Abstract
Replication of the genome of hepatitis delta virus (HDV) requires RNA-directed RNA synthesis using a host polymerase(s). This manuscript reviews the relevant published evidence. It also provides two new studies, both of which made use of transiently transfected Huh7 cells undergoing HDV RNA-directed RNA synthesis. For the first study, RNA transcription inhibitors were added to the transfected cells for periods of 1 to 2 days, after which assays of the effects on the accumulation of processed unit-length genomic HDV RNA were performed. For the second study, nuclei were isolated at 6 days after transfection, and then in vitro runoff transcription was used to assay the effects of RNA transcription inhibitors. Overall, the data support the interpretation that HDV transcription does not require host polymerase I or III (pol I or III) but at least primarily involves an enzyme resembling pol II.
Plant viroids and the human hepatitis delta virus (HDV) have much in common (47). One of the more intriguing similarities is that they both replicate via RNA-directed RNA synthesis using a host RNA polymerase (44). Since there are no known DNA intermediates (5) the most likely explanation is that these agents with their single-stranded RNA genomes are able to redirect a host polymerase that normally uses DNA templates to use RNA as a template. An alternative explanation is that the host cell contains an RNA-directed RNA polymerase that can carry out the transcription. Actually, such a polymerase was first found and purified from plants (40). cDNA cloning of this polymerase (41) revealed that all plants and some animals contain at least one copy of a related gene. However, at this time, no such gene has been detected in mammals, which can be hosts for HDV.
Several different suggestions have surfaced as to which host polymerases are involved. The plant viroids are frequently divided into two families. For one of these families there are data that transcription occurs in association with chloroplasts, using a nucleus-encoded polymerase (34). For the second viroid family, the available evidence implicates the host RNA polymerase II (pol II) (42, 49). Similarly, with HDV there are many claims for pol II as the relevant enzyme (1, 12, 13, 26), although a recent paper has suggested the possibility that more than one host polymerase may be involved (31).
For purposes of evaluation, the evidence for the polymerase(s) involved in HDV transcription may be divided into four different types: (i) circumstantial, (ii) in vivo, (iii) in vitro, and (iv) a combination of in vivo and in vitro.
(i) Consider first the circumstantial evidence. During HDV genome replication the following three RNA species are detected (5): (a) the 1,679-nucleotide (nt) circular RNA genome; (b) its exact complement, the so-called antigenome; and (c) relatively low amounts of a less-than-unit-length antigenomic RNA that contains the open reading frame for the small delta protein, a 195-amino-acid species that is essential for HDV replication. This third RNA is thought to be an mRNA. The 5′ end is capped (17) and corresponds to a discrete location (16), and at the 3′ end is a poly(A) tail (19). There is evidence that the genomic RNA acts as template for the transcription of multimers of antigenomic RNA, some of which are processed to become unit-length RNA circles while others are processed to become the poly(A) species. The site of this polyadenylation is directed by an AAUAAA signal (19). Overall, these features of the third RNA provide circumstantial evidence that it was transcribed and processed just as would be expected for a DNA-directed pol II transcript. Possibly another example of circumstantial evidence is that both in situ hybridization to detect HDV RNAs (9, 45) and immunomicroscopy to detect the small delta protein (3, 9) that is needed for HDV genome replication (20) indicate a nucleoplasmic location, just as is found for pol II and pol III but not for pol I (8).
(ii) Several studies have attempted to use in vivo approaches. The early reports treated cells in which HDV genome replication was occurring with the pol II-specific inhibitor α-amanitin (26). These studies detected inhibition but the shortcoming was that HDV replication had been initiated via the stable transfection of the cells with a multimer of HDV cDNA; it was therefore not possible to separate transcription that was DNA directed from that which was RNA directed. A recent report by Modahl et al. used HDV RNAs transcribed in vitro to transfect cells and initiate genome replication (31). At various times after transfection α-amanitin was added and the accumulation of HDV RNA was measured; the data were interpreted as evidence that pol II is needed for HDV mRNA synthesis but that some other polymerase that is more α-amanitin resistant than pol II carries out the synthesis of the unit-length genomic and antigenomic RNA species (31). The major shortcoming of this study was the lack of controls for DNA-directed transcription by the host polymerases, pol I, II, and III. In addition, an intrinsic limitation of such studies is that they can only assay the accumulation of processed HDV RNAs rather than directly measure HDV transcription. That is, the accumulation detected, for example, by Northern blot analyses, reflects not only RNA transcription but also RNA processing and RNA stability.
(iii) The third experimental approach used has been in vitro, using either cell extracts or sources of purified pol II. Such studies have detected transcription of added HDV RNA that was pol II directed. However, examination of these results shows that in most cases the transcription was no more than a modest 3′ end addition to a linear HDV RNA template (1, 12, 17). In one case, the RNA template somehow underwent an endonucleolytic cut which revealed a 3′ end that was also used for modest 3′-end addition (12). And, except for two recent papers (17, 51), the delta protein that in vivo is essential for genome replication (20) was not even added exogenously to the in vitro transcription. In one of these studies, the exogenous delta protein was reported to have no effect (17), and in another, it was reported to modestly increase the processivity of pol II on DNA and RNA templates (51).
(iv) There is, however, a fourth experimental approach that has not been previously tried but is potentially superior. This approach has two parts. First, HDV genome replication is initiated in vivo by transfecting cells using RNA templates. Second, these cells are disrupted and examined for transcription in vitro in the presence of radioactive precursors, with and without specific polymerase inhibitors. We considered that this strategy might have several advantages relative to the other methods. It allows the use of inhibitor concentrations that could not be used in vivo because of toxic effects on the cell. The effects of a polymerase inhibitor used in this way are more likely to be direct than indirect. Another major advantage is that the HDV template is not exogenously added to the in vitro reaction but is endogenous, and moreover, if the delta protein is needed for such transcription, it is also already present, and possibly present in the appropriate stoichiometry.
The first use of approach iv is described in this manuscript. To make this possible and in parallel to assay the effects of the inhibitors of endogenous DNA-directed transcripts by pol I, II, and III, we had to make some important technical changes to the standard runoff protocols. These are described in Materials and Methods. After these changes, we were able to obtain HDV-specific transcription and to study the effects of α-amanitin on runoff transcription.
As mentioned above, the recent application of approach ii by Modahl et al. offered the interpretation that for the synthesis of the unit-length genomic and antigenomic RNAs the host polymerase used, at least according to α-amanitin sensitivity, was not pol II. As described in this paper, we carried out extensive studies using a very similar approach, with α-amanitin and several other transcription inhibitors. In contrast to Modahl et al., we found no evidence for the involvement of a polymerase other than pol II in the transcription and ultimate accumulation of processed unit-length genomic RNA.
Overall, from an evaluation of the existing data and from our new data using approaches ii and iv, we are left with the interpretation that HDV RNA-directed RNA transcription is at least primarily via an enzyme that behaves like pol II and not like pol I or pol III.
MATERIALS AND METHODS
RNA for transfection.
We used combinations of the three RNAs diagrammed in Fig. 1. RNA1 was an antigenomic RNA of 1.2× the unit length transcribed from plasmid pTW114 (50), which contains an HDV insert flanked by a T7 promoter and terminator, using a T7 RNA polymerase transcription kit (Promega). Similarly, RNA3 was a genomic RNA of 1.2× the unit length transcribed from plasmid pTW101 (50). RNA2 acted as an mRNA containing the open reading frame for the small delta protein. This RNA was both 5′ capped and 3′ polyadenylated. It was transcribed by phage T7 RNA polymerase using an expression PCR product as template and a capped RNA transcription kit (Ambion). The PCR product was such as to place (A)25 at the 3′ end of the T7 transcripts. The T7 polymerase reaction mixtures were treated with DNase and then extracted using Tri Reagent (Molecular Research Center). In the experiments described for Fig. 5, RNA1 was transcribed using T7 polymerase from pTW107, which had been opened at a HindIII site, to obtain transcripts with a 2-nt deletion at the unique EcoRI site to disrupt the open reading frame of the small delta protein (50).
FIG. 1.
Representation of antigenomic and genomic RNA transcripts used to initiate HDV genome replication. RNA1 is a 1.2× linear antigenomic RNA. RNA2 is a capped and polyadenylated mRNA for the small delta protein, with the open reading frame as indicated. RNA3 is a 1.2× linear genomic RNA. Note that RNA1 and RNA2 have two copies of their respective ribozymes (Rz). In the experiment for Fig. 5 we used a form of RNA1 with a 2-nt deletion at the unique EcoRI site (E). The positions of the 5′ and 3′ ends are indicated for RNA1 and RNA3 using the notation of Kuo et al. for the 1,679-nt RNA sequence (21).
FIG. 5.
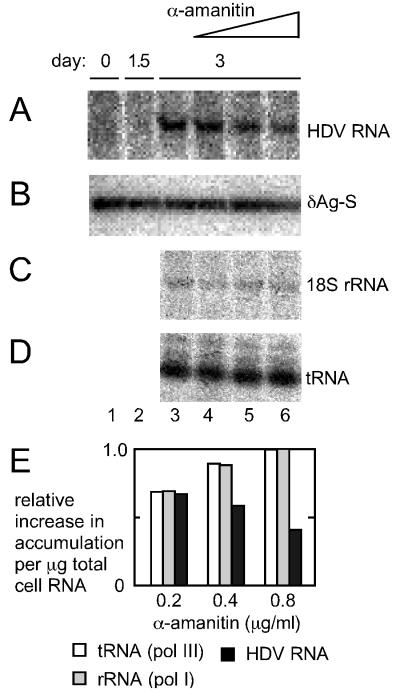
Effect of α-amanitin on the accumulation of HDV genomic RNA in cells transfected with mutated HDV RNA. Replica cultures of Huh7 cells that had been previously stably transfected to express the small form of the delta protein were transfected with an RNA, like the RNA1 in Fig. 1, but with a 2-nt deletion at position E within the open reading frame. Data are shown for an untransfected culture (lane 1) and at days 1.5 (lane 2) and 3 (lane 3) after transfection. For certain cultures α-amanitin was added at day 1.5 at 0.2 (lane 4), 0.4 (lane 5), and 0.8 (lane 6) μg/ml, and the cells were harvested at day 3. For lanes 3 to 6, [3H]uridine was also added at day 1.5 to provide a measure of the rate of host 18S rRNA (C) and tRNA (D) synthesis. Northern blot analyses were used to detect unit-length genomic RNA (A) and immunoblots were used to quantitate expression of the small delta protein (B). For panel E we obtained quantitation of the effects of α-amanitin on accumulation of mutated HDV genomic RNA, just as for Fig. 4
Transfections.
For RNA transfections we used two RNAs, either RNA1 and RNA2 or RNA 2 and RNA3. This two-RNA strategy was a modification of the method described by Modahl and Lai (30). For example, 1 day prior to transfection, Huh7 cells (33) were seeded at 1.7 × 105 per well of a 12-well tissue culture dish (USA Scientific). Transfections were carried out with 0.5 μg of the 1.2× antigenomic RNA and 0.1 μg of the capped delta antigen mRNA, using Lipofectamine Plus reagent (Invitrogen) according to the manufacturer's instructions.
As a variation of the two-RNA procedure, in some cases we transfected just the greater-than-unit-length mutant antigenomic RNA into Huh7 cells that were already stably transfected with pTW198, a pcDNA3.1 construct (Invitrogen) which expresses the small delta protein (50).
As a control for DNA-directed pol II transcription, replica cultures were transfected with the DNA construct pDL541, which uses pol II to transcribe a 1.2-mer of a replication-incompetent form of HDV genomic RNA with a >1,000-nt deletion from the top of the rod-like RNA structure. This RNA is known to be processed by the HDV genomic ribozyme to produce a 348-nt circular RNA species (23).
As a control for transfection efficiency we used a DNA construct (10% amount) that expresses green fluorescent protein. This tended to slow the rate of accumulation of HDV RNA sequences by as much as 1 day. It was omitted from the critical experiment shown in Fig. 4. Within each experiment, for each control and each drug treatment, the transfections were carried out in duplicate, or even triplicate, and processed independently.
FIG. 4.
Effect of α-amanitin on the accumulation of HDV genomic RNA in Huh7 cells transfected with HDV RNAs. For panels A, C, and D, replica cultures were transfected as for Fig. 3. For panel B, replica cultures were transfected with pDL541, a DNA construct that expresses a 1.2-mer of a deleted form of HDV genomic RNA; the transcription is driven by pol II and the transcripts are processed by two copies of the HDV genomic ribozyme to produce 348-nt RNA circles. Lanes 1 to 5, standards as described for Fig. 3; lanes 6 to 9, at 0.2 days α-amanitin was added at 0.025, 0.05, 0.1, and 0.2 μg/ml, respectively. From the gel analyses of the extracted RNAs we detected unit-length HDV genomic RNA (A), the mini-RNA transcribed from a DNA template by pol II (B), 18S rRNA transcribed by pol I (C), and tRNA transcribed by pol III (D). For panel E we used quantitation of the experiment shown in panels A to D, along with optical density measurements on total extracted RNA, to determine, relative to untreated cultures, the amount of accumulation of four different RNA species, as indicated, that was achieved in the presence of four different concentrations of α-amanitin.
Inhibitors and [3H]uridine labeling.
The five inhibitors used were α-amanitin, 5,6-dichloro-1-β-ribofuranosylbenzimidazole (DRB), rifampin, actinomycin D (all from Sigma), and tagetitoxin (Epicenter). The concentrations used and the treatment times are as indicated in the text and/or the figure legends. At the beginning of an in vivo inhibitor treatment, we added 20 μCi of [3H]uridine (Amersham) to label host RNA species, especially rRNA and tRNA, which are transcribed by pol I and pol III, respectively.
RNA extraction and Northern blot analyses.
Total RNA was extracted from transfected cells using Tri Reagent. The RNA fraction was then treated with RQ DNase I (Promega) and reextracted. Aliquots of <5 μg were glyoxalated and assayed for HDV RNA by Northern blot analyses, as previously described (50). However, immediately after the electrotransfer and deglyoxalation, the charged nylon membrane (Zetaprobe; Bio-Rad) was air dried and the 3H-labeled RNA species were detected by placing the filter in direct contact with a special imaging screen (BAS TR2040; Fuji). After appropriate exposure the signal was quantified with a Bio-Imager (Fuji). The filter was then hybridized with strand-specific 32P-labeled RNA probes to detect genomic HDV RNA. Radioactivity was detected with a standard imaging screen and subsequently quantitated using the Bio-Imager.
Protein extraction and immunoblot analyses.
Total proteins were extracted using Tri Reagent. Samples were denatured using Laemmli buffer (22) and analyzed by our standard immunoblot procedures (32), using polyclonal rabbit antiserum against recombinant small delta protein, followed by incubation with 125I-labeled staph A protein (DuPont). Quantitation was done with the Bio-Imager.
Preparation of nuclei and in vitro runoff assays.
Each experimental point required a subconfluent monolayer of Huh7 cells on 100-mm-diameter culture dishes. One day after seeding these cells were cotransfected using Lipofectamine Plus with a combination of the two RNAs (3.2 μg of RNA3 and 0.8 μg of RNA2). HDV replication was allowed to proceed until day 6. In the 15 min prior to preparing the nuclear extracts the cells were treated with 0.2 μg of actinomycin D/ml. Prior studies showed that this dose inhibited rRNA synthesis and accumulation with no detectable effect on HDV, even when present for 1 day (see Fig. 3). Use of this as a pretreatment strategy was suggested by previous studies (35, 37) that used it to partially suppress rRNA synthesis in the subsequent runoff reactions. In our case, such suppression was additionally important because of the known ability of mammalian rRNA to cross-hybridize with HDV RNA (14).
FIG. 3.
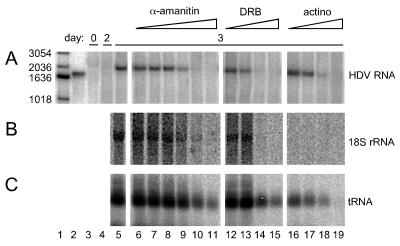
Effect of three polymerase inhibitors on accumulation of HDV genomic RNA in Huh7 cells transfected with HDV RNAs. Replica cultures were transfected as described for Fig. 2 and harvested at day 0 (lane 3), 2 (lane 4), or 3 (lanes 5 to 19), as indicated. For lanes 6 to 19, at day 2 a series of concentrations of one of three inhibitors was added to determine the effect on the accumulation of HDV unit-length genomic RNA (A). Lane 5 represents an untreated control. Also added for lanes 5 to 19, at day 2, was [3H]uridine to provide a measure of the rate of host 18S rRNA (B) and tRNA (C) synthesis. Lanes 6 to 11, α-amanitin added to 0.03, 0.1, 0.3, 1, 3, and 10 μg/ml, respectively; lanes 12 to 15, DRB added to 1, 3, 10, and 30 μg/ml, respectively; lanes 16 to 19, actinomycin D added to 0.03, 0.1, 0.3, and 1 μg/ml, respectively; lane 1, size marker; lane 2, DNA standard of unit-length HDV cDNA.
The runoff procedure used was based on that described by Bishop (http: //www.BioProtocol.com/), but with some important modifications as described below. Cells were removed from the culture dish by trypsinization, washed with cold phosphate-buffered saline, and resuspended at 1.5 × 107 cells/ml in RSB (10 mM Tris [pH 7.5], 10 mM NaCl, 5 mM MgCl2). After 5 min on ice, an equal volume of cold RSB containing 0.5% NP-40 was added and the cells were disrupted to release the nuclei. These were collected by centrifugation at 2,000 rpm (Dynac II centrifuge) for 10 min. The supernatant was removed and the nuclear pellet was resuspended in 2× transcription buffer (50 mM dithiothreitol, 180 mM KCl, 10 mM MgCl2, 20 mM Tris [pH 7.5]), centrifuged once more, and then resupended in the same buffer at 2 × 108 nuclei/ml. The nuclei so prepared were used immediately and not frozen. Aliquots of 50 μl were transferred to 1.5-ml tubes. Reactions were carried out in a final volume of 60 μl. To do this, 10 μl was added to achieve final concentrations of 1 mM for rATP, rGTP, and rCTP along with 20 μCi of [α-32P]rUTP (DuPont; 800 Ci/mM). In some cases, as indicated in the text and figure legends, α-amanitin was also added. Reactions were incubated for 10 min at 37°C, after which RNA was immediately extracted using 1 ml of Tri Reagent and collected by precipitation with isopropanol. This RNA was then treated with alkali to reduce the size and again collected by precipitation with ethanol.
For the hybridizations we applied unlabeled nucleic acids to a charged nylon membrane using a slot device (BRL) exactly as described in the protocol of Bishop. The nucleic acids were fixed to the membrane by UV exposure. The following four nucleic acids were used. (i) To assay pol I activity an antisense nucleic acid to 28S rRNA was transcribed in vitro with T7 polymerase using as template plasmid pES28S (from Joan Steitz) precut with EcoRI. The reaction mixture was treated with DNase I prior to extraction of the RNA product using Tri Reagent. Fifty nanograms was used per slot. (ii) Similarly, to assay pol III activity an antisense nucleic acid to 7S L RNA of the signal recognition particle was transcribed with SP6 polymerase using as template plasmid pSP7SL (from Peter Walter) precut with EcoRI. Five micrograms was used per slot. (iii) As an assay for endogenous transcription by pol II we first isolated poly(A)-containing RNA from untransfected cells [two passages over oligo(dT) were used]. This RNA was reverse transcribed in vitro using an oligo(dT) primer, extracted, treated with RNase A, and extracted again. Four micrograms of cDNA was used per slot. (iv) To detect HDV genomic RNA synthesis we used 1.2× unit-length antigenomic RNA (RNA1 in Fig. 1). Five micrograms was used per slot.
The filters were prehybridized (65°C, 4 h) and hybridized (65°C, 48 h) in Ekono hybridization solution (Research Products International) in heat-sealed plastic bags (Seal-a-Meal; Rival). Hybridizations using all of the alkali-treated runoff product were carried out in 500 μl per filter containing four slots. Subsequently, filters were washed three times for 5 min at room temperature in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) plus 0.1% sodium dodecyl sulfate and then for 1 h at 70°C in 0.1× SSC plus 0.1% sodium dodecyl sulfate. Radioactivity was then detected using the Bio-Imager and subsequent quantitation was performed using the MacBas software (Fuji). Unlike X-ray film, the Bio-Imager plates have a linear response to radioactivity over a 100,000-fold range. Within a given experiment, the various transcription reactions were performed in duplicate. These duplicates were processed independently, each with its own hybridization.
We used Northern blot analyses to monitor the quantity and quality of the unit-length antigenomic RNA, both before and after the runoff transcription. Relative to the intact cells, 83% was recovered in the nuclear preparation. Even after the runoff transcription, at least 57% of this RNA was still in a circular conformation (data not shown).
It is important to note that we found it necessary to assay for genomic HDV RNA rather than antigenomic RNA. One reason was that in vivo studies indicate that genomic RNA is 5 to 20 times more abundant (5). Another reason is that even 6 days after transfection and after nuclei isolation, there can be residual unlabeled RNA from the transfection. In early studies, when we used antigenomic RNA1 to initiate the transfection, the residual antigenomic RNA could hybridize in solution with the radioactive genomic RNA and thereby prevent it from hybridizing to the antigenomic RNA that was immobilized on the filter. This problem was solved by initiating infection with genomic HDV RNA3; then residual RNA did not interfere with the subsequent hybridization to detect radioactive genomic RNA.
RESULTS
HDV RNA-directed RNA synthesis in transfected Huh7 cells.
For the two studies in which we attempted to characterize the host polymerase requirements of HDV transcription we needed cultured cells undergoing HDV genome replication. It is possible to initiate such replication by transient transfection with cDNA constructs (20) and even to select cell clones stably transfected with such constructs (6, 27). However, in such situations the HDV transcription can be both RNA and DNA directed; this could confound studies aimed at characterizing the host polymerase involved in RNA-directed transcription. Therefore, we considered initiation of replication by HDV RNA species. Several such strategies have been described (2, 11, 14, 30). For the present studies we decided that the best choice was that of Modahl and Lai (30) in which cells are transfected using a mixture of two HDV RNAs. As illustrated in Fig. 1, RNA1 is a greater-than-unit-length species of antigenomic RNA and RNA2 is a subgenomic-sized RNA with a 5′ cap and a 3′ poly(A) tail that can be translated to provide the initial source of the essential small delta protein. In a variation of this strategy we replaced RNA1 with RNA3, which is a greater-than-unit-length genomic RNA.
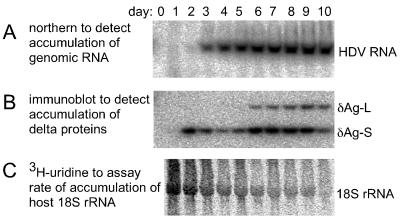
As a preliminary experiment, we transfected cells with RNA1 and RNA2 and measured the kinetics of accumulation of genomic HDV RNA by Northern blot analyses (Fig. 2A) and of delta proteins by immunoblot (Fig. 2B). At early times delta protein was detected before the genomic RNA. We consider that this delta protein was primarily translated from the transfected mRNA. However, after day 4 the levels of HDV RNA and delta antigen underwent a very similar time-dependent increase. Progressively increasing amounts of the large form of the delta protein were seen over time. By day 10, 40% of the total delta protein was of the large form. This large delta protein presumably arose during genome replication by the translation of edited HDV RNA (24).
FIG. 2.
Time course of HDV RNA and protein accumulation following transfection of Huh7 cells. Replica cultures were transfected with two HDV RNAs and at the indicated times were extracted with Tri Reagent to yield RNA samples for Northern blot analysis to detect the accumulation of unit-length genomic HDV RNA (A) or protein for immunoblot to detect the two forms of the delta antigen (δAg-S and δAg-L) (B). At 1 day prior to harvest [3H]uridine was added to the cultures to provide a measure of the rate of host 18S rRNA synthesis (C).
For subsequent studies we needed a measure of host ribosomal and tRNA transcription (pol I and III, respectively). We therefore used [3H]uridine to label the cells for 24 h immediately prior to harvesting. After extraction, the RNAs were subjected to gel electrophoresis, electrotransfer to a nylon membrane, and then detection of 3H using a special bio-imaging plate, with results as shown in Fig. 2C. It can be seen that by the end of the 10-day time course, the cells were synthesizing lower amounts of 18S rRNA. Quantitation showed an overall 10-fold reduction per culture as the cells reached confluence.
Filters were then hybridized using a 32P probe to detect HDV genomic RNA (as in Fig. 2A). These data revealed that HDV RNA accumulation underwent a major burst (at least 14-fold) at around 2 days after transfection. In the following studies, a window of time to day 3 was used to measure the sensitivity of HDV RNA accumulation to in vivo treatment with polymerase inhibitors.
In vivo inhibition of HDV RNA accumulation using approach ii.
We tested the effects of the following five RNA transcription inhibitors. Others have shown that α-amanitin, a mushroom toxin, binds specifically to a region on the large subunit of pol II and promptly inhibits transcription (10). DRB is also reported to be specific for pol II but its mechanism of inhibition is more complicated; this nucleoside analog is believed to act indirectly, causing the inhibition of a kinase whose action on the carboxyl-terminal domain of the largest subunit of pol II is essential for the continued elongation of nascent chains (36). Tagetitoxin has been reported to act on pol III transcription in some systems (43). Rifampin appears to act on the nucleus-encoded mitochondrial RNA polymerase, although there are contrary reports (15). Finally, actinomycin D is considered to be a specific inhibitor of all DNA-directed transcription. RNA-directed transcription by some viral RNA polymerases is resistant to this drug (7, 28, 38). It has been reported that HDV transcription in a stably transfected cell line was also resistant to this drug (25).
Studies with α-amanitin, DRB, and actinomycin D are shown in Fig. 3A. As a control for transcription by pol I and pol III we used [3H]uridine incorporation during the treatment period and assayed the accumulation of labeled 18S rRNA (Fig. 3B) and tRNA (Fig. 3C), respectively.
Consider first the consequences of treatment with actinomycin D (Fig. 3, lanes 16 to 19). With two lower doses (0.03 and 0.1 μg/ml) we were able to detect almost control levels of HDV and tRNA accumulation under conditions where 18S rRNA accumulation was almost totally inhibited. These results confirm previous work showing that HDV RNA transcription and processing continue during actinomycin treatment (25), since actinomycin preferentially inhibits transcription from DNA templates (7, 28, 38). However, the two higher doses (0.3 and 1 μg/ml) were sufficient to block the accumulation of HDV genomic RNA, 18S rRNA, and much of the tRNA. It is possible that this effect on HDV reflected a need for some host DNA-directed transcript. However, we prefer the interpretation that it was a consequence of general covert cell toxicity produced by the actinomycin, because with longer exposures or with higher concentrations cell toxicity revealed itself, as the cells rounded up and many detached from the monolayer.
Consider now the effects of treatment with the other two inhibitors, α-amanitin and DRB. For both of these drugs, as the concentration was increased accumulation of HDV RNA was inhibited (Fig. 3A, lanes 6 to 15). However, we detected almost equal inhibition of accumulation of processed 18S rRNA (Fig. 3B, lanes 6 to 15); tRNA was less affected (Fig. 3C, lanes 6 to 15). From quantitation of this and other experiments, there was an indication that at certain concentrations α-amanitin and not DRB causes a slightly greater inhibition of HDV RNA accumulation than of 18S rRNA and tRNA synthesis (see below and data not shown).
In addition to these three inhibitors we performed similar experiments with a series of doses of tagetitoxin (0.03, 0.1, 0.3, 1, and 3 μM), which has been reported to be an inhibitor of DNA-directed pol III transcription (43). However, we detected no effect on HDV accumulation or the controls for pol I, II, and III (data not shown). Similarly, we tested a series of doses of rifampin (0.025, 0.05, 0.1, and 0.2 mM) that had been claimed to be specific for DNA-directed RNA synthesis in mitochondria by the nuclear-encoded polymerase (15) and similarly saw no effects except at the highest dose, which caused cell toxicity (data not shown). These studies with tagetitoxin and rifampin thus provided no useful information regarding the polymerase(s) needed for HDV transcription.
With these results, we decided for two reasons to concentrate on the effects of α-amanitin and to attempt to find conditions under which it might act specifically on pol II transcription. The first reason, as mentioned above, was that we had an indication that inhibition was more specific for HDV than for 18S rRNA and tRNA. The other reason was that from the studies of others, it was known that α-amanitin acts directly on a subunit of pol II; this toxin binds to the large subunit of pol II, allows the formation of one phosphodiester bond in a dinucleotide-primed reaction, but inhibits further chain elongation by blocking the subsequent translocation step (10). With this in mind, we made two changes in the experimental strategy. (i) The series of drug concentrations was more closely spaced (0.025, 0.05, 0.1, and 0.2 μg/ml). (ii) In addition to transfection with the two HDV RNAs, replica cultures were transfected with a nonreplicating DNA construct as a control for pol II transcription. Based on previous studies (23) this DNA is transcribed in vivo by pol II into an internally deleted genomic RNA 1.2-mer that is subsequently processed by the genomic ribozymes and accumulates as a relatively stable 348-nt circular (nonreplicating) species. In order to detect the effects of α-amanitin treatment on the accumulation of this mini-RNA control for pol II transcription, we added the drug early (0.2 days) after transfection and then harvested the cells at day 2. (As noted in Materials and Methods, for this experiment the green fluorescent protein vector was omitted from the cotransfection, with the consequence that HDV RNA accumulation was detected at day 2, which is earlier than shown in Fig. 2 and 3.)
Some of the experimental results are shown in Fig. 4A to D. Quantitation was carried out from the bio-imager files, with results as represented in Fig. 4E. The data for 0.025 and 0.05 μg/ml indicated that any possible inhibition was <25% relative to untreated cells. However, at 0.1 and 0.2 μg/ml we detected an inhibition that was specific for HDV RNA accumulation and the pol II mini-RNA relative to the controls for pol I and pol III. The bigger difference was with 0.2 μg/ml for which the pol II mini-RNA and HDV were inhibited by 60 and 73%, respectively, while the 18S rRNA and tRNA were inhibited by 26 and 21%, respectively. These data thus supported the interpretation that transcription by pol II was necessary for the continued accumulation of processed HDV RNAs.
In a variation of the above experimental strategy, we also measured the ability of α-amanitin to inhibit the accumulation of HDV RNA when added between days 6 and 8 after transfection. Again we observed specific effects on HDV relative to 18S rRNA and tRNA (data not shown). (Logistically, we could not carry out parallel assays for DNA-directed pol II transcription of the mini-RNA, since it was produced via transient transfection and did not show an increase between days 6 and 8.) Thus, in contrast to the report of Modahl et al., we did not find a time at which the accumulation of processed unit-length HDV RNAs was resistant to α-amanitin (31).
In addition, Modahl et al. have interpreted their studies as evidence that pol II is needed for transcription of the HDV mRNA species but not for the transcription of those RNAs which go on to become processed unit-length RNAs (31). If this were the case, then in the presence of an adequate supply of small delta protein, there would be no inhibition by α-amanitin of the accumulation of full-length HDV RNA. To test this prediction we modified the experiment shown in Fig. 4. We replaced the normal Huh7 cells with a derived line that stably expressed the small form of the delta protein. Furthermore, for the transfection we removed the mRNA (RNA2) and we replaced the greater-than-unit-length antigenomic RNA (RNA1) with a mutated species. Indicated as E in Fig. 1, the mutation in RNA1 was a deletion of 2 nt from the open reading frame of the small delta antigen. Thus, in this modified transfection the only source of small delta protein was that provided by the stable expression; none could be produced by replication of the HDV genome. We tested the α-amanitin sensitivity of this transfection when the drug was added at 1.5 days and the cells were harvested at day 3. As shown in Fig. 5, increasing doses of α-amanitin were able to block the accumulation of HDV RNA (panel A) relative to 18S rRNA (panel C) or tRNA (panel D). As an essential control for this study we also assayed the amounts of small delta protein in the transfected cells, as shown in panel B; the observed levels of accumulation were unchanged by the drug treatments.
Quantitation of the RNA data are shown in Fig. 5E. As for Fig. 4E, the data were normalized relative to untreated cells and expressed per microgram of total cell RNA. Note that the HDV accumulation was reduced by 60% with 0.8 μg of α-amanitin/ml, while at the same dose the 18S rRNA and tRNA synthesis was essentially at control levels. (In the experiments for Fig. 4, inhibition of both host and HDV transcripts was observed with lower doses of inhibitor; we consider this increased sensitivity to be a consequence of adding the inhibitor at only 0.2 days after the transfection was initiated.) We conclude from this experiment that even in the presence of a separate source of delta protein, the accumulation of processed full-length HDV RNA transcripts was sensitive to α-amanitin. Thus, in contrast to Modahl et al. (31), we found no difference in the polymerase requirements for the accumulation of HDV mRNA and unit-length genomic RNA.
In a variation of the experiments for Fig. 5, we used in the RNA transfection the nonmutated HDV antigenomic RNA1. Again the results showed that the accumulation of genomic HDV RNA was sensitive to inhibition by α-amanitin (data not shown).
Overall, our interpretation of these and the earlier data are that independent of HDV mRNA and the availability of small delta protein, α-amanitin treatments still showed a specific inhibition of the accumulation of unit-length HDV RNA relative to an inhibition of 18S rRNA transcribed by pol I and tRNA transcribed by pol III.
In vitro inhibition of HDV runoff transcription using approach iv.
Processed HDV RNAs can accumulate to high levels per infected or transfected cell. For example, in the average liver cell of an infected woodchuck, there can be as many as 300,000 copies of the HDV genome (5). However, the actual rate of RNA transcription can be relatively slow. Also, in transiently transfected cultures replication is not initiated in every cell (maybe 10 to 40% in our experiments). As intimated in the introduction, our aim was to initiate replication in vivo by transfection of Huh7 cells with HDV RNAs and then after 6 days to carry out an in vitro runoff reaction to detect the endogenous transcription of HDV RNAs. In a series of experiments we used the pol II-specific inhibitor α-amanitin, and for controls we assayed for endogenous host transcription by 28S rRNA (pol I), total poly(A)-containing RNA (pol II), and 7S L RNA (pol III) in an attempt to determine which polymerase was needed for HDV genomic RNA transcription.
In nuclear runoff experiments it is known that 45% of the transcripts are typically of rRNA species (29), and independent of which polymerase is used by HDV, we would expect that HDV transcripts would be a minor fraction of the total nuclear transcription. At the outset this became a problem because rRNA species do cross-hybridize with HDV RNA (14). As a solution to this we opted to reduce the overall level of rRNA synthesis by giving the cells a brief treatment with actinomycin D (15 min, 0.2 μg/ml) just prior to nuclear isolation. This strategy has been used by others to suppress rRNA (35, 37) and also we know from our in vivo studies that even a 24-h exposure to similar doses of actinomycin (0.03 and 0.1 μg/ml) did not detectably inhibit HDV RNA synthesis and accumulation (Fig. 3). This strategy was successful and was adopted in all subsequent experiments.
Figure 6A shows four typical examples, in duplicate, of the hybridization data obtained for one experiment. The first example was from using nuclei from untransfected cells. This gave hybridization signals for 28S rRNA, poly(A) RNA, and 7S L RNA. As expected, there was only a minimal background signal for HDV. The second example, with cells transfected by HDV RNAs, gave a signal for all three host RNAs and also for HDV. The third and fourth examples were from when the runoff transcription from HDV-transfected cells was carried out in the presence of two concentrations of α-amanitin (0.1 and 0.3 μg/ml, respectively). These concentrations gave an obvious inhibition of poly(A)-containing RNA and also of HDV genomic RNA.
FIG. 6.
Hybridization of 32P-labeled runoff products to slot-immobilized nucleic acids. Nuclei from untransfected (−) or RNA transfected (+) cells were used in runoff reactions in the absence (0) or presence of α-amanitin at 0.1 and 0.3 μg/ml. For panel A, the 32P-labeled RNAs so obtained were each hybridized to a filter with an array of four specific nucleic acids to assay the endogenous transcripts, as indicated. Detection of 32P was by Bio-Imaging plate, with quantitation summarized in Table 1 and deductions as presented in panel B.
In order to more carefully study the effects of α-amanitin, the data shown in Fig. 6A were quantitated using the Bio-Imager files, with results as summarized in Table 1. Note that for each experimental condition, two separate runoff reactions were performed. Thus, each row in the table represents a single runoff reaction, with quantitation of hybridization to each of the four different type of transcripts.
TABLE 1.
Quantitation of runoff assays
| Source of nuclei and runoff reaction conditionsa | Detected hybridization (arbitrary radioactivity units)b
|
|||
|---|---|---|---|---|
| 28S rRNA (pol I) | poly(A) RNA (pol II) | 7S L RNA (pol III) | Genomic HDV | |
| Nuclei from untransfected cells | 2,937 | 1,320 | 10,450 | 112 |
| 1,484 | 1,173 | 8,320 | 91 | |
| Average | 2,211 | 1,247 | 9,385 | 102 |
| Nuclei from transfected cells | 2,638 | 1,000 | 14,870 | 417 |
| 1,634 | 1,078 | 9,969 | 439 | |
| Average | 2,136 (100) | 1,039 (100) | 12,420 (100) | 326c (100) |
| Plus α-amanitin (0.1 μg/ml) during runoff | 1,608 | 232 | 6,094 | 125 |
| 2,919 | 323 | 5,784 | 224 | |
| Average | 2,264 (106) | 278 (27) | 5,939 (48) | 73c (22) |
| Plus α-amanitin (0.3 μg/ml) during runoff | 1,735 | 192 | 4,000 | 156 |
| 1,542 | 88 | 1,512 | 101 | |
| Average | 1,639 (77) | 140 (13) | 2,756 (22) | 27c (8) |
Transcription was carried out as described in Materials and Methods, with the inhibitor concentrations indicated. For each experimental condition, two separate runoff reactions were performed. The table shows quantitation for the duplicate hybridizations along with the deduced average values.
The hybridized filters described for Fig. 6A were quantitated from the Bio-Imager files, using the radioactive profile and subtraction of a background deduced from the flanking regions on the filter. Numbers in parentheses are the deduced percentages for reactions performed in the presence of α-amanitin and expressed relative to the untreated controls.
Value was determined by subtraction of the background deduced from the runoff reaction performed with untransfected cells (428 − 102 = 326, 175 − 102 =73, 129 − 102 = 27).
Using the quantitation summarized in Table 1, we deduced for each experiment the relative amounts of synthesis in the presence of α-amanitin of the three host RNAs and of the HDV genomic RNA. These values are represented in Fig. 6B. In these experiments the HDV was at least as sensitive to the α-amanitin treatments as poly(A)-containing RNA, which is indicative of pol II. In turn, the poly(A)-containing RNA was more sensitive than the 7S L RNA, indicative of pol III. And finally, the 28S rRNA, indicative of pol I, was the least sensitive. These relative sensitivities were maintained in two other experiments for which we used a higher dose of α-amanitin (1 μg/ml) (data not shown). Therefore, our interpretation of the data shown in Fig. 6B is that HDV transcription is primarily by a host polymerase that resembles pol II in its sensitivity to α-amanitin.
For the above studies we exploited the specificity of the inhibitor α-amanitin to obtain evidence that HDV RNA is transcribed by pol II. For other studies (data not shown) we also tested three more inhibitors: DRB, tagetitoxin, and two mouse monoclonal antibodies (H15 and 8WG12) specific for the carboxy-terminal domain of the large subunit of pol II (48). In our runoff transcription assays we found that none of these inhibitors offered any effect that was specific. (We tested 1- and 10-μg/ml concentrations of DRB and 20 and 60 μM targetitoxin.) This lack of specificity may well be because our runoff assays are for transcription that is already initiated on an endogenous template.
DISCUSSION
We have described here two applications of RNA transcription inhibitors in an attempt to clarify the host polymerase requirements for HDV RNA transcription. In the first application we determined the effect of the inhibitors on the ability of cultured cells transfected with HDV RNAs to continue HDV RNA-directed RNA synthesis and to accumulate processed unit-length HDV RNA species. With low doses of actinomycin we were able to obtain evidence that processed HDV RNAs could accumulate under conditions that blocked host 18S rRNA (Fig. 3). The studies with DRB, rifampin, and tagetitoxin did not provide any evidence as to which host polymerase is involved in the transcription (Fig. 3 and data not shown). However, from the treatment of cells with closely spaced doses of α-amanitin, we were able to determine that HDV RNA accumulation relative to appropriate controls for DNA-directed transcription by pols I, II, and III depended upon pol II (Fig. 3 to 5).
Our results are partially similar to those of Modahl et al. (31), who in some situations found HDV RNA accumulation was sensitive to α-amanitin. However, in other situations these authors reported that HDV accumulation was resistant to as much α-amanitin as 100 μg/ml. We find this additionally puzzling because in our experiments, using the same cells, exposure to more than 10 μg/ml for 24 h was sufficiently toxic that virtually all the cells detached from the monolayer. Even after exposure to 3 or 10 μg/ml for the same period of time we could detect a major inhibition of rRNA accumulation. After exposure to 1 μg/ml we observed by microsopy a rounding up of the cells and a rearrangement of 4′,6′-diamidino-2-phenylindole (DAPI)-stained nuclear DNA (data not shown). Also, by immunostaining to detect either the delta antigen or pol II, we could detect nucleoplasmic rearrangement (data not shown). Others have reported similar effects for cells treated with α-amanitin as well as with DRB and actinomycin (18). While it may not be unreasonable that treatment of cells with such inhibitors produces toxic effects, it raises the concern that the associated inhibition of HDV RNA accumulation might be an indirect rather than a direct effect. Actually, on a wider scale, any inhibition detected in such in vivo studies could be an indirect effect.
In order to avoid such limitations we carried out our second series of experiments. In these we applied nuclear runoff reactions, combined with RNA transcription inhibitors, in an attempt to identify the host polymerase involved in HDV RNA transcription. With α-amanitin we obtained specific inhibition of both HDV RNA transcription and of DNA-directed transcription of host poly(A)-containing RNAs (Fig. 6). We interpret this as evidence that HDV RNA is transcribed by pol II.
It is reasonable to expect that the HDV transcription we detected from the runoff transcription made use of endogenous circular HDV RNA templates. The HDV genome replication was initiated in vivo by transfection of cells with greater-than-unit-length linear genomic RNAs, but it was 6 days later when replication was well under way that we isolated the nuclei for the runoff assays. Furthermore, based on Northern blot analyses of RNA extracted from such cells at day 6, we know that the majority of this de novo antigenomic HDV RNA was both unit-length in size and circular in conformation (4). We think that our experimental strategy avoided potentially artifactual transcriptional results that might arise in an alternative strategy where exogenous linear HDV RNA templates are added to in vitro transcription reactions (1, 12, 17).
We think that our ability to detect HDV runoff transcripts using nuclear extracts rather than having to use permeabilized cells excludes any requirement by HDV of a cytoplasmic polymerase, such as the nucleus-encoded mitochondrial RNA polymerase (15). We should also make two comments on the possible relevance to HDV transcription of the RNA-directed RNA polymerase that is present in all plants and also some animals (41). First, so far this polymerase has not been detected in mammals (41). And second, when this polymerase was purified from plants, it was found to be α-amanitin resistant (39).
In summary, as a consequence of the two studies presented here and an evaluation of earlier studies we are now confident that in vivo HDV RNA-directed transcription at least primarily involves host RNA pol II. We can now move on and determine how this transcription is achieved. The runoff assays used here can be adapted to determine the 5′ ends of the nascent transcripts. Such information will help test the hypothesis that a combination of specific nucleotide sequences and RNA folding might provide an HDV pol II promoter for transcription from an HDV RNA template (1, 16, 46). Also, it may be that the runoff assay system, in conjunction with specific antibodies (other than anti-pol II), can be used to identify the host transcription factors needed for HDV RNA-directed transcription.
ACKNOWLEDGMENTS
This work was supported by grants AI-26522 and CA-06927 from the NIH and by an appropriation from the Commonwealth of Pennsylvania.
We thank Ting-Ting Wu for selection of the stable cell line expressing the small delta protein. Helpful advice on transcription assays was provided by Ken Zaret (Fox Chase), Cheng-Ming Chiang (Case Western Reserve University), and Bob Krug (University of Texas at Austin). A clone for human 7S L was provided by Peter Walter (University of California at San Francisco), and a clone for human 28S rRNA was provided by Joan Steitz (Yale University). Constructive comments on the manuscript were given by Ken Zaret, Glenn Rall, Robert Perry, William Mason, Severin Gudima, and Jinhong Chang.
REFERENCES
- 1.Beard M R, Macnaughton T B, Gowans E J. Identification and characterization of a hepatitis delta virus RNA transcriptional promoter. J Virol. 1996;70:4986–4995. doi: 10.1128/jvi.70.8.4986-4995.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bichko V, Netter H J, Taylor J. Introduction of hepatitis delta virus into animal cell lines via cationic liposomes. J Virol. 1994;68:5247–5252. doi: 10.1128/jvi.68.8.5247-5252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bichko V V, Taylor J M. Redistribution of the delta antigens in cells replicating the genome of hepatitis delta virus. J Virol. 1996;70:8064–8070. doi: 10.1128/jvi.70.11.8064-8070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang J, Moraleda G, Taylor J. Limitations to the replication of hepatitis delta virus in avian cells. J Virol. 2000;74:8861–8866. doi: 10.1128/jvi.74.19.8861-8866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen P-J, Kalpana G, Goldberg J, Mason W, Werner B, Gerin J, Taylor J. Structure and replication of the genome of hepatitis δ virus. Proc Natl Acad Sci USA. 1986;83:8774–8778. doi: 10.1073/pnas.83.22.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng D, Yang A, Thomas H, Monjardino J. Characterization of stable hepatitis delta expressing hepatoma cell lines: effect of HDAg on cell growth. Prog Clin Biol Res. 1993;382:149–153. [PubMed] [Google Scholar]
- 7.Chu P W, Westaway E G. Characterization of Kunjin virus RNA-dependent RNA polymerase: reinitiation of synthesis in vitro. Virology. 1987;157:330–337. doi: 10.1016/0042-6822(87)90275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook P R. The organization of replication and transcription. Science. 1999;284:1790–1795. doi: 10.1126/science.284.5421.1790. [DOI] [PubMed] [Google Scholar]
- 9.Cunha C, Monjardino J, Chang D, Krause S, Carmo-Fonseca M. Localization of hepatitis delta virus RNA in the nucleus of human cells. RNA. 1998;4:680–693. doi: 10.1017/s135583829898013x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Mercoyrol L, Job C, Job D. Studies on the inhibition by α-amanitin of single step addition reactions and productive RNA synthesis catalysed by wheat germ RNA polymerase II. Biochem J. 1989;258:165–169. doi: 10.1042/bj2580165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dingle K, Bichko V, Zuccola H, Hogle J, Taylor J. Initiation of hepatitis delta virus genome replication. J Virol. 1998;72:4783–4788. doi: 10.1128/jvi.72.6.4783-4788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filipovska J, Konarska M M. Specific HDV RNA-templated transcription by pol II in vitro. RNA. 2000;6:41–54. doi: 10.1017/s1355838200991167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu T-B, Taylor J. The RNAs of hepatitis delta virus are copied by RNA polymerase II in nuclear homogenates. J Virol. 1993;67:6965–6972. doi: 10.1128/jvi.67.12.6965-6972.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glenn J S, Taylor J M, White J M. In vitro-synthesized hepatitis delta virus RNA initiates genome replication in cultured cells. J Virol. 1990;64:3104–3107. doi: 10.1128/jvi.64.6.3104-3107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldenthal M, Nishiura J T. Isolation and characterization of a mitochondrial RNA polymerase from Drosophila melanogaster. Biochem Cell Biol. 1987;65:173–182. doi: 10.1139/o87-022. [DOI] [PubMed] [Google Scholar]
- 16.Gudima S, Dingle K, Wu T-T, Moraleda G, Taylor J. Characterization of the 5′ ends for polyadenylated RNAs synthesized during the replication of hepatitis delta virus. J Virol. 1999;73:6533–6539. doi: 10.1128/jvi.73.8.6533-6539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gudima S, Wu S-Y, Chiang C-M, Moraleda G, Taylor J. Origin of the hepatitis delta virus mRNA. J Virol. 2000;74:7204–7210. doi: 10.1128/jvi.74.16.7204-7210.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haaf T, Ward D C. Inhibition of RNA polymerase II transcription causes chromatin decondensation, loss of nucleolar structure, and dispersion of chromosomal domains. Exp Cell Res. 1996;224:163–173. doi: 10.1006/excr.1996.0124. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh S-Y, Chao M, Coates L, Taylor J. Hepatitis delta virus genome replication: a polyadenylated mRNA for delta antigen. J Virol. 1990;64:3192–3198. doi: 10.1128/jvi.64.7.3192-3198.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo M Y-P, Chao M, Taylor J. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J Virol. 1989;63:1945–1950. doi: 10.1128/jvi.63.5.1945-1950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo M Y-P, Goldberg J, Coates L, Mason W, Gerin J, Taylor J. Molecular cloning of hepatitis delta virus RNA from an infected woodchuck liver: sequence, structure, and applications. J Virol. 1988;62:1855–1861. doi: 10.1128/jvi.62.6.1855-1861.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Lazinski D W, Taylor J M. Expression of hepatitis delta virus RNA deletions: cis and trans requirements for self-cleavage, ligation, and RNA packaging. J Virol. 1994;68:2879–2888. doi: 10.1128/jvi.68.5.2879-2888.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo G, Chao M, Hsieh S-Y, Sureau C, Nishikura K, Taylor J. A specific base transition occurs on replicating hepatitis delta virus RNA. J Virol. 1990;64:1021–1027. doi: 10.1128/jvi.64.3.1021-1027.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macnaughton T B, Gowans E J, Jilbert A R, Burrell C J. Hepatitis delta virus RNA, protein synthesis and associated toxicity in stably transfected cell line. Virology. 1990;177:692–698. doi: 10.1016/0042-6822(90)90535-y. [DOI] [PubMed] [Google Scholar]
- 26.Macnaughton T B, Gowans E J, McNamara S P, Burrell C J. Hepatitis delta antigen is necessary for access of hepatitis delta virus RNA to the cell transcriptional machinery but is not part of the transcriptional complex. Virology. 1991;184:387–390. doi: 10.1016/0042-6822(91)90855-6. [DOI] [PubMed] [Google Scholar]
- 27.Macnaughton T B, Gowans E J, Qiao M, Burrell C J. Simultaneous expression from cDNA of markers of hepatitis delta virus and hepadnavirus infection in continuous cell lines. In: Hollonger F B, Lemon S M, Margolis H, editors. Viral hepatitis and liver disease. Baltimore, Md: Williams and Wilkins; 1990. pp. 465–468. [Google Scholar]
- 28.Marzachi C, Accotto G P, d'Aquilio M, Caciagli P, Boccardo G. In vitro transcription of the double-stranded RNA genome of maize rough dwarf virus (Reoviridae) J Gen Virol. 1990;71:707–711. doi: 10.1099/0022-1317-71-3-707. [DOI] [PubMed] [Google Scholar]
- 29.Marzluff W F, Huang R C C. Transcription of RNA in isolated nuclei. In: Hames B D, Higgins S J, editors. Transcription and translation: a practical approach. Oxford, United Kingdom: IRL Press; 1984. pp. 89–129. [Google Scholar]
- 30.Modahl L E, Lai M M C. Transcription of hepatitis delta antigen mRNA continues throughout hepatitis delta virus (HDV) replication: a new model of HDV RNA transcription and regulation. J Virol. 1998;72:5449–5456. doi: 10.1128/jvi.72.7.5449-5456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modahl L E, Macnaughton T B, Zhu N, Johnson D L, Lai M M C. RNA-dependent replication and transcription of hepatitis delta virus RNA involve distinct cellular RNA polymerases. Mol Cell Biol. 2000;20:6030–6039. doi: 10.1128/mcb.20.16.6030-6039.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moraleda G, Dingle K, Biswas P, Chang J, Zuccola H, Hogle J, Taylor J. Interactions between hepatitis delta virus proteins. J Virol. 2000;74:5509–5515. doi: 10.1128/jvi.74.12.5509-5515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cell lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- 34.Navarro J-A, Vera A, Flores R. A chloroplastic RNA polymerase resistant to targetitoxin is involved in replication of avocado sunblotch viroid. Virology. 2000;268:218–225. doi: 10.1006/viro.1999.0161. [DOI] [PubMed] [Google Scholar]
- 35.Perry R P. Selective effects of actinomycin D on the intracellular distribution of RNA synthesis in tissue culture cells. Exp Cell Res. 1963;29:400–406. [Google Scholar]
- 36.Ping Y H, Rana T M. DSIF and NELF interact with RNA polymerase II elongation complex and HIV-1 Tat stimulates P-TEFb-mediated phosphorylation of RNA polymerase II and DSIF during transcription elongation. J Biol Chem. 2000;276:12951–12958. doi: 10.1074/jbc.M006130200. [DOI] [PubMed] [Google Scholar]
- 37.Pombo A, Jackson D A, Hollinshead M, Wang Z, Roeder R G, Cook P R. Regional specialization in human nuclei: visualization of discrete sites of transcription by RNA polymerase III. EMBO J. 1999;18:2241–2253. doi: 10.1093/emboj/18.8.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray J, Fujinami R S. Characterization of in vitro transcription and transcriptional products of measles virus. J Virol. 1987;61:3381–3387. doi: 10.1128/jvi.61.11.3381-3387.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiebel W, Haas B, Marinkovic S, Klanner A, Sanger H L. RNA-directed RNA polymerase from tomato leaves. II. Catalytic in vitro properties. J Biol Chem. 1993;268:11858–11867. [PubMed] [Google Scholar]
- 40.Schiebel W, Haas B, Marinkovik S, Klanner A, Sanger H L. RNA-directed RNA polymerase from tomato leaves. I. Purification and physical properties. J Biol Chem. 1993;268:11851–11857. [PubMed] [Google Scholar]
- 41.Schiebel W, Pelissier T, Riedel L, Thalmeir S, Schiebel R, Kempe D, Lottspeich F, Sanger H L, Wasseneger M. Isolation of an RNA-directed RNA polymerase-specific cDNA from tomato. Plant Cell. 1998;10:2087–2101. doi: 10.1105/tpc.10.12.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schindler I-M, Muhlbach H-P. Involvement of nuclear DNA-dependent RNA polymerases in potato spindle tuber viroid replication: a reevaluation. Plant Sci. 1992;84:221–229. [Google Scholar]
- 43.Steinberg T H, Mathews D E, Durbin R D, Burgess R R. Tagetitoxin: a new inhibitor of eukaryotic transcription by RNA polymerase III. J Biol Chem. 1990;265:499–505. [PubMed] [Google Scholar]
- 44.Taylor J. HDV as a precedent for RNA-directed RNA synthesis by RNA polymerase II. In: Dinter-Gottlieb G, editor. The unique hepatitis delta virus. R. G. Austin, Tex: Landes, Co.; 1995. pp. 47–54. [Google Scholar]
- 45.Taylor J, Mason W, Summers J, Goldberg J, Aldrich C, Coates L, Gerin J, Gowans E. Replication of human hepatitis delta virus in primary cultures of woodchuck hepatocytes. J Virol. 1987;61:2891–2895. doi: 10.1128/jvi.61.9.2891-2895.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor J M. Cis-acting signals on the RNAs of hepatitis delta virus. Semin Virol. 1997;8:212–220. [Google Scholar]
- 47.Taylor J M. Replication of human hepatitis delta virus: influence of studies on subviral plant pathogens. Adv Virus Res. 1999;54:45–60. doi: 10.1016/s0065-3527(08)60365-6. [DOI] [PubMed] [Google Scholar]
- 48.Thompson N E, Steinberg T H, Aronson D B, Burgess R R. Inhibition of in vivo and in vitro transcription by monoclonal antibodies prepared against wheat germ RNA polymerase II that react with the heptapeptide repeat of eukaryotic RNA polymerase II. J Biol Chem. 1989;264:11511–11520. [PubMed] [Google Scholar]
- 49.Warrilow D, Symons R H. Citrus exocortis viroid RNA is associated with the largest subunit of RNA polymerase II in tomato in vivo. Arch Virol. 1999;144:2367–2375. doi: 10.1007/s007050050650. [DOI] [PubMed] [Google Scholar]
- 50.Wu T-T, Netter H J, Lazinski D W, Taylor J M. Effects of nucleotide changes on the ability of hepatitis delta virus to transcribe, process, and accumulate unit-length, circular RNA. J Virol. 1997;71:5408–5414. doi: 10.1128/jvi.71.7.5408-5414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi Y, Filipovska J, Yano K, Furuya A, Inukai N, Narita T, Wada T, Sugimoto S, Konarska M M, Handa H. Stimulation of RNA polymerase II elongation by hepatitis delta antigen. Science. 2001;293:124–127. doi: 10.1126/science.1057925. [DOI] [PubMed] [Google Scholar]