Abstract
Background
We evaluated sociodemographic and clinical predictors of financial toxicity (FT) among patients with breast cancer with higher risk clinical factors warranting regional nodal irradiation (RNI).
Methods
Among 183 participants in a clinical trial of conventional vs. hypofractionated treatment with RNI, 125 (68 %) completed a pilot survey of FT measured using the validated Economic Strain and Resilience in Cancer (ENRICh) instrument, scored from 0 (minimal) to 10 (severe) FT. Associations with predictors were evaluated using Pearson correlation coefficients and Kruskal Wallis, Mann-Whitney U, and Jonckheere-Terpstra tests. Predictors of severe FT (ENRICh≥5) were tested using multivariable logistic regression with odds ratios converted to relative risks (RR).
Results
Of the sample, all received RNI, 92 % chemotherapy, 67 % axillary dissection, 26 % mastectomy without reconstruction, and 32 % mastectomy with reconstruction. At a median follow up of 1.48 years, median FT score was 2.13 (IQR 0.93–4.6), with 20.8 % of patients experiencing severe FT. Unadjusted worse FT score was associated with younger age (P = 0.003), Hispanic ethnicity (P = 0.006), lower income (P = 0.02), shorter interval from diagnosis to FT assessment (P = 0.02), and chemotherapy receipt (P = 0.05), but not with breast surgery type (P = 0.42), axillary surgery type (P = 0.33), or pathologic T (P = 0.68) or N stage (P = 0.47). In multivariable analysis, triple negative subtype was the sole clinical factor predicting severe FT (RR = 3.38; 95 % CI 1.48–4.99; P = 0.01).
Conclusion
Among patients with breast cancer receiving RNI, triple negative subtype was associated with severe FT, suggesting that tumor receptor subtype may help identify a key breast cancer subpopulation for early FT intervention.
Keywords: Breast cancer, Financial toxicity, Triple negative subtype, Radiation therapy
Highlights
-
•
125 patients with breast cancer receiving regional nodal irradiation participated.
-
•
Prevalence of severe financial toxicity (FT) was 20.9 %.
-
•
Younger age, lower income, and Hispanic ethnicity predicted severe FT.
-
•
Triple negative receptor subtype led to a threefold increase in risk of severe FT.
1. Introduction
Financial toxicity (FT) in patients with cancer is comprised of the monetary and psychosocial hardships stemming from financial burdens related to disease and treatment and includes out-of-pocket costs, medical debt, and strain on patients’ financial coping resources [1,2]. Patients experiencing FT suffer from psychosocial distress, bankruptcy, financial ruin, and material deprivation. In addition, they are more likely to miss oncology visits, delay and omit oncology and general medical treatment, and have increased acute oncology care utilization [[3], [4], [5], [6], [7]]. Patients with cancer are susceptible to FT [8]. In the US, a prior analysis demonstrated that 13 % of patients with cancer <65 years old spent at least 20 % of their income on out-of-pocket expenses and approximately 50 % of Medicare beneficiaries (age ≥65 years old) with cancer spent more than 10 % of their income on out-of-pocket expenses [9,10]. In a systematic review specific for patients with breast cancer, the prevalence of FT was 35.3 % in well-resourced countries such as the US. Known risk factors for FT in patients with breast cancer include lower income, younger age, and more advanced cancer stage [11,12].
Given that patients with breast cancer and younger age and more advanced stage commonly receive up to 6 weeks of daily radiation therapy as standard treatment, radiation oncologists and clinical teams are uniquely positioned to screen and connect patients at risk to evidence-based supportive interventions to address FT [8,10,13,14]. Yet among patients with breast cancer with higher risk disease, the impact of specific clinical factors on FT such as breast cancer subtype, which influences both intensity of treatment and risk of adverse clinical outcomes [15], has not been thoroughly defined, impeding the potential benefits of early identification of at risk individuals. This gap in knowledge contributes to gaps in care for patient subgroups who are most likely to benefit from early FT intervention. Our institution has been conducting the Shortening Adjuvant PHoton Irradiation to Reduce Edema (SAPHIRE) trial (NCT02912312), an ongoing investigator-initiated, randomized clinical trial comparing hypofractionation to conventional fractionation in patients with breast cancer with clinical risk factors meriting regional nodal irradiation (RNI). This patient cohort presents a unique opportunity to evaluate predictors of FT among higher-risk patients with breast cancer and includes a diverse population treated in an urban setting. Accordingly, in an analytic cohort of patients participating in the SAPHIRE trial and therefore who exhibited clinical factors meriting RNI, we evaluated for sociodemographic and clinical predictors of FT. Additionally, this analysis sought to evaluate the specific influence of tumor receptor status on FT outcomes, given the singular impact of breast cancer subtype on both cancer treatment and prognosis.
2. Materials and methods
2.1. Study sample
The study sample was derived from the SAPHIRE prospective randomized trial of conventional (50 Gy in 25 fractions±boost of 10–14 Gy in 5–7 fractions) versus hypofractionated regional nodal irradiation (40.05 Gy in 15 fractions±boost of 10–14 Gy in 5–7 fractions). The SAPHIRE trial includes patients age ≥18 years with pathologically-confirmed invasive breast cancer, stage T0-T3, N0-N2a or N3a, M0, treated with curative-intent breast and nodal surgery, for whom the treating radiation oncologist has recommended regional nodal irradiation. Among trial participants diagnosed between March 2016 and May 2019 (before initiation of a multi-center expansion of the trial), individuals were invited to enroll on the Economic Strain and Resilience in Cancer study (NCT04592250), an observational survey study of FT in individuals with cancer and survivors if they were alive at the time of the survey study, able to read the English survey, and were contactable by a US phone number for survey follow-up. All participating patients received breast cancer treatment at either the Main center or regional community-based satellite locations for the institution. A total of 183 trial participants were invited in person or by telephone during standard clinical follow-up or trial follow-up to participate in this cross-sectional survey study of FT. Of those invited, a total of 125 (68 %) completed the survey. This study was approved by the Institutional Review Board.
2.2. Outcome measure: financial toxicity (FT)
The Economic Strain and Resilience in Cancer (ENRICh) instrument is a validated measure of multi-domain FT, containing 15 items that measure patient-reported domains of FT [2,6,[16], [17], [18], [19]]. This includes overall FT scores in addition to sub-scores for the following domains: a) material hardships, b) depletion of coping resources, and c) psychological burdens related to financial hardship. The material, coping, and psychological FT subscores as well as the overall FT score were calculated as an arithmetic weighted average and scored on a scale from 0 to 10 (least to most severe FT). In analyses, the overall FT scores were characterized as a continuous variable or dichotomized at scores of ≥5 vs. <5 based on prior analysis identifying this cutpoint as a marker of severe FT [6].
2.3. Clinical risk factors
Disease- and treatment-related clinical variables were abstracted from the electronic medical record as follows: estrogen receptor (ER), progesterone receptor (PR), HER2, BRCA 1/2 mutation status, pathologic T and N stage, type of breast and reconstructive surgery, type of axillary surgery, and receipt of chemotherapy. For analyses, receptor subtype was coded as ER+ and/or PR + HER2-, HER2+ any ER status, and ER- PR- HER2- (triple negative). Two patients presented with occult breast primary and did not undergo breast surgery; these two were combined with patients undergoing segmental mastectomy for all analyses that included type of breast surgery. As the multi-center SAPHIRE clinical trial is ongoing with continued assessment of primary clinical outcomes and secondary FT outcomes, this study does not include analyses by randomization group or radiation treatment dose/schedule.
2.4. Other covariates
Survey participants reported educational attainment, household income, health insurance type, and race/ethnicity, coded as Black non-Hispanic (herein Black), Hispanic any race (herein Hispanic), White non-Hispanic (herein White), and other. Other demographic covariates abstracted from electronic medical records included age at diagnosis, diagnosis to survey time, and gender. Survey participants also reported financial distress severity, that is, financial worry, using the COmprehensive Score for financial Toxicity (COST) measure [20].
2.5. Statistical analysis
Descriptive statistics were generated to summarize patients' sociodemographic, disease and treatment characteristics. Univariate associations of FT scores and categorical sociodemographic and clinical treatment characteristics were tested using non-parametric tests (Mann-Whitney U test or Kruskal Wallis test). Pearson correlation coefficient was applied to examine the relationship between age and continuous FT scores. Jonckheere-Terpstra test was used to evaluate for trend pattern between the ordinal characteristic of the household income and continuous FT scores. To evaluate the influence of breast cancer subtype and FT, univariate associations between receptor profile category and patients' sociodemographic and treatment characteristic were conducted, using Chi-square test or Fisher's exact test for categorical predictor variables and Kruskal Wallis for continuous predictor variables.
Multivariable logistic regression with backwards selection was used to identify predictors of severe financial toxicity (FT score ≥5). Age was forced into the model given prior literature documenting its importance as a predictor of financial toxicity [11,12,21]. Tumor subtype was also forced into the model given our primary interest in this predictor variable. Other variables were retained in the final model with P < 0.05. Odds ratios were converted to relative risks (RR) using the method of Zhang et al. given that the primary outcome was not rare [22]. SAS Enterprise Guide version 7.1 was used for all statistical analyses.
3. Results
3.1. Patient characteristics
Of respondents, median age at diagnosis was 53 years (interquartile range [IQR] 44 to 63) and one participant was male (0.8 %). Race/ethnicity was as follows: Black (8.8 %), Hispanic (14.4 %), White (72.8 %), and other (4.0 %). Median interval from diagnosis to survey completion was 1.48 years (IQR 1.29–2.05) with 72 % of patients completing their survey within two years of diagnosis.
Breast cancer subtype was as follows: 61.6 % ER+ and/or PR+ and HER2-, 24.0 % HER2+, and 14.4 % triple negative. Regarding treatment, 40.8 % underwent segmental mastectomy, 25.6 % underwent mastectomy without breast reconstruction, 32.0 % underwent mastectomy with breast reconstruction, and 1.6 % did not undergo breast surgery as they presented with an occult breast primary. Of the 40 patients who initiated postmastectomy breast reconstruction, status of reconstruction at the time of survey was as follows: 40 % with tissue expander, 35 % completed tissue-based reconstruction, 7.5 % completed latissimus flap reconstruction, 7.5 % completed permanent breast implant reconstruction, and 10 % had their tissue expander explanted due to complication. A total of 92.0 % of patients in the cohort received any chemotherapy, 82.1 % received neoadjuvant chemotherapy, 36.6 received adjuvant chemotherapy, 74.1 % received an anthracycline, 83.9 % received a taxane, and 27.4 % received trastuzumab (Table 1).
Table 1.
Univariate associations of baseline patient characteristics with financial toxicity.
| N (%) | Median Global ENRICh score | IQR | P | |
|---|---|---|---|---|
| Sociodemographic variables | ||||
| Age (Median, IQR) | 53 (44, 63) | 2.13 | 0.93–4.6 | 0.003a |
| Sex | ||||
| Female | 124 (99.2) | 2.13 | 0.93–4.63 | 0.94 |
| Male | 1 (0.8) | 2.33 | 2.33–2.33 | |
| Race/Ethnicity | ||||
| Black | 11 (8.8) | 2.33 | 0.93–5.36 | 0.006 |
| Hispanic | 18 (14.4) | 4.33 | 2.53–6.73 | |
| White | 91 (72.8) | 1.87 | 0.4–3.73 | |
| Other | 5 (4.0) | 1.73 | 1.6–5.73 | |
| Diagnosis to survey time | ||||
| ≤24 months | 90 (72.0) | 2.47 | 1.13–4.93 | 0.02 |
| >24 months | 35 (28.0) | 1.47 | 0–3.13 | |
| Household income | ||||
| $0 to $9999 | 2 (1.6) | 4.8 | 3.67–5.93 | 0.02b |
| $15,000 to $19,999 | 4 (3.2) | 4.03 | 1.1–6.87 | |
| $20,000 to $34,999 | 5 (4.0) | 2.87 | 1.33–6.73 | |
| $35,000 to $49,999 | 7 (5.6) | 1.67 | 1.6–5.67 | |
| $50,000 to $74,999 | 21 (16.8) | 2.4 | 1.6–4.07 | |
| $75,000 to $99,999 | 19 (15.2) | 2.53 | 0.53–5.27 | |
| $100,000 to $199,999 | 40 (32.0) | 1.73 | 0.7–4.03 | |
| $200,000 or more | 23 (18.4) | 0.93 | 0.07–3.87 | |
| No Response | 4 (3.2) | 3.33 | 2.56–3.83 | |
| Education | ||||
| Less than High School | 1 (0.8) | 5.93 | 5.93–5.93 | 0.89 |
| High School or GED | 13 (10.4) | 2.11 | 0–5.87 | |
| Some College or Trade | 35 (28.0) | 2.13 | 0.27–5.27 | |
| College Degree | 37 (29.6) | 2.13 | 1.07–3.4 | |
| Graduate Degree | 32 (25.6) | 2.13 | 1.07–4.77 | |
| Advanced Degree | 5 (4.0) | 4.67 | 1.27–4.67 | |
| No Response | 2 (1.6) | 2.63 | 1.6–3.67 | |
| Insurance | ||||
| Employer purchased | 80 (64.0) | 2.45 | 0.97–5.31 | 0.28 |
| Family purchased | 10 (8.0) | 1.87 | 0.93–2.2 | |
| Medicaid or Medicare | 9 (7.2) | 3.13 | 2.2–3.67 | |
| Multiple plan | 26 (20.8) | 1.77 | 0.14–3.4 | |
| Breast cancer details | ||||
| Pathologic T stage | ||||
| Tis/T0/T1/T2 | 110 (88.0) | 2.13 | 0.87–4.67 | 0.68 |
| T3/T4 | 15 (12.0) | 2.73 | 1.6–3.8 | |
| Pathologic N stage | ||||
| N0 | 45 (36.0) | 2.8 | 0.73–4.93 | 0.47 |
| N1/N2/N3 | 80 (64.0) | 2.03 | 1–4.1 | |
| Breast Cancer Subtype | ||||
| ER+ and/or PR + HER2- | 77 (61.6) | 1.87 | 0.93–3.8 | 0.07 |
| HER2+, any ER/PR | 30 (24.0) | 2.53 | 1.07–4.67 | |
| Triple negative | 18 (14.4) | 4.93 | 1–6.93 | |
| BRCA1/2 mutation status | 0.92 | |||
| No | 120 (96.0) | 2.17 | 0.93–4.37 | |
| Yes | 5 (4.0) | 1.13 | 1.00–5.73 | |
| Treatment | ||||
| Chemotherapy | ||||
| No | 10 (8.0) | 1.27 | 0–2.2 | 0.05 |
| Yes | 115 (92.0) | 2.33 | 0.93–4.93 | |
| Anthracycline | ||||
| No | 29 (25.9) | 2.50 | 0.73–3.73 | 0.71 |
| Yes | 83 (74.1) | 2.13 | 1.00–4.93 | |
| Taxane | ||||
| No | 18 (16.1) | 1.89 | 0.27–5.40 | 0.46 |
| Yes | 94 (83.9) | 2.37 | 1.07–4.93 | |
| Trastuzumab | ||||
| No | 82 (72.6) | 2.13 | 0.93–4.93 | 0.54 |
| Yes | 31 (27.4) | 2.53 | 1.07–5.27 | |
| Chemotherapy prior to breast surgery | ||||
| No | 20 (17.9) | 2.00 | 0.46–4.33 | 0.44 |
| Yes | 92 (82.1) | 2.40 | 1.07–5.10 | |
| Chemotherapy after last breast surgery | ||||
| No | 71 (63.4) | 2.33 | 1.00–4.67 | 0.48 |
| Yes | 41 (36.6) | 2.20 | 0.87–5.27 | |
| Breast surgery | ||||
| Segmental mastectomy | 51 (40.8) | 1.6 | 0.40–4.00 | 0.42 |
| Mastectomy, no Recon | 32 (25.6) | 2.45 | 1.30–4.17 | |
| Mastectomy with Recon | 40 (32.0) | 2.63 | 0.93–5.33 | |
| None | 2 (1.6) | 2.8 | 2.20–3.40 | |
| Axillary surgery | ||||
| Axillary lymph node dissection | 84 (67.2) | 2.2 | 1.13–4.1 | 0.33 |
| Sentinel node biopsy | 41 (32.8) | 1.6 | 0.4–4.67 | |
| Radiation | ||||
| Received radiation therapy | 125 (100) | 2.13 | 0.93–4.6 | – |
Abbreviations: ER (estrogen receptor), IQR (interquartile range), PR (progesterone receptor), Recon (breast reconstruction).
P-value from Pearson correlation coefficient.
P-value from Jonckheere-Terpstra test All other P-values from Mann-Whitney U test or Kruskal Wallis test.
3.2. Univariate analysis: predictors of financial toxicity
Median ENRICh score for the overall cohort was 2.13 (IQR 0.93 to 4.60) with 20.8 % of patients experiencing severe FT (ENRICh score≥5). Median COST financial distress score was 24.6 (IQR 16.5 to 34.1) with 47.6 % of patients experiencing severe FT (COST score <24) based on this measure. The Pearson correlation coefficient of ENRICh FT score with COST financial distress score was −0.77, indicating a high degree of correlation between the two measures (Supplementary Fig. 1). Of the 26 patients classified with severe FT by ENRICh, 25 (96.2 %) had severe FT by COST (P < 0.001).
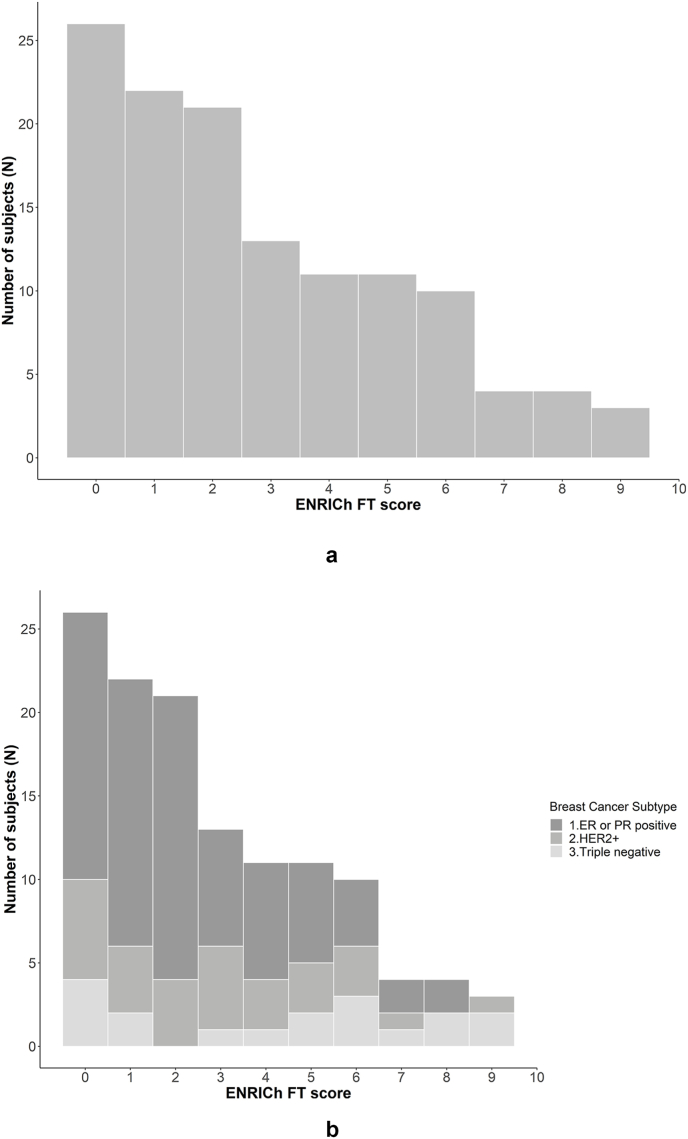
Fig. 1A depicts ENRICh FT score distribution for the overall cohort and Fig. 1B by subtype. Higher ENRICh score was also associated with younger age at diagnosis (P = 0.003), Hispanic ethnicity (P = 0.006), and shorter interval from diagnosis to survey (P = 0.02) but was not associated with type of breast or axillary surgery (P = 0.42 and P = 0.33, respectively). Associations of ENRICh score, when treated as a continuous variable, with receipt of chemotherapy and subtype, were of borderline statistical significance (P = 0.05 and 0.07, respectively) (Table 1). In subset analysis, subtype was associated with significantly higher risk of financial toxicity among patients who received adjuvant chemotherapy and those who received a taxane (Supplementary Tables 1 and 2). Subtype was also strongly associated with severe (ENRICh ≥5) FT (P = 0.02). A total of 23 % (n = 29/125) of patients experienced at least one emergency room visit between diagnosis and date of survey completion. However, ENRICh score was not associated with either any emergency room visit (P = 0.99) or number of emergency room visits (P = 0.93).
Fig. 1A.
Distribution of ENRICh score for the study population
Fig. 1B. Distribution of ENRICh score by breast cancer subtype.
3.3. Univariate analysis: associations of receptor profile with other covariables
Table 2 demonstrates that there was no association between breast cancer subtype and age, race/ethnicity, education, insurance status, or BRCA1/2 mutation status. In contrast, there was a higher likelihood of pathologic N0 status in patients with triple negative and HER2+ breast cancer, reflecting the greater likelihood of nodal pathologic complete response in these subtypes compared to estrogen receptor positive disease when treated with neoadjuvant chemotherapy. Breast cancer subtype was not associated with any emergency room visit (P = 0.59) or number of emergency room visits (P = 0.68).
Table 2.
Univariate associations of covariates with subtype.
| ER or PR Positive N (%) |
HER2+ N (%) |
Triple Negative N (%) |
P | |
|---|---|---|---|---|
| Sociodemographic variables | ||||
| Age(Median, IQR) | 53.2 (44.5–64.1) | 53.1 (44.3–61.2) | 56.4 (35.2–61.6) | 0.93a |
| Sex | 1.00 | |||
| Female | 76 (98.7 %) | 30 (100.0 %) | 18 (100.0 %) | |
| Male | 1 (1.3 %) | 0 (0.0 %) | 0 (0.0 %) | |
| Race/Ethnicity | 0.81 | |||
| Black | 7 (9.1 %) | 2 (6.7 %) | 2 (11.1 %) | |
| Hispanic | 9 (11.7 %) | 5 (16.7 %) | 4 (22.2 %) | |
| White | 58 (75.3 %) | 21 (70.0 %) | 12 (66.7 %) | |
| Other | 3 (3.9 %) | 2 (6.7 %) | 0 (0.0 %) | |
| Diagnosis to survey time | 0.98 | |||
| ≤24 months | 55 (71.4 %) | 22 (73.3 %) | 13 (72.2 %) | |
| >24 months | 22 (28.6 %) | 8 (26.7 %) | 5 (27.8 %) | |
| Household income | 0.73 | |||
| $0 to $9999 | 2 (2.6 %) | 0 (0.0 %) | 0 (0.0 %) | |
| $15,000 to $19,999 | 3 (3.9 %) | 0 (0.0 %) | 1 (5.6 %) | |
| $20,000 to $34,999 | 2 (2.6 %) | 2 (6.7 %) | 1 (5.6 %) | |
| $35,000 to $49,999 | 3 (3.9 %) | 2 (6.7 %) | 2 (11.1 %) | |
| $50,000 to $74,999 | 15 (19.5 %) | 4 (13.3 %) | 2 (11.1 %) | |
| $75,000 to $99,999 | 9 (11.7 %) | 7 (23.3 %) | 3 (16.7 %) | |
| $100,000 to $199,999 | 24 (31.2 %) | 8 (26.7 %) | 8 (44.4 %) | |
| $200,000 or more | 16 (20.8 %) | 6 (20.0 %) | 1 (5.6 %) | |
| No Response | 3 (3.9 %) | 1 (3.3 %) | 0 (0.0 %) | |
| Education | 0.92 | |||
| Less than High School | 1 (1.3 %) | 0 (0.0 %) | 0 (0.0 %) | |
| High School or GED | 8 (10.4 %) | 3 (10.0 %) | 2 (11.1 %) | |
| Some College or Trade | 22 (28.6 %) | 9 (30.0 %) | 4 (22.2 %) | |
| College Degree | 23 (29.9 %) | 8 (26.7 %) | 6 (33.3 %) | |
| Graduate Degree | 20 (26.0 %) | 6 (20.0 %) | 6 (33.3 %) | |
| Advanced Degree | 2 (2.6 %) | 3 (10.0 %) | 0 (0.0 %) | |
| No Response | 1 (1.3 %) | 1 (3.3 %) | 0 (0.0 %) | |
| Insurance | 0.37 | |||
| Employer purchased | 44 (57.1 %) | 23 (76.7 %) | 13 (72.2 %) | |
| Family purchased | 7 (9.1 %) | 2 (6.7 %) | 1 (5.6 %) | |
| Medicaid or Medicare | 7 (9.1 %) | 0 (0.0 %) | 2 (11.1 %) | |
| Multiple plan | 19 (24.7 %) | 5 (16.7 %) | 2 (11.1 %) | |
| Breast cancer details | ||||
| Pathologic T stage | 0.13 | |||
| Tis/T0/T1/T2 | 64 (83.1 %) | 29 (96.7 %) | 17 (94.4 %) | |
| T3/T4 | 13 (16.9 %) | 1 (3.3 %) | 1 (5.6 %) | |
| Pathologic N stage | <0.001 | |||
| N0 | 15 (19.5 %) | 22 (73.3 %) | 8 (44.4 %) | |
| N1/N2/N3 | 62 (80.5 %) | 8 (26.7 %) | 10 (55.6 %) | |
| BRCA1/2 mutation status | 0.24 | |||
| No | 75 (97.4 %) | 29 (96.7 %) | 16 (88.9 %) | |
| Yes | 2 (2.6 %) | 1 (3.3 %) | 2 (11.1 %) | |
| Treatment | ||||
| Received chemotherapy | 0.11 | |||
| No | 9 (11.7 %) | 0 (0.0 %) | 1 (5.6 %) | |
| Yes | 68 (88.3 %) | 30 (100.0 %) | 17 (94.4 %) | |
| Breast surgery | 0.88 | |||
| Segmental Mastectomy | 35 (45.5 %) | 11 (36.7 %) | 7 (38.9 %) | |
| Mastectomy, no recon | 19 (24.7 %) | 9 (30.0 %) | 4 (22.2 %) | |
| Mastectomy with recon | 23 (29.9 %) | 10 (33.3 %) | 7 (38.9 %) | |
| Axillary surgery | 0.37 | |||
| Axillary lymph node dissection | 54 (70.1 %) | 17 (56.7 %) | 13 (72.2 %) | |
| Sentinel node biopsy | 23 (29.9 %) | 13 (43.3 %) | 5 (27.8 %) | |
Abbreviations: ER (estrogen receptor), IQR (interquartile range), PR (progesterone receptor), Recon (breast reconstruction).
P-value from Kruskal Wallis test. All other P-values from Chi-square test or Fisher exact test depending on underlying data distributions.
3.4. Multivariable analysis: predictors of severe financial toxicity
In multivariable logistic regression, risk factors for severe FT (ENRICh ≥5.0) included age less than 60 years, lower educational attainment, non-White race, and triple negative receptor status (Table 3). Specifically, the relative risk of severe FT for individuals with triple negative tumors, as compared to those with ER+ and/or PR + HER2-tumors, was 3.38 (95 % CI 1.48–4.99) (P = 0.01). In contrast, patients with HER2+ tumors, compared to those with ER+ and/or PR + HER2-tumors, did not experience a greater risk of severe FT (risk ratio 1.63, 95 % CI 0.65–3.04; P = 0.27). Neither type of breast surgery nor type of axillary surgery were retained in this model, demonstrating that surgical treatment choices did not substantially impact FT in this cohort.
Table 3.
Multivariable logistic model: Predictors of severe financial toxicity (Economic Strain and Resilience in Cancer [ENRICh] instrument score ≥5).
| RR | 95 % CI | Pa | |
|---|---|---|---|
| Age at Diagnosis | |||
| <40 years | 2.90 | 0.96–4.66 | 0.06 |
| 40–49 years | 2.73 | 0.98–4.28 | 0.05 |
| 50–59 years | 2.88 | 1.08–4.63 | 0.04 |
| 60 years and older | Ref. | ||
| Education | |||
| High School to Some College | 2.64 | 1.24–4.37 | 0.01 |
| College and above | Ref. | ||
| Race | |||
| White | Ref. | ||
| Black/Hispanic/Other | 1.77 | 1.11–2.24 | 0.02 |
| Subtype | |||
| ER+ and/or PR+ | Ref. | ||
| HER2+ | 1.63 | 0.65–3.04 | 0.27 |
| ER- PR- HER2- (triple negative) | 3.38 | 1.48–4.99 | 0.01 |
Abbreviations: CI (confidence interval), RR (risk ratio). Hosmer Lemeshow P-value is 0.52, indicating sufficient goodness of fit for this model.
P-value for adjusted ORs used to approximate the RRs.
3.5. Differences in financial toxicity by systemic therapy and receptor profile
Table 4 presents the distribution of any chemotherapy, neoadjuvant chemotherapy, adjuvant capecitabine, adjuvant trastuzumab emtansine, and adjuvant cyclin-dependent kinase (cdk) 4/6 inhibition by tumor subtype. Of note, 5 of 18 patients (27.8 %) with triple negative breast cancer received adjuvant capecitabine, 5 of 30 patients (16.7 %) with HER2+ disease received adjuvant trastuzumab emtansine, and 5 of 68 patients (6.5 %) with ER+ and/or PR + HER2-disease received adjuvant cdk4/6 inhibition. Table 5 presents median (IQR) ENRICh FT score by type of systemic therapy and receptor profile. Of note, for the patients with triple negative disease who received adjuvant capecitabine, median ENRICh FT score was 7.93 (IQR 6.93–8.57) compared to median ENRICh FT score of 3.20 (IQR 0.27–5.67) for patients with triple negative disease who did not receive adjuvant capecitabine (P = 0.02). In contrast, for patients with ER+ and/or PR + HER2-disease and HER2+ disease, median ENRICh FT scores were numerically similar regardless of systemic therapy type and sequence.
Table 4.
Distribution of systemic therapy by receptor profile.
| Receptor Profile | Any Chemotherapy N (%) |
Neoadjuvant Chemotherapy N (%) |
Adjuvant Capecitabine N (%) |
No Adjuvant Capecitabine N (%) |
Adjuvant T-DM1 N (%) |
Adjuvant CDK4/6 Inhibition N (%) |
|---|---|---|---|---|---|---|
| ER or PR Positive (N = 77) | 68 (88.3 %) | 47 (61.0 %) | 1 (1.3 %) | 76 (98.7 %) | 0 (0) | 5 (6.5 %) |
| Her2+ (N = 30) | 30 (100.0 %) | 28 (93.3 %) | 0 (0) | 30 (100.0 %) | 5 (16.7 %) | 0 (0) |
| Triple negative (N = 18) | 17 (94.0 %) | 17 (94.4 %) | 5 (27.8 %) | 13 (72.2 %) | 0 (0) | 0 (0) |
| Total | 115 | 92 | 6 | 119 | 5 | 5 |
T-DM1 (trastuzumab emtansine).
Table 5.
Median ENRICh Score by breast cancer subtype and Systemic Therapy.
| Receptor Profile | Any Chemotherapy Median (IQR) |
Neoadjuvant Chemotherapy Median (IQR) |
Adjuvant Capecitabine Median (IQR) |
No Adjuvant Capecitabine Median (IQR) |
Adjuvant T-DM1 Median (IQR) |
Adjuvant CDK4/6 Inhibition Median (IQR) |
|---|---|---|---|---|---|---|
| ER or PR Positive (N = 77) | 1.99 (0.93–3.97) | 1.73 (0.93–3.40) | 1.60 (1.60–1.60) | 1.87 (0.90–3.83) | NA | 1.60 (1.33–3.40) |
| Her2+ (N = 30) | 2.53 (1.07–4.67) | 2.70 (1.17–4.97) | NA | 2.53 (1.07–4.67) | 3.73 (3.60–6.00) | NA |
| Triple negative (N = 18) | 4.93 (1–6.93) | 4.93 (1.00–6.93) | 7.93 (6.93–8.57) | 3.20 (0.27–5.67) | NA | NA |
| Total | 115 | 92 | 6 | 119 | 5 | 5 |
Values in parentheses indicated interquartile range for the mean ENRICh score. T-DM1 (trastuzumab emtansine).
4. Discussion
In this study of patients with breast cancer and higher risk clinical factors meriting adjuvant RNI on a clinical trial of conventional versus hypofractionated radiation treatment, risk factors for severe FT involved multiple sociodemographic factors, including younger age (age<60 years), lower educational attainment (high school to some college), and non-White race/ethnicity. In addition, patients with triple negative tumors, in particular those who received adjuvant capecitabine, also experienced high rates of severe FT. In contrast, chemotherapy use was not associated with severe FT, although the high rate of chemotherapy use in this sample (92 %) limited power to measure a difference in FT by chemotherapy receipt.
It is also important to underscore the severity of FT experienced by these patients with triple negative breast cancer who received neoadjuvant chemotherapy, local-regional therapy including surgery and radiation, and adjuvant capecitabine. Their median ENRICh score of 7.93 is higher than any previously measured subgroup in a separate study of 311 patients with cancer undergoing treatment in our health system [19]. Considering that median time from diagnosis to survey completion was 1.48 years, our finding underscores that triple negative breast cancer patients who do not respond well to neoadjuvant chemotherapy suffer from prolonged, severe FT at a level that can place patients at the brink of financial catastrophe and at risk for adverse health outcomes such as excess acute care utilization or treatment non-compliance [6,7].
The association between subtype and FT noted in this study is a novel finding. Of note, sociodemographic characteristics of patients were not significantly different by receptor subtype in our sample of clinical trial participants, suggesting that there could be underlying mechanisms driving elevated risk of FT in triple negative breast cancer independent of sociodemographic patient characteristics. Our finding of particularly elevated FT risk among those patients with triple negative breast cancer who received adjuvant capecitabine suggests that intensity and duration of cytotoxic chemotherapy is one underlying factor that may contribute to FT in this population. This is consistent with prior research identifying active treatment as a key risk factor for FT [7]. Out of pocket costs for oral capecitabine have been estimated at approximately $19.66 annually among women with private insurance, suggesting that out-of-pocket cost is unlikely to be the sole or even primary driving factor of FT among patients receiving capecitabine [23]. We hypothesize that the cumulative effects of pre- and post-operative chemotherapy, coupled with the unique toxicity profile of capecitabine which can impact toxicity, functional, or performance status, could contribute to FT through impairment of ability to work and participate in caregiving roles.
While the specific use of adjuvant capecitabine is currently less favored with adoption of the Keynote 522 regimen for triple negative breast cancer [24], our findings nevertheless support the conceptual model that the addition of potentially efficacious systemic therapies with potentially added clinical toxicities could accentuate the burden of FT in patients with cancer. As treatment regimens for patients with more refractory disease continue to lengthen survival yet also increase risks of toxicity, it is imperative for both researchers and clinicians to be mindful of the associated financial burdens in the settings of both novel treatment development and treatment decision-making.
Several findings of this study conducted within a radiation oncology clinical trial are relevant to radiation oncologists and associated ancillary services. First, it is important for radiation oncologists to be aware of, and sensitive to, the fact that many of their more advanced breast cancer patients are experiencing FT. The daily trips to and from the radiation oncology treatment center exert their own toll of FT as well, both in terms of direct costs to the patient and lost productivity in work and caregiving roles. However, the patient's daily presence in the radiation oncology clinic also provides opportunities to ensconce patients with supportive services, such as social work support and/or financial counseling [2]. Our study findings suggest that younger patients and those with protracted adjuvant and neoadjuvant treatment plans may be most at risk and thus most in need of such resources. Finally, our study findings underscore the importance of studying both the clinical and financial impact of shortening radiation treatment schedules to understand whether FT during the radiation treatment phase can be specifically ameliorated with such strategies.
There are several limitations to consider regarding this study. For example, our sample was relatively small and limited to insured, English-speaking patients participating in a clinical trial at a tertiary cancer center and thus findings could underestimate the burden of FT among the overall population of patients with breast cancer receiving RNI. Second, the survey was only administered cross-sectionally at a median of 1.48 years since diagnosis, and thus study findings cannot document the entire trajectory of financial toxicity or establish the patient's long-term financial status after recovering from initial cancer treatment. Third, the sample was drawn from a relatively uniform cohort all treated with RNI on a clinical trial, and thus findings are not directly applicable to patients with early-stage breast cancer whose treatment burdens differ. Finally, as the sample was drawn from an ongoing randomized trial comparing conventional versus hypofractionation, our Data Safety and Monitoring Board did not allow this study to compare FT by radiation treatment schedule.
5. Conclusion
In summary, in this study of women with breast cancer, triple negative receptor status was associated with more severe FT even after adjustment for sociodemographic factors, whereas type of surgical treatment and T and N stage were not. Further prospective research is needed to delineate detailed underlying mechanisms of FT among the at-risk subpopulation of triple negative breast cancer patients, and whether FT in this group can be mitigated through proactive FT screening and financial navigation or shorter radiation treatment courses.
Ethical Approval
This work was approved by the University of Texas MD Anderson Institutional Review Board and complies with the ethical consent policies of the Journal.
Conflict of interest
Dr G Smith receives research funding from National Association for Proton Therapy and Scripps Health via Genentech and consulting fees from AstraZeneca. Dr B Smith has a royalty and equity interest in Oncora Medical, receives research funding from Artidis, the Cancer Research and Prevention Institute of Texas, and Varian Medical Systems. He previously served on the ASTRO Board of Directors. Dr. Shaitelman receives contracted research support from Artidis and Exact Sciences and has received consulting fees from Lumicell and BD. Dr Chavez-MacGregor has received honoraria from Astra Zeneca, Exact Sciences, Merck, Novartis, and Daichii-Sankyo; she has received travel support from Exact Sciences, Astra Zeneca, and Merck; she participates in Advisory Boards for Genentech and Astra Zeneca; she serves on the ASCO Board, Hope Foundation Board, and Legacy Community Health Board. Dr. Shih receives consulting fees for serving on Sanofi's OncoCollective Advisory Board.
CRediT authorship contribution statement
Grace L. Smith: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. Benjamin D. Smith: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. Simona F. Shaitelman: Data curation, Formal analysis, Writing – review & editing, Conceptualization, Data curation, Writing – review & editing. Kelsey Kaiser: Investigation, Methodology, Writing – review & editing. Julia J. Shi: Data curation, Investigation, Writing – original draft, Writing – review & editing, Investigation, Methodology, Writing – review & editing. Sanjay S. Shete: Data curation, Investigation, Writing – review & editing, Conceptualization, Writing – review & editing, Project administration, Writing – review & editing. Ying-Shiuan Chen: Data curation, Writing – review & editing. Robert J. Volk: Conceptualization, Writing – review & editing. Sharon H. Giordano: Conceptualization, Investigation, Methodology, Writing – review & editing. Karen E. Hoffman: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing, Conceptualization, Writing – review & editing.
Acknowledgments
This study was supported in part by the National Cancer Institute (NIH/NCI K07CA211804 and NIH R01CA247307) (GLS) and by the Rising Tide Foundation (KEH, BDS). Dr Giordano's effort is supported by Komen SAC150061. Dr Chavez-MacGregor's effort is supported by Susan G. Komen SA220221 and The Breast Cancer Research Foundation BCRF23-190. Dr Shih is supported by the National Cancer Institute (NCI R01CA225647). The study was also supported, in part, by The University of Texas MD Anderson Cancer Center and NCI P30 CA016672.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2024.103813.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Altice C.K., Banegas M.P., Tucker-Seeley R.D., et al. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith G.L., Banegas M.P., Acquati C., et al. Navigating financial toxicity in patients with cancer: a multidisciplinary management approach. CA Cancer J Clin. 2022;72:437–453. doi: 10.3322/caac.21730. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey S.D., Bansal A., Fedorenko C.R., et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34:980–986. doi: 10.1200/JCO.2015.64.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren J.L., Mariotto A.B., Stevens J., et al. Association of major adverse financial events and later-stage cancer diagnosis in the United States. J Clin Oncol. 2024;42:1001–1010. doi: 10.1200/JCO.23.01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yabroff K.R., Doran J.F., Zhao J., et al. Cancer diagnosis and treatment in working-age adults: implications for employment, health insurance coverage, and financial hardship in the United States. CA Cancer J Clin. 2024;74:341–358. doi: 10.3322/caac.21837. [DOI] [PubMed] [Google Scholar]
- 6.Shi J.J., Maldonado J.A., Wu C.F., et al. Financial toxicity in cancer patients and subsequent risk of repeat acute care utilization. Front Psychol. 2023;14 doi: 10.3389/fpsyg.2023.1209526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith G.L., Lopez-Olivo M.A., Advani P.G., et al. Financial burdens of cancer treatment: a systematic review of risk factors and outcomes. J Natl Compr Canc Netw. 2019;17:1184–1192. doi: 10.6004/jnccn.2019.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrams H.R., Durbin S., Huang C.X., et al. Financial toxicity in cancer care: origins, impact, and solutions. Transl Behav Med. 2021;11:2043–2054. doi: 10.1093/tbm/ibab091. [DOI] [PubMed] [Google Scholar]
- 9.Bernard D.S., Farr S.L., Fang Z. National estimates of out-of-pocket health care expenditure burdens among nonelderly adults with cancer: 2001 to 2008. J Clin Oncol. 2011;29:2821–2826. doi: 10.1200/JCO.2010.33.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zafar S.Y. Financial toxicity of cancer care: it's time to intervene. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djv370. [DOI] [PubMed] [Google Scholar]
- 11.Ehsan A.N., Wu C.A., Minasian A., et al. Financial toxicity among patients with breast cancer worldwide: a systematic review and meta-analysis. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2022.55388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Offodile A.C., 2nd, Asaad M., Boukovalas S., et al. Financial toxicity following surgical treatment for breast cancer: a cross-sectional pilot study. Ann Surg Oncol. 2021;28:2451–2462. doi: 10.1245/s10434-020-09216-9. [DOI] [PubMed] [Google Scholar]
- 13.Lentz R., Benson A.B., 3rd, Kircher S. Financial toxicity in cancer care: prevalence, causes, consequences, and reduction strategies. J Surg Oncol. 2019;120:85–92. doi: 10.1002/jso.25374. [DOI] [PubMed] [Google Scholar]
- 14.Desai A., Gyawali B. Financial toxicity of cancer treatment: moving the discussion from acknowledgement of the problem to identifying solutions. EClinicalMedicine. 2020;20 doi: 10.1016/j.eclinm.2020.100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukasiewicz S., Czeczelewski M., Forma A., et al. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers. 2021;13 doi: 10.3390/cancers13174287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith G.L., Mendoza T.R., Lowenstein L.M., et al. Financial hardship in survivorship care delivery. J Natl Cancer Inst Monogr 2021. 2021:10–14. doi: 10.1093/jncimonographs/lgaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi J.J., McGinnis G.J., Peterson S.K., et al. Pilot study of a Spanish language measure of financial toxicity in underserved Hispanic cancer patients with low English proficiency. Front Psychol. 2023;14 doi: 10.3389/fpsyg.2023.1188783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu C., Smith G.L., Chen Y.S., et al. Short-form adaptive measure of financial toxicity from the Economic Strain and Resilience in Cancer (ENRICh) study: derivation using modern psychometric techniques. PLoS One. 2022;17 doi: 10.1371/journal.pone.0272804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrigan K.L., Fu S., Chen Y.S., et al. Financial toxicity impact on younger versus older adults with cancer in the setting of care delivery. Cancer. 2022;128:2455–2462. doi: 10.1002/cncr.34220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Souza J.A., Yap B.J., Wroblewski K., et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: the validation of the COmprehensive Score for financial Toxicity (COST) Cancer. 2017;123:476–484. doi: 10.1002/cncr.30369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran T.B., Malhotra G., Razavi M., et al. Emotional distress and financial toxicity in younger adult patients undergoing oncologic surgery. Ann Surg. 2022;276:694–700. doi: 10.1097/SLA.0000000000005593. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J., Yu K.F. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 23.Hager A., Gracia G., Rodin D., et al. Out-of-Pocket costs of treatment among employer-insured women with invasive breast cancer. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmid P., Cortes J., Pusztai L., et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.