Abstract
Macrophages respond to virus infections by rapidly secreting proinflammatory cytokines, which play an important role in the first line of defense. Tumor necrosis factor alpha (TNF-α) is one of the major macrophage-produced cytokines. In this study we have investigated the virus-cell interactions responsible for induction of TNF-α expression in herpes simplex virus (HSV)-infected macrophages. Both HSV type 1 (HSV-1) and HSV-2 induced TNF-α expression in macrophages activated with gamma interferon (IFN-γ). This induction was to some extent sensitive to UV treatment of the virus. Virus particles unable to enter the cells displayed reduced capacity to stimulate TNF-α expression but retained a significant portion which was abolished by HSV-specific antibodies. Recombinant HSV-1 glycoprotein D was able to trigger TNF-α secretion in concert with IFN-γ. Sugar moieties of HSV glycoproteins have been reported to be involved in induction of IFN-α but did not contribute to TNF-α expression in macrophages. Moreover, the entry-dependent portion of the TNF-α induction was investigated with HSV-1 mutants and found to be independent of the tegument proteins VP16 and UL13 and partly dependent on nuclear translocation of the viral DNA. Finally, we found that macrophages expressing an inactive mutant of the double-stranded RNA (dsRNA)-activated protein kinase (PKR) produced less TNF-α in response to infectious HSV infection than the empty-vector control cell line but displayed the same responsiveness to UV-inactivated virus. These results indicate that HSV induces TNF-α expression in macrophages through mechanisms involving (i) viral glycoproteins, (ii) early postentry events occurring prior to nuclear translocation of viral DNA, and (iii) viral dsRNA-PKR.
Herpes simplex virus (HSV) is an enveloped DNA virus that infects through contact with mucosal membranes. Following the initial HSV replication in epithelial cells, the virus is transported to the ganglia where a latent infection is established (31). A primary HSV infection is normally associated with a vigorous host response, including production of a range of cytokines and chemokines (26, 34, 36). Cytokines play an important role in the immune response to HSV infections. In particular, tumor necrosis factor alpha (TNF-α), which is primarily produced by macrophages, is known to be central for control of virus replication (13, 27). However, TNF-α and TNF-α-induced products are also involved in the immunopathology often associated with HSV infections (2, 10).
The virus-derived entities responsible for induction of TNF-α expression have been characterized for a number of viruses (reviewed in reference 22). For instance, the virion surface proteins gp350 and gp120 of Epstein-Barr virus and human immunodeficiency virus type 1 (HIV-1), respectively, stimulate expression of TNF-α (3, 8). Apart from surface glycoproteins other viral proteins primarily with an intracellular location such as the hepatitis B virus (HBV) protein X (HBx), the human T lymphotropic leukemia virus type 1 Tax protein and the HIV-1 Tat protein directly interact with the intracellular signaling machinery, thus leading to TNF-α expression (7, 15). Some viruses are endowed with several components able to stimulate production of specific cytokines. For instance, HIV-1 encodes four different proteins able to induce interleukin 6 (IL-6) expression (3, 24, 29, 33), and the two HBV proteins HBx and HBV core antigen both trigger expression of TNF-α (15, 37). Hence, induction of a specific cytokine by virus infection may involve several viral components. For HSV little is known about the viral factors that bring about cytokine production. One study has demonstrated that the glycoprotein D (gD), which is responsible for interaction with the herpes virus entry mediators A, B, and C, is capable of stimulating alpha interferon (IFN-α) expression (4). Others showed that infection of a permissive murine epithelial cell line with HSV-1 triggered IL-6 secretion, which was sensitive to UV inactivation of the virus (12). Studies from our laboratory have shown that the ability of HSV-2 to induce secretion of the IL-12/IL-23 subunit p40 in murine macrophages is also sensitive to UV and occurs through a mechanism involving the transcription factor nuclear factor κB (17).
In this study we have investigated the mechanisms of TNF-α induction by HSV in macrophages. We show that HSV-1 and HSV-2 induce modest production of TNF-α in resting macrophages and strongly stimulate TNF-α production in IFN-γ-treated macrophages. Our data suggest that the mechanisms involved include interaction of gD with a cellular receptor, early postentry events, and activation of the double-stranded RNA (dsRNA)-activated protein kinase by viral RNA.
MATERIALS AND METHODS
Reagents.
The recombinant murine cytokines used were TNF-α (Genzyme) and IFN-γ (PharMingen). Recombinant HSV-1 gD and HSV-2 gG were obtained from Viral Therapeutics and kindly provided by Sytske Welling-Wester, respectively. The antibodies used were murine monoclonal anti-gD antibody (Virusys), neutralizing polyclonal rabbit anti-mouse TNF-α antibody (Genzyme), horseradish peroxidase (HRP)-conjugated rabbit polyclonal anti-mouse immunoglobulin antibody (Transduction Laboratories), human anti-HSV antibodies (Wellcome Diagnostics), and human immunoglobulin (The Danish State Serum Institute). LumiGLO was purchased at New England BioLabs and the polyvinylidene difluoride membranes were from Novex. Other reagents used were heparin (Leo Pharmacies), N-acetylglucosamine (Sigma), d-mannose (Sigma), G418 (Roche), actinomycin D (Calbiochem), and PNGase (New England Biolabs).
Cell culture and virus infection.
The murine macrophage-like cell line RAW 264.7 was maintained in Dulbecco's modified essential medium with 1% Glutamax I (Life Technologies) supplemented with 200 IU of penicillin per ml, 200 μg of streptomycin per ml, and 5% fetal calf serum (FCS). For experiments the cells were seeded in either 24- or 6-well tissue culture plates at a density of 6 × 105 and 2 × 106 cells/well, respectively, and left 16 to 20 h before further treatment. RAW-pBK-CMV and RAW-PKR-M7 cells (a gift from John A. Corbett) were grown as above except that 200 μg of G418 per ml was added. RAW-pBK-CMV and RAW-PKR-M7 cells are RAW 264.7-derived cell lines stably transfected with pBK and the protein kinase (PKR) mutant M7, respectively (16). Vero and 79VB4 cells were maintained in minimal essential medium supplemented with antibiotics and FCS as above. 79VB4 cells (from Patricia G. Spear), which is a Vero-derived cell line stably expressing gL, were further grown in the presence of 200 μg of G418 per ml. For virus amplification, Vero and 79VB4 cells were seeded at a density of 20 × 106 cells per 175-cm2 tissue culture flask 5 h prior to infection. For plaque assays, the cells were seeded at a density of 2 × 106 cells per 22.5-cm2 tissue culture plate.
The wild-type viruses used in this study were the MS strain of HSV-2 and the HSV-1 strains HFEM, F, KOS, and 17+. gL86 (from Patricia G. Spear) is a gL mutant derived from KOS (23). The VP16 mutant in1814 and rescued virus in1814R (from Chris M. Preston) are on a 17+ genetic back ground (1). The temperature-sensitive mutant tsB7 is derived from HFEM (6), and R7356 is produced from the F strain (28). Both viruses were kindly donated by Bernard Roizman. The virus and mock preparations were produced as previously described (9). The virus infectivity titers were determined by plaque assay in Vero cells. For gL86, virus was quantified by Western blotting using a mouse monoclonal anti-gD antibody for detection. Just before usage, virus was thawed and used as infectious virus, subjected to heat inactivation at 56°C for 30 min, or inactivated by UV light for 20 min. For formaldehyde inactivation, virus was incubated 24 h at 4°C with 3.5% formaldehyde or phosphate-buffered saline (PBS) control before being added to the cells. To deglycosylate the virus, 3 × 106 PFU of virus in PBS were incubated with 1,000 U of PNGase at 37°C for 3 h.
Plaque assay.
Vero cells were seeded and left overnight to settle. Infection was performed by incubating the cells with 100 μl of 10-fold virus dilutions plus 400 μl of medium. The tissue culture dishes were rocked every 15 min to ensure even distribution of the virus. After 1 h, 8 ml of preheated minimal essential medium supplemented with 2% FCS and 0.02% human immunoglobulin was added, and the cells were incubated for 2 to 6 days, depending on the virus strain. The cells were stained with 0.03% methylene blue, and the numbers of plaques were determined.
Experimental procedures.
For examination of TNF-α production, RAW 264.7 cells were seeded at a density of 6 × 105 cells in 2-cm2 wells in a volume of 1 ml and left overnight to settle. The cells were treated with 100 IU of IFN-γ per ml and infected with 3 × 105 PFU of HSV per ml or an equivalent amount of mock virus preparation and incubated at 37°C. Supernatants were harvested after the appropriate period of incubation (as indicated in the individual experiments). Prior to measurement of TNF-α, infectious virus in the supernatants were inactivated by UV irradiation for 20 min. TNF-α activity was measured by a bioassay (see below). To test the influences of heparin, anti-HSV antibodies, d-mannose, and N-acetylglycosamine on the ability of HSV to induce TNF-α expression, the substances were given to the cells immediately prior to addition of virus to the cultures. Formaldehyde-inactivated virus was diluted 300 times to reach a final concentration equivalent to 3 ×105 PFU of virus per ml in the cell cultures. This led to a final formaldehyde concentration of 0.01%, which did not cause any overt toxic effects on the cells.
TNF-α bioassay.
Measurement of TNF-α bioactivity was performed with the L929 cell-based bioassay. L929 cells were seeded (2 × 104 cells/well in 96-well culture plates) and left for 16 to 24 h at 37°C in a humidified atmosphere with 5% CO2. The culture supernatants were removed and substituted with the samples to be assayed for TNF-α content in successive twofold dilutions and incubated at 38.5°C with 1 μg of actinomycin D per ml for 18 h. Cells were fixed in 10% formaldehyde and stained with crystal violet (1 mg/ml). Measurement of light absorbance at 580 nm and comparison with a TNF-α standard dilution series allowed assessment of TNF-α activity. The bioassay was specific for TNF-α since the activity was neutralized with a neutralizing antibody against TNF-α.
Western blotting.
Virus particles and recombinant proteins were denatured in sample buffer (140 mM Tris-HCl [pH 8.5], 10% glycerol, 2% sodium dodecyl sulfate [SDS], 1 mM EDTA, 0.019% Serva Blue G250, 0.06% phenol red) and subjected to SDS-polyacrylamide gel electrophoresis (PAGE) followed by blotting onto a polyvinylidene difluoride membrane and blocking for 1 h in Tris-buffered saline (10 mM Tris, 140 mM NaCl) supplemented with 0.05% Tween 20 and 5% skim milk powder. The anti-gD antibody was added for overnight incubation at 4°C. The membrane was washed 4 × 10 min in washing buffer (Tris-buffered saline plus 0.05% Tween 20) and incubated for 1 h at room temperature with a polyclonal HRP-conjugated antibody against mouse immunoglobulin. The membrane was washed as above and the HRP-conjugated antibody was visualized using enhanced chemiluminescence.
Statistical analysis.
The data shown are means ± standard errors of the means. Statistical significance was estimated with Student's t test for unpaired observations. TNF-α concentrations from repeated experiments were pooled and used for the calculations in order to obtain the largest number of observations available for evaluation of statistical significance. P values of <0.05 were considered significant.
RESULTS
HSV-1 and HSV-2 induce secretion of TNF-α in IFN-γ-treated macrophages.
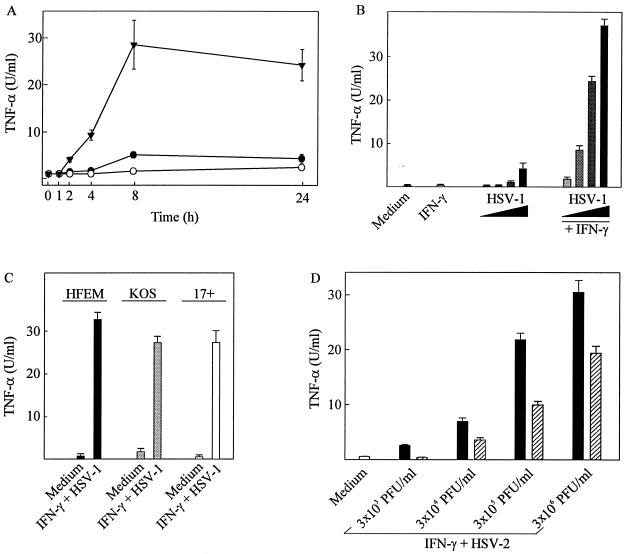
We have previously shown that HSV-2 infection of macrophages induces secretion of TNF-α (9) and that IFN-γ synergistically enhances the response (26). The data in Fig. 1A confirm these findings and show that TNF-α production is detectable in the culture supernatants of the macrophage-like cell line RAW 264.7 already 2 h after infection and reaches a maximum after 8 h. Mock infection did not induce appreciable amounts of TNF-α in resting or IFN-γ-treated macrophages (data not shown). HSV-1 was also able to induce TNF-α expression in macrophages through cooperation with IFN-γ (Fig. 1B). Moreover, by using three HSV-1 strains with different degrees of virulence (HFEM < KOS < 17+) we found that the capacity to induce TNF-α did not correlate with virus virulence (Fig. 1C).
FIG. 1.
TNF-α production in resting and IFN-γ-treated macrophages infected with infectious and UV-inactivated HSV. (A) RAW 264.7 cells were treated with 100 IU of IFN-γ per ml and infected with 3 × 105 PFU (multiplicity of infection [MOI], 0.6) of HSV-2 per ml. After the indicated time points posttreatment, supernatants were harvested and analyzed for TNF-α bioactivity. ○, IFN-γ; ●, HSV-2; ▾, IFN-γ + HSV-2. (B) RAW 264.7 cells were treated with 100 IU of IFN-γ per ml and infected with the following amounts of HSV-1 (KOS): 3 × 103 PFU/ml (MOI, 0.006), 3 × 104 PFU/ml (MOI, 0.06), 3 × 105 PFU/ml (MOI, 0.6), and 3 × 106 PFU/ml (MOI, 6). (C) RAW 264.7 cells were treated with 100 IU of IFN-γ per ml and 3 × 105 PFU (MOI, 0.6) of the three HSV-1 strains HFEM, KOS, and 17+ per ml. (D) RAW 264.7 cells were treated with 100 IU of IFN-γ per ml and infected with 3 × 105 PFU (MOI, 0.6) of HSV-2 (black bars) or an equivalent amount of UV-inactivated virus (hatched bars) per ml. For panels B, C and D, supernatants were harvested 8 h postinfection and analyzed for TNF-α bioactivity. Results are shown as means of two cultures ± standard errors of the means. Similar results were obtained in at least three independent experiments.
In separate experiments it has been observed that treatment of RAW 264.7 macrophages with HSV-2 does lead to viral entry and cellular accumulation of immediate-early, early, and late transcripts and proteins (J. Melchjorsen and S. R. Paludan, unpublished data). To assess the requirement for a functional viral genome in TNF-α induction, we tested the ability of UV-irradiated virus to induce TNF-α secretion. The results show that although such treatment significantly reduced the TNF-α-inducing capacity of the virus, UV irradiation was unable to abolish HSV-2-induced TNF-α production (Fig. 1D). In experiments not presented here we further found that induction of TNF-α by UV-inactivated virus followed the same kinetics as infectious HSV-2. Similar results were obtained in murine peritoneal cells with both HSV-1 and HSV-2 (data not shown). The remaining potential to bring about TNF-α secretion was not due to incomplete inactivation of the virus since UV-treated virus was unable to induce immediate-early viral gene transcription and to replicate in Vero cells (data not shown).
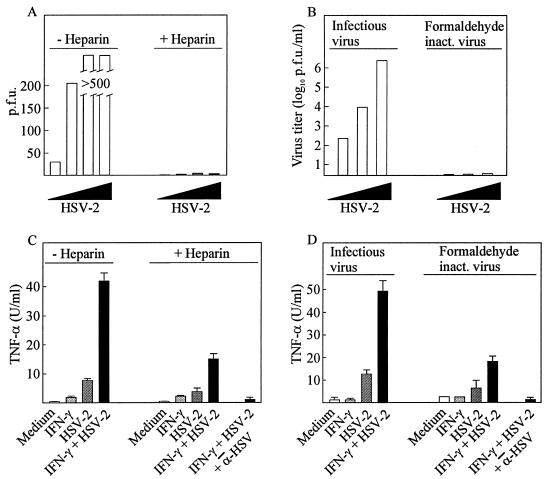
Inhibition of viral entry reduces—but does not abolish—the ability of HSV to induce TNF-α secretion.
The initial step in HSV infections is the interaction between the viral glycoprotein gC and heparan sulfates on the cell surface. Consistently, free heparin has been shown to prevent viral entry presumably by engaging gC molecules on the virus particle (11). In agreement with this we observed that the presence of 100 μg of heparin per ml in the growth medium totally prevented 3 × 105 PFU of HSV-2 from forming plaques in Vero cells (Fig. 2A). Thus, in the presence of saturating amounts of heparin, the interaction between gC and heparan sulfates cannot occur while other viral glycoproteins may still interact with cellular surface proteins. At the level of TNF-α production we found that although heparin reduced the ability of HSV-2 to induce TNF-α secretion by more than 70%, a significant proportion of the TNF-α-inducing potential was retained (Fig. 2C). As in the absence of heparin, a strong synergy between the virus and IFN-γ was observed. The remaining TNF-α-inducing potential of the virus could not be ascribed to nonspecific effects since it was totally inhibited by anti-HSV antibodies. In contrast to TNF-α, induction of interleukin 12 (IL-12) p40 and IL-6 by HSV-2 was fully inhibited by heparin (data not shown).
FIG. 2.
Effects of heparin and formaldehyde inactivation on viral replication and TNF-α production. (A) Confluent Vero cells were infected with increasing amounts of HSV-2 (1 × 101, 3 × 102, 1 × 104, and 3 × 105 PFU) in the presence and absence of 100 μg of heparin per ml. After 48 h of infection, the cells were stained and plaques were counted. (B) HSV-2 was inactivated by incubation with 3.5% formaldehyde for 24 h at 4°C or, as a control, treated with PBS. The virus was titrated on confluent Vero cells. After 48 h of infection, the cells were stained and plaques were counted. (C) RAW 264.7 cells were treated with 100 IU of IFN-γ per ml and infected with 3 × 105 PFU (MOI, 0.6) of HSV-2 per ml in the presence and absence of 100 μg of heparin per ml. (D) RAW 264.7 cells were treated with 100 IU of IFN-γ per ml and infected with 3 × 105 PFU (MOI, 0.6) of HSV-2 per ml or equal amounts of formaldehyde-inactivated virus. Supernatants were harvested 8 h later and analyzed for TNF-α bioactivity. Results are shown as means of two cultures ± standard errors of the means. Similar results were obtained in two independent experiments.
We also examined the TNF-α-inducing potential of formaldehyde-treated virus, which can bind to but not fuse with the cellular membrane. Although formaldehyde treatment completely inactivated the virus, as assessed by replication in Vero cells (Fig. 2B), it did not entirely prevent TNF-α production (Fig. 2D). Approximately 30% of the full TNF-α-inducing capacity was conserved, and this portion could be inhibited if the cells treated with the inactivated virus also received anti-HSV antibodies.
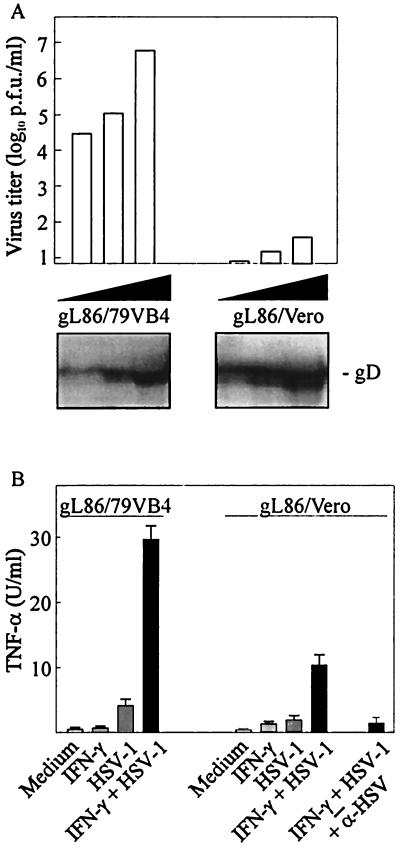
To analyze the requirement for viral entry in TNF-α induction more thoroughly, we used an HSV-1 mutant lacking the glycoprotein gL. This HSV glycoprotein, which forms heterodimers with gH, is essential for fusion of the virus envelope with the cellular membrane (30, 35). Thus, HSV particles deficient in gL are able to interact with cellular-surface heparan sulfates and proteins and to adhere to the cells but fail to fuse with the cellular membrane (32). We confirmed these previous observations using Vero cells (Fig. 3A). As to the ability of these viruses to influence TNF-α expression in RAW 264.7 cells, we found that gL86 virus grown in Vero cells (gL86/Vero), and hence lacking gL, displayed reduced ability to induce TNF-α production (Fig. 3B) compared to the virus grown in gL-expressing Vero cells (gL86/79VB4). However, and in parallel with the results shown in Fig. 2, inhibition of entry did not totally abolish TNF-α production. The presence of anti-HSV antibodies completely abolished induction of TNF-α by gL86/Vero. Collectively these results show that viral entry is not required for induction of TNF-α secretion by macrophages but also demonstrate that postentry events are required for maximal TNF-α production.
FIG. 3.
Replication and induction of TNF-α expression by gL-deficient HSV-1. (A) Confluent 79VB4 and Vero cells were infected with increasing amounts of gL-rescued gL86 (gL86/79VB4) and gL86 (gL86/Vero), respectively. After 48 h of infection, the cells were stained and plaques were counted. Antigen levels of the two virus preparations were compared by Western blotting using a specific gD-directed monoclonal antibody. (B) RAW 264.7 cells were treated with 100 IU of IFN-γ per ml and infected with 3 × 105 PFU (MOI, 0.6) of gL86/79VB4 or equivalent amounts of gL86/Vero per ml. Supernatants were harvested 8 h later and analyzed for TNF-α bioactivity. Results are shown as means of duplicate cultures ± standard errors of the means. Similar results were obtained in three independent experiments.
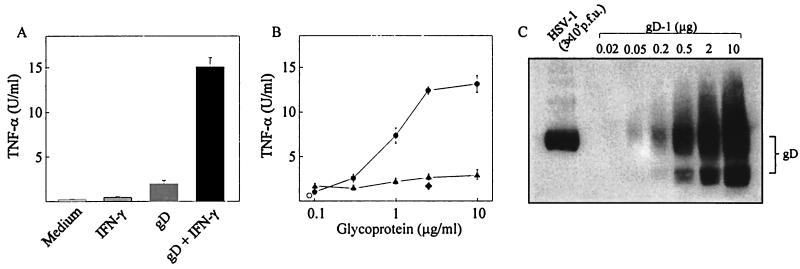
Recombinant gD induces TNF-α secretion in macrophages.
It has previously been shown that HSV-1 gD is able to trigger secretion of IFN-α (4), and signaling cellular receptors for gD are known (18, 23). Given the observation that HSV-1 and HSV-2 can induce TNF-α production independent of entry, we wanted to investigate how gD influenced TNF-α production. To test this, RAW 264.7 cells were cultured in the presence and absence of IFN-γ and recombinant gD and supernatants were assayed for TNF-α bioactivity. As seen in Fig. 4A, gD treatment alone led to a modest increase in TNF-α levels but significantly induced TNF-α secretion if given together with IFN-γ. Three hundred nanograms per ml of gD was sufficient to stimulate TNF-α production in IFN-γ-treated cells, and maximal levels of TNF-α were reached with around 3 μg of gD per ml (Fig. 4B). Another HSV glycoprotein, gG-2, did not trigger TNF-α production in RAW 264.7 macrophage-like cells. When examining the ability of recombinant gD to induce TNF-α production in peritoneal macrophages, we observed that these cells responded to the glycoprotein in a manner similar to that of RAW 264.7 cells (data not shown).
FIG. 4.
Induction of TNF-α production in RAW 264.7 cells by recombinant gD-1. The cells were treated with 100 IU of IFN-γ per ml and either 3 μg/ml of recombinant gD-1 (A) or concentrations of gD-1 and gG-2 ranging from 0.1 to 10 μg/ml (B). Supernatants were harvested 8 h later and analyzed for TNF-α bioactivity. Results are shown as means of duplicate cultures ± standard errors of the means. Symbols: ○, untreated; ●, IFN-γ + gD-1; ▴ IFN-γ + gG-2; ♦ IFN-γ + gD-1 + α-HSV antibody. (C) Semiquantification of gD on 3 × 105 PFU of HSV-1. Virus was loaded onto an SDS-PAGE together with increasing amounts of recombinant gD and subjected to Western blotting using a monoclonal antibody against gD. The level of gD in the sample was estimated by comparison with the recombinant gD standard. Similar results were obtained in two independent experiments.
To assess if the levels of recombinant gD required to induce TNF-α secretion corresponded to the quantity present on 3 × 105 PFU of HSV-1, this amount of virus was loaded onto an SDS-PAGE together with known amounts of recombinant gD and subjected to Western blotting. We found that whereas recombinant gD was present in both unglycosylated and various glycosylated forms, the gD in the virus particle appeared to be uniformly glycosylated as assessed from the mobility in the gel. Moreover, the experiment shows that 3 × 105 PFU of HSV-1 contain approximately 0.5 μg of gD (Fig. 4C) and thus shows that the amounts of recombinant gD capable of inducing TNF-α are comparable to the levels present on 3 × 105 PFU of HSV-1.
Induction of TNF-α by HSV is independent of glycosylation of the virus
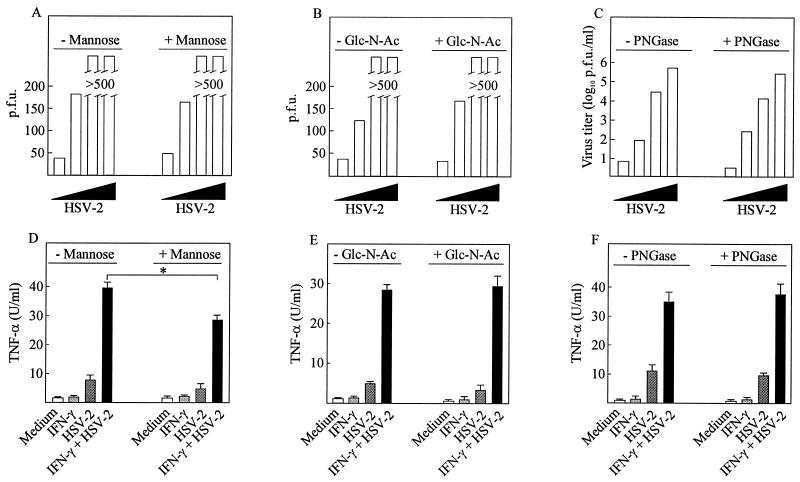
Since previous work from other laboratories has shown that HSV-1-induced IFN-α expression by dendritic cells is inhibited by the presence of free sugars in the growth medium and by neutralizing antibodies against the mannose receptor (20), we wanted to investigate if sugar moieties also were involved in stimulation of TNF-α production in macrophages. When 50 mM d-mannose was present in the growth medium we did observe significant reduction of TNF-α production (Fig. 5D) (P = 0.003), whereas the same amount of N-acetylglucosamine did not affect the induction of TNF-α by HSV (Fig. 5E). The free sugars did not affect viral replication (Fig. 5A and B).
FIG. 5.
Effect of virus glycosylation on TNF-α induction. (A and B) Confluent Vero cells were infected with increasing amounts of HSV-2 (1 × 101, 3 × 102, 1 × 104, and 3 × 105 PFU) in the presence and absence of 50 mM d-mannose (A) or N-acetylglucosamine (B). (C) HSV-2 was treated with 1,000 U of PNGase or PBS at 37°C for 3 h prior to infection in the same doses as above. After 48 h of infection, the cells were stained and plaques were counted. (D to F) RAW 264.7 cells were treated with 100 IU of IFN-γ per ml and 3 × 105 PFU (multiplicity of infection, 0.6) of HSV-2 per ml. The growth medium was supplemented with free sugars (50 mM d-mannose [D] or N-acetylglucosamine [E]) or the virus was deglycosylated with PNGase prior to infection (F). After 8 h of infection, supernatants were harvested and analyzed for TNF-α bioactivity. Results are shown as means of duplicate cultures ± standard errors of the means. Similar results were obtained in three independent experiments. ∗, P < 0.05.
However, since mannose (at least in the doses used in our study) was mildly toxic to the RAW 264.7 cells, we also assessed the involvement of glycoprotein sugar moieties in TNF-α induction by stripping the virus for sugars prior to infection. Incubation of the virus with PNGase for 3 h increased the mobility of gD on SDS-PAGE, showing that the treatment did deglycosylate the virus (data not shown). However, the infectivity was unaltered, as was the TNF-α-inducing potential (Fig. 5C and F). These results indicate that HSV infection of macrophages induces TNF-α expression independently of the glycosylation status of the virus.
Induction of TNF-α is independent of the transactivating potential of VP16 and the viral protein kinase UL13 and partly dependent on nuclear translocation of the HSV genome
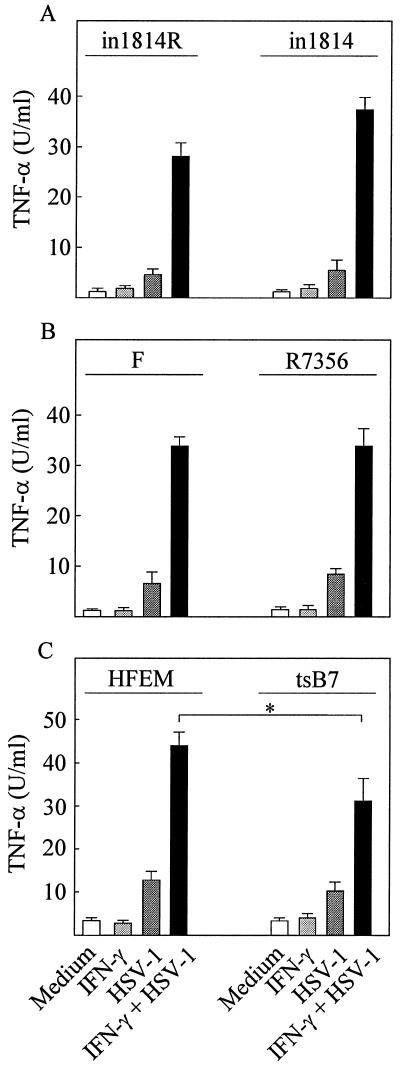
Since full induction of TNF-α secretion requires viral entry we wanted to investigate whether the virion-associated transcription factor VP16, either directly or indirectly via stimulation of immediate-early gene expression, was involved in activation of TNF-α expression. For this purpose we used the VP16 mutant in1814, which is unable to associate with cellular transcriptional coactivators (1). When RAW 264.7 cells were infected with this virus or the VP16-rescued virus (in1814R) we found an unaltered ability to induce TNF-α (Fig. 6A). In fact, we observed that although in1814R was severalfold more infectious than in1814, the TNF-α-inducing capacity correlated with virus quantity (gD levels) rather than the plaque-forming potential (data not shown). Likewise, when examining the ability of the UL13 mutant R7356 to induce TNF-α, we also found no defect relative to the wild-type virus F (Fig. 6B).
FIG. 6.
Induction of TNF-α secretion in RAW 264.7 cells by HSV-1 mutants with defects in VP16 and UL13 or nuclear translocation of viral DNA. (A) Cells were treated with 100 IU of IFN-γ per ml and infected with 3 × 105 PFU (multiplicity of infection [MOI], 0.6) of in1814 or the rescued virus in1814R per ml. (B) The cells were treated with IFN-γ as above and infected with 3 × 105 PFU (MOI, 0.6) of R7356 or wild-type F strain per ml. (C) Cells preheated to 39.5°C were treated with 100 IU of IFN-γ per ml and infected with 3 × 105 PFU (MOI, 0.6) of tsB7 or wild-type HFEM per ml. The cells were further incubated at 39.5°C. (A to C) After 8 h of infection, supernatants were harvested and analyzed for TNF-α bioactivity. Results are shown as means of duplicate cultures ± standard errors of the means. Similar results were obtained in three independent experiments. ∗, P < 0.05.
To further characterize the part of the TNF-α-inducing signal that was dependent on viral entry, we used the HSV-1 mutant tsB7, which is unable to release DNA into the nucleus at 39.5°C (6). This allowed us to dissect the involvement of specific events occurring after viral entry. We found that wild-type HFEM was capable of inducing TNF-α expression at 39.5°C (Fig. 6C). Interestingly, although the tsB7 mutant also induced TNF-α expression, this occurred at significantly lower levels than those observed for the wild-type virus (P = 0.001). At 37°C the abilities of the two viruses to induce TNF-α expression were indistinguishable (data not shown). Thus, VP16 and UL13 are not central players in HSV-induced TNF-α secretion in macrophages, which involves virus-cell interactions occurring both before and after translocation of the viral genome to the nucleus.
The dsRNA-activated protein kinase PKR participates in TNF-α expression in HSV-infected macrophages.
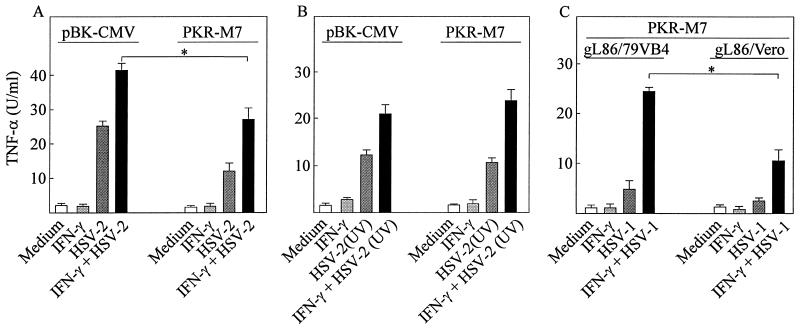
Given the partial sensitivity of TNF-α induction by HSV to UV irradiation of the virus we wanted to investigate if the dsRNA-activated protein kinase (PKR) played a role in TNF-α induction by HSV. Since accumulation of viral mRNA during virus replication is sensitive to UV irradiation and PKR is a potent stimulator of proinflammatory signaling, we speculated that this kinase could potentially contribute to the cellular signaling machinery leading to TNF-α expression. To test this we used a previously described RAW-derived cell line (16) overexpressing a PKR mutant (M7) lacking the first RNA-binding domain of the protein. As a control we used a cell line (RAW-pBK-CMW) stably transfected with the empty pBK vector. This cell line responded to HSV-1 and HSV-2 infection alone with a remarkably high production of TNF-α, which was enhanced by cotreatment with IFN-γ (Fig. 7A and data not shown). RAW-PKR-M7 was also capable of producing TNF-α after HSV-1 and HSV-2 infection but at significantly lower levels (P = 0.01).
FIG. 7.
Effects of PKR inactivation on TNF-α induction by HSV. (A) RAW-pBK-CMV and RAW-PKR-M7 cells were treated with 100 IU of IFN-γ per ml and infected with 3 × 105 PFU (multiplicity of infection [MOI], 0.6) of HSV-2 per ml as indicated. (B) The cells were treated as for panel A with the sole modification that the virus was UV irradiated prior to addition to the cell cultures. (C) RAW-PKR-M7 cells were treated with 100 IU of IFN-γ per ml and 3 × 105 PFU (MOI, 0.6) of gL86/79VB4 per ml or equivalent amounts of gL86/Vero. (A to C) After 8 h of infection the levels of bioactive TNF-α in the supernatants were assayed. Results are shown as means of duplicate cultures ± standard errors of the means. Similar results were obtained in three independent experiments. ∗, P < 0.05.
To assess if the role of PKR was dependent on viral dsRNA we tested how the cell lines responded to UV-inactivated virus. Interestingly, unlike what was observed after treatment of the cells with infectious virus, UV-inactivated virus resulted in comparable levels of TNF-α production in the two cell lines (Fig. 7B). Finally, we wanted to examine if in fact postentry events other than PKR contributed to TNF-α production. To do this we used the PKR mutant cell line used in experiments illustrated in Fig. 7A and B and the above-described viruses gL86/Vero and gL86/79VB4 (Fig. 3). As seen from Fig. 7C, gL86/79VB4 remained a significantly more potent inducer of TNF-α than was gL86/Vero (P = 0.006) despite lack of a functional PKR system in the RAW-PKR-M7 cell line. Thus, these results suggest that signaling through viral dsRNA-activated PKR plays a role in HSV-induced TNF-α expression in macrophages and that entry-dependent events other than PKR are involved.
DISCUSSION
Production of cytokines is an essential part of the early response to viral infection. This induces an antiviral state in infected cells and recruits cells of the immune system to the site of infection. Macrophages play an essential role in the natural immune response to many viruses and among these HSV (13, 21). One of the macrophage-derived products that contribute to inhibition of HSV replication is the cytokine TNF-α (13, 27), which induces a number of antiviral effector mechanisms, notably production of nitric oxide (5, 26). In this study we have investigated the virus-cell interactions that trigger production of TNF-α in murine macrophages.
The ability of HSV infection to stimulate TNF-α expression to any major extent in RAW 264.7 macrophage-like cells was dependent on the presence of IFN-γ. Similar findings have previously been done in another murine macrophage-like cell line (J774A.1) and in murine peritoneal cells (26). Since IFN-γ is known to be produced early during HSV infections by NK cells (19, 25), this observation emphasizes the importance of interplay between different cells of the immune system in order to bring about a robust host response.
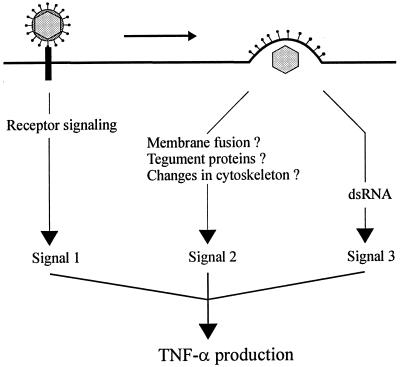
For some viruses it has been shown that more than one mechanism is involved in cytokine induction. HBV is able to stimulate TNF-α production through both HBx and the HBV core antigen (15, 37). Likewise, several HIV-1 proteins promote IL-6 expression (3, 24, 29, 33). We found that the ability of HSV to induce TNF-α expression is dependent on entry-independent events and at least two different postentry events: one independent of release of viral DNA into the nucleus and one dependent on PKR and a functional viral genome. These results are compatible with the model shown in Fig. 8.
FIG. 8.
Model for virus-cell interactions in induction of TNF-α production by HSV. First, interaction between gD and a cellular receptor triggers intracellular signals leading to production of low levels of TNF-α. Second, viral entry also stimulates signaling pathways with a positive effect on TNF-α gene expression. The nature of the viral component or activity responsible for this signal is not known but could involve membrane fusion, viral tegument proteins, or changes in the cytoskeleton. Third, accumulation of viral dsRNA and subsequent activation of PKR also promotes TNF-α production. IFN-γ, which strongly enhances HSV-induced TNF-α expression, is omitted from the figure.
The entry-independent TNF-α induction could be mimicked by recombinant gD. It is possible that the viral glycoprotein interacts with one of the cellular herpes virus entry mediators, which in turn mediates intracellular signaling. In this respect it is interesting that the herpesvirus entry mediator A is a member of the TNF receptor superfamily and is known to activate some of the transcription factors involved in regulation of TNF-α transcription (18). However, it is also possible that gD induces TNF-α expression through binding to receptors other than the entry mediators. In fact, it has been reported that the ability of gD to stimulate IFN-α secretion can be inhibited with antibodies directed against the chemokine receptors CCR3 and CXCR4 (4). The concept of immune stimulation by viral surface proteins is well described in the literature. To illustrate, gp350 of Epstein-Barr virus and gp120 of HIV-1 stimulate expression of TNF-α (3, 8), and it was recently reported that the fusion protein of respiratory syncytial virus is recognized by toll-like receptor 4 and CD14 (14).
Concerning the postentry events involved in TNF-α induction, we found that both UV-sensitive and -insensitive mechanisms are involved. The existence of the entry-dependent UV-insensitive response was documented primarily by the observations (i) that the gL-deficient virus particles (gL86/Vero) induced lower levels of TNF-α than did the gL-containing virions (gL86/79VB7) in RAW-PKR-M7 cells, which lack dsRNA-responsive PKR; (ii) that UV-irradiated virus induced comparable amounts of TNF-α in RAW-PKR-M7 and RAW-pBK-CMV cells; and (iii) that tsB7 was a less potent inducer of TNF-α than HFEM at the nonpermissive temperature, under which condition the mutant is unable to release its DNA into the nuclei of infected cells. The exact nature of the virus-cell interaction responsible for this response is not known but is independent of VP16 and UL13. Potential candidates include fusion of the viral envelope with the cell membrane, tegument proteins other than VP16 and UL13, changes in the cytoskeleton caused by transport of the capsid to the nuclear pores, or sensing by the cell of HSV capsids.
The entry-dependent signal sensitive to UV irradiation of virus involved PKR. This conclusion is based on the finding that the PKR mutant cell line RAW-PKR-M7 produced lower amounts of TNF-α than did the control cell line RAW-pBK-CMV in response to infectious virus but similar levels after treatment with UV-inactivated virus. Since PKR is activated by dsRNA (38) and UV irradiation of virus inhibits transcription from the viral genome, these results suggest that this signal is comprised of PKR activated by HSV mRNA produced during the viral life cycle.
In summary, we have shown that the HSV glycoprotein gD is able to induce secretion of TNF-α in macrophages and that postentry events are required for full induction. Our results suggest that macrophages can react to HSV with three levels of TNF-α secretion: (a) a low response that is induced when the macrophage detects the virus on the cell surface, (b) a stronger response that is initiated when the virus has entered the cell, and (c) full induction when the infecting virus goes through its replication cycle.
ACKNOWLEDGMENTS
We thank Patricia G. Spear for the gL86 HSV-1 mutant and the 79VB4 cell line, Chris M. Preston for the viruses in1814 and in1814R, and Bernard Roizman for the mutant viruses tsB7 and R7356. We are also indebted to Sytske Welling-Wester for providing the recombinant HSV-2 gG. The generous donation of the cell lines RAW-pBK-CMV and RAW-PKR-M7 by John A. Corbett is greatly appreciated. The skillful technical help provided by Birthe Søby and Elin Jakobsen has been invaluable.
This work was supported by grants from the Danish Health Science Research Council (grant number 12-1622), Fonden til Lægevidenskabens Fremme (grant number 00182), and The Leo Research Foundation.
REFERENCES
- 1.Ace C I, McKee T A, Ryan J M, Cameron J M, Preston C M. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J Virol. 1989;63:2260–2269. doi: 10.1128/jvi.63.5.2260-2269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler H, Beland J L, Del-Pan N C, Kobzik L, Brewer J P, Martin T R, Rimm I J. Suppression of herpes simplex virus type 1 (HSV-1)-induced pneumonia in mice by inhibition of inducible nitric oxide synthase (iNOS, NOS2) J Exp Med. 1997;185:1533–1540. doi: 10.1084/jem.185.9.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ameglio F, Capobianchi M R, Castilletti C, Cordiali Fei P, Fais S, Trento E, Dianzani F. Recombinant gp120 induces IL-10 in resting peripheral blood mononuclear cells; correlation with the induction of other cytokines. Clin Exp Immunol. 1994;95:455–458. doi: 10.1111/j.1365-2249.1994.tb07018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ankel H, Westra D F, Welling-Wester S, Lebon P. Induction of interferon-α by glycoprotein D of herpes simplex virus: a possible role of chemokine receptors. Virology. 1998;251:317–326. doi: 10.1006/viro.1998.9432. [DOI] [PubMed] [Google Scholar]
- 5.Baskin H, Ellermann-Eriksen S, Lovmand J, Mogensen S C. Herpes simplex virus type 2 synergizes with interferon-γ in the induction of nitric oxide production in mouse macrophages through autocrine secretion of tumour necrosis factor-α. J Gen Virol. 1997;78:195–203. doi: 10.1099/0022-1317-78-1-195. [DOI] [PubMed] [Google Scholar]
- 6.Batterson W, Furlong D, Roizman B. Molecular genetics of herpes simplex virus. VIII. Further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J Virol. 1983;45:397–407. doi: 10.1128/jvi.45.1.397-407.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowan E P, Alexander R K, Daniel S, Kashanchi F, Brady J N. Induction of tumor necrosis factor alpha in human neuronal cells by extracellular human T-cell lymphotropic virus type 1 Tax. J Virol. 1997;71:6982–6989. doi: 10.1128/jvi.71.9.6982-6989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Addario M, Ahmad A, Morgan A, Menezes J. Binding of the Epstein-Barr virus major envelope glycoprotein gp350 results in the upregulation of the TNF-α gene expression in monocytic cells via NF-κB involving PKC, PI3-K and tyrosine kinases. J Mol Biol. 2000;298:765–778. doi: 10.1006/jmbi.2000.3717. [DOI] [PubMed] [Google Scholar]
- 9.Ellermann-Eriksen S. Autocrine secretion of interferon-α/β1 and tumour necrosis factor-α synergistically activates mouse macrophages after infection with herpes simplex virus type 2. J Gen Virol. 1993;74:2191–2199. doi: 10.1099/0022-1317-74-10-2191. [DOI] [PubMed] [Google Scholar]
- 10.Fujii S, Akaike T, Maeda H. Role of nitric oxide in pathogenesis of herpes simplex virus encephalitis in rats. Virology. 1999;256:203–212. doi: 10.1006/viro.1999.9610. [DOI] [PubMed] [Google Scholar]
- 11.Immergluck L C, Domowicz M S, Schwartz N B, Herold B C. Viral and cellular requirements for entry of herpes simplex virus type 1 into primary neuronal cells. J Gen Virol. 1998;79:549–559. doi: 10.1099/0022-1317-79-3-549. [DOI] [PubMed] [Google Scholar]
- 12.Kanangat S, Babu J S, Knipe D M, Rouse B T. HSV-1-mediated modulation of cytokine gene expression in a permissive cell line: selective upregulation of IL-6 gene expression. Virology. 1996;219:295–300. doi: 10.1006/viro.1996.0250. [DOI] [PubMed] [Google Scholar]
- 13.Kodukula P, Liu T, van Rooijen N, Jager M J, Hendricks R L. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J Immunol. 1999;162:2895–2905. [PubMed] [Google Scholar]
- 14.Kurt-Jones E A, Popova L, Kwinn L, Haynes L M, Jones L P, Tripp R A, Walsh E E, Freeman M W, Golenbock D T, Anderson L J, Finberg R W. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 15.Lara-Pezza E, Majano P L, Gomez-Gonzalo M, Garcia-Monzon C, Moreno-Otero R, Levrero M, Lopez-Cabrera M. The hepatitis B virus X protein up-regulates tumor necrosis factor α gene expression in hepatocytes. Hepatology. 2000;28:1013–1021. doi: 10.1002/hep.510280416. [DOI] [PubMed] [Google Scholar]
- 16.Maggi L B, Jr, Heitmeier M R, Scheuner D, Kaufman R J, Buller R M, Corbett J A. Potential role of PKR in double-stranded RNA-induced macrophage activation. EMBO J. 2000;19:3630–3638. doi: 10.1093/emboj/19.14.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malmgaard L, Paludan S R, Mogensen S C, Ellermann-Eriksen S. Herpes simplex virus type 2 induces interleukin-12 in macrophages through a mechanism involving NF-κB. J Gen Virol. 2000;81:3011–3020. doi: 10.1099/0022-1317-81-12-3011. [DOI] [PubMed] [Google Scholar]
- 18.Marsters S A, Ayres T M, Skubatch M, Gray C L, Rothe M, Ashkenazi A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-κB and AP-1. J Biol Chem. 1997;272:14029–14032. doi: 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- 19.Milligan G N, Bernstein D I. Interferon-γ enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 20.Milone M C, Fitzgerald-Bocarsly P. The mannose receptor mediates induction of IFN-α in peripheral blood dendritic cells by enveloped RNA and DNA viruses. J Immunol. 1998;161:2391–2399. [PubMed] [Google Scholar]
- 21.Mogensen S C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979;43:1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mogensen T H, Paludan S R. Molecular pathways in virus-induced cytokine production. Microbiol Mol Biol Rev. 2001;65:131–150. doi: 10.1128/MMBR.65.1.131-150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 24.Nath A, Conant K, Chen P, Scott C, Major E O. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- 25.Paludan S R, Ellermann-Eriksen S, Malmgaard L, Mogensen S C. Inhibition of NO production in macrophages by IL-13 is counteracted by HSV infection through TNF-α-induced activation of NF-κB. Eur Cytokine Netw. 2000;11:275–282. [PubMed] [Google Scholar]
- 26.Paludan S R, Ellermann-Eriksen S, Mogensen S C. NF-κB activation is responsible for the synergistic effect of herpes simplex virus type 2 infection on interferon-γ-induced nitric oxide production. J Gen Virol. 1998;79:2785–2793. doi: 10.1099/0022-1317-79-11-2785. [DOI] [PubMed] [Google Scholar]
- 27.Pavic I, Polic B, Crnkovic I, Lucin P, Jonjic S, Koszinowski U H. Participation of endogenous tumour necrosis factor α in host resistance to cytomegalovirus infection. J Gen Virol. 1993;74:2215–2223. doi: 10.1099/0022-1317-74-10-2215. [DOI] [PubMed] [Google Scholar]
- 28.Purves F C, Ogle W O, Roizman B. Processing of the herpes simplex virus regulatory protein α 22 mediated by the UL13 protein kinase determines the accumulation of a subset of α and γ mRNAs and proteins in infected cells. Proc Natl Acad Sci USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quaranta M G, Camponeschi B, Straface E, Malorni W, Viora M. Induction of interleukin-15 production by HIV-1 Nef protein: a role in the proliferation of uninfected cells. Exp Cell Res. 1999;250:112–121. doi: 10.1006/excr.1999.4494. [DOI] [PubMed] [Google Scholar]
- 30.Rajcani J, Vojvodova A. The role of herpes simplex virus glycoproteins in the virus replication cycle. Acta Virol. 1998;42:103–118. [PubMed] [Google Scholar]
- 31.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Raven Publishers; 1996. pp. 2231–2295. [Google Scholar]
- 32.Roop C, Hutchinson L, Johnson D C. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993;67:2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roux P, Alfieri C, Hrimech M, Cohen E A, Tanner J E. Activation of transcription factors NF-κB and NF-IL-6 by human immunodeficiency virus type 1 protein R (Vpr) induces interleukin-8 expression. J Virol. 2000;74:4658–4665. doi: 10.1128/jvi.74.10.4658-4665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimeld C, Whiteland J L, Williams N A, Easty D L, Hill T J. Cytokine production in the nervous system of mice during acute and latent infection with herpes simplex virus type 1. J Gen Virol. 1997;78:3317–3325. doi: 10.1099/0022-1317-78-12-3317. [DOI] [PubMed] [Google Scholar]
- 35.Spear P G, Shieh M T, Herold B C, WuDunn D, Koshy T I. Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus. Adv Exp Med Biol. 1992;313:341–353. doi: 10.1007/978-1-4899-2444-5_33. [DOI] [PubMed] [Google Scholar]
- 36.Su Y H, Yan X T, Oakes J E, Lausch R N. Protective antibody therapy is associated with reduced chemokine transcripts in herpes simplex virus type 1 corneal infection. J Virol. 1996;70:1277–1281. doi: 10.1128/jvi.70.2.1277-1281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vingerhoets J, Michielsen P, Vanham G, Bosmans E, Paulij W, Ramon A, Pelckmans P, Kestens L, Leroux-Roels G. HBV-specific lymphoproliferative and cytokine responses in patients with chronic hepatitis B. J Hepatol. 1998;28:8–16. doi: 10.1016/s0168-8278(98)80196-7. [DOI] [PubMed] [Google Scholar]
- 38.Williams B R. The role of the dsRNA-activated protein kinase, PKR, in signal transduction. Semin Virol. 1995;6:191–202. [Google Scholar]