Abstract
Objectives:
This pharmacovigilance analysis was conducted to assess the safety signals of FMS-related tyrosine kinase 3 (FLT3) inhibitors in a real-world setting using the United States Food and Drug Administration Adverse Event Reporting System (FAERS).
Design:
We analyzed adverse event (AE) reports related to FLT3 inhibitors submitted to the FAERS database from the first quarter of 2015 to the fourth quarter of 2022. Disproportionality analysis was used to identify AEs of FLT3 inhibitors in the FAERS database.
Results:
A total of 55,393 AE reports were identified, of which 5938, 44,013, and 5442 were attributed to midostaurin, sorafenib, and gilteritinib, respectively, as primary suspects. Compared to the full database, significant safety signals at the system organ class level were observed for midostaurin (blood and lymphatic system disorders and hepatobiliary disorders), sorafenib (skin and subcutaneous tissue disorders and hepatobiliary disorders), and gilteritinib (investigations, blood and lymphatic system disorders, infections and infestations, and hepatobiliary disorders). All the drugs studied were associated with hepatobiliary disorders. The most prominent AEs associated with midostaurin, sorafenib, and gilteritinib were cytopenia, palmar-plantar erythrodysesthesia syndrome, and increased blast cell count, respectively. Compared with chemotherapy, midostaurin and gilteritinib showed a higher risk of electrocardiogram QT prolongation, gastrointestinal hemorrhage, cerebral hemorrhage, and increased white blood cell count. Gilteritinib had the highest overall death percentage (30.28%), whereas sorafenib had the lowest (23.06%).
Conclusion:
Mining AE signals using the FAERS database provides a method for analyzing the safety of FLT3 inhibitors in post-marketing. We found several significant AE signals that corresponded to previous studies; however, some AE signals were not mentioned in the drug instructions. Our study could provide a direction for follow-up real-world studies to verify the results further.
Keywords: drug safety, FAERS, FLT3 inhibitors, gilteritinib, midostaurin, sorafenib
Plain language summary
A study on the adverse effects of FLT3 inhibitors
Introduction:
The United States Food and Drug Administration Adverse Event Reporting System (FAERS) is an essential tool for the United States Food and Drug Administration (FDA) to detect adverse events (AE). This study explored the safety signals of FMS-related tyrosine kinase 3 (FLT3) inhibitors (midostaurin, sorafenib, and gilteritinib) using the FAERS database.
Research design and methods:
We used reporting odds ratios, proportional reporting ratios, and Bayesian confidence propagation neural network to analyze the safety signals of FLT3 inhibitors by comparing them with the full database and chemotherapy agents from 2015 to 2022.
Results:
A total of 5,938, 44,013, and 5,442 reports were attributed to midostaurin, sorafenib, and gilteritinib, respectively. Based on the analysis results, we observed the following:
• Regarding the analysis of system organ class level compared with the full database, “hepatobiliary disorders” appeared as an important signal in all three drugs. In addition, “blood and lymphatic system disorders” of midostaurin, “skin and subcutaneous tissue disorders” of sorafenib, and “investigations,” “blood and lymphatic system disorders,” and “infections and infestations” of gilteritinib were significant.
• Cytopenia was the most prominent AE associated with midostaurin in comparisons of midostaurin versus the full database, and electrocardiogram QT prolonged was the strongest signal in comparisons of midostaurin versus chemotherapy.
• In both comparisons of FLT3 inhibitors versus the full database and chemotherapy, the strongest safety signals of sorafenib and gilteritinib were palmar-plantar erythrodysesthesia syndrome and increased blast cell count, respectively.
• Gilteritinib exhibited the highest overall mortality rate, whereas sorafenib had the lowest.
Conclusion:
We identified several significant AE signals that corresponded to previous studies. However, some AE signals were not mentioned in the drug instructions. The AE signals should be evaluated further based on real-world data in the future.
Introduction
Acute myeloid leukemia (AML) is a hematopoietic malignancy characterized by cytogenetic and chromosomal aberrations. Among the frequently mutated genes in AML, FMS-related tyrosine kinase 3 (FLT3) stands out, with mutations such as internal tandem duplications (FLT3-ITD) and tyrosine kinase domain mutations occurring in approximately 30% of patients newly diagnosed AML patients.1,2 FLT3 encodes a receptor-type tyrosine kinase critical for hematopoietic stem cell differentiation, proliferation, and survival. 3 Patients with FLT3-ITD mutations have higher relapse rates and inferior survival rates, leading to their classification as adverse-risk according to the 2017 European LeukemiaNet (ELN) risk stratification. 4
FLT3 inhibitors are tyrosine kinase inhibitors that can be classified into first- and second-generation inhibitors based on their kinase specificity and potency. First-generation FLT3 inhibitors, including sorafenib, tandutinib, sunitinib, midostaurin, and lestaurtinib, lack specificity for FLT3 and inhibit multiple downstream receptor tyrosine kinases. 5 Second-generation FLT3 inhibitors, such as gilteritinib, specifically target FLT3 with greater selectivity and fewer off-target effects. To date, four drugs have been clinically used (midostaurin, sorafenib, sunitinib, and gilteritinib). Midostaurin is indicated for the treatment of adult patients with newly diagnosed AML that is FLT3 mutation positive. 6 Besides AML, sorafenib is used for hepatocellular carcinoma and thyroid carcinoma. 6 Gilteritinib, the first United States Food and Drug Administration (FDA) approved second-generation FLT3 inhibitor, is indicated for the treatment of relapsed or refractory AML and is recommended as a first-line treatment of AML in the National Comprehensive Cancer Network (NCCN) guidelines.6,7 Sunitinib is mainly used to treat patients with renal cell carcinoma and no patients with indications of AML were found in the database, which is different from other drugs and could potentially influence the study results. In this study, we investigated the safety signals of midostaurin, sorafenib, and gilteritinib.
An adverse event (AE) refers to any unfavorable and unintended signs, symptoms, or disease temporally associated with the use of a medicine, whether or not related to the medicine. In early drug safety evaluations, midostaurin commonly causes grade 3 or higher AEs, such as anemia and rash. Sorafenib is associated with grade 3 or higher AEs, including fever, diarrhea, bleeding, cardiac events, hand-foot syndrome (HFS), and rash. 8 Conversely, gilteritinib, commonly leads to serious cytopenia-related AEs, including febrile neutropenia, anemia, and thrombocytopenia. 9 With the increasing use of FLT3 inhibitors, more attention is being given to their AEs. Most safety studies on FLT3 inhibitors have been conducted as clinical trials, which may not fully reflect the safety issues that arise in real-world applications owing to their strict study designs.
A better understanding of the real-world safety profile of FLT3 inhibitors would improve patient compliance, reduce treatment interruptions, and lower treatment costs. The Food and Drug Adverse Event Reporting System (FAERS) is a publicly available database designed explicitly for post-marketing safety surveillance of all FDA-approved drugs and therapeutic biological products. 10 It enables the identification of potential associations between drugs and their AEs. 11 Given the increasing use of FLT3 inhibitors in clinical practice and the scarcity of evaluations of their adverse effects in real-world cohorts, we conducted a pharmacovigilance analysis to evaluate the safety profile of available FLT3 inhibitors using the FAERS database.
Methods
Data sources and standardization
A retrospective pharmacovigilance study was conducted using the FAERS database, an essential tool for the FDA to detect AEs, which is recognized as one of the largest sources of drug safety information. FAERS data were downloaded from the website (https://open.fda.gov/data/faers/). The FAERS database comprises seven tables (DEMO, DRUG, REAC, OUTC, PRSR, THER, and INDI) containing demographic information, drug details, and AEs. For our study, we extracted relevant variables, such as primaryid, caseid, drugname, and pt, covering the period from the first quarter of 2015 to the fourth quarter of 2022.
Before the data from FAERS could be utilized appropriately, it was necessary to address issues related to duplicate reports and inconsistent drug nomenclatures. To achieve this, we performed a thorough cleaning and standardization process that involved removing duplicate records, merging data, and employing standardized vocabulary and drug names mapped to the RxNorm concept using Medex_UIMA_1.8.3 and MYSQL (version 15.0) of Oracle corporation. Drug names were categorized into generic names to facilitate a comprehensive search. Our analysis focused on reports where the drug was designated as the primary suspect, indicated by the restricted role code “ps.”
AE outcomes were classified into three categories using the outcome code “OUTC_COD”: Death (DE); major events including Life-Threatening (LT), Hospitalization (HO), and Disability (DS); and other significant medical events (OT). AEs in FAERS were coded using the preferred term (PT) from the standard Medical Dictionary for Regulatory Activities (MedDRA), which encompasses 27 system organ classes (SOCs). However, certain SOCs such as “injury, poisoning, and connective tissue disorders,” “surgical and medical procedures,” and “social circumstances” were excluded as they were considered nondrug-related signals.
Statistical analysis
To identify potential safety signals for FLT3 inhibitors, disproportionality analyses were performed, including reporting odds ratio (ROR), proportional reporting ratio (PRR), and Bayesian confidence propagation neural network (BCPNN) analyses. The satisfaction of all three conditions was regarded as a significant safety signal, which helped mitigate false-positive signals. These analyses utilized two-by-two contingency tables to compare the reported AE counts for FLT3 inhibitors and all other drugs. When the targeted drug was found to have a higher likelihood of causing a specific AE than all other drugs, the ROR and PRR scores were typically higher, owing to greater disproportionality. The BCPNN employs a prior belief that incorporates a beta distribution, and the strength of the association between the drug-AE pair is defined by the information component. 12 Supplemental Tables 1 and 2 present the criteria and equations used for the algorithms mentioned above.
This study conformed to the STROBE statement. 13 All data were imported and extracted using MySQL 15.0 of Oracle Corporation and Navicat Premium 15 of PremiumSoft CyberTech Limited Company, and statistical analyses were performed using Microsoft Excel 2021.
Results
Descriptive analysis
From the first quarter of 2015 to the fourth quarter of 2022, 55,393 AE reports were recorded, of which 5938, 44,013, and 5442 were attributed to midostaurin, sorafenib, and gilteritinib, respectively, as the primary suspected drugs. Table 1 summarizes the clinical characteristics of patients associated with FLT3 inhibitors. The age range of patients using midostaurin and sorafenib was primarily focused on 45–74 years old (42.58% and 66.37%, respectively), whereas gilteritinib was more commonly used in children under 18 years of age than the other two drugs (25.62% vs 3.74% and 1.97%, respectively). Sorafenib was administered to more male patients than female patients (64.90% vs 31.96%). Medical staff constituted the main group of reporters for all three drugs (66.42%, 49.39%, and 65.29%, respectively). Reports on midostaurin and sorafenib were predominantly from the United States, whereas reports on gilteritinib mainly originated in Japan.
Table 1.
Information of patients with reported adverse events associated with FLT3 inhibitors.
| Characteristics | Midostaurin | Sorafenib | Gilteritinib |
|---|---|---|---|
| Number of reports, n | 5938 | 44,013 | 5442 |
| Gender, n (%) | |||
| Male | 2931 (42.6) | 28,565 (64.9) | 2510 (46.1) |
| Female | 2532 (49.4) | 14,065 (32.0) | 2634 (48.4) |
| Unknown or missing | 474 (8.0) | 1374 (3.1) | 298 (5.5) |
| Age (years), n (%) | |||
| <18 | 222 (3.7) | 865 (2.0) | 1394 (25.6) |
| 18–44 | 560 (9.4) | 2450 (6.0) | 710 (13.0) |
| 45–64 | 1606 (27.0) | 15,913 (36.2) | 916 (17.7) |
| 65–74 | 922 (15.5) | 13,298 (30.2) | 882 (16.2) |
| ⩾75 | 611 (10.3) | 6678 (15.2) | 944 (17.4) |
| Unknown or missing | 2017 (34.0) | 4809 (10.9) | 551 (10.1) |
| Reporter, n (%) | |||
| Medical staff | 3943 (66.4) | 21,740 (49.4) | 3553 (65.3) |
| Nonmedical staff | 1358 (22.9) | 17,780 (40.4) | 1633 (30.0) |
| Unknown or missing | 637 (10.7) | 4493 (10.2) | 256 (4.7) |
| Reporting year, n (%) | |||
| 2022 | 789 (13.3) | 2058 (4.7) | 1653 (30.4) |
| 2021 | 1346 (22.7) | 3029 (6.9) | 1930 (35.5) |
| 2020 | 1200 (20.2) | 4055 (9.2) | 1115 (20.5) |
| 2019 | 1086 (18.3) | 5982 (13.6) | 737 (13.5) |
| 2018 | 958 (16.1) | 9518 (21.6) | 7(0.1) |
| 2017 | 557 (9.4) | 7053 (16.0) | — |
| 2016 | 2 (<0.1) | 6161 (14.0) | — |
| 2015 | — | 6160 (14.0) | — |
| Indication, n (%) | |||
| Acute myeloid leukemia | 3017 (50.8) | 1283 (2.9) | 2961 (54.4) |
| Acute myeloid leukemia recurrent/refractory | 12 (0.2) | 214 (0.5) | 1645 (30.2) |
| Systemic mastocytosis | 1176 (19.8) | — | — |
| Hepatocellular carcinoma | — | 29,883 (67.9) | — |
| Others | 394 (6.6) | 11,975 (27.2) | 253 (4.6) |
| Unknown or missing | 1366 (23.0) | 2155 (4.9) | 583 (10.7) |
| Occurred country, n (%) | |||
| Japan | 2 (0.0) | 4525 (10.3) | 3405 (62.6) |
| United States | 2049 (34.5) | 15,148 (34.4) | 771 (14.2) |
| Germany | 364 (6.1) | 418(<0.1) | 144 (2.6) |
FLT3, FMS-related tyrosine kinase 3.
Analysis of SOCs disproportionality
To assess the adverse effects of FLT3 inhibitors, firstly, we compared FLT3 inhibitors with the full database. In the disproportionality analysis of SOCs, significant safety signals were identified. For midostaurin, sorafenib, and gilteritinib, the significant safety signals were blood and lymphatic system disorders (ROR: 6.5, 95% CI: 5.9–7.1) and hepatobiliary disorders (ROR: 2.6, 95% CI: 2.2–3.2); skin and subcutaneous tissue disorders (ROR: 2.5, 95% CI: 2.4–2.6) and hepatobiliary disorders (ROR: 3.1, 95% CI: 2.9–3.3); and investigations (ROR: 3.2, 95% CI: 2.9–3.4), blood and lymphatic system disorders (ROR: 9.8, 95% CI: 9.0–10.5), infections and infestations (ROR: 2.5, 95% CI: 2.3–2.7), and hepatobiliary disorders (ROR: 6.0, 95% CI: 5.2–6.8). These findings are presented in Table 2. Blood and lymphatic system disorders and hepatobiliary disorders were common to midostaurin and gilteritinib. Hepatobiliary disorders were common among the three drugs.
Table 2.
Detected significant safety signals on the system organ class level.
| System organ class | Count, n (%) | ROR (95% CI) | PRR (χ2) | IC (IC025) |
|---|---|---|---|---|
| Midostaurin | ||||
| Blood and lymphatic system disorders | 556 (9.4) | 6.5 (5.9–7.1) | 6.0 (2329.8) | 2.6 (2.4) |
| Hepatobiliary disorders | 123 (2.1) | 2.6 (2.2–3.2) | 2.6 (122.9) | 1.4 (1.1) |
| Sorafenib | ||||
| Skin and subcutaneous tissue disorders | 5464 (12.4) | 2.5 (2.4–2.6) | 2.3 (4205.6) | 1.2 (1.2) |
| Hepatobiliary disorders | 1074 (2.4) | 3.1 (2.9–3.3) | 3.1 (1508.2) | 1.6 (1.5) |
| Gilteritinib | ||||
| Investigations | 945 (17.4) | 3.2 (2.9–3.4) | 2.8 (1150.6) | 1.5 (1.4) |
| Blood and lymphatic system disorders | 733 (13.5) | 9.8 (9.0–10.5) | 8.8 (4976.8) | 3.1 (3.0) |
| Infections and infestations | 676 (12.4) | 2.5 (2.3–2.7) | 2.3 (514.0) | 1.2 (1.1) |
| Hepatobiliary disorders | 248 (4.6) | 6.0 (5.2–6.8) | 5.7 (977.2) | 2.5 (2.3) |
IC, information component; IC025, the lower limit of 95% CI of the IC; PRR, proportional reporting ratio; PRR, proportional reporting ratio; PT, preferred term; ROR, reporting odds ratio; χ2, chi-squared.
AE signal analysis
Further analysis was performed at the PT level to explore the association between each PT and different drugs. The top 50 AE signals, ranked according to frequency, are listed in Table 3. We considered the ROR as an indicator, and we found some AE not listed in the drug specification, such as bone marrow failure, graft-versus-host disease, and tumor lysis syndrome (TLS). In the analysis of drug-AE associations with FLT3 inhibitors, the frequent adverse safety signals for midostaurin included nausea, vomiting, and pyrexia. The highest ROR was observed for cytopenia, bone marrow failure, and graft-versus-host disease. For sorafenib, frequent adverse safety signals included diarrhea, fatigue, and palmar-plantar erythrodysesthesia syndrome, with the highest ROR observed for palmar-plantar erythrodysesthesia syndrome. Gilteritinib showed frequent adverse safety signals, such as platelet count decreased, pyrexia, and hepatic function abnormal. Among these signals, blast cell count increase was the strongest signal observed, despite the limited number of available records (n = 43). This was followed by cytopenia, graft-versus-host disease, and myelosuppression. These results were consistent with the SOC levels, and strong signals were observed for gilteritinib and midostaurin, but not significant for sorafenib.
Table 3.
Top 50 AEs induced by FLT3 inhibitors.

|
 ROR < 2;
ROR < 2;  ROR ≥ 2;
ROR ≥ 2;  ROR ≥ 10;
ROR ≥ 10;  ROR ≥ 20.
ROR ≥ 20.
Represents adverse events which ROR ≥ 2 and not listed in specification.
AE, adverse event; FLT3, FMS-related tyrosine kinase 3; PT, preferred term; ROR, reporting odds ratio.
To further assess the AE associated with FLT3 inhibitors, we compared the significant signals observed in Table 3 with those of chemotherapy. Surprisingly, the signal strengths of electrocardiogram QT prolongation, gastrointestinal hemorrhage, cerebral hemorrhage, and white blood cell count increase associated with midostaurin were found to be significantly higher than those versus the full database. The ROR values of sorafenib versus chemotherapy, compared with sorafenib versus the full database, indicated that none of the signals showed increased signal strength; however, palmar-plantar erythrodysesthesia syndrome, decreased appetite, hypertension, diarrhea, and hepatic function remained significant. The signal strengths of electrocardiogram QT prolongation, renal impairment, increased blood creatine phosphokinase, increased blast cell count, gastrointestinal hemorrhage, increased white blood cell count, and cerebral hemorrhage associated with gilteritinib were found to be significantly higher than those compared to the full database. Regardless of comparing FLT3 inhibitors with the full database and chemotherapy, the strongest safety signals for sorafenib and gilteritinib were palmar-plantar erythrodysesthesia syndrome and increased blast cell count, respectively (Tables 4–6).
Table 4.
Comparisons of midostaurin with the full database and chemotherapy.
| PT of midostaurin | Midostaurin versus full database | Midostaurin versus chemotherapy |
|---|---|---|
| Nausea | 3.2 (2.8–3.7) | 2.0 (1.7–2.2) |
| Vomiting | 3.4 (2.9–4.0) | 2.0 (1.7–2.4) |
| Pyrexia | 4.0 (3.4–4.8) | 2.4 (2.0–2.9) |
| Pneumonia | 3.4 (2.8–4.1) | 2.8 (2.3–3.4) |
| Thrombocytopenia | 9.3 (7.6–11.4) | 2.1 (1.7–2.5) |
| Febrile neutropenia | 14.9 (12.1–18.4) | 1.5 (1.2–1.8) |
| Neutropenia | 6.2 (4.9–7.7) | 1.4 (1.1–1.7) |
| Sepsis | 7.6 (6.0–9.5) | 2.8 (2.2–3.4) |
| Platelet count decreased | 6.1 (4.8–7.8) | 4.1 (3.2–5.3) |
| Anemia | 3.1 (2.3–4.0) | 1.2 (0.9–1.5) |
| Infection | 3.4 (2.6–4.5) | 1.9 (1.4–2.5) |
| Bone marrow failure | 19.1 (14.1–25.9) | 2.5 (1.8–3.4) |
| Pancytopenia | 8.7 (6.4–11.9) | 1.3 (0.9–1.8) |
| Cytopenia | 28.5 (20.6–39.4) | 6.4 (4.6–9.0) |
| White blood cell count decreased | 2.8 (2.0–4.0) | 1.7 (1.2–2.4) |
| Hemoglobin decreased | 3.2 (2.3–4.6) | 3.1 (2.2–4.4) |
| Cardiac failure | 3.56 (2.4–5.2) | 2.3 (1.5–3.4) |
| Electrocardiogram QT prolonged | 6.90 (4.6–10.3) | 18.1 (11.8–27.8) ↑ |
| General physical health deterioration | 2.09 (1.4–3.2) | 1.0 (0.7–1.5) |
| Gastrointestinal hemorrhage | 2.35 (1.5–3.6) | 3.8 (2.4–5.9) ↑ |
| Aspartate aminotransferase increased | 3.43 (2.0–5.8) | 2.1 (1.2–3.6) |
| Neutrophil count decreased | 3.38 (2.0–5.7) | 1.2 (0.7–2.1) |
| Alanine aminotransferase increased | 2.58 (1.5–4.4) | 1.4 (0.8–2.4) |
| Therapeutic response decreased | 2.43 (1.4–4.3) | 1.5 (0.9–2.7) |
| Graft-versus-host disease | 17.0 (9.1–31.6) | 6.1 (3.2–11.5) |
| Myelosuppression | 5.3 (2.8–9.9) | 0.6 (0.3–1.1) |
| Bacteremia | 9.0 (4.9–16.8) | 2.6 (1.4–4.8) |
| Liver disorder | 2.3 (1.2–4.4) | 1.1 (0.6–2.1) |
| Edema | 2.0 (1.1–3.9) | 1.8 (1.0–3.6) |
| Cerebral hemorrhage | 2.6 (1.3–5.1) | 4.3 (2.1–8.8) ↑ |
| Bronchopulmonary aspergillosis | 10.1 (5.1–20.3) | 3.1 (1.5–6.2) |
| White blood cell count increased | 2.1 (1.0–4.4) | 2.4 (1.2–5.2) ↑ |
| Tumor lysis syndrome | 3.3 (1.1–10.3) | 0.5 (0.2–1.6) |
Data are RORs (95% CI) of PT of midostaurin compared with the full database and chemotherapy; Bold text: ROR of midostaurin versus chemotherapy ≥ 2 and the lower limit of 95% CI > 1; ↑: ROR of midostaurin versus chemotherapy higher than midostaurin versus full database.
PT, preferred term; ROR, reporting odds ratio.
Table 5.
Comparisons of sorafenib with the full database and chemotherapy.
| PT of sorafenib | Sorafenib versus full database | Sorafenib versus chemotherapy |
|---|---|---|
| Diarrhea | 3.0 (2.9–3.2) | 2.1 (2.0–2.3) |
| Palmar-plantar erythrodysesthesia syndrome | 59.1 (55.3–63.2) | 5.3 (5.0–5.7) |
| Decreased appetite | 4.9 (4.5–5.2) | 3.9 (3.6–4.2) |
| Hypertension | 3.1 (2.8–3.4) | 3.0 (2.8–3.4) |
| General physical health deterioration | 2.8 (2.4–3.2) | 1.3 (1.2–1.5) |
| Hepatic function abnormal | 4.6 (3.9–5.6) | 2.1 (1.7–2.6) |
| Aspartate aminotransferase increased | 3.3 (2.7–4.0) | 1.9 (1.6–2.4) |
| Alanine aminotransferase increased | 2.5 (2.0–3.1) | 1.3 (1.0–1.6) |
| Liver disorder | 2.9 (2.3–3.5) | 1.4 (1.1–1.8) |
| Bone marrow failure | 3.2 (2.5–4.2) | 0.4 (0.3–0.5) |
| Tumor lysis syndrome | 3.3 (2.2–5.1) | 0.5 (0.3–0.8) |
| Graft-versus-host disease | 2.8 (1.6–5.0) | 1.0 (0.6–1.8) |
Data are RORs (95% CI) of PT of sorafenib compared with the full database and chemotherapy; Bold text: ROR of sorafenib versus chemotherapy ≥ 2 and the lower limit of 95% CI > 1.
PT, preferred term; ROR, reporting odds ratio.
Table 6.
Comparisons of gilteritinib with the full database and chemotherapy.
| PT of gilteritinib | Gilteritinib versus full database | Gilteritinib versus chemotherapy |
|---|---|---|
| Platelet count decreased | 19.7 (17.1–22.8) | 13.7 (11.8–15.6) |
| Pyrexia | 5.42 (4.6–6.4) | 3.2 (2.8–3.8) |
| Hepatic function abnormal | 46.4 (39.2–55.0) | 21.9 (18.2–26.3) |
| Myelosuppression | 60.7 (51.2–71.9) | 7.2 (6.0–8.5) |
| Pneumonia | 4.9 (5.8–4.1) | 4.0 (3.3–4.7) |
| Cytopenia | 97.4 (81.4–116.6) | 23.3 (19.1–28.3) |
| Febrile neutropenia | 19.1 (15.8–23.2) | 1.8 (1.5–2.2) |
| Neutrophil count decreased | 26.5 (21.8–32.2) | 9.7 (7.9–11.8) |
| Infection | 6.0 (4.8–7.4) | 3.4 (2.7–4.3) |
| Anemia | 5.0 (4.0–6.2) | 1.8 (1.5–2.3) |
| Electrocardiogram QT prolonged | 20.1 (15.6–25.8) | 50.3 (37.2–68.1) ↑ |
| Neutropenia | 4.8 (3.7–6.2) | 1.2 (0.9–1.5) |
| White blood cell count decreased | 5.4 (4.2–7.0) | 3.3 (2.5–4.2) |
| Thrombocytopenia | 6.6 (5.1–8.5) | 1.5 (1.2–2.0) |
| Liver disorder | 15.0 (11.5–19.6) | 7.5 (5.6–9.8) |
| Sepsis | 6.1 (4.6–7.9) | 2.2 (1.7–2.9) |
| Pancytopenia | 13.2 (10.1–17.3) | 2.0 (1.5–2.6) |
| Renal impairment | 6.3 (4.8–8.24) | 6.4 (4.8–8.5) ↑ |
| Blood creatine phosphokinase increased | 28.4 (21.4–37.8) | 56.8 (40.0–80.7) ↑ |
| Bone marrow failure | 26.3 (19.6–35.3) | 3.4 (2.5–4.6) |
| Blood lactate dehydrogenase increased | 41.4 (30.6–55.9) | 20.9 (15.1–29.0) |
| Blast cell count increased | 406.7 (556.2–297.4) | 771.2 (328.1–1812.7) ↑ |
| Graft-versus-host disease | 61.5 (43.6–86.7) | 22.6 (15.5–32.9) |
| Aspartate aminotransferase increased | 8.4 (5.9–11.9) | 5.4 (3.8–7.7) |
| Gastrointestinal hemorrhage | 4.8 (3.3–6.8) | 6.8 (4.6–9.8) ↑ |
| Alanine aminotransferase increased | 5.7 (3.9–8.4) | 3.1 (2.1–4.6) |
| White blood cell count increased | 8.6 (5.8–12.7) | 9.8 (6.5–14.8) ↑ |
| General physical health deterioration | 2.4 (1.6–3.6) | 1.1 (0.7–1.6) |
| Blood creatinine increased | 4.4 (2.9–6.6) | 4.1 (2.7–6.2) |
| Cerebral hemorrhage | 9.4 (6.3–14.1) | 15.4 (10.0–23.6) ↑ |
| Bacteremia | 23.6 (15.8–35.2) | 6.6 (4.4–10.0) |
| Tumor lysis syndrome | 27.0 (17.9–40.7) | 4.5 (3.0–6.9) |
| Disseminated intravascular coagulation | 26.1 (17.3–39.3) | 9.2 (6.0–14.0) |
| Cardiac failure | 3.1 (2.0–4.8) | 2.0 (1.2–3.0) |
| Edema | 5.0 (3.2–7.8) | 4.9 (3.1–7.7) |
| Liver function test increased | 7.0 (4.4–11.2) | 1.4 (0.9–2.3) |
| Interstitial lung disease | 4.2 (2.6–6.6) | 1.0 (0.6–1.6) |
| Bronchopulmonary aspergillosis | 24.0 (15.1–38.1) | 7.6 (4.7–12.4) |
| Therapeutic response decreased | 3.9 (2.4–6.2) | 2.4 (1.5–3.8) |
Data are RORs (95% CI) of PT of gilteritinib compared with the full database and chemotherapy; Bold text: ROR of gilteritinib versus chemotherapy ≥ 2 and the lower limit of 95% CI > 1; ↑: ROR of gilteritinib versus chemotherapy higher than gilteritinib versus full database.
PT, preferred term; ROR, reporting odds ratio.
Outcomes and mortality rates associated with FLT3 inhibitors
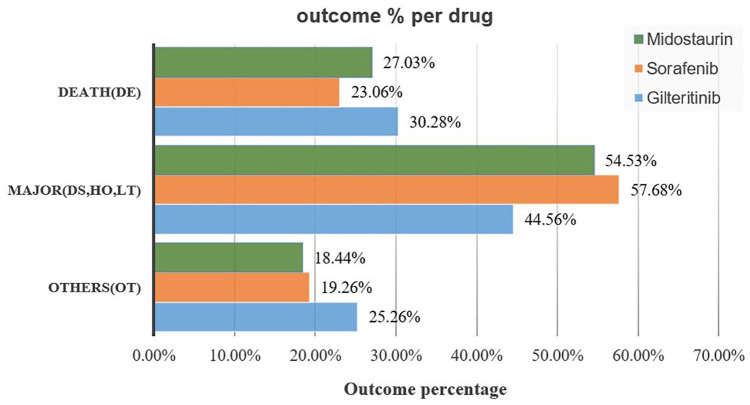
Gilteritinib had the highest percentage of overall deaths among the studied drugs (30.28%), whereas sorafenib had the lowest percentage of overall deaths (23.06%; Figure 1). Safety concerns associated with FLT3 inhibitors prompted further analysis of the potential causes of fatality.
Figure 1.
Outcomes for adverse events associated with FLT3 inhibitors.
DE, death, DS, disability; FLT3, FMS-related tyrosine kinase 3; HO, hospitalization; LT, life-threatening; OT, other.
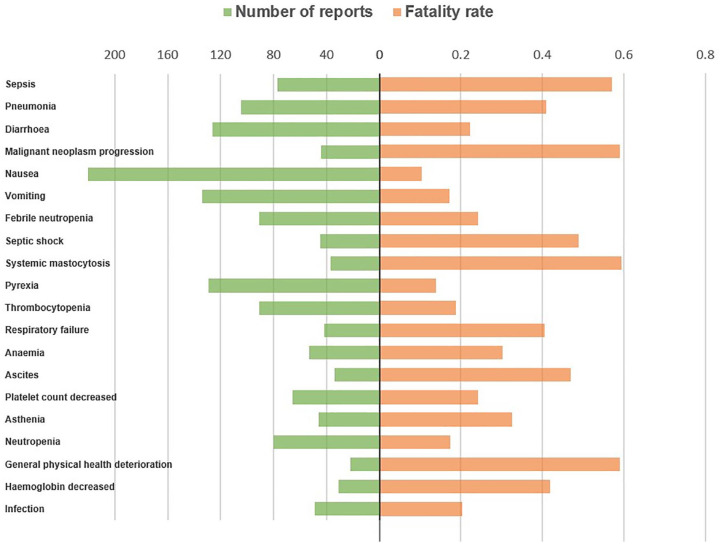
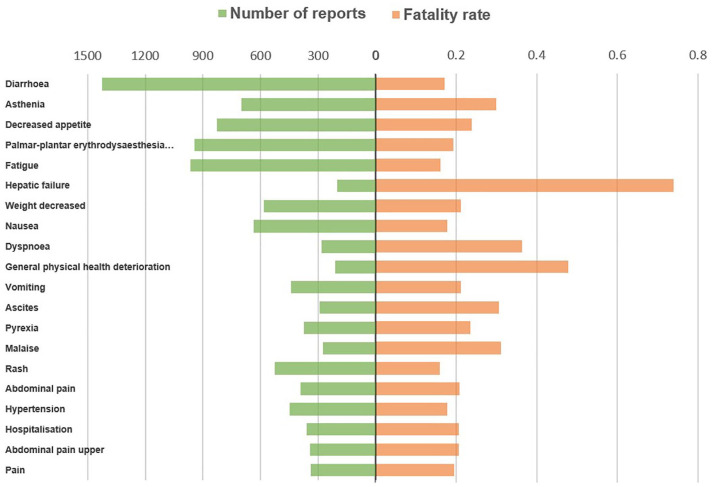
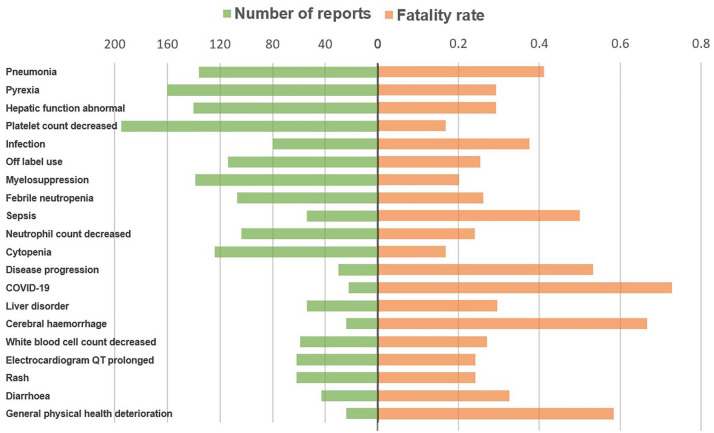
The most common AEs associated with overall death in patients administered midostaurin, sorafenib, and gilteritinib were sepsis, pneumonia, and diarrhea (Figure 2); diarrhea, asthenia, and decreased appetite (Figure 3); and pneumonia, pyrexia, and abnormal hepatic function (Figure 4), respectively.
Figure 2.
Number of reports and fatalities of the top 20 AEs associated with midostaurin according to overall death reports.
AE, adverse event.
Figure 3.
Number of reports and fatalities of the top 20 AEs associated with sorafenib according to overall death reports.
AE, adverse event.
Figure 4.
Number of reports and fatalities of the top 20 AEs associated with gilteritinib according to overall death reports.
AE, adverse event.
Discussion
Pharmacovigilance data mining from the post-marketing AE database could provide valuable Supplemental Information to drug instructions due to the limited preclinical data. 14 Our study first analyzed the potential link between FLT3 inhibitors and AEs based on FAERS and further compared the safety of midostaurin, sorafenib, and gilteritinib. Compared with the full database, the level of organ systems showed that hepatobiliary disorders were associated with all three drugs, while blood and lymphatic system disorders were common to midostaurin and gilteritinib. Sorafenib showed significant safety signals in the skin and subcutaneous tissue disorders.
Regarding hepatobiliary disorders, three drugs all exhibited significant signals of abnormal hepatic function compared with the full database. In a phase III trial of gilteritinib for relapsed or refractory FLT3-mutated AML, 15 41.9% of patients experienced increased alanine aminotransferase levels, and 40.2% of patients experienced increased aspartate aminotransferase levels, which were notably higher than those in the chemotherapy group. Hepatobiliary disease and increased transaminase levels were also the most frequent AEs resulting in the discontinuation of midostaurin. 16 In a drug-induced liver injury (DILI) AE analysis, 17 sorafenib was most frequently associated with DILI based on the Japanese Adverse Event Reporting (JADER) database. The mechanism underlying hepatobiliary disorders remains unclear but may be attributed to the fact that FLT3 inhibitors are primarily metabolized by the liver. Considering that most patients treated with midostaurin and sorafenib were older adults, the concentrations may accumulate in older adults owing to decreased metabolism, increasing the risk of hepatic toxicity. The predominant use of sorafenib in hepatocellular carcinoma may have introduced potential bias in the study results. The age distribution of gilteritinib was more common in children and young people. However, both the comparison of gilteritinib versus the full database and chemotherapy indicated that liver disorders showed a strong signal strength, indicating that gilteritinib probably carries a high risk of hepatotoxicity.
Both midostaurin and gilteritinib demonstrated significant signals for blood system disorders. PT level analysis compared with the full database revealed that cytopenia, febrile neutropenia, bone marrow failure, and graft-versus-host disease were common strong AE signals for midostaurin and gilteritinib, consistent with the results of clinical trials. This study highlights that the main toxic effect of midostaurin and gilteritinib is myelosuppression. Gotlib et al. 18 reported that the most common grade 3 or higher AEs of midostaurin were hematological abnormalities, with neutropenia, anemia, and thrombocytopenia occurring in 41%, 24%, and 29% of patients, respectively. In the phase III ADMIRAL study, 15 the most common grade 3 or higher AEs in the gilteritinib group were febrile neutropenia (45.9%), anemia (40.7%), and thrombocytopenia (22.8%). Notably, the results, compared with those of chemotherapy, showed that midostaurin and gilteritinib had a higher risk of gastrointestinal and cerebral hemorrhage, which may be influenced by cytopenia. Considering that midostaurin and gilteritinib are primarily used to treat AML, blood system disorders may be influenced by primary disease progression.
Furthermore, cardiac disorders are notable AEs associated with midostaurin and gilteritinib. Comparisons of midostaurin and gilteritinib versus the full database and chemotherapy showed strong signal strengths in electrocardiogram QT prolongation. The QTc interval represents the time between depolarization and repolarization of ventricular myocytes, and QT intervals >440 ms are considered pathologically prolonged. 19 QT prolongation has been identified as the dose-limiting toxicity of these drugs, leading to dose modification or treatment discontinuation. 20 McMahon and Perl 21 reported that QT prolongation occurred in 13% of midostaurin-treated patients and 4% of gilteritinib-treated patients. Kim et al. 22 suggested that the cardiotoxicity associated with FLT3 inhibitors may be attributed to the expression of FLT3 and FLT3 ligands in cardiomyocytes and proposed that the activation of FLT3 signaling may serve as a cardioprotective anti-apoptotic system.
Most AEs associated with sorafenib were grade 1–2. Regardless of the comparisons between sorafenib and the full database or sorafenib and chemotherapy, palmar-plantar erythrodysesthesia syndrome remained the strongest AE signal. Palmar-plantar erythrodysesthesia syndrome, also known as HFS, is a dose-limiting cutaneous toxicity of sorafenib. The clinical characteristics of patients with HFS include a decreased sensation of temperature and pain and well-demarcated plaques of palmoplantar erythema and edema. 23 Clinical trials have reported HFS in 10%–62% of sorafenib-treated patients. 24 In addition to inhibiting FLT3, sorafenib is a vascular endothelial growth factor receptor inhibitor, which may contribute to the occurrence of HFS. 25 The correlation of sorafenib with the risk of HFS requires further clinical studies.
In addition to the safety signals shared with midostaurin, gilteritinib exhibited a higher risk of decreased neutrophil count, increased blast cell count, increased blood creatine phosphokinase, disseminated intravascular coagulation (DIC), and TLS. In both comparisons of gilteritinib versus the full database and chemotherapy, the strongest safety signal associated with gilteritinib was increased blast cell count. It is likely related to the progression of the primary disease because gilteritinib is indicated for the treatment of relapsed or refractory AML, which has a higher risk of progress. DIC and TLS were not mentioned in the drug instructions, and they showed significant signals in our results. In a study of gilteritinib in 24 Japanese patients with AML, 26 9 patients experienced increased blood creatine phosphokinase levels, 3 patients developed DIC, and 1 patient in the 120-mg dose group experienced TLS. Increased blood creatine phosphokinase and TLS were found to be dose-dependent, warranting dose reduction or delay when necessary. Gilteritinib was also associated with decreased platelet count (ROR: 19.7, 95% CI: 17.1–22.8). The connection between DIC, TLS, and gilteritinib needed to be verified with more clinical studies.
Some AEs associated with FLT3 inhibitors were fatal, which is a critical concern in clinical practice. In contrast to previous studies that demonstrated that first-generation inhibitors (sorafenib and midostaurin) are less specific for FLT3 and have more off-target toxicities, 27 our study found that sorafenib exhibited greater safety among the three drugs studied, with the lowest overall death percentage (23.06%). In contrast, gilteritinib had the highest percentage (30.28%). However, gilteritinib is approved by the FDA and the European Medicines Agency (EMA) for treating adult patients who have relapsed or refractory AML with an FLT3 mutation, while midostaurin is indicated for treating newly diagnosed AML.6,28 The prognosis in relapsed or refractory AML is generally poor, which may cause the highest fatal rate of gilteritinib. 15 Pneumonia and sepsis were the major causes of overall death associated with midostaurin and gilteritinib. Usuki et al. 26 reported pneumonia as a drug-related AE leading to discontinuation of gilteritinib. In the Tomlinson study, 29 grade 3 infectious events were common, occurring in 42% of patients treated with midostaurin. Both midostaurin and gilteritinib can cause myelosuppression, increasing the risk of infection. A case study by Gozzo et al. 27 reported the death of a 38-year-old female patient who received gilteritinib treatment. The patient developed a fever, and blood analysis revealed severe pancytopenia, eventually leading to carbapenemase-producing Klebsiella pneumoniae bacteremia and multi-organ failure, resulting in the patient’s death 4 months after gilteritinib treatment. For sorafenib, the most common cause of overall death was diarrhea. Cheng et al. 30 reported that diarrhea was the most frequently reported grade 3/4 drug-related AE (6.0%) after sorafenib treatment. Diarrhea can lead to severe complications, including dehydration, malnutrition, renal insufficiency, and an increased risk of infection, posing a life-threatening situation for patients. The pathogenesis of sorafenib-induced diarrhea remains under investigation; however, it is believed to involve multiple mechanisms. One theory suggests that inhibition of epidermal growth factor receptors may inhibit epithelial repair. 31 Probiotics may be effective in preventing or treating chemotherapy- and radiotherapy-induced diarrhea. 32
Limitations
This study had several limitations. First, FAERS is a spontaneous reporting system that does not require a causal relationship between a drug and an adverse drug reaction,14,33 and the ROR and PRR are merely indicators of signal strength and cannot reflect direct causality. 34 Second, AEs influenced by the primary disease could not be eliminated, which may have resulted in the overestimating of certain relevant positive adverse signals. 35 Further, the distribution of the AEs may be affected by the differences in populations using the studied drug. Lastly, owing to the limited marketing time, our study only analyzed and compared the safety signals of midostaurin, sorafenib, and gilteritinib. Therefore, more clinical studies and long-term data are needed to validate these results and further understand the safety profile of FLT3 inhibitors in future research.
Conclusion
In conclusion, utilizing the FAERS database to mine AE signals provides a method for analyzing the safety of FLT3 inhibitors in post-marketing. Our study aimed to identify safety signals linked to FLT3 inhibitors. All the three drugs examined in this study were associated with hepatobiliary disorders. Patients receiving gilteritinib and midostaurin treatment demonstrated a higher susceptibility to blood system disorders, infections, and cardiac disorders, whereas sorafenib use was more likely to result in skin and subcutaneous tissue disorders. Our study showed DIC and TLS might associate with the treatment of gilteritinib. DIC and TLS were not mentioned in the drug instructions, which require further investigation. Our study could increase awareness of the potential drug-related risks associated with the use of FLT3 inhibitors. However, AE signals detected from FAERS require follow-up studies based on real-world data and our study could provide a direction for further research.
Supplemental Material
Supplemental material, sj-docx-1-taw-10.1177_20420986241284105 for A disproportionality analysis for assessing the safety of FLT3 inhibitors using the FDA Adverse Event Reporting System (FAERS) by Jie Zhou, Jinping Zhang, Qiaoyun Wang, Miaoxin Peng, Yun Qian, Fang Wu, Qi Rao, Laji DanZhen, Yonggong Yang, Siliang Wang and Mengying Liu in Therapeutic Advances in Drug Safety
Acknowledgments
None.
Footnotes
ORCID iD: Siliang Wang  https://orcid.org/0000-0003-1228-869X
https://orcid.org/0000-0003-1228-869X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jie Zhou, Department of Pharmacy, Nanjing Drum Tower Hospital, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, China.
Jinping Zhang, Department of Pharmacy, Nanjing Drum Tower Hospital, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, China; Department of Pharmacy, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China.
Qiaoyun Wang, Department of Pharmacy, Nanjing Drum Tower Hospital, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, China.
Miaoxin Peng, Department of Hematology, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China.
Yun Qian, Department of Dermatologic Surgery, Institute of Dermatology, Chinese Academy of Medical Science and Peking Union Medical College, Nanjing, China.
Fang Wu, Department of Pharmacy, Nanjing Drum Tower Hospital, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, China.
Qi Rao, Department of Pharmacy, Nanjing Drum Tower Hospital, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, China.
Laji DanZhen, Department of Pharmacy, Nanjing Drum Tower Hospital, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, China.
Yonggong Yang, Department of Hematology, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, 321 Zhongshan Road, Nanjing 210008, China.
Siliang Wang, Department of Pharmacy, Nanjing Drum Tower Hospital, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, 321 Zhongshan Road, Nanjing 210008, China; Department of Pharmacy, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, 321 Zhongshan Road, Nanjing University, Nanjing, China.
Mengying Liu, Department of Pharmacy, Nanjing Drum Tower Hospital, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, 321 Zhongshan Road, Nanjing 210008, China; Department of Pharmacy, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, 321 Zhongshan Road, Nanjing University, Nanjing, China.
Declarations
Ethics approval and consent to participate: This safety study was exempt from Institutional Review Board approval and informed patient consent because the study was observational, using a global open database (FAERS) with anonymized information, and not involve treatment intervention or collection of human samples.
Consent for publication: Not applicable.
Author contributions: Jie Zhou: Data curation; Writing – original draft.
Jinping Zhang: Resources; Supervision; Writing – review & editing.
Qiaoyun Wang: Methodology; Software; Writing – review & editing.
Miaoxin Peng: Funding acquisition; Project administration; Writing – review & editing.
Yun Qian: Methodology; Writing – review & editing.
Fang Wu: Formal analysis; Writing – original draft.
Qi Rao: Data curation; Resources; Writing – original draft.
Laji Danzhen: Methodology; Writing – review & editing.
Yonggong Yang: Funding acquisition; Investigation; Writing – review & editing.
Siliang Wang: Investigation; Project administration; Writing – review & editing.
Mengying Liu: Funding acquisition; Validation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by funding for Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University [grant number 2022-LCYJ-PY-48 and 2023-LCYJ-MS-32]; Chinese Pharmaceutical Association Hospital Pharmacy Department [grant number CPA-Z05-ZC-2023002]; Jiangsu Research Hospital Association for Precision Medication [grant number JY202113]; Nanjing Medical Insurance Research Association [grant number NJYB2023YL006].
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: We used publicly available data from FAERS that can be accessed at: https://open.fda.gov/data/faers/.
References
- 1. Daver N, Perl AE, Maly J. Venetoclax plus gilteritinib for FLT3-mutated relapsed/refractory acute myeloid leukemia. J Clin Oncol 2022; 40(35): 4048–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu H. Emerging agents and regimens for AML. J Hematol Oncol 2021; 14(1): 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao J, Song Y, Liu D. Gilteritinib: a novel FLT3 inhibitor for acute myeloid leukemia. Biomarker Res 2019; 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu M, Li C, Zhu X. FLT3 inhibitors in acute myeloid leukemia. J Hematol Oncol 2018; 11(1): 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao JC, Agarwal S, Ahmad H, et al. A review of FLT3 inhibitors in acute myeloid leukemia. Blood Rev 2022; 52: 100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. United States Food and Drug Administration. Drug approvals and databases, https://www.fda.gov/drugs (2022, accessed 21 December 2023).
- 7. Pollyea DA, Altman JK, Assi R, et al. Acute myeloid leukemia, Version 3.2023, NCCN Clinical Practice Guidelines in Oncology. J National Comprehensive Cancer Network. 2023; 21(5): 503–513. [DOI] [PubMed] [Google Scholar]
- 8. Gounder MM, Mahoney MR, Van Tine BA, et al. Sorafenib for advanced and refractory desmoid tumors. N Engl J Med 2018; 379(25): 2417–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perl AE, Larson RA, Podoltsev NA, et al. Follow-up of patients with R/R FLT3-mutation-positive AML treated with gilteritinib in the phase 3 ADMIRAL trial. Blood 2022; 139(23): 3366–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aggarwal P. Disproportionality analysis of bullous pemphigoid adverse events with PD-1 inhibitors in the FDA adverse event reporting system. Expert Opin Drug Saf 2019; 18(7): 623–633. [DOI] [PubMed] [Google Scholar]
- 11. Peng L, Xiao K, Ottaviani S, et al. A real-world disproportionality analysis of FDA Adverse Event Reporting System (FAERS) events for baricitinib. Expert Opin Drug Saf 2020; 19(11): 1505–1511. [DOI] [PubMed] [Google Scholar]
- 12. Ang PS, Chen Z, Chan CL, et al. Data mining spontaneous adverse drug event reports for safety signals in Singapore - a comparison of three different disproportionality measures. Expert Opin Drug Saf 2016; 15(5): 583–590. [DOI] [PubMed] [Google Scholar]
- 13. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4(10): 1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wan Q, Li Q, Lai X, et al. Data mining and safety analysis of BTK inhibitors: a pharmacovigilance investigation based on the FAERS database. Front Pharmacol 2022; 13: 995522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med 2019; 381(18): 1728–1740. [DOI] [PubMed] [Google Scholar]
- 16. Dohner H, Weber D, Krzykalla J, et al. Midostaurin plus intensive chemotherapy for younger and older patients with AML and FLT3 internal tandem duplications. Blood Adv 2022; 6(18): 5345–5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamimura H, Setsu T, Kimura N, et al. Analysis of drug-induced liver-related adverse event trend reporting between 1997 and 2019. Hepatol Res 2023; 53(6): 556–568. [DOI] [PubMed] [Google Scholar]
- 18. Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med 2016; 374(26): 2530–2541. [DOI] [PubMed] [Google Scholar]
- 19. Chai S, Zhan JL, Zhao LM, et al. Safety of triazole antifungals: a pharmacovigilance study from 2004 to 2021 based on FAERS. Therap Adv Drug Saf 2022; 13: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perl AE, Altman JK, Cortes J, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol 2017; 18(8): 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McMahon CM, Perl AE. Gilteritinib for the treatment of relapsed and/or refractory FLT3-mutated acute myeloid leukemia. Expert Rev Clin Pharmacol 2019; 12(9): 841–849. [DOI] [PubMed] [Google Scholar]
- 22. Kim L, Fowler B, Campbell CM, et al. Acute cardiotoxicity after initiation of the novel tyrosine kinase inhibitor gilteritinib for acute myeloid leukemia. Cardiooncology 2021; 7(1): 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller KK, Gorcey L, McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol 2014; 71(4): 787–794. [DOI] [PubMed] [Google Scholar]
- 24. Lipworth AD, Robert C, Zhu AX. Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): focus on sorafenib and sunitinib. Oncology. 2009; 77(5): 257–271. [DOI] [PubMed] [Google Scholar]
- 25. Ishak RS, Aad SA, Kyei A, et al. Cutaneous manifestations of anti-angiogenic therapy in oncology: review with focus on VEGF inhibitors. Crit Rev Oncol Hematol 2014; 90(2): 152–164. [DOI] [PubMed] [Google Scholar]
- 26. Usuki K, Sakura T, Kobayashi Y, et al. Clinical profile of gilteritinib in Japanese patients with relapsed/refractory acute myeloid leukemia: an open-label phase 1 study. Cancer Science 2018; 109(10): 3235–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gozzo L, Nardo A, Brancati S, et al. Severe gastrointestinal toxicity following the use of gilteritinib: a case series and analysis of postmarketing surveillance data. Healthcare (Basel) 2023; 11(10): 1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. The European Medicines Agency. Medicines, https://www.ema.europa.eu/en/medicines (2023, accessed 21 December 2023).
- 29. Tomlinson BK, Gallogly MM, Kane DM, et al. A phase II study of midostaurin and 5-azacitidine for untreated elderly and unfit patients with FLT3 wild-type acute myelogenous leukemia. Clin Lymphoma Myeloma Leuk 2020; 20(4): 226–233. [DOI] [PubMed] [Google Scholar]
- 30. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10(1): 25–34. [DOI] [PubMed] [Google Scholar]
- 31. Andreyev J, Ross P, Donnellan C, et al. Guidance on the management of diarrhoea during cancer chemotherapy. Lancet Oncol 2014; 15(10): 447–460. [DOI] [PubMed] [Google Scholar]
- 32. Wei D, Heus P, van de Wetering FT, et al. Probiotics for the prevention or treatment of chemotherapy- or radiotherapy-related diarrhoea in people with cancer. Cochrane Database Syst Rev 2018; 8(8): CD008831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teng C, Reveles KR, Obodozie-Ofoegbu OO, et al. Clostridium difficile infection risk with important antibiotic classes: an analysis of the FDA adverse event reporting system. Int J Med Sci 2019; 16(5): 630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Inoue M, Matsumoto K, Tanaka M, et al. Analysis of chemotherapy-induced peripheral neuropathy using the Japanese Adverse Drug Event Report database. Sci Rep 2021;11: 11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shu Y, He X, Liu Y, et al. A real-world disproportionality analysis of olaparib: data mining of the public version of FDA Adverse Event Reporting System. Clin Epidemiol 2022; 14: 789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taw-10.1177_20420986241284105 for A disproportionality analysis for assessing the safety of FLT3 inhibitors using the FDA Adverse Event Reporting System (FAERS) by Jie Zhou, Jinping Zhang, Qiaoyun Wang, Miaoxin Peng, Yun Qian, Fang Wu, Qi Rao, Laji DanZhen, Yonggong Yang, Siliang Wang and Mengying Liu in Therapeutic Advances in Drug Safety