Abstract
Introduction: This study aimed to assess the effect of repeated irradiations of 660 nm photobiomodulation therapy (PBMT) after photodynamic therapy (PDT) with curcumin on the viability of human gingival fibroblasts (HGFs).
Methods: In this in vitro, experimental study, HGFs were cultured and assigned to five groups: One control group with no intervention and four experimental groups of PDT with curcumin, PBMT (660 nm laser irradiation) immediately after PDT, PBMT immediately and 24 hours after PDT and PBMT immediately and 24 hours and 48 hours after PDT. Cell viability was assessed after 1, 4, and 7 days using the methyl thiazolyl tetrazolium (MTT) assay. Data were analyzed by one-way ANOVA.
Results: On day 1, the control group had no significant difference with one-time (P=1.00), two-time (P=1.00), and three-time (P=0.88) laser irradiation groups. On day 4, the difference between the control and one-time (P<0.001), two-time (P<0.001) and three-time (P=0.02) laser irradiation groups was statistically significant, suggesting more cell viability in test groups. On day 7, the three-time laser irradiation group showed significant cell viability compared to the other two test groups but not with the control group (P=0.98).
Conclusion: PBMT with 660 nm laser irradiation after PDT with curcumin would increase the viability of HGFs by increasing the frequency of irradiation.
Keywords: Human gingival fibroblasts, Photodynamic therapy, Curcumin, Low-level laser, Photobiomodulation
Introduction
Periodontal disease as an oral health dilemma in today’s world is a leading factor in tooth loss. It develops due to the accumulation of microbial plaque on teeth, resulting in the production of microbial byproducts and subsequent inflammation and host response. Several host-related risk factors are also implicated in the progression of periodontal disease. Additionally, various host-related risk factors like age play a role in the advancement of periodontal disease.1
Periodontal cells, such as gingival fibroblasts, osteoblasts, cementoblasts, and periodontal ligament (PDL) fibroblasts, are crucial for periodontal tissue healing and regeneration. Among these cell types, fibroblasts are particularly important as they release various growth factors during the re-epithelialization of wounds and the formation of granulation tissue.2
Periodontal surgery is frequently selected as the preferred treatment for advanced periodontal disease due to its invasive nature, lengthy recovery period, and common occurrence of postoperative pain. In 1967, Endre Mester demonstrated that low-level lasers, now recognized as photobiomodulation therapy (PBMT), can induce biological changes along their path of irradiation. These changes include improved wound healing, increased collagen synthesis, neovascularization, and enhanced enzyme production in the irradiated tissue.3
Photosensitizers (PSs) are used in treatments such as photodynamic therapy (PDT) and produce reactive oxygen species (ROSs) after being irradiated by a proper wavelength of light. ROSs include free radicals and singlet oxygen with antifungal and antibacterial effects.4 PDT is a novel promising antibacterial modality that uses a combination of PS, light, and oxygen for the effective resolution of wound infections, elimination of antibiotic-resistant bacteria, and degradation of cancer cells.4 In this method, irradiation of a specific wavelength of light activates PS molecules in the presence of molecular oxygen and results in the production of ROSs, which can damage the cell membrane and lead to the subsequent leakage of intracellular components and the inactivation of intracellular transport, eventually resulting in the death of microorganisms through a bactericidal effect.5 PDT can eliminate microorganisms lodged in periodontally inaccessible areas. Therefore, it could be utilized as a substitute or supplement in managing periodontitis and peri-implantitis.6 Non-invasiveness, reproducibility, wide-spectrum antimicrobial activity, not generating light-resistant species following multiple treatments, and local application over the target tissue are among the main advantages of PDT.7
Due to toxic effects in high doses, PDT has been applied for cancer treatment as well. Moreover, it can be used to induce cell regeneration and proliferation if combined with low-level laser therapy.8,9 The mechanisms of action of PBMT are highly complex; however, they require the absorption of specific visible red and near infrared wavelengths by the photo-receptors of subcellular components, particularly the mitochondrial electron transfer chain (respiratory cycle). Light absorption by the respiratory cycle components results in short-term activation of the respiratory cycle and oxidation of the NADH pool. The induction of oxidative phosphorylation changes the mitochondrial and cytoplasm redox state and resultantly increases the ATP storage, cytoplasm alkalization, and activation of nucleic acid synthesis. The three aforementioned events stimulate normal cell function and can lead to tissue regeneration in response to PBMT such as a 660 nm laser. The stimulation of cells coated with small amounts of PS by PDT with a light-emitting diode (LED) or laser with a proper wavelength and suitable duration can activate different cascades in proliferation pathways. However, adequate knowledge about PSs, duration of treatment, and suitable target cells is imperative to achieve this goal.10,11
Curcumin, derived from the Curcuma longa L. (turmeric) rhizome, is a potent compound known as diferuloylmethane with the chemical formula C21H20O6. It is composed of two aryl buten-2-one (feruloyl) chromophores connected by a methylene group.12 Despite its low toxicity, Curcumin exhibits various pharmacological benefits including antioxidant, anti-inflammatory, antimicrobial, and anti-cancer properties.
It neutralizes ROSs and protects the natural cells from oxidative damage as such. It has been reported that the antioxidant and free radical-reducing effects of curcumin are attributed to its OH phenolic groups or CH2 group of the beta-diketone part of the molecule. Moreover, it has been confirmed that curcumin can regulate the cellular activity of several cytokines and growth factors.13 Firstly, curcumin down-regulates the epidermal growth factor by the down-regulation of expression and the activity of epidermal growth factor receptors. Secondly, curcumin also down-regulates the activity of human EGFR-2 (neu/2-HER) which is a growth factor receptor with significant correlations with breast cancer, lung cancer, renal cancer, and prostate cancer. Thirdly, curcumin suppresses the activity of interleukin-6 through the down-regulation of STAT3.12,14 It also inhibits the transforming growth factor-B1 and decreases the production of many pro-inflammatory cytokines such as MPC-1 and tumor necrosis factor-alpha. Furthermore, it has been suggested that the beneficial effects of curcumin are further augmented by photo-activation. This statement encouraged the researchers to further focus on the applications of this pigment in photochemical and photobiological fields. Curcumin is activated by an LED in the wavelength range of 300 to 500 nm (maximum 430 nm).14,15
Despite the advancements in the use of lasers for periodontal therapy, there are still numerous unanswered questions surrounding its potential toxic effects on tissues. Factors such as cell type, PS type, PS concentration, light dosage, power, and density, as well as exposure duration, require further investigation.16
The diode laser is frequently utilized in the treatment of periodontal disease due to its ability to positively influence the proliferation and differentiation of fibroblasts. This, in turn, leads to improved wound healing and faster treatment outcomes.17,18
PDT has shown promising results in the treatment of periodontal disease by the elimination of microorganisms lodged in hard-to-reach and inaccessible areas.19 Nevertheless, there is still a dearth of sufficient data concerning its impact on the survival of human gingival fibroblasts (HGFs). Also, soft tissue lasers have shown positive efficacy for the bacterial reduction and regeneration of periodontal tissues. Thus, given that laser therapy can increase the viability of HGFs, it may be used as a novel treatment modality. Therefore, this study aimed to evaluate the concomitant impact of two novel modalities, namely PDT and PBMT, on the viability of HGFs. Accordingly, the obtained knowledge regarding the advantages and disadvantages of this treatment may pave the way for its application in the treatment of patients whose systemic condition does not allow complex and long surgical procedures.
Materials and Methods
This in vitro, experimental study was conducted at the Biotechnology Department of Tehran University of Medical Sciences. According to the available literature regarding fibroblasts, the sample size was calculated to be 45 for 5 groups.
HGFs (IBRC C10459) were acquired from the Cell Bank of Pasteur Institute of Iran. The cells were cultured at 37°C, 95% air, and 5% CO2 in low-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Gibco) and Pen Strep solution (10 000 units/mL penicillin and 10 000 µg/mL streptomycin) to achieve a cell density of 10 000 cells/cm2. The growth medium was replaced every 3 days.
Light exposure was carried out by using an LED unit (WL-090, Dentmate Co., Taiwan) emitting light at a wavelength of 390-480 nm with a peak at 460 nm, a power density of 1000 ± 100 mW/cm2, and an energy density of 60 J/cm2. The power output was assessed by using a power-meter. Before PDT treatment, the culture medium was removed, the cells were washed twice with sterile phosphate buffered saline (pH 7.4), and 100 µL of curcumin at a concentration of 40 µM was added to the microplates. Methylene blue was added to the outer culture plates to block light exposure to the central samples. Subsequently, the samples were placed in a dark, humid environment with 95% air and 5% CO2 for a duration of 5 minutes. The cells were then subjected to LED irradiation with a mean energy density of 60 J/cm2 at room temperature. The LED probe was positioned 1 mm above the surface of microplates on a microphone stub and irradiation was performed in a circular manner. To prevent light reflection from the table surface, black papers were placed below the microplates. The diameter of the irradiated area matched the diameter and dimensions of the bottom surface of the microplates. Subsequently, 100 µL of the culture medium was introduced into each well as previously described. Each group was subjected to the methyl thiazolyl tetrazolium (MTT) assay on days 1, 4, and 7. The samples were then allocated to five groups using simple randomization:
Group 1. PDT: curcumin (40 µM) and LED irradiation with an average dose of 60 J/cm2
Group 2. PDT + PBMT 1 time: Samples in this group were subjected to PDT as explained for group 1 and then underwent immediate irradiation of 660 nm diode laser in continuous-wave mode with 150 mW power, 0.24 W/cm2 power density, and 7.2 J/cm2 irradiation dosage for 30 seconds.
Group 3. PDT + PBMT 2 times: Samples in this group were subjected to PDT as explained for group 1 and then underwent irradiation of 660 nm diode laser with the same parameters mentioned in group 2 immediately and 24 hours after PDT.
Group 4. PDT + PBMT 3 times: Samples in this group were subjected to PDT as explained for group 1 and then underwent irradiation of 660 nm diode laser with the same parameters mentioned in group 2 immediately, 24 hours and 48 hours after PDT.
Group 5. Control: Samples that did not undergo any intervention.
All groups underwent the MTT assay on days 1, 4, and 7.
MTT Assay
For the MTT assay at each time point, the supernatant was discarded and 40 µg of the MTT solution (5 mg/mL in phosphate buffered saline; Sigma, Germany) was added to each well. The plates were incubated again for 3-4 hours at 37 °C and 5% CO2. Finally, the MTT solution was extracted, and 60 µg dimethyl sulfoxide was added. The optical density of each well was then read at a 570 nm wavelength by a microplate reader (BioTek, USA). Finally, the data were analyzed by ANOVA.
Results
The research was carried out on 45 samples divided into 5 groups, with each group consisting of 9 samples. Each group underwent three consecutive assessments. The statistical analysis using two-way ANOVA revealed a significant interaction effect between time and group on cell viability (P < 0.001). Consequently, the results were analyzed separately for each group and assessment time point.
According to the data presented in Table 1, there was no significant disparity in cell viability between the PDT with curcumin and 660 nm laser and the control group on day 1, as indicated by the one-way ANOVA analysis (P = 0.310).
Table 1. Results of the MTT Assay in the Five Groups on Days 1, 4 and 7 .
| Group | Mean±SD | Range |
| Day 1 | ||
| Control | 0.0653 ± 0.05470 | 0.03-0.20 |
| PDT with curcumin | 0.0196 ± 0.0027 | 0.02-0.03 |
| (PDT with curcumin) + 660 nm laser 0 h | 0.0653 ± 0.05470 | 0.03-0.20 |
| (PDT with curcumin) + 660 nm laser 24 h | 0.0741 ± 0.03661 | 0.05-0.16 |
| (PDT with curcumin) + 660 nm laser 48 h | 0.0920 ± 0.01799 | 0.07-0.12 |
| Day 4 | ||
| Control | 0.2959 ± 0.04436 | 0.22-0.35 |
| PDT with curcumin | 0.1743 ± 0.03330 | 0.08-0.36 |
| (PDT with curcumin) + 660 nm laser 0 h | 0.1873 ± 0.00517 | 0.13-0.23 |
| (PDT with curcumin) + 660 nm laser 24 h | 0.2312 ± 0.02083 | 0.18-0.20 |
| (PDT with curcumin) + 660 nm laser 48 h | 0.2959 ± 0.04436 | 0.20-0.26 |
| Day 7 | ||
| Control | 0.2370 ± 0.03820 | 0.19-0.29 |
| PDT with curcumin | 0.1740 ± 0.03395 | 0.10-0.22 |
| (PDT with curcumin) + 660 nm laser 0 h | 0.1676 ± 0.01054 | 0.15-0.19 |
| (PDT with curcumin) + 660 nm laser 24 h | 0.1807 ± 0.00919 | 0.16-0.19 |
| (PDT with curcumin) + 660 nm laser 48 h | 0.2270 ± 0.03820 | 0.21-0.23 |
On day 4, one-way ANOVA showed a significant difference between the PDT with curcumin and 660 nm laser and the control group (P = 0.043). Similarly, on day 7, one-way ANOVA showed a significant difference between the PDT with curcumin and 660 nm laser and the control group (P = 0.020).
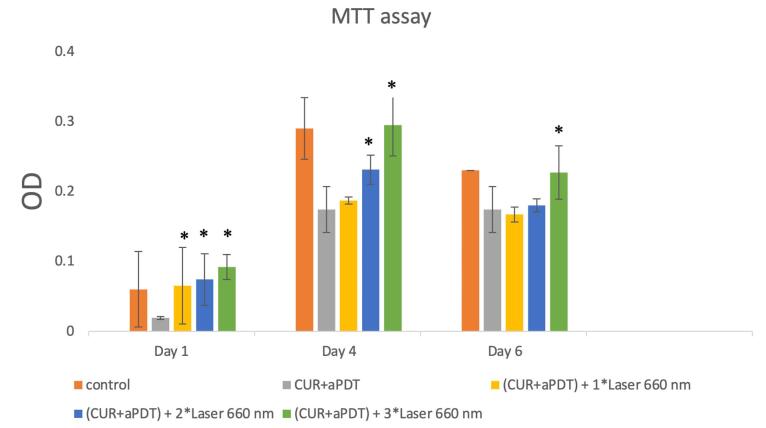
As illustrated in Table 2, on day 1, one-way ANOVA revealed that the control group had no significant difference with one-time (P = 1.00), two-time (P = 1.00), and three-time (P = 0.88) laser irradiation groups. On day 4, the difference between the control and one-time (P < 0.001), two-time (P < 0.001) and three-time (P = 0.02) laser irradiation groups was statistically significant. On day 7, the control group had a significant difference with one-time (P = 0.005) and two-time (P = 0.02) laser irradiation groups but not with the three-time laser irradiation group (P = 0.98) (Figure 1).
Table 2. Comparison of the Results of the MTT Assay Between the Experimental and Control Groups on Days 1, 4 and 7 .
| Groups | Time | P value |
| PDT with curcumin | Day 1 | 0.310 |
| Day 4 | 0.043 | |
| Day 7 | 0.020 | |
| (PDT with curcumin) + 660 nm laser 0 h | Day 1 | 1.000 |
| Day 4 | 0.000 | |
| Day 7 | 0.005 | |
| (PDT with curcumin) + 660 nm laser 24 h | Day 1 | 1.000 |
| Day 4 | 0.001 | |
| Day 7 | 0.020 | |
| (PDT with curcumin) + 660 nm laser 48 h | Day 1 | 0.887 |
| Day 4 | 0.021 | |
| Day 7 | 0.983 |
Figure 1.
Cell Viability Assessment by the MTT Assay in Study Groups. * Significantly different from the PDT group, P < 0.05
Discussion
The impact of PDT using an LED and curcumin in combination with PBMT with 660 nm irradiation on the viability of HGFs in vitro was evaluated in this research. In line with earlier research,20,21 the current findings demonstrated that PDT with a 430 nm LED and curcumin as PS negatively affected the viability of HGFs. Furthermore, exposure to laser irradiation in the visible red light spectrum (660 nm) following PDT enhanced the viability of weakened HGFs, consistent with previous study outcomes.22-24 The present study is among the few on the effects of repeated irradiations of 660 nm laser on HGFs.
The effects of PBMT alone and the comparison of different wavelengths of lasers on cells have been evaluated in some previous studies. Moore et al conducted an experiment to assess the impact of laser irradiation in the visible red spectrum (625, 635, 645, 655, 665, and 675 nm) and an 810 nm laser on the proliferation of fibroblasts and endothelial cells. Through a colorimetric assay, they determined that all visible red wavelengths (625-675 nm) led to increased fibroblast proliferation, with the most significant effect observed at 665 nm and 675 nm wavelengths. Despite variations in culture media, cell preparation, and assay methods between their study and the current findings, both studies demonstrated the beneficial effects of visible red spectrum laser irradiation on fibroblast viability and proliferation. Additionally, Moore et al utilized murine cells, while the present study used HGFs, enhancing the generalizability of the current results to clinical applications.25
Ismiyatin et al conducted a study to assess the impact of different irradiation times on PDL fibroblasts. They exposed the fibroblasts to 650 nm PBMT with a power of 20 mW for durations of 15 and 35 seconds. The researchers concluded that both 15 and 35 seconds of irradiation significantly improved the viability of PDL fibroblasts. Although there were variations in the isolation of fibroblasts and the culture media used, their findings were consistent with the results of the present study. In our study, the laser power, duration of irradiation, and distance between the light source and the culture medium surface were adjusted to increase the dosage of light received by the fibroblasts. Despite receiving a lower dosage of radiation in their study, their results are still aligned with our findings, further confirming the positive effects of PBMT in the visible light spectrum on fibroblast viability.26
The study conducted by Szezerbaty et al in 2018 demonstrated that enhancing the frequency of irradiations (up to 72 hours) and power density (5 J/cm2) resulted in higher fibroblast viability and upregulation of genes associated with the secretion of vascular endothelial growth factor.
They concluded that by an increase in the frequency of irradiations and time passed by 7 days, fibroblasts experienced an ascending trend of viability. Similarly, HGFs in the present study experienced a significant increase in viability in all groups on day 7 compared with day 1.22 Szlasa et al in their in vitro study on the necrotic and apoptotic activity of curcumin demonstrated that fibroblasts were less affected by the activity of curcumin and PDT, which may be due to the stimulation of some pathways related to their regeneration; their findings were in accordance with the present results.27 Choi et al examined the alterations in morphology, cellular function, and HSP90 gene expression in fibroblasts after PBMT. A 660 nm laser was applied at an energy density of 50 mW/cm2 for durations of 15, 30, and 60 minutes. The researchers conducted the MTT assay and observed enhanced fibroblast viability across all time points 24 hours post-laser exposure.28 Based on the studies mentioned earlier, the current research found that the viability of HGFs increased after exposure to 660 nm laser irradiation, as shown by the results of the MTT assay. Despite variations in the duration of laser exposure, energy density, and type of fibroblasts used in the two studies (both involving skin fibroblasts), they both concluded that 660 nm laser irradiation promoted the viability and proliferation of fibroblasts. One distinction between Choi and colleagues’ study and the current one is that they did not investigate the impact of repeated laser irradiations over multiple days.28 A study by Pourhajibagher et al, which is the most recent investigation into the effect of PDT with curcumin, further confirmed the effect of curcumin on the viability of fibroblasts.21 Curcumin can be activated by blue range wavelengths including the blue laser or LED. In a study which compared the efficacy of aPDT by the LED or laser in the disinfection of an implant surface contaminated by Aggregatibacter actinomycetemcomitans, Afrasiabi et al showed that the LED as a light source was more effective in reducing bacteria in comparison with the diode laser.29
It should be noted that only one wavelength and one specific power of laser were applied in the present study, which can be considered as a limitation. Future studies are recommended on the effects of different wavelengths and power densities of lasers, irradiation times, number of irradiations, and time intervals to better elucidate this topic.
Conclusion
According to the present results, it may be concluded that the combination of PDT with curcumin and 660 nm laser irradiation and also increasing the number of laser irradiations (aiming to benefit from its antibacterial properties) can provide a suitable environment for the regeneration of periodontal tissues. Thus, considering the lower invasiveness of this modality compared with other methods, it may be able to serve as a safe alternative for the treatment of periodontal disease.
Authors’ Contribution
Conceptualization: Ardavan Etemadi, Nasim Chiniforush.
Data curation: Morteza Neshandar.
Formal analysis: Seyed Khashayar Koochak Hosseini.
Funding acquisition: Seyed Khashayar Koochak Hosseini.
Investigation: Seyed Khashayar Koochak Hosseini, Nasim Chiniforush.
Methodology: Nasim Chiniforush, Ardavan Etemadi.
Project administration: Ardavan Etemadi.
Resources: Seyed Khashayar Koochak Hosseini.
Software: Morteza Neshandar.
Supervision: Ardavan Etemadi, Nasim Chiniforush.
Validation: Ardavan Etemadi.
Visualization: Morteza Neshandar.
Writing–original draft: Seyed Khashayar Koochak Hosseini, Nasim Chiniforush.
Writing–review & editing: Nasim Chiniforush, Ardavan Etemadi.
Competing Interests
None.
Ethical Approval
This study was approved by the ethics committee of Tehran Medical Sciences, Islamic Azad University.
Funding
None.
Please cite this article as follows: Etemadi A, Koochak Hosseini SK, Neshandar M, Chiniforush N. In vitro effect of photodynamic therapy with curcumin in combination with photobiomodulation therapy by 660 nm on the viability of human gingival fibroblasts. J Lasers Med Sci. 2024;15:e42. doi:10.34172/jlms.2024.42.
References
- 1.Elavarasu S, Naveen D, Thangavelu A. Lasers in periodontics. J Pharm Bioallied Sci. 2012;4(Suppl 2):S260–3. doi: 10.4103/0975-7406.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basso FG, Pansani TN, Turrioni AP, Bagnato VS, Hebling J, de Souza Costa CA. In vitro wound healing improvement by low-level laser therapy application in cultured gingival fibroblasts. Int J Dent. 2012;2012:719452. doi: 10.1155/2012/719452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Negi S, Krishnamurthy M, Ganji KK, Pendor S. Modulatory effects by neodymium-doped yttrium aluminum garnet laser on fibroblast attachment to single rooted tooth surfaces following ultrasonic scaling and root planning: an in vitro study. J Indian Soc Periodontol. 2015;19(1):25–31. doi: 10.4103/0972-124x.145819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajendran M. Quinones as photosensitizer for photodynamic therapy: ROS generation, mechanism and detection methods. Photodiagnosis Photodyn Ther. 2016;13:175–87. doi: 10.1016/j.pdpdt.2015.07.177. [DOI] [PubMed] [Google Scholar]
- 5.Afrasiabi S, Chiniforush N. Antibacterial potential of riboflavin mediated blue diode laser photodynamic inactivation against Enterococcus faecalis: A laboratory investigation. Photodiagnosis Photodyn Ther. 2023;41:103291. doi: 10.1016/j.pdpdt.2023.103291. [DOI] [PubMed] [Google Scholar]
- 6.Vohra F, Akram Z, Safii SH, Vaithilingam RD, Ghanem A, Sergis K, et al. Role of antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: a systematic review. Photodiagnosis Photodyn Ther. 2016;13:139–47. doi: 10.1016/j.pdpdt.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kędzierska E, Knap-Czop K, et al. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–107. doi: 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 8.Pires Marques EC, Piccolo Lopes F, Nascimento IC, Morelli J, Pereira MV, Machado Meiken VM, et al. Photobiomodulation and photodynamic therapy for the treatment of oral mucositis in patients with cancer. Photodiagnosis Photodyn Ther. 2020;29:101621. doi: 10.1016/j.pdpdt.2019.101621. [DOI] [PubMed] [Google Scholar]
- 9.Khalil M, Hamadah O. Association of photodynamic therapy and photobiomodulation as a promising treatment of herpes labialis: a systematic review. Photobiomodul Photomed Laser Surg. 2022;40(5):299–307. doi: 10.1089/photob.2021.0186. [DOI] [PubMed] [Google Scholar]
- 10.Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin. J Med Chem. 2017;60(5):1620–37. doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araújo NC, Fontana CR, Gerbi ME, Bagnato VS. Overall-mouth disinfection by photodynamic therapy using curcumin. Photomed Laser Surg. 2012;30(2):96–101. doi: 10.1089/pho.2011.3053. [DOI] [PubMed] [Google Scholar]
- 12.Santezi C, Reina BD, Dovigo LN. Curcumin-mediated photodynamic therapy for the treatment of oral infections-a review. Photodiagnosis Photodyn Ther. 2018;21:409–15. doi: 10.1016/j.pdpdt.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Nimse S. Nimse SB; Pal DFree radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. doi: 10.1039/c4ra13315c. [DOI] [Google Scholar]
- 14.Choi EJ, Yim JY, Koo KT, Seol YJ, Lee YM, Ku Y, et al. Biological effects of a semiconductor diode laser on human periodontal ligament fibroblasts. J Periodontal Implant Sci. 2010;40(3):105–10. doi: 10.5051/jpis.2010.40.3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh LJ. Walsh LJThe current status of low-level laser therapy in dentistryPart 2Hard tissue applications. Aust Dent J. 1997;42(5):302–6. doi: 10.1111/j.1834-7819.1997.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 16.Corrêa MG, Oliveira DH, Saraceni CH, Ribeiro FV, Pimentel SP, Cirano FR, et al. Short-term microbiological effects of photodynamic therapy in non-surgical periodontal treatment of residual pockets: a split-mouth RCT. Lasers Surg Med. 2016;48(10):944–50. doi: 10.1002/lsm.22449. [DOI] [PubMed] [Google Scholar]
- 17.Pourshahidi S, Ebrahimi H, Bahrami N, Abbasi Javan Z, Ghoreyshi Y, Chiniforush N, et al. In vitro effect of 810 nm and 940 nm diode laser irradiation on proliferation of human gingival fibroblasts and expression of procollagen gene. Photochem Photobiol. 2022;98(6):1441–6. doi: 10.1111/php.13630. [DOI] [PubMed] [Google Scholar]
- 18.Etemadi A, Sadatmansouri S, Sodeif F, Jalalishirazi F, Chiniforush N. Photobiomodulation effect of different diode wavelengths on the proliferation of human gingival fibroblast cells. Photochem Photobiol. 2021;97(5):1123–8. doi: 10.1111/php.13463. [DOI] [PubMed] [Google Scholar]
- 19.Derikvand N, Ghasemi SS, Safiaghdam H, Piriaei H, Chiniforush N. Antimicrobial photodynamic therapy with diode laser and methylene blue as an adjunct to scaling and root planning: a clinical trial. Photodiagnosis Photodyn Ther. 2020;31:101818. doi: 10.1016/j.pdpdt.2020.101818. [DOI] [PubMed] [Google Scholar]
- 20.Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat. 2014;46(1):2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pourhajibagher M, Chiniforush N, Parker S, Shahabi S, Ghorbanzadeh R, Kharazifard MJ, et al. Evaluation of antimicrobial photodynamic therapy with indocyanine green and curcumin on human gingival fibroblast cells: an in vitro photocytotoxicity investigation. Photodiagnosis Photodyn Ther. 2016;15:13–8. doi: 10.1016/j.pdpdt.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Szezerbaty SK, de Oliveira RF, Pires-Oliveira DA, Soares CP, Sartori D, Poli-Frederico RC. The effect of low-level laser therapy (660 nm) on the gene expression involved in tissue repair. Lasers Med Sci. 2018;33(2):315–21. doi: 10.1007/s10103-017-2375-7. [DOI] [PubMed] [Google Scholar]
- 23.Etemadi A, Imani N, Seyed Jafari E, Chiniforush N. In vitro effect of photodynamic therapy with indocyanine green followed by 660 nm photobiomodulation therapy on fibroblast viability. Photochem Photobiol. 2022;98(2):498–503. doi: 10.1111/php.13524. [DOI] [PubMed] [Google Scholar]
- 24.Aghayan S, Yazdanfar A, Seyedjafari E, Noroozian M, Ioana Bordea R, Chiniforush N. Evaluation of indocyanine-mediated photodynamic therapy cytotoxicity in human osteoblast-like cells: an in vitro study. Folia Med (Plovdiv) 2022;64(6):932–7. doi: 10.3897/folmed.64.e67475. [DOI] [PubMed] [Google Scholar]
- 25.Moore P, Ridgway TD, Higbee RG, Howard EW, Lucroy MD. Effect of wavelength on low-intensity laser irradiation-stimulated cell proliferation in vitro. Lasers Surg Med. 2005;36(1):8–12. doi: 10.1002/lsm.20117. [DOI] [PubMed] [Google Scholar]
- 26.Ismiyatin K, Subiyanto A, Tangdan I, Nawawi R, Lina RC, Ernawati R, et al. The effects of different 650 nm laser diode irradiation times on the viability and proliferation of human periodontal ligament fibroblast cells. Dent J (Majalah Kedokteran Gigi) 2019;52(3):142–6. doi: 10.20473/j.djmkg.v52.i3.p142-146. [DOI] [Google Scholar]
- 27.Szlasa W, Supplitt S, Drąg-Zalesińska M, Przystupski D, Kotowski K, Szewczyk A, et al. Effects of curcumin-based PDT on the viability and the organization of actin in melanotic (A375) and amelanotic melanoma (C32) - in vitro studies. Biomed Pharmacother. 2020;132:110883. doi: 10.1016/j.biopha.2020.110883. [DOI] [PubMed] [Google Scholar]
- 28.Choi SH, Chang SY, Biswas R, Chung PS, Mo S, Lee MY, et al. Light-emitting diode irradiation using 660 nm promotes human fibroblast HSP90 expression and changes cellular activity and morphology. J Biophotonics. 2019;12(9):e201900063. doi: 10.1002/jbio.201900063. [DOI] [PubMed] [Google Scholar]
- 29.Afrasiabi S, Barikani HR, Chiniforush N. Comparison of bacterial disinfection efficacy using blue and red lights on dental implants contaminated with Aggregatibacteractinomycetemcomitans. Photodiagnosis Photodyn Ther. 2022;40:103178. doi: 10.1016/j.pdpdt.2022.103178. [DOI] [PubMed] [Google Scholar]