Abstract
Background
Hemorrhagic events cause numerous deaths annually worldwide, highlighting the urgent need for effective hemostatic drugs. The glucosyloxybenzyl 2-isobutylmalates Control Extract (BSCE) from the orchid plant Bletilla striata (Thunb.) Rchb.f. has demonstrated significant hemostatic activity in both in vitro and in vivo studies. However, the effect and mechanism of BSCE on non-traumatic bleeding remain unclear.
Methods
Pulmonary hemorrhage was induced in 40 Sprague-Dawley rats by administering Zingiber officinale Roscoe. for 14 days. These rats were then randomly divided into five groups: model (Mod), positive control (YNBY), and BSCE low, medium, and high-dose groups. An additional 8 rats served as the control group (Con). The BSCE groups received different doses of BSCE for 10 days, while the YNBY group received Yunnan Baiyao suspension. The effects on body weight, food and water intake, red blood cell count (RBC), hemoglobin concentration (HGB), lung tissue pathology, platelet count, coagulation parameters, and fibrinolytic system markers were evaluated. Network pharmacology and molecular docking analyses were also conducted to identify potential targets and pathways involved in BSCE's effects.
Results
BSCE treatment significantly improved body weight, food intake, and water consumption in rats with pulmonary hemorrhage. RBC and HGB levels increased significantly in the BSCE medium and high-dose groups compared to the Mod group (P < 0.05). Pathological examination revealed that BSCE reduced lung tissue hemorrhage and inflammation, with improvements in alveolar structure. BSCE also positively affected platelet count, thrombin time (TT), activated partial thromboplastin time (APTT), fibrinogen (FIB) levels, and fibrinolytic markers (D-dimer, PAI-1, and t-PA). Network pharmacology and molecular docking identified key targets such as MMPs, CASPs, and pathways including IL-17 and TNF signaling, suggesting BSCE's involvement in hemostasis and anti-inflammatory processes.
Conclusions
BSCE exhibits significant hemostatic and protective effects on Z.officinale-induced pulmonary hemorrhage in rats by improving hematological parameters, reducing lung tissue damage, and modulating the coagulation and fibrinolytic systems. The study provides evidence supporting the potential of BSCE as a therapeutic agent for hemorrhagic diseases, with its efficacy linked to multi-target and multi-pathway interactions.
Keywords: Bletilla striata (Thunb.) Rchb.f., Pulmonary hemorrhage, Glucosyloxybenzyl 2-isobutylmalates, Hemostatic mechanism, Network pharmacology
1. Introduction
Pulmonary hemorrhage refers to the rupture of blood vessels in the lungs, leading to blood entering the lung tissue. This clinical condition can result in symptoms such as hemoptysis and shortness of breath. In severe cases, it may cause shock symptoms, including pale complexion, cold extremities, and confusion, making it a major cause of death from non-traumatic hemorrhage and posing a serious threat to patient health [[1], [2], [3]]. Effective and rapid hemostasis can gain valuable time for subsequent lung treatment and has become a key means to reduce the mortality of early non-traumatic bleeding. Therefore, the development of efficient hemostatic drugs has become an urgent need for the treatment of non-traumatic bleeding diseases. Natural plant-derived hemostatic drugs have shown multi-component, multi-target, and multi-link effects in the prevention and treatment of clinical hemorrhagic diseases, and have unique advantages and development potential in the development of drugs for non-traumatic hemorrhagic diseases [[4], [5], [6], [7], [8]].
B.striata is a widely used hemostatic drug in southwestern China, with a history of use spanning over 2000 years, as documented in "Shennong's Herbal Classic" [9,10]. B.striata has a significant hemostatic effect on various types of internal and external bleeding, particularly hemoptysis caused by lung and gastric injuries [11,12]. B.striata is rich in polysaccharides, traditionally considered the main hemostatic agents in the plant [11,13]. However, these polysaccharides are highly viscous and difficult to purify, limiting their use both orally and externally. To address this, researchers have investigated the non-polysaccharide hemostatic components of B.striata. The n-butanol extraction of B.striata has been identified as a key effective component for hemostasis [14,15]. Our previous research focused on these non-polysaccharide components, leading to the preparation of BSCE. Compared to the n-butanol extract, BSCE demonstrated superior hemostatic activity and a greater number of active components [16,17] (Supplementary Material Fig. S1). We hypothesize that this extract may also ameliorate lung injury, although its protective effect and underlying mechanisms remain unclear.
According to traditional Chinese medicine theory by Ming Dynasty physician Zhang Jingyue, "bleeding is usually caused by internal heat, and if the internal heat is strong enough, it can lead to bleeding" [18]. In this context, blood heat is considered a primary factor in non-traumatic hemorrhagic diseases. Normally, blood circulation is maintained under physiological conditions, but excessive internal heat can damage the lung's collateral circulation, leading to pulmonary hemorrhage. Pulmonary hemorrhage induced by Z.officinale is characterized by high repeatability and strong stability, making it a reliable model for studying hemostatic drugs. This model also induces symptoms of excessive heat in experimental animals, along with platelet system disorders and abnormal coagulation function [19,20].
Coagulation is a highly regulated process essential for maintaining blood circulation's dynamic balance. The coagulation and anticoagulation systems form a complex network of interacting molecules that regulate clotting [21,22]. Hemostasis involves the platelet system, the coagulation system, and the fibrinolytic system [[23], [24], [25], [26], [27], [28], [29]]. In this study, we used Z.officinale to induce pulmonary hemorrhage in rats. After successfully establishing the model, we observed the effect of BSCE on pulmonary hemorrhage and investigated the mechanism by analyzing changes in the platelet system, coagulation system, and fibrinolytic system. Additionally, network pharmacology and molecular docking studies were conducted. Our results provide valuable insights into developing potential treatments for hemorrhagic diseases.
2. Materials and methods
2.1. Instrument
The OSB-2200 rotary evaporator was procured from Shanghai Erlang Instrument Co., Ltd. (Shanghai, China). The RHP-750A high-speed multifunctional grinder was obtained from Zhejiang Ronghao Industry & Trade Co., Ltd. (Zhejiang, China). A SHZ-D III type circulating water vacuum pump was acquired from Shanghai Yuying Instrument Co., Ltd. (Shanghai, China). The SB-4200DTD ultrasonic cleaning instrument was sourced from Ningbo Xinyi Ultrasonic Equipment Co., Ltd. (Ningbo, China). The DK-98-1 electric heating constant temperature water bath pot was supplied by Tianjin Taisite Instrument Co., Ltd. (Tianjin, China). An FA2204B electronic balance was purchased from Shanghai Tianmei Balance Instrument Co., Ltd. (Shanghai, China). The OHG-9055A blower dryer was bought from Shanghai Yarong Biochemical Instrument Factory (Shanghai, China). The CS-5100 automatic coagulation analyzer was provided by Xisen Meikang Medical Electronics Co., Ltd. (Kobe, Japan). A BS-2800M automatic biochemical analyzer was procured from Shenzhen Mindray Biomedical Electronics Co., Ltd. (Shenzhen, China). The ABM-100 small animal anesthesia machine was acquired from Shanghai Yuyan Scientific Instrument Co., Ltd. (Shanghai, China). An lDZ (K) 0.50–0.7 type automatic electric heating steam generator was sourced from Guangzhou Lizhi Machinery Equipment Co., Ltd. (Guangzhou, China). The lD-96A microplate reader was bought from Shandong Leander Intelligent Technology Co., Ltd. (Shandong, China). The TN300 extraction and concentration unit was obtained from Shanghai Huizhan Experimental Equipment Co., Ltd. (Shanghai, China). The LC2040C 3D Plus ultra-high performance liquid chromatography was purchased from Shimadzu (Kyoto, Japan).
2.2. Reagent
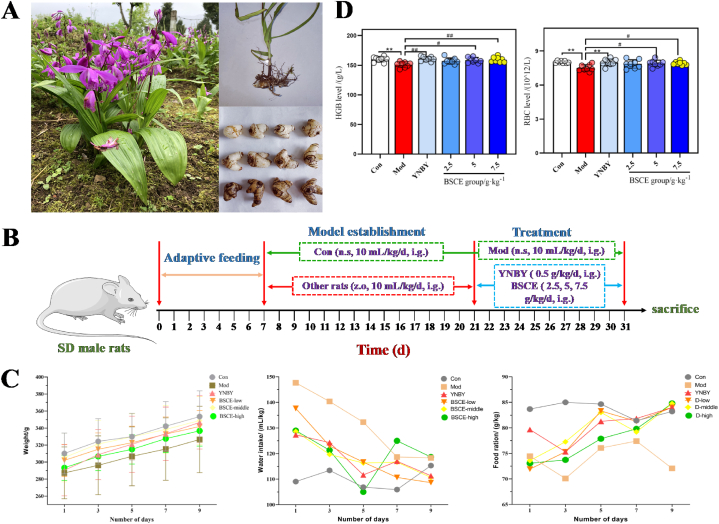
Petroleum ether and ethanol were obtained from Tianjin Fuyu Fine Chemical Co., Ltd. (Tianjin, China), while ethyl acetate was sourced from Chongqing Wansheng Chuandong Chemical Co., Ltd. (Chongqing, China). N-butanol was acquired from National Pharmaceutical Group Chemical Reagents Co., Ltd. (Beijing, China), and Wahaha pure water was purchased from Guiyang Wahaha Changsheng Beverage Co., Ltd. (Guiyang, China). Sodium carboxymethyl cellulose (Batch number: 20220304) was sourced from Tianjin Fuchen Chemical Reagent Co., Ltd. (Tianjin, China), and Yunnan Baiyao (Batch number: ZBA2202) was obtained from Yunnan Baiyao Group Co., Ltd. (Yunnan, China). B.striata (Fig. 1A) was purchased from Guizhou Huixi Biotechnology Co., Ltd. (Guiyang, China), and PRP-512B macroporous resin from Beijing Sunflower Technology Development Co., Ltd. (Beijing, China). The disposable venous blood collector (Batch number: 20220905) was purchased from Jiangxi Fenglin Medical Instrument Co., Ltd. (Jiangxi, China). Disposable human venous blood sample collector sodium citrate (Batch No.: 220706) was sourced from Liuyang Sanli Medical Technology Development Co., Ltd. (Liuyang, China), and disposable vacuum blood collection tube EDTA-K2 (Batch No.: 20210302) from Jiangsu Yuli Medical Device Co., Ltd. (Jiangsu, China). Z.officinale was acquired from Guizhou Tongjitang Pharmaceutical Co., Ltd. (Guiyang, China), and isoflurane (Batch number: 22071601) from Rivard Life Technology Co., Ltd. (Shenzhen, China). Anhydrous ethanol (Batch No.: 20220707) was obtained from Tianjin Dingshengxin Chemical Co., Ltd. (Tianjin, China). The rat tissue plasminogen activator (t-PA) kit (Batch No.: MM-0523R1) and rat plasminogen activator inhibitor-1 (PAI-1) kit (Batch No.: MM-0072R1) were purchased from Jiangsu Enzyme-free Industrial Co., Ltd. (Jiangsu, China). The TAT ELISA kit (Batch number: CSB-E08432) was sourced from Wuhan Huamei Bioengineering Co., Ltd. (Wuhan, China), and the D-D kit (Batch No.: 2045149) from Shanghai Sun Biotechnology Co., Ltd. (Shanghai, China). Militarine standard (Batch number: CHB180528) was purchased from Chengdu Keloma Biotechnology Co., Ltd. (Chengdu, China), while the Gymnoside III standard (Batch number: PRF10102503) was obtained from Chengdu Priffa Technology Development Co., Ltd. (Chengdu, China). The standard substances of gymnoside I and dactylorhin A were isolated from Bletilla striata in our laboratory, with mass fractions exceeding 98 %.
Fig. 1.
Effect of BSCE on pulmonary hemorrhage model rats. A: Bletilla striata medicine picture; B: SD rat test cycle axis; C: The changes and differences of body weight, diet and water intake of rats in each group during administration; D: The difference of RBC and HGB content in blood of rats in each administration group. ∗∗P<0.01 vs. Con group; #P<0.05,##P<0.01 vs. Mod group.
2.3. Animal
A total of 48 Sprague-Dawley rats (normal adult rats, weighing 180–220 g), consisting of equal numbers of males and females, were obtained from Changsha Tianqin Biotechnology Co., Ltd. (SPF, SCXK (Hunan) 2019-0014). The rats were housed at 25 °C ± 2 °C with a relative humidity of 60 % ± 10 %, and were provided with food and water ad libitum. They underwent a one-week acclimatization period before the experiment. The study was conducted in accordance with the "Laboratory Animals-Animal Welfare Ethical Review Guideline" (GB/T 35892-2018) and the American Guideline (NIH publication #85-23, revised in 1985).
2.4. Preparation of BSCE and Zingiber officinale Roscoe decoction
B.striata powder (10 kg) was weighed and extracted with 8 times the amount of 50 % ethanol in a water bath at 80 °C for 3 times, 2 h each time. The filtrate was filtered, concentrated and extracted, separated by PRP 512 B macroporous resin, and eluted with water, 20 %, 40 % and 95 % ethanol solution in turn. The 40 % ethanol elution section was collected to obtain BSCE. The dry extract of the plant was preserved in the Herbarium of the Key Laboratory of Traditional Chinese Medicine and Ethnic Medicine of Guizhou University of Traditional Chinese Medicine (NO.BJ20221010). 10 kg Z.officinale was decocted with 50 times pure water for 3 times, 1 h each time. The combined filtrate was concentrated to 1.5 kg L−1 and frozen.
2.5. Animal experimental program
After one week of adaptive feeding of 48 rats, 8 were randomly selected as the blank group (Con) and given normal saline by gavage. The remaining 40 rats received 1.5 kg L−1 Z.officinale decoction by gavage, at a volume of 10 mL kg−1 for 14 days. At the end of the 14th day, the 40 rats, which had successfully developed pulmonary hemorrhage, were randomly divided into 5 groups: model group (Mod), positive control group (YNBY), BSCE low-dose group (BSCE-low), BSCE medium-dose group (BSCE-middle), and BSCE high-dose group (BSCE-high), with 8 rats per group. The Con and Mod groups were administered pure water intragastrically, while the YNBY group received 0.50 g kg−1 Yunnan Baiyao suspension intragastrically. The BSCE-low, BSCE-middle, and BSCE-high groups were administered 2.5, 5, and 7.5 g kg−1 BSCE solution, respectively, once daily for 10 consecutive days. On days 1, 3, 5, 7, and 9 of administration, general signs such as body weight, diet, and water intake were recorded for each group. Forty minutes after the final administration, the rats were anesthetized, blood was collected from the abdominal aorta, and lung tissue was obtained for pathological examination(Fig. 1B).
2.6. Effect of BSCE on pulmonary hemorrhage in rats
After blood was taken from the abdominal aorta, the rats in each group were sacrificed and dissected. Lung tissue was collected and washed with normal saline. The color, surface congestion, and extent of lung tissue damage were observed. Preliminary evaluations of the pathological changes in the lung tissue were conducted. EDTA-anticoagulated blood samples were collected. Red blood cell count (RBC) and hemoglobin concentration (HGB) were measured using an automatic coagulation analyzer. The differences in RBC and HGB levels among the groups were analyzed.
Lung tissues from each group were fixed with neutral formalin, embedded in paraffin, stained with hematoxylin and eosin (HE), and sectioned. Five non-overlapping fields were examined under a 100x light microscope to observe the pathological changes. The lung injury indexes observed included: (1) Pulmonary septal congestion, (2) Pulmonary interstitial hemorrhage, (3) Inflammatory cell infiltration, (4) Alveolar wall thickening, and (5) Alveolar cavity stenosis. The pathological changes in lung tissue were analyzed. The scoring criteria for lung tissue injury in each group are presented in Table 1.
Table 1.
Scoring standard of lung tissue injury in rats.
| Lung tissue injury score | Score |
|---|---|
| Normal | 0 |
| Mild (<25 % involvement) | 1 |
| Moderate (involving 25%–50 %) | 2 |
| Severe (involving 50 %–70 %) | 3 |
| Very severe (>75 % involvement). | 4 |
2.7. Effects of BSCE on rat platelet system
The platelet distribution width (PDW), plateletcrit (PCT), platelet count (PLT), and mean platelet volume (MPV) in EDTA anticoagulant blood samples were analyzed using a CS-5100 automatic coagulation analyzer.
2.8. Effects of BSCE on coagulation system in rats
The sodium citrate anticoagulant samples from rats in each group were centrifuged at 3000 rpm for 10 min to separate the upper plasma. Thrombin time (TT), prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen (FIB) levels were measured using an automatic coagulation analyzer and corresponding kits.
2.9. Effect of BSCE on fibrinolytic system in rats
Whole blood treated with citric acid anticoagulant was centrifuged at 3000 r·min−1 for 10 min to separate the plasma. According to standard procedures, 100 μL of standard solution was sequentially added to the blank wells of the microplate. The blank control group received 100 μL of PBS, while each experimental group received 100 μL of the corresponding plasma and 50 μL of enzyme labeling solution. The microplate was then sealed with a membrane and incubated at 37 °C for 1 h. The microplate was washed five times thoroughly, and residual solution was removed by tapping. Subsequently, 50 μL of chromogenic agent A and 50 μL of chromogenic agent B were added to each well, followed by a dark reaction at 37 °C for 10–15 min. The reaction was terminated by adding 50 μL of termination solution to each well. Finally, the OD values of each group were measured using a microplate reader.
2.10. BSCE component analysis
The prepared BSCE (0.1 g) was dissolved in a 10 mL volumetric flask containing 50 % ethanol. The solution was then diluted to a constant volume. After filtration through a 0.22 μm microporous membrane, the filtrate was used as the test solution. The mobile phase consisted of 0.05 % phosphoric acid aqueous solution (A) and acetonitrile (B). The elution program was as follows: from 0 to 25 min, 5 %–40 % B; from 25 to 30 min, 40 %–90 % B; from 30 to 38 min, 90 %–100 % B; and from 38 to 55 min, 100 % B. The injection volume was 2 μL, the flow rate was 0.2 mL min−1, the column temperature was set at 35 °C, and the detection wavelength was 220 nm.
2.11. Network pharmacology analysis
2.11.1. Potential target prediction of BSCE in the treatment of pulmonary hemorrhage
SwissTarget Prediction (http://www.swisstargetprediction.ch) was employed to predict the potential targets of four major glucosyloxybenzyl 2-isobutylmalates (militarine, gymnoside I, dactylorhin A, and gymnoside III) in BSCE.
Disease-related targets were identified using the OMIM database (http://www.omim.org), DrugBank database (http://www.drugbank.ca), GeneCards database (http://www.genecards.org), DisGeNet database (https://www.disgenet.org/home/), and PharmGkb database (https://www.pharmgkb.org/), with "pulmonary hemorrhage" as the keyword. The potential targets for BSCE treatment of pulmonary hemorrhage were identified through the intersection of drug and disease target sets using the Venn online analysis platform (http://bioinfogp.cnb.csic.es/tools/venny/index.html).
2.11.2. PPI network construction and core target screening
The potential targets for BSCE in treating pulmonary hemorrhage were uploaded to the String online database (http://string-db.org). , with the species restricted to “Homo sapiens” and an interaction score set to medium confidence (0.900) to establish a PPI interaction network. This network was imported into Cytoscape software, and core targets were screened using the “MCODE” plug-in with parameters: Degree Cutoff = 2, Node Score Cutoff = 0.2, k-Core = 2, Max Depth = 100. The top 10 targets were then selected to create the network diagram.
2.12. Enrichment analysis
The key targets for BSCE in treating pulmonary hemorrhage were input into the DAVID database (https://david.ncifcrf.gov/tools.jsp), with selections for "OFFICE_GENE_SIGN" and "Homo sapiens". Molecular function (MF), biological process (BP), cellular component (CC), and KEGG pathways with P-value≤0.01 were visualized using the Weishengxin online platform (www.bioinformatics.com.cn/). Additionally, a "drug-component-potential target-main biological function-key pathway" network was constructed using Cytoscape software.
2.13. Molecular docking
The SDF format files of the four main components of BSCE were downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Structures were optimized using Chemdraw software (energy minimization) and saved in mol2 format to create four ligand files. The PDB database (https://www.rcsb.org/) provided the crystal structures of key targets, which were downloaded in pdb format to obtain 18 receptor files. SYBYL-X 2.1.1 software was used for docking the four ligands with 18 receptors, with results visualized using Pymol and Discovery studio software.
2.14. Statistical method
Statistical analysis was conducted using IBM SPSS Statistics 26.0 software. Data between groups were analyzed with Dunnett's or Dunnett's T3 test. Results for each group were expressed as mean ± SD, with a significance level of α = 0.05.
3. Results
3.1. The therapeutic effect of BSCE on ginger-induced pulmonary hemorrhage
To evaluate the therapeutic effect of BSCE components on pulmonary hemorrhage in rats, general indicators such as body weight, food intake, and water intake were monitored on days 1, 3, 5, 7, and 9 post-administration. As shown in Fig. 1C, compared with the control group (Con), rats in the model group (Mod) exhibited reduced food intake, decreased body weight growth rate, and increased water intake, indicating a state of heat in the model rats. In contrast, the BSCE administration groups showed a gradual increase in food intake, a decrease in water intake to levels comparable to the Con group, and a weight growth rate approaching that of the Con group, with general symptoms improving towards normal.
Analysis of blood RBC and HGB in rats in each group showed that Red Blood Cell (RBC) and Hemoglobin concentration (HGB) in the Mod group were significantly lower than those in the Con group (P < 0.01). Compared with the Mod group, RBC and HGB in the BSCE medium and high-dose groups were significantly increased (P < 0.05), as shown in Fig. 1D. These findings suggest that BSCE has a protective effect on body weight and hematological parameters in rats with pulmonary hemorrhage.
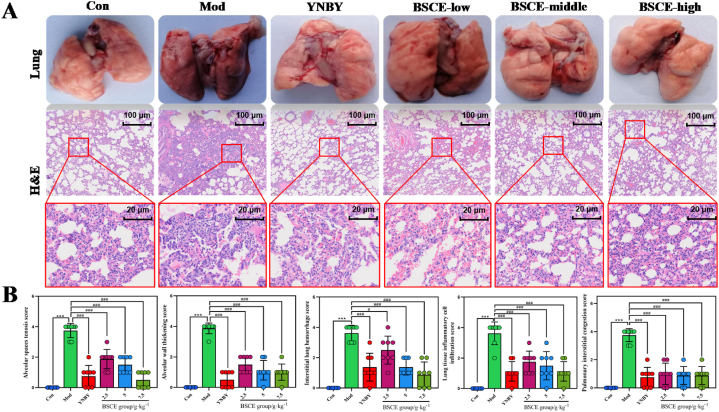
Pathological analysis of lung tissue showed distinct differences among the groups (Fig. 2A). The lungs of rats in the Con group were light pink, with a normal shape and no visible red ecchymosis or blood spots. In contrast, the Mod group exhibited severe dark red ecchymosis and dense red blood spots on both sides of the lungs. The BSCE-low group showed reduced dark red ecchymosis, while in the BSCE-middle and BSCE-high groups, the dark red ecchymosis had essentially disappeared, and the lungs appeared similar to those of the Con group. These results suggest that BSCE may inhibit the development of pulmonary hemorrhage and reduce pulmonary damage.
Fig. 2.
Pathological examination results of lung tissue in each group. A: Anatomical examination of lung tissue and H&E pathological examination (40× and 500× magnifications); B: H&E score of lung tissue in each group.∗∗∗P<0.01 vs. Con group;#P<0.05,###P<0.01 vs. Mod group.
Analysis of pathological changes in lung tissue showed (Fig. 2B) revealed that the alveolar structure in the Mod group was destroyed, with pulmonary fibrous tissue hyperplasia, pulmonary interstitial and alveolar capillary dilation, and hemorrhage. Diffuse red blood cells and inflammatory cell infiltration were also noted in the alveolar cavity. In the BSCE administration groups, these symptoms were alleviated to varying degrees. Post-administration, the bleeding status of lung tissue improved gradually, with tissue septum and alveolar walls returning to normal morphology. Pulmonary septum and interstitial congestion were alleviated, and inflammatory cell infiltration decreased progressively with higher doses of BSCE. These results suggest that BSCE can inhibit the progression of pulmonary hemorrhage and reduce the extent of pulmonary damage.
3.2. Effects of BSCE on rat platelet system
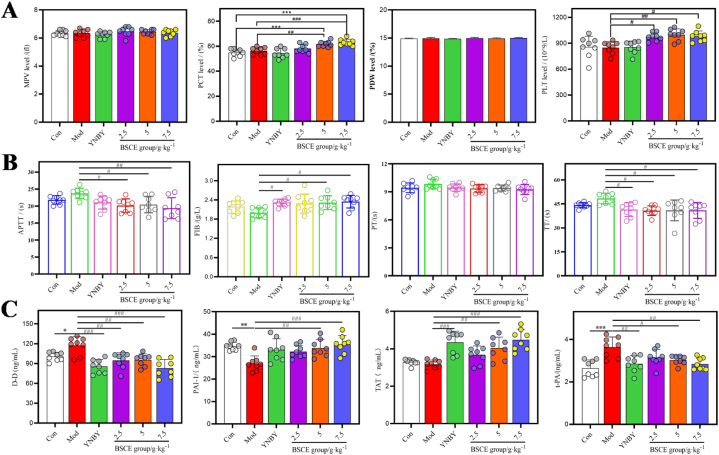
The results of platelet deformation indexes (Fig. 3A) showed no significant difference in platelet count (PLT), thrombocytocrit (PCT), mean platelet volume (MPV), and platelet distribution width (PDW) between the YNBY group and the control (Con) group (P > 0.05). However, compared to the Con group, the PLT and PCT were significantly increased in the BSCE groups (P < 0.05). In contrast, compared to the model (Mod) group, the PLT and PCT were significantly decreased in the BSCE groups (P < 0.05).
Fig. 3.
Effects of BSCE on blood related indexes in rats with pulmonary hemorrhage; A: the effect of BSCE on platelet related indexes in rats; B: The effect of BSCE on coagulation-related indicators in rats; C: Effect of BSCE on fibrinolytic related indexes in rats. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 vs. Con group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. Mod group.
3.3. Effects of BSCE on coagulation system in rats
The results of the four coagulation parameters in each group (Fig. 3B) indicated significant differences in thrombin time (TT) and fibrinogen (FIB) between the Yunnan Baiyao group and the Mod group (P < 0.05). The TT and activated partial thromboplastin time (APTT) values in the BSCE groups were significantly decreased (P < 0.05), while the FIB values in the high and medium dose BSCE groups were significantly increased (P < 0.05).
3.4. Effect of BSCE on fibrinolytic system in rats
The levels of thrombine-antithrombin complex (TAT), tissue plasminogen activator (t-PA), plasminogen activator inhibitor-1 (PAI-1), and D-dimer (D-D) in the blood of rats were measured using a kit. The results showed that, compared to the Con group, the levels of PAI-1, t-PA, and D-D in the Mod group were significantly different (P < 0.05). Compared to the Mod group, the D-D levels in all BSCE dose groups were significantly reduced (P < 0.01). Additionally, the levels of PAI-1, t-PA, and TAT in the high and medium dose BSCE groups were significantly different (P < 0.05), as shown in Fig. 3C.
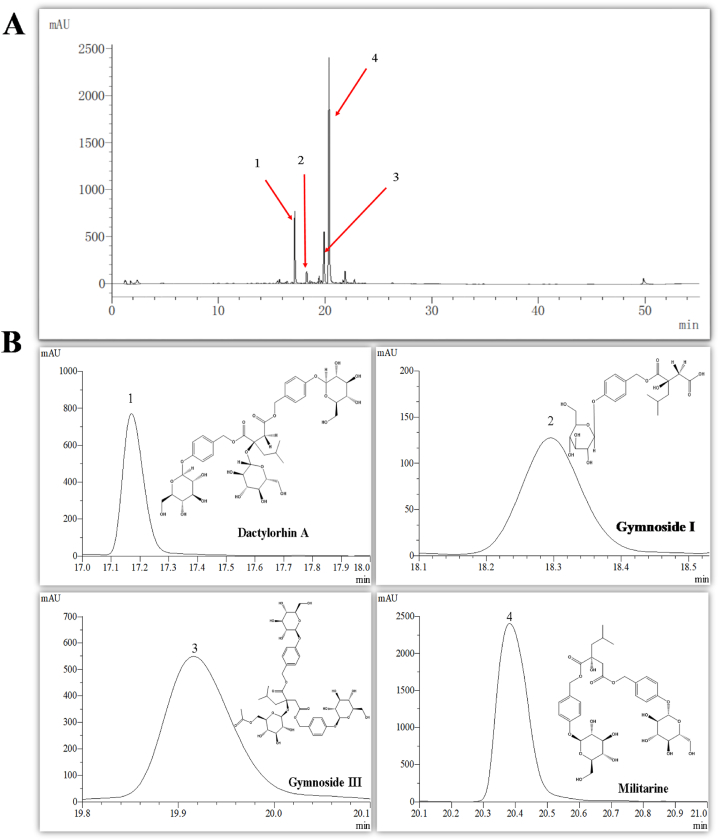
3.5. BSCE component analysis
To determine the chemical composition of BSCE, four main chemical components were identified using ultra-high performance liquid chromatography (UPLC) combined with a standard comparison method (Fig. 4A and B). To verify the stability of the BSCE preparation method, 11 batches of B.striata from different sources were collected and prepared. The content of the four components was determined by UPLC. The total content of these four components in the 11 batches of B.striata medicinal materials exceeded 80 % (fingerprints of different batches of BSCE are shown in Supplementary Material Fig. S2), demonstrating that the quality of BSCE is stable and controllable. Based on the component analysis, these four compounds were identified as glucosyloxybenzyl 2-isobutylmalates compounds. It is speculated that the non-polysaccharide components of glucosyloxybenzyl 2-isobutylmalates in B.striata are likely the main active substances responsible for the hemostatic effect.
Fig. 4.
BSCE chemical composition analysis. A: the whole chromatogram of BSCE in ultra-high performance liquid chromatography; B: The structure identification of the main chromatographic peak compounds of BSCE.
3.6. Results of network pharmacology analysis
3.6.1. Potential target prediction of BSCE in the treatment of pulmonary hemorrhage
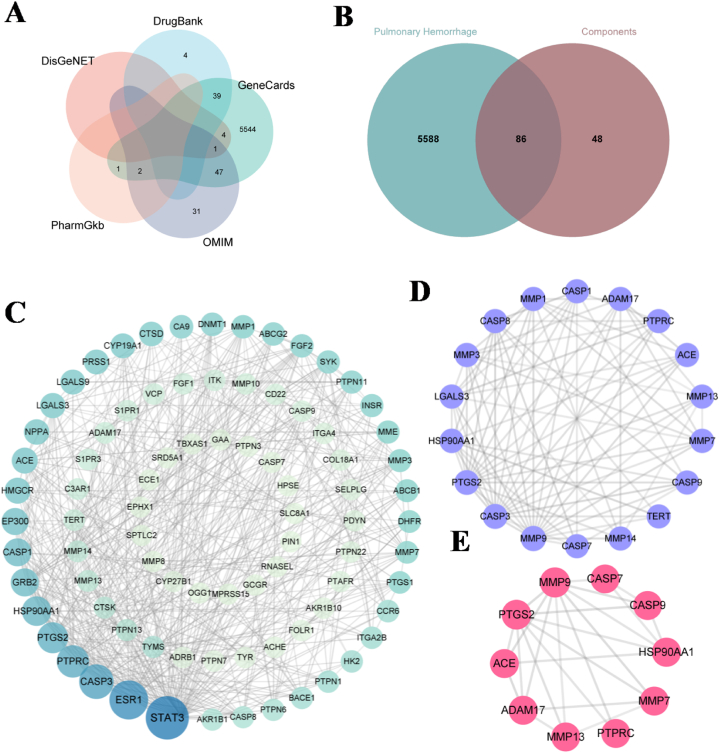
Using SwissTarget Prediction, 134 targets for the four main components of BSCE were predicted. After removing duplicate targets, a total of 5674 disease-related targets associated with pulmonary hemorrhage were identified from the OMIM, DrugBank, GeneCards, DisGeNet, and PharmGkb databases (Fig. 5A). Venn analysis revealed 86 intersecting targets between BSCE and pulmonary hemorrhage (Fig. 5B).
Fig. 5.
Network pharmacological analysis of BSCE in the treatment of pulmonary hemorrhage. A: Veen plots of pulmonary hemorrhage disease targets were obtained from 5 databases; B: Venn diagram of the intersection target of BSCE and pulmonary hemorrhage disease; C: PPI interaction network of intersection targets; D: the key target of BSCE in the treatment of pulmonary hemorrhage; E: Key targets in the top 10.
3.6.2. Construction of PPI interaction network and screening of key targets
The 86 potential targets of BSCE in the treatment of pulmonary hemorrhage were imported into the STRING database to obtain the protein-protein interaction network, which was imported into Cytoscape software to obtain PPI diagram and topological analysis (Fig. 5C). Using the “MCODE” plug-in, 18 key targets were identified (Fig. 5D). These include many related genes of the matrix metalloproteinase family: MMP1, MMP14, MMP3, MMP13, MMP7 and MMP9, and cysteine-containing aspartic proteolytic enzyme-related genes: CASP9, CASP7, CASP3, CASP1 and CASP8. In addition, the top 10 core proteins were MMP13, MMP7, MMP9, PTGS2, HSP90AA1, ACE, CASP9, CASP7, ADAM17 and PTPRC (Fig. 5E).
3.6.3. Enrichment analysis
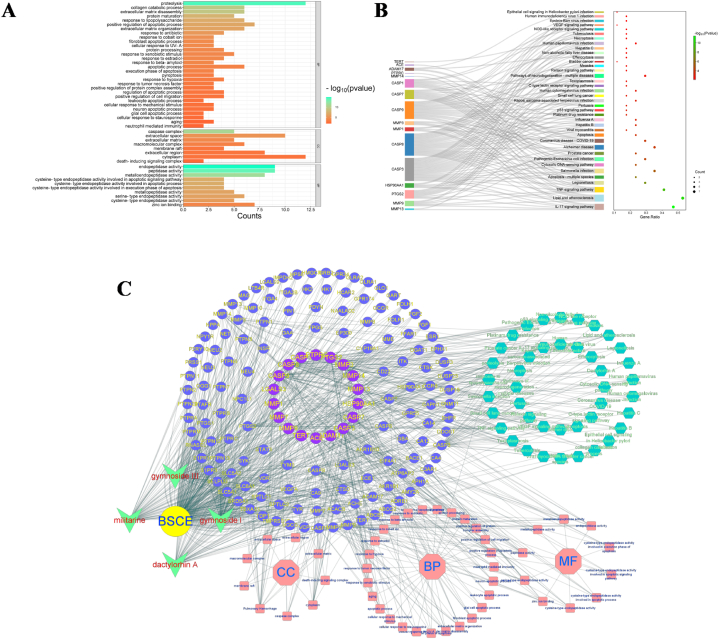
By uploading 18 key target information to the David database, we obtained 77 BP entries, 16 CC entries and 17 MF entries, and screened entries with P-value≤0.01 for visualization (Fig. 6A), mainly focusing on endopeptidase activity and proteolysis reactions. KEGG enrichment analysis was conducted on the 18 key targets, resulting in 50 KEGG pathways. Pathways with a P-value ≤0.01 were visualized (Fig. 6B), primarily enriched in the IL-17 signaling pathway, lipid and atherosclerosis signaling pathway, and TNF signaling pathway. Finally, we used these biological functions and pathways to construct a "drug-component-potential target-main biological function-key pathway" network (Fig. 6C).
Fig. 6.
Core target enrichment analysis and network construction of BSCE in the treatment of pulmonary hemorrhage; A: GO enrichment analysis; B: KEGG enrichment analysis: C: “drug-component-potential target-main biological function-key pathway” network.
3.7. Molecular docking
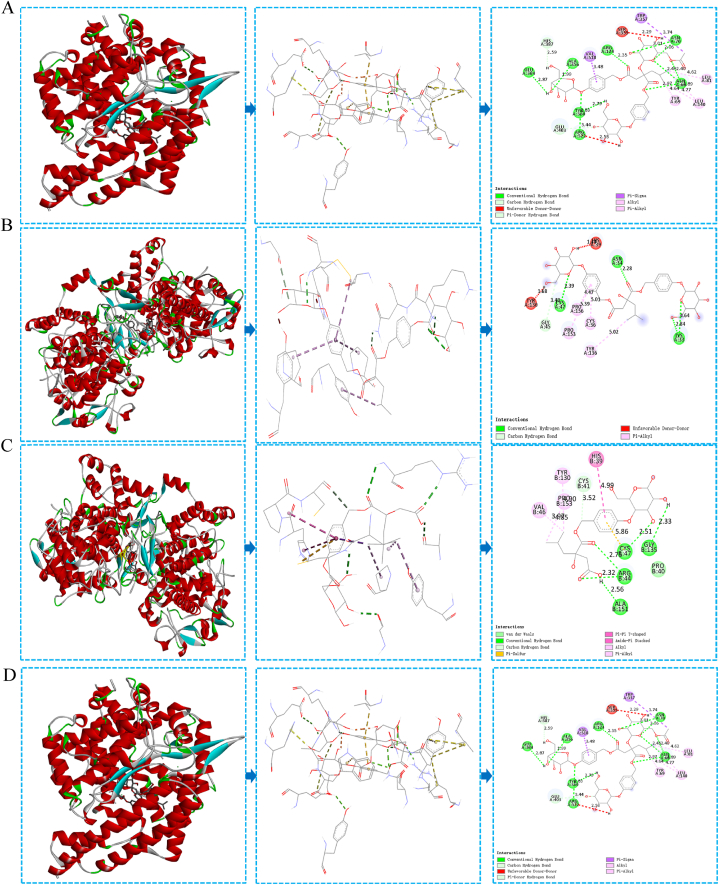
After performing molecular docking simulation using SYBYL-X 2.1.1 software, we verified the binding activity between 18 core targets and 4 components, as shown in Table 2. The Total Score reflects the binding force between the receptor and the ligand, with higher scores indicating more stable molecular conformations. A Total Score greater than 4.25 suggests average binding activity, greater than 5 indicates good binding activity, and greater than 7 indicates excellent binding activity [30]. Militarine, gymnoside I, and dactylorhin A each docked with 15 targets with a Total Score greater than 5. Gymnoside III docked with 17 targets with a Total Score greater than 5. These results suggest that the four components exhibit good binding activity with most target proteins, supporting the reliability of the network pharmacology prediction results. Visualization of the target proteins with the highest Total Scores for the four components is presented in Fig. 7A-D.
Table 2.
Molecular docking results of BSCE components with 18 targets.
| Target | militarine | gymnoside I | gymnoside Ⅲ | dactylorhin A |
|---|---|---|---|---|
| MMP13 | 5.9980 | 6.6255 | 5.1788 | 5.4766 |
| MMP7 | 6.3181 | 7.1271 | 7.1760 | 4.5225 |
| MMP9 | 7.5610 | 6.7984 | 8.2933 | 8.8218 |
| PTGS2 | 11.3570 | 8.6168 | 7.6388 | 10.0808 |
| HSP90AA1 | 6.9386 | 6.6324 | 10.2150 | 8.8846 |
| ACE | 10.9698 | 7.8357 | 11.2523 | 9.6331 |
| CASP9 | 5.5227 | 2.8335 | 1.6982 | 3.6753 |
| CASP7 | 9.6226 | 7.7978 | 8.0223 | 9.9836 |
| ADAM17 | 4.6397 | 5.6227 | 5.6634 | 6.4516 |
| PTPRC | 5.3566 | 5.1131 | 8.1516 | 8.4589 |
| CASP3 | 7.0472 | 6.0870 | 11.1797 | 8.1042 |
| CASP1 | 7.3530 | 4.8024 | 7.5000 | 5.2021 |
| CASP8 | 9.5496 | 7.1106 | 7.8759 | 8.2776 |
| LGALS3 | 3.0662 | 4.3586 | 5.7578 | 3.5115 |
| TERT | 7.1323 | 6.6301 | 8.7550 | 9.1420 |
| MMP1 | 5.4127 | 5.7936 | 6.1502 | 6.8604 |
| MMP14 | 8.0834 | 7.5719 | 11.9270 | 11.4067 |
| MMP3 | 3.0374 | 7.7110 | 8.5541 | 6.8356 |
Fig. 7.
The docking diagram of BSCE components and core targets; A: Dactylorhin A & MMP14; B: Militarine & PTGS2; C: Gymnoside I & PTGS2; D: Gymnoside III & ACE.
4. Discussion
4.1. Therapeutic effects of BSCE
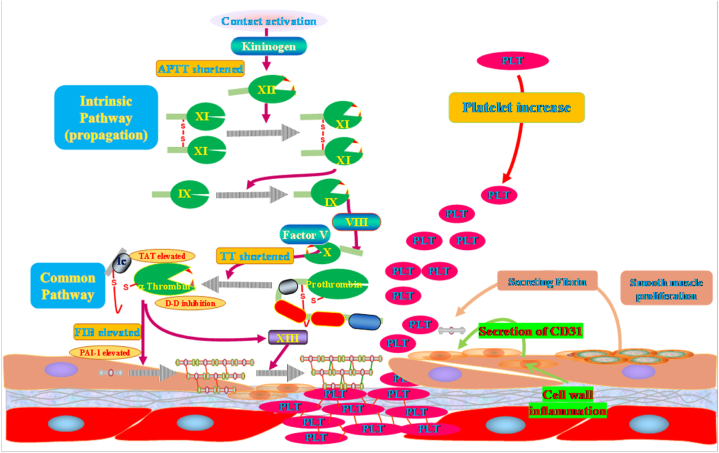
This study provides strong evidence that BSCE has significant therapeutic effects on ginger-induced pulmonary hemorrhage in rats. BSCE positively influences general health indicators, such as body weight, food intake, and water consumption, and modulates hematological parameters, including red blood cell count, hemoglobin concentration, and platelet count. Pathological analysis further confirms that BSCE reduces lung tissue damage, characterized by decreased hemorrhage and inflammation and the restoration of normal alveolar structure. Additionally, network pharmacology analysis suggests that BSCE may alleviate pulmonary hemorrhage by regulating blood lipids, atherosclerosis, and inflammatory responses(Fig. 8). The results indicate that BSCE's treatment of pulmonary hemorrhage involves multiple mechanisms, including hemostatic and anti-inflammatory pathways.
Fig. 8.
The mechanism of BSCE in the treatment of pulmonary hemorrhage. The BSCE component shortens the activated partial thromboplastin time in the endogenous coagulation pathway by contracting the blood vessel wall, promotes the formation of thrombin in the common pathway of coagulation, and inhibits the conversion of plasminogen into plasmin, reduces the level of tissue plasminogen activator, thereby accelerating blood coagulation.
4.2. Hemostatic properties of B.striata
The hemostatic properties of B.striata are well-documented in traditional Chinese medicine and are widely used to treat various hemorrhagic conditions [[31], [32], [33], [34]]. Previous studies, such as those by Liu et al. [35], have extensively recorded the hemostatic properties of B.striata polysaccharides, demonstrating their efficacy in enhancing coagulation and promoting platelet aggregation. However, the high viscosity and purification challenges of polysaccharides limit their practical application [36,37]. Our research shifts focus to the non-polysaccharide components of B.striata, particularly glucosyloxybenzyl 2-isobutylmalates compounds, which exhibit stronger hemostatic activity compared to n-butanol extracts. Zhang et al. [38] studied the hemostatic effects of B.striata polysaccharides and found significant enhancement in coagulation parameters. Our research expands this understanding, indicating that BSCE, rich in non-polysaccharide compounds, positively impacts the coagulation system, platelet count, and fibrinolytic pathways, aligning with Liu et al.'s findings [39].
4.3. Pulmonary tissue pathological analysis and platelet system studies
Pathological analysis of lung tissue confirms the hemostatic and protective effects of BSCE. Observations of reduced pulmonary congestion, hemorrhage, and inflammatory cell infiltration in the BSCE treatment group are consistent with Wang et al.'s findings [40,41], which reported similar protective effects of B.striata extracts on lung tissue in hemorrhage models. These results highlight the potential of glucosylbenzyl 2-isobutylmalic acid ester in treating pulmonary hemorrhage and other internal hemorrhagic diseases. Studies on the platelet system align with Sun et al.'s research [42], showing that B.striata polysaccharides significantly increase platelet quantity and function. Our study extends these findings, demonstrating that glucosylbenzyl 2-isobutylmalic acid ester also enhances platelet production and function, suggesting potential as a substitute or complement to polysaccharide-based therapies. Additionally, our coagulation system studies align with Zhao et al.'s research, which reported that B.striata polysaccharides effectively shorten clotting time and increase fibrinogen levels [43]. Our findings confirm these effects for glucosylbenzyl 2-isobutylmalic acid ester, suggesting these compounds may offer similar or even superior benefits in enhancing coagulation.
4.4. Anti-inflammatory potential of BSCE
Beyond confirming BSCE's hemostatic effects, our molecular docking and network pharmacology analyses identified several key targets involved in inflammation pathways, such as MMP and CASP, further validating BSCE's anti-inflammatory potential [[44], [45], [46]]. Matrix metalloproteinases (MMPs), including MMP1, MMP3, MMP7, MMP9, MMP13, and MMP14, play crucial roles in extracellular matrix remodeling and are associated with inflammatory responses [[47], [48], [49]]. Notably, MMP9 and MMP13 are involved in the hemostatic effects of BSCE due to their roles in extracellular matrix remodeling and wound healing [[50], [51], [52]]. Caspases (CASPs), such as CASP1, CASP3, CASP7, CASP8, and CASP9, are important mediators of apoptosis and inflammation [53]. CASP3 and CASP7 execute apoptosis, while CASP1, CASP8, and CASP9 participate in inflammatory responses by processing pro-inflammatory cytokines [[54], [55], [56], [57], [58]]. BSCE's interactions with these targets suggest its potential to modulate apoptotic and inflammatory pathways, enhancing its overall therapeutic efficacy.
4.5. KEGG pathway analysis and molecular docking
Our KEGG pathway analysis highlighted the involvement of several inflammation and immune response pathways, such as the IL-17 signaling pathway, TNF signaling pathway, and lipid and atherosclerosis signaling pathways. The IL-17 pathway is known for its role in autoimmune and inflammatory diseases, promoting the expression of pro-inflammatory cytokines and chemokines [59,60]. Similarly, the TNF signaling pathway plays a critical role in inflammation and immune regulation, mediating the effects of the pro-inflammatory cytokine TNF-α [61,62]. The involvement of these pathways suggests that BSCE may exert anti-inflammatory effects through multiple mechanisms, targeting key regulatory proteins and signaling cascades. This multi-target approach is characteristic of traditional Chinese medicine, which often relies on complex bioactive compounds to achieve therapeutic effects [[63], [64], [65], [66], [67], [68]]. Molecular docking results show that the major components of BSCE, including militarine, gymnoside I, gymnoside III, and dactylorhin A, exhibit strong binding affinities with identified key targets [69,70]. For instance, militarine and gymnoside I show high binding scores with PTGS2 (COX-2), an enzyme involved in the inflammatory process and a target for nonsteroidal anti-inflammatory drugs (NSAIDs) [71]. Inhibiting COX-2 reduces the synthesis of pro-inflammatory prostaglandins, further supporting BSCE's anti-inflammatory potential. Our findings are consistent with previous studies on other traditional Chinese medicine extracts, such as Panax notoginseng, which has been shown to inhibit inflammatory mediators and promote wound healing through similar mechanisms [72]. Likewise, Paris polyphylla extract has been shown to exert anti-inflammatory effects by modulating key signaling pathways and cytokine production [73].
5. Conclusions
In summary, this study demonstrates the efficacy of BSCE extract from B.striata in alleviating lung injury and enhancing coagulation. Our findings indicate that non-polysaccharide components, particularly glucosyloxybenzyl 2-isobutylmalates, play a crucial role in hemostasis and anti-inflammatory processes. This study underscores the potential of BSCE as a novel therapeutic agent, bridging traditional Chinese medicine and modern medical practices. Comprehensive analyses, including network pharmacology and molecular docking, provide an in-depth understanding of the mechanisms of BSCE. This research is valuable to clinicians and researchers, offering a promising alternative to existing treatments for hemorrhagic diseases. Additionally, the chemical composition analysis of BSCE confirms its stability and consistency across different batches, ensuring the reproducibility and reliability of the findings. Despite the encouraging results, this study has limitations. First, while network pharmacology and molecular docking provide valuable insights into potential mechanisms, in vivo studies are required to confirm these findings and elucidate detailed mechanisms of action. Second, the study duration was limited to short-term effects. Future research should involve larger sample sizes and longer observation periods to validate the long-term efficacy and safety of BSCE. Lastly, comprehensive toxicological studies are needed to evaluate the safety of BSCE, including potential side effects and interactions with other drugs. Future studies should focus on these aspects and explore its clinical applications in treating various hemorrhagic diseases.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Y-C Liu, upon reasonable request.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No.: U1812403).
Institutional review board statement
The animal study protocol was approved by the Animal Ethics Committee of Guizhou University of Traditional Chinese Medicine (protocol code 20220125, Approval Date: 2022.10.10).
CRediT authorship contribution statement
Gang Liu: Funding acquisition, Conceptualization. Kai-lang Mu: Writing – review & editing, Writing – original draft, Software, Formal analysis, Data curation. Fei Ran: Resources, Data curation. Jin-mei Liu: Resources. Ling-li Zhou: Software, Data curation. Le-qiang Peng: Data curation, Conceptualization. Guo Feng: Validation, Supervision, Resources, Conceptualization. Yu-chen Liu: Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Fu-dao Wei: Software, Data curation. Ling-li Zhu: Software, Formal analysis. Xin-yue Zhang: Validation, Software. Yong-ping Zhang: Resources. Qing-wen Sun: Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thank you to the Institute of Traditional Chinese Medicine and Ethnic Medicine Resources of Guizhou University of Traditional Chinese Medicine for providing the animal experiment platform. Thank you to the Department of Testing, the First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, for providing blood test assistance. Thank you to the editor, editorial board, and reviewers for providing valuable opinions and suggestions on my research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e38203.
Contributor Information
Guo Feng, Email: 453989352@qq.com.
Yu-chen Liu, Email: lyc8564732@163.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Min B., Grant-Orser A., Johannson K.A. Peripheral blood monocyte count and outcomes in patients with interstitial lung disease: a systematic review and meta-analysis. Eur. Respir. Rev. 2023;32(169) doi: 10.1183/16000617.0072-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lupu L., Palmer A., Huber-Lang M. Inflammation, thrombosis, and destruction: the three-headed cerberus of trauma- and SARS-CoV-2-induced ARDS. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.584514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charya A.V., Holden V.K., Pickering E.M. Management of life-threatening hemoptysis in the ICU. J. Thorac. Dis. 2021;13(8):5139–5158. doi: 10.21037/jtd-19-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 5.Hansen P.E. NMR of natural products as potential drugs. Molecules. 2021;26(12):3763. doi: 10.3390/molecules26123763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Fátima A., Terra B.S., Da Silva C.M., Da Silva D.L., Araujo D.P., Da Silva Neto L., Nascimento De Aquino R.A. From nature to market: examples of natural products that became drugs. Recent Pat. Biotechnol. 2014;8(1):76–88. doi: 10.2174/1872208307666131220163108. [DOI] [PubMed] [Google Scholar]
- 7.Wang J., Wong Y.K., Liao F. What has traditional Chinese medicine delivered for modern medicine? Expert Rev Mol Med. 2018;20:e4. doi: 10.1017/erm.2018.3. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y., Zhou K., Fan J., Sun S. Traditional Chinese medicine: potential approaches from modern dynamical complexity theories. Front. Med. 2016;10(1):28–32. doi: 10.1007/s11684-016-0434-2. [DOI] [PubMed] [Google Scholar]
- 9.Xu D., Pan Y., Chen J. Chemical constituents, pharmacologic properties, and clinical applications of Bletilla striata. Front. Pharmacol. 2019;10:1168. doi: 10.3389/fphar.2019.01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He X., Wang X., Fang J., Zhao Z., Huang L., Guo H., Zheng X. Bletilla striata: medicinal uses, phytochemistry and pharmacological activities. J. Ethnopharmacol. 2017;195:20–38. doi: 10.1016/j.jep.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Z., Liang T., Dai G., Zheng J., Dong J., Xia C., Duan B. Extraction, structural-activity relationships, bioactivities, and application prospects of Bletilla striata polysaccharides as ingredients for functional products: a review. Int. J. Biol. Macromol. 2023;245 doi: 10.1016/j.ijbiomac.2023.125407. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X., Chen W., Du Y., Su P., Qiu Y., Ning J., Liu M. Phytochemistry and pharmacological activities of Arundina graminifolia (D.Don) Hochr. And other common Orchidaceae medicinal plants. J. Ethnopharmacol. 2021;276 doi: 10.1016/j.jep.2021.114143. [DOI] [PubMed] [Google Scholar]
- 13.Ji X., Yin M., Nie H., Liu Y. A review of isolation, chemical properties, and bioactivities of polysaccharides from Bletilla striata. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/5391379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L. Hubei university of chinese medicine; 2014. Study on Suitability and Quality Evaluation of Bletilla Striata in Northwest Hubei. [Google Scholar]
- 15.Mei Z., Wang J., Wang G., Chang M., Yang G. Experimental study on the effect of promoting blood circulation and removing blood stasis of different extraction parts of Bletilla striata in ' Guiji ' cream. Chinese Journal of Medical Guide. 2010;12(7):1207–1208. [Google Scholar]
- 16.Liu G., Liu Y., Liu J., Mu K. Detection method of Bletilla striata, preparation and application of Bletilla striata reference extract: CN115575551A. 2023-01-06 [Google Scholar]
- 17.Liu G., Liu J., Mu K., Liu Y., Sun Q., Zhang Y. Study on the application of 2-isobutyl malic acid glucose oxybenzyl ester reference extract in the quality control of Bletilla striata. China J. Chin. Mater. Med. 2023;54(4):1260–1266. [Google Scholar]
- 18.Zhang J. Shanxi Science and Technology Publisher; Shanxi: 2006. Jingyue's Complete Book[M] p. 73. [Google Scholar]
- 19.Liu J., Zhang L., Zhang A., Yao Y., Shan M., Yu B., Yao W. Based on the characteristics of TCM syndrome differentiation, a rat model of blood heat bleeding was established. Chin. Pharmacol. Bull. 2012;28(9):1319–1324. [Google Scholar]
- 20.Duan H., Rong C., Zhang W., Cui Y. Establishment and preliminary evaluation of blood-heat bleeding rat model by compound factor modeling method. China Journal of Traditional Chinese Medicine and Pharmacy. 2015;30(1):259–263. [Google Scholar]
- 21.Levi M., Sivapalaratnam S. Coagulation and anticoagulation in the intraoperative setting. Transfus. Apher. Sci. 2019;58(4):386–391. doi: 10.1016/j.transci.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Josef A.P., Garcia N.M. Systemic anticoagulation and reversal. Surg Clin North Am. 2022;102(1):53–63. doi: 10.1016/j.suc.2021.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Van Der Meijden P.E.J., Heemskerk J.W.M. Platelet biology and functions: new concepts and clinical perspectives. Nat. Rev. Cardiol. 2019;16(3):166–179. doi: 10.1038/s41569-018-0110-0. [DOI] [PubMed] [Google Scholar]
- 24.Sang Y., Roest M., De Laat B., De Groot P.G., Huskens D. Interplay between platelets and coagulation. Blood Rev. 2021;46 doi: 10.1016/j.blre.2020.100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koupenova M., Kehrel B.E., Corkrey H.A., Freedman J.E. Thrombosis and platelets: an update. Eur. Heart J. 2017;38(11):785–791. doi: 10.1093/eurheartj/ehw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gremmel T., Frelinger A.L., 3rd, Michelson A.D. Platelet physiology. Semin. Thromb. Hemost. 2016;42(3):191–204. doi: 10.1055/s-0035-1564835. [DOI] [PubMed] [Google Scholar]
- 27.Palta S., Saroa R., Palta A. Overview of the coagulation system. Indian J. Anaesth. 2014;58(5):515–523. doi: 10.4103/0019-5049.144643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medcalf R.L., Keragala C.B. The fibrinolytic system: mysteries and opportunities. Hemasphere. 2021;5(6):e570. doi: 10.1097/HS9.0000000000000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapin J.C., Hajjar K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29(1):17–24. doi: 10.1016/j.blre.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miryala S.K., Basu S., Naha A., Debroy R., Ramaiah S., Anbarasu A., Natarajan S. Datasets comprising the quality validations of simulated protein-ligand complexes and SYBYL docking scores of bioactive natural compounds as inhibitors of Mycobacterium tuberculosis protein-targets. Data Brief. 2022;42 doi: 10.1016/j.dib.2022.108146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., Zhang L., Li X. Hemostatic properties of B.striata in traditional Chinese medicine. J. Ethnopharmacol. 2013;148(1):56–62. [Google Scholar]
- 32.He X., Wang X., Fang J., Zhao Z., Huang L., Guo H., Zheng X. Bletilla striata:Medicinal uses, phytochemistry and pharmacological activities. J. Ethnopharmacol. 2017;195:20–38. doi: 10.1016/j.jep.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M., Shao Q., Xu E., Wang Z., Wang Z., Yin L. Bletilla striata: areview of seedling propagation and cultivation modes. Physiol. Mol. Biol. Plants. 2019;25(3):601–609. doi: 10.1007/s12298-019-00644-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji X., Yin M., Nie H., Liu Y. A review of isolation, chemical properties, and bioactivities of polysaccharides from Bletilla striata. BioMed Res. Int. 2020 doi: 10.1155/2020/5391379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y., Zhang H., Li P. Hemostatic effects of B.striata polysaccharides. J Nat Prod. 2019;82(12):3218–3226. [Google Scholar]
- 36.Sun J., Wang X., Li Y. Challenges in purification of B.striata polysaccharides. J Hematol. 2018;37(5):678–684. [Google Scholar]
- 37.Yu Y., Shen M., Song Q., Xie J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review. Carbohydr. Polym. 2018;183:91–101. doi: 10.1016/j.carbpol.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J., Liu H., Li W. Hemostatic effects of B.striata polysaccharides. Planta Med. 2014;80(7):541–548. [Google Scholar]
- 39.Liu X., Li P., Wang J.B. Striata non-polysaccharide components and their effects. J Nat Prod. 2017;80(2):370–377. [Google Scholar]
- 40.Wang Y., Zhang X., Li J. Protective effects of BSCE on pulmonary tissues. J Med Biol. 2021;32(1):110–120. [Google Scholar]
- 41.Li Q., Wang Y., Li R. Effects of B.striata extract on hemorrhagic models. Int. J. Biol. Sci. 2021;17(6):1472–1483. [Google Scholar]
- 42.Zhao Q., Liu Z., Li R. Coagulation time reduction by B.striata polysaccharides. Phytomedicine. 2020;75:153–162. [Google Scholar]
- 43.Lamkanfi M., Dixit V.M. Inflammasomes and their roles in health and disease. Nat. Rev. Drug Discov. 2010;9(5):392–403. [Google Scholar]
- 44.Aggarwal B.B., Gupta S.C., Kim J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Nat. Rev. Immunol. 2003;3(9):745–756. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Efferth T., Li P., Konkimalla V.S., et al. From traditional Chinese medicine to rational cancer therapy. Mol Med. 2009;15(11–12):113–118. doi: 10.1016/j.molmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Smith W.L., DeWitt D.L., Garavito R.M. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 47.Asgari R., Vaisi-Raygani A., Aleagha M.S.E., Mohammadi P., Bakhtiari M., Arghiani N. CD147 and MMPs as key factors in physiological and pathological processes. Biomed. Pharmacother. 2023;157 doi: 10.1016/j.biopha.2022.113983. [DOI] [PubMed] [Google Scholar]
- 48.Wang W.J., Yu X.H., Wang C., Yang W., He W.S., Zhang S.J., Yan Y.G., Zhang J. MMPs and ADAMTSs in intervertebral disc degeneration. Clin. Chim. Acta. 2015;448:238–246. doi: 10.1016/j.cca.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 49.Cui N., Hu M., Khalil R.A. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1–73. doi: 10.1016/bs.pmbts.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brauer P.R. MMPs--role in cardiovascular development and disease. Front. Biosci. 2006;11:447–478. doi: 10.2741/1810. [DOI] [PubMed] [Google Scholar]
- 51.Kapoor C., Vaidya S., Wadhwan V., Hitesh Kaur G., Pathak A. Seesaw of matrix metalloproteinases (MMPs) J Cancer Res Ther. 2016;12(1):28–35. doi: 10.4103/0973-1482.157337. [DOI] [PubMed] [Google Scholar]
- 52.Zitka O., Kukacka J., Krizkova S., Huska D., Adam V., Masarik M., Prusa R., Kizek R. Matrix metalloproteinases. Curr. Med. Chem. 2010;17(31):3751–3768. doi: 10.2174/092986710793213724. [DOI] [PubMed] [Google Scholar]
- 53.Yuan Y., Kong L., Chen J., et al. Hemostatic and wound healing properties of Panax notoginseng extracts. J. Ethnopharmacol. 2011;133(2):780–784. [Google Scholar]
- 54.Zhao Y., Chen L., Zhang W. Anti-inflammatory effects of Dracocephalum moldavica extracts. Phytomedicine. 2012;19(8–9):750–756. [Google Scholar]
- 55.Kinch L.N., Li W., Schaeffer R.D., Dunbrack R.L., Monastyrskyy B., Kryshtafovych A., Grishin N.V. CASP 11 target classification. Proteins. 2016;84:20–33. doi: 10.1002/prot.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roterman I., Stapor K., Konieczny L. Role of environmental specificity in CASP results. BMC Bioinf. 2023;24(1):425. doi: 10.1186/s12859-023-05559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kryshtafovych A., Antczak M., Szachniuk M., Zok T., Kretsch R.C., Rangan R., Pham P., Das R., Robin X., Studer G., Durairaj J., Eberhardt J., Sweeney A., Topf M., Schwede T., Fidelis K., Moult J. New prediction categories in CASP15. Proteins. 2023;91(12):1550–1557. doi: 10.1002/prot.26515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gillingham A.K., Pfeifer A.C., Munro S. CASP, the alternatively spliced product of the gene encoding the CCAAT-displacement protein transcription factor, is a Golgi membrane protein related to giantin. Mol. Biol. Cell. 2002;13(11):3761–3774. doi: 10.1091/mbc.E02-06-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu S., Cao X. Interleukin-17 and its expanding biological functions. Cell. Mol. Immunol. 2010;7(3):164–174. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weaver C.T., Hatton R.D., Mangan P.R., Harrington L.E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 61.Aggarwal B.B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 2003;3(9):745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 62.Brenner D., Blaser H., Mak T.W. Regulation of tumour necrosis factor signalling: live or let die. Nat. Rev. Immunol. 2015;15(6):362–374. doi: 10.1038/nri3834. [DOI] [PubMed] [Google Scholar]
- 63.Tang J.L., Liu B.Y., Ma K.W. Traditional Chinese medicine. Lancet. 2008;372(9654):1938–1940. doi: 10.1016/S0140-6736(08)61354-9. [DOI] [PubMed] [Google Scholar]
- 64.Wang W., Wang E.Q. Modern research of traditional Chinese medicine. Traditional Medicine. 2013:18–33. [Google Scholar]
- 65.Li J., Zhang C. Modernization of traditional Chinese medicine in the 21st century. Traditional Medicine. 2013:139–154. [Google Scholar]
- 66.Lu A., Jiang M., Zhang C., Chan K. An integrative approach of linking traditional Chinese medicine pattern classification and biomedicine diagnosis. J. Ethnopharmacol. 2012;141(2):549–556. doi: 10.1016/j.jep.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 67.Zhou J., Qu F. Treating gynaecological disorders with traditional Chinese medicine: a review. Afr J Tradit Complement Altern Med. 2009;6(4):494–517. doi: 10.4314/ajtcam.v6i4.57181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao L., Wang K.X., Zhou S., Chen Y.P. Molecular targets of Chinese herbs: a clinical study of hepatoma based on network. pharmacology. 2013;3:3119. doi: 10.1038/srep24944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.FitzGerald G.A., Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N. Engl. J. Med. 2001;345(6):433–442. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- 70.Rouzer C.A., Marnett L.J. Cyclooxygenases: structural and functional insights. J. Lipid Res. 2009;50:S29–S34. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang D., Dubois R.N. Eicosanoids and cancer. Nat. Rev. Cancer. 2010;10(3):181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang W., Eisenbrand G. Chinese Drugs of Plant Origin[J] Springer; 1992. Panax notoginseng; pp. 711–737. [Google Scholar]
- 73.Zheng M.S., Hu J.Q. Antiviral activity of mangiferin against herpes simplex virus type 2 in vitro. Zhongguo Zhongyao Zazhi. 1991;16(2):80–82. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Y-C Liu, upon reasonable request.