Abstract
Fat embolism syndrome is a rare clinical entity. The diagnosis is largely clinical, with the imaging studies supporting the clinical diagnosis. Here we present the case of a 19-year-old boy who presented with a tibial fracture and developed sudden onset shortness of breath on the following day. His clinical and investigation findings were suggestive of acute respiratory distress syndrome with fever, tachycardia, and tachypnea along with acute hemoglobin and platelet drop with positive fat globules. According to two clinical criteria, his diagnosis of fat embolism was established. The diagnostic dilemma arose when S1Q3T3 was seen in the electrocardiogram raising a doubt whether it could be a pulmonary embolism.
Keywords: Fat embolism, acute respiratory distress, pulmonary embolism mimics, acute shortness of breath, major fractures
Introduction
Fat embolism syndrome (FES) is a rare syndrome that typically occurs 24–72 h usually after long bone or pelvic fractures. However, this condition can occur in non-orthopedic-related patients such as those following burn injuries, lung transplantation, bone marrow biopsy or transplant, sickle cell anemia, acute pancreatitis, and liposuction. It has become a rare phenomenon because of the diagnostic difficulties but can cause high mortality rates if not timely diagnosed.1,2 The classic triad of symptoms of FES includes respiratory distress, neurologic changes, and petechial rash. 3 FES, which was first described by Zenker in 1861, is commonly associated with long bone fractures and pelvic fractures and frequently presents as a manifestation of the above-mentioned triad of symptoms. 4 Fat embolism occurs following pelvic bone fracture in 5%–10% of cases, following femur fracture in 0.5%–2% of cases, and following tibial fracture it is 0.15% of cases which shows that it is relatively a rare phenomenon to get fat embolism following tibial fracture.4,5 The severity of the condition can vary, most cases are self-limiting, but mortality has been reported as high as 5%–15%, especially with acute respiratory distress syndrome (ARDS) but the severity of the condition is unpredictable from patient to patient. 6 Diagnosis of FES is mainly done clinically and currently, we use two clinical criteria for the diagnosis, Gurd and Wilson 7 criteria and Schonfeld fat embolism index. Treatment is mainly supportive but the early fixation of the long-bone fracture with early immobilization of fractures and operative correction rather than conservative management is important to prevent or to decrease the severity of FES. 7 FES is a clinical entity that is underdiagnosed or misdiagnosed and it is proven by the fact that clinically detected cases of fat embolism are <1%, whereas incidents of postmortem findings of fat embolism are 20%. 1
Case report
This 19-year-old boy presented to the accident service unit after a motorbike accident and following the accident had an open left-side tibial mid-shaft fracture. He did not have other impacts and injuries other than the left-sided lower limb. On the same day, manipulation under anesthesia, cleaning, suturing, and dressing were applied to the wound. Then, above knee plaster of Paris was applied, and the patient was transferred to the surgical ward. His vitals were normal, and the peripheral circulation was normal without any evidence of compartment syndrome.
The following day of the accident and immobilization, the patient was mobilized, with support and after arriving at the bed developed sudden onset shortness of breath (SOB) and was immediately connected to the cardiac monitor. He had severe tachycardia with a pulse rate of 150 beats per minute, tachypnoea with a 56 respiratory rate, and saturation recorded low in room air. Blood pressure was 130/80 mmHg and the patient was conscious but drowsy, with on-and-off agitation and complaints of headache.
His respiratory examination showed a mild reduction of air entry to the right-side lower zone, and he was immediately connected to the oxygen supply with a non-rebreather mask of 15 L, and saturation improved to 92%. Urgent bed-sided arterial blood gas (ABG) and electrocardiogram (ECG) were done. ECG showed S1, Q3, and T3 patterns with sinus tachycardia. ABG showed type 1 respiratory failure with severe hypoxemia. D-dimer values were significantly high and even though the immobilization duration was short, the other factors were in favor of pulmonary embolism (PE). With time, he started to get high-grade fever without cough and other sites of infection.
The patient was immediately transferred to the intensive care unit (ICU) and connected to high-flow nasal oxygen. Due to his ECG changes, an urgent computed tomography pulmonary angiogram (CTPA) was arranged to rule out a PE. His CTPA was negative for pulmonary emboli and his lungs showed generalized ground glass appearance and lower zone bilateral consolidations. The features were mainly suggestive of ARDS in both lungs. His basic investigations were sent at the time he got distressed and were repeated 8 h after the acute process (Table 1).
Table 1.
Investigations.
| Investigation | At the time of SOB | Following day | In 2 days | Reference range |
|---|---|---|---|---|
| White blood cells | 15,010 | 8890 | 10,000 | 4000–11,000 µL |
| Hemoglobin | 14.5 | 9.4 | 11 | 12–16 g/dL |
| Hematocrit | 40.1% | 26.7% | 30.4% | 37%–54% |
| Platelets | 210,000 | 135,000 | 189,000 | 150,000–450,000 µ/L |
| Creatinine | 52.4 | 70–110 µmol/L | ||
| Blood urea | 3.1 | 2.8–7.2 mmol/L | ||
| Aspartate aminotransferase | 10.4 | <50 U/L | ||
| Alanine aminotransferase | 7.6 | <50 U/L | ||
| Blood picture | Normochromic normocytic red cells with a mild reduction in number. White blood cell changes are of infective inflammatory process. No evidence of microangiopathic hemolytic anemia (MAHA) | |||
| Procalcitonin | 0.22 (Possible localized mild infection) | <0.07 ng/ml | ||
| Creatine phosphokinase | 199.8 | <171 U/L | ||
| Total bilirubin | 6.81 | <21 µmol/L | ||
| Direct bilirubin | 1.53 | <3.4 µmol/L | ||
| C-reactive protein | 83.3 | <5 mg/L | ||
| Sodium | 144 | 135–145 mmol/L | ||
| Potassium | 3.4 | 3.5–4.5 mmol/L | ||
| Chloride | 124.4 | 101–109 mmol/L | ||
| PT/INR | 1.38 | 0.8–1.1 | ||
| COVID-19 rapid antigen and polymerase chain reaction (PCR) | Negative | |||
| Urine fat globules | Positive | |||
| Sputum fat globules | Not done | |||
| Erythrocyte sedimentation rate | 35 | mm/1st hour | ||
| D-dimer levels | 8.16 | <1 mg/L | ||
| CTPA | No evidence of pulmonary embolism. Bibasal consolidations, bilateral pleural effusions, and bilateral patchy ground glass chances are in favor of ARDS. | |||
| Chest X-ray | Bilateral lower zone opacities | |||
| pH | 7.429 | 7.42 | 7.44 | 7.35–7.45 |
| PCO2 | 26.8 | 29.5 | 35.5 | 35–45 mmHg |
| PO2 | 69.7 (85% FiO2) (NRBM 15L) | 99.2 (40% FiO2) | 124 (40% FiO2) | 75–100 mmHg in room air |
| HCO3− | 17.94 | 19.4 | 24.7 | 22–26 mmol/L |
| PO2/FiO2 | 82 | 247.9 | 310 | 300–500 mmHg |
| Lactate | 1.4 | <1 mmol/L | ||
| ECG | S1, Q3, and T3 patterns with sinus tachycardia and right bundle branch block | |||
| 2D echo | Ejection fraction 60% with normal cardiac function | |||
| Fasting blood sugar | 89 | 70–100 mg/dl | ||
ARDS: acute respiratory distress syndrome; CECT: contrast-enhanced computerized tomography; ECG: electrocardiogram; SOB: shortness of breath.
In the ICU, he was given non-invasive ventilation (NIV) through bilevel positive airway pressure mode, with inspiratory positive airway pressure-12 mmHg and expiratory positive airway pressure-8 mmHg with fraction of inspiratory oxygen (FiO2) 40% and high flow nasal oxygen therapy initially 60% FiO2 and 60L oxygen alternatively. When clinically recovering tailing off was done with 50% FiO2 50L and 40% FiO2 40L and to the face mask oxygen. Since he responded to NIV, mechanical intubation was not done. Intravenous (IV) antibiotics were started, IV Ticarcillin 3.2 g, eight hourly with IV clindamycin 600 mg eight hourly. IV hydration was given along with other supportive care. His bilateral lung basal air entry was poor and he was constantly on oxygen support. A subcutaneous enoxaparin prophylactic dose was initiated as the patient was not mobilizing. Chest physiotherapy and early rehabilitation processes were stated in the ICU stay along with the alternative NIV. With supportive care and 7 days of ICU stay, the patient recovered from ARDS without other complications and went home after complete recovery. The patient was subsequently followed up in two weekly intervals and there were no other clinical complications (Figure 1).
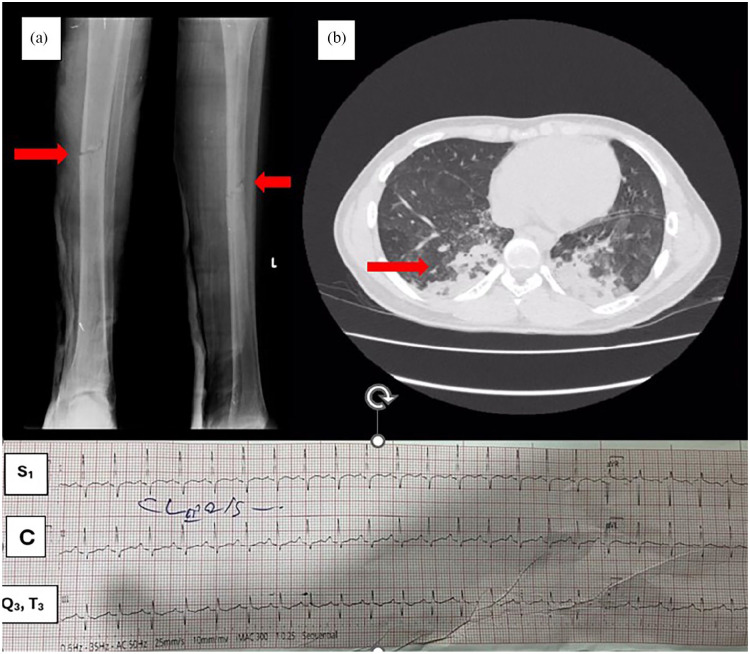
Figure 1.
(a) Left-side tibial mid-shaft fracture causing exposure of the bone marrow anteroposterior and lateral views. (b) Contrast-enhanced computerized tomography of the lung shows bilateral basal consolidations and ground glass opacifications suggestive of acute respiratory distress syndrome C-ECG showing S1, Q3, and T3 patterns.
Discussion
FES is a life-threatening disease affecting multiple organ systems. It results from fat emboli entering the bloodstream causing tissue damage. 8 Pathophysiology remains uncertain, but two mechanisms are often described: the mechanical theory, where intramedullary pressure increases and forces marrow to pass into injured venous sinusoids, causing fat droplets to be released into the venous system and then to the lungs and systemic vasculatures. The obstruction of pulmonary capillaries causes interstitial hemorrhage and alveolar collapse and it is the cause for hypoxemic vasoconstriction. Besides lung injury, these fat emboli enter the arterial circulation and can damage the other organs causing neurological and dermatological manifestations. The second theory is biochemical, based on a proinflammatory state where local hydrolysis of triglyceride emboli by tissue lipase produces glycerol and free fatty acids, which leads to the development of vasogenic and cytotoxic edema and hemorrhage responsible for acute lung injury because of their toxicity to endothelial cells and pneumocytes. 9
Major and minor diagnostic criteria for FES were proposed by Gurd and Wilson (Table 2). 7 Using this system, a diagnosis of FES could be made if one major feature and four minor features. 7 In our patient’s scenario, there was ARDS proven by contrast-enhanced computerized tomography of the chest (major) and tachycardia, tachypnoea, fever, sudden hemoglobin drop from 14.5 mg/dl to 9.5 mg/dl and sudden thrombocytopenia from 210,000 to 135,000 and urine fat globulins present. So, he fulfilled Gurd’s criteria. Schonfeld proposed the fat embolism index to aid in diagnosing FES (Table 3). 7 A cumulative score of five or more over the first 3 days of hospitalization corresponds with a diagnosis of FES. 4 Our patient had a score of 10 and fulfilled the Schonfeld index too. However, the sensitivity and specificity of these diagnostic criteria remain unknown but these are accepted worldwide. 10
Table 2.
Gurd and Wilson’s criteria.
| Major | Minor |
|---|---|
| Petechiae | Tachycardia |
| Respiratory symptoms (ARDS) with radiological changes | Pyrexia |
| Central nervous system signs unrelated to trauma or other condition | Retinal changes like fat or petechiae |
| Renal abnormalities like lipid urea, oliguria, or anuria | |
| Acute thrombocytopenia | |
| Acute decrease in hemoglobin | |
| High erythrocyte sedimentation rate | |
| Fat globules in sputum |
Table 3.
Schonfeld index.
| Feature | Points |
|---|---|
| Diffuse petechiae | 5 |
| Alveolar infiltrated | 4 |
| Hypoxemia (PaO2 <70 mmHg) | 3 |
| Confusion | 1 |
| Fever (>38°C) | 1 |
| Heart rate >120/min | 1 |
| Respiratory rate >30/min | 1 |
Laboratory test results are nonspecific for the diagnosis of FES. They may show acute anemia, thrombocytopenia, and ARDS. Identification of fat droplets in macrophages obtained during broncho-alveolar lavage may be a rapid method for diagnostic confirmation. 11
Prevention of FES includes early stabilization of long bone fractures and prophylactic corticosteroids. Treatment of FES is mainly supportive. The use of corticosteroids and heparin remains controversial. Possible beneficial effects of corticosteroids include stabilization of the pulmonary capillary membrane, thus reducing interstitial edema, blunting the inflammatory response, stabilizing complement system activation, and retarding platelet aggregation. Heparin is known to clear lipemic serum by stimulating lipase activity and hydrolyze the fat emboli in the lung, but it can potentiate and augment the pathogenesis of fat embolism by increasing free fatty chain production. So, giving heparin in fat embolism remains controversial.6,12
Our main differential diagnosis was PE with initial S1, Q3, T3 ECG patterns and sinus tachycardia, tachypnoea, and low saturation with positive D-dimers and the patient was started on unfractionated heparin, and oxygen support was given in the ICU setting. After the negative CTPA, a prophylactic dose was continued after weighing the risks vs benefits as the patient was not mobilizing. 12 Since there is no proven benefit, corticosteroids were not administered to our patient.
Laboratory tests are not specific for the diagnosis of FES. The mainstay of management of FES is organ support like supplying oxygen if the patient is hypoxic, maintaining blood volume with transfusions when necessary, and correcting hemodynamic status to control lung injury. NIV plays a major role in the management of fat embolism and if the initial trial of NIV is successful prognosis is better. If NIV is failing should consider mechanical ventilation without a delay.13,14 Patients with neurologic damage require close monitoring of intracranial pressure. 15
Fat embolism is a rare clinical manifestation as shown in a case-controlled study of the Japan Trauma Data Bank from 2004 to 2017, and the incidence of FES in trauma patients was 0.1%. 16 This case was a clinical conundrum as our patient presented with acute SOB with a typical ECG of S1, Q3, and T3 patterns, positive D-dimers, and without skin petechiae. But fat embolism also can rarely present with that type of ECG pattern. 14 Even though it is not very well identified in fat embolism, it can appear secondary to acute bronchospasms (in this case ARDS) or corpulmonale. 17 So high clinical suspicion should be there to diagnose fat embolism when respiratory distress occurs in a day or more after major trauma or orthopedic surgery to focus on the management of FES.
The outcome of patients with FES is generally favorable and pulmonary, neurological, and dermatologic manifestations of FES generally resolve completely. With supportive care, mortality has decreased with advances in supportive care and is <10% currently. 9
Conclusion
Without specific tests and validated clinical criteria, diagnosis of FES is challenging. One should have a high degree of suspicion despite small fractures not usually associated with FES. Although most patients recover fully, if not timely intervened mortality rates are high. Early diagnosis and treatment of symptoms are of paramount importance for a successful outcome.
Acknowledgments
We extend our thanks to the patient and her family who provided us with the consent to publish this case report.
Footnotes
Author contributions: C.M. is a physician trainee under the supervision of T.M., a consultant internal medicine specialist. R.W. is a consultant intensivist and A.K. is a consultant orthopedic surgeon who managed the patient during the ICU stay and in the ward. All the authors are involved in the management of the patient and the composition of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iDs: Chathuri Munagama  https://orcid.org/0009-0000-3506-8580
https://orcid.org/0009-0000-3506-8580
Rasanee Wanigasuriya  https://orcid.org/0009-0006-3685-7825
https://orcid.org/0009-0006-3685-7825
References
- 1. Bentaleb M, Abdulrahman M, Ribeiro-Junior MAF. Fat embolism: the hidden murder for trauma patients! Rev Col Bras Cir 2024; 51: e20243690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cleveland Clinic. Fat embolism syndrome: causes, symptoms & treatment, https://my.clevelandclinic.org/health/diseases/23995-fat-embolism-syndrome (2022, accessed 19 September 2024).
- 3. Rothberg DL, Makarewich CA. Fat embolism and fat embolism syndrome. J Am Acad Orthop Surg 2019; 27(8): e346–e355. [DOI] [PubMed] [Google Scholar]
- 4. Gregorakos L. Prolonged coma due to cerebral fat embolism: report of two cases. Emerg Med J 2000; 17(2): 144–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsai I-T, Hsu C-J, Chen Y-H, et al. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc 2010; 73(8): 407–410. [DOI] [PubMed] [Google Scholar]
- 6. Mellor A, Soni N. Fat embolism. Anaesthesia 2001; 56(2): 145–154. [DOI] [PubMed] [Google Scholar]
- 7. Grigorakos L, Nikolopoulos I, Stratouli S, et al. Fat embolism syndrome—Three case reports and review of the literature. J Trauma Inj 2017; 30(3): 107–111. [Google Scholar]
- 8. Pell ACH, Hughes DL, Keating JG, et al. Fulminating fat embolism syndrome caused by paradoxical embolism through a patent Foramen Ovale. N Engl J Med 1993; 329(13): 926–929. [DOI] [PubMed] [Google Scholar]
- 9. Kosova E, Bergmark B, Piazza G. Fat embolism syndrome. Circulation 2015; 131(3): 317–320. [DOI] [PubMed] [Google Scholar]
- 10. Scarpino M, Lanzo G, Lolli F, et al. From the diagnosis to the therapeutic management: cerebral fat embolism, a clinical challenge. Int J Gener Med 2019; 12: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chastre J, Fagon JY, Soler P, et al. Bronchoalveolar lavage for rapid diagnosis of the fat embolism syndrome in trauma patients. Ann Intern Med 1990; 113(8): 583–583. [DOI] [PubMed] [Google Scholar]
- 12. Soni KD, Aggarwal R, Jalwal G. Management of fat embolism co-existing with thromboembolism may be challenging! Burns Trauma 2014; 2(4): 206–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tucci MR, Costa ELV, Nakamura MAM, et al. Noninvasive ventilation for acute respiratory distress syndrome: the importance of ventilator settings. J Thorac Dis 2016; 8(9): E982–E986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ashok R, Kumar RV, Saravanan K, et al. Fat embolism syndrome managed by non-invasive ventilation–A case report. J Indian Med Assoc 2012; 110(2): 123–124. [PubMed] [Google Scholar]
- 15. Berdai AM, Shimi A, Khatouf M. Le syndrome d’embolie graisseuse post traumatique. Pan Afr Med J 2014; 17: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. www.uptodate.com . (n.d.). UpToDate. https://www.uptodate.com/contents/fat-embolism-syndrome#:~:text=Fat%20embolism%20syndrome%20(FES)%20is (accessed 1 May 2024).
- 17. Ali OM, Masood AM, Siddiqui F. Bedside cardiac testing in acute cor pulmonale. Case Rep 2014; 2014: bcr2013200940. [DOI] [PMC free article] [PubMed] [Google Scholar]