Abstract
ABSTRACT
Objective
Colonoscopy-related adverse events increase the burden of colorectal cancer (CRC) screening. This cross-sectional study evaluates adverse events during and after colonoscopy in a large, randomised CRC screening trial in Norway comparing sigmoidoscopy to immunochemical testing for faecal blood.
Methods
We included all individuals who underwent colonoscopy at two screening centres between 2012 and 2020. From medical records, we retrieved data on adverse events during and within 30 days after colonoscopy and classified them according to the American Society for Gastrointestinal Endoscopy lexicon for endoscopic adverse events. Multivariable logistic regression models were fitted to identify risk factors for adverse events.
Results
Of the 10 244 included individuals, 242 (2.4%) had at least one adverse event that was possibly, probably, or definitively related to the colonoscopy. 188 (1.8%) had mild adverse events, 50 (0.49%) had moderate, 3 (0.03%) had severe, and 1 had a fatal adverse event. The most frequent adverse events were lower gastrointestinal bleeding (0.86%), abdominal pain (0.48%), vasovagal reaction (0.39%), postpolypectomy syndrome (0.20%), and perforation (0.08%). 23 (0.22%) individuals had non-gastrointestinal adverse events. Risk factors associated with adverse events were older age, female sex, screening centre, anticoagulant therapy, number of polypectomies, size of lesion removed, presence of proximal lesion, and adenocarcinoma. Adverse event rates per endoscopist ranged from 0% to 4.9%.
Conclusion
Adverse events after colonoscopy of screening positives occurred in about 2 out of 100 procedures. Three-quarters of events were mild. Awareness of risk factors may help endoscopists to mitigate the risk.
Trial registration number
Keywords: Colonoscopy, Colorectal cancer screening, Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Adverse events after screening colonoscopies may increase burden of screening to participants, public scepticism to screening, and financial burden to society; however, few large colorectal cancer screening trials and programmes quantify adverse events in detail.
WHAT THIS STUDY ADDS
We found that 2.4% of colonoscopy attenders had adverse events, with considerable variation in frequency from 0% to 4.9% between screening centres and endoscopists.
High-risk subgroups include individuals with older age, on anticoagulant therapy, and with multiple, large, or right-sided polyps.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Screening participants of older age and on anticoagulants should be informed of the increased risk of adverse events prior to colonoscopy.
Endoscopists should be aware of and inform participants on the risk of adverse events in case of removal of multiple, large, or right-sided polyps.
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer death worldwide, with more than 900 000 deaths annually.1 In most cases, CRC arises from benign colorectal polyps.2 CRC screening programmes may reduce CRC mortality by identification of early cancers and may reduce incidence and mortality by identification and removal of polyps.3
CRC screening is a delicate balance between benefits and harms. Few screening participants benefit from a screening programme, but all are at risk for burdens (eg, time expenditure, medicalisation), overtreatment, and adverse events following diagnostic procedures and treatment.4 5
Colonoscopy is the final common pathway in CRC screening, either as the primary screening procedure or as follow-up after another positive screening test (eg, sigmoidoscopy, faecal immunochemical test (FIT), and guaiac faecal occult blood test (gFOBT)).3
Adverse events occur in 0–3% of colonoscopies,6,8 and therapeutic colonoscopy carries a higher risk than diagnostic colonoscopy.9 Frequently reported adverse events associated with colonoscopy after positive FIT or gFOBT screening test are bleeding (0.3–1.7%)10,12 and perforation (0.02–0.13%).10,13 Rare adverse events include myocardial infarction, heart failure, arrhythmia, and ischaemic stroke.13 14 Death after colonoscopy following positive FIT or gFOBT is observed in approximately 0.01%.15 16
No common standard for adverse event identification after colonoscopy exists. Both endoscopists and quality registers under-report adverse events, including fatal events.15 16 Non-gastrointestinal (GI) adverse events are often difficult to identify and attribute to the colonoscopy in register-based studies.3
To improve reporting of adverse events in endoscopic procedures, the American Society for Gastrointestinal Endoscopy (ASGE) has proposed a lexicon to standardise the nomenclature and definitions of adverse events.17
The aim of the present study was to evaluate adverse events according to the ASGE lexicon after colonoscopy in a randomised CRC screening trial comparing two screening methods (sigmoidoscopy vs FIT), and to provide an analysis of risk factors associated with adverse events.
Methods
Study design and setting
We analysed adverse events occurring during or after colonoscopy in a randomised, population-based CRC screening trial between 2012 and April 2020. A user representative has been involved in the screening trial and in the planning of the present study.
Details of the randomised trial have been reported elsewhere.18 In brief, 139 291 individuals 50–74 years old living in two geographical areas in Southeast Norway were randomly assigned in 2012 to either a once-only sigmoidoscopy or biennial FIT for a maximum of four rounds. A positive sigmoidoscopy was defined as detection of any polyp ≥10 mm, ≥3 adenomas, an adenoma with high-grade dysplasia or ≥25% villous components, or CRC. Polyps <10 mm were removed at sigmoidoscopy. The threshold for positive FIT was 15 µg haemoglobin/g faeces.
All individuals with a positive sigmoidoscopy or FIT were referred for colonoscopy. All colonoscopies were performed at dedicated screening units imbedded in two community hospitals, one hospital in each of the two trial areas. To increase endoscopy capacity, trainees without or with limited endoscopy experience were recruited specifically for this screening trial. Trainees were trained under 1:1 supervision for 3–6 months. Consultants who had completed train-the-colonoscopy-trainer courses were responsible for the training, but also other experienced gastroenterologists supervised the trainees. Endoscopists had access to their own continuously updated individual key performance indicators (KPIs) and results were discussed biannually at staff meetings. There is no formal accreditation of colonoscopists in Norway, but allowance to perform unsupervised colonoscopies and polypectomies is granted on an individual basis by chief of endoscopy units. Detailed description of training and trainees’ colonoscopy performance have been published previously.19 Trainees performed high-quality colonoscopies and were superior to experienced gastroenterologists for a number of KPIs.19 Colonoscopies were routinely performed with analgesics and sedatives administered on demand.
We used the screening trial database to identify all screening participants with at least one colonoscopy after a positive screening test (FIT or sigmoidoscopy) between the start of the trial in 2012 and the end of April 2020, and included all in the study cohort.
Data collection
Data from the colonoscopy procedures were registered prospectively in the screening trial database by the endoscopist (bowel cleansing quality, colonic lesions (size, location, histology) and therapy (biopsy forceps, snare, clipping)). Medical history (coronary heart disease, cerebrovascular disease, diabetes mellitus) and use of medication (antiplatelet therapy, anticoagulant therapy) were self-reported.
Adverse event recording and classification
Individuals with possible adverse events were identified through either an adverse event registered in the screening trial database or by having had any contact with the hospital within 30 days after colonoscopy. All Norwegian residents are assigned one local hospital, to which medical emergencies are referred. Any contacts were identified using queries in the electronic medical records at the hospitals affiliated with the screening centres.
One author (ØBR) scrutinised hospital electronic medical records. Unclear information was discussed with two or three of the coauthors to reach consensus (KRR, GH, ØH). If more than one condition was treated during the hospital stay, the most severe diagnosis was chosen. For individuals transferred between hospitals, the combined length of stay in days was used. In cases where no definite diagnosis was reached, the dominant symptom was chosen (eg, chest pain, abdominal pain). Hospital admission included referral to the emergency department, or referral for repeat endoscopy after discharge from the endoscopy unit. Minor events treated successfully during colonoscopy without compromising the completion of the procedure were not defined as adverse events. Preprocedural adverse events (eg, related to bowel preparation) were not registered.
Adverse events were classified according to the ASGE lexicon.17 The ASGE lexicon includes parameters on severity of adverse event, attribution of adverse event to a procedure, timing of adverse event to the procedure, and diagnosis of adverse event. An event was considered an adverse event if it prevented completion of the colonoscopy, resulted in hospital admission or outpatient contact at the hospital. Mild adverse events include aborted procedures, postprocedure outpatient contact, or hospital admission for up to three nights. Moderate adverse events include hospital admission for 4–10 nights, intensive care unit (ICU) admission for one night, red blood cell transfusion, repeat endoscopy, or interventional radiology. Severe adverse events include hospital admission for more than 10 nights, ICU admission for more than one night, surgery for adverse events, or permanent disability.
Outcomes
The primary study outcome was the proportion of individuals having at least one adverse event of any severity grade ‘possibly’, ‘probably’, or ‘definitely’ attributable to the colonoscopy according to the ASGE lexicon. Secondary outcomes included proportion of individuals with at least one moderate, severe, or fatal adverse event according to the ASGE lexicon, postprocedural bleedings, and perforation, and identification of risk factors for adverse events.
Variables
Possible risk factors for adverse events were selected a priori based on reported literature. These included age, sex, number of polyps removed (0, 1, 2, 3, 4, ≥5), largest diameter of removed lesion (1–5, 6–10, 11–20, >20 mm), histology of lesion (serrated lesion (yes/no), adenoma (yes/no), adenocarcinoma (yes/no)), location of lesion (distal to the splenic flexure, or proximal to and including the splenic flexure at colonoscopy), bowel cleansing quality (good, acceptable, partly poor, poor), history of heart disease, cerebrovascular disease, diabetes mellitus, antiplatelet use, and anticoagulant use.6 7 10 14 15 20 Polypectomy included all lesions removed by snare polypectomy, and removed lesions included all lesions removed by forceps or snare polypectomy.
Postpolypectomy syndrome was defined as an individual presenting with abdominal pain in the region of polypectomy with two out of three additional parameters: (1) temperature >38.0°C, (2) elevated C reactive protein or leucocytes, or (3) radiology finding indicating postpolypectomy syndrome. One individual had polypectomy with missing information on largest diameter of removed lesion and was assigned to the polyp size category of 1–5 mm. In individuals with multiple colonoscopies, the bowel cleansing quality of the first colonoscopy was used. Results for individual endoscopists were reported for endoscopists with more than 100 colonoscopies in the trial. One endoscopist had performed colonoscopies at both screening centres and was assigned to the centre where the endoscopist performed the most colonoscopies.
Statistical methods
Adverse event rates were reported per participant unless otherwise stated. In case of multiple adverse events, the most severe was chosen.
At univariable analysis, we used the χ2 test for dichotomous factors and the Cochran-Armitage test for trend for ordinal factors. When we analysed the adverse events by colonoscopy, we used logistic regression analysis with robust variance estimation for cluster-correlated data, since some individuals had more than one colonoscopy. Colonoscopies were nested within individuals.
We fitted multivariable logistic models for the two outcomes of interest: any adverse event and moderate, severe, or fatal adverse event. We decided a priori to adjust all models for age and sex. We then included other covariates using a backward stepwise selection with a cut-off p value >0.20 for exclusion. We imputed missing data by running a multivariable missing imputation model, including age, sex, pre-existing coronary heart disease, cerebrovascular disease, diabetes mellitus, anticoagulant use, and antithrombotic use. In the multivariable models, we treated age, maximum diameter of removed lesion, and number of polypectomies as continuous variables. Other variables were treated as dichotomous variables. It was not possible to include endoscopists as a variable in the multivariable models because of the low number of events per endoscopist. The number of colonoscopies per individual was not included in the multivariable models because of the risk of reverse causation. As a sensitivity analysis, we ran a complete-case analysis.
ORs were reported with 95% CIs. Results with a p value <0.05 were considered statistically significant. All analyses were performed using Stata statistical software V.17.0 (StataCorp, College Station, Texas, USA).
The trial is registered at ClinicalTrials.gov (NCT01538550).
Results
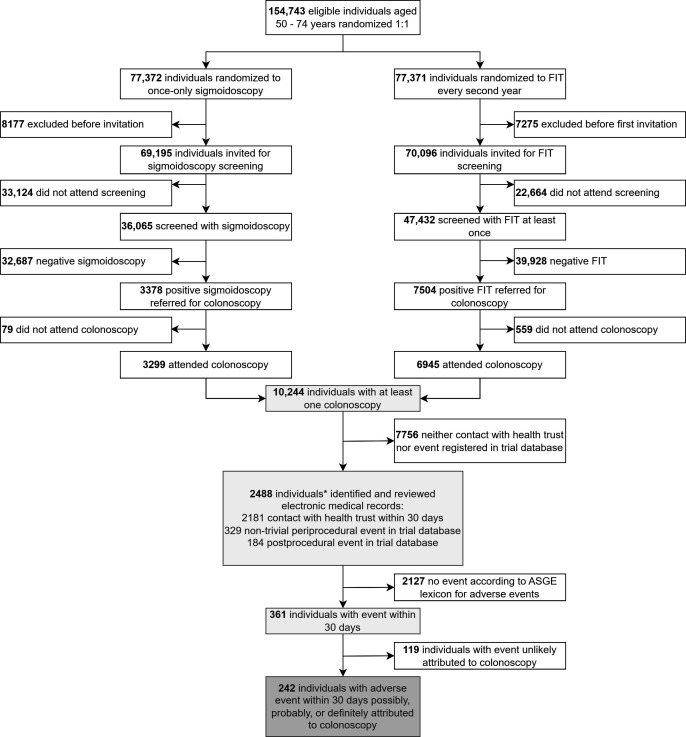
Of 139 291 individuals invited for sigmoidoscopy or FIT screening, 10 244 underwent at least one colonoscopy after a positive screening test (figure 1): 3299 (32.2%) were referred after positive sigmoidoscopy and 6945 (67.8%) after a positive FIT. In total, 19 426 polyps were removed, averaging 1.9 per individual. Mean age at colonoscopy was 65.6 years (range 49.9–79.7) and 42.4% of individuals were female (table 1).
Figure 1. Study flow chart of patients included for analysis. *The sum may exceed the total number because of the possibility of individuals being identified both from health trust register, and periprocedural and postprocedural adverse event registration in the trial database. ASGE, American Society for Gastrointestinal Endoscopy; FIT, faecal immunochemical test.
Table 1. Individuals’ baseline characteristics and association with occurrence of adverse events.
| Total (col %) | Any adverse events (row %) | P value | Moderate, severe, or fatal adverse events (row %) | P value | |
| Total | 10 244 (100%) | 242 (2.4%) | 54 (0.5%) | ||
| Age | <0.01* | <0.01* | |||
| 50–55 | 862 (8.4%) | 8 (0.9%) | 0 (0.0%) | ||
| 56–60 | 1985 (19.4%) | 37 (1.9%) | 7 (0.4%) | ||
| 61–65 | 2212 (21.6%) | 50 (2.3%) | 11 (0.5%) | ||
| 66–70 | 2652 (25.9%) | 61 (2.3%) | 13 (0.5%) | ||
| >70 | 2533 (24.7%) | 86 (3.4%) | 23 (0.9%) | ||
| Sex | 0.13† | 0.42† | |||
| Female | 4345 (42.4%) | 114 (2.6%) | 20 (0.5%) | ||
| Male | 5899 (57.6%) | 128 (2.2%) | 34 (0.6%) | ||
| Screening group | 0.12† | 0.68† | |||
| FIT | 6945 (67.8%) | 153 (2.2%) | 38 (0.5%) | ||
| Sigmoidoscopy | 3299 (32.2%) | 89 (2.7%) | 16 (0.5%) | ||
| Screening centre | <0.01† | 0.02† | |||
| 1 | 5465 (53.3%) | 108 (2.0%) | 20 (0.4%) | ||
| 2 | 4779 (46.7%) | 134 (2.8%) | 34 (0.7%) | ||
| Colonoscopies per person, n | <0.01* | <0.01* | |||
| 1 | 9428 (92.0%) | 151 (1.6%) | 41 (0.4%) | ||
| 2 | 704 (6.9%) | 69 (9.8%) | 7 (1.0%) | ||
| ≥3 | 112 (1.1%) | 22 (19.6%) | 6 (5.4%) | ||
| Polypectomies per person, n | <0.01* | <0.01* | |||
| 0 | 3487 (34.0%) | 33 (0.9%) | 4 (0.1%) | ||
| 1 | 2608 (25.5%) | 49 (1.9%) | 12 (0.5%) | ||
| 2 | 1581 (15.4%) | 33 (2.1%) | 6 (0.4%) | ||
| 3 | 918 (9.0%) | 28 (3.1%) | 8 (0.9%) | ||
| 4 | 531 (5.2%) | 20 (3.8%) | 7 (1.3%) | ||
| ≥5 | 1119 (10.9%) | 79 (7.1%) | 17 (1.5%) | ||
| Max diameter of removed lesion, mm | <0.01* | <0.01* | |||
| No lesion removed | 2522 (24.6%) | 27 (1.1%) | 3 (0.1%) | ||
| 1–5 | 2449 (23.9%) | 25 (1.0%) | 5 (0.2%) | ||
| 6–10 | 2685 (26.2%) | 58 (2.2%) | 16 (0.6%) | ||
| 11–20 | 2043 (19.9%) | 86 (4.2%) | 17 (0.8%) | ||
| >20 | 545 (5.3%) | 46 (8.4%) | 13 (2.4%) | ||
| Serrated lesion | <0.01† | 0.01† | |||
| No | 6591 (64.3%) | 115 (1.7%) | 26 (0.4%) | ||
| Yes | 3653 (35.7%) | 127 (3.5%) | 28 (0.8%) | ||
| Adenoma | <0.01† | <0.01† | |||
| No | 3690 (36.0%) | 47 (1.3%) | 6 (0.2%) | ||
| Yes | 6554 (64.0%) | 195 (3.0%) | 48 (0.7%) | ||
| Adenocarcinoma | <0.01† | 0.35† | |||
| No | 9762 (95.3%) | 220 (2.3%) | 50 (0.5%) | ||
| Yes | 482 (4.7%) | 22 (4.6%) | 4 (0.8%) | ||
| Distal lesion | <0.01† | 0.02† | |||
| No | 3819 (37.3%) | 63 (1.6%) | 12 (0.3%) | ||
| Yes | 6425 (62.7%) | 179 (2.8%) | 42 (0.7%) | ||
| Proximal lesion | <0.01† | <0.01† | |||
| No | 5435 (53.1%) | 70 (1.3%) | 11 (0.2%) | ||
| Yes | 4809 (46.9%) | 172 (3.6%) | 43 (0.9%) | ||
| Bowel cleansing quality‡ | 0.41* | 0.81* | |||
| Good | 7343 (71.7%) | 170 (2.3%) | 40 (0.5%) | ||
| Acceptable | 1998 (19.5%) | 49 (2.5%) | 9 (0.5%) | ||
| Partly poor | 582 (5.7%) | 12 (2.1%) | 3 (0.5%) | ||
| Poor | 162 (1.6%) | 7 (4.3%) | 1 (0.6%) | ||
| Coronary heart disease§ | <0.01† | <0.01† | |||
| No | 8730 (85.2%) | 184 (2.1%) | 38 (0.4%) | ||
| Yes | 1341 (13.1%) | 51 (3.8%) | 15 (1.1%) | ||
| Cerebrovascular disease§ | 0.21† | 0.14† | |||
| No | 9563 (93.4%) | 219 (2.3%) | 48 (0.5%) | ||
| Yes | 508 (5.0%) | 16 (3.1%) | 5 (1.0%) | ||
| Diabetes§ | 0.13† | 0.37† | |||
| No | 9266 (90.5%) | 210 (2.3%) | 47 (0.5%) | ||
| Yes | 805 (7.9%) | 25 (3.1%) | 6 (0.7%) | ||
| Antiplatelet§ | 0.03† | 0.30† | |||
| No | 7993 (78.0%) | 173 (2.2%) | 39 (0.5%) | ||
| Yes | 2078 (20.3%) | 62 (3.0%) | 14 (0.7%) | ||
| Anticoagulant§ | <0.01† | <0.01† | |||
| No | 9158 (89.4%) | 197 (2.2%) | 39 (0.4%) | ||
| Yes | 913 (8.9%) | 38 (4.2%) | 14 (1.5%) |
Cochran -Armitage test for trend.
Chi-squareΧ2 test.
Missing value for each variable: 159.
Missing value for each variable: 173.
FITfaecal immunochemical test
Adverse events
A total of 361 individuals (3.5% of all individuals undergoing colonoscopy) had an adverse event during or within 30 days after colonoscopy, of which 119 (1.2%) had events assessed as unlikely related to the colonoscopy and not included in further analyses (online supplemental table S1 and figure S1).
We identified 242 (2.4%) individuals with at least one adverse event possibly, probably, or definitely attributed to the colonoscopy. Of 816 (8.0%) individuals having more than one colonoscopy, eight had two colonoscopies with adverse events registered. Thus, there were 250 colonoscopies (2.2%) with adverse events among 11 206 colonoscopies performed (online supplemental table S2). The adverse event rate during or after colonoscopy was 2.7% in the sigmoidoscopy group and 2.2% in the FIT group (p=0.12; table 1).
Severity
Among the 242 individuals with adverse events, 188 (1.8%) had a mild adverse event, 50 (0.49%) had a moderate adverse event, 3 (0.03%) had a severe adverse event, and 1 (0. 01%) had a fatal adverse event (online supplemental table S3).
Of the severe adverse events, there was one cerebrovascular event, one perforation after polypectomy, and one appendicitis. One individual died.
Time of adverse event
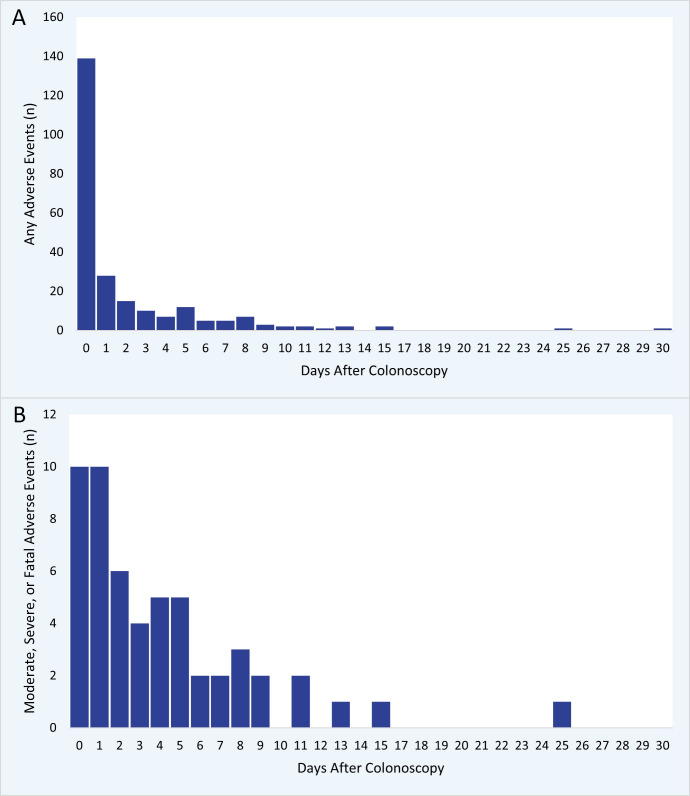
The distribution of adverse events categorised according to occurrence on the day of colonoscopy, within 2, and 7 days after colonoscopy was 57.4%, 75.2%, and 91.3%, respectively (figure 2). The admission/readmission rate per colonoscopy was 1.3% within 1 week, and 1.5% both within 14 and 30 days (online supplemental figure S2).
Figure 2. Days from colonoscopy to adverse event. For all events (A) and moderate, severe, or fatal adverse events (B).
Adverse event diagnoses
The majority of adverse events were GI (online supplemental table S3). Lower GI bleeding occurred in 88 (0.86%), abdominal pain in 49 (0.48%), vasovagal reaction in 40 (0.39%), postpolypectomy syndrome in 20 (0.20%), and perforation in 8 (0.08%) individuals. All but one bleeding occurred after polypectomy. Repeat endoscopy was performed in 30 individuals, 15 were treated with transfusion of packed red blood cells, and two had interventional radiology. Out of the 40 (0.39%) individuals with a vasovagal reaction, 34 had the procedure aborted, 3 had an outpatient visit and 3 were admitted for one night. The consequence of postpolypectomy syndrome was an outpatient visit in seven individuals and admission for up to 4 days for 13 individuals. All perforations occurred after polypectomy. One perforation was treated surgically, one was acknowledged during the colonoscopy and was treated with through-the-scope clips, and the remainder were treated conservatively with antibiotics.
We identified 23 (0.23%) non-GI adverse events including cardiovascular disease, pulmonary disease, venous thromboembolic disease, infectious disease, non-abdominal pain, and electrolyte imbalance or dehydration (online supplemental tables S1 and S3), and two were classified as severe. The nine individuals with cardiovascular events included cardiac arrhythmias, cardiac arrest, myocardial infarction, cerebrovascular disease, and exacerbation of congestive heart disease. Eight of the nine had pre-existing cardiovascular disease or used antithrombotic or anticoagulant medication prior to the colonoscopy.
Risk factors for adverse events
We identified increasing age, female sex, screening centre, number of polypectomies, size of lesion removed, presence of adenocarcinoma, presence of proximal lesion, and use of anticoagulant therapy as independent risk factors for adverse events (table 2). For moderate, severe, or fatal adverse events, independent risk factors were increasing age, screening centre, number of polypectomies, size of polyp removed, presence of proximal lesion, and use of anticoagulant therapy (table 2). The analysis of all individuals with complete data led to very similar results (online supplemental table S4).
Table 2. Multivariable logistic regression models of risk factors for adverse events.
| Outcome | Any adverse eventOR (95% CI) | Moderate, severe, or fatal adverse eventOR (95% CI) |
| Variablecategory | ||
| Age | ||
| 50–55 years | Reference | Reference |
| Every 5-year increase | 1.15 (1.03 to 1.29) | 1.32 (1.04 to 1.67) |
| Sex | ||
| Male | Reference | |
| Female | 1.57 (1.20 to 2.05) | |
| Screening centre | ||
| 1 | Reference | Reference |
| 2 | 1.35 (1.04 to 1.75) | 1.80 (1.02 to 3.18) |
| Number of polypectomies | ||
| No polypectomy | Reference | Reference |
| Every additional polypectomy | 1.06 (1.04 to 1.08) | 1.04 (1.01 to 1.07) |
| Maximum diameter of removed lesion | ||
| No lesion removed | Reference | Reference |
| Every 5 mm increase | 1.29 (1.22 to 1.37) | 1.35 (1.23 to 1.49) |
| Serrated lesion | ||
| No | Reference | |
| Yes | 1.19 (0.89 to 1.58) | |
| Adenocarcinoma | ||
| No | Reference | |
| Yes | 1.65 (1.03 to 2.63) | |
| Proximal lesion | ||
| No | Reference | Reference |
| Yes | 1.61 (1.17 to 2.21) | 2.42 (1.21 to 4.83) |
| Coronary heart disease | ||
| No | Reference | |
| Yes | 1.40 (0.95 to 2.08) | |
| Antiplatelet therapy | ||
| No | Reference | Reference |
| Yes | 1.36 (0.97 to 1.90) | 1.49 (0.79 to 2.81) |
| Anticoagulant therapy | ||
| No | Reference | Reference |
| Yes | 1.83 (1.19 to 2.81) | 3.61 (1.87 to 6.97) |
One model for any adverse event and one model for moderate, severe, or fatal adverse event.
Covariates selected after backward stepwise selection approach for any adverse event and moderate, severe, or fatal adverse event. Missing values are imputed.
Performance variation
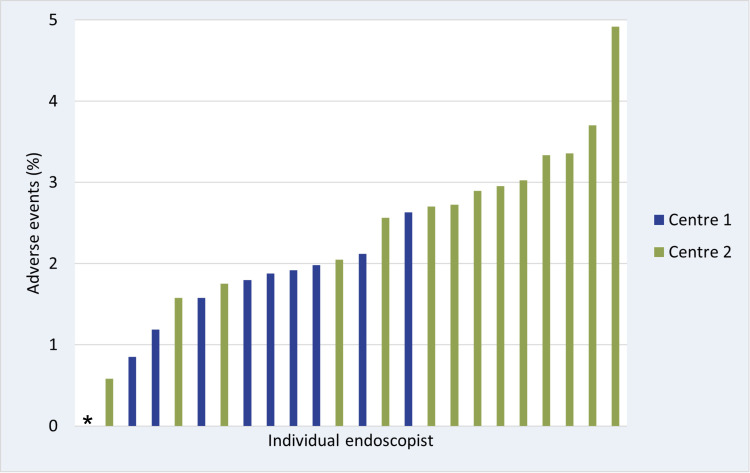
The adverse event rates at the two screening centres were 2.0% and 2.8% (p<0.01). To explore the higher adverse event rate at screening centre 2, we performed post hoc analyses. There was no difference between the centres for use of clip at the colonoscopy (online supplemental table S5). Screening centre 2 had a higher rate of colonoscopies with polypectomy (66.3% vs 62.8%, p<0.001), and a higher rate of large polyps removed compared with screening centre 1 (5.9% vs 4.2% of colonoscopies with lesion over 20 mm removed) (online supplemental table S5), but the difference was still statistically significant in multivariable models accounting for these confounders. We explored temporal trends in adverse event rates, and except from the year 2020, screening centre 2 had higher adverse event rates each year of the trial (online supplemental figure S3). We also addressed the adverse event rates for the 24 endoscopists with more than 100 colonoscopies in the trial, and adverse event rates ranged from 0.9% to 2.6% and from 0.0% to 4.9% for endoscopists at centres 1 and 2, respectively (figure 3).
Figure 3. Unadjusted adverse event rates per endoscopist with more than 100 colonoscopies in the trial. Screening centre 1 blue, screening centre 2 orange. *One endoscopist from centre 2 had no adverse events.
Discussion
We report an overall risk for adverse events after colonoscopy in FIT or sigmoidoscopy CRC screening of 2.4% per individual, or 2.2% per colonoscopy, using the ASGE lexicon. This translates to a number needed to harm of 42 for individuals attending colonoscopies after positive screening test. The adverse event rate was 1.7 per 1000 individuals invited to screening, and 2.8 per 1000 individuals screened with sigmoidoscopy or FIT. Bleeding (0.9%), abdominal pain (0.5%), vasovagal reaction (0.4%), postpolypectomy syndrome (0.2%), and perforation (0.1%) were the most common adverse events. Three-quarters of the adverse events were mild. Risk factors for adverse events were age, sex, polypectomy, size of removed lesion, proximal location of lesion, and anticoagulant therapy.
The overall adverse event rate is higher in our trial (2.4%) than in two FIT screening programmes reporting adverse events after colonoscopy according to the ASGE lexicon in the Netherlands (1.0%)9 and Alsace in France (1.9%).15 The two studies had similar age and sex distribution compared with ours, whereas the FIT positivity thresholds were 47 and 30 μg haemoglobin/g faeces in the Netherlands and France, respectively, higher than our threshold of 15 μg haemoglobin/g faeces. One would expect that a lower FIT threshold results in a lower adverse event rate, since negative colonoscopies are more frequent with lower FIT thresholds.21 Despite similar definition of adverse events, there were possible explanations for the differences in adverse event rates.
First, we report the overall adverse event rate per individual, which is slightly higher than per colonoscopy. Nass et al and Denis et al report adverse events per colonoscopy, which is commonly reported.11 22 We argue that the adverse event rate per individual is a more valid measure of the burden in a CRC screening programme. The number of colonoscopies needed to achieve clean colon (colon without polyps) varies between endoscopists, centres and countries.23 Our study includes all colonoscopies needed for an individual to achieve clean colon, for which 8% required more than one colonoscopy.
Second, to report adverse events, these must be acknowledged and identified. Nass et al used adverse events registered in a nationwide register (Dutch Registration of Complications in Endoscopy, DRCE).9 The study by Denis et al identified adverse events by gastroenterologist reporting and by postal survey every other year to individuals undergoing colonoscopy.15 Kooyker et al found incomplete reporting of screening colonoscopy-related deaths in DRCE compared with the national death register,16 and we have previously identified incomplete reporting of adverse events to the Norwegian National Quality Assurance network for endoscopy (Gastronet).24 Denis et al state that 15% of adverse events were not reported by the gastroenterologist. In addition, only 50% of the target group responded to the survey performed up to 2 years after the procedure, increasing the risk of having forgotten the adverse event or not responding at all.15 Data from the Danish Colorectal Cancer Screening Database identified only 29% of true adverse events in the Danish CRC screening programme.25 In order to identify all individuals with adverse events and ensure data completeness, we combined the screening trial database and the health trust register. We identified more non-severe adverse events than Nass et al report, indicating that our approach for identifying adverse events was probably more sensitive and accurate.
The European Society of Gastrointestinal Endoscopy (ESGE) recommends a minimum standard of ≤0.5% for 7-day readmission rate after any colonoscopy.26 Colonoscopy attenders after FIT screening have higher prevalence of advanced adenomas and colorectal polyps than the general colonoscopy population, resulting in more endoscopic resection procedures and increasing risk of adverse events.11 Other FIT-based screening programmes have reported rates of hospitalisation of 0.7% up to 1.2% per colonoscopy, reflecting our rate of 1.5% admitted to the emergency department or hospitalised per colonoscopy in the FIT cohort, but also higher than the ESGE-recommended readmission rate.12 15 25 If we define admission to a hospital to include only individuals with at least one overnight admission, our 7-day admission/readmission rate is 0.8% in the FIT cohort.
Denis et al have argued that with increasing numbers of polypectomies in FIT-based screening programmes, we should regard only hospitalisations >24 hours as a clinically relevant adverse event.15 With this definition, we find a 0.5% 7-day admission/readmission rate and an overall adverse event rate of 0.5%. We argue to include all events requiring hospitalisation as adverse events, as hospitalisation irrespective of length in hours or days poses harm and inconvenience to the patient and adds financial burden to society associated with CRC screening.
We showed a significant difference in adverse event rates between the screening centres in the multivariable model. The threshold for performing repeat endoscopy in case of lower GI bleeding differed between the centres, but that should not influence the overall adverse event rate as observation at the emergency department or admission to hospital was considered a complication in the same way as repeated endoscopy. The centre with the highest adverse event rate serves a population with a higher socioeconomic status compared with the other centre, but whether people of higher socioeconomic status have a lower threshold for contacting the health service after screening colonoscopy is unknown.
Variations of endoscopist performance in screening colonoscopy have been documented for outcomes such as participant pain reporting, or adenoma detection rate, but rarely for adverse events.27 The variation of adverse event rates between endoscopists from nil to almost 5% was larger than we expected. We could not include the individual endoscopist in multivariable analysis due to low number of events per endoscopist, but it is unlikely that adjusting for covariates would explain the difference between endoscopists. Raising awareness to adverse events by reporting at individual endoscopist level might improve colonoscopy quality.
Along with the trial, an endoscopy school providing structured train-the-trainee courses was initiated from 2011, and formally established in 2014. Thus, the training provided on-site has changed over time, but we found no change in adverse event rates per year throughout the trial (online supplemental figure S3).
Our study design also has limitations. One individual only has reviewed the medical records. To ensure agreement with the ASGE lexicon, we have collectively discussed as described over 100 cases, but we cannot exclude some degree of subjectivity in judgement of events.
Even though we have used both the screening trial database and the local hospital trust registers, we cannot rule out undetected events. For instance, reporting of aborted procedures might not be complete, as the threshold for an endoscopist to consider an aborted colonoscopy as an adverse event may differ between endoscopists. Also, we lack data from primary care physicians. However, we have previously shown that the quality and reporting of colonoscopy performance reaches international target standards in the screening trial,19 and we do not believe under-reporting is a major issue comparing our rate of mild adverse events to other studies.
The study does not include a no-screening group. Therefore, the true background rate of non-GI-related events unrelated to screening colonoscopy is unknown. Some events are difficult to attribute to the colonoscopy and may have happened irrespective of the colonoscopy. Yet, other events we have attributed as unlikely related to the colonoscopy might be a result of the colonoscopy. Online supplemental figure S1 illustrates a stable number of adverse events unlikely attributed to colonoscopy throughout the 30 days after colonoscopy, making us more confident in the attribution judgements.
Several studies report overall adverse events after colonoscopy in CRC screening, but with varying definitions of adverse events.11 28 29 The lack of a common, used gold standard for identifying and defining adverse events complicates comparisons between reports on the subject, reflecting the high degree of heterogeneity and low quality of evidence in meta-analyses.11 22
The concept of applying the ASGE lexicon including severity, timing, diagnosis, and attribution to all events within 30 days after the colonoscopy yields a detailed, structured, and standardised description of all adverse events. Several studies on colonoscopy have used the lexicon with various local modifications and with varying degrees of transparency on how adverse events are identified.730,34 Few CRC screening studies have used the full version of the lexicon.,9 13 15 and our rigorous methodology may serve as benchmarks for other studies and policymakers.
Conclusion
We identified an adverse event rate of 2.4% after colonoscopy in a CRC screening trial, with more mild and moderate adverse events than other studies. Variation in endoscopist adverse event rates was considerable, from nil to almost 5%.
supplementary material
Acknowledgements
We thank Ellen Pahle Anker at Østfold Hospital Trust and Erik Natvig at the Cancer Registry of Norway for help with data management.
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Funding: ØBR is supported by a research grant from the South-Eastern Norway Regional Health Authority (grant number 2020057). The screening trial is funded by the Norwegian Parliament.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by the Regional Committee for Medical Research Ethics in Southeast Norway (2011/1272). Participants gave informed consent to participate in the study before taking part.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability free text: Access to research data for external investigators, or use outside of the current protocol, will require approval from the Norwegian Regional Committee for Medical and Health Research Ethics and the bowel cancer screening in Norway steering committee (information available on the project website, https://www.kreftregisteret.no/screening/Tarmscreeningpiloten/). Research data are not openly available because of the principles and conditions set out in articles 6[1] (e) and 9[2] (j) of the General Data Protection Regulation (GDPR). Questions regarding data access should be directed to the corresponding author.
Contributor Information
Øyvind Bakken Rognstad, Email: oyvind.bakken.rognstad@gmail.com.
Edoardo Botteri, Email: edbo@kreftregisteret.no.
Geir Hoff, Email: hofg@kreftregisteret.no.
Michael Bretthauer, Email: michael.bretthauer@medisin.uio.no.
Elisabeth Gulichsen, Email: elisabeth.haagensen.gulichsen@so-hf.no.
Svein Oskar Frigstad, Email: svosfr@vestreviken.no.
Øyvind Holme, Email: oyvind.holme@sshf.no.
Kristin Ranheim Randel, Email: kran@kreftregisteret.no.
Data availability statement
No data are available.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin . 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan BA, Noujaim M, Roper J. Cause, Epidemiology, and Histology of Polyps and Pathways to Colorectal Cancer. Gastrointest Endosc Clin N Am. 2022;32:177–94. doi: 10.1016/j.giec.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin JS, Perdue LA, Henrikson NB, et al. Screening for Colorectal Cancer. JAMA. 2021;325:1978. doi: 10.1001/jama.2021.4417. [DOI] [PubMed] [Google Scholar]
- 4.van Dam L, Bretthauer M. Ethical issues in colorectal cancer screening. Best Pract Res Clin Gastroenterol. 2014;28:315–26. doi: 10.1016/j.bpg.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Kim GY, Walker JG, Bickerstaffe A, et al. The CRISP-Q study: Communicating the risks and benefits of colorectal cancer screening. Aust J Gen Pract. 2018;47:139–45. doi: 10.31128/AFP-04-17-4195. [DOI] [PubMed] [Google Scholar]
- 6.Causada-Calo N, Bishay K, Albashir S, et al. Association Between Age and Complications After Outpatient Colonoscopy. JAMA Netw Open. 2020;3:e208958. doi: 10.1001/jamanetworkopen.2020.8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benazzato L, Zorzi M, Antonelli G, et al. Colonoscopy-related adverse events and mortality in an Italian organized colorectal cancer screening program. Endoscopy. 2021;53:501–8. doi: 10.1055/a-1228-9225. [DOI] [PubMed] [Google Scholar]
- 8.Laanani M, Coste J, Blotière P-O, et al. Patient, Procedure, and Endoscopist Risk Factors for Perforation, Bleeding, and Splenic Injury After Colonoscopies. Clin Gastroenterol Hepatol. 2019;17:719–27. doi: 10.1016/j.cgh.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Nass KJ, van der Schaar PJ, van der Vlugt M, et al. Continuous monitoring of colonoscopy performance in the Netherlands: first results of a nationwide registry. Endoscopy. 2022;54:488–95. doi: 10.1055/a-1556-5914. [DOI] [PubMed] [Google Scholar]
- 10.Hsu W-F, Chang C-Y, Chang C-C, et al. Risk of colonoscopy-related complications in a fecal immunochemical test-based population colorectal cancer screening program. Endoscopy. 2022;54:290–8. doi: 10.1055/a-1328-5126. [DOI] [PubMed] [Google Scholar]
- 11.Chandan S, Facciorusso A, Yarra P, et al. Colonoscopy-Related Adverse Events in Patients With Abnormal Stool-Based Tests: A Systematic Review of Literature and Meta-analysis of Outcomes. Am J Gastroenterol. 2022;117:381–93. doi: 10.14309/ajg.0000000000001614. [DOI] [PubMed] [Google Scholar]
- 12.Arana-Arri E, Imaz-Ayo N, Fernández MJ, et al. Screening colonoscopy and risk of adverse events among individuals undergoing fecal immunochemical testing in a population-based program: A nested case-control study. United European Gastroenterol J. 2018;6:755–64. doi: 10.1177/2050640618756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen L, Sorensen N, Lindorff-Larsen K, et al. Colonoscopy adverse events: are we getting the full picture? Scand J Gastroenterol. 2020;55:979–87. doi: 10.1080/00365521.2020.1792541. [DOI] [PubMed] [Google Scholar]
- 14.Ladabaum U, Mannalithara A, Desai M, et al. Age-Specific Rates and Time-Courses of Gastrointestinal and Nongastrointestinal Complications Associated With Screening/Surveillance Colonoscopy. Am J Gastroenterol. 2021;116:2430–45. doi: 10.14309/ajg.0000000000001531. [DOI] [PubMed] [Google Scholar]
- 15.Denis B, Gendre I, Weber S, et al. Adverse events of colonoscopy in a colorectal cancer screening program with fecal immunochemical testing: a population-based observational study. Endosc Int Open. 2021;9:E224–32. doi: 10.1055/a-1324-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kooyker AI, Toes-Zoutendijk E, Opstal-van Winden AWJ, et al. Colonoscopy-Related Mortality in a Fecal Immunochemical Test-Based Colorectal Cancer Screening Program. Clin Gastroenterol Hepatol. 2021;19:1418–25. doi: 10.1016/j.cgh.2020.07.066. [DOI] [PubMed] [Google Scholar]
- 17.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Randel KR, Schult AL, Botteri E. Colorectal Cancer Screening With Repeated Fecal Immunochemical Test Versus Sigmoidoscopy: Baseline Results From a Randomized Trial. Gastroenterology. 2021;160:1085–96. doi: 10.1053/j.gastro.2020.11.037. [DOI] [PubMed] [Google Scholar]
- 19.Schult AL, Hoff G, Holme Ø, et al. Colonoscopy quality improvement after initial training: A cross-sectional study of intensive short-term training. Endosc Int Open. 2023;11:E117–27. doi: 10.1055/a-1994-6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanaclocha-Espi M, Ibáñez J, Molina-Barceló A, et al. Risk factors for severe complications of colonoscopy in screening programs. Prev Med. 2019;118:304–8. doi: 10.1016/j.ypmed.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Li SJ, Seedher T, Sharples LD, et al. Impact of changes to the interscreening interval and faecal immunochemical test threshold in the national bowel cancer screening programme in England: results from the FIT pilot study. Br J Cancer. 2022;127:1525–33. doi: 10.1038/s41416-022-01919-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reumkens A, Rondagh EJA, Bakker CM, et al. Post-Colonoscopy Complications: A Systematic Review, Time Trends, and Meta-Analysis of Population-Based Studies. Am J Gastroenterol. 2016;111:1092–101. doi: 10.1038/ajg.2016.234. [DOI] [PubMed] [Google Scholar]
- 23.Juul FE, Garborg K, Nesbakken E, et al. Rates of repeated colonoscopies to clean the colon from low-risk and high-risk adenomas: results from the EPoS trials. Gut. 2023;72:951–7. doi: 10.1136/gutjnl-2022-327696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoff G, de Lange T, Bretthauer M, et al. Patient-reported adverse events after colonoscopy in Norway. Endoscopy. 2017;49:745–53. doi: 10.1055/s-0043-105265. [DOI] [PubMed] [Google Scholar]
- 25.Mikkelsen EM, Thomsen MK, Tybjerg J, et al. Colonoscopy-related complications in a nationwide immunochemical fecal occult blood test-based colorectal cancer screening program. Clin Epidemiol. 2018;10:1649–55. doi: 10.2147/CLEP.S181204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaminski MF, Thomas-Gibson S, Bugajski M, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2017;49:378–97. doi: 10.1055/s-0043-103411. [DOI] [PubMed] [Google Scholar]
- 27.Bretthauer M, Kaminski MF, Løberg M, et al. Population-Based Colonoscopy Screening for Colorectal Cancer: A Randomized Clinical Trial. JAMA Intern Med. 2016;176:894–902. doi: 10.1001/jamainternmed.2016.0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forbes N, Boyne DJ, Mazurek MS, et al. Association Between Endoscopist Annual Procedure Volume and Colonoscopy Quality: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2020;18:2192–208. doi: 10.1016/j.cgh.2020.03.046. [DOI] [PubMed] [Google Scholar]
- 29.Mazurek M, Murray A, Heitman SJ, et al. Association Between Endoscopist Specialty and Colonoscopy Quality: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:1931–46. doi: 10.1016/j.cgh.2021.08.029. [DOI] [PubMed] [Google Scholar]
- 30.Rajasekhar PT, Clifford GM, Lee TJW, et al. Bowel cancer screening is safe, detects earlier stage cancer and adenomas in 50% of cases: experience of the prevalent round of screening from two first wave centres in the North East of England. Frontline Gastroenterol. 2012;3:10–5. doi: 10.1136/flgastro-2011-100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barros RA, Monteverde MJ, Dumonceau J-M, et al. Cold snare polypectomy without submucosal injection: safety and efficacy in 615 large serrated lesions. Endosc Int Open. 2021;9:E1421–6. doi: 10.1055/a-1517-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nogueira PB, Albuquerque W, Nascimento RC, et al. Underwater endoscopic mucosal resection of adenomas and colorectal serrated lesions: a prospective clinical study. Ann Gastroenterol. 2021;34:552–8. doi: 10.20524/aog.2021.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riverso M, Perbtani YB, Shuster JJD, et al. Carbon dioxide insufflation is associated with increased serrated polyp detection rate when compared to room air insufflation during screening colonoscopy. Endosc Int Open. 2017;5:E905–12. doi: 10.1055/s-0043-116382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perbtani YB, Riverso M, Shuster JJ, et al. Does carbon dioxide insufflation impact adenoma detection rate? A single-center retrospective analysis. Endosc Int Open. 2016;4:E1275–9. doi: 10.1055/s-0042-118177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data are available.