Abstract
Background and Aims: Clonality is characterized by the formation of independent individuals of the same genotype that are capable of reproducing and propagating vegetatively. Although clonality is an important mechanism that facilitates the persistence of a population, its extensive use can lead to negative impacts on sexual reproduction due to trade-offs in the investment of resources. Therefore, studies on the sexual reproduction of species that exhibit clonality can provide information about resilience to environmental changes, information about fecundity, the risk of the absence of pollinators and the ability to persist in unfavourable conditions and to successfully occupy new areas. Here, we investigated the role of clonal propagation and sexual reproduction in Daphnopsis filipedunculata (Thymelaeaceae), a dioecious species distributed only in Serra dos Carajás. Methods: We evaluated the extent of clonality in this species using molecular tools and anatomical analyses of the underground system responsible for developing new ramets. Furthermore, we analysed the sexual system and its contribution to reproductive success through morphometric analyses of floral types and pollination experiments in the field. Key Results: Overall, we found that clonal propagation plays an important role in maintaining the population of D. filipedunculata. Specifically, we demonstrated that this species presents functional male and female plants, indicating that D. filipedunculata is an obligate xenogamous species but has low reproductive success. We also showed that clonal vegetative propagation is the main form of asexual reproduction in this species, with roots responsible for clonal growth. Finally, our results indicated that this species presents an intermediate phalanx–guerrilla clonal architecture. Conclusions: Our study provides the first insights into sexual reproduction and clonal propagation in D. filipedunculata and can inform management practices, conservation and the restoration of endemic species.
Keywords: Campo rupestre on canga, clonality, Daphnopsis filipedunculata, dioecy, endemic species, root anatomy, sexual reproduction, vegetative propagation
In this study, we integrate molecular tools, anatomical and morphometric analyses and pollination experiments to investigate the importance of clonal propagation and sexual reproduction in Daphnopsis filipedunculata population, an endemic species from the Eastern Brazilian Amazon. We found that clonal propagation plays an important role in the D. filipedunculata population. Specifically, we demonstrated that this species presents female and functional male phenotypes, indicating that D. filipedunculata is an obligate xenogamous species but has low reproductive success. We also showed that clonal propagation is the main mode of reproduction in D. filipedunculata and that roots are the organ responsible for clonal growth. Finally, our results indicated that D. filipedunculata presents an intermediate phalanx–guerrilla clonal architecture. Our findings have important implications for the conservation of this endangered, highly endemic species.
Introduction
Clonality is characterized by the formation of independent individuals of the same genotype capable of reproducing and propagating vegetatively (Harper 1977); this form of asexual reproduction is common in 80 % of angiosperm species (Klimes et al. 1997). Clonal growth can be accomplished by several morphological organs (i.e. rhizome, stolons, roots), which are not functionally equivalent and can be triggered by different drivers (Klimešová et al. 2017; Herben and Klimešová 2020). Most clonal plants emit ramets from underground organs—roots and/or stems, which can be distinguished anatomically (see Klimešová et al. 2019). Compared to the stems, the roots are deeper, protecting the buds that form the new ramets and can reach greater distances (Klimešová et al. 2019), interfering with the distance between the genets. Furthermore, roots are directly associated with nutrient acquisition and root sprouting constitutes an independent route to clonal propagation (Klimešová et al. 2017; Herben and Klimešová 2020). Contrary to stem-based clonality that does not evolve to promote species occurrence in disturbed habitats, clonal growth throughout root sprouting is an important trait for response to disturbance (Klimešová et al. 2017; Herben and Klimešová 2020).
Furthermore, clonal growth organs can display different spatial arrangements of ramets (i.e. clonal architecture). For instance, tillers, bulbs and rhizomes tend to generate ramets relatively close to the parent, whereas stolons and runners emit ramets placed at greater distances from their parents (Barrett 2015). It characterizes two contrasting ecological strategies: (a) the phalanx strategy, which results in a close aggregation of ramets, usually packed around the parental shoot and (b) the guerrilla strategy, which is characterized by an extensive intermingling of ramets from different genets (Ye et al. 2006; Barret et al. 2015). The guerrilla strategy is associated with resource-heterogeneous or disturbed habitats, while phalanx strategy is more related to homogeneous and less disturbed habitats (Ye et al. 2006). Regarding its importance, more detailed analyses of belowground organs responsible for clonality should not be overlooked because it may have a direct impact on clonal architecture and consequently on the benefits and disadvantages of clonality (Charpentier 2001; Thomas and Hay 2010; Klimešová et al. 2017).
An increasing number of studies have shown that asexual reproduction through clonal propagation has significant benefits for population growth (Klimes et al. 1997; Xiao et al. 2011; Chen et al. 2015). As clonality requires a lower investment when compared to reproduction via seeds, it can boost population growth, favouring species persistence when sexual reproduction is restricted (Barrett 2015; Klimešová et al. 2021). Moreover, physiological integration amongst ramets can be advantageous due to the sharing of multiple resources (e.g. water, carbohydrates and mineral nutrients) and information (signalling molecules) as well as the potential division of labour within the genet (Callaghan 1984; Dong 1996; reviewed by Liu et al. 2016). It can reduce the likelihood of the death of genets, allowing them to cope with environmental heterogeneity (Dong 1996; Liu et al. 2016).
Although clonality is an important mechanism to facilitate population persistence regardless of sexual reproduction (Barrett 2015; Hu et al. 2017), extensive clonality may increase susceptibility to diseases and other disturbances due to a lack of genetic variation (Lei 2010) and affect sexual reproduction in a manner that decreases plant fitness (Barrett 2015). Trade-offs between clonality and sexual reproduction are mainly due to (a) resource investment, as clonal reproduction may limit resource allocation to flowering and seed production (Barrett 2015; Hewitt 2020), and (b) the effects of clonal architecture on mating availability (Charpentier 2001; Honnay and Jacquemyn 2008; Thomas and Hay 2010; Barrett 2015). In fact, low fruit sets and overall lower reproductive success have been reported in clonal species (Sydes and Peakall 1998; Faria et al. 2006; Herben et al. 2012; Franklin et al. 2021). Clonality may enhance geitonogamy, increasing fitness costs in self-compatible species due to higher selfing rates and reduction of genetic diversity (Ushimaru and Kikuzawa 1999; Honnay and Jacquemyn 2008; Dering et al. 2015). This selective pressure imposed by geitonogamy on the mating system can explain the correlated evolution of self-incompatibility and clonality (Charpentier 2001; Honnay and Jacquemyn 2008).
Nevertheless, self-incompatible, dioecious and other sexual polymorphic species can experience more severe consequences from clonal propagation, which, in some cases, leads to a disruption of sexual reproduction (Barrett 2015; Hu et al. 2017). First, clonality can lead to a sex-biased population, usually male-biased, due to the low reproductive costs often attributed to male over female plants (Delph 1999; Sinclair et al. 2012; Field et al. 2013; Khanduri et al. 2019). This might favour the occurrence of single-sex monoclonal patches where clonal propagation is the only reproductive mechanism, significantly reducing population viability (Honnay and Bossuyt 2005; Barrett 2015). Furthermore, various combinations of clonal architecture and clone size may have important implications for mating, fertility and persistence of self-incompatible and sexual polymorphic species (Barrett 2015). For example, spatially clustered ramets (phalanx growth form) are advantageous for optimizing resource capture and space occupation. However, this growth form is expected to decrease mate availability in self-incompatible species as clonal patches increase (Barrett 2015). Moreover, dioecious or self-incompatible species with a guerrilla growth form can benefit from clonality, which can help to maintain genetic diversity when combined with an outcrossing mating system (Vallejo-Marín and O’Brien 2006; Hu et al. 2017).
Plants have repeatedly evolved asexual reproduction in tandem with sexual reproduction (Klimes et al. 1997), a combined strategy proposed to ensure the transmission of well-adapted genes while simultaneously providing the genetic variability necessary to colonize new habitats and survive future environmental changes (Niklas and Cobb 2017). Investigating the multiple outcomes in the trade-off between sexual reproduction and vegetative propagation can provide relevant information about the resilience of species to environmental changes, such as fertility, the risk of pollination failure and the persistence of a species under unfavourable conditions or the ability to successfully occupy new sites (Sydes and Peakall 1998; Adam and Williams 2001; Butcher et al. 2011; Hu et al. 2017). These issues might be critical for threatened species, as extensive clonality in these species can have important implications for their conservation status (Sydes and Peakall 1998; Hu et al. 2017). Indeed, clonality can contribute to extinction debt, and the current distribution of a species might not reflect the ecological viability of its populations (Eriksson and Ehrlén 2001).
Thymelaeaceae is a family with several species with clonal reproduction that has received particular attention from researchers examining mating system evolution given its variable sexual expression (Herber 2003; Beaumont et al. 2006). Thymelaeaceae has approximately 800 species and is split into 2 subfamilies, Octolepidoideae and Thymelaeoideae (Rogers 2010), which mainly include hermaphroditic, dioecious and gynodioecious species (Herber 2003). Approximately one-third of the genera in Thymelaeoideae have unisexual flowers, and most of their species are sexually dimorphic (Beaumont et al. 2006). Most studies on sexual expression have been carried out with gynodioecious species of Daphne (Medrano et al. 2005; Alonso et al. 2007; Alonso and Herrera 2011; Shibata et al. 2018, 2021), Gnidia (Beaumont et al. 2006; Smith 2009) and Pimelea (Merrett 2007) or with Thymelaea hirsuta, an interesting species with a tetramorphic sexual system (Dommée et al. 1990, 1995; Shaltout and El-Keblawy 1992; El-Keblawy et al. 1996; Minuto et al. 2005); these species occur in Australian, Ethiopian and Palaeartic regions. Neotropical genus, such as Daphnopsis (the largest genus in the New World), have received less attention in studies of mating systems (Bullock 1985; Ramírez and Briceño 2021).
Daphnopsis is an exclusively dioecious genus that predominantly has morphologically unisexual flowers (organs of one sex are functional, whereas those of the other are deformed/modified or vestigial, sensuMayer and Charlesworth 1992), especially species of the subgenus Daphnopsis (Nevling Jr. 1959, 1963). However, some plants of D. americana have flowers that appear to be functionally bisexual and produce fruits (Nevling Jr. 1959). According to this author, there are also reports of functionally male plants with flowers in which the ovary contains a relatively well-formed seed. In the subgenus Neivira, pistillate flowers usually have a staminode, and staminate flowers usually have a well-developed pistillode (Nevling Jr. 1959). However, there have been no further investigations into the seed production capacity of staminate flowers with developed pistillodes in this genus. Daphnopsis filipedunculata Nevling & Barringer (Thymelaeaceae) is a dioecious, rare and endangered species endemic to Brazil with distribution restricted to the Serra dos Carajás in the eastern Amazon (Nevling Jr. and Barringer 1993; Mota and Giulietti 2016; Watanabe et al. 2018; Rossi 2020). Although D. filipedunculata has been recorded as a dioecious species (Mota and Giulietti 2016; Watanabe et al. 2018), the specifics of its reproductive system have never been investigated. Previous studies have reported that some sites of occurrence of D. filipedunculata are formed by clumps of individuals, with some presenting clonal behaviour similar to other Thymelaeaceae species (Rogers 2009; Watanabe et al. 2018). Moreover, the currently monitored population exhibit a male-biased population structure with a low frequency of female plants (Watanabe et al. 2018).
Here, we investigated the importance of clonal propagation and sexual reproduction in D. filipedunculata population. We hypothesized that if, on the one hand, clonality is the dominant mechanism in D. filipedunculata, most individuals in the population will consist of the same genotype. On the other hand, predominantly sexual reproduction would result in most individuals presenting unique genotypes. To test these predictions, first, we investigated the presence and extent of clonality using genomic data to identify potential clones. In addition, we performed anatomical analyses to identify from which organ the new ramets develop and the occurrence of reserve substances. Since stem and root sprouting have different triggers, it might have different implications for the explanation of clonality patterns in D. filipedunculata. Second, we analysed the sexual system and its contribution to reproductive success. We performed morphometric analyses of the two floral types to assess the existence of sexual dimorphism. Through pollination experiments on both floral types, we verified whether staminate flowers with developed pistillodes act as functional males or as true hermaphrodites, potentially generating fruits and whether these flowers can perform self-fertilization or whether the species rely solely on outcrossing. Finally, we discussed the implications of the balance between sexual reproduction and vegetative propagation for the conservation of this treelet species.
Materials and Methods
Study area
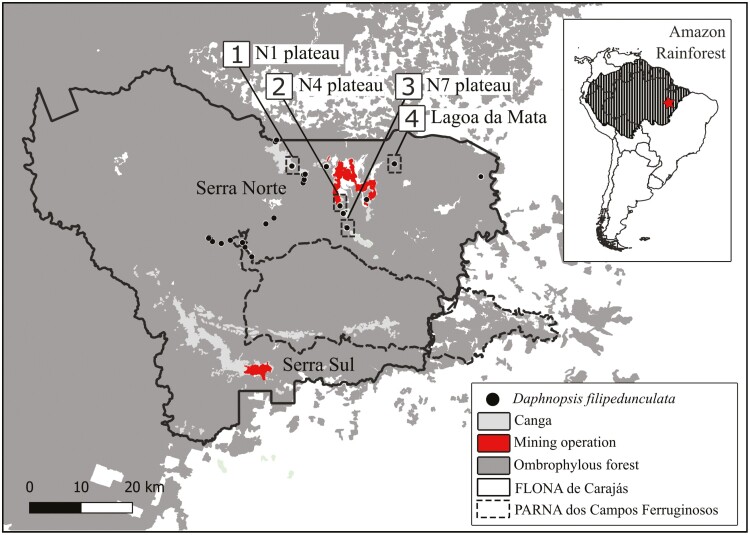
The study was conducted in the Floresta Nacional de Carajás (FLONA de Carajás), a protected area with sustainable use of natural resources of 411.949 ha located in the Eastern Brazilian Amazon (IBAMA 2003; Fig. 1). FLONA Carajás is composed of ironstone outcrops (known as campo rupestre on ‘canga’), a unique ecosystem limited by iron concentration in the soils, nestled within ombrophilous dense or open forests (Fig. 1). Daphnopsis filipedunculata, a treelet species (1–8 m in hight), grows in the transition zone between the ‘canga’ ecosystem and the ombrophilous forest (Nevling Jr. and Barringer 1993), and more recently was recorded within the ombrophilous forest (Amplo Engenharia 2021). We carried out most of the experiments in the transition zone between the N1 plateau (‘canga’ ecosystem) and the ombrophilous forest (Fig. 1) to control for any differences due to environmental conditions. This is the area where the species was first recorded growing (Nevling Jr. and Barringer 1993) and presents a high abundance of individuals (personal communications). To increase the sample size for the experiments on the sexual system, we also sampled individuals from other transition zones that present similar environmental conditions (Lagoa da Mata, the N4 plateau and the N7 plateau, Fig. 1).
Figure 1.
Study area, sampling sites and distribution range of D. filipedunculata in the FLONA de Carajás and PARNA dos Campos Ferruginosos, eastern Amazon, Brazil. Sampling sites are in the transition zone between ‘cangas’ and ombrophilous dense forest in the N1 plateau (1), Lagoa da Mata (2), N4 plateau (3) and N7 plateau (4).
Identification of clonemates using high-throughput sequencing
To determine potential clonal individuals, that is, those that present the same multilocus genotype, we covered the entire known distribution area of D. filipedunculata in the transition zone between the N1 plateau and the ombrophilous forest in 2021 (SISBIO collection permit N. 76784-1) and sampled 49 individuals, without delimiting a minimum distance for sampling. The sampled individuals had different sizes. There is no information on whether the height of the plants is related to the age of the individuals, but we found reproductive individuals measuring less than one metre. These samples corresponded to 70 % of the known individuals on the southern edge of the N1 plateau at that time, and their distance ranged from less than a metre to 247 m. From each individual, we collected leaflet samples for genotype analysis that were preserved in CTAB and stored at −20 °C until DNA extraction. The geographic location of each sample was recorded with a GPS device. Among the sampled individuals, 36 were classified as male plants, 3 as female plants and 10 as undetermined (due to a lack of reproductive structures).
Total DNA was extracted with the Qiagen DNeasy Plant Kit and quantified with the Qubit High Sensitivity Assay Kit (Invitrogen), and its degradation was assessed by electrophoresis in 1.2 % agarose gels. Samples were then shipped to Ecomol Consultoria (https://ecomolconsultoria.com.br/) for sequencing. We performed genotype-by-sequencing using the protocol described by Elshire et al. (2011), which consists of reducing genome complexity using restriction enzymes. Here, all samples were digested using the PstI enzyme. Then, the DNA fragments were ligated to adapters containing specific barcodes for each individual. The restriction-ligation products were pooled and enriched through PCR. The library was then sequenced with two lanes on an Illumina NovaSeq 6000 instrument using an SP 100 cycle (1 × 100 bp) kit.
We sequenced a mean of 2 530 789.7 reads per sample (min. 595 441 and max. 5 769 606 reads). The raw sequence reads were de novo assembled in ipyrad software (Eaton and Overcast 2020) using a pipeline for species without a reference genome, and 99.96 % of reads passed ipyrad filtering procedures. The de novo assembly resulted in a mean of 16 053 locus per sample (min. 7243 and max. 21 384). We set the analysis parameters as suggested by the authors, except for the following: clust_threshold = 0.95, filter_adapters = 2 (stricter), max_Indels_locus = 4. VCFTools was further used to obtain a final dataset of biallelic SNPs (--remove-indels, --max-alleles 2 and --min-alleles 2) without missing data (--max-missing 1) and with minimum allele frequency of 2 % (--maf 0.02) and a minimum depth of 10 (--minDP 10). We did not filter our dataset for one SNP per locus. However, our dataset presents a low mean number of SNPs per locus (= 1.23 SNPs/locus), thus it is unlikely to affect the genetic diversity results.
To identify potential clones, we used the ‘poppr’ R package (Kamvar et al. 2014), which creates a genetic distance matrix, calculates the minimum genetic distance among the different multilocus genotypes (i.e. threshold) and collapses the genotypes into distinct multilocus genotypes. For that, we used the function cut-off to define the threshold [see Supporting Information—Fig. S1] needed to cluster individuals into the same multilocus genotypes (hereafter clones) or distinct multilocus genotypes. Based on Euclidean genetic distance, we estimated the value was 10.30 to account for possible genetic variation among clones due to library preparation, sequencing error and somatic mutations. After the definition of the potential clones using the function mgl.filter, we estimated the geographic distance between them and between distinct multilocus genotypes, and the genotypic diversity (the number of genotypes divided by the number of ramets, G/N). We randomly sampled one clone individual to estimate the geographic distance between distinct multilocus genotypes. We used the Kolmogorov–Smirnov test to check for differences in the distance distributions between clone individuals and individuals with distinct multilocus genotypes.
Fine-scale genetic structure and genetic diversity
To investigate the genetic divergence amongst sampled individuals of D. filipedunculata, we calculated the proportion of shared alleles using ‘adegenet’ R package (Jombart 2008), then plotted the results as a heatmap and a dendrogram showing individuals clustering using the ‘dartR’ R package (Gruber et al. 2018). We also tested for within-population genetic structure using the Bayesian analysis implemented in fastSTRUCTURE (Raj et al. 2014) with the full individuals dataset (49 clonal and non-clonal individuals) and the subset of 26 non-clonal individuals. The best K value was assessed from values between 1 and 10, as determined by their likelihood values using StructureSelector (Li and Liu 2018). Finally, we evaluated the fine-scale genetic structure (FSGS; i.e., the non-random spatial distribution of genotypes within populations) with both datasets using a spatial autocorrelation analysis as implemented in SPAGeDi (v. 1.5 software) (Hardy and Vekemans 2002). To do so, we set Fij as the kinship coefficient among individuals described in Loiselle et al. (1995) and defined 10 distance intervals. We permuted individuals’ spatial positions 1000 times to test for the significance of FSGS in each distance class.
We characterized the genetic diversity from the full dataset and the subset of non-clonal individuals using the number of alleles (A), observed (HO) and expected (HE) heterozygosities, and the inbreeding coefficient (FIS) with their confidence intervals based on 1000 bootstrap using the ‘diveRsity’ R packages (Keenan et al. 2013).
Anatomical analysis
During fieldwork, we observed the underground structures of mature D. filipedunculata individuals (ca. 1 m in height, connected through small branches) and found that underground organs emitted new aerial branches. Thus, to identify these structures, we performed comparative anatomical analyses between the underground organs and the aerial branches. For this purpose, samples were collected from individuals (n = 3; with a distance of at least 5 m between each collection point) in the transition zone between the N1 plateau and the ombrophilous forest. Sampling of clonal structures followed Klimešová et al. (2019), in which adult plants without physical damage or signs of disease and nutritional deficiency were sampled. Sampled material was fixed in FAA 70 (37 % formaldehyde, glacial acetic acid, 70 % ethanol, 1:1:18 (v/v); Johansen 1940) and stored in 70 % ethanol. Subsequently, small fragments of aerial stems and underground organs were obtained, dehydrated in an n-butyl alcohol series and embedded in historesin (Leica Historesin Embedding Kit). Cross sections (5–7 µm thick) were obtained with a rotary microtome (Leica, RM 2255), stained with periodic acid–Schiff’s reagent (PAS) and toluidine blue (O’Brien et al. 1964; Feder and O’Brien 1968) and mounted on permanent slides using Entellan (Merck). The slides were analysed using a light microscope (Zeiss, Axio Scope A1) with an attached camera (Zeiss, AxioCam ICc 5) and AxioVision (version 4.8.3.0) software. Cross sections of the underground organs were sliced by hand and their general characterization was carried out under a stereomicroscope (Zeiss, SteREO Discovery V12) with an attached camera (Zeiss, AxioCam 712 colour) and ZEN 3.4 (blue edition) software. Histochemical tests were performed with Sudan III for lipids (Sass 1951) and ferric chloride for phenolic compounds (Johansen 1940).
Sexual reproductive system
To verify whether D. filipedunculata reproduces sexually, we first analysed the floral morphology of individuals located in the transition zones between the ‘canga’ ecosystem and the ombrophilous forest (21 male and 10 female plants at the transition zone nearest to the N1 plateau, 2 female plants at the transition zone nearest to the N4 plateau, 1 female plant at the transition zone nearest to the N7 plateaus and 19 male plants at the transition zone nearest to the Lagoa da Mata; Fig. 1). Most individuals from N1 plateau are the same sample for genetic analysis; however, it is not possible to match them because they were sampled at different times and the individuals for genetic analysis were not marked. Since male and female plants are vegetatively similar, we first confirmed the sexuality of flowers from individuals that were flowering at the study sites from June to August 2022, observing the presence of androecium and gynoecium with a hand-magnifying lens. Additional individuals were analysed in June and July 2023 to increase sample size of female plants. To assess the existence of sexual dimorphism between floral types, we performed morphometric analyses of the perianth (hypanthium + corolla; length and width) and the gynoecium (length and width of the ovary, style length and stigma width). We collected 3 flowers from each of the 40 male plants and the 13 female plants, totalling 159 flowers analysed. The samples were fixed in FAA 70 and stored in 70 % ethanol. To perform the measurements, we took photographs on a stereomicroscope with a digital camera (the same equipment as that used in the anatomical analyses). We processed the images in ZEN 3.4 (blue edition) software.
We analysed the morphometric data using linear mixed models with a Gaussian distribution and the identity link function. Floral type (staminate or pistillate flowers) was considered a fixed factor, and the individual was considered a random factor to control pseudo-replicate effects. The best model was selected using likelihood ratio tests (Zuur et al. 2009), and the influence of the fixed factors on the response variable was analysed using post hoc Tukey tests. We performed all statistical analyses in R (R Core Team 2020) using the packages ‘lme4’ (Bates et al. 2015) and ‘emmeans’ (Lenth 2020).
To evaluate the functionality of flowers, we performed pollination tests (Richards 1997; Sage et al. 2005) on both floral types. For staminate flowers with developed pistillodes, we used the 40 male plants and marked 6 flower per individual, 1 for each test and performed the following treatments [see Supporting Information—Table S1]: (a) spontaneous self-pollination, in which preanthesis buds were isolated with fabric bags, preventing pollination by external agents; (b) manual self-pollination, in which preanthesis buds were bagged and, during anthesis, pollinated with their own pollen; (c) geitonogamy, in which preanthesis buds were emasculated and bagged, and then flowers at anthesis were pollinated with pollen from flowers of the same individual; (d) cross-pollination, in which preanthesis buds were emasculated and bagged, and at the time of anthesis, the flowers were pollinated with pollen from flowers of different individuals; (e) pollen supplementation in which we performed manual outcross pollen addition without bagging the flowers and (f) control (open pollination), in which preanthesis buds were marked and during anthesis were exposed to local environmental conditions. We performed all crosses on the first day of anthesis with fresh pollen. We carried out manual pollinations (treatments b–e) with the help of wooden toothpicks, replacing them in each flower to avoid contamination. For treatments (d) and (e), we used individuals as pollen donors, whose preanthesis flowers were bagged to avoid pollen loss. We collected pollen from individuals at least 10 m away from the individuals that would be pollen recipients. According to distance and flower availability, different sets of individuals were used as pollen donors.
For pistillate flowers, we used 13 plants and marked 4 flowers per individual 1 for each test, and performed the following treatments: (a) apomixis, in which preanthesis buds were isolated with fabric bags, preventing pollination by external factors; (b) cross-pollination, in which preanthesis buds were bagged and at anthesis, the flowers were pollinated using pollen from individuals with staminate flowers; (c) pollen supplementation, similar to the corresponding condition described above and (d) control, similar to the corresponding condition described above.
We followed all the reproductive phases of the individuals in the population, such as floral buds, flowers at anthesis and immature and mature fruits (Fig. 2). In general, we verified the possible formation of fruits (berry-like, containing one seed) 1 month after we carried out the treatments in the field. Differences in fruit formation among treatments were compared with chi-squared tests, and P values ≤ 0.05 were considered to indicate a significant difference.
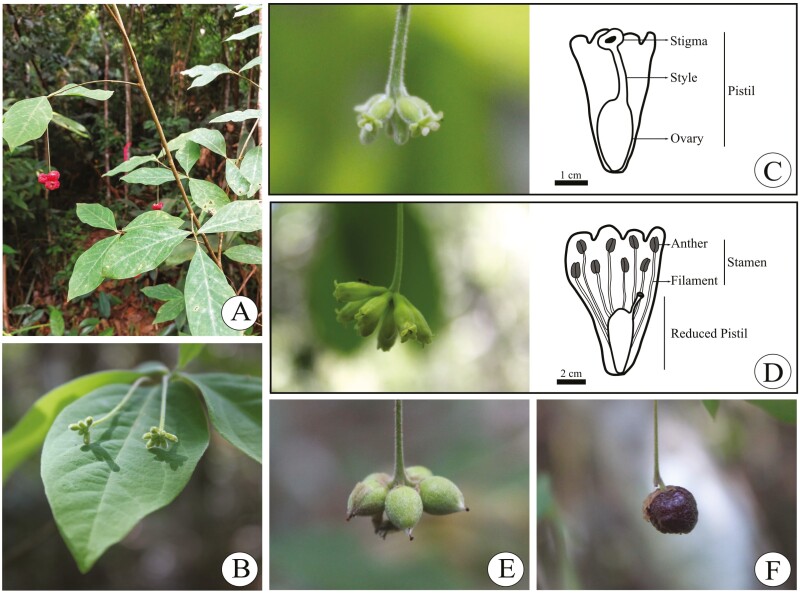
Figure 2.
Characterization of D. filipedunculata. (A) Habit, (B) nflorescences with buds, (C) pistillate flowers, (D) staminate flowers, (E) immature fruits and (F) ripe fruits of D. filipedunculata.
Results
Identification of clonemates using high-throughput sequencing
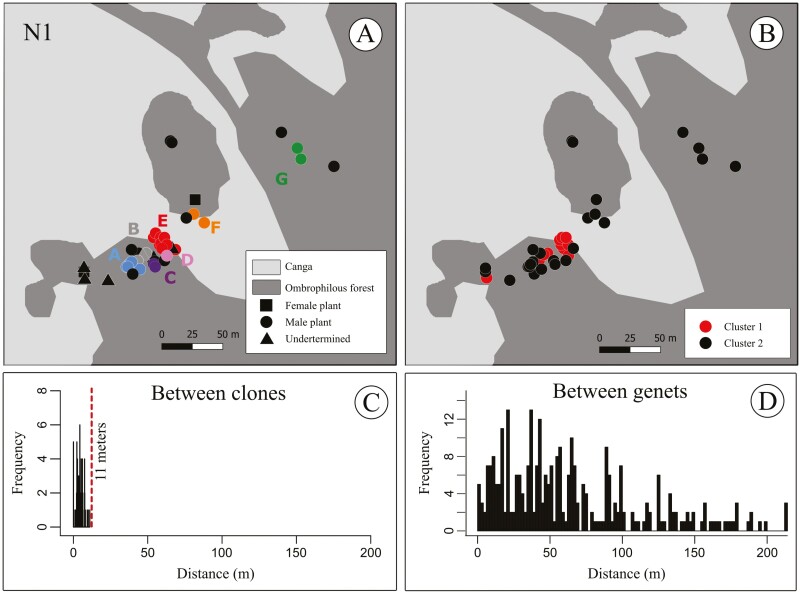
We retained 1122 SNPs across 49 individuals of D. filipedunculata for clonal identification. The number of distinct multilocus genotypes found across the 49 individuals was 26, and the G/N ratio was 0.53 (Supporting Information—Table S2, Fig. 3A). The number of individuals by multilocus genotypes is A = 5, B = 3, C = 2, D = 2, E = 12, G = 3 and F = 3. The geographic distance between clonal individuals ranged from less than a metre to 11 m, with a median of 4 m (Fig. 3C). In contrast, the geographic distance between distinct multilocus genotypes ranged from 0 to 213 m, with a median of 51 m (Fig. 3D). These distance distributions were significantly different (Kolmogorov–Smirnov test, D = 0.85, P < 2.2e-16). Interestingly, we found one case of identical multilocus genotypes (clones) presenting female and male individuals [Supporting Information—Table S2].
Figure 3.
Fine-scale distribution and geographic distance among sampled individuals of D. filipedunculata in the transition zone between N1 canga plateau and the ombrophilous forest, FLONA de Carajás, Eastern Amazon, Brazil. (A) Spatial distribution of clonal and non-clonal individuals. Distinct letters (A–G) and colours correspond to individuals identified as clones based on genetic analysis using the dataset of 1122 SNPs. (B) Geographic distance between D. filipedunculata individuals with the same multilocus genotypes (clonal), (C) and between individuals belonging to distinct multilocus genotypes (non-clonal).
Fine-scale genetic structure and genetic diversity
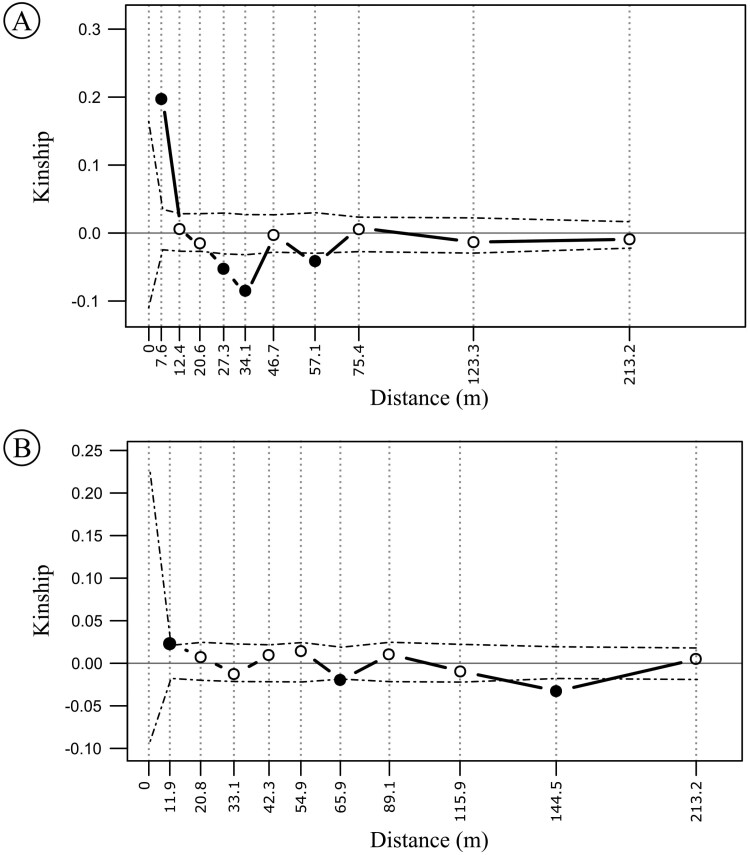
While non-clonal individuals share 80.03–92.69 % of the sampled alleles, distinct individuals identified as belonging to the same genet (clones) have 97.77–99.95 % of identity [Supporting Information—Fig. S2]. The analysis performed in fastSTRUCTURE from the full dataset of individuals recovered two distinct genetic clusters mixed in the geographical space (Fig. 3B, Supporting Information—Fig. S3A). The individuals with the greatest genetic mixture were those with distinct multilocus genotypes in the population (Supporting Information—Fig. S3, individuals from 18 to 32). Such substructure within the sampled area was not corroborated by the analysis performed with the dataset of non-clonal individuals [see Supporting Information—Fig. S3B]. The spatial autocorrelation analysis performed from the full dataset showed a pattern of sharp decrease of kinship with increasing geographical distance, up to the sixth distance class (Fig. 4A). When clonal individuals are not included in the analysis, such trend disappears (i.e. kinship is not different from the expected by chance for any but three distance class, Fig. 4B). We found no evidence of inbreeding (FIS = −0.027 [−0.064 to −0.003] or −0.0069 [−0.0419 to 0.0122], including or not the clonal individuals, respectively) and a moderate level of genetic diversity (HE = 0.180 and 0.190, including or not clonal individuals, respectively) in D. filipedunculata [see Supporting Information—Table S3].
Figure 4.
FSGS of D. filipedunculata in the transition zone between N1 canga plateau and the ombrophilous forest, FLONA de Carajás, eastern Amazon, Brazil. (A) Correlogram for the spatial autocorrelation analyses for 10 distance intervals using kinship index calculated between all pairs of clonal and non-clonal individuals. (B) and between the subset of all pairs of non-clonal individuals. Kinship above or below permuted 95 % confidence intervals (dashed lines) are represented by filled symbols.
Comparison of root and stem anatomy
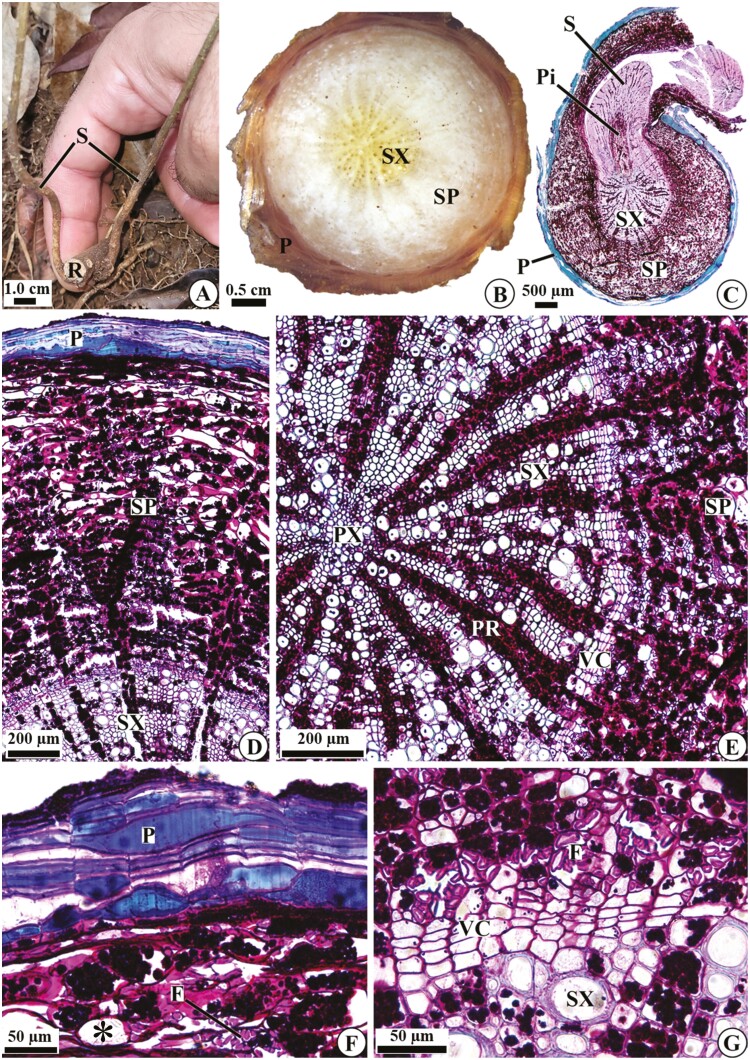
We found that the clonal propagation of D. filipedunculata occurs through the formation of new sprouts (aerial branches) from underground organs (Fig. 5A and C). Our observations revealed differences in the structure of the aerial branches and underground system, which allowed the characterization of underground organs as roots (absence of a pith and centripetal maturation of the primary xylem). Roots in the secondary structure consist of periderm and secondary phloem and xylem (Fig. 5B–D). The outer layers of the periderm (suber) have a loose arrangement, with evident lacunae and cells containing phenolic compounds and suberized walls (Fig. 5D and F). Secondary phloem occurs between the vascular cambium and periderm, characterized mainly by parenchymatous cells containing starch grains and fibres (Fig. 5D–G); idioblasts containing crystals may occur (Fig. 5F—asterisk). The vascular cambium has three to four layers of flat, thin-walled cells (Fig. 5E and G). Regarding the xylem, the secondary structure is quite evident compared to the primary structure, presenting conducting cells and many parenchymatous rays, with cells storing starch grains (Fig. 5E and G). The aerial branches are connected to the vascular cambium and secondary xylem (Fig. 5C); these sprouts develop in regions where the parenchyma rays of the secondary xylem are wider (Fig. 6A), connecting the reserve tissues of the root and stem.
Figure 5.
Plant architecture (A) and root anatomy (B–G) of D. filipedunculata shown in cross sections. (A) Vegetative structure; note the two sprouts originating from one root. (B–C) General aspects: in C, a sprout is connected to the vascular system. (D) Details of the periderm, secondary phloem and secondary xylem. (E) Details of the secondary phloem, vascular cambium and secondary and primary xylem. (F) Details of the periderm and part of the secondary phloem. (G) Details of the vascular cambium and part of the secondary phloem and secondary xylem. F, fibres; P, periderm; Pi, pith; PR, parenchymatous ray; PX, primary xylem; R, root; S, stem; SP, secondary phloem; SX, secondary xylem; VC, vascular cambium; *, idioblast.
Figure 6.
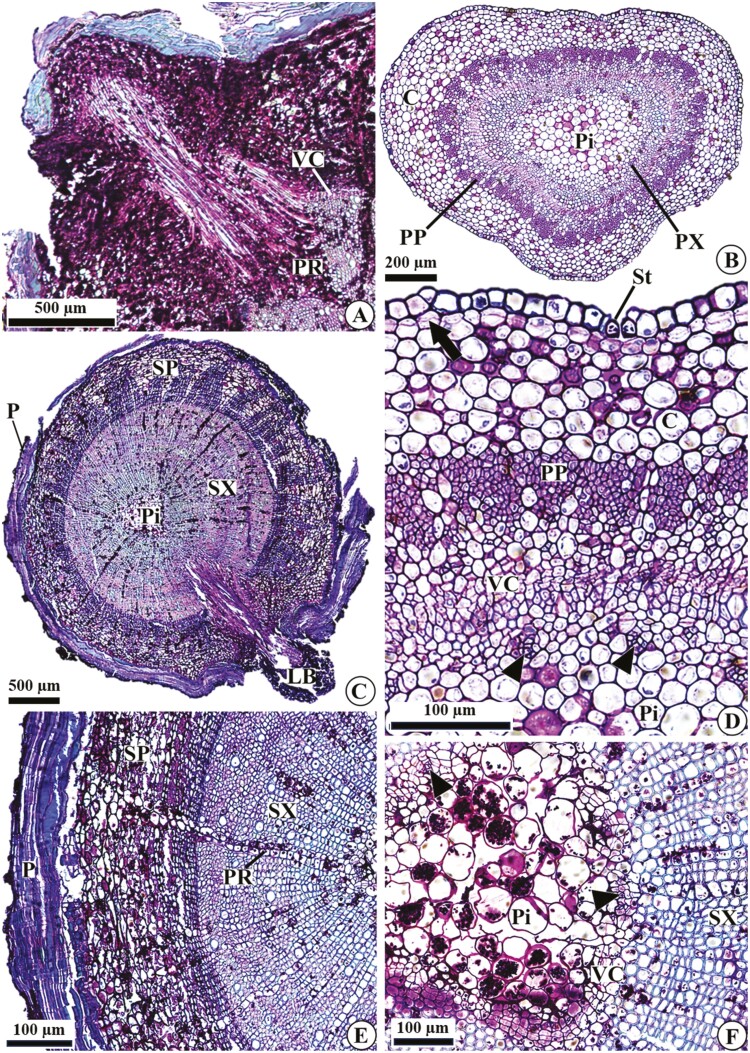
Stem anatomy of D. filipedunculata in longitudinal (A) and cross (B–F) sections. (A) Part of the sprout connected to the root, associated with a wide parenchymatous ray. (B–C) General aspects in the apical and basal regions. (D) Details of the primary structure, with the establishment of phellogen below the epidermis. (E) Details of the secondary structure, with periderm, secondary phloem and secondary xylem. (F) Details of the pith in a stem with secondary growth. Arrow, phellogen; arrowheads, primary xylem; C, cortex; P, periderm; Pi, pith; PP, primary phloem; PR, parenchymatous ray; PX, primary xylem; SP, secondary phloem; St, stomata; SX, secondary xylem; VC, vascular cambium.
The stem anatomy differs from that of the root mainly due to the presence of a parenchymatous pith (Figs 5C and 6B and C). In the apical portion, the stem exhibits a primary structure or initiates secondary growth (Fig. 6B and D); a single-layered epidermis and a parenchymatous cortex are still present. In the basal portion, the stem is composed of periderm, vascular cambium and secondary phloem and xylem (Fig. 6C and E), with characteristics similar to those of the roots; the only difference is the narrower parenchymatous rays in the secondary xylem of the stems (Fig. 6E). In stems with secondary growth, the pith starts to store starch grains, and some of its cells rupture, forming intercellular spaces (Fig. 6F).
Sexual reproductive system
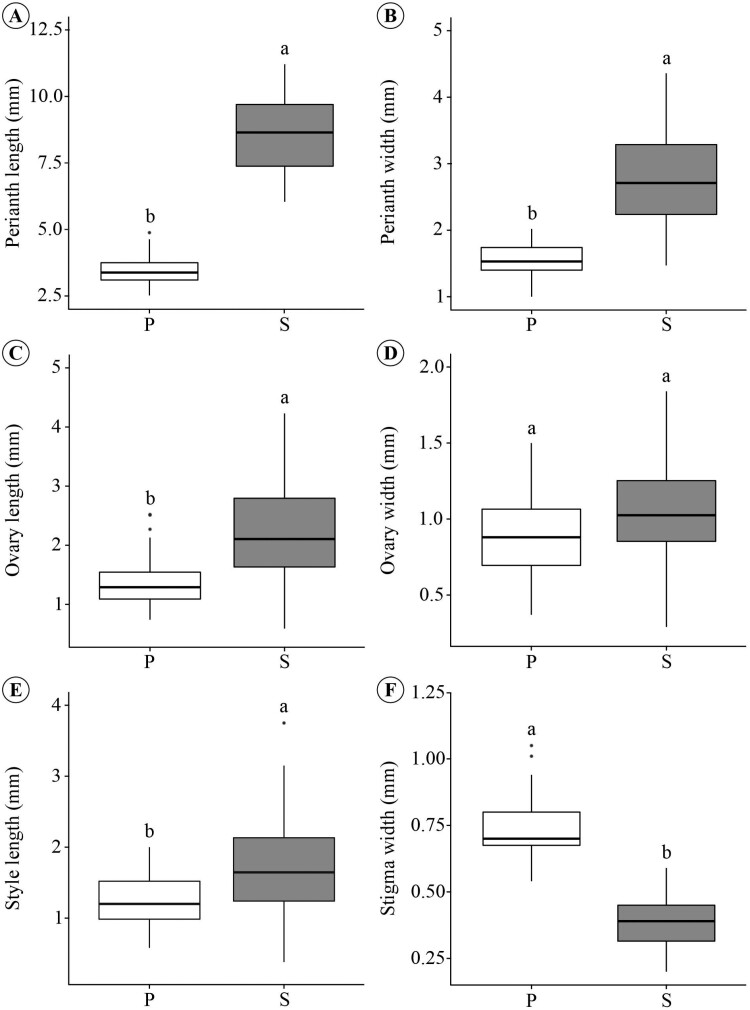
The floral types of D. filipedunculata have morphometric differences [see Supporting Information—Table S4]. The perianth of staminate flowers with developed pistillodes is larger than that of pistillate flowers (Fig. 7A and B). The gynoecium also differs between floral types, with the length of the ovary and style greater in staminate flowers with developed pistillodes (Fig. 7C and E), while the width of the stigma was greater in pistillate flowers (Fig. 7F). Ovary width did not differ between floral types (Fig. 7D). Pistillate flowers do not have staminodes. The androecium of staminate flowers with developed pistillodes consists of 8 (6–12) stamens that have fillets fused with the perianth; the anthers are arranged at two heights (see Fig. 2).
Figure 7.
Floral morphometry of D. filipedunculata. (A) Perianth length. (B) Perianth width. (C) Ovary length. (D) Ovary width. (E) Style length. (F) Stigma width. Averages followed by the same lowercase letter indicate floral traits that did not differ significantly (at P ≤ 0.05) between floral types according to Tukey’s post hoc test. P, pistillate flower; S, staminate flower with developed pistillode.
Daphnopsis filipedunculata staminate flowers with developed pistillodes did not produce fruits in any of the applied treatments [see Supporting Information—Table S1]. Pistillate flowers did not produce fruit by apomixis or in the pollen supplementation treatment, and fruitification in the other treatments was low; open pollination led to the highest reproductive success (23.1 %) [see Supporting Information—Table S1]. However, there were no significant differences in fruit formation among the treatments in pistillate flowers (X2 = 4.25; P = 0.23).
Discussion
Sexual reproduction is vital to ensure variation within species and promote adaptation to novel environmental conditions, while asexual reproduction, a common feature of plants, can help some species escape failure of sexual reproduction and potentially preserve well-adapted phenotypes in environmentally stable sites (Silvertown 2008; Niklas and Cobb 2017; Tandon et al. 2020). Here, we showed that clonal propagation plays an important role in the D. filipedunculata population. Specifically, we demonstrated that this species presents female and functional male phenotypes, indicating that D. filipedunculata is an obligate xenogamous species but has low reproductive success. We also showed that clonal propagation is the main mode of reproduction in D. filipedunculata and that roots are the organ responsible for clonal growth. Finally, our results indicated that D. filipedunculata presents an intermediate phalanx–guerrilla clonal architecture, as the distance between ramets from the same genet was usually short, but the spatial distribution of individuals showed a mixture of ramets from different genotypes. Our findings have important implications for the conservation of this endangered, highly endemic species and throughout the discussion, we have suggested future studies to carry out with this species.
The flowers of D. filipedunculata exhibit dimorphisms in primary (pistil) and secondary (perianth) sexual characteristics (Sakai and Weller 1999). Dimorphic species generally exhibit staminate flowers that have a larger perianth than that of pistillate flowers (Freeman et al. 1997; Eckhart 1999), as observed here. This difference may be associated with the attraction of pollinators, considering that the fitness of staminate flowers depends more strongly on mating success (Bell and Hamilton 1985; Eckhart 1999). In relation to the gynoecium, the size of the pistillode of staminate flowers may be similar to or larger than that of the pistil of pistillate flowers, a common characteristic in the subgenus Neivira (Nevling Jr. 1959). This morphological similarity could be mistakenly interpreted as evidence of gynodioecy (hermaphrodite and female individuals), a sexual expression present in other genera of Thymelaeaceae (Medrano et al. 2005; Beaumont et al. 2006; Merrett 2007; Shibata and Kudo 2017). However, the loss of female function in the staminate flower occurred due to the atrophy of the pistillode stigma, which we confirmed by the absence of fruiting in all pollination tests carried out in this floral type. Thus, D. filipedunculata, as well as the other species of the subgenus Neivira that have a well-developed pistillode (Nevling Jr. 1959; Barringer and Pruski 2005), is cryptic dioecious (sensuMayer and Charlesworth 1991), differing from the other species of the genus that have reduced pistillodes (Nevling Jr. 1959, 1963; Nevling and Barringer 1986; Breedlove and de la Luz 1989) and are morphologically dioecious (sensuMayer and Charlesworth 1992).
The existence of cryptic dioecy in D. filipedunculata plays an important role in inbreeding avoidance (Mayer and Charlesworth 1991), as confirmed by the inbreeding coefficient equal to zero and moderate genetic diversity calculated from our genomic dataset. These values of genetic diversity are similar to those found in other obligate xenogamous endemic plant (Ipomoea cavalcantei, Lanes et al. 2018), but smaller than those observed in endemic species with wider distributions in this region (Brasilianthus carajensis and Monogereion carajensis, Carvalho et al. 2019). According to Mayer and Charlesworth (1991), non-functional sexual parts may be found in flowers because insufficient evolutionary time has passed to lead to their suppression, indicating that these populations are transitioning to morphological dioecy. Dioecy in D. filipedunculata must have originated with a hermaphrodite ancestor through an intermediate gynodioecious stage, a route also proposed for other species in the family (Burrows 1960; Mayer 1990; Alonso and Herrera 2011; Shibata et al. 2018). The fact that staminate flowers with developed pistillodes morphologically resemble a bisexual flower reinforces this idea. Dioecious species that evolved by this route should neither have individuals who change sex in response to increasing age or heterogeneous environments nor should there be spatial segregation between male and female plants (Freeman et al. 1997). Nevertheless, genetic analyses revealed one case of identical multilocus genotypes presenting female and male individuals; thus, further investigation is needed to confirm that D. filipedunculata is sexually labile. Sex change in individuals is expected to be a random, nonadaptive and rare event in dioecious species that evolved via gynodioecy (Freeman et al. 1997), with this change better defined as sexual inconstancy (sensuLloyd and Bawa 1984).
We observed a male-biased ramet sex ratio in D. filipedunculata, corroborating the findings of Watanabe et al. (2018), who reported a lower frequency of female individuals than male individuals. Most of the clones detected in our study were male plants, but these data must be interpreted with caution, as we found few female plants in the area (personal observation). Moreover, the number of individuals varies among clones and may be related to age, fitness or habitat, factors that were not tested in our study. Dioecious species typically present male-biased populations (e.g. Queenborough et al. 2013; Khanduri et al. 2019), although female-biased populations can occur (see Wang et al. 2013). The prevalence of male-biased populations is associated with the higher reproductive costs involved in the production of fruit and seeds by female plants, which are often associated with reduced survival rates, thus affecting population sex ratios (Obeso 2002; Barrett and Hough 2013; Lin et al. 2015). Differences in investment in sexual reproduction can also explain the rates of clonal propagation between sexes (Field et al. 2013). Male plants invest less in reproduction and thus likely have more resources for clonal growth, favouring a male-biased ramet sex ratio (Sinclair et al. 2012; Field et al. 2013). Interestingly, in a gynodioecious Gnidia species, Beaumont et al. (2006) observed that female plants invested 7.3 % more energy in reproduction than hermaphrodite plants. In contrast to observations in Gnidia, D. filipedunculata staminate flowers with developed pistillodes (morphologically hermaphrodite) did not produce fruits, which can increase the availability of resources for male plants, increasing their fertility, growth or survival (Mayer and Charlesworth 1991). Further investigations are needed to identify whether reproductive costs are related to the higher frequency of clonal male ramets in D. filipedunculata.
The absence of fruiting by apomixis in D. filipedunculata indicates that this dioecious species is dependent on pollination vectors for fruit formation to occur, in contrast to other species in the family, which are hermaphrodite and apomictic (Williams 2004; Graves 2008). Moreover, the lack of fine-spatial genetic structure among non-clonal individuals likely indicates that dispersal by pollen or seeds is not limited by distance in this species. Thymelaeaceae species are visited by insects from different orders, such as Coleoptera, Diptera, Hymenoptera, Hemiptera, Lepidoptera and Thysanoptera (de la Bandera and Traveset 2006; Roccotiello et al. 2009; Rodríguez-Pérez and Traveset 2011; Sakata and Nakahama 2018), and are possibly an ecologically and functionally generalist pollination system (sensuOllerton et al. 2007); however, there are also some cases of pollination by moths (Makholela and Manning 2006; Okamoto et al. 2008; Chen et al. 2016). Nevertheless, fruit set tends to be <50 % (Williams 2004; Roccotiello et al. 2009; Rodríguez-Pérez and Traveset 2011; Sakata and Nakahama 2018; Shibata et al. 2021), similar to the rates observed in this study, and there may be annual variation (Schulz et al. 2004; Roccotiello et al. 2012). Pollen limitation due to the lack of compatible mates and pollinator behaviour have been identified as key factors explaining the low fruitification among dioecious and self-incompatible species (e.g. Wilcock and Neiland 2002; Faria et al. 2006; Vallejo-Marín et al. 2010; Barros et al. 2013; Ferreira et al. 2022). However, pollen supplementation did not favour fruit set in D. filipedunculata and we found markedly higher genotypic diversity (G/N = 0.53 for all individuals and G/N = 0.44 after removing female and undetermined ramets). As the studied population is male-biased, the high G/N ratio could reflect a high diversity of males for pollen donation. Thus, such results are the opposite of those expected in dioecious species. Other factors may be related to the low fruiting rate, such as plant size, light availability (Schulz et al. 2004), the short duration of pollen grain viability or stigmatic receptivity (Roccotiello et al. 2009).
Asexual reproduction in angiosperms can be achieved via clonal propagation and apomixis (Barrett 2015). Since we did not observe fruit formation by apomixis, we concluded that clonality is the main form of asexual reproduction in D. filipedunculata. Patterns of clonal propagation, such as clonal architectures and growth forms, are expected to affect mating systems and sexual reproductive success because they are the main factors responsible for the spatial distribution of ramets (Vallejo-Marín et al. 2010; Barrett 2015). Here, we found that roots are the main organ responsible for vegetative propagation in D. filipedunculata and that the distance between ramets from the same genet is usually short resulting in fine-scale genetic structure within the N1 transition area. Nevertheless, the spatial distribution of individuals showed a mixture of ramets from different genotypes. These observations suggest that D. filipedunculata presents an intermediate phalanx–guerrilla clonal architecture (Vallejo-Marín et al. 2010). Our results also show two genetic clusters when clonal and non-clonal individuals are included in genetic structure analysis, showing that although there is a mixture of ramets from different genotypes, some clonal groups are genetically very similar. This pattern disappears when only non-clonal individuals are analysed, suggesting that the inclusion of individuals with the same genotypes forces the formation of more genetic groups.
Clonal propagation, especially through underground organs, represents a general strategy of resource storage (Klimešová et al. 2018; Franklin et al. 2021), which may include starch, as seen in D. filipedunculata. Interestingly, root sprouting is frequently associated with environmental disturbances such as windthrows and fires (Klimešová et al. 2017). We found that the gemmiferous roots of D. filipedunculata exhibit regular secondary growth (establishment of a single vascular cambium), with the cambium forming a large proportion of the parenchyma tissue storing starch grains; thus, these roots are characterized as tuberous (Appezzato da Glória 2015). Gemmiferous roots can develop two types of buds, additional and reparative, with the former associated with undisturbed environments and the latter associated with physical damage (i.e. injuries and fire). While the reparative buds have an exogenous origin from external tissues of the root, the additional buds have an endogenous origin and may develop from internal tissues such as the secondary phloem and vascular cambium, leaving traces contiguous with the centre of the root (Appezzato da Glória 2015). Although we did not carry out an ontogenetic study to characterize the root buds, we observed a continuity of vascular tissues between roots and new ramets, which suggests that they are additional buds. This indicates that in D. filipedunculata, clonal propagation occurs naturally, that is, not mediated by any root damage.
Besides the importance of disturbance events (Klimešová et al. 2017), the natural formation of bud by roots has been previously reported (e.g. Klimešová and Martínková 2004), and environmental heterogeneity has been pointed as a key trigger of root sprouting (see Klimešová et al. 2017). Daphnopsis filipedunculata occurs in the transition zone between ironstone outcrops and ombrophilous forests (Watanabe et al. 2018), which is a more heterogeneous habitat than those from mature forests where the species was recently found (unpubl. data). Thus, resource sharing (evidenced by starch grains stored in the stems and roots of ramets of a single genet) represents an efficient strategy for clonal survival, avoiding or minimizing intraindividual competition, especially in the understory, where resources such as light, water and soil nutrients are heterogeneously distributed throughout the habitat (Lei 2010; Guo et al. 2011; Duchoslavová and Herben 2020). Compared to the stems, the roots are deeper, protecting the buds that form the new ramets (Klimešová et al. 2019). Yet, buds can originate from different portions of the root system with no morphological limitations (i.e. by node positions as in rhizomatous and stoloniferous species), and new shoots possess its own root system with no time limitations such as those from rhizomatous and stoloniferous plants, where the formation of adventitious root system is often delayed (Klimešová and Klimeš 2008; Martínková et al. 2018). The costs and constraints in root bud formation and resource sharing in this manner will need further investigation, but the functional integration observed between taller ramets and new sprouts might be a key step in the success of clonality in D. filipedunculata, as it prolongs the life of clonal individuals and reduces the risk of mortality of these new ramets in the area (Lei 2010; Duchoslavová and Herben 2020; Shi et al. 2021).
We found low fruit production that pollen supplementation did not increase and a moderate proportion of clones in the studied population. The expansion of clonality can impact seed production by limiting the resources allocated to flowering (Van Drunen and Dorken 2012) and by constraining mating opportunities in self-incompatible species (Honnay and Jacquemyn 2008; Hu et al. 2017) or in species with sexual polymorphism (Widén and Widén 1990; Wang et al. 2005; Barrett 2015), as observed in our study. Despite the impact of clonality on fruit production, clonal propagation has been identified as a driver of population abundance and persistence in species with low fruit and seed sets (Faria et al. 2006). Such trade-offs between low seed production and clonal propagation as well as compensatory responses have been reported for self-incompatible and dioecious species (Herben et al. 2012; Van Drunen and Dorken 2012; Barrett 2015; Hu et al. 2017). Environmental factors seem to drive the balance between sexual and asexual reproduction (Honnay and Bossuyt 2005; Binks et al. 2015; Hewitt 2020); thus, future studies of D. filipedunculata should investigate whether the balance between sexual reproduction and clonal propagation differs throughout its distribution range, conducting detailed analyses of the costs associated with these types of reproduction (e.g. Lei 2010; Franklin et al. 2021).
Conservation implications
Our results have important implications for the management and conservation of D. filipedunculata, a highly restricted endemic species. First, the extensive clonal growth indicates that population size estimates for the species should consider that many apparently distinguishable individuals may actually represent a single genet. Second, the finding that individuals within 11 m from each other are more likely to be clones implies that one should avoid sampling more than one plant within such a radius threshold for ex situ conservation, such as the establishment of genetically representative ex situ collections, seed banks or translocated populations (Bragg et al. 2020). It is also important to consider, for ex situ collection, sampling plants during their reproductive period to ensure collection from both sexes. Our data also provide the first insights into the relative importance of sexual reproduction and clonal propagation in D. filipedunculata, an important aspect that should inform the management, conservation and restoration practices implemented for threatened species (Sydes and Peakall 1998; Cascante-Marín et al. 2020). Nevertheless, further research evaluating the extent of clonality in other habitats where the species occurs naturally, as well as the impact of clonality versus sexual reproduction on population growth rates, is needed since such patterns may vary over time and may be dependent on the environmental context (e.g. Dorken and Eckert 2001; Xie et al. 2001; Kimura et al. 2013). Notably, prolonged and nearly exclusive clonal growth could ultimately lead to local sexual extinction in D. filipedunculata with significant consequences for population viability (see Honnay and Bossuyt 2005). The lack of genetic variation due to increased clonality could also constrain long-term adaptation to environmental changes (Lynch 1995; Barrett and Schluter 2008) and needs to be extensively investigated across the entire distribution range of the species.
Supporting Information
The following additional information is available in the online version of this article –
Table S1. Pollination test results of floral types of Daphnopsis filipedunculata in the FLONA de Carajás, Brazil.
Table S2. Multilocus genotypes that occurred in more than one individual of Daphnopsis filipedunculata in the FLONA de Carajás, Brazil.
Table S3: Characterization of the genetic diversity of Daphnopsis filipedunculata based on 1122 SNPs sampled in 49 clonal and non-clonal individuals (full dataset) and a subset of 26 non-clonal individuals.
Table S4. Summaries of the linear mixed models used to analyse floral traits of Daphnopsis filipedunculata in the FLONA de Carajás, Brazil.
Figure S1. Threshold of Euclidean genetic distance (vertical line) among individuals of Daphnopsis filipedunculata in the FLONA de Carajás, Brazil.
Figure S2. Heatmap (A) and histogram (B) showing the proportion of shared alleles amongst 49 individuals of Daphnopsis filipedunculata.
Figure. S3. Barplot showing fastSTRUCTURE results for the full dataset of 49 clonal and non-clonal individuals and a subset of 26 non-clonal individuals of Daphnopsis filipedunculata.
Contributor Information
Carolina da Silva Carvalho, Instituto Tecnologico Vale—Desenvolvimento Sustentável, Rua Boaventura da Silva 955, Belém, Pará 66055-090, Brazil.
Lucas Erickson Nascimento da Costa, Instituto Tecnologico Vale—Desenvolvimento Sustentável, Rua Boaventura da Silva 955, Belém, Pará 66055-090, Brazil.
Bárbara Simões Santos Leal, Instituto Tecnologico Vale—Desenvolvimento Sustentável, Rua Boaventura da Silva 955, Belém, Pará 66055-090, Brazil.
Kleber Resende Silva, Instituto Tecnologico Vale—Desenvolvimento Sustentável, Rua Boaventura da Silva 955, Belém, Pará 66055-090, Brazil.
Adriano Valentin-Silva, Instituto Tecnologico Vale—Desenvolvimento Sustentável, Rua Boaventura da Silva 955, Belém, Pará 66055-090, Brazil.
Ana Carolina Galindo Costa, Instituto Tecnologico Vale—Desenvolvimento Sustentável, Rua Boaventura da Silva 955, Belém, Pará 66055-090, Brazil.
Lourival Tyski, Plano de Gestão da Biodiversidade de Carajás (PGBio), Gerência de Estudos Técnicos de Longo Prazo, Vale S.A., Rodovia Raimundo Mascarenhas Km 26, s/n, Núcleo Urbano de Carajás, Parauapebas, Pará 64516-000, Brazil.
Fernando Marino Gomes dos Santos, Gerência de Licenciamento Ambiental, Vale S/A, Alameda Oscar Niemeyer 132—Edifício Concórdia, Nova Lima, Minas Gerais 34006-049, Brazil.
Mauricio Takashi Coutinho Watanabe, Instituto Tecnologico Vale—Desenvolvimento Sustentável, Rua Boaventura da Silva 955, Belém, Pará 66055-090, Brazil.
Acknowledgements
We thank Fábia Cavalcante for assistance in the field logistics, and Manoel Lopes for help in the laboratory.
Sources of Funding
Funding was provided by Instituto Tecnológico Vale.
Contributions by the Authors
C.S.C., L.E.N.C., B.S.S.L., K.R.S., A.C.G.C. and M.T.C.W. conceived the ideas; C.S.C. and M.T.C.W. acquired funding and administered the project; C.S.C., L.E.N.C., B.S.S.L., K.R.S., A.V.S., A.C.G.C., L.T., F.M.G.S. and M.T.C.W. collected the data; C.S.C., L.E.N.C., B.S.S.L., K.R.S., A.V.S. and A.C.G.C. analyzed the data; C.S.C., L.E.N.C., B.S.S.L., K.R.S. and A.V.S. wrote the original draft; and C.S.C., L.E.N.C., B.S.S.L., K.R.S., A.V.S., A.C.G.C., L.T., F.M.G.S. and M.T.C.W. reviewed and edited the manuscript.
Conflict of Interest Statement
None declared.
Data Availability
Floral traits and mating system data, and geographic coordinates in decimal degrees and the genotypes in Variant Call Format are provided in figshare: 10.6084/m9.figshare.26836693. Raw reads have been deposited in the NCBI SRA database (BioProject ID: PRJNA1153566).
Literature Cited
- Adam P, Williams G.. 2001. Dioecy, self-compatibility and vegetative reproduction in Australian subtropical rainforest trees and shrubs. Cunninghamia 7:89–100. [Google Scholar]
- Alonso C, Herrera CM.. 2011. Back‐and‐forth hermaphroditism: phylogenetic context of reproductive system evolution in subdioecious Daphne laureola. Evolution 65:1680–1692. [DOI] [PubMed] [Google Scholar]
- Alonso C, Mutikainen P, Herrera CM.. 2007. Ecological context of breeding system variation: sex, size and pollination in a (predominantly) gynodioecious shrub. Annals of Botany 100:1547–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amplo Engenharia. 2021. Estudo de Impacto Ambiental do Projeto Mina N3. Carta resposta ao ofício IBAMA N° 471/2021/COMIP/CGTEF/DILIC. Anexo V - Informações adicionais sobre a distribuição de Daphnopsis filipedunculata (protocolo SEI 11552798).
- Appezzato da Glória B. 2015. Morfologia de sistemas subterrâneos de plantas. 3rd edn. Belo Horinzonte: 3 Editora. [Google Scholar]
- Barrett SC. 2015. Influences of clonality on plant sexual reproduction. Proceedings of the National Academy of Sciences of the United States of America 112:8859–8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SC, Hough J.. 2013. Sexual dimorphism in flowering plants. Journal of Experimental Botany 64:67–82. [DOI] [PubMed] [Google Scholar]
- Barrett RD, Schluter D.. 2008. Adaptation from standing genetic variation. Trends in Ecology & Evolution 23:38–44. [DOI] [PubMed] [Google Scholar]
- Barringer K, Pruski JF.. 2005. Two new cauliflorous species of Daphnopsis (Thymelaeaceae) from French Guiana and Surinam. Novon 15:50–54. [Google Scholar]
- Barros ECDO, Webber AC, Machado IC.. 2013. Limitação de polinizadores e mecanismo de autoincompatibilidade de ação tardia como causas da baixa formação de frutos em duas espécies simpátricas de Inga (Fabaceae-Mimosoideae) na Amazônia Central. Rodriguésia 64:37–47. [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S.. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67:1–48. [Google Scholar]
- Beaumont AJ, Edwards TJ, Smith FR.. 2006. The first record of gynodioecy in a species of Gnidia (Thymelaeaceae) from South Africa. Botanical Journal of the Linnean Society 152:219–233. [Google Scholar]
- Bell G, Hamilton WD.. 1985. On the function of flowers. Proceedings of the Royal Society of London. Series B. Biological Sciences 224:223–265. [Google Scholar]
- Binks RM, Millar MA, Byrne M.. 2015. Contrasting patterns of clonality and fine-scale genetic structure in two rare sedges with differing geographic distributions. Heredity 115:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg JG, Cuneo P, Sherieff A, Rossetto M.. 2020. Optimizing the genetic composition of a translocation population: incorporating constraints and conflicting objectives. Molecular Ecology Resources 20:54–65. [DOI] [PubMed] [Google Scholar]
- Breedlove DE, de la Luz JLL.. 1989. A new species of Daphnopsis (Thymelaeaceae) from Baja California Sur, Mexico. Madroño 36:266–270. [Google Scholar]
- Bullock SH. 1985. Breeding systems in the flora of a tropical deciduous forest in Mexico. Biotropica 17:287–301. [Google Scholar]
- Burrows CJ. 1960. Studies in Pimelea I—the breeding system. Transaction of the Royal Society of New Zealand: Botany 88:29–45. [Google Scholar]
- Butcher PA, Bradbury D, Krauss SL.. 2011. Limited pollen-mediated dispersal and partial self-incompatibility in the rare ironstone endemic Tetratheca paynterae subsp. paynterae increase the risks associated with habitat loss. Conservation Genetics 12:1603–1618. [Google Scholar]
- Callaghan TV. 1984. Growth and translocation in a clonal southern hemisphere sedge, Uncinia meridensis. The Journal of Ecology 72:529–546. [Google Scholar]
- Carvalho CS, Lanes EC, Silva AR, Caldeira CF, Gastauer M, Imperatriz-Fonseca VL, Nascimento Júnior W, Oliveira G, Siqueira JO, Viana PL, et al. 2019. Habitat loss does not always entail negative genetic consequences. Frontiers in Genetics 10:455095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascante-Marín A, Trejos C, Madrigal R, Fuchs EJ.. 2020. Genetic diversity and reproductive biology of the dioecious and epiphytic bromeliad Aechmea mariae-reginae (Bromeliaceae) in Costa Rica: implications for its conservation. Botanical Journal of the Linnean Society 192:773–786. [Google Scholar]
- Charpentier A. 2001. Consequences of clonal growth for plant mating. Evolutionary Ecology 15:521–530. [Google Scholar]
- Chen XS, Li YF, Xie YH, Deng ZM, Li X, Li F, Hou ZY.. 2015. Trade-off between allocation to reproductive ramets and rhizome buds in Carex brevicuspis populations along a small-scale elevational gradient. Scientific Reports 5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Liu C, Sun W.. 2016. Pollination and seed dispersal of Aquilaria sinensis (Lour.) Gilg (Thymelaeaceae): an economic plant species with extremely small populations in China. Plant Diversity 38:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Bandera MC, Traveset A.. 2006. Breeding system and spatial variation in the pollination biology of the heterocarpic Thymelaea velutina (Thymelaeaceae). Plant Systematics and Evolution 257:9–23. [Google Scholar]
- Delph LF. 1999. Sexual dimorphism in life history. In: Geber MA, Dawson TE, Delph LF, eds. Gender and sexual dimorphism in flowering plants. Berlin: Springer Verlag, 149–173. [Google Scholar]
- Dering M, Chybicki IJ, Rączka G.. 2015. Clonality as a driver of spatial genetic structure in populations of clonal tree species. Journal of Plant Research 128:731–745. [DOI] [PubMed] [Google Scholar]
- Dommée B, Bompar JL, Denelle N.. 1990. Sexual tetramorphism in Thymelaea hirsuta (Thymelaeaceae): evidence of the pathway from heterodichogamy to dioecy at the infraspecific level. American Journal of Botany 77:1449–1462. [Google Scholar]
- Dommée B, Biascamano A, Denelle N, Bompar JL, Thompson JD.. 1995. Sexual tetramorphism in Thymelaea hirsuta (Thymelaeaceae): morph ratios in open-pollinated progeny. American Journal of Botany 82:734–740. [Google Scholar]
- Dong M. 1996. Clonal growth in plants in relation to resource heterogeneity: foraging behavior. Journal of Integrative Plant Biology 38:828–835. [Google Scholar]
- Dorken ME, Eckert CG.. 2001. Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythraceae). Journal of Ecology 89:339–350. [Google Scholar]
- Duchoslavová J, Herben T.. 2020. Effect of clonal growth form on the relative performance of species in experimental communities over time. Perspectives in Plant Ecology, Evolution and Systematics 44:125532. [Google Scholar]
- Eaton DA, Overcast I.. 2020. ipyrad: interactive assembly and analysis of RADseq datasets. Bioinformatics 36:2592–2594. [DOI] [PubMed] [Google Scholar]
- Eckhart VM. 1999. Sexual dimorphism in flowers and inflorescences. In: Geber MA, Dawson TE, Delph LF, eds. Gender and sexual dimorphism in flowering plants. Berlin: Springer-Verlag, 123–148. [Google Scholar]
- El-Keblawy A, Lovett-Doust J, Lovett-Doust L.. 1996. Gender variation and the evolution of dioecy in Thymelaea hirsuta (Thymelaeaceae). Canadian Journal of Botany 74:1596–1601. [Google Scholar]
- Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE.. 2011. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One 6:e19379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson O, Ehrlén J.. 2001. Landscape fragmentation and the viability of plant populations. In: Silvertown J, Anotonovics J, eds. Integrating ecology and evolution in a spatial context. Oxford: Blackwell, 157–175. [Google Scholar]
- Faria APG, Matallana G, Wendt T, Scarano FR.. 2006. Low fruit set in the abundant dioecious tree Clusia hilariana (Clusiaceae) in a Brazilian restinga. Flora 201:606–611. [Google Scholar]
- Feder N, O’Brien TP.. 1968. Plant microtechnique: some principles and new methods. American Journal of Botany 55:123–142. [Google Scholar]
- Ferreira INM, Cavalcante RKDO, Borges JPR, Teixeira TPDO, Silva DP, Sá T, Franceschinelli EV.. 2022. Two dioecious Simarouba species with a specialized pollination system and low reproductive efficacy in Central Brazil. Rodriguésia 73:e02002020. [Google Scholar]
- Field DL, Pickup M, Barrett SC.. 2013. Comparative analyses of sex‐ratio variation in dioecious flowering plants. Evolution 67:661–672. [DOI] [PubMed] [Google Scholar]
- Franklin S, Alpert P, Salguero-Gómez R, Janovský Z, Herben T, Klimešová J, Douhovnikoff V.. 2021. Next-gen plant clonal ecology. Perspectives in Plant Ecology, Evolution and Systematics 49:125601. [Google Scholar]
- Freeman D, Doust JL, El-Keblawy A, Miglia KJ, McArthur ED.. 1997. Sexual specialization and inbreeding avoidance in the evolution of dioecy. The Botanical Review 63:65–92. [Google Scholar]
- Graves WR. 2008. Habitat and reproduction of Dirca mexicana (Thymelaeaceae). Rhodora 110:365–378. [Google Scholar]
- Gruber B, Unmack PJ, Berry OF, Georges A.. 2018. dartR: an R package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Molecular Ecology Resources 18:691–699. [DOI] [PubMed] [Google Scholar]
- Guo W, Song YB, Yu FH.. 2011. Heterogeneous light supply affects growth and biomass allocation of the understory fern Diplopterygium glaucum at high patch contrast. PLoS One 6:e27998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy OJ, Vekemans X.. 2002. SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Molecular Ecology Notes 2:618–620. [Google Scholar]
- Harper JL. 1977. Population biology of plants. London: Academic Press, 892. [Google Scholar]
- Herben T, Klimešová J.. 2020. Evolution of clonal growth forms in angiosperms. The New Phytologist 225:999–1010. [DOI] [PubMed] [Google Scholar]
- Herben T, Nováková Z, Klimešová J, Hrouda L.. 2012. Species traits and plant performance: functional trade‐offs in a large set of species in a botanical garden. Journal of Ecology 100:1522–1533. [Google Scholar]
- Herber BE. 2003. Thymelaeaceae. In: Kubitzki K, Bayer C, eds. Flowering plants·dicotyledons: malvales, capparales and non-betalain caryophyllales. Berlin: Springer Berlin Heidelberg, 373–396. [Google Scholar]
- Hewitt A. 2020. Genetic and environmental factors in the trade‐off between sexual and asexual reproduction of a rare clonal angiosperm. Austral Ecology 45:187–194. [Google Scholar]
- Honnay O, Bossuyt B.. 2005. Prolonged clonal growth: escape route or route to extinction? Oikos 108:427–432. [Google Scholar]
- Honnay O, Jacquemyn H.. 2008. A meta-analysis of the relation between mating system, growth form and genotypic diversity in clonal plant species. Evolutionary Ecology 22:299–312. [Google Scholar]
- Hu AQ, Gale SW, Kumar P, Saunders RM, Sun M, Fischer GA.. 2017. Preponderance of clonality triggers loss of sex in Bulbophyllum bicolor, an obligately outcrossing epiphytic orchid. Molecular Ecology 26:3358–3372. [DOI] [PubMed] [Google Scholar]
- IBAMA, Instituto Brasileiro do Meio Ambiente e Recursos Naturais. 2003. Plano de manejo para uso múltiplo da Floresta Nacional de Carajás, Brasilia, Brazil. [Google Scholar]
- Johansen DA. 1940. Plant microtechnique. New York: McGraw Hill Book. [Google Scholar]
- Jombart T. 2008. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24:1403–1405. [DOI] [PubMed] [Google Scholar]
- Kamvar ZN, Tabima JF, Grünwald NJ.. 2014. Poppr: an R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2:e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K, McGinnity P, Cross TF, Crozier WW, Prodöhl PA.. 2013. diveRsity: An R package for the estimation and exploration of population genetics parameters and their associated errors. Methods in Ecology and Evolution 4:782–788. [Google Scholar]
- Khanduri VP, Sukumaran A, Sharma CM.. 2019. Male-skewed sex ratio in Myrica esculenta: a dioecious tree species. Trees 33:1157–1165. [Google Scholar]
- Kimura MK, Kabeya D, Saito T, Moriguchi Y, Uchiyama K, Migita C, Chiba Y, Tsumura Y.. 2013. Effects of genetic and environmental factors on clonal reproduction in old-growth natural populations of Cryptomeria japonica. Forest Ecology and Management 304:10–19. [Google Scholar]
- Klimes L, Klimesov J, Hendriks R, van Groenendael JM, de Kroon H.. 1997. Clonal plant architecture: a comparative analysis of form and function. In: de Kroon H, van Groenendael JM, eds. The ecology and evolution of clonal plants. Leiden: Backhuys, 1–29. [Google Scholar]
- Klimešová J, Klimeš L.. 2008. Clonal growth diversity and bud banks of plants in the Czech flora: an evaluation using the CLO-PLA3 database. Preslia 80:255–275. [Google Scholar]
- Klimešová J, Martínková J.. 2004. Intermediate growth forms as a model for the study of plant clonality functioning: an example with root sprouters. Evolutionary Ecology 18:669–681. [Google Scholar]
- Klimešová J, Herben T, Martínková J.. 2017. Disturbance is an important factor in the evolution and distribution of root-sprouting species. Evolutionary Ecology 31:387–399. [Google Scholar]
- Klimešová J, Martínková J, Ottaviani G.. 2018. Belowground plant functional ecology: towards an integrated perspective. Functional Ecology 32:2115–2126. [Google Scholar]
- Klimešová J, Martínková J, Pausas JG, de Moraes MG, Herben T, Yu FH, Puntieri J, Vesk PA, de Bello F, Janeček S, et al. 2019. Handbook of standardized protocols for collecting plant modularity traits. Perspectives in Plant Ecology, Evolution and Systematics 40:125485. [Google Scholar]
- Klimešová J, Ottaviani G, Charles-Dominique T, Campetella G, Canullo R, Chelli S, Janovský Z, Lubbe FC, Martínková J, Herben T.. 2021. Incorporating clonality into the plant ecology research agenda. Trends in Plant Science 26:1236–1247. [DOI] [PubMed] [Google Scholar]
- Lanes EC, Pope NS, Alves R, Carvalho Filho NM, Giannini TC, Giulietti AM, Imperatriz-Fonseca VL, Monteiro W, Oliveira G, Silva AR, et al. 2018. Landscape genomic conservation assessment of a narrow-endemic and a widespread morning glory from Amazonian Savannas. Frontiers in Plant Science 9:343731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei SA. 2010. Benefits and costs of vegetative and sexual reproduction in perennial plants: a review of literature. Journal of the Arizona-Nevada Academy of Science 42:9–14. [Google Scholar]
- Lenth R. 2020. Emmeans: estimated marginal means, aka least-squares means. R package version 1.5.0. https://CRAN.R-project.org/package=emmeans [accessed 13 March 2023] [Google Scholar]
- Li YL, Liu JX.. 2018. StructureSelector: a web‐based software to select and visualize the optimal number of clusters using multiple methods. Molecular Ecology Resources 18:176–177. [DOI] [PubMed] [Google Scholar]
- Lin SY, Nol E, Dorken ME.. 2015. Spatial dynamics of pollination in dioecious Shepherdia canadensis (Elaeagnaceae). Plant Ecology 216:1213–1223. [Google Scholar]
- Liu F, Liu J, Dong M.. 2016. Ecological consequences of clonal integration in plants. Frontiers in Plant Science 7:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DG, Bawa KS.. 1984. Modification of gender of seed plants in varying conditions. Evolutionary Biology 17:255–338. [Google Scholar]
- Loiselle BA, Sork VL, Nason J, Graham C.. 1995. Spatial genetic structure of a tropical understory shrub, Psychotria officinalis (Rubiaceae). American Journal of Botany 82:1420–1425. [Google Scholar]
- Lynch M. 1995. A quantitative-genetic perspective on conservation issues. In: Avise JC, Hamrick JL, eds. Conservation genetics: case histories from nature. New York: Chapman & Hall, 471–501. [Google Scholar]
- Makholela T, Manning JC.. 2006. First report of moth pollination in Struthiola ciliata (Thymelaeaceae) in southern Africa. South African Journal of Botany 72:597–603. [Google Scholar]
- Martínková J, Klimeš A, Klimešová J.. 2018. No evidence for nutrient foraging in root-sprouting clonal plants. Basic and Applied Ecology 28:27–36. [Google Scholar]
- Mayer SS. 1990. The origin of dioecy in Hawaiian Wikstroemia (Thymelaeaceae). Memoirs of the New York Botanical Garden 55:76–82. [Google Scholar]
- Mayer SS, Charlesworth D.. 1991. Cryptic dioecy in flowering plants. Trends in Ecology and Evolution 6:320–325. [DOI] [PubMed] [Google Scholar]
- Mayer SS, Charlesworth D.. 1992. Genetic evidence for multiple origins of dioecy in the Hawaiian Wikstroemia (Thymelaeaceae). Evolution 46:207–215. [DOI] [PubMed] [Google Scholar]
- Medrano M, Alonso C, Herrera CM.. 2005. Mating system, sex ratio, and persistence of females in the gynodioecious shrub Daphne laureola L. (Thymelaeaceae). Heredity 94:37–43. [DOI] [PubMed] [Google Scholar]
- Merrett MF. 2007. Sex ratios, fruit set and size-class structure in the threatened, gynodioecious, sand-dune species Pimelea arenaria (Thymelaeaceae) from New Zealand. Australian Journal of Botany 55:554–560. [Google Scholar]
- Minuto L, Casazza G, Profumo P.. 2005. Sexual polymorphism and spatial segregation of Thymelaea hirsuta in Liguria (north-west Italy). Plant Biosystems 139:234–240. [Google Scholar]
- Mota NFO, Giulietti AM.. 2016. Flora das cangas da Serra dos Carajás, Pará, Brasil: Thymelaeaceae. Rodriguésia 67:1481–1484. [Google Scholar]
- Nevling LI Jr. 1959. A revision of the genus Daphnopsis. Annals of the Missouri Botanical Garden 46:257–358. [Google Scholar]
- Nevling LI Jr. 1963. Notes on Daphnopsis. Journal of the Arnold Arboretum 44:402–410. [Google Scholar]
- Nevling LI Jr, Barringer K.. 1986. New and noteworthy species of Daphnopsis (Thymelaeaceae) from Mexico and Central America. Phytologia 61:361–366. [Google Scholar]
- Nevling LI Jr, Barringer K.. 1993. A new species of Daphnopsis (Thymelaeaceae) from Brazil. Brittonia 45:335–336. [Google Scholar]
- Niklas KJ, Cobb ED.. 2017. The evolutionary ecology (evo-eco) of plant asexual reproduction. Evolutionary Ecology 31:317–332. [Google Scholar]
- Obeso JR. 2002. The costs of reproduction in plants. The New Phytologist 155:321–348. [DOI] [PubMed] [Google Scholar]
- OʼBrien TP, Feder N, McCully ME.. 1964. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373. [Google Scholar]
- Okamoto T, Kawakita A, Kato M.. 2008. Floral adaptations to nocturnal moth pollination in Diplomorpha (Thymelaeaceae). Plant Species Biology 23:192–201. [Google Scholar]
- Ollerton J, Killick A, Lamborn E, Watts S, Whiston M.. 2007. Multiple meanings and modes: on the many ways to be a generalist flower. Taxon 56:717–728. [Google Scholar]
- Queenborough SA, Humphreys AM, Valencia R.. 2013. Sex-specific flowering patterns and demography of the understorey rain forest tree Iryanthera hostmannii (Myristicaceae). Tropical Conservation Science 6:637–652. [Google Scholar]
- R Core Team. 2020. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Raj A, Stephens M, Pritchard JK.. 2014. fastSTRUCTURE: variational inference of population structure in large SNP data sets. Genetics 197:573–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez N, Briceño H.. 2021. Pollination types and plant reproductive systems of two areas of Venezuelan cloud forests. International Journal of Plant Reproductive Biology 13:82–103. [Google Scholar]
- Richards AJ. 1997. Plant breeding systems. London: Chapman and Hall. [Google Scholar]
- Roccotiello E, Casazza G, Galli L, Cornara L, Moncalvo A, Minuto L.. 2009. The flower biology of Daphne gnidium L. (Thymelaeaceae). Plant Systematics and Evolution 279:41–49. [Google Scholar]
- Roccotiello E, Casazza G, Cornara L, Moncalvo A, Minuto L.. 2012. Reproductive success in Daphne gnidium (Thymelaeaceae). Bollettino dei Musei e degli Istituti Biologici 74:22–37. [Google Scholar]
- Rodríguez-Pérez J, Traveset A.. 2011. Influence of reproductive traits on pollination success in two Daphne species (Thymelaeaceae). Journal of Plant Research 124:277–287. [DOI] [PubMed] [Google Scholar]
- Rogers ZS. 2009. A revision of Malagasy Gnidia (Thymelaeaceae, Thymelaeoideae). Annals of the Missouri Botanical Garden 96:324–369. [Google Scholar]
- Rogers ZS. 2010. Nomenclatural notes on American Thymelaeaceae. Novon: A Journal for Botanical Nomenclature 20:448–462. [Google Scholar]
- Rossi L. 2020. Thymelaeaceae in Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. https://floradobrasil2020.jbrj.gov.br/FB32927 (9 October 2022). [Google Scholar]
- Sage TL, Husband BC, Routley MB.. 2005. Intrinsic attributes of the breeding system. In: Dafni A, Kevan PG, Husband BC, eds. Practical pollination biology. Cambridge: Enviroquest, 30–55. [Google Scholar]
- Sakai AK, Weller SG.. 1999. Gender and sexual dimorphism in flowering plants: a review of terminology, biogeographic patterns, ecological correlates, and phylogenetic approaches. In: Geber MA, Dawson TE, Delph LF, eds. Gender and sexual dimorphism in flowering plants. Berlin: Springer-Verlag, 1–31. [Google Scholar]
- Sakata Y, Nakahama N.. 2018. Flexible pollination system in an unpalatable shrub Daphne miyabeana (Thymelaeaceae). Plant Species Biology 33:239–247. [Google Scholar]
- Sass JE. 1951. Botanical Microtechnique. 2nd edn. Ames: The Iowa State College Press. [Google Scholar]
- Schulz K, Zasada J, Nauertz E.. 2004. Annual, local, and individual variation in the inflorescence and fruit production of eastern leatherwood (Dirca palustris L. Thymelaeaceae). Journal of the Torrey Botanical Society 131:292–304. [Google Scholar]
- Shaltout KH, El-Keblawy AA.. 1992. Sex expression in Egyptian Thymelaea hirsuta (Thymelaeaceae) populations. Plant Systematics and Evolution 181:133–141. [Google Scholar]
- Shi J, Mao S, Wang L, Ye X, Wu J, Wang G, Chen F, Yang Q.. 2021. Clonal integration driven by source-sink relationships is constrained by rhizome branching architecture in a running bamboo species (Phyllostachys glauca): a 15n assessment in the field. Forest Ecology and Management 481:118754. [Google Scholar]
- Shibata A, Kudo G.. 2017. Size-dependent sex allocation and reproductive investment in a gynodioecious shrub. AoB Plants 9:plw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata A, Kameyama Y, Kudo G.. 2018. Restricted female function of hermaphrodites in a gynodioecious shrub, Daphne jezoensis (Thymelaeaceae). Journal of Plant Research 131:245–254. [DOI] [PubMed] [Google Scholar]
- Shibata A, Kameyama Y, Kudo G.. 2021. Low seed fertility of hermaphrodites is maintained in a gynodioecious species throughout the distribution range in Japan. Plant Systematics and Evolution 307:55. [Google Scholar]
- Silvertown J. 2008. The evolutionary maintenance of sexual reproduction: evidence from the ecological distribution of asexual reproduction in clonal plants. International Journal of Plant Sciences 169:157–168. [Google Scholar]
- Sinclair JP, Emlen J, Freeman DC.. 2012. Biased sex ratios in plants: theory and trends. The Botanical Review 78:63–86. [Google Scholar]
- Smith FR. 2009. Sex-ratio variation in the gynodioecious shrub Gnidia wikstroemiana (Thymelaeaceae). South African Journal of Botany 75:591–593. [Google Scholar]
- Sydes MA, Peakall ROD.. 1998. Extensive clonality in the endangered shrub Haloragodendron lucasii (Haloragaceae) revealed by allozymes and RAPDs. Molecular Ecology 7:87–93. [Google Scholar]
- Tandon R, Koul M, Shivanna KR.. 2020. Reproductive ecology of flowering plants: an introduction. In: Tandon R, Shivanna KR, Koul M, eds. Reproductive ecology of flowering plants: patterns and processes. Berlin: Springer, 1–24. [Google Scholar]
- Thomas RG, Hay MJ.. 2010. The role of nodal roots in prostrate clonal herbs:‘phalanx’versus ‘guerrilla’. Evolutionary Ecology 24:1489–1504. [Google Scholar]
- Ushimaru A, Kikuzawa K.. 1999. Variation of breeding system, floral rewards, and reproductive success in clonal Calystegia species (Convolvulaceae). American Journal of Botany 86:436–446. [PubMed] [Google Scholar]
- Vallejo-Marín M, O’Brien HE.. 2006. Correlated evolution of self-incompatibility and clonal reproduction in Solanum (Solanaceae). New Phytologist 173:415–421. [DOI] [PubMed] [Google Scholar]
- Vallejo-Marín M, Dorken ME, Barrett SC.. 2010. The ecological and evolutionary consequences of clonality for plant mating. Annual Review of Ecology, Evolution, and Systematics 41:193–213. [Google Scholar]
- Van Drunen WE, Dorken ME.. 2012. Trade-offs between clonal and sexual reproduction in Sagittaria latifolia (Alismataceae) scale up to affect the fitness of entire clones. The New Phytologist 196:606–616. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang QF, Guo YH, Barrett SC.. 2005. Reproductive consequences of interactions between clonal growth and sexual reproduction in Nymphoides peltata: a distylous aquatic plant. The New Phytologist 165:329–335. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang C, Zhao X, Gadow KV.. 2013. Limitations to reproductive success in the dioecious tree Rhamnus davurica. PLoS One 8:e81140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe MTC, Mota NFO, Pastore M, Santos FMG, Zappi DC.. 2018. Completing the jigsaw: the first record of the female plant of Daphnopsis filipedunculata (Thymelaeaceae), an endemic species from the Brazilian Amazon. PhytoKeys 109:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widén B, Widén M.. 1990. Pollen limitation and distance-dependent fecundity in females of the clonal gynodioecious herb Glechoma hederacea (Lamiaceae). Oecologia 83:191–196. [DOI] [PubMed] [Google Scholar]
- Wilcock C, Neiland R.. 2002. Pollination failure in plants: why it happens and when it matters. Trends in Plant Science 7:270–277. [DOI] [PubMed] [Google Scholar]
- Williams CE. 2004. Mating system and pollination biology of the spring‐flowering shrub, Dirca palustris. Plant Species Biology 19:101–106. [Google Scholar]
- Xiao Y, Tang J, Qing H, Zhou C, Kong W, An S.. 2011. Trade-offs among growth, clonal, and sexual reproduction in an invasive plant Spartina alterniflora responding to inundation and clonal integration. Hydrobiologia 658:353–363. [Google Scholar]
- Xie ZW, Lu YQ, Ge S, Hong DY, Li FZ.. 2001. Clonality in wild rice (Oryza rufipogon, Poaceae) and its implications for conservation management. American Journal of Botany 88:1058–1064. [PubMed] [Google Scholar]
- Ye XH, Yu FH, Dong M.. 2006. A trade-off between guerrilla and phalanx growth forms in Leymus secalinus under different nutrient supplies. Annals of Botany 98:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Savaliev AA, Smith GM.. 2009. Mixed effects models and extensions in ecology with R. NY: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Floral traits and mating system data, and geographic coordinates in decimal degrees and the genotypes in Variant Call Format are provided in figshare: 10.6084/m9.figshare.26836693. Raw reads have been deposited in the NCBI SRA database (BioProject ID: PRJNA1153566).