Significance
Transcription factor (TF) encoding genes, which temporally control development in multiple cell types, can have tens of enhancers that regulate their expression. The NR2F1 TF promotes caudal and ventral cortical regional fates. Here, we epigenomically compared the activity of Nr2f1’s enhancers during mouse cortical development with their activity in a transgenic assay; we identified at least six that are likely to be important in prenatal cortical development. We studied the function of two robust enhancers by deleting them singly or together. They have distinct and overlapping functions in driving Nr2f1’s regional and laminar cortical expression. Thus, these two enhancers, in combination with the others that we defined epigenetically, precisely tune Nr2f1’s regional, cell type, and temporal expression during corticogenesis.
Keywords: Nr2f1, enhancer, cortical development, epigenomics
Abstract
There is evidence that transcription factor (TF) encoding genes, which temporally control development in multiple cell types, can have tens of enhancers that regulate their expression. The NR2F1 TF developmentally promotes caudal and ventral cortical regional fates. Here, we epigenomically compared the activity of Nr2f1’s enhancers during mouse cortical development with their activity in a transgenic assay. We identified at least six that are likely to be important in prenatal cortical development, with three harboring de novo mutants identified in ASD individuals. We chose to study the function of two of the most robust enhancers by deleting them singly or together. We found that they have distinct and overlapping functions in driving Nr2f1’s regional and laminar expression in the developing cortex. Thus, these two enhancers, probably in combination with the others that we defined epigenetically, precisely tune Nr2f1’s regional, cell type, and temporal expression during corticogenesis.
Understanding the genetic regulation of neural development is critical for understanding the molecular mechanisms guiding the formation, evolution, and diseases of the brain. All central nervous system (CNS) regions begin as neuroepithelial ventricular zone (VZ) stem cells, which sequentially differentiate into subventricular zone (SVZ) secondary progenitors and subsequently into neurons and glia that migrate into the overlying mantle zone (MZ) and white matter layers. Development of the pallium (cerebral cortex) has been studied in detail.
In mammals, it has six neuronal layers and is subdivided into multiple functional regions (1). The past 30 y have seen the discovery of key transcriptional mechanisms guiding its development, particularly of transcription factors (TF) that control cortical regionalization and lamination (1, 2). More recently, attention has shifted to how the noncoding genome, particularly elements called enhancers, control when and where critical genes are expressed.
Cortical enhancer discovery was accelerated using epigenetic and transgenic assays leading to the generation of the VISTA database showing human and mouse enhancer activity at embryonic day 11.5 (E11.5) (3) (https://enhancer.lbl.gov/). This rich resource identified over 150 enhancers which are active in different parts of the cortical VZ, SVZ, and MZ (4). Many of these enhancers are ultraconserved during evolution (5, 6). Stable mouse transgenics made from 10 pallial enhancers, each with distinct regional activity at E11.5, enabled the fate mapping of the cortical protomap (7).
Epigenetic assays in human and mouse prenatal cortex have greatly amplified the number of candidate cortical enhancers to over 50,000 (2, 8–10). Genetic variation in enhancers is linked to brain evolution (11–15) and human neuropsychiatric disorders (16). For instance, we identified mutations in a candidate enhancer of the Autism risk gene SLC6A1 that alter its activity; it remains to be proven whether these mutations are pathogenic (8).
Definitive evidence for enhancer in vivo function requires mutational analysis, such as an enhancer loss of function mutation. An attempt to identify a forebrain histological phenotype in mice from an Arx enhancer deletion failed, potentially because of genetic redundancy between enhancers (there are at least two Arx pallial enhancers) (17). Subsequently, more detailed molecular and histological analyses identified pallial phenotypes in single and double Arx enhancer mutants (18). Similarly, deletions of enhancers near Dmrt3, Emx2, Fezf2, Gli3, and Tbr1 demonstrate their importance for driving expression of their adjacent gene (19–23). Likewise, deletions of enhancers regulating subcortical genes (e.g., Dlx1,2,5&6 and Tcf12) have also identified phenotypes (24–26). Furthermore, the replacement of the mouse Dlx enhancer allele with a human allele bearing a mutation identified in an Autistic individual also generated a phenotype (26). Targeting inhibitory Crispr (CrisprI) to enhancers also provided evidence for their requirement, as for several Sp9 enhancers (27).
Here, we have investigated the in vivo function of two pallial enhancers identified in the human and mouse Nr2f1 locus. Nr2f1 encodes a TF that has a major role in cortical development through its function in progenitors and immature neurons, where it promotes caudal and ventral regional fates (2, 28–32). Humans haploinsufficient for Nr2f1 have Bosch–Boonstra–Schaff optic atrophy syndrome with intellectual disability and cortical polymicrogyria (32). Mice with spontaneous 53 kb deletion near Nr2f1 have reduced Nr2f1 expression in the ear, suggesting that the deletion included an enhancer active during ear development (33). Furthermore, Nr2f1 is part of the cortical regionalization TF network (CRTFN) (2). This network includes at least 38 TFs. Nr2f1 regulates regionalization in part through its function in the VZ (2) and through modulating the epigenomic signature of CRTFN enhancers (2).
Multiple Nr2f1 enhancers have been identified (see VISTA browser). The activity of one (hs1172) has been studied in some detail (7).Here, by making stable deletions of two Nr2f1 VISTA enhancers (hs1172 and hs271) in mice, we provide evidence that they have distinct and overlapping functions in driving Nr2f1 expression during cortical development.
Results
Nr2f1 Candidate Enhancers.
We studied the putative enhancers identified at embryonic day 11.5 (E11.5) in transgenic mice (8) [VISTA Enhancer Browser(4): https://enhancer.lbl.gov/] around Nr2f1 in the 4 Mb chromatin region of topologically associated domain (TAD) (34) (Fig. 1A and SI Appendix, Fig. S1). In the telencephalon, Nr2f1 has pallial caudorostral and ventrodorsal gradiential expression and subpallial regional expression in the LGE, MGE, and CGE (LGE: lateral ganglionic eminence, MGE: medial ganglionic eminence, CGE: caudal ganglionic eminence). This suggests that multiple enhancers may be required to generate this complex expression pattern (Fig. 1 D1–D3) (30, 31, 35–38). Indeed, there are 35 VISTA-tested regions in the Nr2f1 TAD domain, 8 of which show activity (LacZ expression) in the telencephalon (Fig. 1B and Dataset S1). The activity of these enhancers resembles components of Nr2f1 RNA expression, including 7 pallial (cortical) enhancers (mm1556, mm881, hs271, hs1172, hs1049, hs1577, and hs952) (note: mm: denotes mouse genome, hs: denotes human genome) and 7 subpallial enhancers (mm1556, mm881, hs217, hs1172, hs1049, hs269, and hs1577) (Fig. 1C and SI Appendix, Fig. S1). hs271 and hs1172 stand out as having a pattern closely resembling Nr2f1’s pallial RNA expression.
Fig. 1.
Nr2f1 telencephalic enhancers. Selected Nr2f1 enhancers show telencephalic activity closely resembling Nr2f1 expression at E11.5. (A) Schema showing a 4 Mb genomic region surrounding the Nr2f1 locus (mm10, chr13:76762978-80762978) (red bar: Nr2f1 gene body. (B) Bar graph showing 35 Vista enhancers around the Nr2f1 locus; 8 are active in the telencephalon (red bars). 15 are inactive (black bars), 7 are active but not in the forebrain (green bars), and 5 are active in the forebrain but not in the telencephalon (blue bars) (Dataset S1). (C) 6 enhancers driving LacZ activity (blue) in the mouse pallium; whole mounts at E11.5 (a-g). These embryos were coronally sectioned; representative sections show LacZ activity in telencephalic regions. Because of its low background, we highlighted the circumference of mm881’s sections in black. In situ hybridization of Nr2f1 RNA (D1-D3). Black arrows indicate regions with either LacZ activity or Nr2f1 RNA. DP: dorsal pallium, LP: lateral pallium, VP: ventral pallium, MP; medial pallium, LGE: lateral ganglionic eminence, MGE: medial ganglionic eminence, CGE: caudal ganglionic eminence, Th: thalamus, POA: preoptic area. Magnification bar: 0.5 mm.
Next, we assessed whether the six Nr2f1 pallial enhancers loop in proximity to the Nr2f1 promoter in the E12.5 cortex using a Plac-seq assay (2). Interactions were detected between the TSS (Transcription Start Site) and hs271, hs1172, and hs1049 (Fig. 2D). From this point on, we focused on six enhancers with the clearest pallial activity: mm1556, mm881, hs271, hs1172, hs1049, and hs1577.
Fig. 2.
Nr2f1 locus organization and epigenetic analyses at E12.5. (A) The location of six pallial Nr2f1 VISTA enhancers (red) and their genomic distance to Nr2f1 gene are shown in the WashU genome browser. Other Nr2f1 VISTA enhancers are indicated in blue. The location of other genes is shown to the right of RefSq gene. (B) EMX2, LHX2, NR2F1, PAX6, and PBX1 TF ChIP-seq data in E12.5 cortex are displayed (black rectangles surround called peaks) (2). (C) Epigenetic (H3K27ac, H3K27me3, and ATAC) modifications to enhancers in the E12.5 cortex are shown for wildtype and Pax6−/−, Emx2−/−, and Nr2f1−/−(2). Red rectangles indicate gain of peak height in the mutants and green rectangles indicate loss of peak heights in the mutants. (D) Plac-seq data shows enhancer interactions with Nr2f1 promoter (2). (E) Locations of CRISPR deletions of hs271 and hs1172 enhancers are shown as red bars. Abbreviations: 27ac: H3K27ac, 27me3: H3K27me3, Mut: mutant. WT: wild type, pRE: putative regulatory element.
Nr2f1 Enhancers Are Occupied by Specific Cortical TFs and Have Distinct Epigenetic Sensitivity to Mutations in Emx2, Nr2f1, and Pax6.
We used TFs ChIP-seq to assess whether Nr2f1’s candidate pallial enhancers (mm1556, mm881, hs271, hs1172, hs1049, and hs1577) were bound in the E12.5 cortex by 5 TFs known to regulate pallial regional patterning and/or Nr2f1 expression: EMX2, LHX2, NR2F1, PAX6, and PBX1 (Fig. 2B and Dataset S1). These TF ChIP-seq experiments were performed in a previous study (2). NR2F1 bound all 6 enhancers; EMX2, LHX2, and NR2F1 bound 5 enhancers; EMX2, LHX2, NR2F1, and PAX6 bound 4 enhancers, and all 5 TFs bound 3 enhancers (Fig. 2B and Dataset S1).
We next assayed the epigenetic states of the Nr2f1 pallial enhancers in pallial VZ cells purified using FlashTag (2), by performing ATAC-seq (open chromatin), H3K27ac ChIP-seq (active chromatin), H3K27me3 ChIP-seq (repressed chromatin) (Fig. 2C and Dataset S1) (2). At E12.5, 5/6 pallial enhancers (except hs1049) had ATAC-seq peaks, 3/6 enhancers (mm1556, hs271, and hs1172) had H3K27ac peaks, and 1/6 enhancers (mm881) had H3K27me3 peaks (Fig. 2C and Dataset S1). On the other hand, only 5/27 of the enhancers that were inactive in the telencephalon had an ATAC-seq peak, and 2/27 had an H3K27ac peak (Dataset S1). These results suggest that enhancers which are active in the pallium are more likely to have ATAC and H3K27ac peaks than enhancers with no pallial activity.
Then, we tested whether the epigenetic state of the pallial enhancers was altered in the pallial VZ of mice lacking TFs (Emx2, Pax6, and Nr2f1) that regulate pallial regionalization at E12.5. The binary peak calls were annotated as Gain, Loss, or no change of peak enrichment in the mutant (2). In the Pax6 mutant, we observed no epigenetic changes at enhancers. In the Emx2 mutant, enhancers mm1556, hs271, and hs1172 had loss of H3K27ac and gain of H3K27me3 (see red and green boxes in Fig. 2C and Dataset S1). In the Nr2f1 mutant, we observed a loss in H3K27me3 in the regions flanking hs1172. Chromatin accessibility (ATAC-seq) was unchanged in all three mutants. Over the Nr2f1 gene body and promoter, there was a loss of H3K27ac in the Pax6 mutant; a gain of H3K27me3 in the Emx2 mutant; and a loss of H3K27ac and H3K27me3 in the Nr2f1 mutant, though these would be confounded by the partial deletion of the Nr2f1 gene. Epigenetic changes at the mm1556, hs271, and hs1172 enhancers and the Nr2f1 gene body are coherent with the reduction in Nr2f1 expression in these mutants (2, 39).
This provided evidence that the epigenetic state and perhaps the activity of these three enhancers are sensitive to the loss of Emx2 and Nr2f1. Furthermore, it opens the door to the possibility that these enhancers may participate in pallial regionalization, a hypothesis which we test later in the paper.
The Epigenetic States of Nr2f1 Pallial Enhancers Change During Development.
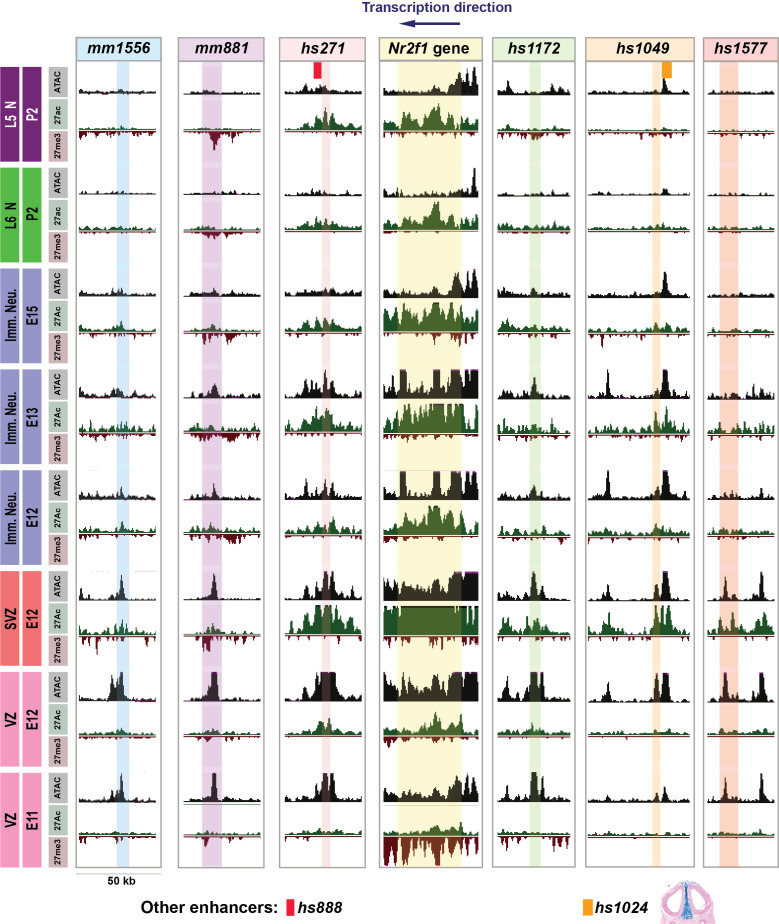
To identify cortical cell subtypes in which Nr2f1 pallial enhancers were active, we purified five cortical cell types by FACS sorting (VZ, SVZ, immature neurons, neonatal Layer 6 and Layer 5 neurons). We performed epigenetic assays (ATAC-seq, H3K27ac, and H3K27me3 ChIP-seq) on these cells at E11.5-through postnatal day 2 (P2) (Fig. 3). We analyzed the developmental chromatin landscape for the six pallial enhancers and the Nr2f1 gene body.
Fig. 3.
Developmental course of epigenetic states for six Nr2f1 pallial enhancers and Nr2f1 gene body in cortical VZ, SVZ, and immature neurons and deep layer neurons. ATAC-seq (ATAC in gray), H3K27ac (27ac in green), and H3K27me3 (27me3 in red) epigenetic marks are shown in cortical cell subtypes at different developmental ages. Each row represents a distinct cortical cell type at a specific age including VZ, SVZ, prenatal Immature Neurons, P2 Layer 6 Neurons (L6N), and P2 Layer 5 Neurons (L5N) (specified in text boxes on the left of the figure). Colored columns represent the genomic regions of each pallial enhancer as well as the Nr2f1 gene body (specified in text boxes at the top of the figure); blue for mm1556, purple for mm881, pink for hs271, yellow for Nr2f1 gene, green for hs1172, orange for hs1049, red for hs1577. Two additional VISTA enhancers with genomic coordinates close to the pallial enhancers are shown in the L5N row: hs888 in red near hs271 whose region of activity is unknown and hs1024 in orange near hs1049 with activity in intertelencephalic mesenchyme at E11.5 (see sectioned brain at the bottom of hs1049 column). The genomic region shown for each enhancer and the Nr2f1 gene body is 50 kb.
The Nr2f1 gene body has robust ATAC-seq and H3K27ac-seq peaks from E11.5-P2 (Fig. 3), consistent with its RNA expression during this period (Figs. 1 D1–D3, 4 A–A4, and 5 A–A4 and SI Appendix, Fig. S15 A–A5). H3K27me3 peaks were present on the gene body in VZ cells at E11.5 but not at E12.5. We found that ATAC-seq peaks were present in VZ and SVZ cells for all pallial enhancers. These ATAC peaks persisted in immature neurons at E12.5 and E13.5 for all enhancers except hs1577, albeit peaks appeared much smaller. ATAC peaks were only present in P2 Layer 5 neurons in hs271. As Nr2f1 expression in the cortex continues postnatally, we observed multiple ATAC peaks in P2 Layer 5 and Layer 6 neurons (SI Appendix, Fig. S2).
Fig. 4.
Changes in Nr2f1 pallial and subpallial expression in hs1172, hs271, and hs1172/ hs271 mutants at E12.5. Nr2f1 in situ RNA expression in telencephalic coronal hemisections (rostral to caudal) in WT (A–A4), hs1172−/− (B–B4), hs271−/−(C–C4), and hs1172−/−/ hs271−/−(D–D4) at E12.5. NR2F1 immunochemistry in WT and mutants (A5–D5). Black arrows indicate pallial locations where Nr2f1 is expressed in WT. (E and F) Quantitative analysis of Nr2f1 expression levels in the lateral and dorsal rostral pallium. Purple rectangles indicated the areas where Nr2f1 in situ signal density was measured. Measurements were made in four brain sections using ImageJ. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (mean ± SD). Between the two green arrowheads are pallial regions with reduced Nr2f1 expression. Red arrowheads indicate reduced Nr2f1 expression in the pallial VZ. Orange arrowheads indicate reduced Nr2f1 expression in LGE, MGE, and CGE. Blue arrowheads indicate preserved Nr2f1 expression in a superficial layer of the pallium. Blue arrowheads also indicate preserved NR2F1 expression in a superficial layer of the pallium (A5–D5). Abbreviations: CP, cortical plate; OE, olfactory epithelium; Hyp: hypothalamus; for the rest of the abbreviations, see legend to Fig. 1. Magnification bar: 0.5 mm.
Fig. 5.
Changes in Nr2f1 pallial and subpallial expression in hs1172, hs271, and hs1172/ hs271 mutants at E14.5. Nr2f1 in situ RNA expression in telencephalic coronal hemisections (rostral to caudal) in WT (A–A4), hs1172−/− (B–B4), hs271−/−(C–C4), and hs1172−/−/ hs271−/−(D–D4). NR2F1 immunochemistry in WT and mutants (A5–D5) at E14.5. Black arrows indicate pallial locations where Nr2f1 is expressed in WT. Between the two green arrowheads are pallial regions with reduced Nr2f1 expression. Red arrowheads indicate reduced Nr2f1 expression in the pallial VZ. Orange arrowheads indicate reduced Nr2f1 expression in LGE, MGE, and CGE. Blue arrowheads indicate preserved Nr2f1 expression in the pallial SVZ of the hs271−/−and hs1172−/−/ hs271−/−despite the reduction in the VZ. Blue arrowheads also indicate preserved NR2F1 expression in the pallial SVZ of the hs271−/−and hs1172−/−/ hs271−/−(A5–D5). Abbreviations: PTh: prethalamus; for the rest of the abbreviations, see legends to Figs. 1 and 4. Magnification bar: 0.5 mm.
H3K27ac active marks were present on the hs271 enhancer in VZ cells at E12.5, SVZ cells at E12.5, immature neurons at E12.5–E15.5 and Layer 6/5 neurons at P2 (SI Appendix, Fig. S2). hs1049 had H3K27ac marks in SVZ cells at E12.5 and very small “called” H3K27ac peaks in immature neurons at E12.5–E15.5 and lacked H3K27ac at P2 (SI Appendix, Fig. S2). mm1556, mm881, hs1172, and hs1577 all had small H3K27ac peaks in VZ cells at E12.5, large H3K27ac peaks in SVZ cells at E12.5 and then the peaks became small in immature neurons at E12.5–E15.5. Only one enhancer (mm881) had H3K27me3 repressive marks (in all cell types and at all developmental times); note that this enhancer had the smallest region of pallial activity at E11.5 (Fig. 1 B1–B3). Thus, among all these pallial enhancers, hs271 had the strongest evidence for being active and accessible in the VZ, SVZ, and neurons. Furthermore, its epigenetic profile closely resembled that of the Nr2f1 gene body, while the epigenetic profile of other enhancers, such as hs1172, only partially resembled that of Nr2f1 in VZ, SVZ, and immature neurons.
Additional enhancers in the region surrounding Nr2f1 are also likely to contribute to Nr2f1 transcriptional regulation in subsets of differentiating cortical cell types. For example, we’ve highlighted in beige some putative enhancers (SI Appendix, Fig. S2). pRE1 is a predicted L5N enhancer since it is both accessible (ATAC peaks) and active (H3K27ac peak) in L5N. pRE2 and pRE3 have putative activity (ATAC and H3K27ac peaks) in all cortical cell subtypes, except L6N for pRE2, and the VZ for pRE3. pRE4 is adjacent to hs271; it contains VZ and SVZ ATAC peaks, but no H3K27ac peaks. pRE5 is in 3′ of Nr2f1 and shows high levels of accessibility as well as a combination of active and repressive marks. Finally, pRE6 is predicted to be accessible and active in all assayed cell types.
Motif Analysis of Telencephalic and Nontelencephalic Nr2f1 Enhancers.
To identify putative regulators of the mouse Nr2f1 pallial enhancers, we searched for conserved TF binding motifs using simple enrichment analysis (SEA, version 5.5.4) from the MEME Suite (https://meme-suite.org/meme/tools/sea) (40). We found 14 known motifs with an E-value < 10 compared to shuffled input sequences. These motifs belonged to five TF families including E2F, Homeobox, Nuclear receptor, Zinc Finger, and Forkhead (SI Appendix, Fig. S3A), members of whom are known regulators of cortical development. Next, we performed de novo motif analysis of mouse hs1172 and hs271 using MEME and TomTom (SI Appendix, Fig. S4 C and D). We uncovered the same motifs as with SEA and also found a Paired motif in hs1172 and a T-box motif (TBX20) and a CBF motif in hs271 (Runx1) (SI Appendix, Fig. S4 C and D).
Next, we extended the SEA to human versions of these enhancers. Human hs1172 shares 89% base-pair identity with mouse hs1172; likewise human hs271 shares 93% base-pair identity with mouse hs271; these variations were scattered throughout the enhancers and the mouse versions had 1–2% gaps. In addition to the motifs identified in the mouse enhancers, we also found Paired Domain (i.e., PAX6) and Heat Shock Protein (i.e., HSFY2) motifs (SI Appendix, Fig. S4A). SEA of motifs in Nr2f1 nontelencephalic enhancers showed enrichment of only two motifs: a homeobox motif (NKX6-3) and a zinc finger motif (ZNF384) (SI Appendix, Fig. S4B).
Next, we mapped the locations of the TF motifs in mouse hs1172 and hs271 (SI Appendix, Fig. S3B). Based on the EMX2, LHX2, and PAX6 ChIP-seq analyses (2) (Fig. 2B), we identified the location of the EMX2, LHX2, and PAX6 motifs (SI Appendix, Fig. S3B). We next mapped the motifs discovered using SEA (SI Appendix, Fig. S3 B and C). Motif analysis showed that hs1172 possessed motifs from a variety of TF families. In contrast, hs271 had fewer known motifs, suggesting that its main regulators may be homeobox TFs (including EMX2 and LHX2) and possibly a Forkhead and/or a Zinc Finger TF.
Deletion of hs271 and hs1172 Differentially Affects Nr2f1 Pallial and Subpallial Expression.
We chose to study the function of hs271 and hs1172 based on multiple features: Their activity closely matches Nr2f1 pallial expression (Fig. 1C), they are both bound by 4 to 5 of the pallial TFs (Fig. 2B and Dataset S1), their epigenetic sensitivity to loss of Emx2 and Nr2f1 (Fig. 2C and Dataset S1), and the chromosomal looping which puts them in proximity to Nr2f1 (Fig. 2D).
We made an hs1172 deletion allele and a hs271+hs1172 pairwise deletion allele using CRISPR–Cas9 (Fig. 2E, SI Appendix, Fig. S6, and Dataset S2). We then generated a mutant that only had the hs271 deletion by screening for mice that had a recombination event which separated the hs271+hs1172 double deletion alleles (SI Appendix, Fig. S6). All of these mutant mice lines were viable and fertile and showed no obvious morphological phenotypes. We went on to phenotype these mice by comparing Nr2f1 RNA levels using in situ hybridization (ISH) in WT, hs271−/−, hs1172−/−, and hs271−/−; hs1172−/− at E12.5, E14.5, and P5.
First, we did not observe a clear change in Nr2f1 expression in hs1172+/− and hs271+/− heterozygotes at E12.5. On the other hand, the hs1172+/−; hs271+/− double heterozygotes showed reduced Nr2f1 expression in the VZ of the rostral pallium (red arrow in SI Appendix, Fig. S8 E and E1).
We quantified the changes in Nr2f1 expression in the rostrodorsal and rostrolateral pallium (Fig. 4 E and F). In hs1172−/−, there was a localized reduction of Nr2f1 VZ expression in rostral regions of the dorsomedial pallium (green arrows show the domain with the reduced Nr2f1). Thus, there was a caudoventral shift in Nr2f1 regional VZ expression (Fig. 4 B–B2 and E). In hs271−/−, there was a massive reduction of Nr2f1 VZ expression (red arrows, Fig. 4 C–C2) in most of the rostrolateral pallium (green arrows; Fig. 4 C–C2, E, and F).
Surprisingly, superficial laminar expression was preserved (blue arrow; Fig. 4 C–C2). hs271−/−; hs1172−/− double mutants were even more severe than hs271−/−, because the reduction of Nr2f1 VZ expression extended into the caudal pallium (Fig. 4 D–D4, E, and F). NR2F1 protein immunochemistry confirmed the reduction in Nr2f1 RNA expression (Fig. 4 A5–D5 and SI Appendix, Fig. S9).
Of note, both hs271−/− and hs271−/−; hs1172−/− mutants had reduced VZ expression in the LGE and CGE (orange arrow; Fig. 4 C2, C3, and D2–D4). This is consistent with the observation that hs1172 and hs271 have subpallial TF DLX2 binding sites; hs271 especially has multiple subpallial TF binding sites (ARX, DLX2, NKX2-1, OTX2, and SP9) (SI Appendix, Fig. S5) (41).
Thus, at E12.5, hs1172 and h271 have different roles in regulating Nr2f1. hs1172 promotes its regional expression in the rostrodorsomedial pallium. On the other hand, hs271 has a stronger function; rostral Nr2f1 VZ pallial expression is reduced along the entire dorsoventral dimension. Finally, hs271−/−; hs1172−/−had the greatest Nr2f1 reduction, providing evidence that these enhancers have at least partially redundant functions.
Next, we studied the phenotypes at E14.5; we quantified Nr2f1 expression in 10 laminar bins (SI Appendix, Fig. S13). We did not observe a large change in Nr2f1 expression in hs1172+/−, hs271+/− and hs1172+/−; hs271+/− heterozygotes (SI Appendix, Fig. S11). In hs1172−/− mutants, there was a subtle reduction in Nr2f1 expression in the upper SVZ (Fig. 5 B–B4 and SI Appendix, Fig. S13). hs271−/− mice had reduced Nr2f1 expression in the VZ, SVZ, and cortical plate (red arrow; Fig. 5 C–C4 and SI Appendix, Fig. S13). The reduced Nr2f1 RNA expression was confirmed using NR2F1 immunochemistry (Fig. 5 A5–D5 and SI Appendix, Fig. S12). The superficial pallial expression which was preserved at E12.5 (Fig. 4 C–C2 and D–D4) appears in a position that could either be subplate or SVZ (blue arrow; Fig. 5 C–C4); TBR2 coexpression showed that these cells were SVZ/secondary progenitors (SI Appendix, Fig. S12). At E14.5, the phenotypes of hs271−/− and the double mutant (hs271−/−; hs1172−/−) appeared indistinguishable (Fig. 5 D–D4 and SI Appendix, Fig. S13). Interestingly, we found Nr2f1 reduction in the rostrodorsomedial cortex of “sensitized” hs1172−/−; hs271+/− compared with hs1172+/−; hs271+/− (green arrow; SI Appendix, Fig. S11 E and F).
Previous enhancer deletion studies show that analyses using a sensitized background can reveal/amplify phenotypes (42). Thus, using an Nr2f1+/− background, we studied sensitized hs271+/−, hs1172+/−, and hs271+/−hs1172+/− mutants (SI Appendix, Figs. S7, S10, and S14). In each case, the Nr2f1+/− sensitized enhancer mutant showed reduced Nr2f1 expression compared to Nr2f1+/− and unsensitized controls (SI Appendix, Figs. S7 B–D, S10 B–D, and S14); the results were qualitatively stronger than that in the enhancer null mutants.
At postnatal day 5, we did not find strong evidence that hs271 or hs1172 continue to regulate Nr2f1 expression. Thus, hs271 and hs1172 are essential regulators of Nr2f1 expression at E12.5 and E14.5 and much less so at P5 (SI Appendix, Fig. S15).
Deletion of hs1172 and/or hs271 Results in Postnatal Neocortical Regional Patterning Defects.
As Nr2f1 regulates cortical patterning (31, 34), and as Nr2f1 expression is reduced at E12.5 and E14.5 in hs1172−/− and hs271−/− mutants, we hypothesized that these mutations may also alter cortical patterning. Thus, we assessed cortical regional properties at P5 using expression of NT3 and Lmo4, markers which distinguish cortical areas (43–45). We performed in situ hybridization on coronal sections of P5 brains. To amplify the effects of enhancer deletion in heterozygotes, we also tested NT3 and Lmo4 expression in these mutants in a Nr2f1+/− sensitized background.
In the neonatal cortex, NT3 is expressed in the secondary motor area/dorsalmedial prefrontal cortex (Mos/dmPFC) and along the dorsomedial cortex, including the cingulate and retrospenial cortex (Cg and Rs) (Fig. 6 A–A5) (44). We did not detect a change in NT3 expression in the hs1172−/− mutant (Fig. 6 B–B5), whereas there was a mild dorsal to ventral expansion in Nr2f1+/−/hs1172+/− double heterozygotes compared with Nr2f1+/− (SI Appendix, Fig. S16 B2, B3, C2, and C3). There was an obvious caudal expansion of NT3’s PFC expression in the hs271−/− and the hs1172−/−/hs271−/− mutants (Fig. 6 C–C3 and D–D3).
Fig. 6.
Deletion of Nr2f1 enhancers results in a caudal expansion of rostral cortical properties marked by NT3. NT3 in situ RNA expression in telencephalic coronal hemisections (rostral to caudal) in WT (A–A5), hs1172−/− (B–B5), hs271−/−(C–C5), and hs1172−/−/ hs271−/−(D–D5) at P5. NT3 strongly marks the PFC (lateral and dorsal parts), Cg and RS, is weak in the MCx, and not detectable in the SSCx (delimited by two green arrowheads). hs1172−/− shows no phenotype, whereas the hs271−/−and hs1172−/−/ hs271−/− lack the NT3-negative SSCx area in rostral sections. Abbreviation: Mos/mdPFC: secondary motor area/dorsomedial prefrontal cortex, vmPFC: ventromedial prefrontal cortex, AI: agranular insular, ORBI: orbital areas, ICx: insular cortex, SSCx: somatosensory cortex, MCx: motor cortex; Cg: cingulate cortex, RS: retrosplenial cortex. Magnification bar: 0.5 mm.
Lmo4 is expressed in the secondary motor area/dorsalmedial prefrontal cortex (Mos/dmPFC), ventralmedial prefrontal cortex (vmPFC), agranular insular (AI), motor cortex (MCx), visual cortex (VCx), auditory cortex (ACx), and dorsomedial cortex (Cg and Rs), but negative in the somatosensory cortex (SSCx) (between green arrows; Fig. 7 A–A6) (43, 44).
Fig. 7.
Lmo4 expression patterning showed Nr2f1 enhancers mutants had rostral/caudal cortical reginal patterning defects. Lmo4 in situ RNA expression in telencephalic coronal hemisections (rostral to caudal) in WT (A–A6), hs1172−/− (B–B6), hs271−/−(C–C6), and hs1172−/−/ hs271−/−(D–D6) at P5. Lmo4 marks the PFC (lateral, dorsal, medial and orbital parts), MCx, Cg, RS, VCx, and ACx but is weak in the SSCx (delimited by two green arrowheads). hs1172−/− shows no phenotype in rostral cortex, whereas caudally there is an expansion of the Lmo4-negative SSCx area. hs271−/−and hs1172−/−/ hs271−/− lack the Lmo4-negative SSCx area in rostral sections, caudally there is a robust expansion of the Lmo4-negative SSCx area. Abbreviations: ACx: auditory cortex, for the rest of the abbreviations, see legend to Fig. 6. Magnification bar: 0.5 mm.
In hs1172−/−, there was a caudal expansion of the Lmo4-negative SSCx (Fig. 7 B4–B6) (but, Lmo4 expression is normal in the rostral cortex). The hs1172+/−; Nr2f1+/− double heterozygotes had an even stronger caudal expansion of the SSCx (SI Appendix, Fig. S17 C5 and C6). In the hs271−/− and hs1172−/−/hs271−/− mutants, there was a caudal expansion of the Lmo4+ PFC and a caudal expansion of the SSCx domain (Fig. 7 C1–D6).
The hs271+/−/Nr2f1+/− double heterozygotes also exhibited a caudal expansion of the PFC (SI Appendix, Fig. S17 D–D4). The hs271+/−/hs1172−/−/Nr2f1+/− had the strongest caudal expansion (SI Appendix, Fig. S17 E4–E6).
De Novo Human Mutations in VISTA Enhancers Near Nr2f1.
As Nr2f1 haploinsufficiency leads to Bosch–Boonstra–Schaff optic atrophy syndrome (32) and 80% of those individuals also exhibited ASD (Autism spectrum disorder) (46). We performed WGS (family-based whole-genome sequencing) analysis to identify putative de novo mutations in 5 Nr2f1 enhancers (mm1556, mm881, hs271, hs1172, and hs1049).
Seven de novo mutations were identified in whole-genome sequencing data of 8,626 ASD cases (six mutations) and 4,461 sibling controls (one mutation) in VISTA elements near Nr2f1 (Fig. 8). In hs1172, a de novo single nucleotide variant (SNV) was observed in an ASD case (MT_195.4) from a multiplex family at a highly conserved position (PhyloP: 2.97, vertebrate 100-way) while a de novo SNV in a sibling (SSC08514) was at a weakly conserved position (PhyloP: 1.16). Three de novo SNVs were observed in three different ASD cases in the hs271 element. All three SNVs are highly conserved (PhyloP: 2.09, 5.70, 4.71). Finally, in mm881, a de novo indel (GAG>TTT, PhyloP: 2.22) and de novo SNV (PhyloP: −0.26) were identified in two ASD cases, both in weakly conserved positions. No de novo mutations were observed in hs1049 or mm1556. While noncoding de novo mutations in these elements are observed more frequently in cases than controls, the difference is not significant (p = 0.25, Chi-squared).
Fig. 8.
De novo mutations in VISTA elements near human NR2F1. VISTA regulatory elements (purple) and genes (blue) in the genomic region around NR2F1 are shown at the bottom. PhyloP conservation scores for the region based on 100-way vertebrate analysis are shown under the genes. Three inserts show the location of seven de novo mutations observed in 8,626 ASD cases (red) and 4,461 sibling controls (blue). Labels show the sample size, genomic position of the first nucleotide, and the nucleotide change (VCF format).
Discussion
Herein, phenotypic analysis of mouse lines with deletion of two Nr2f1 pallial enhancers provides evidence that each enhancer has essential and distinct functions. The Nr2f1 locus has at least six potential pallial enhancers based on transgenic assays (Fig. 1C and SI Appendix, Fig. S1B) (VISTA—https://enhancer.lbl.gov/) and epigenetic analyses (Fig. 2 and Dataset S1) (2). This is very likely an underestimate, particularly as each assay is restricted to one age (E11.5, VISTA) and one tissue (cortex) (2). Thus, this paper, which focuses on enhancers hs271 and hs1172, largely focuses on the function of Nr2f1 enhancers that have known activity in the cortical VZ.
Our epigenetic analysis of enhancer activity over time in the VZ, SVZ, immature prenatal neurons, and in P2 layers 5 and 6 (Fig. 3) enabled us to follow the properties of the six Nr2f1 cortical enhancers and to identify additional candidate enhancer regions, some adjacent to the enhancers which we focused on (i.e., hs271 and hs1172) (SI Appendix, Fig. S2). Future studies should investigate enhancers that control Nr2f1 expression in differentiating and mature pallial neurons in all six cortical layers.
The major findings herein are that both hs271 and hs1172 are essential for normal Nr2f1 expression in the E12.5 and E14.5 cortex. hs271 seems to be a stronger enhancer, as Nr2f1 expression is greatly reduced in the VZ of the rostral pallium at both E12.5 and E14.5 (Figs. 4 C–C5 and 5 C–C5 and SI Appendix, Figs. S7 C–C4 and S10 C–C4). The hs1172 mutant largely reduces Nr2f1 expression in dorsal regions of the rostral pallium, implying that its function is primarily important in refining rostrodorsal aspects of the regionalization process (Fig. 4 B–B2 and SI Appendix, Fig. S7 B–B5). On the other hand, the hs271 mutant has a global reduction in VZ Nr2f1 expression in the rostral pallium, showing that it is a powerful regulator of Nr2f1 neuroepithelial expression. The hs271/ hs1172 double mutant further reduces Nr2f1 expression but largely in the pattern of hs271, showing that these two enhancers have partially redundant functions that are dominated by hs271.
It is of note that the caudal pallium is relatively normal in the single mutants, implying that other enhancer(s) drive Nr2f1 expression in these regions. Normal Nr2f1 expression is in a high caudal-low rostral gradient (31); thus, the hs271 and hs1172 mutations affect pallial regions with lower Nr2f1 expression. Likewise, Nr2f1 expression is high ventral-low dorsal (30); and thus, the hs1172 phenotype affects dorsal regions with lower Nr2f1 expression. Therefore, regions with lower Nr2f1 expression are particularly sensitive to these enhancer deletions.
Further specificity is shown in the hs271 mutant, where Nr2f1 expression is greatly reduced in the VZ but inappreciably altered in the SVZ at E12.5 and E14.5 (Figs. 4 and 5 and SI Appendix, Figs. S7 and S10), implying that there must be a different enhancer(s) that drives Nr2f1 expression in the SVZ.
At P5, cortical plate Nr2f1 expression is remarkably normal in both the single and double enhancer mutants (SI Appendix, Fig. S15), aside from some alterations which might reflect earlier cortical patterning changes (Figs. 6 and 7). Thus, it is very likely that there are other enhancers that drive Nr2f1 expression in the postnatal cortex.
Nr2f1 null mutants have a striking expansion of the rostral cortex and a corresponding retraction of the caudal cortex (31). Given the reduced Nr2f1 expression at E12.5 and E14.5, we were not surprised to find evidence for a qualitatively similar regional patterning defect at P5 in the enhancer mutants (Figs. 6 and 7). It is noteworthy, however, that this phenotype occurs despite the relatively normal Nr2f1 expression at P5. This suggests that once the patterning defect is generated by reduced Nr2f1 at E12.5–E14.5, it cannot be rescued by neonatal Nr2f1 expression.
Our analyses of the enhancers’ nucleotide sequences, in conjunction with TF ChIP-seq, identified likely motifs that may be bound in vivo by TFs (Fig. 2 and SI Appendix, Fig. S3). Mutagenesis studies are needed to verify the function of these motifs. Human vs. mouse Nr2f1 enhancer sequence variations show that prominent evolutionary changes have taken place in hs271 and hs1172. Future studies are needed to evaluate whether these variations have functional significance in pallial activity of these enhancers, particularly as Nr2f1 haploinsuffiency causes intellectual disability, developmental delay, and cortical polymicrogyria (32, 47). Finally, we have found allelic variation in hs271 and hs1172 in individuals with ASD, with some mutations in highly conserved nucleotides (Fig. 8). While it is not yet possible to ascribe deleterious effects of these mutations on human cortical development/function without more patient data and functional tests of the mutant enhancers, it does raise the possibility of Nr2f1 enhancer mutations may contribute to cognitive disorders, in addition to inner ear defects (33).
In sum, the complexity of Nr2f1 developmental expression is encoded by multiple enhancers with distinct and partial overlapping regional and temporal functions. Their redundancies provide genetic buffering against function-altering mutations, which may have deleterious effects on development and/or may open the door for modifications that increase evolutionary fitness.
Materials and Methods
All experiments were performed according to the University of California San Francisco Laboratory Animal Research Center (LARC) protocol (number: AN195095). Details of materials and methods of generation of single and double hs1172 and hs271 enhancers deletion mutant mice, histology, quantification of in situ signal density, WGS analysis, TF ChIP-seq (Chromatin Immunoprecipitation followed by DNA sequencing), histone ChIP-seq and ATAC-seq, and motif analysis can be found in SI Appendix. ChIP Sequencing data have been previously described (2).
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (PDF)
Acknowledgments
This work was supported by the research grants to J.L.R. from: Nina Ireland and NINDS RO1 NS099099. D.E.D., A.V., and L.A.P. were supported by NIH Grants R01MH117106 and R01HG003988. Research conducted at the E.O. Lawrence Berkeley National Laboratory was performed under U.S. Department of Energy Contract DE-AC02-05CH11231, University of California (UC). This work was also supported by grants to S.J.S. from the NIMH (R01 MH129751 and U01 MH122681). Epigenetic data in Fig. 2 were analyzed by Justin Lim (UCSF data scientist in John Rubenstein’s lab). Epigenetic data in SI Appendix, Fig. S2was previously analyzed by Rinaldo Catta-Preta and Alex Nord (UC Davis) in published experiments.
Author contributions
Z.L., A.R.Y., and J.L.R. designed research; Z.L., A.R.Y., E.M.-P., D.E.D., S.J.S., L.A.P., and A.V. performed research; D.E.D., S.J.S., L.A.P., A.V., and J.L.R. contributed new reagents/analytic tools; Z.L., A.R.Y., S.J.S., S.D., L.A.P., A.V., and J.L.R. analyzed data; and Z.L., A.R.Y., and J.L.R. wrote the paper.
Competing interests
J.L.R. is cofounder, stockholder, and currently on the scientific board of Neurona, a company studying the potential therapeutic use of interneuron transplantation. J.L.R has stock in Neurona.
Footnotes
This article is a PNAS Direct Submission. J.D.M. is a guest editor invited by the Editorial Board.
Data, Materials, and Software Availability
Epigenetics data will be deposited in GEO.
Supporting Information
References
- 1.Chen B., Kwan K. Y., Rubenstein J. L. R., Rakic P., Patterning and Cell Type Specification in the Developing CNS and PNS: Comprehensive Developmental Neuroscience (Academic Press, Amsterdam, The Netherlands, ed. 2, 2020), pp. xxiv, 1098pp. [Google Scholar]
- 2.Ypsilanti A. R., et al. , Transcriptional network orchestrating regional patterning of cortical progenitors. Proc. Natl. Acad. Sci. U.S.A. 118, e2024795118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visel A., Minovitsky S., Dubchak I., Pennacchio L. A., VISTA Enhancer Browser–a database of tissue-specific human enhancers. Nucleic Acids Res. 35, D88–D92 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visel A., et al. , A high-resolution enhancer atlas of the developing telencephalon. Cell 152, 895–908 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bejerano G., et al. , Ultraconserved elements in the human genome. Science 304, 1321–1325 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Visel A., et al. , Ultraconservation identifies a small subset of extremely constrained developmental enhancers. Nat. Genet. 40, 158–160 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pattabiraman K., et al. , Transcriptional regulation of enhancers active in protodomains of the developing cerebral cortex. Neuron 82, 989–1003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nord A. S., et al. , Rapid and pervasive changes in genome-wide enhancer usage during mammalian development. Cell 155, 1521–1531 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Torre-Ubieta L., et al. , The dynamic landscape of open chromatin during human cortical neurogenesis. Cell 172, 289–304.e218 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markenscoff-Papadimitriou E., et al. , A chromatin accessibility atlas of the developing human telencephalon. Cell 182, 754–769.e718 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capra J. A., Erwin G. D., McKinsey G., Rubenstein J. L., Pollard K. S., Many human accelerated regions are developmental enhancers. Philos. Trans. R Soc. Lond. B, Biol. Sci. 368, 20130025 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reilly S. K., et al. , Evolutionary genomics. Evolutionary changes in promoter and enhancer activity during human corticogenesis. Science 347, 1155–1159 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girskis K. M., et al. , Rewiring of human neurodevelopmental gene regulatory programs by human accelerated regions. Neuron 109, 3239–3251.e3237 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whalen S., et al. , Machine learning dissection of human accelerated regions in primate neurodevelopment. Neuron 111, 857–873.e858 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd J., et al. , Human-chimpanzee differences in a FZD8 enhancer alter cell-cycle dynamics in the developing neocortex. Curr. Biol. 25, 772–779 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luquette L. J., et al. , Single-cell genome sequencing of human neurons identifies somatic point mutation and indel enrichment in regulatory elements. Nat. Genet. 54, 1564–1571 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahituv N., et al. , Deletion of ultraconserved elements yields viable mice. PLoS Biol. 5, e234 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickel D. E., et al. , Ultraconserved enhancers are required for normal development. Cell 172, 491–499.e415 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shim S., Kwan K. Y., Li M., Lefebvre V., Šestan N., Cis-regulatory control of corticospinal system development and evolution. Nature 486, 74–79 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suda Y., et al. , The same enhancer regulates the earliest Emx2 expression in caudal forebrain primordium, subsequent expression in dorsal telencephalon and later expression in the cortical ventricular zone. Development 137, 2939–2949 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Theil T., Aydin S. I., Koch S., Grotewold L., Rüther U., Wnt and Bmp signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development 129, 3045–3054 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Hasenpusch-Theil K., et al. , Transcriptional analysis of Gli3 mutants identifies Wnt target genes in the developing hippocampus. Cerebr. Cortex 22, 2878–2893 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenger A. M., et al. , The enhancer landscape during early neocortical development reveals patterns of dense regulation and co-option. PLOS Genet. 9, e1003728 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandberg M., et al. , Genomic analysis of transcriptional networks directing progression of cell states during MGE development. Neural. Dev. 13, 21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darbandi S., et al. , Functional consequences of I56ii Dlx enhancer deletion in the developing mouse forebrain. Dev. Biol. 420, 32–42 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Darbandi S., et al. , Increased sociability in mice lacking intergenic Dlx enhancers. Front. Neurosci. 15, 718948 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindtner S., et al. , Genomic resolution of DLX-orchestrated transcriptional circuits driving development of forebrain GABAergic neurons. Cell Rep. 28, 2048–2063.e2048 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alfano C., Magrinelli E., Harb K., Hevner R., Studer M., Postmitotic control of sensory area specification during neocortical development. Nat. Commun. 5, 5632 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Tomassy G., et al. , Area-specific temporal control of corticospinal motor neuron differentiation by COUP-TFI. Proc. Natl. Acad. Sci. U.S.A. 107, 3576–3581 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faedo A., et al. , COUP-TFI coordinates cortical patterning, neurogenesis, and laminar fate and modulates MAPK/ERK, AKT, and beta-catenin signaling. Cereb. Cortex 18, 2117–2131 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armentano M., et al. , COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat. Neurosci. 10, 1277–1286 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Bertacchi M., et al. , NR2F1 regulates regional progenitor dynamics in the mouse neocortex and cortical gyrification in BBSOAS patients. EMBO J. 39, e104163 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarchini B., et al. , A spontaneous mouse deletion in Mctp1 uncovers a long-range cis-regulatory region crucial for NR2F1 function during inner ear development. Dev. Biol. 443, 153–164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonev B., et al. , Multiscale 3D genome rewiring during mouse neural development. Cell 171, 557–572.e524 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borello U., et al. , Sp8 and COUP-TF1 reciprocally regulate patterning and Fgf signaling in cortical progenitors. Cereb. Cortex 24, 1409–1421 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Touzot A., Ruiz-Reig N., Vitalis T., Studer M., Molecular control of two novel migratory paths for CGE-derived interneurons in the developing mouse brain. Development 143, 1753–1765 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Hu J. S., et al. , Coup-TF1 and Coup-TF2 control subtype and laminar identity of MGE-derived neocortical interneurons. Development 144, 2837–2851 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng J., et al. , COUP-TFI specifies the medial entorhinal cortex identity and induces differential cell adhesion to determine the integrity of its boundary with neocortex. Sci. Adv. 7, eabf6808 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muzio L., Mallamaci A., Emx1, emx2 and pax6 in specification, regionalization and arealization of the cerebral cortex. Cereb. Cortex 13, 641–647 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Timothy L. B., Charles E. G., SEA: Simple enrichment analysis of motifs. bioRxiv [Preprint] (2021). 10.1101/2021.08.23.457422 (Accessed 24 August 2021). [DOI]
- 41.Rinaldo C.-P., et al. , Combinatorial transcription factor binding encodes cis-regulatory wiring of forebrain GABAergic neurogenesis. Dev. Cell, in press.
- 42.Osterwalder M., et al. , Enhancer redundancy provides phenotypic robustness in mammalian development. Nature 554, 239–243 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bishop K. M., Goudreau G., O’Leary D. D., Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science 288, 344–349 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Golonzhka O., et al. , Pbx regulates patterning of the cerebral cortex in progenitors and postmitotic neurons. Neuron 88, 1192–1207 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cholfin J. A., Rubenstein J. L., Frontal cortex subdivision patterning is coordinately regulated by Fgf8, Fgf17, and Emx2. J. Comp. Neurol. 509, 144–155 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rech M. E., et al. , Phenotypic expansion of Bosch–Boonstra–Schaaf optic atrophy syndrome and further evidence for genotype–phenotype correlations. Am. J. Med. Genet., Part A 182, 1426–1437 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Kaplanis J., et al. , Evidence for 28 genetic disorders discovered by combining healthcare and research data. Nature 586, 757–762 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (PDF)
Data Availability Statement
Epigenetics data will be deposited in GEO.