ABSTRACT
BACKGROUND:
Patient-generated data are a cornerstone of individualized multiple sclerosis (MS) treatment. MyMS, an interface for patient-reported outcomes (PROs) was developed by the Finnish MS Register to enable systematic collection of PROs.
METHODS:
MyMS collects data on demographics, lifestyle factors, disease-related factors, and validated questionnaires, including the Quality of Life Questionnaire (15D), the Multiple Sclerosis Impact Scale (MSIS-29), and the Fatigue Severity Scale (FSS). At the end of 2020, the patient-reported Expanded Disability Status Scale (PREDSS), the EuroQOL-5 Dimension (EQ-5D), the Fatigue Scale for Motor and Cognitive Functions (FSMC), and the Multiple Sclerosis Neuropsychological Questionnaire (MSNQ) were added.
RESULTS:
As of January 1, 2023, 1201 individuals with MS (79% female) have added data to MyMS. Of the validated PRO measures (PROMs), the 15D, MSIS-29, and FSS are the most used. The mean PREDSS score is 3.0 and median disease duration is 6.4 years. According to the existing PROMs, patients report mildly compromised quality of life and problems with fatigue and cognition.
CONCLUSIONS:
The patient interface of the Finnish MS Register consists of data from 17 of 21 counties with well-being services. The interface is used by 10% of Finnish individuals with MS. The addition of the PREDSS, EQ-5D, FSMC, and MSNQ to the interface has increased health care professional and patient interest in the use of PROMs. We suggest that PROs should be integrated into electronic health records to improve shared decision-making and diminish documentation burden.
Keywords: patient-reported outcomes, Finnish MS Register, patient-generated data
High-quality patient registers are needed to improve the monitoring and treatment of progressive diseases sucha as multiple sclerosis (MS). MS registers are in use in many European countries,1 and the need to further develop register-based data collection is recognized worldwide.2 Registers allow individualized follow-up by providing systematic data on disease history, real-world data on the natural history of the disease, as well as data on treatment effectiveness, tolerability, and safety.3 They may also be used to predict an individual’s risk of conversion to secondary progressive multiple sclerosis (SPMS).4 Optimally, registers combine patient-reported data with data contributed by health care professionals (HCPs).
Along with the development of clinician-based registers, there is a growing need to implement patient engagement options, combining objective disease data with subjective patient-reported outcomes (PROs). This would promote patient autonomy, shared decision-making, and cost-effective individualized care.5-7 A PRO is any report of the status of an individual’s condition that comes directly from the individual, without interpretation by anyone, such as a clinician. PRO measures (PROMs) are standardized tools such as surveys, scales, or single-item measures. It is important that HCPs and the treatment team know how individuals perceive their disease, quality of life (QOL), treatment effects, and adverse events, and that means measuring outcomes that matter most to people with MS.8 Systematic collection, storage, and use of PRO data are likely to improve disease management and help the treatment team and the patient determine the best treatment options together.
Finland’s national MS register was launched in 20149 to enable systematic disease monitoring as well as to monitor disease incidence, prevalence, and progression. A PRO interface was added in 2017 to enhance patient-centeredness and autonomy. Individuals are able to record data on medication usage, suspected relapses, lifestyle factors, and QOL, as well as keep track of disease severity, symptoms, and impact. The interface allows patients to follow their disease in a visual format and acts as a communication tool with the treatment team.
The objective of this study was to describe the contents and status of MyMS, the PRO interface of the Finnish national MS register. We illustrate the core variables and data collected by MyMS at the beginning of 2023. We also discuss future challenges, perspectives, and needs related to PRO-based data collection.
METHODS
The Finnish MS Register is a browser-based register for public health care organizations.9 During the patient visit, it is the neurologist’s primary user interface, integrated into the hospital’s electronic patient record (EPR) system. HCPs log on to the MS register via the hospital’s EPR system with a single sign-on identifier, which automatically redirects them to the individual’s registry view. It is also possible for the structured patient narrative to automatically transfer to the neurology interleaf of the EPR, which avoids having to record patient information in 2 different systems. The PRO data in the patient interface are displayed directly on the clinician’s interface.
The use of the register is voluntary, and each county with well-being services decides whether to acquire it. The development of the register, the steering committee, and the core features of the clinician-based register are described elsewhere.9 As of January 2023, 17 of 21 of Finland’s counties with well-being services, which includes all 5 university hospitals, use the register, and most of them have integrated it into their EPR system. In January 2023, the number of patients in the register was 11,349, which was approximately 90% of individuals with MS in Finland. Register funding is based on licensing fees paid by the counties to the software service provider, StellarQ Ltd (stellarq.com).
The interface for PROs is available to all patients who have enrolled in the national MS register. MyMS is a secure log-in service with a user-friendly and graphically illustrative interface (FIGURE S1). It includes the following PROs: identification data (name, date of birth, sex), background data (heredity, education, employment), lifestyle factors (smoking, use of alcohol), suspected relapse notation, medications, comorbidities, and requests for assistance and rehabilitation, as well as standardized questionnaires on disease severity, impact, symptoms, disability, and QOL. The standardized PROMs include the patient-reported Expanded Disability Status Scale (PREDSS),10 the EuroQOL-5 Dimension (EQ-5D),11 the Quality of Life Questionnaire (15D),12 the Multiple Sclerosis Impact Scale-29 (MSIS-29),13,14 the Fatigue Severity Scale (FSS),15,16 the Fatigue Scale for Motor and Cognitive Functions (FSMC),17,18 and the Multiple Sclerosis Neuropsychological Questionnaire (MSNQ).18,19 In case of interruption, it is possible to complete a questionnaire within 24 hours. At the end of 2020, the PREDSS, the EQ-5D, the FSMC, and the MSNQ were added to the register; all the other measures were present from the launch of the interface. Approximately 10% of Finnish individuals with MS use MyMS.
FIGURE S1.
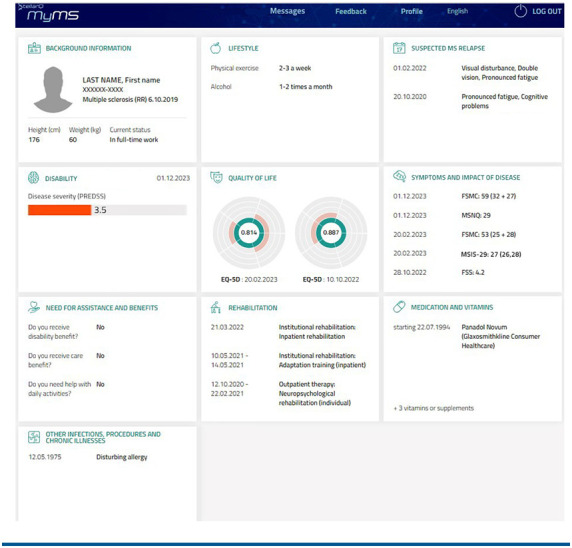
MyMS Interface
Individuals with MS learn about MyMS mainly from MS nurses working in hospital MS clinics. The nurses are provided with written instructions on how to introduce the interface and the PROs, and written instructions are also available as handouts. The Finnish MS associations also tell their members about using MyMS to follow their disease and participate in their treatment.
Ethical Approval and Data Analyses
National level patient-reported data were collected from the real-time pseudonymous database. Patients permit data utilization for study purposes, as long as data are reported in aggregated, anonymous form. This is ensured via a formal consent that is collected through MyMS when the patient begins to use the interface. Data extraction also includes date of MS diagnosis, which is mandatory clinical information to get access to the patient interface. StellarQ is the data processor for all data extracted.
Data extraction included patient-reported data from January 1, 2017, through December 31, 2022. Data counts and percentages of patients with data in MyMS as well as mean, standard deviation, median, and quartiles for the latest scores were calculated using nonmissing data. No imputation was needed. Date of first data entry in the patient interface was considered the index date to calculate age, disease duration, and user count progression. All data analyses were done using RStudio (version 2023.03.0).
RESULTS
A total of 11,349 MS patients (G35) and 692 patients with unspecified demyelinating disease of the central nervous system (G37.9) were registered with the Finnish MS Register as of January 2023. There are 4 well-being services counties that do not use the MS register (FIGURE S2; coded as 1, 7, 8, and 19). In 12 counties, MyMS is considered inactive (ie, coverage under 10%). There are 3 counties (3, 6, 14) with MyMS coverage exceeding 20%.
FIGURE S2.
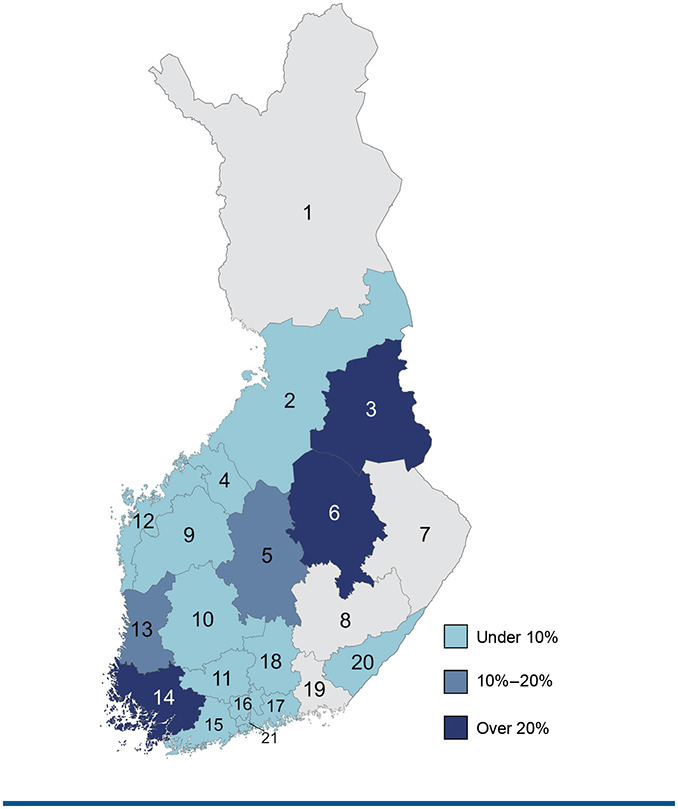
MyMS Coverage by Well-Being Service County
MyMS was used by 1201 patients in January 2023. The number of MyMS users has increased linearly since it was launched in 2017. FIGURE shows the cumulative progression of MyMS users as well as the cumulative progression of filled documents from 2017 to the beginning of 2023.
FIGURE.
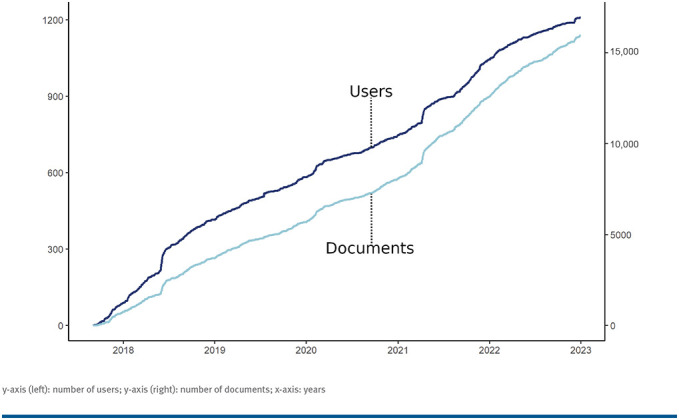
MyMS Users and Data Entries 2017-2023
y-axis (left): number of users; y-axis (right): number of documents; x-axis: years
Of the 1201 MyMS users, 79% are women, and there was a median disease duration of 6.4 years and 13.9 mean years of education based on self-report (TABLE 1). Table 1 describes the MyMS variables, how many times the variable has been recorded in the register, and the percentage of patients who have the information included in their data file. The most actively reported PROs were lifestyle factors and background information. The PROMs that were included since the launch of the patient interface have been used the most; 74.6% of respondents with MS have filled out the 15D, 52% the MSIS-29, and 51% the FSS. The scales added in the register later have been filled out by individuals with MS as follows: the PREDSS by 39.4%, the FSMC by 35.0%, the EQ-5D by 31.6%, and the MSNQ by 18.1%.
TABLE 1.
Patient Demographics and Data Counts in the Finnish MyMS Register
| Variables | All patients (N = 1201) | ||
|---|---|---|---|
| Age, years (mean SD) | 43.7 (10.72) | ||
| Female, n (%) | 946 (78.8%) | ||
| Education, years (mean SD) | 13.9 (2.75) | ||
| Disease duration, years (median Q1-Q3) | 6.4 (1.2, 13.4) | ||
| Variables | Data counts | Coverage, n (%) | Recordings, mean |
| Background information | |||
| Heritage | 670 | 670 (55.8%) | 1.0 |
| Education | 829 | 829 (69.0%) | 1.0 |
| Employment | 816 | 816 (67.9%) | 1.0 |
| Lifestyle factors | |||
| Smoking | 1062 | 1062 (88.4%) | 1.0 |
| Alcohol use | 1047 | 1047 (87.2%) | 1.0 |
| Others | |||
| Patient-reported relapses | 734 | 340 (28.3%) | 2.2 |
| Patient-reported medications | 2334 | 676 (56.3%) | 3.5 |
| Patient-reported comorbidities | 521 | 282 (23.5%) | 1.8 |
| Need for assistance | 782 | 782 (65.1%) | 1.0 |
| Rehabilitation | 345 | 208 (17.3%) | 1.7 |
| Disease questionnaires | |||
| PREDSS score | 703 | 473 (39.4%) | 1.5 |
| EQ-5D score | 503 | 380 (31.6%) | 1.3 |
| 15D score | 1483 | 896 (74.6%) | 1.7 |
| MSIS-29 total score | 971 | 624 (52.0%) | 1.6 |
| FSS score | 964 | 613 (51.0%) | 1.6 |
| FSMC total score | 639 | 420 (35.0%) | 1.5 |
| MSNQ score | 341 | 217 (18.1%) | 1.6 |
15D, Quality of Life Questionnaire; EQ-5D, EuroQOL-5 Dimension; FSMC, Fatigue Scale for Motor and Cognitive Functions; FSS, Fatigue Severity Scale; MSIS-29, Multiple Sclerosis Impact Scale; MSQN, Multiple Sclerosis Neuropsychological Questionnaire; PREDSS, patient-reported Expanded Disability Status Scale; SD, Standard Deviation.
The mean PREDSS score (n = 473) is 3.0, which means the average patient has no walking ability limitations, but has other significant MS-related problems that limit daily activities (TABLE 2). The mean EQ-5D (n = 380) and 15D scores (n = 896) were both 0.8, indicating mildly compromised QOL.11,12 The mean MSIS-29 scores (n = 624) were between 26.2 and 28.5, which indicates few problems13 or mild disease impact.20 The mean FSS score (n = 613) was 4.2, equating to mild self-perceived fatigue.15 In contrast, the FSMC scores (n = 420) seemed to indicate severe overall fatigue (mean score 63.1) as well as moderate motor (means core 31.4) and cognitive fatigue (mean score 31.7).17 The mean MSNQ score (n = 217) was 35.3, which means that the patient perceives problems with cognitive functions.19
TABLE 2.
Latest Scores on the Questionnaires
| Variables | Mean (SD) | Median (Q1, Q3) |
|---|---|---|
| PREDSS score | 3.0 (1.71) | 3.0 (2.0, 4.0) |
| EQ-5D score | 0.8 (0.19) | 0.8 (0.7, 0.9) |
| 15D score | 0.8 (0.12) | 0.8 (0.7, 0.9) |
| MSIS-29 total score | 26.9 (20.95) | 23.3 (9.5, 41.4) |
| Physical scale score | 26.2 (22.67) | 21.2 (6.2, 42.5) |
| Psychological scale score | 28.5 (21.88) | 25.0 (11.1, 41.7) |
| FSS score | 4.2 (1.87) | 4.6 (2.6, 5.7) |
| FSMC total score | 63.1 (21.83) | 67.5 (47.8, 80.0) |
| Motor fatigue score | 31.4 (10.97) | 33.0 (24.0, 40.2) |
| Cognitive fatigue score | 31.7 (11.33) | 33.5 (24.0, 40.0) |
| MSNQ score | 35.3 (9.06) | 35.0 (28.0, 41.0) |
15D, Quality of Life Questionnaire; EQ-5D, Euro Quality of Life-5 Dimension; FSMC, Fatigue Scale for Motor and Cognitive Functions; FSS, Fatigue Severity Scale; MSIS-29, Multiple Sclerosis Impact Scale; MSNQ, Multiple Sclerosis Neuropsychological Questionnaire; PREDSS, patient-reported Expanded Disability Status Scale.
Note: PREDSS scale is 0 to 9, where 0 stands for no disability and 9 for bedridden most of the time. Higher scores refer to better quality of life in EQ-5D and 15D (range, 0-1). Higher MSIS-29 scores indicate more prominent disease impact (range, 0-100). Higher FSS scores indicate more severe symptoms/problems with fatigue (range, 1-7). The FSMC has a total score range of 20 to 100 with a subscore range from 10 to 50; higher scores indicate more impairment. The MSNQ has a total score range of 0 to 60 with higher scores indicating more impact.
DISCUSSION
Seven years after its launch, the Finnish MS Register covers the majority of Finnish MS patients. It is increasingly being adopted as a part of clinical practice following the national treatment guidelines,21 and efforts to achieve complete coverage are ongoing. PROMs, especially standardized and validated ones, are growing in importance because individualized patient-centered treatment is the gold standard of high-quality care.21 The 21st Century Steering Group22 has highlighted the need to improve communication between patients and HCPs to promote patient participation and self-management, as well as to enable access to high-quality information.
There are several national MS registries in Europe that differ from each other with respect to objectives, structure, collected data, and patient and clinician involvement.23 The registries were established between 1956 (Denmark) and 2014 (Finland). In 2017, the number of patients in the registries varied from 1000 to approximately 50,000.1 Registries are typically kept by academic research institutions, patient organizations, or health care organizations.1 Based on a review published in 2014, physician-based outcome measures such as the Expanded Disability Status Scale (EDSS) were used in all 20 identified European registries, whereas data from patients’ perspectives were only collected in 6 registries.23 According to results of a more recent survey published in 2019, 7 of 19 identified MS registries include patient-derived measures.1 The MSIS-29 was used in all 7 registries, and 6 registries included PROMs on fatigue, 4 of those the FSS. The North American Research Committee on Multiple Sclerosis (NARCOMS) registry is one of the few patient-driven registries. In NARCOMS, patients fill out questionnaires online or by mail to a coordinating center.24 In contrast to the patient interface of the Finnish national MS registry, data collection in NARCOMS initially occurred only at enrollment, and longitudinal semiannual data collection began in 2020.
As stated by Brichetto and Zaratin,8 electronic health technologies could play an increasing role in the systematic use of PRO data. The patient interface of the Finnish MS Register is a good example of an e-health solution that enables systematic collection of patient experience. Active use of the data in MyMS together with that in the clinician-based MS register is 1 way to improve shared decision-making. Systematic self-report is a way to empower patients to take responsibility for their disease and commit to treatment, rehabilitation, and beneficial lifestyle choices.8,21,22 When the patient uses MyMS, the treatment team can compare the clinician-rated EDSS25 score with the corresponding PROM and the PREDSS10 and then discuss any discrepancies with the patient. The PREDSS has shown high correlation with the clinician-rated EDSS.26 Moreover, the information on patient-perceived symptoms and impact of the disease is readily available to the team, as it is displayed on the clinician interface.
Because many MS-related symptoms are invisible and difficult to diagnose in their early phases, patient reports are critical. Based on a European registry sample of almost 14,000 individuals with MS, Kobelt and colleagues27 showed that self-reported fatigue and cognitive symptoms reduce working capacity. In MyMS, patients have the opportunity to fill out the MSNQ18,19 to evaluate and follow their cognitive symptoms, the FSS15 to evaluate fatigue severity, and the FSMC17,18 to evaluate the characteristics of their self-perceived fatigue. The clinician can combine the MSNQ with the results on the objective measure of information processing speed, the Symbol Digit Modalities Test,18,28 which is recorded in the clinician-based register. Thus, MyMS may help identify patients who need support to manage invisible symptoms such as fatigue and cognitive problems. At best, longitudinal and systematic patient reporting is a pathway to react to treatment urgencies without delay.
The present sample represents most of the counties in Finland with well-being services, although the coverage is uneven. Women are more active MyMS users than men. MyMS users reported mild disability as rated with the PREDSS (mean score 3.0). QOL was reported by both the 15D and the EQ-5D to be mildly compromised. The MSIS-29 showed mild physical and psychological disease impact. Contrary to the FSS showing mild self-perceived fatigue, the FSMC showed severe overall fatigue and moderate motor and cognitive fatigue.18 At the end of 2020, new validated instruments were implemented into MyMS mainly to help evaluate invisible symptoms, such as fatigue with the FSMC and cognitive problems with the MSNQ. It is possible that patients who filled out the measures added in 2020, the PREDSS,10 the FSMC,17,18 the EQ-5D,11 and the MSNQ,18,19 have more pronounced cognitive and fatigue concerns, which would explain why the FSMC shows more severe fatigue than the FSS. Further, the FSMC covers cognitive and physical aspects of fatigue equally, whereas the FSS focuses on physical aspects of fatigue. This may be another reason for the discrepancies in the results of the fatigue questionnaires.
Patients can use MyMS whenever they want. The interest in self-reporting is growing alongside the need for and interest in early intervention and shared decision-making, especially due to the increasing number of treatments. Patients increasingly look to PROMs to convey their lived experiences and multifaceted challenges. The lack of knowledge on the use of PROMs and difficulties utilizing PROM data in often fast-paced clinical decision-making may have previously hindered the use of patient reporting.
Efforts to improve user experience, including the development of a mobile application, are ongoing. By adding feedback and guidance on the validated questionnaires, MyMS could become a source of reliable information and a self-management tool. Implementation of patient-reported experience measures to evaluate treatment satisfaction is also a future milestone in the development of the patient interface.
As yet, there is no systematic national procedure to present MyMS to MS patients in neurology clinics. Some clinics do have a systematic approach, particularly in southwestern and eastern Finland, and these clinics also ask patients to update PROs, such as the PREDSS and QOL measures, annually to provide insights into long-term changes. This may have led to the higher use of MyMS in these areas, and there are efforts to spread these practices nationwide via education and educational materials.
Successful treatment of MS relies on evidence-based medicine. High-quality health information is built from the knowledge and experience of HCPs combined with data from the lived experience of patients. The Finnish MS Register with MyMS is a clinical and patient interface, a unique e-health platform for this kind of data collection, and this is the first paper to report on it. There are numerous opportunities to further develop the collection and utilization of patient-generated data to improve the quality of care and increase patient participation. As more individuals with MS use the platform, the generalizability of the results increases, and research questions regarding the self-perceived symptomatology, disability, usage experiences, and other factors that are crucial in shared decision-making can be more efficiently addressed.
PRACTICE POINTS
The Finnish MS Register includes an interface to promote patient contribution called MyMS, which includes validated patient-reported outcome measures (PROMs) on disease severity, symptoms, and impact, as well as quality of life.
Of the 21 Finnish counties with well-being services, 17 use the register and have the opportunity to collect PROMs. At present, although 90% of Finnish individuals with MS are on the register, only approximately 10% of them use MyMS.
MyMS offers a great opportunity to increase the use of PROMs because it is easily available to individuals as well as health care professionals.
ACKNOWLEDGMENTS:
The authors gratefully acknowledge the contribution of the StellarQ IT team that built and continuously refines the Finnish MS Register platform. All members of the steering committee of the Finnish MS registry as well as patients who have participated in the development of MyMS are gratefully acknowledged.
Funding Statement
FINANCIAL DISCLOSURES: The study was financially supported by Janssen-Cilag Oy. The funder had no role in or influence on any aspect of the study, including data collection, data analysis, writing of the manuscript, and decision to publish. Preparation of this manuscript was funded in part by the Strategic Research Council within the Academy of Finland (funding No. 358415). Efforts to increase the use of the Finnish MS registry have received funding from Biogen Idec, Merck, Novartis, Sanofi Genzyme, Roche, Teva, and Business Finland.
Footnotes
FINANCIAL DISCLOSURES: The study was financially supported by Janssen-Cilag Oy. The funder had no role in or influence on any aspect of the study, including data collection, data analysis, writing of the manuscript, and decision to publish. Preparation of this manuscript was funded in part by the Strategic Research Council within the Academy of Finland (funding No. 358415). Efforts to increase the use of the Finnish MS registry have received funding from Biogen Idec, Merck, Novartis, Sanofi Genzyme, Roche, Teva, and Business Finland.
CONFLICTS OF INTEREST: Päivi Hämäläinen and Matias Viitala are employees of StellarQ Ltd. The other authors of this manuscript do not declare any conflicts of interest related to this study.
REFERENCES
- 1.Glaser A, Stahmann A, Meissner T, et al. Multiple sclerosis registries in Europe: an updated mapping survey. Mult Scler Relat Disord. 2019;27:171–178. doi: 10.1016/j.msard.2018.09.032. doi: [DOI] [PubMed] [Google Scholar]
- 2.Bebo BF, Jr, Fox RJ, Lee K, Utz U, Thompson AJ. Landscape of MS patient cohorts and registries: recommendations for maximizing impact. Mult Scler. 2018;24(5):579–586. doi: 10.1177/1352458517698250. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziemssen T, Hillert J, Butzkueven H. The importance of collecting structured clinical information on multiple sclerosis. BMC Med. 2016;14:81. doi: 10.1186/s12916-016-0627-1. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manouchehrinia A, Zhu F, Piani-Meier D, et al. Predicting risk of secondary progression in multiple sclerosis: a nomogram. Mult Scler. 2019;25(8):1102–1112. doi: 10.1177/1352458518783667. doi: [DOI] [PubMed] [Google Scholar]
- 5.Hoogervorst EL, Eikelenboom MJ, Uitdehaag BM, Polman CH. One year changes in disability in multiple sclerosis: neurological examination compared with patient self report. J Neurol Neurosurg Psychiatry. 2003;74(4):439–442. doi: 10.1136/jnnp.74.4.439. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuusisto H, Apila S, Saranto K. Information provision and quality. A pilot study on shared decision-making in multiple sclerosis. Stud Health Technol Inform. 2022;295:179–182. doi: 10.3233/SHTI220691. doi: [DOI] [PubMed] [Google Scholar]
- 7.Rahn AC, Solari A, Beckerman H, et al. “I will respect the autonomy of my patient”: a scoping review of shared decision making in multiple sclerosis. Int J MS Care. 2020;22(6):285–293. doi: 10.7224/1537-2073.2020-027. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brichetto G, Zaratin P. Measuring outcomes that matter most to people with multiple sclerosis: the role of patient-reported outcomes. Curr Opin Neurol. 2020;33(3):295–299. doi: 10.1097/WCO.0000000000000821. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laakso SM, Viitala M, Kuusisto H, et al. Multiple sclerosis in Finland 2018: data from the national register. Acta Neurol Scand. 2019;140(5):303–311. doi: 10.1111/ane.13145. doi: [DOI] [PubMed] [Google Scholar]
- 10.Kobelt G, Berg J, Lindgren P, Jönsson B. Costs and quality of life in multiple sclerosis in Europe: method of assessment and analysis. Eur J Health Econ. 2006;7(suppl 2):S5–S13. doi: 10.1007/s10198-006-0365-y. doi: [DOI] [PubMed] [Google Scholar]
- 11.EuroQol Group EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. doi: [DOI] [PubMed] [Google Scholar]
- 12.Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med. 2001;33(5):328–336. doi: 10.3109/07853890109002086. doi: [DOI] [PubMed] [Google Scholar]
- 13.Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124(pt 5):962–973. doi: 10.1093/brain/124.5.962. doi: [DOI] [PubMed] [Google Scholar]
- 14.Rosti-Otajärvi E, Hämäläinen P, Wiksten A, Hakkarainen T, Ruutiainen J. Validity and reliability of the Finnish version of the Multiple Sclerosis Impact Scale-29. Brain Behav. 2017;7(7):e00725. doi: 10.1002/brb3.725. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krupp LB, Rocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. doi: [DOI] [PubMed] [Google Scholar]
- 16.Rosti-Otajärvi E, Hämäläinen P, Wiksten A, Hakkarainen T, Ruutiainen J. Validity and reliability of the Fatigue Severity Scale in Finnish multiple sclerosis patients. Brain Behav. 2017;7(7):e00743. doi: 10.1002/brb3.743. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penner IK, Raselli C, Stöcklin M, Opwis K, Kappos L, Calabrese P. The Fatigue Scale for Motor and Cognitive Functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Mult Scler. 2009;15(12):1509–1517. doi: 10.1177/1352458509348519. doi: [DOI] [PubMed] [Google Scholar]
- 18.Hämäläinen P, Leo V, Therman S, Ruutiainen J. Validation of the Finnish version of the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) and evaluation of the applicability of the Multiple Sclerosis Neuropsychological Questionnaire (MSNQ) and the Fatigue Scale for Motor and Cognitive Functions (FSMC) Brain Behav. 2021;11(6):e02087. doi: 10.1002/brb3.2087. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benedict RH, Munschauer F, Linn R, et al. Screening for multiple sclerosis cognitive impairment using a self-administered 15-item questionnaire. Mult Scler. 2003;9(1):95–101. doi: 10.1191/1352458503ms861oa. doi: [DOI] [PubMed] [Google Scholar]
- 20.Forbes A, While A, Mathes L, Griffiths P. Health problems and health-related quality of life in people with multiple sclerosis. Clin Rehabil. 2006;20(1):67–78. doi: 10.1191/0269215506cr880oa. doi: [DOI] [PubMed] [Google Scholar]
- 21.The Finnish Medical Society Duodecim Käypä hoito. [Accessed March 10, 2024];MS-tauti. https://www.kaypahoito.fi/hoi36070 [Google Scholar]
- 22.Rieckmann P, Centonze D, Elovaara I, et al. MS in the 21st Century Steering Group. Unmet needs, burden of treatment, and patient engagement in multiple sclerosis: a combined perspective from the MS in the 21st Century Steering Group. Mult Scler Relat Disord. 2018;19:153–160. doi: 10.1016/j.msard.2017.11.013. doi: [DOI] [PubMed] [Google Scholar]
- 23.Flachenecker P, Buckow K, Pugliatti M, et al. Multiple sclerosis registries in Europe: results of a systematic survey. Mult Scler. 2014;20(11):1523–1532. doi: 10.1177/1352458514528760. doi: [DOI] [PubMed] [Google Scholar]
- 24.Marrie RA, Cutter GR, Fox RJ, Vollmer T, Tyry T, Salter A. NARCOMS and other registries in multiple sclerosis: issues and insights. Int J MS Care. 2021;23(6):276–284. doi: 10.7224/1537-2073.2020-133. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. doi: [DOI] [PubMed] [Google Scholar]
- 26.Romeo AR, Rowles WM, Schleimer ES, et al. An electronic, unsupervised patient-reported Expanded Disability Status Scale for multiple sclerosis. Mult Scler. 2021;27(9):1432–1441. doi: 10.1177/1352458520968814. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobelt G, Langdon D, Jönsson L. The effect of self-assessed fatigue and subjective cognitive impairment on work capacity: the case of multiple sclerosis. Mult Scler. 2019;25(5):740–749. doi: 10.1177/1352458518769837. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith A. Symbol Digit Modalities Test: Manual. Western Physiological Services; 1982. [Google Scholar]


