Abstract
Background & Aims
Individuals with alcohol use disorder (AUD) are at risk of liver disease. There is scarce information on the effectiveness of screening for liver fibrosis on alcohol consumption. Thus, we evaluated the efficacy of a screening program for liver fibrosis on alcohol consumption in individuals with AUD.
Methods
We performed a prospective interventional study in the Hospital Clinic of Barcelona. The screening cohort included individuals with AUD from the addiction unit who underwent screening for liver fibrosis with transient elastography and counselling on lifestyle habits in the liver unit. The control cohort included individuals with similar characteristics who attended the same unit in a previous period but did not undergo screening. Effects on alcohol consumption were evaluated at 6 months, after clinical follow-up, with clinical assessment by addiction specialists and urine ethyl glucuronide monitoring.
Results
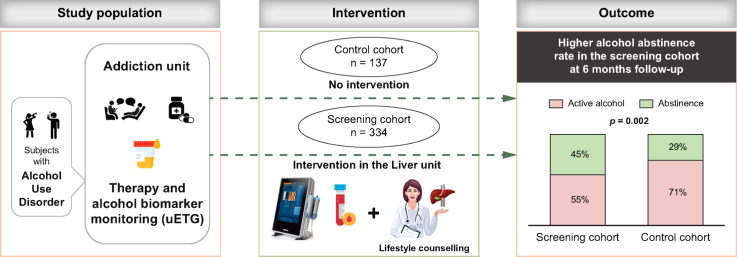
In the screening cohort, 149/334 (45%) individuals were abstinent at 6 months (68% confirmed with urine ethyl glucuronide). Alcohol abstinence was higher in the screening cohort than in the control cohort (40/137 [29%], p = 0.002). Factors associated with alcohol abstinence in the multivariate analysis of the two combined cohorts (n = 471) were: receiving AUD medications (odds ratio [OR] 1.72, 95% CI 1.11-2.67), absence of illicit drug use (OR 0.50, 95% CI 0.31-0.80) and participating in the screening program (OR 1.77, 95% CI 1.14-2.74). In the screening cohort, 40 (12%) individuals had increased liver stiffness (≥8 kPa), which was associated with obesity (p = 0.03), arterial hypertension (p = 0.03), gamma-glutamyltransferase (p <0.001) and platelet levels (p = 0.001).
Conclusions
This study shows that an integrated screening program for liver fibrosis associated with counselling on alcohol consumption in individuals with AUD allows for early diagnosis of alcohol-associated liver disease and is associated with alcohol abstinence.
Impact and implications:
Individuals with high alcohol consumption are at higher risk of liver disease compared to the general population. The potential beneficial effects of screening for liver disease in this population have scarcely been studied. We show that a screening program for liver fibrosis together with a lifestyle counselling intervention favoured alcohol abstinence among individuals with alcohol use disorder attending an addiction unit at 6 months, compared to a matched cohort who did not undergo screening. These findings suggest that screening programs for liver fibrosis have a therapeutic role in individuals with alcohol use disorder, supporting the implementation of these programs in addiction units.
Keywords: Elastography, Stiffness, Alcohol-related Liver Disease, Addiction, Biomarker
Graphical abstract
Highlights:
-
•
Liver fibrosis screening improves abstinence in patients with alcohol use disorder.
-
•
Steatosis and increased liver stiffness are prevalent (43% and 12%, respectively).
-
•
Increased liver stiffness is associated with metabolic risk factors and gamma-glutamyltransferase levels.
-
•
These results support the implementation of fibrosis screening in addiction units.
Introduction
Consumption of alcohol is a common preventable risk factor for premature death and it is associated with over 200 diseases including cancers, neuropsychiatric disorders, cardiovascular disease, cirrhosis and infectious diseases.1 In recent years, different measures have been implemented by governments, policy makers and healthcare providers to reduce the impact of alcohol consumption on global health.[2], [3], [4] In spite of this, morbidity and mortality associated with alcohol have increased globally over the past decades and are expected to increase further in the future.5
Alcohol consumption is the main etiological factor for alcohol-associated liver disease (ALD), the most frequent and burgeoning cause of liver disease and liver-related death. Chronic liver diseases are characterized by the presence of fibrosis deposition in the liver that increases in a stepwise manner from early stages to advanced stages of the disease. The prevalence of significant liver fibrosis in individuals with high alcohol consumption is markedly higher than that of the general population.6 Clinical practice guidelines on liver diseases recommend targeted screening for liver fibrosis in at-risk groups to improve early detection and propose measures to stop disease progression.[7], [8], [9] However, the impact of these screening programs on the modifiable causes of liver disease, such as alcohol consumption, is largely unknown.
To date, several studies have been performed to assess disease prevalence and develop strategies for early detection of liver disease, the majority of them focusing on the primary care setting.10,11 However, due to the underreporting of alcohol consumption in this setting it is likely that many individuals may not be identified as at risk of liver disease and may be left out of the screening strategies. Within this context, addiction units represent the ideal setting to perform early diagnosis of liver fibrosis specifically targeting populations with high-risk alcohol consumption.
The final goal of screening programs for early detection of a condition is to improve patients’ health-related outcomes.12 To date, the cost-effectiveness of screening programs for chronic liver disease, and specifically of ALD, has been investigated based on theoretical models and assumptions as to the effect of the interventions.13 In patients with ALD, alcohol abstinence is one of the main drivers of prognosis both at early and advanced stages.14,15 Few studies have investigated the effect of screening for liver disease on alcohol abstinence in populations with high-risk alcohol consumption. These studies have shown some potential beneficial effects of screening increasing alcohol abstinence, improving engagement to rehabilitation programs or reducing the amounts of alcohol consumed. Nevertheless, due to the inclusion of a low number of individuals, the lack of a control group or of detailed monitoring of alcohol consumption, these studies have left some questions unanswered.[16], [17], [18]
In this setting, the aim of our study was to investigate the effectiveness of a screening program for liver fibrosis with transient elastography (TE) on alcohol consumption in a population of individuals with alcohol use disorder (AUD) undergoing specialized addiction follow-up, compared with a control cohort that did not undergo screening for liver disease.
Patients and methods
Prospective intervention study performed in the liver unit and the addiction unit of the Hospital Clínic of Barcelona, Catalonia, Spain.
Screening cohort
The screening cohort included individuals with AUD attending the addiction unit from the 1st July 2019 to 31st December 2022. These individuals were invited to participate in a screening program for liver disease and were prospectively included. Inclusion criteria were as follows: age 18-80 years-old and high-risk alcohol consumption, defined as a weekly intake of at least 21/14 standard drinks of alcohol (1 SD = 10 g of alcohol) for men/women for at least 1 year and/or at least one episode of binge drinking per month for more than 6 months.19 Those with a previous diagnosis of chronic liver disease or ongoing specialized follow-up in a liver unit, with no significant alcohol consumption and/or without written informed consent were excluded.
Control cohort
To test the effect of the screening program on alcohol abstinence, the screening cohort was compared with a historical control cohort of consecutive patients with AUD, who attended the addiction unit of the Hospital Clinic of Barcelona in the years prior to the implementation of the screening program (1st January 2017 to 31st December 2018). Individuals in the control cohort were matched with those in the screening cohort with respect to the main confounding factors, such as age, gender, duration and quantity of alcohol consumption and active AUD medications (Table S1).
Study endpoints
The primary endpoint was the effect of a screening program for liver disease with counselling in an addiction unit on alcohol abstinence at 6 months. Secondary endpoints included the prevalence of and risk factors for liver fibrosis in this population.
Sample size calculation
To calculate the sample size, as neither pilot studies nor previously published cohorts with similar characteristics were available, the control cohort of the current study was used for the calculation of the sample sizes. In this cohort, the percentage of patients achieving alcohol abstinence at 6 months was 29%. Assuming that the effect of the screening program would increase the alcohol abstinence rate to at least 40% in the screening cohort, an alpha error of 5% and a statistical power of 80%, we calculated a sample size of at least 250 individuals – to identify a group of at least 100 patients reaching alcohol abstinence at the end of the study period – would be needed for statistical analyses.
Study design
Individuals who underwent a first visit (V0) in the addiction unit in the study period were eligible for the study. These individuals subsequently attended the liver unit (V1) where data regarding medical history, comorbidities, socio-economic status and family history were investigated. A detailed enquiry on alcohol consumption in terms of qualitative and quantitative intake and duration and pattern of consumption (e.g. binge drinking) including the Alcohol Use Disorder Identification Test (AUDIT), was performed. Physical examination with measurement of BMI was performed and written informed consent was obtained. After V1 all individuals in the screening cohort underwent the following tests: 1) Blood tests with routine parameters including liver tests (LTs); 2) TE (M or XL probes) with liver stiffness measurement (LSM), performed after at least 6 h of fasting in all cases. Controlled attenuation parameter (CAP) was also measured.
At the second visit in the Liver Unit (V2) the results of blood tests and TE were shared and discussed with the patients and counselling on alcohol consumption was performed by the hepatologist (EA, JGG, EP).
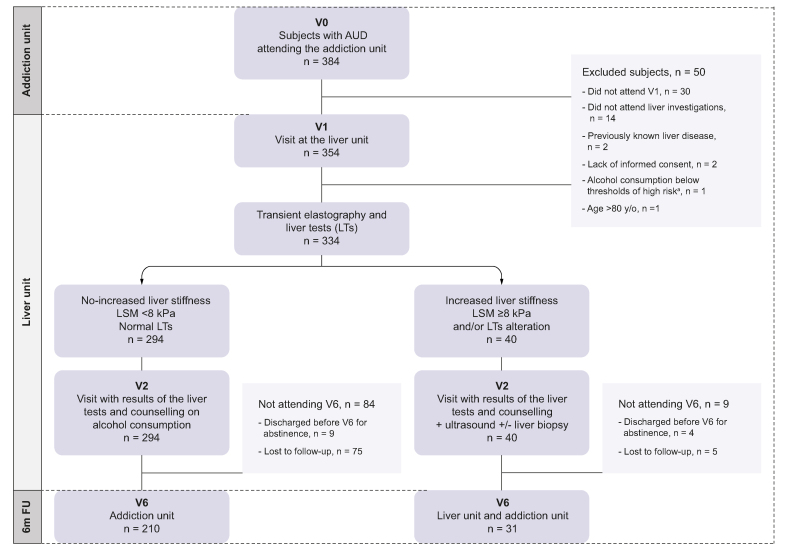
Based on TE and LT values the screening cohort was divided into two groups: 1) No increased LSM: discharged from the liver unit, continued treatment and follow-up in the addiction unit, with assessment of abstinence at 6 months (V6); 3) Increased LSM: this group was further investigated with abdominal ultrasound and a liver biopsy was recommended when liver disease was suspected. These patients continued with clinical follow-up in the liver and addiction units, with assessment of abstinence at 6 months (V6). A summary of the study design is shown in Fig. 1.
Fig. 1.
Study design and flowchart of the individuals included in the study.
V0 = first visit in the addiction unit; V1 = first visit in the liver unit; V2 = second visit in the liver unit; V6 = 6-month follow-up visit. aThreshold of high-risk: weekly intake of at least 21/14 SD (1 SD = 10 g of alcohol) for men/women for at least 1 year and/or persistent binge drinking (>6 months). AUD, alcohol use disorder; LSM, liver stiffness measurement; LTs, liver tests; SD, standard drink of alcohol.
As the study period included pre-COVID (1st July 2019 - 14th March 2020) and COVID (15th March 2020 - 31st December 2022) eras, we performed a subanalysis to explore the effect of the COVID pandemic on the main endpoints.
Definitions and interventions
Increased LSM was defined as LSM ≥8 kPa, corresponding to a Metavir fibrosis grade ≥2 as described in current guidelines.7 Advanced liver fibrosis was defined by LSM ≥15 kPa corresponding to a Metavir fibrosis grade ≥3.20 A subanalysis was performed dividing the screening cohort into four groups based on aspartate aminotransferase (AST) and bilirubin levels, and using the corresponding LSM cut-offs for fibrosis grade F ≥2 in each group, as described in the meta-analysis of Nguyen-Khac and colleagues.21
Significant alteration of LTs suggestive of liver fibrosis were defined by thrombocytopenia (platelets <150,000/ul) and/or at least a two-fold increase of AST and/or alanine aminotransferase.
Liver steatosis was defined by CAP ≥250 dB/m; individuals with CAP ≥250 dB/m and normal LSM or no other signs of chronic liver disease were discharged from the liver unit and provided with recommendations on lifestyle changes and a report for the general practitioner.
Counselling on alcohol consumption in the liver unit consisted of briefing between the hepatologist and the patients in which the following elements were discussed: feedback on the person’s alcohol use; clarification as to what constitutes low-risk alcohol consumption; information on the harms associated with risky alcohol use; benefits of reducing intake and motivational enhancement.22
Intervention in the addiction unit was done following standard clinical practice. Personalized therapy was offered to each patient depending on patients’ features and included either a psychosocial intervention alone or the combination of a psychosocial intervention and pharmacological therapies. All the following were included in the psychosocial interventions: motivational interviewing, contingency management, psychotherapy such as cognitive behaviour therapy and peer support groups and family therapies, performed both in person and/or online.
Medications for AUD were prescribed when indicated by the addiction specialist following standard clinical practice guidelines and included the following: disulfiram (if absence of significant liver disease), baclofen, gabapentin, naloxone, nalmefene and acamprosate.
Ethical approval for this study (protocol code: Alcofib; reference: HCB/2019/0407) was provided by the Ethical Committee of the Hospital Clínic of Barcelona, Spain on 05th June 2019. All research was conducted in accordance with both the Declarations of Helsinki and Istanbul. Written informed consent was obtained from each patient included in the study.
Assessment of alcohol abstinence
Data on alcohol consumption was obtained from 1) addiction specialist by direct interview with the participants and families and, 2) measurement of ethyl glucuronide in urine (uETG). Medical interviews were performed weekly or monthly depending on the patient’s need, as indicated by the addiction specialist, and were in person except in cases where remote interviews were needed. uETG was prescribed by the addiction specialist to all participants once or twice per week as part of the program. When uETG at this frequency was not feasible (i.e. long distance to the addiction Unit from home, poor transport connections or incompatibility with working hours) a monthly uETG measurement during the study period was performed; uETG was not measured in the remaining cases. Alcohol abstinence was assessed at 6 months (V6) and defined as negative uETG measurements and/or alcohol abstinence, reported by the addiction specialist for at least 3 consecutive months. Patients who were discharged from the rehabilitation program before V6 due to sustained alcohol abstinence and at low risk of alcohol relapse were also considered abstinent. Individuals with active alcohol consumption based on positive uETG measurement or the addiction specialist’s records at 6 months were considered as active alcohol consumers. Per protocol, those who were lost to follow-up before V6 were also considered as active alcohol consumers, assuming the worst-case scenario.
Liver biopsy assessment
Liver biopsy was indicated for LSM ≥8 kPa or significant alterations of LTs and/or ultrasound. Percutaneous liver biopsy was preferred to transjugular liver biopsy in the majority of cases, which was performed in individuals with signs of portal hypertension. In cases where the transjugular approach was used, hepatic venous pressure gradient (HVPG) was measured.20 Liver biopsy specimens were formalin-fixed, paraffin-embed, stained and analysed by an expert pathologist of the Hospital Clínic of Barcelona (AD). The grade of fibrosis was defined by the Metavir scale;23 presence and degree of steatosis and features of alcohol-associated hepatitis were assessed.24
Statistical analysis
Categorical variables were reported as frequencies and percentages and compared using the Pearson χ2 or Fisher's exact test. Quantitative variables were reported as median (IQR). Variables with normal and non-normal distribution were analysed through the Student's t test and the Wilcoxon rank-sum test, respectively. Factors associated with fibrosis and abstinence were analysed through univariate logistic regression analysis and multivariate logistic regression analysis with a backward stepwise approach (Wald). Spearman’s rank correlation was computed to assess the relationship between LSM and histological grades of fibrosis. p <0.05 was considered significant. SPSS software (version 24.0) was used.
Results
Characteristics of the screening and the control cohort
During the study period, 384 patients attending the addiction unit were invited to participate in the screening program. Among those, 50 were excluded, mainly for not attending V1 (n = 30) or not undergoing liver investigations (n = 14). Therefore, 334 patients were included in the screening cohort (see Fig. 1). Most participants were men (68%), with a median age of 51 years and a median BMI of 25 kg/m2. Fifty-six (17%) patients had obesity, 136 (41%) had dyslipidaemia and 27 (8%) had type 2 diabetes, with 73 (22%) meeting criteria for metabolic syndrome per the NCEP ATP III definition.25 The majority (n = 192, 57%) were active smokers and one-third were active consumers of illicit drugs (n = 89, 27%). Median alcohol consumption in the year prior to the inclusion was 70 SD/week and median duration of high-risk consumption was 20 years (95% CI 11-30). The median value of AUDIT was 20 (CI, 14-25) and 104/197 (53%) had valid tests classified as severe AUD (AUDIT ≥20). Approximately one-quarter of the population received pharmacological therapy for AUD (n = 89, 27%). In the laboratory analyses, median levels of LTs were within the normal range. Baseline characteristics of the screening cohort are summarized in Table 1. In the sensitivity analysis comparing baseline characteristics of individuals included in the pre-COVID vs. COVID eras, the only significant differences were that the former showed a higher median alcohol consumption and higher AUDIT values (Table S2).
Table 1.
Baseline characteristics of the screening cohort.
| Screening cohort (n = 334) | |
|---|---|
| Age (years) | 51 (42-59) |
| Gender (male) | 228 (68) |
| Diabetes mellitus | 28 (8) |
| Dyslipidemia | 136 (41) |
| Arterial hypertension | 73 (22) |
| BMI (kg/m2)° | 25 (23-29) |
| Obesity (BMI ≥30 kg/m2)° | 56 (17) |
| Metabolic syndrome∗ | 73 (22) |
| Psychiatric comorbidity | 159 (48) |
| Tobacco consumption | 192 (58) |
| Illicit drugs (active) | 89 (27) |
| Alcohol consumption (SD/week) | 70 (47-105) |
| Previous year alcohol consumption (SD/week) | 70 (41-114) |
| Binge drinking | 94 (28) |
| Duration of alcohol consumption (years) | 20 (11-30) |
| Alcohol Use Disorder medication | 89 (27) |
| Family history of high-risk alcohol consumptiona | 188 (56) |
| Low incomeb | 123 (37) |
| AUDIT^,c | 20 (14-25) |
| Low-risk consumption (1-7 points) | 5 (2) |
| Hazardous alcohol consumption (8-15 points) | 51 (26) |
| Moderate Alcohol Use Disorder (16-19 points) | 37 (19) |
| Severe Alcohol Use Disorder (≥20 points) | 104 (53) |
| Creatinine (mg/dl) | 0.9 (0.8-1.0) |
| Glucose (mg/dl) | 93 (86-103) |
| AST (U/L) | 24 (19-34) |
| ALT (U/L) | 27 (18-43) |
| GGT (U/L) | 33 (20-64) |
| Bilirubin (mg/dl) | 0.6 (0.4-0.7) |
| ALP (U/L) | 77 (64-94) |
| Serum sodium (mEq/L) | 140 (139-142) |
| Hemoglobin (g/dl) | 14 (13-15) |
| Platelets (109/μl) | 233 (198-279) |
| Ferritin (ng/ml) | 105 (57-170) |
| INR | 0.9 (0.9-1.00) |
| Total cholesterol (mg/dl) | 202 (171-227) |
| Triglycerides (mg/dl) | 121 (82-161) |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUDIT, Alcohol Use Disorder Identification Test; GGT, gamma-glutamyltransferase; INR, international normalized ratio; SD, standard drink of alcohol.
Variables: median (Q1-Q3) and numbers (percentages).
Family history of high-risk alcohol consumption referred by the patient during the hepatologist interview.
Low income: who struggles to make ends meet, assessed on self-report at the hepatologist interview.
AUDIT questionnaire performed at V1 in the liver unit. Missing values: °n = 80; ∗n = 3; ^n = 137.
To test the effect of the screening program on alcohol abstinence, the screening cohort was compared with a control cohort of 137 individuals with similar characteristics, visited in the same unit in the period right before the beginning of the screening program. Of note, there were no differences between the two cohorts in terms of age, gender, illicit drug use, duration of alcohol consumption or use of AUD medications. The two cohorts were only different in terms of the amount of alcohol consumption in the previous year, this was higher in the screening cohort (70 SD/week, 95% CI 42–98, vs. 50 SD/week, 95% CI 35–84, p = 0.04) (Table S1).
Effect of the screening program on alcohol abstinence
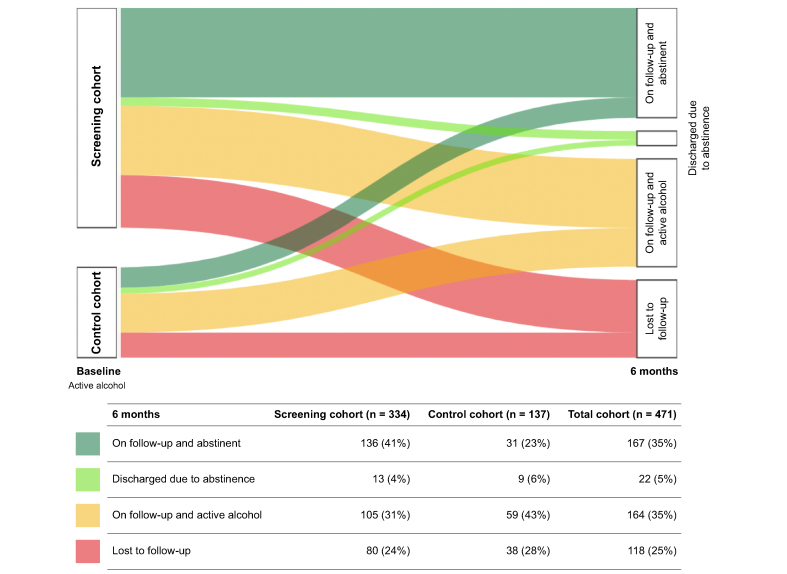
After 6 months of follow-up in the addiction unit, 149/334 (45%) patients achieved alcohol abstinence. Among those, 101 (68%) had negative uETG in urine, 35 (23%) were considered abstinent based on the specialist medical records and 13 (9%) were discharged before V6 due to sustained abstinence with low risk of relapse and were considered abstinent at the end of follow-up. One hundred and eighty-five (55%) patients were categorized as active alcohol consumers: 67 (36%) had positive uETG in urine, 38 (21%) based on medical records and 80 (43%) dropped-out of the program during the study period and were considered lost to follow-up (Fig. 2 and Table 2). Compared to those who completed the study (n = 254), individuals lost to follow-up (n = 80) were younger (47 years-old, 95% CI 38-56, vs. 51 years-old, 95% CI 43-60, p = 0.01), mostly men (79% vs. 65%, p = 0.03) and had higher median albumin levels (46 g/L, 95% CI 44-49, vs. 45 g/L, 95% CI 44-47, p = 0.001) (Table S3).
Fig. 2.
Alcohol consumption at baseline and at 6 months’ follow-up in the screening and control cohorts.
Table 2.
Alcohol abstinence at 6 months follow-up in the screening and control cohort.
| Screening cohort (n = 334) | Control cohort (n = 137) | p value | |
|---|---|---|---|
| Follow-up 6 months (yes) | 241 (72) | 90 (66) | 0.18 |
| Abstinence at 6 months (yes) | 149 (45) | 40 (29) | 0.002 |
| Clinical follow-up with uETG | 101 (68) | 27 (68) | 0.43 |
| Clinical follow-up without uETG | 35 (23) | 4 (10) | 0.25 |
| Discharged for abstinence before 6 months | 13 (9) | 9 (22) | 0.23 |
| Active alcohol 6 months (yes) | 185 (55) | 97 (71) | 0.002 |
| Clinical follow-up with uETG | 67 (36) | 42 (43) | 0.25 |
| Clinical follow-up without uETG | 38 (21) | 17 (18) | 0.15 |
| Lost to follow-up | 80 (43) | 38 (39) | 0.41 |
| uETG 6 months (yes) | 172 (51) | 73 (53) | 0.76 |
| Alcohol use disorder medication at 6 months | 73 (22) | 24 (18) | 0.43 |
| Alcohol consumption among active consumers (SD/week) | 39 (31-47) | 45 (27-62) | 0.49 |
SD, standard drink of alcohol; uETG, ethylglucuronide in urine.
Variables: median (Q1-Q3) and numbers (percentages). Comparisons: Student's t test for “alcohol consumption”; Pearson χ2 or Fisher's exact test for categorical variables. Significance p <0.05 (bold numbers).
Mean alcohol consumption at V6 among active consumers was 39 SD/week, indicating a significant reduction from baseline (70 SD/week) (p <0.001). When analysing the changes in WHO risk drinking levels,26 among active consumers at 6 months from baseline, there was a significant increase in the high/very high WHO levels in both the screening (from 62% at V1 to 24% at V6, p <0.001) and the control cohort (from 64% at V1 to 24% at V6, p = 0.002) with no differences between the two cohorts (p = 0.27) (Fig. S1).
In the univariate regression analysis, age, use of AUD medications, gamma-glutamyltransferase (GGT), alkaline phosphatase, albumin, total cholesterol and serum sodium levels were associated with alcohol abstinence at V6 (Table 3). In the multivariate analysis, older age (OR 1.03, 95% CI 1.01-1.05), AUD medication intake (OR 2.00, CI 1.18-3.38) and cholesterol levels (OR 0.99, 95% CI 0.98-0.99) were independent predictors of alcohol abstinence (Table 3).
Table 3.
Factors associated with alcohol abstinence in the screening cohort.
| A) Variables | Active consumption (n = 185) | Abstinence∗ (n = 149) | p value | |||
|---|---|---|---|---|---|---|
| Age (years) | 48 (41-59) | 52 (44-60) | 0.04 | |||
| Gender (male) | 127 (69) | 101 (68) | 0.91 | |||
| BMI (kg/m2) | 26 (23–29) | 25 (23–29) | 0.79 | |||
| Tobacco consumption | 103 (56) | 89 (60) | 0.50 | |||
| Illicit drugs (active) | 57 (31) | 32 (22) | 0.06 | |||
| Family history of alcohol consumption | 88 (54) | 81 (59) | 0.48 | |||
| Maximum alcohol consumption (SD/week) | 70 (44-104) | 78 (50-126) | 0.06 | |||
| Duration of alcohol consumption (years) | 20 (10–31) | 22 (11–31) | 0.46 | |||
| Alcohol use disorder medication | 42 (22) | 47 (32) | 0.05 | |||
| LSM ≥8 kPa | 19 (10) | 21 (14) | 0.31 | |||
| CAP dB/m | 246 (208-288) | 229 (198-270) | 0.07 | |||
| GGT (U/L) | 37 (23-74) | 29 (18-48) | 0.001 | |||
| ALP (U/L) | 80 (67-98) | 74 (63-97) | 0.05 | |||
| Albumin (g/L) | 46 (44-48) | 45 (44-47) | 0.03 | |||
| Serum sodium (mEq/L) | 140 (138-141) | 140 (139-142) | 0.04 | |||
| Total cholesterol (mg/dl) | 205 (175-234) | 193 (169-218) | 0.02 | |||
| AUDIT ^ | 20 (14–24) | 21 (15–25) | 0.22 | |||
| Low-risk consumption (1-7 points) | 3 (3) | 2 (2) | 1.00 | |||
| Hazardous alcohol consumption (8-15 points) | 28 (27) | 23 (25) | 0.75 | |||
| Moderate alcohol use disorder (16-19 points) | 20 (19) | 17 (18) | 0.86 | |||
| Severe alcohol use disorder (≥20 points) |
52 (51) |
52 (55) |
0.57 |
|||
| B)° |
Univariate analysis |
Multivariate analysis |
||||
|
Variables |
p value |
OR |
95% CI |
p value |
OR |
95% CI |
| Age (years) | 0.04 | 1.02 | 0.99-1.04 | 0.01 | 1.03 | 1.01-1.05 |
| Alcohol use disorder medication (yes) | 0.01 | 1.65 | 1.11-2.44 | 0.01 | 2.00 | 1.18-3.38 |
| GGT (U/L) | 0.04 | 0.99 | 0.99-1.00 | |||
| ALP (U/L) | 0.03 | 0.99 | 0.98-0.99 | |||
| Albumin (g/L) | 0.01 | 0.90 | 0.83-0.97 | |||
| Sodium (mEq/L) | 0.05 | 1.10 | 1.00-1.20 | 0.06 | 1.1 | 0.99-1.21 |
| Total cholesterol (mg/dl) | 0.01 | 0.99 | 0.98-0.99 | 0.002 | 0.99 | 0.98-0.99 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUDIT, Alcohol Use Disorder Identification Test; CAP, controlled attenuation parameter; GGT, gamma-glutamyltransferase; OR, odds ratio; SD, standard drink of alcohol.
(A) Baseline characteristics of active consumers and abstinent individuals at 6 months follow-up in the screening cohort. (B) Univariate and multivariate logistic regression. Variables: median (Q1-Q3) and numbers (percentages). Comparisons: continuous variables by Student's t test (normal distribution) and Wilcoxon rank-sum test (non-normal distribution); categorical variables by Pearson χ2 or Fisher's exact test. Univariate logistic regression analysis and multivariate logistic regression analysis (backward stepwise approach, Wald). Significance p <0.05 (bold numbers). ∗Abstinence was defined as total abstinence for at least 3 consecutive months before the 6 months follow-up visit. ^Missing values n = 137. °The table shows only factors with p <0.05 at binary logistic regression.
In the subanalysis based on COVID eras, the pre-COVID cohort was associated with a significantly higher rate of alcohol abstinence (40/70, 57%) compared to the COVID cohort (109/264, 41%, p = 0.02). The COVID cohort was associated with a lower abstinence rate in both the univariate analysis (OR 0.53, 95% CI 0.31-0.90, p = 0.02) and the multivariate analysis (Table S4).
Comparison of alcohol abstinence between the screening and the control cohort
The proportion of individuals who reached alcohol abstinence in the screening cohort was higher than in the control cohort (45% vs. 29%, p = 0.02) (Table 2). When abstinence was analysed in the two combined cohorts (n = 471), enrolment in the screening program (p = 0.01, OR 1.77), lack of consumption of illegal drugs (p = 0.004, OR 0.50) and being under active treatment with AUD medications (p = 0.02, OR 1.72) were found to be independent predictors of abstinence at V6 visit (Table S5).
Prevalence of and risk factors for increased liver stiffness in the screening cohort
Using the cut-off of LSM ≥8 kPa, the prevalence of increased LSM was 12% (40 out of 334). Of these patients, almost half (19/40, 48%) had LSM ≥15 kPa, suggestive of chronic advanced liver disease. Median LSM values did not differ among abstinent vs. non-abstinent patients at the time of LSM measurement (LSM = 4.9 kPa, 95% CI 4.1-6.1 vs. LSM = 4.8 kPa, 95% CI 3.9-6.0, respectively, p = 0.47). There were no differences in terms of prevalence of increased LSM in the sensitivity analysis of the pre-COVID vs. COVID cohorts (LSM ≥8 kPa in 9% vs. 13%, p = 0.32).
Using the Nguyen-Kach correction to investigate the prevalence of LSM, 285 out of 334 individuals were classified into four groups described based on AST and bilirubin levels.21 Increased LSM (defined as F ≥2) was present in 26 out of 285 (9%) individuals, showing a prevalence of high LSM similar to that obtained using the cut-off of 8 kPa. When applying this correction, a total of seven individuals were reclassified: 4/260 (2%) with LSM <8 kPa were reclassified as F ≥2; 3/25 (12%) with LSM ≥8 kPa were reclassified as F <2 (Table S6).
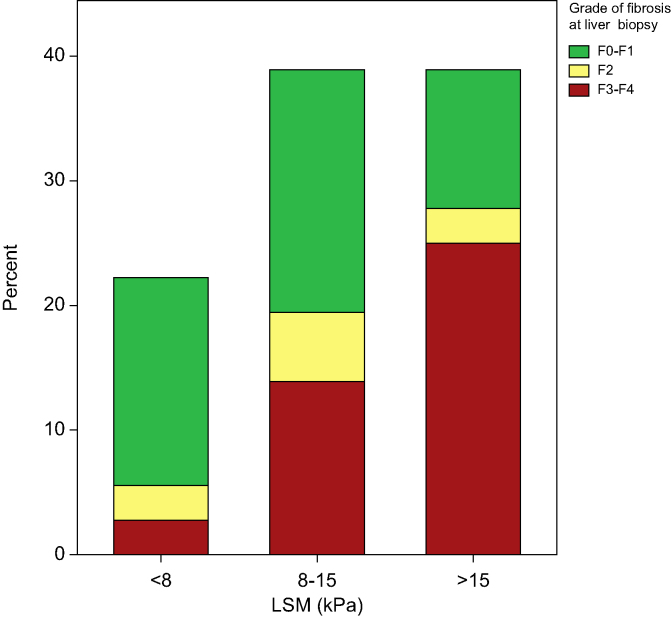
Liver biopsy was performed in 36/334 (11%) patients with the main indication being an increased LSM. Most biopsies were performed using the percutaneous approach (n = 26, 72%); in those who underwent transjugular liver biopsy (n = 10, 28%) the median HVPG was 6.5 mmHg (3.9-10.4) and three patients had clinically significant portal hypertension (HVPG ≥10 mmHg). Liver fibrosis was assessed by histology using the Metavir scale, with 19/36 (53%) showing liver fibrosis stage ≥F2. Of those, 15 (42%) were in a stage of advanced liver fibrosis (F3–F4). There was a positive correlation between LSM and histological grades of fibrosis at the Spearman’s rank correlation test, r34 = 0.57, p <0.001 (Fig. 3).
Fig. 3.
Grade of liver fibrosis using the Metavir scale for patients with liver stiffness <8 kPa, 8-15 kPa and >15 kPa.
LSM, liver stiffness measurement.
Factors associated with the presence of increased LSM on univariate regression analysis are shown in Table 4A. Of the variables related to the history of alcohol consumption, duration of alcohol consumption was the only factor associated with the presence of increased LSM, whilst the majority of metabolic comorbidities, including arterial hypertension, type 2 diabetes, obesity and metabolic syndrome were associated with increased LSM. In the multivariate analysis, obesity (p = 0.03), arterial hypertension (p = 0.03), GGT (p <0.001) and platelet levels (p = 0.001) were the only factors independently associated with increased LSM (Table 4B).
Table 4.
Factors associated with increased liver stiffness (LSM ≥8 kPa) in the screening cohort.
| A) Variables | LSM <8 kPa (n = 294) | LSM ≥8 kPa (n = 40) | p value | |||
|---|---|---|---|---|---|---|
| Age (years) | 49 (42-57) | 60 (52-64) | <0.001 | |||
| Gender (male) | 196 (67) | 32 (80) | 0.10 | |||
| Diabetes mellitus | 19 (7) | 9 (23) | 0.003 | |||
| Dyslipidemia | 117 (40) | 19 (48) | 0.39 | |||
| Arterial hypertension | 55 (19) | 18 (45) | <0.001 | |||
| Metabolic syndrome ∗ | 56 (19) | 17 (44) | 0.002 | |||
| BMI (kg/m2) ° | 25 (23–28) | 29 (24–32) | 0.002 | |||
| Obesity (BMI ≥30 kg/m2) ° | 41(17) | 15 (45) | <0.001 | |||
| Tobacco consumption | 176 (60) | 16 (40) | 0.03 | |||
| Illicit drugs (active) | 82 (28) | 7(18) | 0.11 | |||
| Maximum alcohol consumption (SD/week) | 70 (48-119) | 70 (44-86) | 0.71 | |||
| Duration of alcohol consumption (years) | 20 (10–30) | 30 (15-40) | 0.01 | |||
| Alcohol use disorder medication | 83 (28) | 6 (15) | 0.06 | |||
| Alcohol abstinence ≥1 week at transient elastography performance | 147 (50) | 19 (48) | 0.87 | |||
| Glucose (mg/dl) | 92 (86-102) | 97 (88-108) | 0.05 | |||
| AST (U/L) | 23 (19–31) | 47 (24-75) | <0.001 | |||
| ALT (U/L) | 24 (17-39) | 46 28-76) | <0.001 | |||
| GGT (U/L) | 31 (20-49) | 93 (38-424) | <0.001 | |||
| Bilirubin (mg/dl) | 0.6 (0.4-0.7) | 0.7 (0.5-0.9) | 0.002 | |||
| ALP (U/L) | 76 (64-91) | 93 (74-117) | <0.001 | |||
| Platelets (109/μl) | 236 (203-281) | 208 (146-257) | 0.001 | |||
| Ferritin (ng/ml) | 100 (54-158) | 154 (86-278) | 0.001 | |||
| INR | 0.9 (0.9-1.0) | 1 (0.9-1.1) | <0.001 | |||
| AUDIT ^ | 20 (14–25) | 19 (14–24) | 0.33 | |||
| Low-risk consumption (1–7) | 4 (3) | 1 (4) | 0.47 | |||
| Hazardous alcohol consumption (8–15) | 44 (25) | 7 (30) | 0.61 | |||
| Moderate alcohol use disorder (16–19) | 33 (19) | 4 (17) | 1.00 | |||
| Severe alcohol use disorder (≥20) |
93 (53) |
11 (49) |
0.66 |
|||
|
B)∗∗ Variables |
Univariate analysis |
Multivariate analysis |
||||
|
p value |
OR |
p value |
OR |
p value |
OR |
|
| Age (years) | <0.001 | 1.06 | 1.03-1.09 | |||
| Diabetes mellitus | 0.001 | 4.20 | 1.75-10.09 | 0.07 | 3.00 | 0.91-9.80 |
| Arterial hypertension | <0.001 | 3.56 | 1.79-7.08 | 0.03 | 3.13 | 1.12-8.77 |
| Metabolic syndrome ∗ | 0.001 | 3.26 | 1.62-6.54 | |||
| Obesity (BMI ≥30 kg/m2) ° | <0.001 | 4.07 | 1.90-8.72 | 0.03 | 3.13 | 1.12-7.78 |
| Tobacco consumption | 0.02 | 0.45 | 0.23-0.88 | |||
| Duration of alcohol consumption (years) | 0.007 | 1.03 | 1.01-1.06 | |||
| Glucose (mg/dl) | 0.03 | 1.01 | 1.00-1.01 | |||
| AST (U/L) | 0.01 | 1.01 | 1.01-1.02 | |||
| GGT (U/L) | <0.001 | 1.01 | 1.00-1.01 | <0.001 | 1.01 | 1.01-1.02 |
| Bilirubin (mg/dl) | 0.004 | 3.63 | 1.50-8.80 | |||
| ALP (U/L) | <0.001 | 1.03 | 1.01-1.04 | 0.10 | 1.02 | 0.99-1.03 |
| Platelets (109/μl) | 0.001 | 0.99 | 0.98-1.00 | 0.049 | 0.99 | 0.98-1.00 |
| Ferritin (ng/ml) | 0.001 | 1.01 | 1.00-1.01 | |||
| INR | 0.009 | 10.12 | 1.78-57.54 | |||
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUDIT, Alcohol Use Disorder Identification Test; GGT, gamma-glutamyltransferase; INR, international normalized ratio; OR, odds ratio; SD, standard drink of alcohol.
(A) Baseline characteristics of individuals with LSM <8 kPa and LSM ≥8 kPa. (B) Univariate and multivariate logistic regression. Variables: median (Q1-Q3) and numbers (percentages). Comparisons: continuous variables by Student's t test (normal distribution) and Wilcoxon rank-sum test (non-normal distribution); categorical variables by Pearson χ2 or Fisher's exact test. Univariate logistic regression analysis and multivariate logistic regression analysis (backward stepwise approach, Wald). Significance p <0.05 (bold numbers). Missing values: ∗n = 3; °n = 80; ^n = 137. ∗∗The table shows only factors with p <0.05 at binary logistic regression.
Of note, at the time of undergoing TE (a median of 49 days after V0 at the addiction unit), 166/334 (50%) were abstinent and 168/334 (50%) were active alcohol consumers. When comparing abstinent individuals vs. active alcohol consumers there were no significant differences in median LSM values (LSM = 4.9 kPa, 95% CI 4.1-6.1 and LSM = 4.8 kPa, 95% CI = 3.9-6.0, respectively, p = 0.47), nor in the prevalence of increased LSM (11% vs. 13%, p = 0.77). In the univariate analysis, ongoing alcohol consumption at TE performance was not associated with higher LSM values (p = 0.08). Finally, there were no significant differences in alcohol abstinence at 6 months between patients with increased LSM compared to those with normal LSM (53% vs. 47%, p = 0.31).
Median CAP in the screening cohort was 240 dB/m (95% CI 205-283) with 144 (43%) patients having CAP ≥250 dB/m suggestive of steatosis. CAP values were not associated with alcohol abstinence in the screening cohort (p = 0.07 at the univariate analysis). As previously reported,27 CAP values were highly dependent on active alcohol consumption at the time of TE in our cohort: median CAP values were significantly higher among active consumers at the time of TE compared to those that had already reached alcohol abstinence (252 dB/m, 95% CI 213-290 vs. 227 dB/m, 95% CI 196-273, respectively, p = 0.001).
Discussion
This prospective interventional study performed in individuals with AUD at risk of ALD shows that a program of screening for liver fibrosis together with counselling on alcohol consumption and healthy lifestyle habits is associated with increased alcohol abstinence at 6 months.
Few studies to date have investigated the effects of screening for liver disease on alcohol consumption in patients with AUD. A previous study from Nottingham community alcohol services performed in 87 participants screened for liver disease with TE showed a positive effect of the screening program, with a significant reduction in self-reported alcohol intake.22 Despite the low number of individuals included and the lack of a control group, this was the first study suggesting a positive influence of screening for liver disease on alcohol consumption. Recently, a feasibility clinical trial showed positive effects of a screening program for liver disease in addiction units: the intervention consisted of TE together with counselling on alcohol consumption and videos of previous experiences of individuals achieving alcohol abstinence.17 The study found that this type of intervention was feasible and increased engagement to the addiction program among the individuals undergoing active screening, setting the stage for the development of new studies assessing the effectiveness of screening of liver disease in the population with AUD. The results of the current study are in keeping with these previous observations. In fact, the main finding is that participating in the screening program for liver fibrosis increases the probability of being abstinent at 6 months regardless of other important factors such as AUD medication use. However, there remain unanswered questions regarding the efficacy of screening. First, we cannot rule out that those who decided to participate in the screening were indeed those with higher motivation to undergo therapy for AUD and achieve alcohol abstinence. Nonetheless, previous studies showed that being aware of liver disease may act as a motivation for alcohol abstinence per se.28 It is very likely that attending the screening program for liver disease may act as an extra motivation for lifestyle changes and alcohol abstinence and the current study seems to support this assertion.
In addition to the importance of the screening programme for liver disease, the current study enhances the fundamental role of pharmacological treatment for AUD. In our cohort one-third of individuals were under pharmacological treatment for AUD, and AUD medications were independently associated with abstinence at 6 months. Furthermore, the commonly used AUD medications have previously demonstrated good safety and efficacy profiles, even in patients with advanced liver disease.29 This study supports a more extensive use of AUD medication in addiction units, even in patients with liver disease.
In this screening program, we found that 12% of the participants had liver fibrosis, of whom 5% had chronic advanced liver disease. This proportion is consistent with a previous study on screening for liver disease in populations with high-risk alcohol consumption in primary care.11 Compared to a seminal biopsy-controlled study including a population with AUD, the prevalence of significant liver fibrosis is slightly lower in the current study (12% vs. 17%); this difference can be partially explained by the lower median alcohol intake of the population (70 SD/week vs. 168 SD/week) and the lower prevalence of metabolic comorbidities.16 Of note, obesity, arterial hypertension, GGT, and platelet levels were the main factors associated with presence of increased LSM. Metabolic risk factors have been associated with an increased risk and severity of ALD both at early and advanced stages of liver disease in previous studies.10,30,31 The early identification of this subpopulation with metabolic risk factors and high-risk alcohol consumption with screening programs would allow for the implementation of multidisciplinary clinics involving hepatologists, addiction specialists, general practitioners, nurses, nutritionists and endocrinologists to control risk factors and prevent progression of liver disease.
Another interesting finding is that, in the sensitivity analysis of the pre-COVID vs. COVID cohorts, the COVID period was associated with lower probability of reaching alcohol abstinence despite no significant differences in the baseline characteristics of both cohorts. This could be due in part to shortcomings and difficulties in the follow-up and treatment of these patients in addiction units during the COVID era.32
Our study has some limitations that should be acknowledged. First, the period of 6 months may be considered too short to test effects on sustained alcohol abstinence, sustained remission of AUD or on clinical outcomes. However, the 6-month period was selected based on previous studies with pharmacological treatments for AUD in patients with ALD.29,33,34 Second, the use of uETG as an alcohol biomarker could underestimate alcohol consumption, given its short detection window, and phosphatidylethanol would be more sensitive to uncover long-term alcohol exposure. Nonetheless, as this was a pragmatic study, all the patients were treated following the standards of clinical practice of our addiction unit that currently include uETG as the alcohol biomarker. Finally, the results of our intervention cohort were compared with a historical cohort, so patients were not randomized to receive the intervention under investigation and thus potential biases cannot be entirely excluded. In this regard, as screening for liver fibrosis in individuals with high alcohol consumption is currently recommended by clinical practice guidelines and is currently implemented in our unit, we opted to include a control cohort with similar characteristics who did not undergo screening for liver fibrosis in the period immediately prior.
In conclusion, our study provides new evidence on the beneficial effects of screening programs not only to identify early stages of ALD, but also to improve alcohol abstinence and thus forming part of therapy.
Abbreviations
ALD, alcohol-associated liver disease; AST, aspartate aminotransferase; AUD, alcohol use disorders; AUDIT, Alcohol Use Disorder Identification Test; GGT, gamma-glutamyltransferase; HVPG, hepatic venous pressure gradient; LTs, liver tests; LSM, liver stiffness measurement; SD, standard drinks of alcohol; TE, transient elastography; uETG, ethylglucuronide in urine.
Financial support
The work was funded by a grant PI22/00910 (PI EP) from the Plan Nacional I+D+I and co-funded by ISCIII-Subdirección General de Evaluación and European Regional Development Fund FEDER. PG was funded by grant number PI20/00579, from Plan Nacional I+D+I and co-funded by ISCIII-Subdirección General de Evaluación and European Regional Development Fund FEDER. JGG was funded by a Río Hortega grant (CM21/00095), ISCIII - Acción Estratégica en Salud (AES). EA, MPG, JGG, EP and PG have been funded by a grant from AGAUR 2021-SGR01331. HLP, LN, NF, AL, PB have been funded by Instituto Carlos III, el Fondo Europeo de Desarrollo regional y el Plan de recuperación transformación y resiliencia (código RD21/0009/0010).
Conflicts of interest
EA, JGG, MPG, ABR, QH, MC, RN, MCA, NF, PB, AL, LO, AL, LN, NF, MTP, AD, RB, HLP and EP have no interests to declare related to this paper. PG has received research funding from Gilead & Grifols. PG has consulted or attended advisory boards for Gilead, RallyBio, SeaBeLife, Merck, Sharp and Dohme (MSD), Ocelot Bio, Behring, Roche Diagnostics International and Boehringer Ingelheim, and received speaking fees from Pfizer.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
Conceptualization: EA, EP. Data collection: EA, JGG, MPG, ABR, MC, RN, MCA, NF, PB, AL, LO, AL, LN, NF, MTP. Data analysis: EA, JGG, AD. Supervision: EP, PG, RB, HLP. Funding acquisition: EP. Revision: EA, EP, JGG, QH.
Data availability statement
Due to the sensitive nature of the data collected in this study, raw data would remain confidential and would not be shared.
Acknowledgements
Assistance with the study: we would like to acknowledge Nicola van Berckel for the orthographic revision of the contents. Presentation: preliminary data of the current study were presented at the International EASL Congress 2023 in Vienna in poster format.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2024.101165.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Griswold M.G., Fullman N., Hawley C., et al. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlsen T.H., Sheron N., Zelber-Sagi S., et al. The EASL–Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399(10319):61–116. doi: 10.1016/S0140-6736(21)01701-3. [DOI] [PubMed] [Google Scholar]
- 3.Bataller R., Cabezas J., Aller R., et al. Alcohol-related liver disease. Clinical practice guidelines. Consensus document sponsored by AEEH. Gastroenterol Hepatol. 2019;42(10):657–676. doi: 10.1016/j.gastrohep.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . WHO press; 2018. Thirteenth general programme of work 2019–2023; p. 50.https://www.who.int/about/what-we-do/thirteenth-general-programme-of-work-2019-2023 [2023 Nov 21];(April 2018) [Google Scholar]
- 5.Abbafati C., Abbas K.M., Abbasi-Kangevari M., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caballería L., Pera G., Arteaga I., et al. High prevalence of liver fibrosis among European adults with unknown liver disease: a population-based study. Clin Gastroenterol Hepatol. 2018;16(7):1138–1145. doi: 10.1016/j.cgh.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 7.Berzigotti A., Tsochatzis E., Boursier J., et al. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J Hepatol. 2021;75(3):659–689. doi: 10.1016/j.jhep.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Thursz M., Gual A., Lackner C., et al. EASL clinical practice guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69(1):154–181. doi: 10.1016/j.jhep.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Jophlin L.L., Singal A.K., Bataller R., et al. ACG clinical guideline: alcohol-associated liver disease. Am J Gastroenterol. 2024;119(1):30–54. doi: 10.14309/ajg.0000000000002572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pose E., Pera G., Torán P., et al. Interaction between metabolic syndrome and alcohol consumption, risk factors of liver fibrosis: a population-based study. Liver Int. 2021;41(7):1556–1564. doi: 10.1111/liv.14830. [DOI] [PubMed] [Google Scholar]
- 11.Kjaergaard M., Lindvig K.P., Thorhauge K.H., et al. Using the ELF test, FIB-4 and NAFLD fibrosis score to screen the population for liver disease. J Hepatol. 2023;79(2):277–286. doi: 10.1016/j.jhep.2023.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Ginès P., Castera L., Lammert F., et al. Population screening for liver fibrosis: toward early diagnosis and intervention for chronic liver diseases. Hepatology. 2022;75(1):219–228. doi: 10.1002/hep.32163. [DOI] [PubMed] [Google Scholar]
- 13.Asphaug L., Thiele M., Krag A., et al. GALAXY Consortium Cost-effectiveness of noninvasive screening for alcohol-related liver fibrosis. Hepatology. 2020;71(6):2093–2104. doi: 10.1002/hep.30979. [DOI] [PubMed] [Google Scholar]
- 14.Altamirano J.E., L Opez-Pelayo H., Michelena J., et al. Alcohol abstinence in patients surviving an episode of alcoholic hepatitis: prediction and impact on long-term survival. Hepatology. 2017;66(6):1842–1853. doi: 10.1002/hep.29338. [DOI] [PubMed] [Google Scholar]
- 15.Lackner C., Spindelboeck W., Haybaeck J., et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol. 2017;66(3):610–618. doi: 10.1016/j.jhep.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Thiele M., Detlefsen S., Sevelsted Møller L., et al. Transient and 2-dimensional shear-wave elastography provide comparable assessment of alcoholic liver fibrosis and cirrhosis. Gastroenterology. 2016;150(1):123–133. doi: 10.1053/j.gastro.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 17.Subhani M., Enki D.G., Knight H., et al. Does knowledge of liver fibrosis affect high-risk drinking behaviour (KLIFAD): an open-label pragmatic feasibility randomised controlled trial. EClinicalMedicine. 2023;61 doi: 10.1016/j.eclinm.2023.102069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjaergaard M., Lindvig K.P., Thorhauge K.H., et al. Screening for fibrosis promotes life-style changes. A prospective cohort study in 4,796 individuals. Clin gastroenterol Hepatol. 2023;26 doi: 10.1016/j.cgh.2023.12.018. S1542-3565(23)01053-4. [DOI] [PubMed] [Google Scholar]
- 19.National Institute of Alcohol Abuse and Alcoholism (NIAAA) 2017. Alcohol facts and statistics.https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics [Google Scholar]
- 20.De Franchis R., Bosch J., Garcia-Tsao G., et al. Baveno VII – renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959–974. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen-Khac E., Thiele M., Voican C., et al. Non-invasive diagnosis of liver fibrosis in patients with alcohol-related liver disease by transient elastography: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2018;3(9):614–625. doi: 10.1016/S2468-1253(18)30124-9. [DOI] [PubMed] [Google Scholar]
- 22.Subhani M., Harman D.J., Scott R.A., et al. Transient elastography in community alcohol services: can it detect significant liver disease and impact drinking behaviour? Biomedicines. 2022;10(2):1–9. doi: 10.3390/biomedicines10020477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bedossa P., Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24(2):289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 24.Yip W.W., Burt A.D. Alcoholic liver disease. Semin Diagn Pathol. 2006;23(3–4):149–160. doi: 10.1053/j.semdp.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Grundy S.M., Cleeman J.I., Daniels S.R., et al. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 26.Shmulewitz D., Aharonovich E., Witkiewitz K., et al. The world health organization risk drinking levels measure of alcohol consumption: prevalence and health correlates in nationally representative surveys of U.S. Adults, 2001–2002 and 2012–2013. Am J Psychiatry. 2021;178(6):548–559. doi: 10.1176/appi.ajp.2020.20050610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiele M., Rausch V., Fluhr G., et al. Controlled attenuation parameter and alcoholic hepatic steatosis: diagnostic accuracy and role of alcohol detoxification. J Hepatol. 2023;68(5):1025–1032. doi: 10.1016/j.jhep.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 28.Kann A.E., Jepsen P., Madsen L.G., et al. Motivation to reduce drinking and engagement in alcohol misuse treatment in alcohol-related liver disease: a national health survey. Am J Gastroenterol. 2022;117(6):918–922. doi: 10.14309/ajg.0000000000001616. [DOI] [PubMed] [Google Scholar]
- 29.Gratacós-Ginès J., Bruguera P., Pérez-Guasch M., et al. Medications for alcohol use disorder promote abstinence in alcohol-related cirrhosis: results from a systematic review and meta-analysis. Hepatology. 2024 Feb 1;79(2):368–379. doi: 10.1097/HEP.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 30.Åberg F., Puukka P., Salomaa V., et al. Combined effects of alcohol and metabolic disorders in patients with chronic liver disease. Clin Gastroenterol Hepatol. 2020;18(4):995–997. doi: 10.1016/j.cgh.2019.06.036. [DOI] [PubMed] [Google Scholar]
- 31.Naveau S., Giraud V., Borotto E., et al. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25(1):108–111. doi: 10.1002/hep.510250120. [DOI] [PubMed] [Google Scholar]
- 32.Ostinelli E.G., Smith K., Zangani C., et al. COVID-19 and substance use disorders: a review of international guidelines for frontline healthcare workers of addiction services. BMC Psychiatry. 2022 Mar 31;22(1):228. doi: 10.1186/s12888-022-03804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higuera-de la Tijera F., Servín-Caamaño A.I., Serralde-Zúñiga A.E., et al. Metadoxine improves the three-and six-month survival rates in patients with severe alcoholic hepatitis. World J Gastroenterol. 2015 Apr 28;21(16):4975–4985. doi: 10.3748/wjg.v21.i16.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bajaj J.S., Gavis E.A., Fagan A., et al. A randomized clinical trial of fecal microbiota transplant for alcohol use disorder. Hepatology. 2021 May;73(5):1688–1700. doi: 10.1002/hep.31496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the sensitive nature of the data collected in this study, raw data would remain confidential and would not be shared.