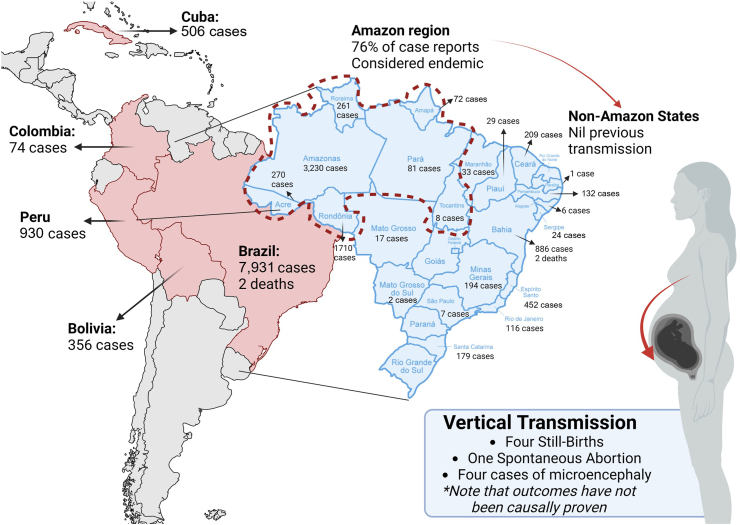
Oropouche fever, caused by the Oropouche orthobunyavirus (OROV), represents a significant yet often overlooked public health issue in South and Central America, particularly in northern Brazil.1 The virus has been reported in Brazil, Bolivia, Colombia, Cuba, and Peru, totalling 9852 cases as of September 6, 2024, with Brazil accounting for over 80.5% (7931) of those cases (Fig. 1).2 Up to September 7, 2024, the Ministry of Health of Brazil has reported 7931 cases of OROV infection, 3230 of them in the Amazonas state (40.72%), 1710 in Rondonia state (21.56%), and 886 in Bahia (11.17%).3 Furthermore, OROV belongs to the Simbu serogroup within the genus Orthobunyavirus, the largest genus of RNA viruses with over 170 named viruses across 18 serogroups and 48 species complexes. The Simbu serogroup includes 22 recognised viruses grouped into seven species complexes: Akabane, Manzanilla, Oropouche, Sathuperi, Simbu, Shamonda, and Shuni. Phylogenetic analysis of the S RNA, which encodes the nucleocapsid protein, has identified four major OROV genotypes (I-IV). Genotype I has been reported in Acre, Amazonas, Maranhão, and Pará in Brazil, as well as in French Guiana. Genotype II occurs in Amapá, Brazil. Genotype III is present in Pará, Brazil, and Panama, while Genotype IV exists in Rondônia, Brazil. Genomic reassortment, where related viruses exchange genetic segments, plays a key role in orthobunyavirus biodiversity. Typically, the S and L segments (coding for nucleocapsid protein and RNA polymerase) are inherited together, while the M segment (coding for viral glycoproteins) mutates more easily under selective pressure.4
Fig. 1.
Oropouche virus in Latin America has led to reported cases, including two deaths in Brazil. Vertical transmission has been observed, with one fetal death, one miscarriage, and four instances of microcephaly in newborns, although no causal link has been confirmed. This remains to be confirmed. Additionally, there are three more fetal deaths in Pernambuco state, with two cases still under investigation. These instances of vertical transmission complicate the understanding of new infection routes, potentially contributing to emerging epidemic patterns, and suggest further research into their implications for maternal and neonatal health.
OROV has caused several outbreaks in the region, with clinical manifestations ranging from mild symptoms to rare neurological events closely resembling those of dengue and chikungunya, likely leading to underreporting of OROV infections in Brazil. The introduction of the chikungunya virus in Brazil in 2014 further complicated clinical diagnosis due to symptom similarity with OROV. Oropouche virus can present additional notable symptoms. These include rash, conjunctival injection, diarrhoea, severe abdominal pain, hemorrhagic symptoms, and petechiae. A retrospective study in Amapá from August 2014 to May 2015 noted that 10.24% of febrile patients had neutralising antibodies for OROV, indicating previous exposure.5,6 This makes Oropouche fever a neglected tropical disease in Brazil. Diagnostic confusion involving other arboviruses, Brazilian spotted fever, viral hantaviruses, and hepatitis poses ongoing public health challenges.
Transmission reaches its highest levels during the rainy season when the number of insect vectors increases. However, outbreaks can also happen during dry seasons if the density of vectors stays high. Understanding these patterns of transmission is essential for implementing successful public health interventions. Transmission of OROV by the biting midge Culicoides paraensis involves biological transmission to susceptible hosts after feeding on viremic individuals, with infection rates reaching up to 80% and transmission rates 25–83%. OROV assembly and budding occur at Golgi cisternae, attracting the endosomal sorting complex required for transport (ESCRT) proteins necessary for viral morphogenesis and egress. This highlights a unique host cell hijacking mechanism for efficient viral particle production.7
The ongoing outbreak includes 10 Brazilian non-Amazonian states where no autochthonous transmission has been reported in 2024. The first two fatal cases linked to OROV infection were also reported from Brazil.8 The first case involved a 24-year-old woman from Valença, Bahia, who developed severe symptoms on 23 March 2024, sought medical care multiple times, and died four days later. RT-PCR confirmed OROV, with other arboviruses testing negative.9 The second case was a 21-year-old woman from Camamú, Bahia, who exhibited severe symptoms, including fever, rash, bleeding, and vomiting, and died three days later on 9 May 2024. RT-PCR confirmed OROV in serum samples collected on 10 May 2024, with negative results for other arboviruses (Dengue, Zika, Chikungunya, and Mayaro).9
Perhaps the emerging hypothesis of vertical transmission and foetal effects is the most problematic of the current outbreak. The presence of OROV in placenta samples was confirmed in at least one case, and the potential vertical transmission and unfavourable fetal outcomes were recently reported on July 25, 2024, in Brazil.9 Additionally, spontaneous abortion was observed in 2 of 9 pregnant women with a serological diagnosis of OROV infection during the second month of gestation in an investigation in the 1980s.9, 10, 11 More studies are needed, but this question should be promptly addressed and cases in pregnancy managed in a cautionary way. Recent cases in Brazil included one fetal death and one miscarriage, both in Pernambuco state, as well as four cases of microcephaly in newborns in Acre and Pará states. Additionally, there were three more fetal deaths in Pernambuco state, with two of these cases still under investigation for vertical transmission (Fig. 1). These vertical transmissions add to the complexity of potential new routes of infection, could contribute to the development of new epidemic patterns, and highlight the importance of further research into its implications for maternal and neonatal health.12
Case studies from Colombia and French Guiana illustrate the emergence of Oropouche fever in new regions with significant attack rates and a range of symptoms. In French Guiana, an outbreak in Saül in 2020 had an attack rate of 43.2% among residents, with persistent tiredness reported by 73.2% of patients. In Colombia, a study from 2019 to 2022 found OROV to be an emerging cause of acute febrile illness, with 10.9% of cases testing positive for OROV by RT-qPCR.13,14
The nonspecific symptoms of Oropouche fever can be like those of other arbovirus infections, making it difficult to diagnose and predict using clinical models. The disease ranks as the second most prevalent arboviral infection in Brazil, following Dengue, and has a significant capacity for causing epidemics.15 The absence of targeted antiviral therapies or a vaccine highlights the importance of employing vector control tactics and implementing personal protection measures.16 The potential for vertical transmission, indicating the silent propagation of the virus within mosquito populations, complicates outbreak control and epidemiological understanding. Increased surveillance and research are essential to determine the actual burden of Oropouche fever and develop effective public health strategies.11
Contributors
RS, SS, RM drafted the first version, SRK, SK, PS, AM contributed with literature review, CF, CL, AJRM reviewed regional and national epidemiological data, JF, and VA developed the figure. All authors contributed with subsequent versions of the manuscript. All authors approved the final version of the manuscript submitted.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
None.
Acknowledgements
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Sah R., Srivastava S, Kumar S, et al. Oropouche fever outbreak in Brazil: an emerging concern in Latin America. Lancet Microbe. 2024 doi: 10.1016/S2666-5247(24)00136-8. [DOI] [PubMed] [Google Scholar]
- 2.Pan American Health Organization/World Health Organization . 2024. Epidemiological update Oropouche in the americas region - 6 september 2024. Washington, D.C.https://www.paho.org/en/documents/epidemiological-update-oropouche-americas-region-6-september-2024 [Google Scholar]
- 3.Brazilian Ministry of Health (Ministerio da Saude) 2024. Oropouche Epidemiological panel. Brasilia, Brazil.https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/o/oropouche/painel-epidemiologico [Google Scholar]
- 4.Da Rosa J.F.T., de Souza WM, Pinheiro FP, et al. Oropouche virus: clinical, epidemiological, and molecular aspects of a neglected Orthobunyavirus. Am J Trop Med Hyg. 2017;96(5):1019. doi: 10.4269/ajtmh.16-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lima R.C., Dias HG, de Souza TMA, et al. Oropouche virus exposure in febrile patients during chikungunya virus introduction in the state of amapa, amazon region, Brazil. Pathogens. 2024;13(6):469. doi: 10.3390/pathogens13060469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domaradzki J., Walkowiak D. Knowledge and attitudes of future healthcare professionals toward rare diseases. Front Genet. 2021;12 doi: 10.3389/fgene.2021.639610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbosa N.S., Concha JO, daSilva LLP, Crump CM, Graham SC. Oropouche virus glycoprotein topology and cellular requirements for glycoprotein secretion. J Virol. 2023;97(1) doi: 10.1128/jvi.01331-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan American Health Organization/World Health Organization . 2024. Epidemiological alert Oropouche in the region of the americas - 1 August 2024. Washington, D.C.https://www.paho.org/en/documents/epidemiological-alert-oropouche-region-americas-1-august-2024 [Google Scholar]
- 9.Pan American Health Organization/World Health Organization . 2024. Public health risk assessment related to Oropouche virus (OROV) in the region of the americas general risk statement, 3 August 2024. Washington, D.C.https://www.paho.org/en/documents/public-health-risk-assessment-related-oropouche-virus-orov-region-americas-3-august-2024 [Google Scholar]
- 10.Borborema C.A., Pinheiro FP, Albuquerque BC, da Rosa AP, da Rosa JF, Dourado HV. [1st occurrence of outbreaks caused by Oropouche virus in the State of Amazonas] Rev Inst Med Trop Sao Paulo. 1982;24(3):132–139. [PubMed] [Google Scholar]
- 11.Huits R., Waggoner J.J., Castilletti C. New insights into Oropouche: expanding geographic spread, mortality, vertical transmission, and birth defects. J Trav Med. 2024 doi: 10.1093/jtm/taae117. [DOI] [PubMed] [Google Scholar]
- 12.Taylor L. Oropouche fever: Latin America on high alert for virus that can cause stillbirths. BMJ. 2024;386:q1667. doi: 10.1136/bmj.q1667. [DOI] [PubMed] [Google Scholar]
- 13.Gaillet M., Pichard C, Restrepo J, et al. Outbreak of Oropouche virus in French Guiana. Emerg Infect Dis. 2021;27(10):2711–2714. doi: 10.3201/eid2710.204760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciuoderis K.A., Berg MG, Perez LJ, et al. Oropouche virus as an emerging cause of acute febrile illness in Colombia. Emerg Microb Infect. 2022;11(1):2645–2657. doi: 10.1080/22221751.2022.2136536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohapatra R.K., Mishra S, Satapathy P, Kandi V, Tuglo LS. Surging Oropouche virus (OROV) cases in the Americas: a public health challenge. New Microbes New Infect. 2024;59:101243. doi: 10.1016/j.nmni.2024.101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durango-Chavez H.V., Toro-Huamanchumo CJ, Silva-Caso W, et al. Oropouche virus infection in patients with acute febrile syndrome: is a predictive model based solely on signs and symptoms useful? PLoS One. 2022;17(7) doi: 10.1371/journal.pone.0270294. [DOI] [PMC free article] [PubMed] [Google Scholar]