Highlights
-
•
Integration of artificial intelligence (AI) in interventional radiotherapy (IRT) is explored to enhance patient care in cancer treatment.
-
•
Systematic review of 78 relevant papers from 2002 to 2024 reveals significant advancements in AI-driven contouring, treatment planning, outcome prediction, and quality assurance.
-
•
AI applications offer potential benefits in reducing procedural times, personalizing treatments, and improving treatment outcomes for oncological patients undergoing IRT.
-
•
Despite challenges in validation and quality assurance, AI presents a transformative opportunity to optimize IRT workflows and meet evolving patient needs.
-
•
This study underscores the promising role of AI in IRT and its potential to revolutionize cancer treatment by improving efficiency and efficacy while prioritizing patient-centric care.
Keywords: Artificial intelligence, Interventional radiotherapy, Brachytherapy, Machine learning, Deep learning
Abstract
This review explores the integration of artificial intelligence (AI) in interventional radiotherapy (IRT), emphasizing its potential to streamline workflows and enhance patient care. Through a systematic analysis of 78 relevant papers spanning from 2002 to 2024, we identified significant advancements in contouring, treatment planning, outcome prediction, and quality assurance. AI-driven approaches offer promise in reducing procedural times, personalizing treatments, and improving treatment outcomes for oncological patients. However, challenges such as clinical validation and quality assurance protocols persist. Nonetheless, AI presents a transformative opportunity to optimize IRT and meet evolving patient needs.
Introduction
Artificial Intelligence (AI) is a rapidly advancing field within computer science, striving to develop machines capable of performing tasks typically associated with human intelligence. This encompasses a range of techniques, including machine learning (ML), deep learning (DL), and natural language processing (NLP), which are literally revolutionizing the healthcare systems [1]. AI is exerting a burgeoning influence across all scientific domains, and it has already made inroads into clinical oncology practice, yet ongoing and intensified efforts are essential to fully unleash its potential [2]. AI holds potential in at least four distinct areas within the workflow of radiotherapy such as clinical decision support, “multi-omics” data integration, streamlining repetitive tasks to optimize time efficiency and overall treatment quality, and modeling behaviors in diverse contexts [3]. Despite advancements in technology, a significant portion of the radiotherapy workflow still relies on labor-intensive, manual tasks performed by a team of diverse and scarce healthcare professionals, including radiation oncologists, medical physicists, radiologists, medical dosimetrists, nurses, and radiation therapists [4].
Unlike external beam radiotherapy (EBRT) which has faced tremendous technological advancements, including the preliminary integration of AI applications, interventional radiotherapy (brachytherapy − IRT) continues to rely heavily on the expertise and technique of the physician rather than technological innovations [5], [6], [7]. In fact, when dealing with IRT, it is fundamental to consider at least three different areas of possible issues namely the need to perform time consuming tasks, the need to consider a learning curve and the scantiness of highly trained personnel [8].
Moreover, automation holds the potential for substantial reductions in healthcare costs and increase in generalizability and reproducibility of IRT. These attributes are applicable across all stages of the IRT workflow: first patient consultation, implant, delineation, planning and treatment delivery [9]. The aim of this review is to highlight how AI could bridge the gap between technical innovations and patient-centered care, ensuring that AI advancements address the specific needs and improve the overall experience of patients undergoing IRT.
Materials and methods
We performed a systematic literature search including a combinations of the terms “artificial intelligence”, “machine learning”, “deep learning”, “brachytherapy” and “interventional radiotherapy” trough titles and abstracts indexed on PubMed from its inception until March 2024. The abstracts that were obtained were manually sorted to ensure relevance. Additionally, cross-references from pertinent articles were gathered from sources outside of PubMed, with duplicates removed. The inclusion criteria encompassed original research papers focusing on AI and brachytherapy. We opted to exclude reviews, letters to the Editor, papers not written in English, or contributions not primarily addressing AI and IRT. The complete texts of all chosen publications underwent screening for inclusion.
Two researchers, each with a minimum of 10 years of clinical expertise in IRT, independently conducted the search (BF, EP). If there were multiple publications related to a topic, we selected the most recent ones or those with the largest subject pool for discussion. Another dedicated team composed by an interventional radiation oncologist expert in large databases and AI, a medical physicist expert in AI and a physicist expert in real world data analysis (MdR, LS, SP) extracted the data presented in this review with articles grouped into clusters based on the major areas of interest examined. The team pinpointed three distinct topics to explore from the patients' viewpoint, where AI could effectively aid in supporting both patients and physicians in fostering a therapeutic alliance.
Firstly, we examined the importance of patient empowerment. Secondly, we delved into the requirement for treatment personalization. Lastly, we addressed the necessity for quality assurance (QA) throughout the whole treatment process.
Finally, a third team, composed of experts in physics, IRT, EBRT and radiology (KT, JMHL, FAS, LB, MAG, MDS, ES, LT), supervised and reviewed the entire process and validated the final version of the manuscript.
Results
We identified 129 papers from 2002 to 2024 and we did not consider reviews (14 articles) or papers not focusing specifically on IRT and AI as primary topic (37 contributions).
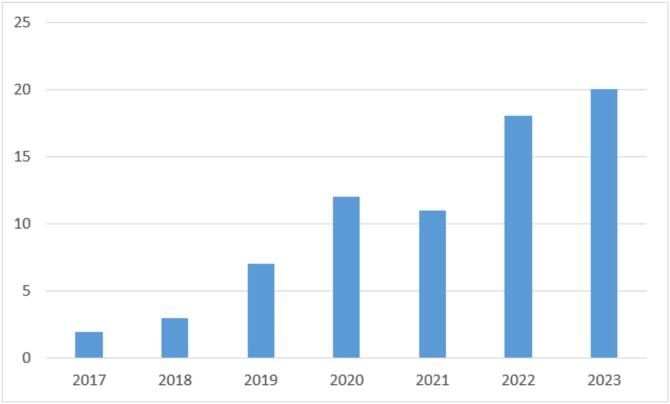
There were only a handful of papers released prior to 2017, but the number of papers published per year has been steadily increasing since then, as depicted in Fig. 1.
Fig. 1.
Number of papers published per year.
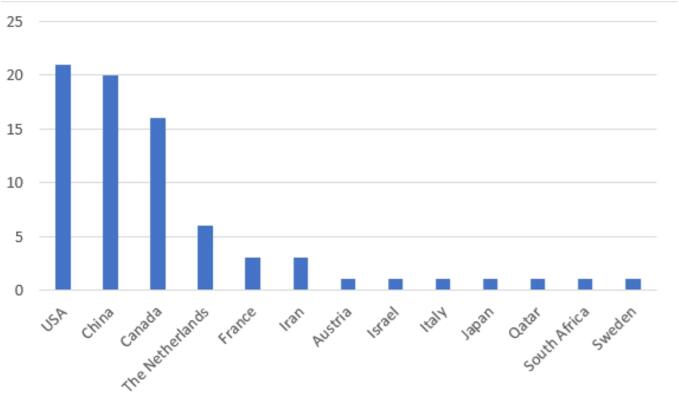
The countries which contributed most were the US, China and Canada. Fig. 2 presents a detailed list of all nations involved.
Fig. 2.
Number of papers published per country.
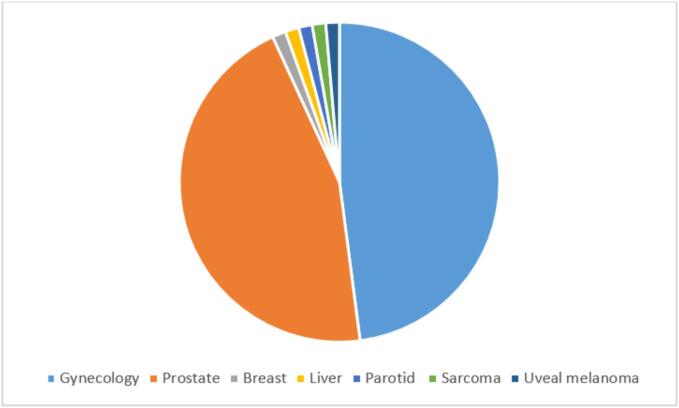
The most common treatment sites were gynecological and prostate cancers, with other tumor sites reported in Fig. 3.
Fig. 3.
Number of papers published per topic.
We categorized the 78 papers into four main categories: contouring (24 articles), treatment planning (38 articles), treatment outcome prediction (11 articles) and QA (5 articles). More specifically, referring to the treatment planning, authors referred to high-dose rate (HDR) in 75 % of the papers, whereas to low-dose rate (LDR) in 25 %, with no paper specifically dealing with pulsed dose rate (PDR).
When analyzing such groups with a focus on the potential advantage within the clinical workflow we came to the conclusion that AI has a major impact in terms of time-saving for repetitive tasks such as contouring and all the phases of the treatment planning (especially for catheter reconstruction where some author report up to 75 % of time saving compared to manual task).
Regarding the articles about treatment outcome prediction, aimed at both predicting local control or toxicity, the most relevant contribution is to provide clinicians with a useful tool which could be used in the decision-making process.
Lastly, the relevance of papers addressing the issue of QA are articles of paramount relevance because in IRT literature such topic is unfortunately underrepresented even though is crucial for the accurate treatment delivery.
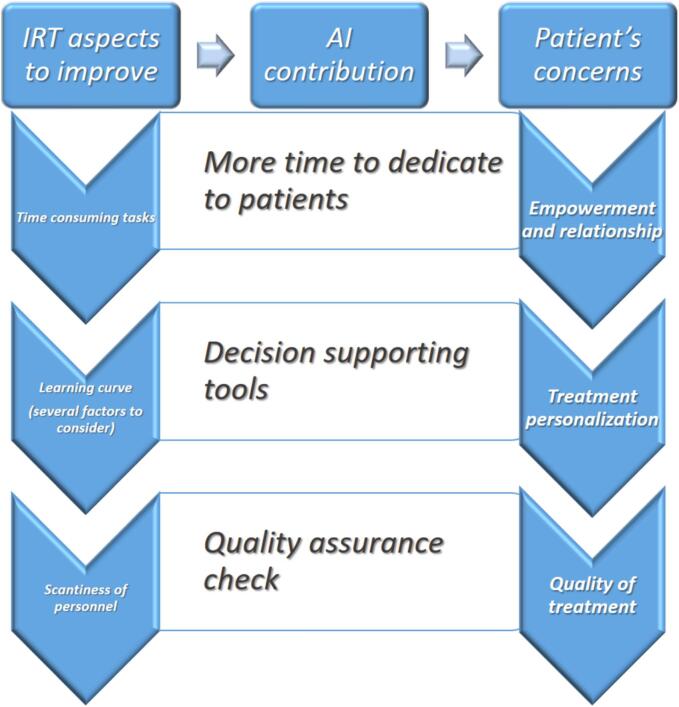
At this stage, our patient-centered approach allowed us to connect the primary issues identified in the current IRT clinical setting with the potential benefits linked to AI usage and the ultimate advantage from the patients’ perspective, as outlined in Fig. 4.
Fig. 4.
AI contribution in bridging the gap between IRT and patients’ needs (from the patients’ perspective).
For the first point we focused on time-consuming tasks, such as catheter reconstruction and dose distribution, which could definitively benefit from AI. In fact, in the clinical scenario of IRT patients typically wait for the treatment delivery with the applicator/needles placed within the site to treat, therefore the time saving has multiple potential benefits for the patients. In fact, in case of procedures not requiring anesthesia this shall allow for a better compliance of the interventional procedure itself. In cases where general anesthesia is required the benefit is of course a shorter duration and therefore reduced peri-interventional anesthesiologic complications. The great addition would however still be with the possibility to have more time available to foster the relationship with the patient, adequately informing and supporting his/her choices.
Regarding the second issue, namely the need for dedicated training in IRT and, therefore, the inevitable presence of a learning curve, the aid provided by AI could support the radiation oncologist by predicting the expected oncological outcomes and possible side effects. This point is of paramount relevance because the possibility to discuss with the patient the risk of relapse and/or of specific toxicities could actually lead to pursue a real treatment personalization which adequately takes into account the therapeutic choices shared with the patients.
The third aspect to consider is the relative lack of adequately trained specialists in IRT to ensure a proper independent check of another specialist on all the interventional procedures; with this regard, the role of AI is crucial because it could intercept potential deviations from the standard clinical practice warranting the need for patient Quality Assurance.
Furthermore a detailed list of the potential benefits for patients deriving from the use of AI in IRT and corresponding with the clinical workflow phase and the specific sub-topic is presented in Table 1.
Table 1.
Main categories and topics in the articles included.
| Benefit for patients | Workflow phase | Specific subtopic | Articles |
|---|---|---|---|
|
More time to dedicate to patients |
Contouring | −Image registration/fusion (US, CT, MRI) −Clinical target volume identification −Organs at risk contouring |
[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33] |
| Treatment planning | −Applicator choice and reconstruction −Needles number and reconstruction −Source activation and dwelling times −Seeds number and positions |
[34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71] | |
|
Decision supporting tool |
Recurrence prediction | −Factors associated with recurrence (dosimetric, histopathological and clinical) −Follow-up personalization (timing) |
[72], [73], [74], [75], [76], [77], [78] |
| Toxicity prediction | −Factors associated with toxicity (dosimetric and clinical) −Follow-up personalization (timing) |
[79], [80], [81], [82] |
|
| Quality assurance check | Quality assurance | −Deviation from standard values −Inconsistencies among the parameters |
[83], [84], [85], [86], [87] |
Regarding contouring, the identified papers focused on image registration across various imaging modalities such as US, CT, and MRI. Several authors explored AI's role in identifying clinical target volumes, while most articles focused on contouring organs at risk.
In treatment planning, AI made contributions in both applicator selection and reconstruction. Needle positioning and reconstruction were also widely investigated. For HDR IRT, significant attention was given to source activation and dwelling times; conversely, LDR analysis focused on seed number and placement.
Regarding treatment outcomes, some authors focused on factors associated with recurrence after treatment, including dosimetric, histopathological, and clinical features. Others concentrated on predicting toxicity, suggesting AI could offer valuable contributions for personalized follow-up, particularly regarding timing.
Finally, a few articles addressed quality assurance, offering insights into detecting inconsistencies among parameters and deviations from expected values.
Discussion
Cancer patients encounter various obstacles throughout their treatment journey, including: physical hurdles (including pain, fatigue, stress, toxicity, hair loss, symptom management, and more), psychosocial challenges (involving existential crises, anxiety, depression, questioning of faith/hope, coping with social stigma, changes in social dynamics, diminished self-esteem, disruption of personal life plans) and organizational difficulties (including fragmented care, financial burdens, lifestyle changes) [88], [89].
Patient empowerment encompasses three elements that are pertinent to the formulation of specific strategies: first, the intrapersonal component, which is concerned with self-perception; second, the interaction aspect pertains to individuals' resources available to achieve their objectives; finally, the interaction component bridges perceived control with actual behavior, constituting the behavioral dimension of PE, which includes active coping behaviors and participation [90]. Empowerment and awareness play a vital role as patients must grasp and assimilate disease-related information and medical instructions to make informed decisions about their treatment options and effectively navigate through the oncological care system [91].
It is crucial to establish a multi-dimensional approach where patients can explore various aspects of their health, such as physiological, psychological, social, and spiritual dimensions. In pursuit of this goal, participatory medicine should align with value-based healthcare principles, wherein patient engagement occurs through clinicians understanding, involving, and providing a coherent narrative of care that is validated within the professional relationship and through the care experience [92]. Several tools are available to oncology teams to address these challenges, including art therapy [93] and AI-driven technologies [94]. These tools serve as adjuncts to standard care, offering additional support while enhancing the patient's care experience with empathy and upliftment [95].
As we showed in Table 1 it is possible to categorize the different steps of the IRT workflow into contouring, treatment planning, recurrence prediction, toxicity prediction and quality assurance.
With regard to contouring and treatment planning they are by far the most time-consuming phases of the IRT workflow; during these phases patients are regularly supervised by the nursing staff while radiotherapy technologists, medical physicists and radiation oncologists perform the several steps. First of all, the radiation oncologist delineates the organs at risk (OARs) and target volumes. Another radiation oncologist should verify these delineations. Shortly after the radiotherapy technologist (or medical physicist) reconstructs the applicator and catheters, independently checked by another operator.
Upon approval of delineations and reconstructions, a treatment plan is proposed by the technologist (or medical physicist) and subsequently reviewed or optimized by the radiation oncologist. Ideally before final approval the plan should be independently checked by another radiation oncologist. In a recent article focusing on 56 cervical cancer IRT procedures these steps accounted for an average of 3 h [96].
Additionally, the gap in radiation therapy knowledge and difference in experience between well-equipped and under-resourced healthcare systems represents one of the most significant global disparities in cancer care. Several experiences have specifically emphasized the existence of a learning curve in IRT procedures [97]. As an illustration, in a study encompassing 42 patients undergoing IRT for prostate cancer, the duration of operating room procedures decreased from 3.6 to 2.4 h, and the time required for treatment planning reduced from 2.0 to 1.3 h as the operators gained experience [98].
Moreover, the increasing complexity of these interactions between physicians’ skills and modern technology, coupled with the rising prevalence of cancer, has resulted in shortages of radiation oncology personnel worldwide and a rise in the variability of care quality. Importantly, discrepancies in the radiotherapy treatment planning process have been demonstrated to have adverse effects on overall survival, even within clinical trials where efforts are made to standardize approaches [99].
Applicator digitization errors significantly affect treatment planning dosimetry. DL reliably automates applicator reconstruction in about 20 s, cutting observer errors and planning time.
As mentioned before about the time consumption for repetitive tasks, a recent study aimed at assessing the viability of employing a deep-learning algorithm for the semi-automatic reconstruction of interstitial catheters within an MR-only workflow. In about 20 gynecological patients, the mean reconstruction time for the AI approach turned out to be about 11 min, compared with 46 min for the expert planners [50].
Another important aspect to consider is the prediction of recurrence and toxicity, where clinical experience plays a critically important role. Regarding the learning curve, the support coming from the use of AI has been proven to be useful in several aspects. A first possible application could be to guide the clinicians in the choice of the best applicator for the treatment volumes [54]; another possible support could come from providing radiation oncologists with indications related to the prognosis of the disease after the IRT treatment [77]; it is noteworthy that crucial information could also be related to the possible presence of side effects based on the dose received by the OARs [79]. All these use cases would benefit from building a retrospective digitized collection of IRT procedures, that would then be used 'digital avatar' for various educational purposes.
When dealing with precision medicine, it's crucial to consider both patient health outcomes and overall health status. Creating and executing this approach hinges on cooperation among patients, patient organizations and healthcare professionals. Patient health outcomes should be evaluated based on the aspects that hold the greatest significance to patients, including recovery, functional enhancement, physical well-being, emotional health, and the capacity to carry out daily activities [100].
The last category to consider, which could significantly benefit from artificial intelligence, is quality assurance—an essential area that stands to gain further optimization in everyday clinical practice. With regard to the contribution of AI in QA there are several experiences in literature fostering the introduction of automated programs supporting the clinicians in the workflow assessment. In one report, among about 4729 fractions delivered 7 events potentially associated with patient safety were identified and the authors conclude that 57 % of such events could have been detected by using additional programs supporting the clinicians check [101]. Also in this case there is evidence that AI could reduce the time of the independent check with an average reduction time of 16 min in one recent report [102].
Some authors have underlines that even though deep learning applications for radiotherapy have undergone great development they are yet to be validated from a clinical point of view in the light of some uncertainties and therefore strict QA protocols and close human supervision will still be needed in the near future [103].
With regard to QA programs, they are aimed at facilitating treatment standardization and equipping clinicians with data regarding optimal equipment and human resource utilization. Additionally, it aids in establishing uniform procedures for the effective management of the radiotherapy workflow, aiming to enhance local control and survival rates on a broader scale, while mitigating the severity of treatment-related complications and optimizing the access to the therapies [104].
Summarizing both possible potential advantages and possible drawback emerge in the current scientific literature for IRT. With regard to the benefits treatment planning could be significantly enhanced, leading to more precise and personalized radiation dose distributions. AI algorithms can analyze complex patient data to optimize the placement of radioactive sources, minimizing exposure to healthy tissues while maximizing the dose to the tumor. This could result in improved treatment outcomes and reduced side effects. Furthermore, AI can streamline workflow, reduce planning time, and assist in real-time decision-making during procedures, ultimately increasing the efficiency and accuracy of IRT treatments [105].
On the other hand, at the moment, the clinical implementation of AI in IRT still needs to be validated by expert clinicians to ensure clinical safety and efficacy. In fact AI systems, though capable of processing vast amounts of data and identifying patterns that may elude human analysis, are not infallible. They can be subject to biases inherent in their training datasets, potentially leading to skewed or suboptimal treatment recommendations. Furthermore, AI lacks the ability to understand and incorporate the full spectrum of clinical subtleties, such as a patient's comorbidities, preferences, and real-time physiological responses. Expert radiation oncologists bring a depth of experience and critical thinking that AI currently cannot match. They can interpret AI outputs within the broader context of each patient's unique medical situation, ensuring that treatment plans are not only technically sound but also holistically appropriate. Additionally, the ethical considerations and responsibilities inherent in oncology care necessitate human oversight to maintain accountability, patient trust, and adherence to established medical standards. Thus, the collaborative integration of AI and expert human judgment is essential to harness the full potential of AI while safeguarding patient health and upholding the integrity of clinical practice [106], [107].
Conclusion
Artificial intelligence tools have the potential to offer significant support to modern interventional radiation therapy (brachytherapy). They can streamline time-consuming tasks, provide decision support tools, and aid clinicians in ensuring QA. These advantages directly align with the needs of oncological patients who are seeking empowerment in their treatment journey, are interested in personalized therapeutic approaches, and prioritize treatment quality. By integrating AI into the workflow, the ultimate goal is to enhance the patient experience, ensuring that treatments are not only more efficient but also tailored to individual patient needs and preferences, thereby improving overall care and outcomes.
Authors’ contributions
All Authors have revised the manuscript for intellectual content and approved the final version to be published.
CRediT authorship contribution statement
Bruno Fionda: Conceptualization, Supervision, Data curation, Formal analysis. Elisa Placidi: Data curation, Formal analysis. Mischa de Ridder: Visualization, Writing - review & editing. Lidia Strigari: Visualization, Writing - review & editing. Stefano Patarnello: Validation, Visualization, Writing - review & editing. Kari Tanderup: Visualization, Writing - review & editing. Jean-Michel Hannoun-Levi: Visualization, Writing - review & editing. Frank-André Siebert: Validation, Visualization, Writing - review & editing. Luca Boldrini: Visualization. Maria Antonietta Gambacorta: Supervision, Validation. Marco De Spirito: Supervision, Validation. Evis Sala: Supervision, Validation. Luca Tagliaferri: Conceptualization, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Alowais S.A., Alghamdi S.S., Alsuhebany N., Alqahtani T., Alshaya A.I., Almohareb S.N., et al. Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Med Educ. 2023;23(1):689. doi: 10.1186/s12909-023-04698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luchini C., Pea A., Scarpa A. Artificial intelligence in oncology: current applications and future perspectives. Br J Cancer. 2022;126(1):4–9. doi: 10.1038/s41416-021-01633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tagliaferri L, Fionda B, Masiello V, Siebert FA, Martínez-Monge R, Damiani A. Artificial Intelligence and Radiotherapy: Impact on Radiotherapy Workflow and Clinical Example. In: Cesario, A., D'Oria, M., Auffray, C., Scambia, G. (eds) Personalized Medicine Meets Artificial Intelligence. 2023. Springer, Cham. doi: 10.1007/978-3-031-32614-1_11.
- 4.Huynh E., Hosny A., Guthier C., Bitterman D.S., Petit S.F., Haas-Kogan D.A., et al. Artificial intelligence in radiation oncology. Nat Rev Clin Oncol. 2020;17(12):771–781. doi: 10.1038/s41571-020-0417-8. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee S, Goyal S, Mishra S, Gupta D, Bisht SS, K V, Narang K, Kataria T. Artificial intelligence in brachytherapy: a summary of recent developments. Br J Radiol. 2021 Jun 1;94(1122):20200842. doi: 10.1259/bjr.20200842. [DOI] [PMC free article] [PubMed]
- 6.Strolin S., Santoro M., Paolani G., Ammendolia I., Arcelli A., Benini A., et al. How smart is artificial intelligence in organs delineation? Testing a CE and FDA-approved Deep-Learning tool using multiple expert contours delineated on planning CT images. Front Oncol. 2023;2(13):1089807. doi: 10.3389/fonc.2023.1089807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santoro M., Strolin S., Paolani G., Della Gala G., Bartoloni A., Giacometti C., et al. Recent applications of artificial intelligence in radiotherapy: where we are and beyond. Appl Sci. 2022;12(7):3223. doi: 10.3390/app12073223. [DOI] [Google Scholar]
- 8.Tagliaferri L., Vavassori A., Lancellotta V., De Sanctis V., Barbera F., Fusco V., et al. Can brachytherapy be properly considered in the clinical practice? Trilogy project: The vision of the AIRO (Italian Association of Radiotherapy and Clinical Oncology) Interventional Radiotherapy study group. J Contemp Brachytherapy. 2020;12(1):84–89. doi: 10.5114/jcb.2020.92765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fionda B., Boldrini L., D'Aviero A., Lancellotta V., Gambacorta M.A., Kovács G., et al. Artificial intelligence (AI) and interventional radiotherapy (brachytherapy): state of art and future perspectives. J Contemp Brachytherapy. 2020;12(5):497–500. doi: 10.5114/jcb.2020.100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraus A.C., Iqbal Z., Cardan R.A., Popple R.A., Stanley D.N., Shen S., et al. Prospective evaluation of automated contouring for CT-based brachytherapy for gynecologic malignancies. Adv Radiat Oncol. 2023;9(4) doi: 10.1016/j.adro.2023.101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hampole P., Harding T., Gillies D., Orlando N., Edirisinghe C., Mendez L.C., et al. Deep learning-based ultrasound auto-segmentation of the prostate with brachytherapy implanted needles. Med Phys. 2024;51(4):2665–2677. doi: 10.1002/mp.16811. [DOI] [PubMed] [Google Scholar]
- 12.King M.T., Kehayias C.E., Chaunzwa T., Rosen D.B., Mahal A.R., Wallburn T.D., et al. Observer preference of artificial intelligence-generated versus clinical prostate contours for ultrasound-based high dose rate brachytherapy. Med Phys. 2023;50(10):5935–5943. doi: 10.1002/mp.16716. [DOI] [PubMed] [Google Scholar]
- 13.Peng T, Dong Y, Di G, Zhao J, Li T, Ren G, Zhang L, Cai J. Boundary delineation in transrectal ultrasound images for region of interest of prostate. Phys Med Biol. 2023 Sep 20;68(19). doi: 10.1088/1361-6560/acf5c5. [DOI] [PubMed]
- 14.Rodríguez Outeiral R., González P.J., Schaake E.E., van der Heide U.A., Simões R. Deep learning for segmentation of the cervical cancer gross tumor volume on magnetic resonance imaging for brachytherapy. Radiat Oncol. 2023;18(1):91. doi: 10.1186/s13014-023-02283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duprez D., Trauernicht C., Simonds H., Williams O. Self-configuring nnU-Net for automatic delineation of the organs at risk and target in high-dose rate cervical brachytherapy, a low/middle-income country's experience. J Appl Clin Med Phys. 2023;24(8):e13988. doi: 10.1002/acm2.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z.Y., Yue J.H., Wang W., Wu W.J., Zhou F.G., Zhang J., et al. Deep learning-based two-step organs at risk auto-segmentation model for brachytherapy planning in parotid gland carcinoma. J Contemp Brachytherapy. 2022;14(6):527–535. doi: 10.5114/jcb.2022.123972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Chen Y, Tu Y, Xie H, Chen Y, Luo L, Zhou P, Tang Q. Evaluation of auto-segmentation for brachytherapy of postoperative cervical cancer using deep learning-based workflow. Phys Med Biol. 2023 Feb 23;68(5). doi: 10.1088/1361-6560/acba76. [DOI] [PubMed]
- 18.Huang M, Feng C, Sun D, Cui M, Zhao D. Segmentation of clinical target volume from CT images for cervical cancer using deep learning. Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338221139164. doi: 10.1177/15330338221139164. [DOI] [PMC free article] [PubMed]
- 19.Salehi M, Vafaei Sadr A, Mahdavi SR, Arabi H, Shiri I, Reiazi R. Deep Learning-based non-rigid image registration for high-dose rate brachytherapy in inter-fraction cervical cancer. J Digit Imaging. 2023 Apr;36(2):574-587. doi: 10.1007/s10278-022-00732-6. Epub 2022 Nov 23. PMID: 36417026; PMCID: PMC10039214. [DOI] [PMC free article] [PubMed]
- 20.Li Z., Zhu Q., Zhang L., Yang X., Li Z., Fu J. A deep learning-based self-adapting ensemble method for segmentation in gynecological brachytherapy. Radiat Oncol. 2022;17(1):152. doi: 10.1186/s13014-022-02121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ecker S., Zimmermann L., Heilemann G., Niatsetski Y., Schmid M., Sturdza A.E., et al. Neural network-assisted automated image registration for MRI-guided adaptive brachytherapy in cervical cancer. Z Med Phys. 2022;32(4):488–499. doi: 10.1016/j.zemedi.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng T., Wu Y., Qin J., Wu Q.J., Cai J. H-ProSeg: Hybrid ultrasound prostate segmentation based on explainability-guided mathematical model. Comput Methods Programs Biomed. 2022;219 doi: 10.1016/j.cmpb.2022.106752. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y., Vassantachart A., Ragab O., Bian S., Mitra P., Xu Z., et al. Automatic segmentation of high-risk clinical target volume for tandem-and-ovoids brachytherapy patients using an asymmetric dual-path convolutional neural network. Med Phys. 2022;49(3):1712–1722. doi: 10.1002/mp.15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang X., Wang F., Chen Y., Yan S. RefineNet-based automatic delineation of the clinical target volume and organs at risk for three-dimensional brachytherapy for cervical cancer. Ann Transl Med. 2021;9(23):1721. doi: 10.21037/atm-21-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luximon D.C., Abdulkadir Y., Chow P.E., Morris E.D., Lamb J.M. Machine-assisted interpolation algorithm for semi-automated segmentation of highly deformable organs. Med Phys. 2022;49(1):41–51. doi: 10.1002/mp.15351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y., Xing L., Yu L., Liu W., Pooya Fahimian B., Niedermayr T., et al. MR to ultrasound image registration with segmentation-based learning for HDR prostate brachytherapy. Med Phys. 2021;48(6):3074–3083. doi: 10.1002/mp.14901. [DOI] [PubMed] [Google Scholar]
- 27.Mohammadi R., Shokatian I., Salehi M., Arabi H., Shiri I., Zaidi H. Deep learning-based auto-segmentation of organs at risk in high-dose rate brachytherapy of cervical cancer. Radiother Oncol. 2021;159:231–240. doi: 10.1016/j.radonc.2021.03.030. [DOI] [PubMed] [Google Scholar]
- 28.Girum K.B., Lalande A., Hussain R., Créhange G. A deep learning method for real-time intraoperative US image segmentation in prostate brachytherapy. Int J Comput Assist Radiol Surg. 2020;15(9):1467–1476. doi: 10.1007/s11548-020-02231-x. [DOI] [PubMed] [Google Scholar]
- 29.Wang F., Xing L., Bagshaw H., Buyyounouski M., Han B. Deep learning applications in automatic needle segmentation in ultrasound-guided prostate brachytherapy. Med Phys. 2020;47(9):3797–3805. doi: 10.1002/mp.14328. [DOI] [PubMed] [Google Scholar]
- 30.Orlando N., Gillies D.J., Gyacskov I., Romagnoli C., D'Souza D., Fenster A. Automatic prostate segmentation using deep learning on clinically diverse 3D transrectal ultrasound images. Med Phys. 2020;47(6):2413–2426. doi: 10.1002/mp.14134. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Lei Y., Qiu R.L.J., Wang T., Wang H., Jani A.B., et al. Multi-needle localization with attention U-net in US-guided HDR prostate brachytherapy. Med Phys. 2020;47(7):2735–2745. doi: 10.1002/mp.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karimi D., Zeng Q., Mathur P., Avinash A., Mahdavi S., Spadinger I., et al. Accurate and robust deep learning-based segmentation of the prostate clinical target volume in ultrasound images. Med Image Anal. 2019;57:186–196. doi: 10.1016/j.media.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Lee E.K., Fung A.Y., Brooks J.P., Zaider M. Automated planning volume definition in soft-tissue sarcoma adjuvant brachytherapy. Phys Med Biol. 2002;47(11):1891–1910. doi: 10.1088/0031-9155/47/11/305. [DOI] [PubMed] [Google Scholar]
- 34.Berumen F, Ouellet S, Enger S, Beaulieu L. Aleatoric and epistemic uncertainty extraction of patient-specific deep learning-based dose predictions in LDR prostate brachytherapy. Phys Med Biol. 2024 Apr 9;69(8). doi: 10.1088/1361-6560/ad3418. [DOI] [PubMed]
- 35.Dickhoff LRM, Scholman RJ, Barten DLJ, Kerkhof EM, Roorda JJ, Velema LA, Stalpers LJA, Pieters BR, Bosman PAN, Alderliesten T. Keeping your best options open with AI-based treatment planning in prostate and cervix brachytherapy. Brachytherapy. 2024 Mar-Apr;23(2):188-198. doi: 10.1016/j.brachy.2023.10.005. [DOI] [PubMed]
- 36.Wang Y., Jian W., Zhu L., Cai C., Zhang B., Wang X. Attention-gated deep-learning-based automatic digitization of interstitial needles in high-dose-rate brachytherapy for cervical cancer. Adv Radiat Oncol. 2023;9(1) doi: 10.1016/j.adro.2023.101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grigo J, Karius A, Hanspach J, Mücke L, Laun FB, Huang Y, Strnad V, Fietkau R, Bert C, Putz F. Toward a deep learning-based magnetic resonance imaging only workflow for postimplant dosimetry in I-125 seed brachytherapy for prostate cancer. Brachytherapy. 2024 Jan-Feb;23(1):96-105. doi: 10.1016/j.brachy.2023.09.009. [DOI] [PubMed]
- 38.Li Z., Yang Z., Lu J., Zhu Q., Wang Y., Zhao M., et al. Deep learning-based dose map prediction for high-dose-rate brachytherapy. Phys Med Biol. 2023;68(17) doi: 10.1088/1361-6560/acecd2. [DOI] [PubMed] [Google Scholar]
- 39.Zhu J., Wang C., Teng S., Lu J., Lyu P., Zhang P., et al. Embedding expertise knowledge into inverse treatment planning for low-dose-rate brachytherapy of hepatic malignancies. Med Phys. 2024;51(1):348–362. doi: 10.1002/mp.16627. [DOI] [PubMed] [Google Scholar]
- 40.Podgorsak AR, Venkatesulu BP, Abuhamad M, Harkenrider MM, Solanki AA, Roeske JC, Kang H. Dosimetric and workflow impact of synthetic-MRI use in prostate high-dose-rate brachytherapy. Brachytherapy. 2023 Sep-Oct;22(5):686-696. doi: 10.1016/j.brachy.2023.05.005. [DOI] [PubMed]
- 41.Xie H., Wang J., Chen Y., Tu Y., Chen Y., Zhao Y., et al. Automatic reconstruction of interstitial needles using CT images in post-operative cervical cancer brachytherapy based on deep learning. J Contemp Brachytherapy. 2023;15(2):134–140. doi: 10.5114/jcb.2023.126514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berumen F., Enger S.A., FastDM B.L. Mcalculation in LDR brachytherapy using deep learning methods. Phys Med Biol. 2023;68(11) doi: 10.1088/1361-6560/accd42. [DOI] [PubMed] [Google Scholar]
- 43.Yuan A., Podder T., Yuan J., Zheng Y. Using a deep learning approach for implanted seed detection on fluoroscopy images in prostate brachytherapy. J Contemp Brachytherapy. 2023;15(1):69–74. doi: 10.5114/jcb.2023.125512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barten DLJ, Pieters BR, Bouter A, van der Meer MC, Maree SC, Hinnen KA, Westerveld H, Bosman PAN, Alderliesten T, van Wieringen N, Bel A. Towards artificial intelligence-based automated treatment planning in clinical practice: A prospective study of the first clinical experiences in high-dose-rate prostate brachytherapy. Brachytherapy. 2023 Mar-Apr;22(2):279-289. doi: 10.1016/j.brachy.2022.11.013. [DOI] [PubMed]
- 45.Cortes KG, Kallis K, Simon A, Mayadev J, Meyers SM, Moore KL. Knowledge-based three-dimensional dose prediction for tandem-and-ovoid brachytherapy. Brachytherapy. 2022 Jul-Aug;21(4):532-542. doi: 10.1016/j.brachy.2022.03.002. [DOI] [PubMed]
- 46.Weishaupt LL, Sayed HK, Mao X, Choo R, Stish BJ, Enger SA, Deufel C. Approaching automated applicator digitization from a new angle: Using sagittal images to improve deep learning accuracy and robustness in high-dose-rate prostate brachytherapy. Brachytherapy. 2022 Jul-Aug;21(4):520-531. doi: 10.1016/j.brachy.2022.02.005. [DOI] [PubMed]
- 47.Liu D., Tupor S., Singh J., Chernoff T., Leong N., Sadikov E., et al. The challenges facing deep learning-based catheter localization for ultrasound guided high-dose-rate prostate brachytherapy. Med Phys. 2022;49(4):2442–2451. doi: 10.1002/mp.15522. [DOI] [PubMed] [Google Scholar]
- 48.Yoganathan S.A., Paul S.N., Paloor S., Torfeh T., Chandramouli S.H., Hammoud R., et al. Automatic segmentation of magnetic resonance images for high-dose-rate cervical cancer brachytherapy using deep learning. Med Phys. 2022;49(3):1571–1584. doi: 10.1002/mp.15506. [DOI] [PubMed] [Google Scholar]
- 49.Pu G., Jiang S., Yang Z., Hu Y., Liu Z. Deep reinforcement learning for treatment planning in high-dose-rate cervical brachytherapy. Phys Med. 2022;94:1–7. doi: 10.1016/j.ejmp.2021.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Shaaer A., Paudel M., Smith M., Tonolete F., Ravi A. Deep-learning-assisted algorithm for catheter reconstruction during MR-only gynecological interstitial brachytherapy. J Appl Clin Med Phys. 2022;23(2):e13494. doi: 10.1002/acm2.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boussion N., Schick U., Dissaux G., Ollivier L., Goasduff G., Pradier O., et al. A machine-learning approach based on 409 treatments to predict optimal number of iodine-125 seeds in low-dose-rate prostate brachytherapy. J Contemp Brachytherapy. 2021;13(5):541–548. doi: 10.5114/jcb.2021.109789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lei Y., Wang T., Fu Y., Roper J., Jani A.B., Liu T., et al. Catheter position prediction using deep-learning-based multi-atlas registration for high-dose rate prostate brachytherapy. Med Phys. 2021;48(11):7261–7270. doi: 10.1002/mp.15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu H., Yang Q., Li J., Wang P., Tang B., Wang X., et al. Deep learning applications in automatic segmentation and reconstruction in CT-based cervix brachytherapy. J Contemp Brachytherapy. 2021;13(3):325–330. doi: 10.5114/jcb.2021.106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stenhouse K., Roumeliotis M., Ciunkiewicz P., Banerjee R., Yanushkevich S., McGeachy P. Development of a machine learning model for optimal applicator selection in high-dose-rate cervical brachytherapy. Front Oncol. 2021;5(11) doi: 10.3389/fonc.2021.611437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Younes H., Troccaz J., Voros S. Machine learning and registration for automatic seed localization in 3D US images for prostate brachytherapy. Med Phys. 2021;48(3):1144–1156. doi: 10.1002/mp.14628. [DOI] [PubMed] [Google Scholar]
- 56.Rigaud B., Anderson B.M., Yu Z.H., Gobeli M., Cazoulat G., Söderberg J., et al. Automatic segmentation using deep learning to enable online dose optimization during adaptive radiation therapy of cervical cancer. Int J Radiat Oncol Biol Phys. 2021;109(4):1096–1110. doi: 10.1016/j.ijrobp.2020.10.038. [DOI] [PubMed] [Google Scholar]
- 57.Andersén C., Rydén T., Thunberg P., Lagerlöf J.H. Deep learning-based digitization of prostate brachytherapy needles in ultrasound images. Med Phys. 2020;47(12):6414–6420. doi: 10.1002/mp.14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang D., Yang Z., Jiang S., Zhou Z., Meng M., Wang W. Automatic segmentation and applicator reconstruction for CT-based brachytherapy of cervical cancer using 3D convolutional neural networks. J Appl Clin Med Phys. 2020;21(10):158–169. doi: 10.1002/acm2.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y., Tian Z., Lei Y., Wang T., Patel P., Jani A.B., et al. Automatic multi-needle localization in ultrasound images using large margin mask RCNN for ultrasound-guided prostate brachytherapy. Phys Med Biol. 2020;65(20) doi: 10.1088/1361-6560/aba410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yusufaly TI, Kallis K, Simon A, Mayadev J, Yashar CM, Einck JP, Mell LK, Brown D, Scanderbeg D, Hild SJ, Covele B, Moore KL, Meyers SM. A knowledge-based organ dose prediction tool for brachytherapy treatment planning of patients with cervical cancer. Brachytherapy. 2020 Sep-Oct;19(5):624-634. doi: 10.1016/j.brachy.2020.04.008. [DOI] [PubMed]
- 61.Dai X., Lei Y., Zhang Y., Qiu R.L.J., Wang T., Dresser S.A., et al. Automatic multi-catheter detection using deeply supervised convolutional neural network in MRI-guided HDR prostate brachytherapy. Med Phys. 2020;47(9):4115–4124. doi: 10.1002/mp.14307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mao X., Pineau J., Keyes R., Enger S.A. RapidBrachyDL: rapid radiation dose calculations in brachytherapy via deep learning. Int J Radiat Oncol Biol Phys. 2020;108(3):802–812. doi: 10.1016/j.ijrobp.2020.04.045. [DOI] [PubMed] [Google Scholar]
- 63.Golshan M., Karimi D., Mahdavi S., Lobo J., Peacock M., Salcudean S.E., et al. Automatic detection of brachytherapy seeds in 3D ultrasound images using a convolutional neural network. Phys Med Biol. 2020;65(3) doi: 10.1088/1361-6560/ab64b5. [DOI] [PubMed] [Google Scholar]
- 64.Jung H., Shen C., Gonzalez Y., Albuquerque K., Jia X. Deep-learning assisted automatic digitization of interstitial needles in 3D CT image based high dose-rate brachytherapy of gynecological cancer. Phys Med Biol. 2019;64(21) doi: 10.1088/1361-6560/ab3fcb. [DOI] [PubMed] [Google Scholar]
- 65.Jung H, Gonzalez Y, Shen C, Klages P, Albuquerque K, Jia X. Deep-learning-assisted automatic digitization of applicators in 3D CT image-based high-dose-rate brachytherapy of gynecological cancer. Brachytherapy. 2019 Nov-Dec;18(6):841-851. doi: 10.1016/j.brachy.2019.06.003. [DOI] [PubMed]
- 66.Nosrati R, Song WY, Wronski M, Pejović-Milić A, Morton G, Stanisz GJ. Feasibility of an MRI-only workflow for postimplant dosimetry of low-dose-rate prostate brachytherapy: Transition from phantoms to patients. Brachytherapy. 2019 Nov-Dec;18(6):863-874. doi: 10.1016/j.brachy.2019.06.004. [DOI] [PubMed]
- 67.Zaffino P., Pernelle G., Mastmeyer A., Mehrtash A., Zhang H., Kikinis R., et al. Fully automatic catheter segmentation in MRI with 3D convolutional neural networks: application to MRI-guided gynecologic brachytherapy. Phys Med Biol. 2019;64(16) doi: 10.1088/1361-6560/ab2f47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen C., Gonzalez Y., Klages P., Qin N., Jung H., Chen L., et al. Intelligent inverse treatment planning via deep reinforcement learning, a proof-of-principle study in high dose-rate brachytherapy for cervical cancer. Phys Med Biol. 2019;64(11) doi: 10.1088/1361-6560/ab18bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nosrati R., Soliman A., Safigholi H., Hashemi M., Wronski M., Morton G., et al. MRI-based automated detection of implanted low dose rate (LDR) brachytherapy seeds using quantitative susceptibility mapping (QSM) and unsupervised machine learning (ML) Radiother Oncol. 2018;129(3):540–547. doi: 10.1016/j.radonc.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Nicolae A., Morton G., Chung H., Loblaw A., Jain S., Mitchell D., et al. Evaluation of a machine-learning algorithm for treatment planning in prostate low-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2017;97(4):822–829. doi: 10.1016/j.ijrobp.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 71.Nicolae A, Semple M, Lu L, Smith M, Chung H, Loblaw A, Morton G, Mendez LC, Tseng CL, Davidson M, Ravi A. Conventional vs machine learning-based treatment planning in prostate brachytherapy: Results of a Phase I randomized controlled trial. Brachytherapy. 2020 Jul-Aug;19(4):470-476. doi: 10.1016/j.brachy.2020.03.004. [DOI] [PubMed]
- 72.Valdes G., Chang A.J., Interian Y., Owen K., Jensen S.T., Ungar L.H., et al. Salvage HDR brachytherapy: multiple hypothesis testing versus machine learning analysis. Int J Radiat Oncol Biol Phys. 2018;101(3):694–703. doi: 10.1016/j.ijrobp.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 73.Yilmaz E.C., Harmon S.A., Belue M.J., Merriman K.M., Phelps T.E., Lin Y., et al. Evaluation of a deep learning-based algorithm for post-radiotherapy prostate cancer local recurrence detection using biparametric MRI. Eur J Radiol. 2023;168 doi: 10.1016/j.ejrad.2023.111095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdalvand N, Sadeghi M, Mahdavi SR, Abdollahi H, Qasempour Y, Mohammadian F, Birgani MJT, Hosseini K. Brachytherapy outcome modeling in cervical cancer patients: A predictive machine learning study on patient-specific clinical, physical and dosimetric parameters. Brachytherapy. 2022 Nov-Dec;21(6):769-782. doi: 10.1016/j.brachy.2022.06.007. [DOI] [PubMed]
- 75.Xu Z., Yang L., Liu Q., Yu H., Chen L. Machine learning of dose-volume histogram parameters predicting overall survival in patients with cervical cancer treated with definitive radiotherapy. J Oncol. 2022;14(2022):2643376. doi: 10.1155/2022/2643376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reijtenbagh D., Godart J., de Leeuw A., Seppenwoolde Y., Jürgenliemk-Schulz I., Mens J.W., et al. Multi-center analysis of machine-learning predicted dose parameters in brachytherapy for cervical cancer. Radiother Oncol. 2022;170:169–175. doi: 10.1016/j.radonc.2022.02.022. [DOI] [PubMed] [Google Scholar]
- 77.Luo J., Chen Y., Yang Y., Zhang K., Liu Y., Zhao H., et al. Prognosis prediction of uveal melanoma after plaque brachytherapy based on ultrasound with machine learning. Front Med (Lausanne) 2022;21(8) doi: 10.3389/fmed.2021.777142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoffer O., Rabin T., Nir R.R., Brzezinski R.Y., Zimmer Y., Gannot I. Automated thermal imaging monitors the local response to cervical cancer brachytherapy. J Biophotonics. 2023;16(1):e202200214. doi: 10.1002/jbio.202200214. [DOI] [PubMed] [Google Scholar]
- 79.Tohidinezhad F., Willems Y., Berbee M., Limbergen E.V., Verhaegen F., Dekker A., et al. Prediction models for brachytherapy-induced rectal toxicity in patients with locally advanced pelvic cancers: a systematic review. J Contemp Brachytherapy. 2022;14(4):411–422. doi: 10.5114/jcb.2022.119427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Z., Chen K., Yang Z., Zhu Q., Yang X., Li Z., et al. A personalized DVH prediction model for HDR brachytherapy in cervical cancer treatment. Front Oncol. 2022;30(12) doi: 10.3389/fonc.2022.967436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian Z, Yen A, Zhou Z, Shen C, Albuquerque K, Hrycushko B. A machine-learning-based prediction model of fistula formation after interstitial brachytherapy for locally advanced gynecological malignancies. Brachytherapy. 2019 Jul-Aug;18(4):530-538. doi: 10.1016/j.brachy.2019.04.004. [DOI] [PubMed]
- 82.Chen J., Chen H., Zhong Z., Wang Z., Hrycushko B., Zhou L., et al. Investigating rectal toxicity associated dosimetric features with deformable accumulated rectal surface dose maps for cervical cancer radiotherapy. Radiat Oncol. 2018;13(1):125. doi: 10.1186/s13014-018-1068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakanishi K., Yamamoto S., Yabe T., Yogo K., Noguchi Y., Okudaira K., et al. Estimating blurless and noise-free Ir-192 source images from gamma camera images for high-dose-rate brachytherapy using a deep-learning approach. Biomed Phys Eng Express. 2023;10(1) doi: 10.1088/2057-1976/ad0bb2. [DOI] [PubMed] [Google Scholar]
- 84.Reijtenbagh D.M.W., Godart J., de Leeuw A.A.C., Jürgenliemk-Schulz I.M., Mens J.M., Huge M., et al. Multi-center dosimetric predictions to improve plan quality for brachytherapy for cervical cancer treatment. Radiother Oncol. 2023;182 doi: 10.1016/j.radonc.2023.109518. [DOI] [PubMed] [Google Scholar]
- 85.Linares Rosales H.M., Couture G., Archambault L., Beddar S., Després P., Beaulieu L. On the use of machine learning methods for mPSD calibration in HDR brachytherapy. Phys Med. 2021;91:73–79. doi: 10.1016/j.ejmp.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 86.Fan J., Xing L., Yang Y. Independent verification of brachytherapy treatment plan by using deep learning inference modeling. Phys Med Biol. 2021;66(12) doi: 10.1088/1361-6560/ac067f. [DOI] [PubMed] [Google Scholar]
- 87.Götz T.I., Lahmer G., Strnad V., Bert C., Hensel B., Tomé A.M., et al. A tool to automatically analyze electromagnetic tracking data from high dose rate brachytherapy of breast cancer patients. PLoS One. 2017;12(9):e0183608. doi: 10.1371/journal.pone.0183608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mateo J., Steuten L., Aftimos P., André F., Davies M., Garralda E., et al. Delivering precision oncology to patients with cancer. Nat Med. 2022;28(4):658–665. doi: 10.1038/s41591-022-01717-2. [DOI] [PubMed] [Google Scholar]
- 89.Hoeben A., Joosten E.A.J., van den Beuken-van Everdingen M.H.J. Personalized medicine: recent progress in cancer therapy. Cancers (Basel) 2021;13(2):242. doi: 10.3390/cancers13020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zimmerman M.A. Psychological empowerment: issues and illustrations. Am J Community Psychol. 1995;23(5):581–599. doi: 10.1007/BF02506983. [DOI] [PubMed] [Google Scholar]
- 91.Ziegler E, Hill J, Lieske B, Klein J, dem OV Knesebeck, Kofahl C. Empowerment in cancer patients: does peer support make a difference? A systematic review. Psychooncology. 2022 May;31(5):683-704. doi: 10.1002/pon.5869. [DOI] [PubMed]
- 92.Di Capua B., Iervolino M., Rocconi A., Bracci S., Marconi E., Dinapoli L., et al. SUPeRO: a multidimensional approach to prevent and manage oncological frailty in a radiation oncology unit. J Clin Med. 2022;11(22):6768. doi: 10.3390/jcm11226768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tagliaferri L., Dinapoli L., Casà C., Colloca G.F., Marazzi F., Cornacchione P., et al. Art and digital technologies to support resilience during the oncological journey: the Art4ART project. Tech Innov Patient Support Radiat Oncol. 2022;1(24):101–106. doi: 10.1016/j.tipsro.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Casà C., Dinapoli L., Marconi E., Chiesa S., Cornacchione P., Beghella Bartoli F., et al. Integration of art and technology in personalized radiation oncology care: experiences, evidence, and perspectives. Front Public Health. 2023;23(11):1056307. doi: 10.3389/fpubh.2023.1056307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fionda B., Piras A., D'Aviero A., Venuti V., Casà C., Preziosi F., et al. The, “PC-WIRED” study: patient centred evolution of websites of italian radiotherapy departments. Patient Educ Couns. 2021;104(9):2152–2153. doi: 10.1016/j.pec.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 96.van Vliet-Pérez S.M., van Paassen R., Wauben L.S.G.L., Straathof R., Berg N.J.V., Dankelman J., et al. Time-action and patient experience analyses of locally advanced cervical cancer brachytherapy. Brachytherapy. 2024;S1538–4721(24):00011–00014. doi: 10.1016/j.brachy.2024.01.007. [DOI] [PubMed] [Google Scholar]
- 97.Buus S, Rylander S, Hokland S, Søndergaard CS, Pedersen EM, Tanderup K, Bentzen L. Learning curve of MRI-based planning for high-dose-rate brachytherapy for prostate cancer. Brachytherapy. 2016 Jul-Aug;15(4):426-434. doi: 10.1016/j.brachy.2016.03.011. [DOI] [PubMed]
- 98.Jääskeläinen E, Kärkkäinen H, Palmgren JE, Tolmunen T, Kraav SL, Anttila M. MRI-guided brachytherapy for locally advanced cervical cancer: Program initiation, learning curve and dose delivery results in Kuopio University Hospital. Brachytherapy. 2021 Jul-Aug;20(4):738-747. doi: 10.1016/j.brachy.2021.02.006. [DOI] [PubMed]
- 99.Wilkinson D.A. High dose rate (HDR) brachytherapy quality assurance: a practical guide. Biomed Imaging Interv J. 2006;2(2):e34. doi: 10.2349/biij.2.2.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lassen U.N., Makaroff L.E., Stenzinger A., Italiano A., Vassal G., Garcia-Foncillas J., et al. Precision oncology: a clinical and patient perspective. Future Oncol. 2021;17(30):3995–4009. doi: 10.2217/fon-2021-0688. [DOI] [PubMed] [Google Scholar]
- 101.Damato A.L., Devlin P.M., Bhagwat M.S., Buzurovic I., Friesen S., Hansen J.L., et al. Independent brachytherapy plan verification software: improving efficacy and efficiency. Radiother Oncol. 2014;113(3):420–424. doi: 10.1016/j.radonc.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 102.Cai B, Altman MB, Reynoso F, Garcia-Ramirez J, He A, Edward SS, Zoberi I, Thomas MA, Gay H, Mutic S, Zoberi JE. Standardization and automation of quality assurance for high-dose-rate brachytherapy planning with application programming interface. Brachytherapy. 2019 Jan-Feb;18(1):108-114.e1. doi: 10.1016/j.brachy.2018.09.004. [DOI] [PubMed]
- 103.van den Berg C.A.T., Meliadò E.F. Uncertainty assessment for deep learning radiotherapy applications. Semin Radiat Oncol. 2022;32(4):304–318. doi: 10.1016/j.semradonc.2022.06.001. [DOI] [PubMed] [Google Scholar]
- 104.Bernier J., Horiot J.C., Poortmans P. Quality assurance in radiotherapy: from radiation physics to patient- and trial-oriented control procedures. Eur J Cancer. 2002;38(Suppl 4):S155–S158. doi: 10.1016/s0959-8049(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 105.Zhao JZ, Ni R, Chow R, Rink A, Weersink R, Croke J, Raman S. Artificial intelligence applications in brachytherapy: A literature review. Brachytherapy. 2023 Jul-Aug;22(4):429-445. doi: 10.1016/j.brachy.2023.04.003. [DOI] [PubMed]
- 106.Jarrett D., Stride E., Vallis K., Gooding M.J. Applications and limitations of machine learning in radiation oncology. Br J Radiol. 2019;92(1100):20190001. doi: 10.1259/bjr.20190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kolla L., Parikh R.B. Uses and limitations of artificial intelligence for oncology. Cancer. 2024;130(12):2101–2107. doi: 10.1002/cncr.35307. [DOI] [PMC free article] [PubMed] [Google Scholar]