Abstract
Transient antiretroviral treatment with tenofovir, (R)-9-(2-phosphonylmethoxypropyl)adenine, begun shortly after inoculation of rhesus macaques with the highly pathogenic simian immunodeficiency virus (SIV) isolate SIVsmE660, facilitated the development of SIV-specific lymphoproliferative responses and sustained effective control of the infection following drug discontinuation. Animals that controlled plasma viremia following transient postinoculation treatment showed substantial resistance to subsequent intravenous rechallenge with homologous (SIVsmE660) and highly heterologous (SIVmac239) SIV isolates, up to more than 1 year later, despite the absence of measurable neutralizing antibody. In some instances, resistance to rechallenge was observed despite the absence of detectable SIV-specific binding antibody and in the face of SIV lymphoproliferative responses that were low or undetectable at the time of challenge. In vivo monoclonal antibody depletion experiments demonstrated a critical role for CD8+ lymphocytes in the control of viral replication; plasma viremia rose by as much as five log units after depletion of CD8+ cells and returned to predepletion levels (as low as <100 copy Eq/ml) as circulating CD8+ cells were restored. The extent of host control of replication of highly pathogenic SIV strains and the level of resistance to heterologous rechallenge achieved following transient postinoculation treatment compared favorably to the results seen after SIVsmE660 and SIVmac239 challenge with many vaccine strategies. This impressive control of viral replication was observed despite comparatively modest measured immune responses, less than those often achieved with vaccination regimens. The results help establish the underlying feasibility of efforts to develop vaccines for the prevention of AIDS, although the exact nature of the protective host responses involved remains to be elucidated.
The typical course of infection observed in susceptible hosts infected with pathogenic primate immunodeficiency viruses is characterized by persistent progressive infection with continuous unrelenting viral replication. Various factors and mechanisms have been invoked to explain this, including the capability of these viruses to integrate into the host genome; to establish latent infection; to evade immune responses by mutation, concealment of key antigenic determinants, downregulation of major histocompatibility complex proteins, or other strategies to thwart immune mechanisms; and to attack, kill, or disable cells critically involved in coordinating the antiviral host immune response (5, 6, 25, 26). We and others have proposed that compromise of host immune responses during the critical interval of early infection results in an inability to establish an immune response capable of clearing or controlling the infection in the long term (1, 2, 15, 19, 29, 42, 43, 45). In particular, the loss of effective CD4 help for establishment of virus-specific memory CD8 cells capable of persisting even in the face of declining or very low levels of antigen is proposed as a key feature contributing to the establishment of persistent, progressive infection (1, 2, 15, 19, 42, 43, 45). According to this model, although it may take years for the process to progress to clinically manifest disease, much of the outcome may be largely determined during the critical first few weeks of infection, when the dynamic equilibrium between viral replication and host responses is established (16, 23, 36, 41).
To evaluate the hypothesis that the dynamics of the virus-host interaction during the early stages of initial infection exert a profound influence on the subsequent immunologic, virologic, and clinical course of infection, we recently conducted a study using transient postinoculation antiretroviral treatment of simian immunodeficiency virus (SIV)-infected macaques to modulate viral replication during the immediate postinoculation period (15). Prior studies had established that certain regimens of short-term postinoculation (p.i.) antiretroviral treatment could prevent the emergence of measurable plasma viremia following cessation of drug administration (39, 40), but the detailed viral dynamics and host immune responses, particularly cellular immune responses, underlying this protection remained unexplored. Similarly, it remained unclear whether protection from emergent viremia following termination of antiretroviral treatment in this model was associated with protection from rechallenge with infectious pathogenic SIV.
We addressed these questions in a recent study and demonstrated that in rhesus macaques inoculated with a highly pathogenic strain of SIV, certain transient postinoculation treatment regimens prevented the emergence of measurable plasma viremia following discontinuation of the treatment (15). Interestingly, this protective effect was associated with the development of SIV-specific lymphoproliferative responses, despite the absence of measurable amounts of virus or seroconversion during treatment or in the initial period following treatment (15). Strikingly, animals that appeared to have established control of their infections following only transient drug treatment also resisted a subsequent intravenous homologous rechallenge with SIV 6 weeks after discontinuation of drug treatment. Both the delay to initiation of treatment and the duration of treatment affected the outcome. However, even in animals in which measurable plasma virus was observed after treatment termination, peak levels of virus were often markedly blunted in comparison to those in untreated control animals. Many animals showed spontaneous progressive declines in plasma viremia, eventually to below the level of quantitation (100 copy Eq/ml of plasma), in the absence of any further experimental manipulation (15). In aggregate, these results strongly suggested that limitation of virus replication by drug treatment during the critical first weeks of infection permitted a more effective sensitization of the host than occurs during typical untreated infection. This drug-induced suppression of viral replication appeared to have allowed the establishment of host responses capable of controlling the infection, at least in the near term, despite the comparatively low levels of immune responses measured in the peripheral blood.
Important questions that were not addressed in this earlier study include the durability of this control of viral replication, the durability of the resistance to rechallenge, the breadth of resistance to rechallenge with heterologous virus strains, and the underlying mechanism(s) responsible for control of the infection and resistance to rechallenge. We have conducted extended follow-up and further testing on a subset of animals from our previous study. The results clearly indicate that the protective effects observed extend for more than a year following the initial infection and drug treatment, that at least partial protection is conferred against rechallenge with highly heterologous virus isolates, and that control of the infection is mediated at least in part by CD8+ T lymphocytes. In aggregate, these results strongly suggest that limitation of virus replication by drug treatment during the critical first weeks of infection can permit a more effective sensitization of the immune system, allowing the establishment of an immune response capable of controlling the infection and resisting rechallenge. These findings have important implications both for understanding the pathogenesis of the primate lentiviruses and for developing vaccines for the prevention of AIDS.
MATERIALS AND METHODS
Overall study design.
In our previous study of rhesus macaques infected with SIVsmE660 (8), we demonstrated the ability of transient postinoculation antiretroviral treatment to facilitate the development of antiviral cellular immune responses, sustained control of viral replication following drug withdrawal, and resistance to homologous rechallenge 6 weeks following discontinuation of treatment (15). To assess the durability, breadth, and mechanism(s) of these effects, we conducted extensive follow-up testing of a subset of animals from this previous study, with virologic and immunologic monitoring and homologous (SIVsmE660) and heterologous (SIVmac239) rechallenge at various intervals following the initial infection, along with in vivo depletion of CD8+ lymphocytes using monoclonal antibody treatment. Animals for follow-up testing were selected based on evidence of sustained effective control of plasma viremia (SIV RNA < 100 copy Eq/ml for more than 16 weeks and at the time of proposed rechallenge) and included three of four animals from the original study that were treated with the optimal regimen of 28 days of tenofovir, begun 1 day p.i., and one animal each from original groups of four that received 28 or 63 days of tenofovir treatment, beginning 3 days p.i. (15). Experimental interventions are summarized in Table 1.
TABLE 1.
Experimental interventions used in this study
| Animal | Initial inoculationa | Initial treatment (start/duration)b | Time of initial homologous rechallenge (wk after initial inoculation) | Time of second homologous rechallenge (wk after initial inoculation) | Time of heterologous rechallenge (wk after initial inoculation) | Time of CD8 depletionc (wk after initial inoculation) |
|---|---|---|---|---|---|---|
| Experimental animals | ||||||
| Rh 120 | E660 | PMPA (24 h/28 days) | 10 | 75 | 98 | 86 |
| Rh 300 | E660 | PMPA (24 h/28 days) | 10 | 75 | 98 | 86 |
| Rh 009 | E660 | PMPA (24 h/28 days) | 10 | 75 | 85 | 98 |
| Rh 092 | E660 | PMPA (72 h/63 days) | 65 | NA | 75 | 90 |
| Rh 155 | E660 | PMPA (72 h/28 days) | 65 | NA | 75 | 90 |
| Control animals | ||||||
| Rh 128 | E660 | None | NA | NA | NA | NA |
| Rh 058 | E660 | None | NA | NA | NA | NA |
| Rh 106 | E660 | None | NA | NA | NA | NA |
| Rh 294 | E660 | None | NA | NA | NA | NA |
| Rh 226 | E660 | None | NA | NA | NA | NA |
| Rh 169 | E660 | None | NA | NA | NA | NA |
| Rh 399 | 239 | None | NA | NA | NA | NA |
| Rh 405 | 239 | None | NA | NA | NA | NA |
E660, intravenous inoculation with 30 MID50 of SIVsmE660; 239, intravenous inoculation with 100 MID50 of SIVmac239.
PMPΔ, treatment with tenofovir (PMPΔ; 30 mg/kg, subcutaneously, once daily).
CD8 depletion, treatment with the anti-CD8 MAb cMT807, 10 mg/kg subcutaneously followed by 5 mg/kg, intravenously, 2 and 6 days later.
Since the natural history of intravenous infection with SIVsmE660 and with SIVmac239 in SIV-naive, untreated rhesus macaques is well established (10), and to conserve nonhuman primate resources, we used a minimal number of SIV-naive, untreated animals as controls for each challenge or rechallenge phase of the present study (n = 1 or 2), primarily to confirm the in vivo infectivity in untreated, SIV-naive animals of the challenge virus stocks used. Comparisons of viral replication patterns between treated animals and comparably inoculated untreated animals were based on these control animals from the present study (n = 6 for SIVsmE660 and n = 2 for SIVmac239), supplemented by data for six additional animals comparably inoculated with SIVmac239 in a separate study (Desrosiers et al., unpublished data).
Animal care specimens and specimen processing.
The 13 female rhesus macaques (Macaca mulatta) used for this study ranged in age from 3 to 5 years and in weight from 3.5 to 5 kg at the time of study initiation. The animals were negative for simian T-cell leukemia virus type 1 and simian retrovirus based on serological and PCR testing, prior to study initiation (15). All animal housing and care, and research performed was in conformance with the Guide for the Care and Use of Laboratory Animals (22a), according to a protocol approved by the National Cancer Institute Animal Care and Use Program, which is fully accredited by Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC International). Blood and lymph node specimens were obtained as described previously (15), and peripheral blood mononuclear cells (PBMC) or lymph node mononuclear cells (LNMC) were isolated by density centrifugation on Ficoll-Hypaque from blood or single-cell suspensions prepared from lymph node biopsy specimens. Plasma samples for SIV RNA analysis were cryopreserved at −70°C until analysis.
Drug treatment.
Animals received 30 mg of tenofovir [(R)-9-(2-phosphonylmethoxypropyl)adenine (PMPA); Gilead Sciences, Foster City, Calif.] per kg subcutaneously, once daily, for the indicated periods.
Viral load measurements.
Particle-associated SIV RNA in plasma was quantitated using a modification of a previously described real-time reverse transcription-PCR (RT-PCR) assay for SIV gag RNA, on a Prism 7700 sequence detection system (PE Biosystems, Foster City, Calif.). Specimen preparation and reverse transcription with random priming were as previously described (37). For PCR amplification of the resulting cDNA, the following primers and biterminally labeled and 3′-blocked probe were used: forward primer (SGAG21), 5′-gTC TgC gTC ATP Tgg TgC ATT C-3′; reverse primer (SGAG22), 5′-CAC TAg KTg TCT CTg CAC TAT PTg TTT Tg-3′; and probe (P-SGAG23), 5′-(FAM)CTT CPT CAg TKT gTT TCA CTT TCT CTT CTg Cg(TAMRA) 3′, where P and K are modified bases (Glen Research catalog no. 10-1047-90 and 10-1048-90, respectively), introduced to minimize the impact of potential sequence mismatches at positions of described heterogeneity among SIV isolates (Los Alamos HIV sequence database, http://hiv-web.lanl.gov/.), and FAM and TAMRA indicate the reporter fluorochrome 6-carboxy-fluorescein and the quencher fluorochrome 6-carboxy-tetramethylrhodamine, respectively. After 10 min at 95°C to activate the Taq Gold polymerase, 45 cycles of amplification were performed (consisting of 95°C for 15 and 60°C for 60 s), and the nominal SIV gag copy number for test specimens was determined by interpolation of the average measured threshold cycle number for duplicate determinations onto a standard curve of threshold cycle number versus known input template copy number for a purified in vitro transcript control template, essentially as described previously (37). The threshold sensitivity of the assay is 100 copy Eq/ml of plasma, with an average interassay coefficient of variation of <25%.
SIV-induced lymphoproliferation and β-chemokine production assays.
To measure SIV specific lymphoproliferative responses, mononuclear cells were cultured for 5 days at 105 per ml with aldrithiol-inactivated SIV (300 ng of p28CA equivalent per ml, prepared as described previously [3, 31]) in triplicate wells containing 200 μl of RPMI 1640 with 10% human AB serum. The cells were labeled with [3H]thymidine (1 μCi/well) during the last 16 h, and [3H]thymidine incorporated into DNA was quantitated by liquid scintillation counting. The results are expressed as stimulation index, calculated as cpm in stimulated cultures/cpm in control cultures. Stimulation indices of >2.5 are considered positive (15). Supernatants from identical unlabeled replicate wells were harvested for measurement of the representative β-chemokine macrophage inflammatory protein 1β (MIP-1β) by enzyme-linked immunosorbent assay (ELISA), using commercial antibodies for capture and detection (Pharmingen, San Diego, Calif.). The assay had a threshold for detection of 50 pg/ml.
Serological assays.
SIV-specific serum antibody was measured by ELISA and confirmed by Western blot analysis, as described previously (15). Neutralization was measured against SIVmac239, SIVsmE660, and a neutralization-sensitive culture-adapted strain of SIVmac251, using an indicator cell line with production of secreted alkaline phosphatase by infected cells as the readout, as described previously (20).
mAb treatment.
For in vivo depletion of CD8+ cells, animals received three doses of the mouse/human chimeric anti-human CD8 MAb cM-T807, with 10 mg/kg given subcutaneously on day 0 and 5-mg/kg doses administered intravenously on days 3 and 7 (32, 33).
Flow cytometry.
EDTA anticoagulated whole blood was stained with fluorescence-labeled control antibodies (immunoglobulin G1 clone MOPC21, immunoglobulin G3 clone J606) or with fluorescence-labeled antibodies (anti-CD4, clone M-T424; anti-CD8, clone SK1; anti-CD3, clone SP34; anti-CD20, clone 2H7; anti-HLA-DR, clone L243 [all from Pharmingen]) for 20 mins. Red cells were lysed, and samples were read using a FacsCalibur flow cytometer (Becton-Dickinson, San Jose, Calif.). Data were analyzed using FloJo software (Tree Star, Inc., San Carlos, Calif.). For evaluation of in vivo depletion of CD8+ cells, we confirmed that the results obtained with labeled SK1 anti-CD8 antibody were unaffected by prior incubation of target cells with the depleting antibody cM-T807, to ensure that failure to detect CD8 cells in in vivo-derived specimens after treatment reflected depletion as opposed to epitope masking.
Enzyme-linked immunospot (ELISPOT) assay.
To measure the frequency of in vitro-activatable SIV-specific CD8+ T cells, mononuclear cells were isolated by Ficoll-Hypaque density centrifugation. The cells were stimulated with 3 μg (SIV p28CA equivalent) of purified aldrithiol-2-inactivated SIV virions per ml in complete medium for 4 days. Following this culture period, nonadherent cells enriched for CD8+ cells were obtained using negative selection with anti-CD4 magnetic beads (Miltenyi Biotec, Auburn, Calif.). CD8-enriched T cells (>90% CD8+ by flow cytometry) were then plated in triplicate for 24 h on Millipore HA membrane 96-well plates coated with anti-gamma interferon antibody (clone MD-1; BioSource International, Inc., Camarillo, Calif.). The cells were lysed, and the plates were developed with biotinylated rabbit, anti-rhesus gamma interferon antibody (BioSource International), streptavidin-alkaline phosphatase (Sigma, St. Louis, Mo), and BCIP/NBT substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md). Spots corresponding to gamma interferon-secreting cells were counted manually by an observer blinded to the identity of the test wells, using an Olympus dissecting microscope equipped with a charge-coupled device video camera.
Virus isolation.
For virus isolation, cocultures of PBMC or LNMC were activated with phytohemagglutinin (1:50; Gibco, Grand Island, N.Y.) and recombinant human interleukin-2 (rHu-IL-2)(100 U/ml; Roche, Nutley, N.J.) for 3 days and then depleted of CD8+ cells on immunoaffinity columns (Miltenyi Biotec). The resulting populations were routinely >90% CD4+ by flow cytometry. Quadruplicate cocultures were then performed using 106 to 107 CD8-depleted, activated mononuclear cells and 106 cells of the highly SIV-susceptible cell line AA2, clone 5 (38), in RPMI 1640 with 10% heat-inactivated human AB serum, l-glutamine, and antibiotics (complete medium), supplemented with 100 U of rHu-IL-2 per ml. Cultures were maintained for 4 weeks with weekly medium changes. Viral replication was assessed by measurement of SIV p28CA or particle-associated SIV RNA in the culture supernatant, by capture immunoassay (AIDS Vaccine Program, National Cancer Institute at Frederick, Frederick, Md.), or by real-time RT-PCR.
Genotyping.
Sequence comparison of the amplification products derived from SIVmac239 and SIVsmE660 and specified by the primers SGAG03 and SGAG04 showed several restriction enzyme polymorphisms that could be exploited to differentiate the viruses (Los Alamos HIV Sequence Database). Viral RNA prepared from selected plasma specimens was subjected to RT-PCR as described above but with modifications of the amplification primers SGAG03 and SGAG04 extended at the 5′ ends to contain an EcoRI and a BamHI restriction site, respectively. Primer sequences were matched for both SIVmac239 and SIVsmE660 viral templates. Amplification products were purified using a commercially available kit (Qiagen, Santa Clarita, Calif.), restricted with EcoRI and BamHI, and repurified. These products were ligated to similarly digested pGEM3zf(+) (Promega Corp., Madison, Wis.), which was also treated with calf intestinal phosphatase to reduce vector-only religation events. Ligations were transformed into Escherichia coli DH5α (Life Technologies, Gaithersburg, Md.). Recombinant plasmid DNAs were prepared from randomly selected transformant colonies (Quantum Prep HT/96; Bio-Rad, Hercules, Calif.), digested with ApoI, and separated by gel electrophoresis to evaluate the restriction patterns. The relative proportional representation of SIVsmE660 and SIVmac239 in the product amplified by RT-PCR from plasma samples was estimated by scoring of at least 20 independent recombinant DNA clones as either SIVsmE660 or SIVmac239 based on the presence or absence of an additional ApoI site in the virus-derived sequences. (For a subset of test DNAs, the validity of the ApoI polymorphism was confirmed by evaluation of a second polymorphism for the restriction site, AflIII.)
RESULTS
Treatment period, initial off-treatment period, and early homologous rechallenge.
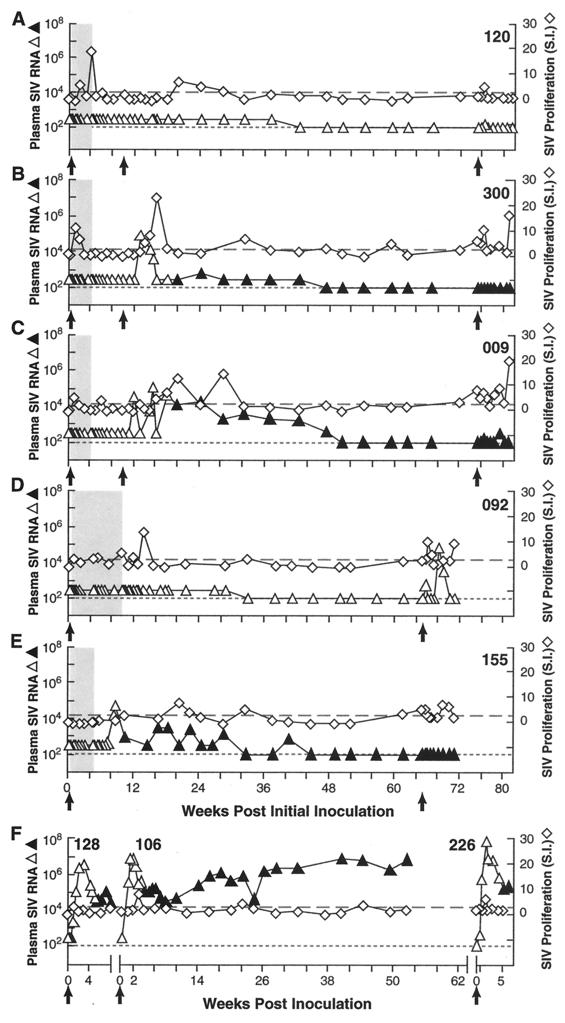
The animals studied either received antiretroviral treatment for 28 days, beginning 24 h after intravenous inoculation with 50% monkey infectious doses (MID50) of SIVsmE660 (Rh 009, Rh 120, and Rh 300), or for 28 days (Rh 155) or 63 days (Rh 092), beginning 72 h p.i. (Table 1, Fig. 1) (15). With the exception of Rh 155 (Fig. 1E), all of the treated animals had undetectable levels of plasma SIV RNA during the treatment and initial posttreatment periods and showed modest but measurable SIV-specific lymphoproliferative responses transiently during and/or immediately following the period of antiretroviral treatment. The animals remained seronegative over this interval (Fig. 1 and 2). Presumably, despite the absence of detectable plasma viremia, these proliferative responses reflected cellular immunological sensitization from a low level of ongoing viral replication in the face of less than complete suppression of viral replication by drug treatment. Only one animal (Rh 155) showed measurable plasma viremia during the initial off-treatment period (Fig. 1E). However, in striking contrast to the viremia seen in identically inoculated untreated control animals (Fig. 1F), the off-treatment rebound viremia seen in Rh 155 was blunted, reaching a peak level of 81,000 SIV RNA copy Eq/ml before declining spontaneously over a period of months, through a series of low-level oscillations to below 100 copy Eq/ml, where it remained. Interestingly, this progressive resolution of the plasma viremia in Rh 155 was associated with a similar series of modest peaks in SIV-specific proliferative responses.
FIG. 1.
Control of viremia, SIV lymphoproliferative responses, and resistance to early and late homologous SIV rechallenge. Plasma SIV RNA levels (triangles; open symbols prior to seroconversion, solid symbols after seroconversion [antibody status not evaluated at every time point]) and SIV-specific lymphoproliferative responses (open diamonds) are shown for macaques infected intravenously with 30 MID50 of SIVsmE660 and then transiently treated with tenofovir to block viral replication. Black arrows indicate initial inoculation and subsequent homologous rechallenges. (A to E) The interval of drug treatment is indicated by the shaded box and consisted of 28 days, beginning 24 h p.i. in Rh 120 (A), Rh 300 (B), and Rh 009 (C), or either 28 days (Rh 155) (D) or 63 days (Rh 092) (E) of treatment, beginning 72 h p.i. (F) Results for a representative, contemporaneously inoculated, SIV-naive, untreated control animal, for each separate challenge. Note the control of plasma viremia and increased lymphoproliferative responses for animals receiving early transient treatment relative to control animals. Dotted line indicates threshold sensitivity for RNA assay, 300 copy Eq/ml (early time points) or 100 copy Eq/ml (later time points); dashed line indicates threshold for positive lympoproliferative responses, stimulation index (S.I.) ≥ 2.5.
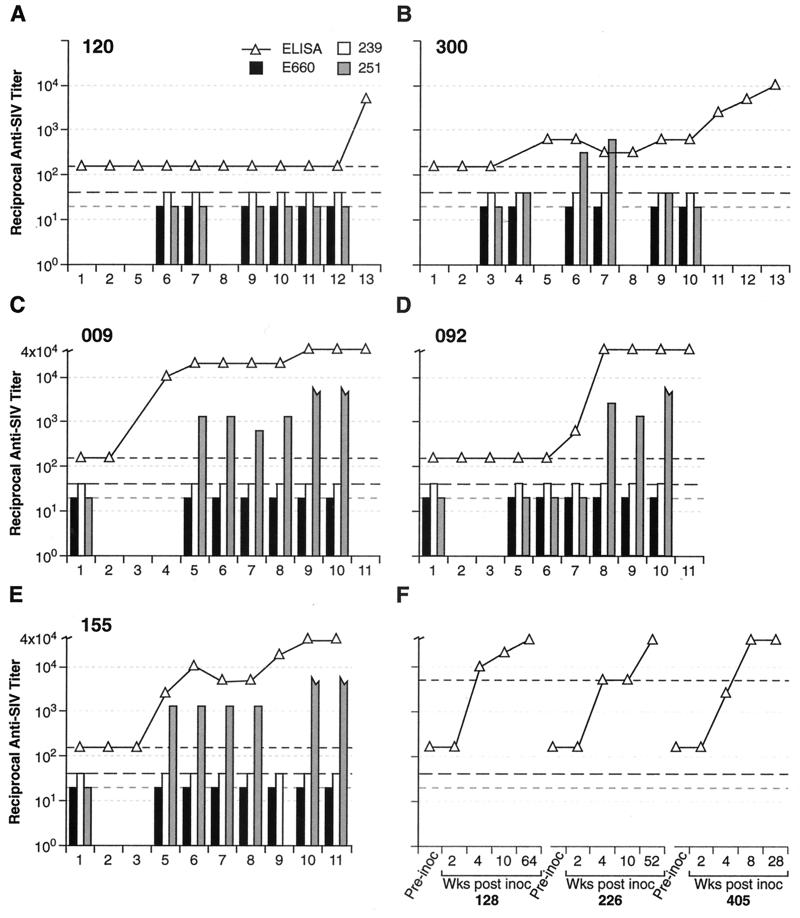
FIG. 2.
Humoral responses. Results shown are reciprocal titers for SIV-binding antibody (determined by ELISA; open triangles, range from <160 to >40,000) and neutralizing antibody against SIVsmE660 (black bars), SIVmac239 (white bars), and neutralization-sensitive culture-adapted SIVmac251 (gray bars) for animals Rh 120 (A), Rh 300 (B), Rh 009 (C), Rh 092 (D), and Rh 155 (E) and representative untreated, SIVsmE660-infected (Rh 128 and Rh 226) or SIVmac239-infected (Rh 405) control animals (F). Dashed lines indicate assay threshold levels (160 for ELISA binding antibody [short black dashes], 20 for SIV smE660 neutralizing antibody [short gray dashes], and 40 for SIVmac239 and SIVmac251 neutralizing antibody [long black dashes]), Jagged-ended gray bars indicate an SIVmac251 reciprocal neutralizing titer of >5,000. For panels A (Rh 120) and B (Rh 300); 1, preinoculation; 2, last day on PMPA treatment; 3, pre-early SIVsmE660 rechallenge; 4, 2 weeks post-early SIVsmE660 rechallenge; 5, 10 weeks post-early SIVsmE660 rechallenge; 6, pre-late SIVsmE660 rechallenge; 7, 2 weeks post-late SIVsmE660 rechallenge; 8, 10 weeks post-late SIVsmE660 rechallenge; 9, pre-CD8 depletion; 10, 2 weeks post-CD8 depletion; 11, pre-late SIVmac239 rechallenge; 12, 2 weeks post-SIVmac239 rechallenge; 13, 4 weeks post-SIVmac239 rechallenge. For panels C (Rh 009), D (Rh 092), and E (Rh 155): 1, preinoculation; 2, last day on PMPA treatment, 3, 2 weeks post-PMPA treatment; 4, pre-early SIVsmE660 rechallenge; 5, pre-late SIVsmE660 rechallenge; 6, 2 weeks post-late SIVsmE660 rechallenge; 7, pre-SIVmac239 rechallenge; 8, 2 weeks post-SIVmac239 rechallenge; 9, pre-CD8 depletion; 10, 2 weeks post-CD8 depletion; 11, 3 weeks post-CD8 depletion. For panel F, bleeds are as labelled. Open triangles show ELISA binding-antibody titers. Both SIVsmE660 and SIVmac239 are very difficult to neutralize; neutralizing titers in chronically infected animals are generally <1:40 and are often not measurable (Desrosiers et al., unpublished). For chronically SIV-infected macaques, neutralizing titers against the cell line-adapted SIVmac251 are typically in the range of 1:10,000.
Three macaques (Rh 120, Rh 300, and Rh 009), which were all aviremic during the initial off-treatment period and had shown SIV-specific lymphoproliferative responses during and/or after treatment, received an intravenous homologous rechallenge with 30 MID50 of SIVsmE660, 10 weeks p.i., 6 weeks after discontinuation of antiretroviral treatment. As shown in Fig. 1A, Rh 120 completely resisted this rechallenge. In Rh 300 and Rh 009 (Fig. 1B and C, respectively), there were readily detectable peaks of plasma viremia following rechallenge, which were associated with seroconversion (Fig. 1B and C and 2). However, these peaks were blunted relative to the peaks achieved in untreated control animals inoculated identically and simultaneously (Fig. 1F, note Rh 106). Strikingly, the plasma viremia in Rh 300 and Rh 009 resolved spontaneously over a period of weeks to below the threshold for quantitation, in conjunction with low levels of anti-SIV lymphoproliferative responses and without further intervention.
Extended follow-up period and late homologous rechallenge.
During a subsequent period of prolonged follow-up, plasma viremia remained undetectable regardless of whether the animals had never shown prior viremia (Rh 120 and Rh 092) or whether such viremia was detected only transiently after rechallenge (Rh 009 and Rh 300) or transiently in the off-treatment period without rechallenge (Rh 155). These results and the associated immunological findings seemed consistent with the hypothesis that transient early antiviral treatment had allowed the development of host responses capable of existing durable control of the infection and of resisting early rechallenge.
To determine the durability of resistance to homologous rechallenge, all five animals were challenged intravenously with 30 MID50 of SIVsmE660, 65 to 75 weeks after the original inoculation. Just prior to this challenge, animals Rh 300, Rh 009, and Rh 155 had been clearly infected, as judged by conventional criteria of seroconversion (Fig. 1B, C, and E and 2B, C, and E) and prior evidence of measurable plasma viremia (Figure 1B, C, and E). Based on the known infectious inoculum administered intravenously and the presence of anti-SIV lymphoproliferative responses, animals Rh 120 and Rh 092 were considered to be presumptively infected, despite the absence of seroconversion, the absence of demonstrable plasma viremia (Fig. 1A and D and 2A and D), and the inability to recover virus (data not shown). As shown in Fig. 1A to E, all five animals showed either complete (Rh 300 and Rh 155) or partial (Rh 120, Rh 009, and Rh 092) resistance to this late homologous rechallenge, as reflected by plasma SIV RNA measurements. Modest transient boosting of SIV-specific lymphoproliferative responses was observed during the post-rechallenge period. Even the three animals that showed transient measurable viremia following the rechallenge (Rh 120, Rh 009, and Rh 092) showed blunted and spontaneously resolving peaks of plasma virus, dramatically different from the profiles in untreated control animals (Fig. 1F [note Rh 226] and other data not shown [10]). After the late homologous rechallenge, Rh 155 maintained unmeasurable levels of SIV RNA in plasma (Fig. 1E). Levels exceeding the assay threshold of 100 copy Eq/ml were present only transiently in Rh 120, Rh 009, and Rh 092, in striking contrast to accumulated experience with SIVsmE660 infection, in which peak plasma RNA levels typically exceed 107 copy Eq/ml and stabilize at levels in excess of 105 copy Eq/ml (Fig. 1F and data not shown [average peak levels for six comparably inoculated SIV-naive control animals and standard deviation, 1.6 × 108 ± 3.3 × 108 copy Eq/ml; range, 3.3 × 106 to 8.4 × 108]) (10).
Depletion of CD8+ lymphocytes and heterologous rechallenge.
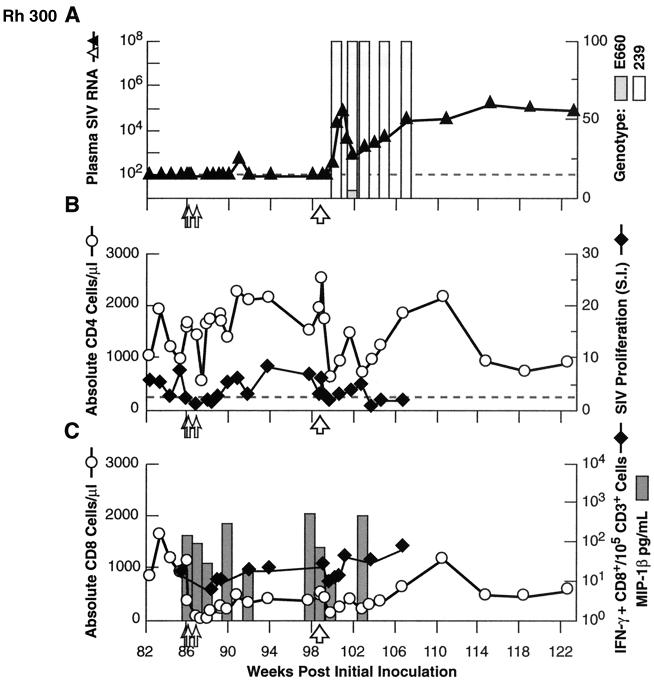
To evaluate the potential contribution of CD8+ lymphocytes (14) to the apparent control of viral replication observed, Rh 120 and Rh 300 received three doses of the anti-CD8 MA6 cM-T807. This antibody effectively mediates transient near-complete depletion of CD8+ lymphocytes in vivo in rhesus macaques (32, 33). We monitored total CD8+ cells by flow cytometry, SIV-specific CD8+ cells by ELISPOT assay, and SIV-inducible secretion of the representative β-chemokine MIP-1β by ELISA. Effective depletion of total and SIV-specific CD8+ cells from PBMC was achieved (Fig 3C and 4C). While depletion of CD8+ T cells in SIV- or simian/human immunodeficiency virus (SHIV)-infected macaques with measurable plasma viremia has been reported to produce dramatic, transient increases in viral load (1, 11, 18, 21, 32, 33), we saw only a modest transient increase to detectable levels following depletion of CD8+ cells in Rh 300 (Fig. 4A); the plasma viral load remained below the threshold for quantitation in Rh 120 (Fig. 3A), despite the depletion of CD8+ cells. However, the supernatant of a virus isolation culture of LNMC, from a biopsy specimen taken from Rh 120 after two doses of anti-CD8 MAb, was weakly positive, whereas previous attempts at virus isolation had been negative. Flow cytometric analysis of the lymph node biopsy specimens confirmed substantial, albeit incomplete, depletion of CD8+ cells. Overall, these results are consistent with release from a controlling effect on viral replication mediated by CD8+ cells, although the levels of viral replication evinced during the brief period of maximal depletion of CD8+ cells were very modest relative to previous reports (1, 11, 18, 21, 32, 33) perhaps because of the presumably very low levels of viral replication present in these animals prior to antibody treatment.
FIG. 3.
Results for in vivo depletion of CD8+ cells (doses indicated by small open arrows) and heterologous (SIVmac239; 100 MID50 intravenously) virus rechallenge (large open arrow) in animal Rh 120. (A) Plasma viral load (triangles) and viral genotype (bars; SIVsmE660 [grey] or SIVmac239 [white]), based on characterization of at least 20 independent clones derived from RT-PCR-amplified plasma viral RNA for each indicated time point. (B) Absolute CD3+ CD4+ cell counts per microliter of of peripheral blood (circles) and SIV-specific proliferative responses (diamonds). (C) Absolute CD3+ CD8+ cell counts per microliter of peripheral blood (circles), SIV-specific CD8+ cells/105 total CD3+ CD8+ cells (diamonds), and SIV-inducible MIP-1β secretion (grey bars). LNMC from a biopsy specimen obtained after 2 of 3 doses of anti-CD8 MAb showed 15% residual CD3+ CD8+ cells, compared to 4% in matched PBMC. IFN-γ, gamma interferon.
FIG. 4.
Results for in vivo depletion of CD8+ cells (doses indicated by small open arrows) and heterologous (SIVmac239; 100 MID50 intravenously) virus rechallenge (large open arrow) in animal Rh 300. Graphing conventions are as for Fig. 3. LNMC from a biopsy specimen obtained after two of three doses of anti-CD8 MAb showed 9% residual CD3+ CD8+ cells, compared to 3% in matched PBMC.
At 13 weeks after the first dose of anti-CD8 MAb, we evaluated the breadth of protective effects by performing an intravenous challenge with the highly pathogenic and sequence-divergent heterologous virus isolate SIVmac239 (10, 28, 44). As shown in Fig. 4A, Rh 300 showed some resistance to this challenge, with a blunted peak viremia and postpeak levels lower than typically observed in naive control macaques (mean peak plasma RNA for eight comparably inoculated SIV-naive control animals, 4.2 × 107 ± 4.6 × 107 copy Eq/ml; range, 6.6 × 106 to 1.40 × 108). In contrast, Rh 120 (Fig. 3A) showed a viral load profile following SIVmac239 challenge that was similar to profiles observed in challenged naive animals. Genotyping demonstrated that the plasma virus pool following this challenge was composed essentially of only SIVmac239, since SIVsmE660 was not detected in samples from Rh 120 and was found only rarely in samples from Rh 300 (Fig. 3A and 4A).
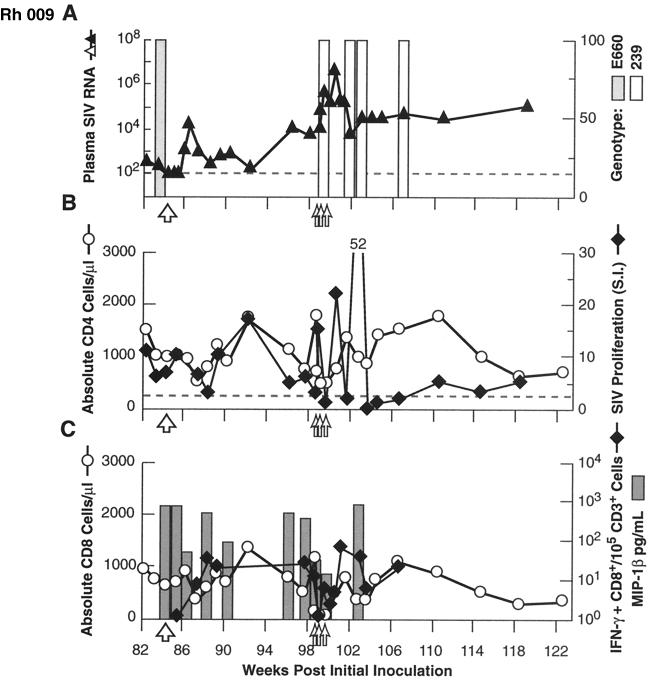
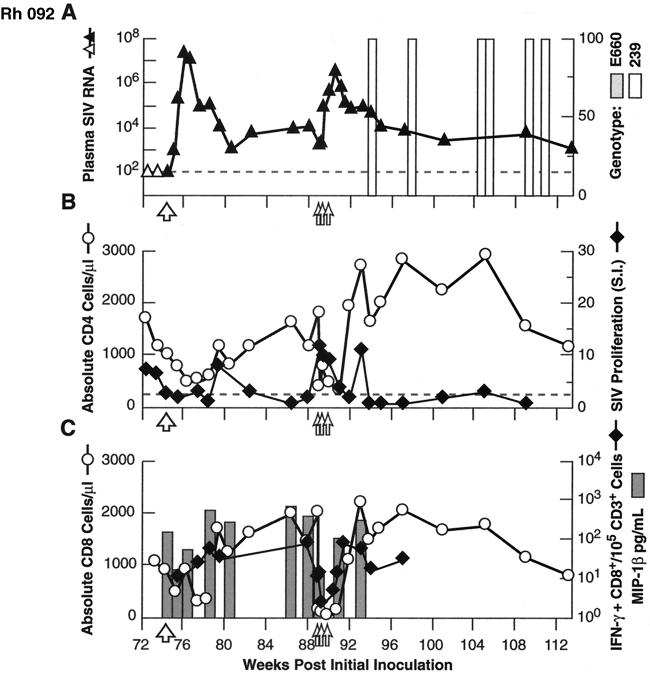
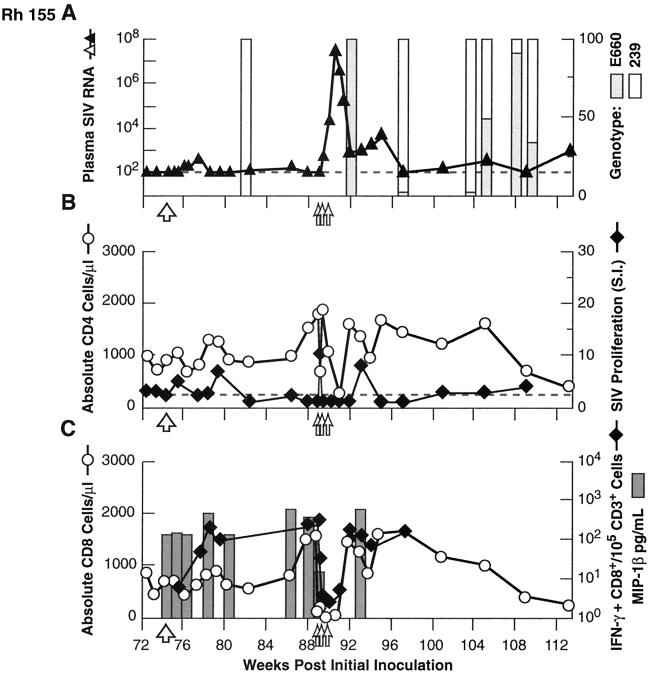
Similar experiments to assess the breadth, and mechanism(s) of control of infection and resistance to rechallenge were performed in Rh 009, Rh 092, and Rh 155, although the order of experiments varied from those described above. As noted above, all three animals showed substantial to complete protection from a late homologous rechallenge with SIVsmE660, performed 65 to 75 weeks after the initial inoculation, with postchallenge boosting of SIV-specific lymphoproliferative responses (Fig. 1C to E). Rh 092, Rh 009, and Rh 155 then received an intravenous challenge with the highly heterologous virus isolate SIVmac239. As shown in Fig. 5A, 6A, and 7A, all three animals showed substantial control of the virus following this challenge. Although peak plasma virus levels exceeded 107 copy Eq/ml in Rh 092 (Fig. 6A), similar to those in untreated control animals (range, 6.6 × 106 to 1.4 × 108 [also see reference 10]), the levels rapidly declined in Rh 092, equilibrating between 103 and 104 copy Eq/ml. Untreated control animals inoculated intravenously with SIVmac239 typically stabilize at a level in excess of 106 (10). Rh 009 showed even better control, with the levels peaking at 1.6 × 104 copy Eq/ml and equilibrating between 103 and 104 copy Eq/ml (Fig. 5A), while Rh 155 showed the most robust resistance to challenge with SIVmac239, with the transient plasma viremia peaking at only 5.4. × 102 copy Eq/ml before declining to below the threshold for quantitation (Fig. 7A). For all three animals, genetic analysis demonstrated that the virus present in the plasma immediately following the SIVmac239 challenge was SIVmac239, without SIVsmE660 detected (Fig. 5A, 6A, and 7A). Containment of the heterologous challenge virus was observed despite the absence of neutralizing antibody, only modest levels of SIV-specific binding antibody, and SIV-specific cellular immune responses that were low or unmcasurable at the time of challenge (Fig. 2, 5B, 6B, and 7B).
FIG. 5.
Results for heterologous (SIVmac239; 100 MID50 intravenously) virus rechallenge (large open arrow) and in vivo depletion of CD8+ cells (doses indicated by small open arrows) in animal Rh 009. Note the reversal of the order of anti-CD8 MAb depletion and heterologous rechallenge, relative to animals Rh 120 and Rh 300 (Fig. 3 and 4). Graphing conventions are as for Fig. 3. LNMC from a biopsy specimen obtained after two of three doses of anti-CD8 MAb showed 24% residual CD3+ CD8+ cells, compared to 6% in matched PBMC.
FIG. 6.
Results for heterologous (SIVmac239; 100 MID50 intravenously) virus rechallenge (large open arrow) and in vivo depletion of CD8+ cells (doses indicated by small open arrows) in animal Rh 092. Graphing conventions are as for Fig. 3. LNMC from a biopsy specimen obtained after two of three doses of anti-CD8 MAb showed 14% residual CD3+ CD8+ cells, compared to 1% in matched PBMC.
FIG. 7.
Results for heterologous (SIVmac239; 100 MID50 intravenously) virus rechallenge (large open arrow) and in vivo depletion of CD8+ cells (doses indicated by small open arrows) in animal Rh 155. Graphing conventions are as for Fig. 3. LNMC from a biopsy specimen obtained after two of three doses of anti-CD8 MAb showed 14% residual CD3+ CD8+ cells, compared to 3% in matched PBMC.
To assess the contribution of CD8+ lymphocytes to the impressive control of viral replication demonstrated in these animals, including after heterologous challenge, the anti-CD8 MAb cM-T807 was administered. Effective depletion of both total and SIV-specific CD8+ lymphocytes from PBMC was achieved, and this depletion was associated with a prompt and dramatic increase in plasma SIV RNA levels (Fig. 5A and C, 6A and C, and 7A and C). Strikingly, after the completion of antibody treatment, with the return toward pretreatment levels of circulating CD8+ cells, including SIV-specific CD8+ lymphocytes, plasma viral load levels returned to approximately pretreatment levels as well. Plasma viral RNA levels in Rh 155 declined to below the threshold of detection (Fig. 7A). As an indicator of CD8+ cell-derived soluble factors that can inhibit SIV via noncytolytic mechanisms, we measured the representative β-chemokine MIP-1β (7). SIV-inducible secretion of MIP-1β declined roughly in parallel with the declines in CD8+ lymphocyte numbers, then increased as circulating CD8+ cell numbers normalized (Fig. 5C, 6C, and 7C). Interestingly, SIV-specific lymphoproliferative responses showed an initial increase as plasma virus levels first started to increase with depletion of CD8+ cells (Fig. 5B, 6B, and 7B). However, with further increases in plasma viral load, proliferative responses then declined, rising again only after viremia had been brought under relative control coincident with the return of circulating CD8+ cells. Only Rh 155 showed appreciable SIVsmE660 genotype in the plasma virus compartment after depletion of CD8+ lymphocytes (Fig. 7A).
DISCUSSION
Transient antiretroviral treatment, begun shortly after inoculation, profoundly modulated the virologic, immunologic, and clinical course of SIV infection. In the well-established natural history of intravenous infection of rhesus macaques with SIVsmE660, animals typically (i) show peak acute viremia in excess of 106 copy Eq/ml; (ii) develop post-acute plasma RNA levels in excess of 105 copy Eq/ml; (iii) demonstrate little or no evidence of SIV-specific lympoproliferative responses during primary infection; and (iv) eventually manifest progressive infection, ultimately necessitating euthanasia (10). In striking contrast, the treated animals in this study showed measurable SIV-specific lymphoproliferative responses, sustained control of plasma viremia (often to below detectable limits), resisted both homologous and heterologous (SIVmac239) rechallenge, and remained clinically well. Even given the limitations of the small number of animals studied, the results for resistance to rechallenge are particularly noteworthy in view of the difficulty in achieving cross-protection between SIVsmE660 and SIVmac239 using conventional vaccine approaches, even with live attenuated virus vaccination (44). Following the initial rechallenge with either SIVsmE660 or SIVmac239, plasma SIV RNA levels were significantly lower in animals that had received transient p.i. treatment than in comparably inoculated untreated control animals at 2 and 8 weeks postchallenge and for the peak value measured over the first 8 weeks postchallenge (P = 0.0078, P = 0.0345, and P = 0.0043 for SIVsmE660, and P = 0.0478, P = 0.0186, and P = 0.0475 for SIVmac239, respectively [Wilcoxon rank sum test]). Depletion of CD8+ lymphocytes in vivo by MAb treatment confirmed that at least a part of the control of viremia was mediated by this cell population.
The implication of these results is that transient antiretroviral treatment during the early stages of primary infection modulated viral replication sufficiently to allow an immunological sensitization more effective than that which typically occurs during untreated infection. This immune response, mediated at least in part by CD8+ lymphocytes, appears to be capable of controlling the infection and conveying variable degrees of resistance to rechallenge, including heterologous rechallenge, more than 1 year following the initial challenge. In this regard, the results are reminiscent of those obtained with live attenuated strains of SIV that have been evaluated as candidate vaccines (12, 13). The findings are also similar to recently reported results of other p.i. transient antiretroviral treatment studies (22, 30, 35).
Our results from MAb depletion studies, showing a key role for CD8+ lymphocytes in controlling viral replication, are consistent with observations implicating this population in maintaining control of attenuated vaccine strains of SIV (21). Perhaps the most remarkable finding in the present study is that prior to the depletion of CD8+ cells, some of these animals were manifesting sustained effective control of viral replication despite being demonstrably coinfected with two of the more highly replicating and consistently pathogenic strains of SIV known (Fig. 5 to 7) (10).
The contribution of neutralizing humoral immunity to this control is probably modest, since no neutralizing antibodies to either SIVsmE660 or SIVmac239 were demonstrable at any time (Fig. 2). However, it is possible that low-titer neutralizing antibody (<1:20) may have been present without being detected in the assay used (20). In some instances, animals appeared to be protected from rechallenge in the absence of SIV-binding antibody, detectable via whole-virus ELISA or Western blot analysis or even using an ultrasensitive immuoprecipitation/Western blot assay for gp120-reactive antibody (see, for example, early [Fig. 1A to C] and late [Fig. 1A and D] homologous rechallenges). For other examples of resistance to rechallenge, binding antibody was present, suggesting that antibody is not essential for protection in all instances but may contribute to protection through nonneutralizing mechanisms that remain to be elucidated (Fig. 1 and 2). Serum antibody capable of neutralizing a highly neutralization-sensitive culture-adapted strain of SIVmac251 (20) was demonstrated. However, this neutralizing antibody generally correlated with overall binding antibody titers and, absent any measurable neutralization for SIVsmE660 or SIVmac239, seems unlikely to contribute significantly to virus control, particularly in view of the results of the experiments involving depletion of CD8+ lymphocytes (Fig. 3 to 7).
The findings do not appear to reflect any intrinsic resistance to infection or any lack of permissiveness for viral replication in vivo among the animals that controlled their infections. Although different susceptibilities to infection have been described for PBMC from different animals, with in vitro susceptibility testing predicting in vivo viral replication patterns (8, 9, 16, 34), the animals studied here were prescreened to have approximately comparable susceptibilities to SIV infection, as assessed by in vitro infectivity assays using PBMC. Furthermore, the robust viral replication seen following depletion of CD8+ cells also argues against a role for differences in intrinsic resistance between animals at the cellular level, unless such resistance were mediated indirectly through effects of CD8+ cells. In a similar way, although it is formally possible that the control of viral replication observed may reflect in vivo selection of an attenuated strain of virus, the robust viral replication seen on depletion of CD8+ cells makes this unlikely. It should be noted that the anti-CD8 MAb used for depletion in the present study depletes both CD3+ CD8+ T cells and the CD3− CD8+ cells believed to comprise at least part of the natural killer cell population in rhesus macaques (4). Future studies with more specific depleting MAbs may allow dissection of the respective contributions of both populations to the control of viral replication observed. Future studies may also allow assessment of the respective contributions of cytolytic and noncytolytic mechanisms mediated by CD8+ lymphocytes to the suppression of viral replication seen in this system.
It is possible that the serial challenge design of the present experiments may have contributed to the protection from late rechallenge we observed through intermediate boosting of immune responses associated with interim homologous challenges. However, in some instances (Fig. 1D and E) we observed resistance to homologous rechallenge more than 1 year after the initial inoculation, without an earlier rechallenge in the intervening period. In addition, in light of the difficulty to date in demonstrating heterologous cross-neutralization or cross-protection between SIVsmE660 and SIVmac239 in other systems (24, 44), the current results of partial control are impressive even if the serial challenges did contribute a boosting effect. It will be of interest in future studies to see how early the resistance to heterologous challenge can be demonstrated in this system and to assess the contribution of CD8+ T cells to such early protection against both homologous and heterologous challenge strains.
While it is likely that animals with measurable plasma viremia will eventually show signs of progressive SIV infection, lower plasma viral loads can be associated with substantially delayed disease progression (10). However, it should be clearly acknowledged that vaccine studies have shown that there is potential for late breakthrough of high-level viral replication, even in animals that initially show good control (27). For these reasons, the animals are continuing to be monitored.
Perhaps the most significant aspect of the present results is the implication that a substantial fraction of hosts may be able to establish sustained effective control of viral replication by primate immunodeficiency viruses if compromise of the immune response during the key period of initial immunological sensitization through primary infection can be avoided (1, 2, 15, 19, 29, 42, 43, 45). These findings have important implications for understanding basic aspects of the pathogenesis of the primate immunodeficiency viruses. The observation that this control of viral replication is associated with resistance to rechallenge, including heterologous rechallenge, has important implications for vaccine development. Indeed, particularly in view of recent results suggesting that early treatment of HIV infection may facilitate antiviral immune responses and the ability to control viral replication when treatment is stopped (1, 2, 17, 29), the current results speak encouragingly to the conceptual feasibility of developing an effective vaccine for the prevention or control of HIV infection. A striking feature of the present study is the impressive control of viral replication and the extent of resistance to even heterologous rechallenge achieved in the setting of comparatively modest measured immune responses. Indeed, the immune responses measured in the present study are much less impressive than those achieved in many vaccine studies that have demonstrated less impressive control of viral replication or resistance to challenge. It will be of great interest to characterize in greater detail the nature of the immune responses associated with protection in this model and to ascertain whether designed vaccination regimens that induce similar responses produce comparable levels of protection in a prophylactic vaccination setting.
ACKNOWLEDGMENTS
We thank L. Arthur for many helpful discussions; C. Whistler for preparation of the figures; G. Heissler for expert assistance with animal care; J. Bess, Jr., M. Grimes, and W. Bohn for preparation of inactivated viruses used as stimulating antigens in immunological assays; R. Means for ultrasensitive anti-SIV env antibody assays; and G. Alvord for assistance with statistical analyses.
This project has been funded with Federal funds from the National Cancer Institute, National Institutes of Health, under contract NO1-CO-56000, and by NIH grants R24 RR 160001 and R01 RR 13150.
Footnotes
Dedicated to the memory of our colleague Kalachar Suryanarayana.
REFERENCES
- 1.Altfeld M, Rosenberg E S. The role of CD4+ T helper cells in the cytotoxic T lymphocyte response to HIV-1. Curr Opin Immunol. 2000;12:375–380. doi: 10.1016/s0952-7915(00)00103-5. [DOI] [PubMed] [Google Scholar]
- 2.Altfeld M, Rosenberg E S, Shankarappa R, Mukherjee J S, Hecht F M, Eldridge R L, Addo M M, Poon S H, Phillips M N, Robbins G K, Sax P E, Boswell S, Kahn J O, Brander C, Goulder P J, Levy J A, Mullins J I, Walker B D. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169–180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur L O, Bess J W, Jr, Chertova E N, Rossio J L, Esser M T, Benveniste R E, Grimes M K, Henderson L E, Lifson J D. Simian immunodeficiency virus: production, inactivation, and characterization. AIDS Res Hum Retroviruses. 1998;14(Suppl. 3):S311–S319. [PubMed] [Google Scholar]
- 4.Carter D L, Shieh T M, Blosser R L, Chadwick K R, Margolick J B, Hildreth J E, Clements J E, Zink M C. CD56 identifies monocytes and not natural killer cells in rhesus macaques. Cytometry. 1999;37:41–50. [PubMed] [Google Scholar]
- 5.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV 1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:398–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 6.Desrosiers R C. Strategies used by human immunodeficiency virus that allow persistent viral replication. Nat Med. 1999;5:723–735. doi: 10.1038/10439. [DOI] [PubMed] [Google Scholar]
- 7.Garzino-Demo A, DeVico A L, Gallo R C. Chemokine receptors and chemokines in HIV infection. J Clin Immunol. 1998;18:243–255. doi: 10.1023/A:1027329721892. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein S, Elkins W R, London W T, Hahn A, Goeken R, Martin J E, Hirsch V M. Immunization with whole inactivated vaccine protects from infection by SIV grown in human but not macaque cells. J Med Primatol. 1994;23:75–82. doi: 10.1111/j.1600-0684.1994.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein S, Brown C R, Dehghani H, Lifson J D, Hirsch V M. Intrinsic susceptibility of rhesus macaque peripheral CD4+ T cells to simian immunodeficiency virus in vitro is predictive of in vivo viral replication. J Virol. 2000;74:9388–9395. doi: 10.1128/jvi.74.20.9388-9395.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch V M, Lifson J D. Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment and prevention. Adv Pharmacol. 2000;49:437–477. doi: 10.1016/s1054-3589(00)49034-4. [DOI] [PubMed] [Google Scholar]
- 11.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson R P, Desrosiers R C. Protective immunity induced by live attenuated simian immunodeficiency virus. Curr Opin Immunol. 1998;10:436–443. doi: 10.1016/s0952-7915(98)80118-0. [DOI] [PubMed] [Google Scholar]
- 13.Johnson R P, Lifson, Czajak S C, Cole K S, Manson K H, Glickman R, Yang J, Montefiori D C, Montelaro R, Wyand M S, Desrosiers R C. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relation of degree of protection with level of attenuation. J Virol. 1999;73:4952–4961. doi: 10.1128/jvi.73.6.4952-4961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Letvin N L, Schmitz J E, Jordan H L, Seth A, Hirsch V M, Reimann K A, Kuroda M J. Cytotoxic T lymphocytes specific for the simian immunodeficiency virus. Immunol Rev. 1999;170:127–134. doi: 10.1111/j.1600-065x.1999.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 15.Lifson J D, Rossio J L, Arnaout R, Li L, Parks T L, Schneider D K, Kiser R F, Coalter V J, Walsh G, Imming R J, Fisher B, Flynn B M, Bischofberger N, Piatak M, Jr, Hirsch V M, Nowak M, Wodarz D. Containment of SIV infection: cellular immune responses and protection from re-challenge following transient post-inoculation antiretroviral treatment. J Virol. 2000;74:2584–2593. doi: 10.1128/jvi.74.6.2584-2593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lifson J D, Nowak M A, Goldstein S, Rossio J L, Kinter A, Vasquez G, Wiltrout T A, Brown C, Schneider D, Wahl L, Lloyd A, Elkins W R, Fauci A S, Hirsch V M. The extent of early viral replication is a critical determinant of the natural history of AIDS virus infection. J Virol. 1997;75:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lori F, Lewis M G, Xu J, Varga G, Zinn D E, Jr, Crabbs C, Wagner W, Greenhouse J, Silvera P, Yalley-Ogunro J, Tinelli C, Lisziewicz J. Control of SIV rebound through structured treatment interruptions during early infection. Science. 2001;290:1591–1593. doi: 10.1126/science.290.5496.1591. [DOI] [PubMed] [Google Scholar]
- 18.Matano T, Shibata R, Siemon C, Connors M, Lane H C, Martin M A. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMichael A J, Ogg G, Wilson J, Callan M, Hambleton S, Appay V, Kelleher T, Rowland-Jones S. Memory CD8+ T cells in HIV infection. Philos Trans R Soc Lond Ser B. 2000;355:363–367. doi: 10.1098/rstb.2000.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Means R E, Greenough T, Desrosiers R C. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metzner K J, Jin X, Lee F V, Gettie A, Bauer D E, Di Mascio M, Perelson A S, Marx P A, Ho D D, Kostrikis L G, Connor R I. Effects of in vivo CD8(+) T cell depletion on virus replication in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J Exp Med. 2000;191:1921–1932. doi: 10.1084/jem.191.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori K, Yasutomi Y, Sawada S, Villinger F, Sugama K, Rosenwirth B, Heeney J L, Uberla K, Yamazaki S, Ansari A A, Rubsamen-Waigmann H. Suppression of acute viremia by short term postexposure prophylaxis of simian/human immunodeficiency virus SHIV-RT-infected monkeys with a novel reverse transcriptase inhibitor ( GW420867) allow for the development of potent antiviral immune responses resulting in containment of infection. J Virol. 2000;74:5747–5753. doi: 10.1128/jvi.74.13.5747-5753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.National Research Council. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 23.Nowak M A, Lloyd A L, Vasquez G, Wiltrout T A, Bischofberger N, Williams J, Kinter A, Fauci A S, Hirsch V M, Lifson J D. Viral dynamics of primary viremia and antiretroviral therapy in simian immunodeficiency virus infection. J Virol. 1997;71:7518–7525. doi: 10.1128/jvi.71.10.7518-7525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ourmanov I, Bilska M, Hirsch V M, Montefiori D C. Recombinant modified vaccinia virus ankara expressing the surface gp120 of simian immunodeficiency virus (SIV) primes for a rapid neutralizing antibody response to SIV infection in macaques. J Virol. 2000;74:2960–2965. doi: 10.1128/jvi.74.6.2960-2965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pantaleo G, Fauci A S. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 26.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 27.Polacino P S, Stallard V, Klaniecki J E, Pennathur S, Montefiori D C, Langlois A J, Richardson B A, Morton W R, Benveniste R E, Hu S L. Role of immune responses against the envelope and the core antigens of simian immunodeficiency virus SIV mne in protection against homologous cloned and uncloned virus challenge in macaques. J Virol. 1999;73:8201–8215. doi: 10.1128/jvi.73.10.8201-8215.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regier D A, Desrosiers R C. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990;6:1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg E S, Altfeld M, Poon S H, Phillips M N, Wilkes B M, Eldridge R L, Robbins G K, D'Aquilla R Y, Goulder P J R, Walker B D. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 30.Rosenwirth B, ten Haaft P, Bogers W M, Nieuwenhuis I G, Niphuis H, Kuhn E M, Bischofberger N, Heeney J L, Uberla K. Antiretroviral therapy during primary immunodeficiency virus infection can induce persistent suppression of virus load and protection from heterologous challenge in rhesus macaques. J Virol. 2000;74:1704–1711. doi: 10.1128/jvi.74.4.1704-1711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossio J L, Esser M T, Suryanarayana K, Schneider D K, Bess J W, Jr, Vasquez G M, Wiltrout T A, Chertova E, Grimes M K, Sattentau Q J, Arthur L O, Henderson L E, Lifson J D. Inactivation of HIV-1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz J E, Simon M A, Kuroda M J, Lifton M A, Ollert M W, Vogel C W, Racz P, Tenner-Racz K, Scallon B J, Dalesandro M, Ghrayeb J, Rieber E P, Sasseville V G, Reimann K A. A nonhuman primate model for the selective elimination of CD8+ lymphocytes using a mouse-human chimeric monoclonal antibody. Am J Pathol. 1999;154:1923–1932. doi: 10.1016/S0002-9440(10)65450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seman A L, Pewen W F, Fresh L F, Martin L N, Murphey-Corb M. The replicative capacity of rhesus macaque peripheral blood mononuclear cells for simian immunodeficiency virus in vitro is predictive of the rate of progression to AIDS in vivo. J Gen Virol. 2000;8:2441–2449. doi: 10.1099/0022-1317-81-10-2441. [DOI] [PubMed] [Google Scholar]
- 35.Spring M, Stahl-Henning C, Stolte N, Bischofberger N, Heeney J, ten Haaft P, Tenner-Racz K, Racz P, Lorenzen D, Hunsmann G, Dittmer U. Enhanced cellular immune response and reduced CD8+ lymphocyte apoptosis in acutely SIV-infected rhesus macaques after short-term antiretroviral treatment. Virology. 2001;279:221–232. doi: 10.1006/viro.2000.0720. [DOI] [PubMed] [Google Scholar]
- 36.Staprans S L, Dailey P J, Rosenthal A A, Horton C, Grant R M, Lerche N, Feinberg M B. Simian immunodeficiency virus disease course is predicted by the extent of virus replication during primary infection. J Virol. 1999;73:4829–4839. doi: 10.1128/jvi.73.6.4829-4839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suryanarayana K, Wiltrout T A, Vasquez G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load by real time quantification of product generation in RT PCR. AIDS Res Hum Retroviruses. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 38.Tsai C C, Folis K E, Yarnall M, Deaver L E, Benveniste R E, Sager P R. In vitro screening for antiretroviral agents against simian immunodeficiency virus (SIV) Antiviral Res. 1990;14:87–98. doi: 10.1016/0166-3542(90)90046-a. [DOI] [PubMed] [Google Scholar]
- 39.Tsai C C, Emau P, Follis K E, Beck T W, Benveniste R E, Bischofberger N, Lifson J D, Morton W R. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl)adenine treatment for prevention of persistent simian immunodeficiency virus STVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai C C, Follis K E, Sabo A, Beck T W, Grant R F, Bischofberger N, Benveniste R E, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 41.Watson A, Ranchalis J, Travis B, McClure B J, Sutton W, Johnson P R, Hu S L, Haigwood N L. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wodarz D, Page K M, Arnaout R A, Thomsen A R, Lifson J D, Nowak M A. A new theory of cytotoxic T-lymphocyte memory: implications for HIV treatment. Philos Trans R Soc Lond Ser B. 2000;355:329–343. doi: 10.1098/rstb.2000.0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wodarz D, Arnaout R A, Nowak M A, Lifson J D. Transient post-inoculation antiretroviral treatment facilitates long term control of SIV infection, Philos. Trans R Soc Lond Ser B. 2000;355:1021–1029. doi: 10.1098/rstb.2000.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wyand M S, Manson K, Montefiori D C, Lifson J D, Johnson R P, Desrosiers R C. Protection by live attenuated simian immunodeficiency virus against heterologous challenge. J Virol. 1999;73:8356–8363. doi: 10.1128/jvi.73.10.8356-8363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zajac A J, Murali-Krishna K, Blattman J: N, Ahmed R. Therapeutic vaccination against chronic viral infection: the importance of cooperation between CD4+ and CD8+ T cells. Curr Opin Immunol. 1998;10:444–449. doi: 10.1016/s0952-7915(98)80119-2. [DOI] [PubMed] [Google Scholar]