analysis, were contacted by their treating physician to obtain informed consent for use of their baseline demographics, treatment characteristics, and clinical follow-up data. As this study was retrospective, it did not fall under the scope of the Dutch Medical Research Involving Human Subjects Act (WMO) and was therefore waived from study approval by an accredited medical ethics committee. An independent quality check was carried out to ensure compliance with legislation and regulations (regarding Informed Consent procedure, data management, privacy aspects and legal aspects). Demographic information, details regarding halo fixation and radiation treatment plans were extracted from patients’ medical records and recorded in Castor electronic data capture [11].
Patient selection
All included patients visited the combined radiotherapy/orthopedic surgery outpatient clinic for elective consultation and the treatment plan was established through shared decision making. Halo fixation and radiotherapy was offered to patients presenting with multiple cervical/cervicothoracic metastases, requiring stabilization due to instability, deformity and/or mechanical pain, but where internal fixation was not preferred, because of the considerable loss of mobility after multi-level fixation. Additionally, patients had to be neurologically intact, or present with minor non-progressive neurological deficits, had to have a prognosis of ≥ 3 months to allow for reossification and to be fit enough for this treatment combination.
Halo fixation and radiotherapy
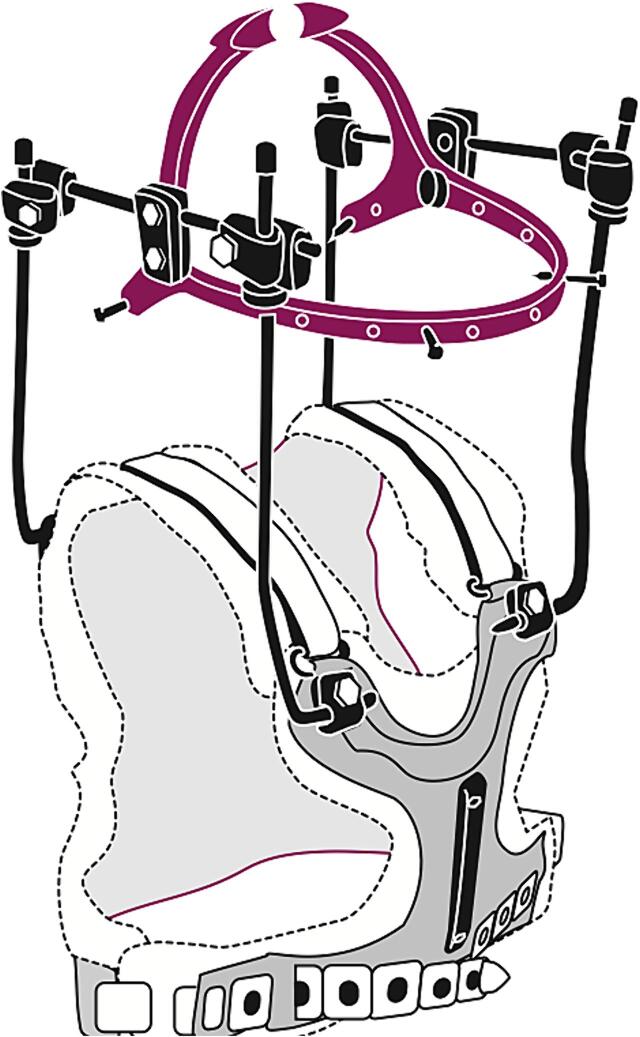
All patients received halo fixation (Fig. 1) for an intended duration of up to three months to provide sufficient time for reossification of the vertebrae after radiotherapy. It is recommended to obtain a CT skull before application of the halo frame to rule out bone metastasis at the intended site of the skullpins. The halo frame was applied under local anesthesia with lidocaine/adrenaline. With the halo frame in place, careful adjustments of the head relative to the torso could be performed for re-alignment of the cervical spine and to allow for a horizontal gaze. Patients were allowed to ambulate immediately and most were able to go home on the same day. Follow up in term of how the halo frame was tolerated, and check-up/retightening of the pins was performed one day and one week after application and every two weeks thereafter by the casting technician. If patients received systemic therapy during halo fixation, they generally received a peripherally inserted central catheter which did not interfere with the halo vest.
Fig. 1.
Schematic representation of a halo frame. Illustration by Ingrid Janssen.
External beam conventional radiotherapy was administered with a fractionation scheme best suited to the histological origin of the tumor and with the highest chance of reossification, at the discretion of the treating radiation oncologist. Treatment planning and delivery was performed in vacuum cushion immobilization and the standard planning format could be used. In some patients it was necessary to make adjustments to the halo frame during the radiotherapy treatment, they had to undergo a new computed tomography (CT) scan to adjust the planning of the radiotherapy treatment of the remaining fractions. After removal of the halo frame, a weaning collar was applied for 7–14 days upon patient preference. In most patients, volumetric modulated arc therapy (VMAT) was used to deliver the conventional radiotherapy dose, in which 95% of the planning target volume (PTV) had to receive at least 95% of the prescribed dose. In four patients, single or multiple CT-guided fields were used for radiotherapy planning, in which the vertebral body received at least 80% of the prescribed dose. Dexamethasone was prescribed around the time of radiation delivery at the discretion of the radiation oncologist. In patients newly diagnosed with metastasized breast cancer, the oncologist was asked to start bisphosphonates before initiation of radiotherapy as is standard practice for this category of patients. Previous research indicates that when there are visible changes in the vertebrae indicating remineralization on radiographs/CT-scans, this is reflected in Hounsfield Units measurements [9]. The halo frame was removed after a CT-scan or plain radiographs of the cervical spine confirmed acceptable alignment and visible changes indicating ongoing reossification and pain was managed adequately.
Data collection
Study endpoints included neck pain (using the numeric rating scale, NRS), neurological status as indicated by American Spinal Injury Association Impairment Scale (ASIA) [12], patient reported neck motion, Karnofsky Performance Score (KPS) [13], Spinal Instability Neoplastic Score (SINS) [14], and semi-quantitative assessment of reossification on CT images or radiographs at time of halo frame removal, and last follow-up as assessed by the treating physicians. The ASIA impairment scale is used to assess the degree of impairment due to neurological deficits. It ranges from A, complete paralysis, to E, where no neurological deficits are present [12]. We classified patient reported neck motion into full range of motion (ROM), minor limitation in ROM, major limitation in ROM, not assessable due to premature removal of the halo frame, and not reported. The SINS score is a tool to aid physicians in determining spinal stability. A score between 0 and 18 results from the sum of one clinical and five radiological components. A score > 6 indicates potential instability requiring a consultation from a spine surgeon [14]. Follow up time was defined as time between application of halo frame and date of data analysis or death. The patient vital status was recovered through governmental database.
Statistical analysis
Descriptive statistics were used to describe patient and treatment characteristics. Continuous variables were expressed as median and ranges and proportions as percentages. Data was analyzed using SPSS version 25.0.
Results
Demographics
Between September 2013 and July 2023, 20 patients were treated with halo fixation and radiotherapy. Metastases originated mostly from radiosensitive tumors (15/20 patients). In this cohort, radiosensitive primary tumors included multiple myeloma, prostate- and breast cancer. At baseline the majority of patients (18/20) had mechanical pain, multiple vertebrae affected (median 4.5; range, 1–9) and high SINS scores (median SINS 14; range, 8–17). C1 and/or C2 were affected in 16/20 patients. Median KPS was 70 at the start of halo fixation (range, 50–100). All patients presented with no or mild neurological deficits (ASIA E or non-progressive ASIA D) at baseline (Table 1). Median follow-up was 28 months (range, 1–124). At 3 months after start of treatment three patients had died and after 6 months one additional patient had died. At time of analysis nine patients were alive, 1- and 2-year overall survival was 70% (95% CI 46–88%) and 60% (95% CI 36–81%) respectively.
Table 1.
Baseline characteristics of twenty participants treated with halo fixation and radiotherapy.
| Patients (N=20) | |
|---|---|
| Gender, n (%) | |
| Male | 9 (45 %) |
| Female | 11 (55 %) |
| Age | |
| Median (range) | 61 (45–82) |
| Primary cancer site, n (%) | |
| Breast | 6 (30 %) |
| Multiple myeloma | 5 (25 %) |
| Prostate | 4 (20 %) |
| Lung | 1 (5 %) |
| Other* | 4 (20 %) |
| SINS score, n (%) | |
| SINS 8–12 | 3 (15 %) |
| SINS 13 | 5 (25 %) |
| SINS 14 | 5 (25 %) |
| SINS 15 | 5 (25 %) |
| SINS 16–17 | 2 (10 %) |
| Radiation treatment | |
| 8 Gy in 1 fraction | 5 (25 %) |
| 30 Gy in 10 fractions | 10 (50 %) |
| Other fractionation scheme | 5 (25 %) |
| KPS, n (%) | |
| 50 | 6 (30 %) |
| 60–70 | 6 (30 %) |
| 80–100 | 8 (40 %) |
| ASIA Impairment Scale, n (%) | |
| ASIA D | 7 (35 %) |
| ASIA E | 13 (65 %) |
| Pain score before halo-fixation | |
| Median (range) | 8 (0–10) |
| Missing | 4 |
| Use of dexamethason, n (%) | |
| Yes | 16 (80 %) |
| No | 4 (20 %) |
SINS: Spinal instability neoplastic score, KPS: Karnofsky performance score, ASIA: American Spinal Injury Association
*other histologies included vaginal cancer, urothelial carcinoma and cholangiocarcinoma.
Treatment
The median period of immobilization with halo fixation was 83 days (range, 41–132 days). In three patients, the halo frame was removed prematurely (after 41, 56 and 61 days) due to rapidly progressive systemic disease. One patient died due to perforated diverticulitis 43 days after start of treatment, before completion of the intended halo fixation period. Radiotherapy was initiated on average 7 days after halo fixation (range, −35–118 days). Three patients initially received radiotherapy only, but due to mechanical pain and/or occurrence of a pathological fracture halo fixation was applied 3–35 days after radiotherapy. Most patients received long course radiotherapy (typically 30 Gy in 10 fractions) to increase the probability of reossification (Table 1) [9]. None of the patients were treated with stereotactic body radiotherapy.
Clinical outcomes
The median NRS for neck pain at baseline was 8 (range, 0–10). At time of halo frame removal, the median NRS for neck pain was 0 (range, 0–4). At last follow-up, median NRS for neck pain remained 0 (range, 0–4) (Table 2). One patient received re-irradiation of a recurring painful C1 vertebra 8 months after start of halo fixation for the purpose of pain palliation.
Table 2.
Pain and KPS outcomes at baseline and during follow up.
| N* | Pain or KPS Score, median (range) | |
|---|---|---|
| NRS neck pain | ||
| Before halo frame fixation | 16 | 8 (0–10) |
| After halo frame removal | 17 | 0 (0–4) |
| At last follow up | 17 | 0 (0–4) |
| KPS | ||
| Before halo frame fixation | 20 | 70 (50–100) |
| After halo frame removal | 18 | 80 (60–90) |
| At last follow up | 17 | 80 (40–100) |
NRS: Numeric rating scale, KPS: Karnofsky performance score.
*number of patients with outcome recorded.
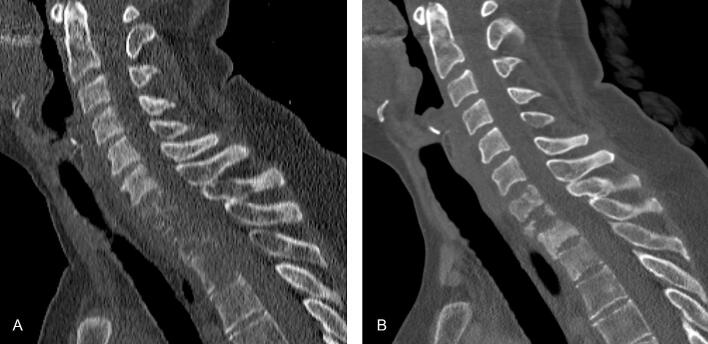
Two patients had their halo frame removed before imaging could be obtained to assess reossification, because of rapidly progressive systemic disease: one patient had urothelial carcinoma and one patient with multiple myeloma became severely ill from a perforated diverticulitis during treatment and died. The majority of patients (17/18) showed some or substantial reossification of the cervical/cervicothoracic vertebrae at the time of halo removal. An example of reossification of vertebral bodies C7–T4 after radiotherapy of metastases originating from breast cancer is shown in Fig. 2, Fig. 3, Fig. 4. In one patient, a pathological fracture of the odontoid process remained unstable after treatment with halo fixation followed by radiotherapy and this patient ultimately underwent spondylodesis of C1-C4, 112 days after radiotherapy. At last follow-up, no new pathological fractures requiring surgery had occurred in the cohort.
Fig. 2.
Patient 1 with metastases originating from breast cancer. A, Sagittal reconstruction of computed tomography (CT) scan of the cervicothoracic spine showing lytic lesions of C7 and Th1–4 at baseline. B, Sagittal view of CT scan of the cervicothoracic spine showing reossification of the lytic lesions 71 days after start of radiotherapy (10x 3 Gy).
Fig. 3.
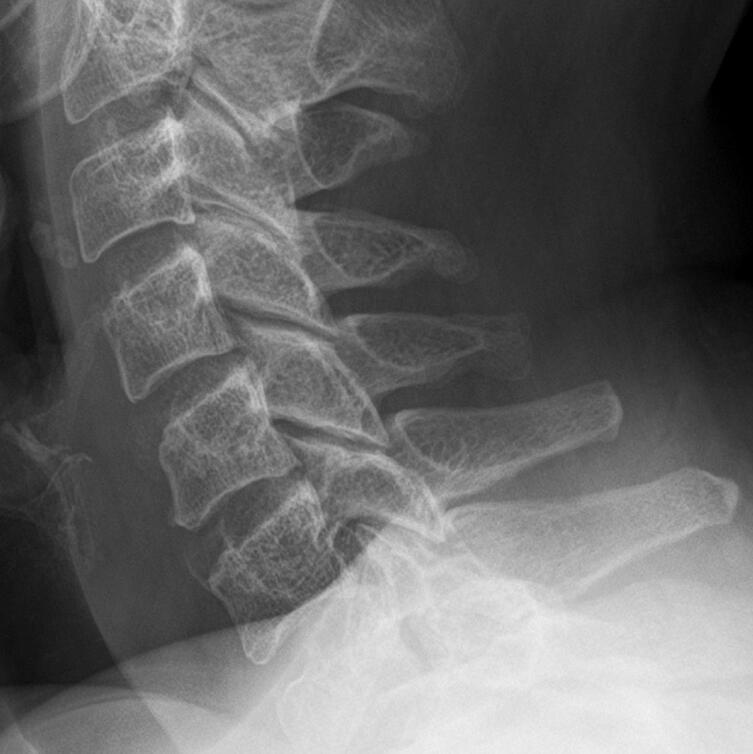
Plain lateral radiograph of the cervical spine of Patient 1, almost one year after treatment showing persistent reossification and near normal alignment.
Fig. 4.
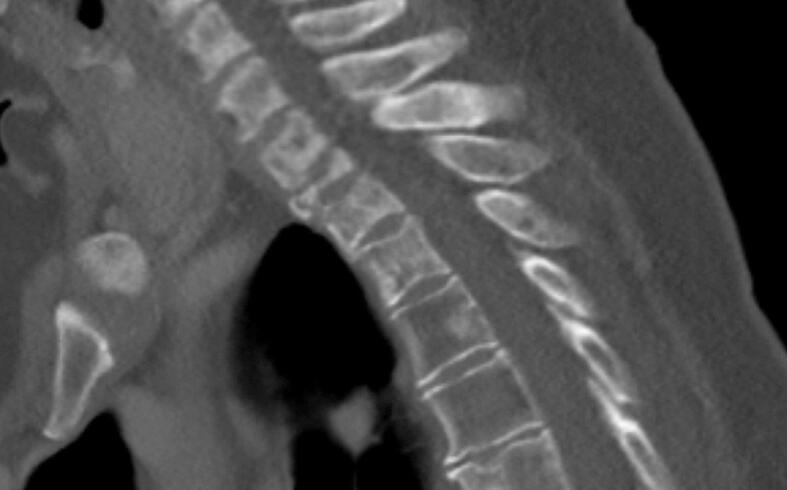
Sagittal reconstruction of CT scan of the cervicothoracic junction of Patient 1, showing evidence of reossification on multiple levels and near normal alignment 16 months after start of treatment.
No patient deteriorated in neurological function during treatment or follow-up. The KPS at time of removal of the halo frame had improved in 11/18 patients (61 %) or was stable in 4/18 patients (22 %). At last follow-up, for 12/17 patients (71 %) the KPS remained the same or had improved compared to the time of halo frame removal (Table 2). The majority of patients self-reported either full ROM or minor limitations in ROM at last follow-up (Table 3 and Table A1 in the appendix).
Table 3.
Neck motion at last follow up.
| Neck motion at last follow up, N (%) | |
|---|---|
| Full range of motion | 6 (30 %) |
| Minor limitation in range of motion | 8 (40 %) |
| Major limitation in range of motion | 1 (5 %) |
| Not assessable due to premature removal | 4 (20 %) |
| Not reported | 1 (5 %) |
Adverse events
Eleven out of twenty patients (55%) experienced adverse events (Table 4). One patient suffered from an intestinal perforation and pneumonia during systemic treatment for multiple myeloma. Another patient experienced a bilateral pneumonia and a urinary tract infection for which intensive care unit admittance was needed. Additionally, one patient presented with urosepsis and developed neoplastic fever after which the halo frame was removed early. One patient presented with urinary retention. Hepatitis and benign paroxysmal positional vertigo was diagnosed in one patient after starting immunotherapy. Another patient died 43 days after start of halo fixation due to perforated diverticulitis.
Table 4.
Adverse events during treatment with halo fixation and radiotherapy.
| Related to treatment | Removal of a halo pin due to metastasis |
| Loosening of a halo pin | |
| Temporary dysphagia after radiotherapy | |
| Possibly related to treatment | Pneumonia |
| Unrelated to treatment | Intestinal perforation |
| Urinary tract infection | |
| Urosepsis/Neoplastic fever | |
| Perforated diverticulitis | |
| Early removal due to rapidly progressive disease | |
| Hepatitis and benign paroxysmal positional vertigo |
In three patients complications occurred directly related to the application of the halo frame or radiotherapy. In one patient a pin of the halo ring had to be removed due to a nearby metastasis in the skull and another patient experienced loosening of the pins at the end of the treatment period. Lastly, one patient experienced temporary dysphagia after radiation therapy.
Patient experience
Patients described the application of the halo fixation as hard and difficult, especially when tightening the skull pins. However, the majority of patients characterized the treatment, afterwards, as an overall acceptable experience because they appreciated the rapid decline in mechanical neck pain and long term preserved range of motion after halo removal.
Discussion
For patients with multiple unstable cervical spinal metastases, long-construct spondylodesis is the standard of care [15], [16] which results in immediate mechanical stability but also in a substantially reduced range motion of the neck in the long term. Our study shows that temporary halo fixation and radiotherapy is an alternative for long-construct spinal fixation by immediately relieving mechanical pain without compromising range of motion substantially in the long term. The majority of patients were free of neck pain or had substantial pain reduction during/after halo frame removal with sustained favorable outcomes at longer follow-up. In addition, new neurological deficits did not develop and the majority of patients showed some or substantial reossification of the cervical spine after treatment. Another possible advantage of this treatment combination is that radiotherapy can be initiated quickly after halo frame application and is not delayed due to wound healing after surgery, subsequently systemic therapy can also be initiated earlier.
Previous studies have reported on reossification and increased bone density after palliative radiotherapy for bone metastases [8], [9], [17], [18]. The occurrence of reossification after radiotherapy allows for this novel treatment combination, since halo fixation provides immediate stability and correction of posture, giving the cervical/cervicothoracic spinal column the opportunity to re-ossify after radiotherapy in an optimized anatomical position. Hyper fractionation is hypothesized to induce reossification (especially in breast cancer patients) by inducing destruction of tumor cells, while osteoclasts and osteoblasts remain after radiation and the bone turnover will return (close) to normal. However, previous research yielded conflicting results. Two small RCTs compared single fraction radiotherapy with multiple fraction radiotherapy and showed significantly higher remineralization in the multiple fractionation group [19], [20]. In a previous study at our hospital we found a higher degree of remineralization in patients who received more than 5 fractions compared to patients who received 1–5 fractions, although this difference was not statistically significant [9]. Other studies did not find a significant difference in remineralization between short course and long course radiotherapy [21], [22]. In addition, it is hypothesized that administration of bone-modifying agents (further) improve reossification [8], [9], [17], [23], [24], but more research on remineralization after radiotherapy in combination with bone-modifying agents is needed to clearly demonstrate this.
To the best of our knowledge, this is the first study reporting on clinical outcomes after halo fixation and radiotherapy. Literature on the outcomes after surgery for cervical spinal metastases is relatively scarce. In an international, multicenter, prospective observational study (the EPOSO cohort) initiated by the AO Spine Knowledge Forum Tumor, 38 patients with cervical metastases underwent surgical intervention [25]. The majority of patients had a posterior surgical approach, and 44% of patients received adjuvant radiotherapy. In this group, there were statistically significant improvements in pain (mean NRS) compared with baseline at 6 weeks, 3 and 6 months (7.4 vs 4.5, 4.7 and 4.5, respectively) after surgery. Postoperative adverse events, including thromboembolic events, wound complications, infections and neurologic deterioration, occurred in 17 out of 38 patients (45%). In four patients (11%) intraoperative events occurred, including occurrence of a dural tear, implant-related complication and massive blood loss. In a retrospective study of a prospective database including patients with spinal metastases, 47 patients with cervical spinal metastases received surgical decompression, tumor resection, and spinal fixation [26]. The majority had a posterior surgical approach. Postoperatively, 89% experienced pain relief. In 10 patients, adverse events occurred (4% infections, 12% medical complications, and 4% surgical complications). In a small prospective cohort [27], 26 patients with metastases of the cervical or cervicothoracic spine underwent palliative decompression and stabilization surgery via an anterior (18 patients), posterior (7 patients) or combined approach (1 patient). The average pain VAS score decreased from 6.9 preoperatively to 1.5 postoperatively and improvement in pain was maintained at all subsequent follow-up appointments up to 1 year. There were no wound healing problems or infections reported. One patient became paraplegic and died on the 8th postoperative day.
With a mean age of 57 year, a mean SINS score of 13, and 90% of patients experiencing mechanical neckpain at baseline, the patients from the EPOSO study [25] are broadly comparable to our cohort. Also, the patients from the study of Truong et al. [26] (mean age 60 year) and from the study of Quan et al. [27] (mean age 63 year, mean KPS of 66, and mean VAS pain score of 6.9) are comparable. With caution, the outcomes of our approach of halo fixation combined with radiotherapy appear no worse compared to long-construct surgical fixation, with the advantage of the relatively preserved range of motion. Of note, the patients in the aforementioned cohorts had higher ASIA impairment scores, indicating more neurological symptoms at baseline. For patients with clinically relevant neurological impairment (i.e. ASIA impairment score of C or worse) the halo fixation approach is not suitable, as this condition requires surgical decompression.
Our initial experiences indicate that the proposed treatment might be best suited to younger patients and/or patients with metastases originating from radiosensitive tumors (without serious neurological deficits). However, older patients and patients with rather radioresistant metastases (vaginal cancer, urothelial carcinoma and cholangiocarcinoma) also reported a median pain score of 0 at halo frame removal with signs of reossification in the majority of patients. This treatment combination may be especially suited for patients with multiple cervical spinal metastases as long-construct spinal fixations could be avoided.
This study has some limitations. Since the retrospective nature of the study, we do not know how many patients would have been eligible for halo fixation and radiotherapy, but were not referred to the combined radiotherapy/orthopedic surgery outpatient clinic. This might have introduced some selection bias.
Also due to the retrospective nature of this study we did not prospectively collect information on clinical outcomes leading to some missing values. For example, although we retrieved the majority of pain scores from the medical records, the use of concomitant pain medication was not always documented at the same time points. Pain progression could have been masked if the pain medication was increased simultaneously. However, patients in this cohort generally experienced mechanical pain, because they were selected based on the presence of spinal metastases requiring stabilization. It is often difficult to treat mechanical pain adequately with pain medication. The application of a halo frame (rapidly) relieves a large component of the mechanical pain of patients with unstable cervical metastasis, which is consequently the most important analgetic treatment. Therefore we believe that missing data on analgetic treatment will not have affected our study largely.
The relatively preserved range of motion in the neck is the main advantage of the combined halo fixation and radiotherapy treatment compared to long-construct spinal fixation. Minor or no limitations in range of motion were expressed by the majority of our included patients following halo frame removal and at last follow up. To our knowledge, articles reporting outcomes after surgery for multiple unstable cervical metastases do not report on motion of the neck post-surgery. In this study, we report qualitatively on the range of neck motion, as collected from the medical records or reported by patients at last follow up (by phone). Due to the retrospective nature of our study we could, however, not perform a systematic assessment with metrics, or use a validated questionnaire at time of treatment. We expect patients treated with the combined halo fixation and radiotherapy treatment to have a larger quantitative range of motion compared to patients treated with a long construct spinal fixation.
Conclusions
In conclusion, in patients with multiple unstable cervical/cervicothoracic metastases, complete pain response or substantial pain reduction, reossification and a preserved range of motion was observed after treatment with temporary halo fixation and radiotherapy. In selected patients without serious neurological deficits, this approach might be a reasonable alternative to long-construct spinal fixation. Halo fixation combined with radiotherapy for patients with unstable cervical metastases needs to be evaluated prospectively with regards to pain, mechanical stability, neurological status, reossification and complications, before it can be routinely applied.
Patient consent statement
Some patients were participant of a prospective cohort of patients with bone metastases (Prospective Evaluation of interventional StudiEs on boNe meTastases (PRESENT) (13-261/C)(10) and we used cohort data for the current study. Patients who were not included in the PRESENT cohort and were alive at the time of data analysis, were contacted by their treating physician to obtain informed consent for use of their baseline demographics, treatment characteristics, and clinical follow-up data. As this study was retrospective, it did not fall under the scope of the Dutch Medical Research Involving Human Subjects Act (WMO) and was therefore waived from study approval by an accredited medical ethics committee. An independent quality check was carried out to ensure compliance with legislation and regulations (regarding Informed Consent procedure, data management, privacy aspects and legal aspects).
CRediT authorship contribution statement
E.H. Huele: Methodology, Formal analysis, Data curation, Writing – original draft. J.M. van der Velden: Methodology, Formal analysis, Data curation, Writing – original draft. H.M. Verkooijen: Writing – review & editing, Methodology. N. Kasperts: Conceptualization, Resources, Writing – review & editing. J.J. Verlaan: Conceptualization, Resources, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
E.H. Huele, Email: e.h.huele-3@umcutrecht.nl.
J.M. van der Velden, Email: J.M.vanderVelden@umcutrecht.nl.
Appendix.
Table A1.
Neck motion as described in the medical records.
| Case number | Neck motion | Information from the medical records | Follow up time (months) |
|---|---|---|---|
| 1 | Minor limitation in range of motion | Painless, full range of motion except for neck extension. | 57 |
| 2 | Full range of motion | Range of motion of cervical spine almost 100%. | 31 |
| 3 | Full range of motion | Range of motion of cervical spine almost 100%. Some stiffness of the neck. | 48 |
| 4 | Not reported | Not reported due to progression of systemic disease. | NA |
| 5 | Minor limitation in range of motion | Limited range of motion, in particular for neck extension and rotations. | 42 |
| 6 | Full range of motion | Full rotations possible in the neck. | 6 |
| 7 | Minor limitation in range of motion | Limited range of motion due to general neck stiffness. | 11 |
| 8 | Minor limitation in range of motion | Limited range of motion in rotations and extensions of the neck. | 47 |
| 9 | Early removal | Early removal due to rapid progression of disease. | NA |
| 10 | Full range of motion | No complaints in neck movement. | 8 |
| 11 | Minor limitation in range of motion | Limited range of motion in neck extension. | 31 |
| 12 | Major limitation in range of motion | Limited range of motion due to pain during movement. (this patient received spondylodesis surgery) | 27 |
| 13 | Early removal | Early removal due to rapid progression of disease. | NA |
| 14 | Full range of motion | Full range of motion of the neck. | 78 |
| 15 | Early removal | Patient died 43 days after halo fixation due to perforated diverticulitis. | NA |
| 16 | Minor limitation in range of motion | Range of motion somewhat limited in rotations. | 115 |
| 17 | Minor limitation in range of motion | Hesitant to move beyond current limited range of motion. | 17 |
| 18 | Minor limitation in range of motion | Limited mobility of the neck during daily activies, for example navigating traffic. | 20 |
| 19 | Full range of motion | Range of motion of cervical spine almost 100%. | 8 |
| 20 | Early removal | Early removal due to rapid progression of disease. | NA |
References
- 1.van der Velden J.M., van der Linden Y.M., Versteeg A.L., Verlaan J.J., Sophie Gerlich A., Pielkenrood B.J., et al. Evaluation of effectiveness of palliative radiotherapy for bone metastases: a prospective cohort study. J Radiat Oncol. 2018;7(4):325–333. doi: 10.1007/s13566-018-0363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Velden J., Willmann J., Spałek M., Oldenburger E., Brown S., Kazmierska J., et al. ESTRO ACROP guidelines for external beam radiotherapy of patients with uncomplicated bone metastases. Radiother Oncol. 2022;173:197–206. doi: 10.1016/j.radonc.2022.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Weinfurt K.P., Li Y., Castel L.D., Saad F., Timbie J.W., Glendenning G.A., et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol. 2005;16(4):579–584. doi: 10.1093/annonc/mdi122. [DOI] [PubMed] [Google Scholar]
- 4.Jenis L.G., Dunn E.J., An H.S. Metastatic disease of the cervical spine. A review. Clin Orthop Relat Res. 1999;359:89–103. doi: 10.1097/00003086-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Dea N., Versteeg A., Fisher C., Kelly A., Hartig D., Boyd M., et al. Adverse events in emergency oncological spine surgery: a prospective analysis. J Neurosurg Spine. 2014;21(5):698–703. doi: 10.3171/2014.7.SPINE131007. [DOI] [PubMed] [Google Scholar]
- 6.Dunning E.C., Butler J.S., Morris S. Complications in the management of metastatic spinal disease. World J Orthop. 2012;3(8):114. doi: 10.5312/wjo.v3.i8.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luksanapruksa P., Buchowski J.M., Zebala L.P., Kepler C.K., Singhatanadgige W., Bumpass D.B. Perioperative complications of spinal metastases surgery. Clin Spine Surg. 2017;30(1):4–13. doi: 10.1097/BSD.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 8.Kito M., Tsukahara Y., Okamoto M., Fukazawa A., Ikegami S., Tanaka A., et al. Does re-ossification after palliative radiotherapy for spinal bone metastases help maintain vertebral body height? Spine J. 2023 doi: 10.1016/j.spinee.2023.06.389. [DOI] [PubMed] [Google Scholar]
- 9.Pielkenrood B.J., Visser T.F., van Tol F.R., Foppen W., Eppinga W.S., Verhoeff J.J., et al. Remineralization of lytic spinal metastases after radiotherapy. Spine J. 2023;23(4):571–578. doi: 10.1016/j.spinee.2022.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Prospective Evaluation of Interventional Studies on Bone Metastases – the PRESENT Cohort. : ClinicalTrials.gov NCT02356497. ; [Available from: https://clinicaltrials.gov/ct2/show/NCT02356497.
- 11.Castor EDC. (2019). Castor Electronic Data Capture. [online] Available at: https://castoredc.com.
- 12.Maynard FM, Bracken MB, Creasey G, Jr JFD, Donovan WH, Ducker TB, et al. International standards for neurological and functional classification of spinal cord injury. Spinal Cord. 1997;35(5):266–74. [DOI] [PubMed]
- 13.Karnofsky DA. The clinical evaluation of chemotherapeutic agents in cancer. Evaluation of chemotherapeutic agents. 1949:191–205.
- 14.Fisher CG, DiPaola CP, Ryken TC, Bilsky MH, Shaffrey CI, Berven SH, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976). 2010;35(22):E1221–9. [DOI] [PubMed]
- 15.Al Farii H., Aoude A., Al Shammasi A., Reynolds J., Weber M. Surgical management of the metastatic spine disease: a review of the literature and proposed algorithm. Global Spine J. 2023;13(2):486–498. doi: 10.1177/21925682221146741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spratt D.E., Beeler W.H., de Moraes F.Y., Rhines L.D., Gemmete J.J., Chaudhary N., et al. An integrated multidisciplinary algorithm for the management of spinal metastases: an International Spine Oncology Consortium report. Lancet Oncol. 2017;18(12):e720–e730. doi: 10.1016/S1470-2045(17)30612-5. [DOI] [PubMed] [Google Scholar]
- 17.Foerster R., Eisele C., Bruckner T., Bostel T., Schlampp I., Wolf R., et al. Bone density as a marker for local response to radiotherapy of spinal bone metastases in women with breast cancer: a retrospective analysis. Radiat Oncol. 2015;10:62. doi: 10.1186/s13014-015-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen G.L., Gaddipati R., Hammonds K.P., Morrow A., Swanson G.P. Bone density changes following radiotherapy to vertebral metastases. Cureus. 2021;13(6):e15417. doi: 10.7759/cureus.15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koswig S, Budach V. [Remineralization and pain relief in bone metastases after after different radiotherapy fractions (10 times 3 Gy vs. 1 time 8 Gy). A prospective study]. Strahlenther Onkol. 1999;175(10):500–8. [DOI] [PubMed]
- 20.El-Shenshawy H KA, El-Essawy S. . The effect of a single fraction compared to multiple fractions radiotherapy on painful bone metastases with evaluation of computed tomography bone density. . Bull Alex Fac Med 2006;2(389–91).
- 21.Chow E., Holden L., Rubenstein J., Christakis M., Sixel K., Vidmar M., et al. Computed tomography (CT) evaluation of breast cancer patients with osteolytic bone metastases undergoing palliative radiotherapy—a feasibility study. Radiother Oncol. 2004;70(3):291–294. doi: 10.1016/j.radonc.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Sprave T., Hees K., Bruckner T., Foerster R., Bostel T., Schlampp I., et al. The influence of fractionated radiotherapy on the stability of spinal bone metastases: a retrospective analysis from 1047 cases. Radiat Oncol. 2018;13(1):134. doi: 10.1186/s13014-018-1082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harada H., Katagiri H., Kamata M., Yoshioka Y., Asakura H., Hashimoto T., et al. Radiological response and clinical outcome in patients with femoral bone metastases after radiotherapy. J Radiat Res. 2010;51(2):131–136. doi: 10.1269/jrr.09096. [DOI] [PubMed] [Google Scholar]
- 24.Nakata E., Sugihara S., Kataoka M., Yamashita N., Furumatsu T., Takigawa T., et al. Early response assessment of re-ossification after palliative conventional radiotherapy for vertebral bone metastases. J Orthop Sci. 2019;24(2):332–336. doi: 10.1016/j.jos.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Bond M.R., Versteeg A.L., Sahgal A., Rhines L.D., Sciubba D.M., Schuster J.M., et al. Surgical or radiation therapy for the treatment of cervical spine metastases: results from the epidemiology, process, and outcomes of spine oncology (EPOSO) cohort. Global Spine J. 2020;10(1):21–29. doi: 10.1177/2192568219839407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Truong V.T., Al-Shakfa F., Phan P., Newman N., Boubez G., Shedid D., et al. Does the region of the spine involved with metastatic tumor affect outcomes of surgical treatments? World Neurosurg. 2021;156:e139–e151. doi: 10.1016/j.wneu.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Quan G.M., Vital J.M., Pointillart V. Outcomes of palliative surgery in metastatic disease of the cervical and cervicothoracic spine. J Neurosurg Spine. 2011;14(5):612–618. doi: 10.3171/2011.1.SPINE10463. [DOI] [PubMed] [Google Scholar]