Abstract
Immediate treatment of acute human immunodeficiency virus type 1 (HIV-1) infection has been associated with subsequent control of viremia in a subset of patients after therapy cessation, but the immune responses contributing to control have not been fully defined. Here we examined neutralizing antibodies as a correlate of viremia control following treatment interruption in HIV-1-infected individuals in whom highly active antiretriviral therapy (HAART) was initiated during early seroconversion and who remained on therapy for 1 to 3 years. Immediately following treatment interruption, neutralizing antibodies were undetectable with T-cell-line adapted strains and the autologous primary HIV-1 isolate in seven of nine subjects. Env- and Gag-specific antibodies as measured by enzyme-linked immunosorbent assay were also low or undetectable at this time. Despite this apparent poor maturation of the virus-specific B-cell response during HAART, autologous neutralizing antibodies emerged rapidly and correlated with a spontaneous downregulation in rebound viremia following treatment interruption in three subjects. Control of rebound viremia was seen in other subjects in the absence of detectable neutralizing antibodies. The results indicate that virus-specific B-cell priming occurs despite the early institution of HAART, allowing rapid secondary neutralizing-antibody production following treatment interruption in a subset of individuals. Since early HAART limits viral diversification, we hypothesize that potent neutralizing-antibody responses to autologous virus are able to mature and that in some persons these responses contribute to the control of plasma viremia after treatment cessation.
Results of passive-antibody studies in nonhuman primate models of pathogenic simian immunodeficiency virus (SIV) and simian-human immunodeficiency viruses (SHIV) have shown that neutralizing antibodies can block infection completely when present at the time of virus exposure or shortly thereafter (9, 14, 23, 24, 37). The same may be true for human immunodeficiency virus type 1 (HIV-1) infection in humans. It remains less certain whether neutralizing antibodies exert a clinically beneficial impact on the virus after infection has been established. For example, neutralizing-antibody production is delayed in HIV-1-infected individuals to an extent that it is not detected until weeks or months after the initial downregulation in peak plasma viremia that occurs during primary infection (2, 16, 27, 33, 34). Existing information on the virologic and immunologic profile of primary HIV-1 infection strongly indicate that virus-specific CD8+ cytotoxic T lymphocytes (CTL) are the major mediators of early viremia control (7, 10, 16, 31), but these CTL ultimately fail to prevent immunologic suppression and AIDS in the absence of antiretroviral therapy. Any means of augmenting the neutralizing-antibody response to episodes of increased viremia, whether in the acute or chronic phase of HIV-1 infection, might have a clinical benefit when added to the antiviral CTL response.
Early effective treatment with highly active antiretroviral therapy (HAART) influences the immune response to HIV-1 infection. This leads to augmented T-helper-cell responses (21, 30, 36), presumably by limiting or preventing the immunologic dysfunction caused by infection (3, 30, 38). HIV-1-specific helper T cells are associated with viremia control in nontreated individuals (36), probably through augmentation of CTL responses. Anecdotal reports and now prospective trials showing at least temporary virus containment following treatment interruption (19, 29) have led to a growing interest in immune-based interventions as an adjunct to HAART.
Efforts to develop effective immune intervention strategies for HIV-1 would benefit from a more complete understanding of the functional immune responses that correlate with viremia control following treatment interruption. In this regard, little is know about the effect of HAART on the virus-specific neutralizing-antibody response. The observation that HAART can preserve and restore normal B-cell functions (12, 28) suggests a possible benefit for the virus-specific antibody response. Contrary to this notion, however, current evidence suggests that the B-cell response wanes when HAART is initiated during chronic infection and fails to mature when HAART is begun early in infection (17, 20, 22, 28). In the few cases where neutralizing antibodies were examined, widely disparate effects of HAART have been observed (6, 13, 19, 29). Interestingly, anecdotal cases of an improved neutralizing-antibody response in a small number of HIV-1-infected individuals who were intermittently nonadherent to HAART have been reported (6, 29).
Recently, a group of HIV-1-infected individuals in whom HAART was initiated early in infection and who later underwent one or more supervised treatment interruptions exhibited a spontaneous reduction in their rebound viremia (35). This viremia control was associated with maintenance of virus-specific T-helper-cell responses and an increase in virus-specific CD8+ T-cell responses. Moreover, analysis of autologous virus in these individuals indicated that early treatment impaired viral diversification, suggesting that at least some of the improved control in these individuals might be due to a more homogeneous virus population with less opportunity for immune escape (1). Here we establish that in addition to cellular immunity, neutralizing antibodies are associated with viremia control in a subset of individuals treated in the earliest stages of acute infection and in whom therapy is discontinued. Since early therapy prevents viral diversification (1), we hypothesize that treatment allowed the maturation of a strain-specific response that was able to control the homogeneous virus population that emerged when therapy was stopped.
MATERIALS AND METHODS
Subjects.
Nine individuals who were identified with symptomatic acute HIV-1 infection and in whom HAART was initiated at the time of their diagnosis (35) were selected for study. These subjects belonged to a larger cohort and were selected because they showed evidence of viremia control following one or more structured treatment interruptions. Antiretroviral regimens consisted of two nucleoside reverse transcriptase inhibitors combined with a protease inhibitor (Table 1). HAART was discontinued for the first time after 1 to 3 years of treatment and reinitiated if plasma viral loads exceeded either 5,000 RNA copies/ml for three consecutive weeks or 50,000 RNA copies/ml at one time point. All individuals suppressed plasma viremia to a level below the limit of detection (<50 RNA copies/ml) for a minimum of 8 months prior to treatment interruption and showed no evidence of mutations conferring drug resistance (35). Moreover, all subjects had HIV-1-specific T-helper-cell function that exceeded a stimulation index of 10 and net counts per minute of ≥800 prior to treatment interruption (35).
TABLE 1.
Description of subjects
| Subject | No. of HIV-1 RNA copies at entry | ELISA | Medicationsa | Phenotype of virusb |
|---|---|---|---|---|
| AC-01 | 250,000 | Negative | ZDV/3TC/IND | R5 |
| AC-02 | 4,850,000 | Negative | ZDV/3TC/NFV | R5 |
| AC-04 | 10,000,000 | Negative | ZDV/3TC/NFV | X4 |
| AC-05 | 1,100,000 | Weak | ZDV/3TC/NFV | R5 |
| AC-06 | 8,180,000 | Negative | D4T/3TC/NFV | R5 |
| AC-10 | 40,700 | Weak | D4T/3TC/IND | R5 |
| AC-13 | 730,000 | Negative | D4T/3TC/IND | R5 |
| AC-14 | 95,000 | Positive | D4T/3TC/IND | R5 |
| AC-16 | 337,000 | Negative | ZDV/3TC/IND | R5 |
D4T, stavudine; 3TC, lamivudine; IND, indinavir, ZDV, zidovudine; NFV, nelfinavir.
R5, CCR5 coreceptor usage; X4, CXCR4 coreceptor usage.
Virus isolation and phenotyping.
HIV-1 was isolated from each subject by peripheral blood mononuclear cell (PBMC) coculture as described previously (25). Virus-containing culture fluids collected at the time of peak p24 Gag antigen production were made cell free by 0.45-μm-pore-size filtration and stored at −80°C in 1-ml aliquots. All assays were performed with virus either in the original isolation fluids or after a single passage in PBMC. Biologic phenotypes were assigned in accordance with established nomenclature (4) and were based on the differential infection of MT-2, U87-CD4-CCR5, and U87-CD4-CXCR4 cells (15) as measured by p24 Gag protein synthesis. Virus was isolated prior to the first treatment-interruption from subjects AC-10, AC-06, AC-16, AC-14 and AC-02. A second isolate from subject AC-10 was obtained during a brief episode of rebound plasma viremia following treatment interruption (1.5 months). Virus from the remaining subjects was isolated 1.5 months into the second treatment phase (AC-13), 1 week after the third treatment interruption (AC-05), 3 weeks after the second treatment interruption (AC-04), and 4.7 months into the fourth treatment phase (AC-01). All viruses exhibited an R5 biologic phenotype with the exception of the virus from subject AC-04, which exhibited an X4 phenotype.
Neutralizing-antibody assays.
Antibody-mediated neutralization of the subjects' HIV-1 isolates was assessed in phytohemagglutinin (PHA)-stimulated PBMC by using a reduction in p24 production as described previously with minor modifications (25). Briefly, cell-free virions (500 50% tissue culture infectious doses) were incubated with various dilutions of plasma for 1 h at 37°C in triplicate prior to the addition of 1-day-old PHA-stimulated PBMC. The cells were washed 4 h later and incubated in fresh interleukin-2-containing growth medium. The concentration of HIV-1 p24 Gag antigen in culture supernatants was measured in an antigen capture enzyme-linked immunosorbent assay (ELISA) (DuPont/NEN) at a time when p24 production in the absence of test plasma was in an early linear phase of increase (usually 3 to 4 days), which is when optimal sensitivity is achieved (39). Neutralization was considered positive when p24 production was reduced by at least 80% relative to the corresponding dilution of a negative control plasma sample from a healtly, noninfected individual. Neutralization of HIV-1IIIB and HIV-1MN was measured in MT-2 cells as described previously (26). Neutralization titers in the MT-2 assay were defined as the plasma dilution at which 50% of cells were protected from virus-induced killing as measured by neutral red uptake. A 50% protection from cell killing corresponds to an approximate 90% reduction in p24 Gag antigen synthesis in the MT-2 assay (8).
ELISA.
HIV-1 Env- and Gag-specific binding antibodies were assessed in Immuno Plates (MaxiSorb F96) (Nunc, Roskilde, Denmark) coated with either baculovirus-derived HIV-1MNgp120 or HIV-1IIIBp24 (Quality Biologicals, Inc., Gaithersburg, Md.) at 0.5 μg/ml as described previously (11).
RESULTS
Neutralizing antibodies prior to treatment interruption in treated acute infection.
Neutralizing antibodies were first examined with T-cell-line-adapted (TCLA) strains and the autologous primary HIV-1 isolate. Initial results with TCLA strains gave a strong indication that the concentration of antiretroviral drugs in plasma samples was sufficient to inhibit HIV-1 in vitro, an activity that could be mistaken for the presence of neutralizing antibodies. Specifically, plasma samples from subjects who were on therapy inhibited HIV-1IIIB and HIV-1MN at high dilutions (1:100 to 1:2,000) despite having low-level or absent MNgp120-specific antibodies as detected by ELISA (data not shown). Furthermore, their plasma had little or no antiviral activity within 14 days following treatment interruption but the activity returned and was sustained soon after HAART was reinitiated (data not shown), indicating the presence in plasma of drug that was affecting the measurement of neutralizing antibodies.
To distinguish between the activity of antiretroviral drugs and neutralizing antibodies, all subsequent assays were performed on plasma samples obtained during treatment interruption, and it is only these data that are presented here. The earliest plasma sample following the first treatment interruption (2 to 35 days) in six of eight subjects had no detectable neutralizing antibodies even though these individuals had been infected for at least 1 to 3 years (Fig. 1 and 2). This was true for neutralizing antibodies measured with either the autologous virus isolate or highly neutralization-sensitive TCLA strains of HIV-1. Moreover, these samples had little or no detectable gp120-specific and p24-specific binding antibodies (Fig. 3). These results suggest that HAART uniformly suppressed the virus-specific antibody response in those subjects. One additional subject in the treatment group had a low neutralizing-antibody titer to HIV-1 MN (AC-13), while another subject had detectable neutralizing antibodies to all three TCLA strains and his autologous virus (AC-01). Serum samples were unavailable for the ninth individual (AC-02) following the first interruption of therapy. The overall poor antibody responses in most subjects was in marked contrast to their T-helper-cell and CTL responses, which were clearly detectable in all (35). Negative neutralization results in many subjects confirmed the clearance of antiretroviral drugs by the time the first samples were obtained post-therapy (minimum of 2 days [AC-16]).
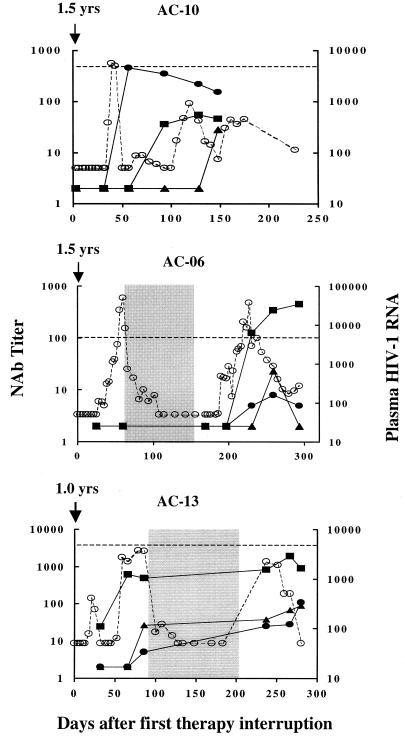
FIG. 1.
Cases where autologous neutralizing-antibody levels correlated with viremia control following treatment interruption. The HIV-1 RNA level in plasma was measured at closely spaced intervals, usually every 3 to 7 days, during the periods of treatment and treatment interruption. Neutralizing-antibody levels were measured at various intervals while the subjects remained off therapy. Intervals when the subjects were on therapy are shaded. Plasma HIV-1 RNA levels (copies per milliliter) are shown as open circles, where a value of 5,000 is indicated by a horizontal dashed line. Titers of neutralizing antibodies are shown as solid circles (autologous virus), solid squares (HIV-1MN), and solid triangles (HIV-1IIIB). Titers of neutralizing antibodies that were below the limit of detection (<1:4 for autologous viruses and <1:20 for HIV-1MN and HIV-1IIIB) were assigned a value of 2. It should be noted that neutralization assays were performed with all viruses for each plasma sample, such that negative values at early time points overlap in the figure.
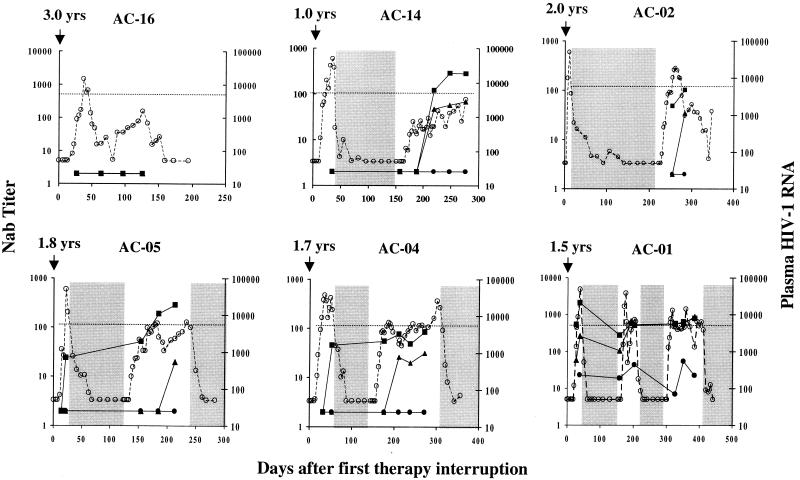
FIG. 2.
Cases where autologous neutralizing-antibody levels did not correlate with viremia control following treatment interruption. Plasma RNA and neutralizing-antibody levels were assessed at intervals described in the legend to Fig. 1. Symbols are as in Fig. 1.
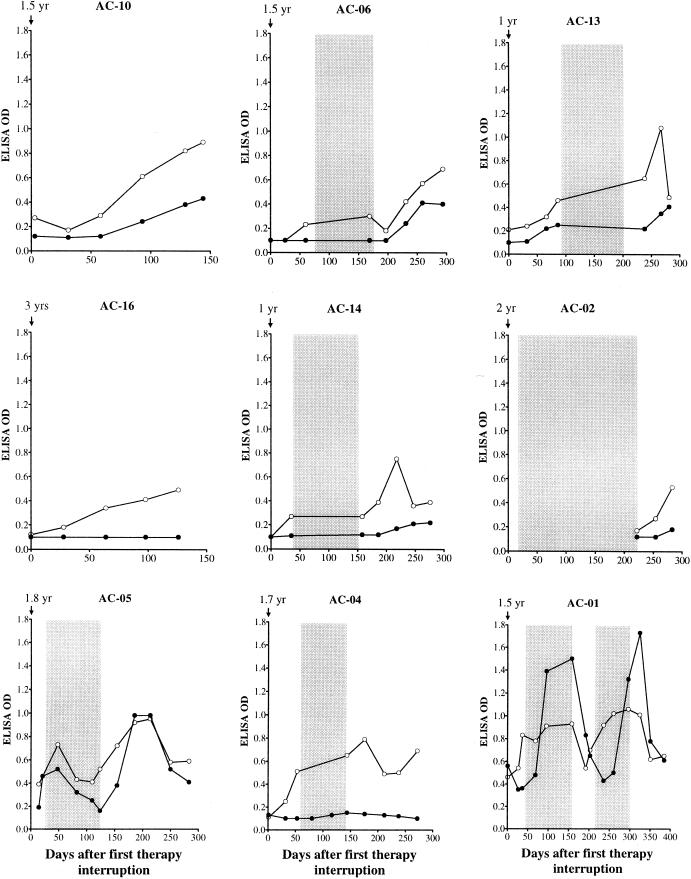
FIG. 3.
ELISA reactivity over time in subjects receiving HAART. Plasma samples were assessed at multiple time points for reactivity to MNgp120 and IIIBp24. Intervals when subjects were on therapy are shaded. Anti-Env antibodies are shown as solid circles; anti-Gag antibodies are shown as open circles.
Neutralizing antibodies in persons in whom viremia was controlled following initial treatment cessation.
Most previous studies of untreated acute HIV infection failed to detect neutralizing antibodies at the time when peak viremia was brought under control (2, 16, 18, 27, 33, 34). The presence of detectable CTL has been used as evidence that cellular immunity is largely contributing to viral containment in most cases (7, 10, 16, 31). We first examined an individual (AC-10) who achieved viral control after a single treatment interruption. This person was selected for initial analysis because only a modest and slowly developing CTL response directed against a single epitope was detected (35). Titers of autologous neutralizing antibodies in this individual were undetectable immediately following treatment cessation but soon rose to a titer of greater than 1:300 as peak viremia began to decline (Fig. 1). Interestingly, the presence of these autologous neutralizing antibodies was not predicted by MNgp120-specific ELISA reactivities, which were negative at the peak of the neutralizing-antibody response and rose only slightly therafter (Fig. 3). The strong autologous neutralizing activities of plasma samples from subject AC-10 shown in Fig. 1 were measured with virus isolated at the time of rebound viremia. These plasma samples had similar neutralization potencies when assayed with the virus variant isolated at the time of diagnosis (1.5 years earlier [data not shown]), suggesting that little or no changes occurred in the neutralization determinants of this subject's virus while he was on HAART. Of note, no neutralizing antibodies were detected with TCLA strains until approximately 6 weeks after the first detection of autologous neutralizing antibodies (Fig. 1). The coincident increase of autologous neutralizing antibodies at the time the viral load was decreasing is consistent with the hypothesis that these antibodies contributed to virus suppression in this individual. Moreover, the development of a strong secondary neutralizing-antibody response and the kinetics of this response indicate that it was primed during the initial infection.
Because plasma from subject AC-10 had potent autologous virus-specific neutralizing activity, we tested whether the sample could cross-neutralize heterologous primary HIV-1 isolates. The plasma failed to neutralize HIV-1 isolates from subjects AC-06, AC-16, AC-05, and AC-04 when tested at a 1:4 dilution and, in an extended analysis, neutralized 5 of an additional 50 heterologous primary isolates when tested at a 1:20 dilution (data not shown). These results indicate that the neutralizing antibodies had minimal cross-reactivity.
Autologous neutralizing antibodies were examined in a second subject (AC-16) in whom viremia was controlled following the first cessation of HAART, but the pattern was quite different in this person. This individual developed strong and broadly directed CTL responses with treatment interruption, and the increase in CTL responses was coincident with the drop in viremia (35). In contrast, neutralizing antibodies to TCLA viruses as well as autologous virus were undetectable at all time points tested (Fig. 2), as were Env-specific binding antibodies (Fig. 3). Low but gradually increasing levels of Gag-specific binding antibodies were observed (Fig. 3) as additional evidence that virus was replicating at low levels. An early peak in viremia was associated with an increase in the breadth and magnitude of CTL responses (35), but despite continued low-level viral replication, there were no detectable neutralizing antibodies. Thus, these data indicate that prolonged control of viremia can occur in the absence of detectable neutralizing antibodies, suggesting that cellular immune responses alone can control viremia under certain circumstances.
Neutralizing-antibody detection following multiple treatment interruptions.
Seven of the nine persons in this study did not show control of their viremia with the first treatment interruption and therefore underwent additional courses of therapy and interruptions. Because therapy was reinitiated in these subjects, it is not known if the virus would have been controlled after a longer period of treatment cessation. Nonetheless, four of these individuals (AC-06, AC-13, AC-14, and AC-02) went on to show control of viremia after the second treatment interruption. In three of these persons for whom assays were completed during the first treatment interruption, no neutralizing antibodies were detected in two (AC-06, and AC-14) and only low titers of neutralizing antibodies to HIV-1MN and the autologous virus were detected in one (AC-13) prior to the reinitiation of treatment (Fig. 1 and 2). In contrast, with the second interruption, two subjects developed autologous neutralizing-antibody responses (AC-06 and AC-13) that coincided with the drop in viremia and persisted as viremia continued to be controlled. These responses peaked at titers of 1:8 and 1:25, respectively, and thus were less robust than those seen in subject AC-10. However, in both persons they increased as the viral load decreased. The rebound in viremia and the appearance of detectable neutralizing antibodies were accompanied by an increase in ELISA reactivity to HIV-1 Env and Gag in all four subjects, and the magnitude of Env-specific reactivity was greatest in AC-06 and AC-13 (Fig. 3).
Two additional subjects (AC-05 and AC-04) exhibited a transient downregulation in their rebound viremia during a second period of treatment interruption despite the absence of autologous neutralizing antibodies. Both subjects exhibited an increase in the level of Gag-specific antibodies coincident with rebound viremia (Fig. 3). An increase in the level of Env-specific antibodies was most notable in subject AC-05, who developed high-titer neutralizing antibodies to HIV-1MN. The lack of autologous neutralizing antibodies suggests that CTL (35) or other immune responses played a major antiviral role in these two subjects.
A final subject (AC-01) underwent three treatment interruptions, the last two being accompanied by a lower level of rebound viremia suggestive of increasing immune control. This individual possessed autologous neutralizing antibodies during each treatment interruption (Fig. 2) and his serum had strong ELISA reactivity to HIV-1 Env and Gag, which appeared to boost during therapy (Fig. 3). It is notable that the neutralizing antibodies were always detected with a late virus isolate (4.7 months into the fourth treatment phase, which is after the last plasma sample examined here). The ability of early plasma samples to neutralize a later virus isolate suggests that the neutralization determinants remained unchanged during the course of study. Alternatively, this individual might possess neutralizing antibodies to a highly conserved neutralization determinant.
DISCUSSION
The results presented here indicate that immediate treatment of acute HIV-1 infection not only results in low levels of HIV-1-specific binding antibodies detected by ELISA but also retards the development of detectable neutralizing-antibody responses. Typically, much stronger seroconversion is seen within 1 year of infection in the absence of HAART, including the presence of detectable neutralizing antibodies (27, 33, 34). We conclude that early HAART has a suppressive effect on the normal antibody response to HIV-1, presumably by limiting the concentration of viral antigens needed to drive virus-specific B-cell maturation.
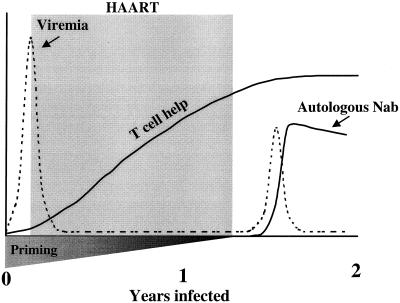
Despite weak antibody responses while on HAART, autologous neutralizing-antibody production commenced rapidly and correlated with a downregulation in rebound viremia following treatment interruption in three individuals. The fact that autologous neutralizing antibodies are rarely detected as peak viremia is downregulated during primary infection in patients who do not receive HAART (2, 16, 18, 27, 33, 34) suggests that a secondary neutralizing-antibody response was operating in these three subjects. A secondary antibody response was most probably primed early in infection, before viremia was brought under control by HAART. The preservation and possible augmentation of this priming while on HAART, combined with a lack of viral evolution, might explain the rapid secondary neutralizing-antibody response to rebound viremia (Fig. 4). The ability of those antibodies to neutralize the autologous virus isolate makes it possible that they contributed to immune control of viremia.
FIG. 4.
Proposed model for a secondary neutralizing-antibody response following treatment interruption in a subset of study subjects.
The potency of the secondary neutralizing-antibody response varied considerably among the three subjects mentioned above, as did the magnitude and longevity of viremia control. The net immunologic effect on the virus most probably reflects combined activities of virus-specific CTL, neutralizing antibodies, and other inducible antiviral mechanisms, such as cytokine and chemokine production. While we cannot be certain of the relative contributions of CTL and neutralizing antibodies to the control of viremia in these subjects, it is worth noting that a potent autologous neutralizing-antibody response was seen in subject AC-10, who had limited virus-specific CTL and maintained low virus loads for at least 230 days following treatment interruption. Subjects AC-06 and AC-13, who had less potent autologous neutralizing antibodies but strong CTL, also maintained low virus loads for an extended period.
Rebound viremia failed to generate a secondary autologous neutralizing-antibody response in other subjects. In these cases, the control of viremia may be attributed to a vigorous virus-specific CD8+ CTL response (35). Antibodies that neutralize TCLA strains of virus were sometimes detected in these latter individuals, but it is doubtful that such antibodies were effective (32). It is not clear why autologous neutralizing antibodies were detected in some individuals but not others. We saw no evidence that neutralizing-antibody induction was related to the magnitude of rebound viremia. Other possible explanations include (i) the extent of B-cell priming before and during HAART; (ii) the degree of immune function preservation, restoration, and augmentation while on HAART; (iii) the relative immunogenicity of the major neutralization determinant(s) on the emerging virus variant; and (iv) host genetic factors. HIV-1 Gag-specific T-helper-cell responses in all study participants were either maintained or augmented while they were on HAART (35), and although not investigated here, it is possible that similar Env-specific T-helper-cell responses played a role in neutralizing-antibody induction. Is has been suggested, however, that Gag-specific but not Env-specific antibody responses are T-cell dependent (5). Clearly, additional work is needed to clarify the role of T-cell help in eliciting HIV-1-specific neutralizing antibodies.
Our observations suggest that appropriate virus-specific B-cell priming can lead to rapid and effective secondary neutralizing-antibody production in response to episodes of viremia in HIV-1-infected individuals. This may be especially true in cases where immune cell functions are preserved, virus specific T-cell responses are generated, and viral genetic diversification has been limited. Efforts to boost the neutralizing-antibody response by therapeutic exposure to appropriate HIV-1 Env immunogens in patients on HAART seem warranted, especially in cases where HAART is intiated early in infection. These results also support the notion that virus-specific B-cell priming, combined with CD8+ CTL induction, may be beneficial for HIV-1 vaccines that aim to suppress viremia in the absence of complete protection to prevent disease and reduce the rate of virus transmission.
ACKNOWLEDGMENTS
We gratefully acknowledge the HIV-1-infected individuals who participated in this study.
This work was funded by National Institutes of Health grants AI40237 (D.C.M.) and AI01541 (E.S.R.), with further support from the Doris Duke Charitable Foundation (B.D.W. and E.S.R.)
REFERENCES
- 1.Altfeld M, Rosenberg E S, Shankarappa R, Mukherjee J S, Hecht F M, Eldridge R L, Addo M M, Poon S H, Phillips M N, Robbins G K, Sax P E, Boswell S, Kahn J O, Brander C, Goulder P J R, Levy J A, Mullins J I, Walker B D. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169–180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ariyoshi K, Harwood E, Chiengsong-Popov R, Weber J. Is clearance of HIV-1 viremia at seroconversion mediated by neutralizing antibodies? Lancet. 1992;340:1257–1258. doi: 10.1016/0140-6736(92)92953-d. [DOI] [PubMed] [Google Scholar]
- 3.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 4.Berger E A, Doms R W, Fenyö E M, Korber B T M, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J, Weiss R A. HIV-1 phenotypes classified by co-receptor usage. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 5.Binley J M, Klasse P J, Cao Y, Jones I, Markowitz M, Ho D D, Moore J P. Differential regulation of the antibody response to Gag and Env proteins of human immunodeficiency virus type 1. J Virol. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binley J M, Trkola A, Ketas T, Schiller D, Clas B, Little S, Richman D, Hurley A, Markowitz M, Moore J P. The effect of highly active antiretroviral therapy on binding and neutralizing antibody responses to human immunodeficiency virus type 1 infection. J Infect Dis. 2000;182:945–949. doi: 10.1086/315774. [DOI] [PubMed] [Google Scholar]
- 7.Borrow P, Lewicki H, Wei X, Horowitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B A, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 8.Bures R, Gaitan A, Zhu T, Graziosi C, McGrath K M, Tartaglia J, Caudrelier P, El Habib R, Klein M, Lazzarin A, Stablein D M, Deers M, Corey L, Greenberg M L, Schwartz D H, Montefiori D C. Immunization with recombinant canarypox vectors expressing membrane-anchored gp120 followed by gp160 protein boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 2000;16:2019–2035. doi: 10.1089/088922200750054756. [DOI] [PubMed] [Google Scholar]
- 9.Clements J E, Montelaro R C, Zink M C, Martin-Amedee A, Miller S, Trichel A M, Jagerski B, Hauer D, Martin L N, Bohm R P, Murphey-Corb M. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J Virol. 1995;69:2737–2744. doi: 10.1128/jvi.69.5.2737-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connick E, Marr D G, Zhang X-Q, Clark S J, Saag M S, Schooley R T, Curiel T J. HIV-specific cellular and humoral immune responses in primary HIV infection. AIDS Res Hum Retroviruses. 1996;12:1129–1140. doi: 10.1089/aid.1996.12.1129. [DOI] [PubMed] [Google Scholar]
- 11.Crawford J M, Earl P L, Moss B, Reimann K A, Wyand M S, Manson K H, Bilska M, Zhou J T, Pauza C D, Parren P W H I, Burton D R, Sodroski J G, Letvin N L, Montefiori D C. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J Virol. 1999;73:10199–10207. doi: 10.1128/jvi.73.12.10199-10207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David D, Pirès R, Treilhou M-P, DuPont B, Joussemet M, Pialoux G, Thèze J, Bouvet J-P. Downregulation of the expression of the main immunoglobulin VH family in HIV-infected patients: modulation by triple combination therapy. AIDS Res Hum Retroviruses. 1999;15:315–316. doi: 10.1089/088922299311510. [DOI] [PubMed] [Google Scholar]
- 13.Dreyer K, Kallas E G, Planelles V, Montefiori D, McDermott M P, Hasan M, Evans T G. Primary isolate neutralization by HIV-1 infected patient sera in the era of highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 1999;15:1563–1571. doi: 10.1089/088922299309856. [DOI] [PubMed] [Google Scholar]
- 14.Foresman L, Jia F, Li Z, Wang C, Stephens E B, Sahni M, Narayan O, Joag S V. Neutralizing antibodies administered before, but not after, virulent SHIV prevent infection in macaques. AIDS Res Hum Retroviruses. 1998;14:1035–1043. doi: 10.1089/aid.1998.14.1035. [DOI] [PubMed] [Google Scholar]
- 15.Hill C M, Deng H, Unutmaz D, Kewalramani V N, Bastiani L, Gorny M K, Zolla-Pazner S, Littman D R. Envelope glycoproteins from human immunodeficiency virus types 1 and 2 and simian immunodeficiency virus can use human CCR5 as a coreceptor for viral entry and make direct CD4-dependent interactions with this chemokine receptor. J Virol. 1997;71:6296–6304. doi: 10.1128/jvi.71.9.6296-6304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D A. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafeuillade A, Poggi C, Tamalet C, Profizi N, Tourres C, Costes O. Effects of a combination of zidovudine, didanosine, and lamivudine on primary human immunodeficiency virus type 1 infection. J Infect Dis. 1997;175:1051–1055. doi: 10.1086/516442. [DOI] [PubMed] [Google Scholar]
- 18.Lathey J L, Pratt R D, Spector S A. Appearance of autologous neutralizing antibody correlates with reduction in virus load and phenotype switch during primary infection with human immunodeficiency virus type 1. J Infect Dis. 1997;175:231–232. doi: 10.1093/infdis/175.1.231. [DOI] [PubMed] [Google Scholar]
- 19.Lisziewicz J, Rosenberg E, Leiberman J, Jessen H, Lopalco L, Siliciano R, Walker B, Lori F. Control of HIV despite the discontinuation of antiretroviral therapy. N Engl J Med. 1999;340:1683–1684. doi: 10.1056/NEJM199905273402114. [DOI] [PubMed] [Google Scholar]
- 20.Luzuriaga K, McManus M, Catalina M, Mayack S, Sharkey M, Stevenson M, Sullivan J L. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: control of viral replication and absence of persistent HIV-1-specific immune response. J Virol. 2000;74:6984–6991. doi: 10.1128/jvi.74.15.6984-6991.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malhotra U, Berry M M, Huang Y, Markee J, Brown D J, Ap S, Musey L, Schacker T, Corey L, McElrath M J. Effect of combination antiretroviral therapy on T-cell immunity in acute human immunodeficiency virus type 1 infection. J Infect Dis. 2000;181:121–131. doi: 10.1086/315202. [DOI] [PubMed] [Google Scholar]
- 22.Markowitz M, Vesanen M, Tenner-Racz K, Cao Y, Binley J M, Talal A, Hurley A, Ji X, Chaudry R, Yaman M, Frankel S, Heath-Chiozzi M, Leonard J M, Moore J P, Racz P, Nixon D F, Ho D D. The effect of commencing combination antiretroviral therapy soon after human immunodeficiency virus type 1 infection on viral replication and antiviral immune responses. J Infect Dis. 1999;179:525–537. doi: 10.1086/314628. [DOI] [PubMed] [Google Scholar]
- 23.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C R, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mascola J R, Stiegler G, VanCott T C, Katinger H, Carpenter C B, Hanson C E, Beary H, Hayes D, Frankel S S, Birx D L, Lewis M G. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 25.Montefiori D C, Pantaleo G, Fink L M, Zhou J T, Zhou J Y, Bilska M, Miralles G D, Fauci A S. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J Infect Dis. 1996;173:60–67. doi: 10.1093/infdis/173.1.60. [DOI] [PubMed] [Google Scholar]
- 26.Montefiori D C, Robinson W E, Jr, Schuffman S S, Mitchell W M. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J Clin Microbiol. 1988;26:231–235. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moog C, Fleury H J A, Pellegrin I, Kirn A, Aubertin A M. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris L, Binley J M, Clas B A, Bonhoeffer S, Astill T P, Kost R, Hurley A, Cao Y, Markowitz M, Ho D D, Moore J P. HIV-1-antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med. 1998;188:233–245. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortiz G M, Nixon D F, Trkola A, Binley J, Jin X, Bonhoeffer S, Kuebler P J, Donahoe S M, Demoitie M A, Kakimoto W M, Ketas T, Clas B, Heymann J J, Zhang L, Cao Y, Hurley A, Moore J P, Ho D D, Markowitz M. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J Clin Investig. 1999;104:677–682. doi: 10.1172/JCI7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oxenius A, Price D A, Eastbrook P J, O'Callaghan C A, Kelleher A D, Whelan J A, Sontag G, Sewell A K, Phillips R E. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci USA. 2000;97:3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pantaleo G, Demarest J F, Soudeyns H, Graziosi C, Denis F, Adelsberger J W, Borrow P, Saag M S, Shaw G M, Sekaly R P, Fauci A S. Major expansion of CD8+ T cells with a predominant Vβ usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- 32.Parren P W H I, Moore J P, Burton D R, Sattentau Q J. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS. 1999;13(Suppl. A):S137–S162. [PubMed] [Google Scholar]
- 33.Pellegrin I, Legrand E, Neau D, Bonot P, Masquelier B, Pellegrin J-L, Ragnaud J-M, Bernard N, Fleury H J A. Kinetics of appearance of neutralizing antibodies in 12 patients with primary or recent HIV-1 infection and relationship with plasma and cellular viral loads. J Acquired Immune Defic Syndr. 1996;11:438–447. doi: 10.1097/00042560-199604150-00003. [DOI] [PubMed] [Google Scholar]
- 34.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg E S, Altfeld M, Poon S H, Phillips M N, Wilkes B M, Eldridge R L, Robbins G K, D'Aquila R T, Goulder P J R, Walker B D. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 37.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho M W, Martin M A. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 38.Smith D, Berrey M M, Robertson M, Mehrotra D, Markowitz M, Perrin L, Clumeck N, Lazzarin A, Burckhardt B, Weber R, Corey L, Cooper D A. Virologic and immunologic effects of combination antiretroviral therapy with zidovudine, lamivudine, and indinavir during primary human immunodeficiency virus type 1 infection. J Infect Dis. 2000;182:950–954. doi: 10.1086/315753. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J Y, Montefiori D C. Antibody-mediated neutralization of primary isolates of human immunodeficiency virus type 1 in peripheral blood mononuclear cells is not affected by the initial activation state of the cells. J Virol. 1997;71:2512–2517. doi: 10.1128/jvi.71.3.2512-2517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]