Abstract
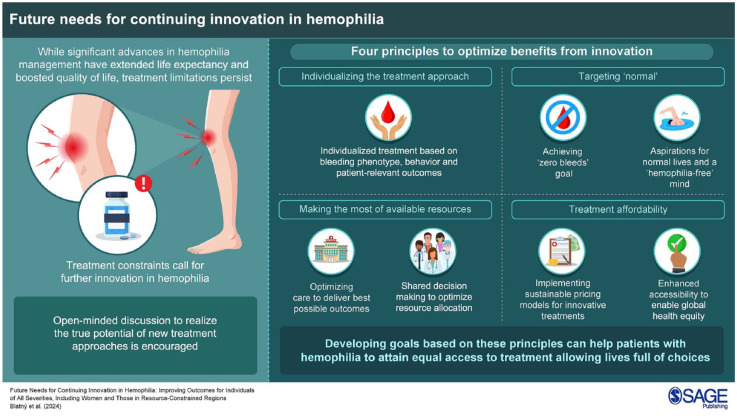
Over recent decades, management of people with hemophilia (PwH) has been greatly improved by scientific advances that have resulted in a rich and varied therapeutic landscape. Nevertheless, treatment limitations continue to drive innovation, and emerging options have the potential to realize further improvement. We advocate four general principles to optimize benefits from innovation: individualizing the treatment approach, targeting ‘normal,’ making the most of available resources, and considering treatment affordability. Ultimately, all PwH—men and women, of all ages and severities, and worldwide—should have access to treatment that fully prevents bleeding, while allowing personal, social, family, and professional lives of choice. Clearly, we are not there yet, but developing goals/milestones based on the principles we describe may help to achieve this.
Keywords: hemophilia, innovation, management, outcomes, quality of life
Graphical abstract.
Introduction
Tremendous progress in hemophilia management over recent decades has created a rich and varied therapeutic landscape. Scientific advances have increased life expectancy, while decreasing disease-related morbidity and treatment burden, with substantial improvements in quality of life and participation (in sport/work/school, etc.). The need to address limitations of previous and current therapies has driven innovation. Emerging treatment options have the potential to offer new possibilities to tailor therapeutic approaches to individuals’ specific needs. Each person has his/her own unique characteristics, aspirations, and expectations, irrespective of age and sex. Goals and ambitions vary, for example, in individuals with inhibitors, those with comorbidities, persons engaged in high-level physical activity/sports, and patients living in resource-constrained countries.
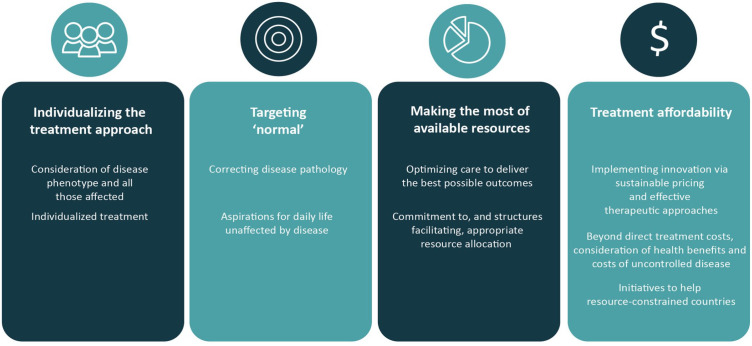
We advocate four general principles to optimize benefit from innovation for all patients (Figure 1). These are generic and can be achieved in different ways. With these principles, our intention is to stimulate open-minded discussion to realize the real potential of new treatment approaches.
Figure 1.
General principles to guide goals for continuing innovation.
Principle 1: Individualizing the treatment approach
Historically, hemophilia has been classified based on residual plasma levels of factor VIII (FVIII) and factor IX (FIX) for hemophilia A and B, respectively. 1 Severe, moderate, and mild diseases are defined by laboratory levels of <1%, 1%–5%, and >5%–<40%, respectively. 1 This classification has been used to guide treatment, but has limitations,2,3 As within these categories individuals can exhibit marked clinical heterogeneity with varied bleeding phenotypes. While acknowledging ongoing debate,2–4 we advocate a gradual change of treatment paradigm, moving from consideration of factor levels to phenotype. This should encompass all people with hemophilia (PwH), including women for whom prophylaxis is appropriate, and not just individuals conventionally categorized as having severe hemophilia. In addition, differences between hemophilia A and B should also be recognized. 5 We support the view that a restrictive classification may not be fit for purpose, and a more inclusive and comprehensive way of assessing patients’ clinical journeys and therapeutic needs is required.
Prophylactic treatment has traditionally relied on converting hemophilia from a severe to ‘moderate’ state, 6 improving hemostasis by increasing FVIII/FIX levels. Historically, aspirations of prophylaxis were modest—to generate measurable levels of FVIII/FIX and reduce bleeding. Initial regimens were not very flexible or individualized. This perhaps reflected short half-lives of earlier products, concerns over plasma safety, supply limitations, burden of frequent intravenous injections, and not taking inter-individual differences in pharmacokinetics into account. While the need for higher factor levels has been acknowledged, including a more individualized approach and extension of prophylaxis to some patients with nonsevere hemophilia (in whom joint damage can occur7,8), there is still a lack of consensus on optimal treatment for all PwH. This may impede true improvements in outcomes.
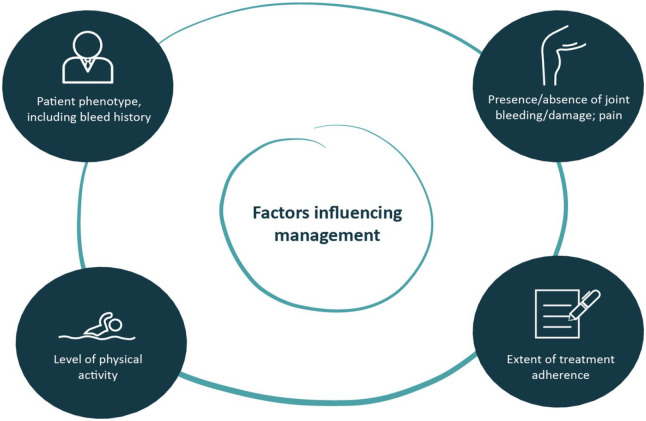
The development of extended half-life (EHL) recombinant factor products has increased flexibility of product administration, facilitating tailored prophylaxis via individualized treatment. Moreover, the first subcutaneously administered nonreplacement therapy, emicizumab, is also available. 9 The principle of individualized hemophilia treatment 10 should be generally accepted and extended to guide patient management, with empirical evidence based on product data. 11 To optimize patient outcomes and resource allocation, decision-making aimed at increasing protection should involve a range of considerations, allowing for patients’ individual characteristics, with regard to both phenotype and behavior (Figure 2). Taking into account patient-reported outcomes helps to personalize care, and improvement of patient-relevant tools is required. 12
Figure 2.
Considerations affecting treatment decision-making.
In the near future, it may be possible to offer therapies targeting hemostasis in the non-hemophilia range, hence defining outcomes beyond peak and trough factor levels, supporting individuals’ goals, preventing future morbidity, and removing restrictions in everyday life activities.
Principle 2: Targeting ‘normal’
Therapeutic progress in hemophilia has broadened product choice to include EHL factors, as well as nonfactor treatment and gene therapy. Nonfactor treatment targeting anticoagulant pathways 13 may become more widely available. Advances have ameliorated the burden of prophylaxis and delivered more sustained protection against bleeds, making the prospect of ‘zero bleeds’ a reality for many more PwH. ‘Zero bleeds’ should include asymptomatic joint bleeds (‘silent bleeds’ or microbleeds 14 ). Increasing evidence supports the relevance of asymptomatic bleeds as a cause of joint damage in patients with no clinically evident joint bleeds, and to avoid this progression, synovitis, and bone/cartilage damage should be detected via ultrasonography. 14 This approach underlies the importance of preventing all joint bleeds to achieve optimal outcomes.15,16 Of note, further research is needed to define the optimal treatment for otherwise asymptomatic joint disease detected only by imaging.
Currently, prophylaxis targeting FVIII trough levels in the mild range (e.g., 8%–12% 17 ), or mean FVIII activity >40% for around 4 days after weekly administration, 18 has achieved further benefits, including for joint health and quality of life. With data demonstrating that FVIII/FIX levels >30% are needed to abolish the residual risk of joint bleeds in PwH, 19 novel approaches may benefit joint health. Data from gene therapy trials show that factor levels >40% can be achieved in some trial participants, and they can remain bleed-free during clinical trial periods.20–23 However, long-term results have yet to be obtained, and it is not possible to predict results for individual patients, resulting in complex decision-making. 24
The goal of hemophilia treatment should be compared with other diseases involving deficient or missing proteins (e.g., diabetes or hypothyroidism), where the aim is to achieve similar levels of protein activity to unaffected people. The concept of targeting the physiologically normal range has only more recently been promoted for hemophilia with the advent of novel therapeutic modalities. Indeed, recent advances have raised expectations of ‘health equity’ and ‘functional cure.’ 25 PwH can now live relatively normal lives, 26 beyond concerns of bleed risk. ‘Normal’ participation, including sporting activity as appropriate, with freedom from joint damage/deterioration and pain, reduced treatment burden and mental well-being are the new targets.
With continuing innovation, particularly reducing prophylactic burden while still delivering functionally ‘normal’ factor levels, concepts of normal hemostasis, and a ‘hemophilia-free mind’ (free from constraints, fears and restrictions 27 ) are becoming reality.
Principle 3: Making the most of available resources
Within the constraints of any healthcare budget, resource use should be optimized. Ideally, this should be a joint venture between payers, healthcare professionals, patients, and caregivers, maximizing outcomes in an environment of mutual respect and responsibility.
If resources are limited, primary low/intermediate dose prophylaxis can provide cost-effective hemophilia care.28,29 In an era of evolving treatment, this approach provides better outcomes/joint protection than on-demand therapy but will not prevent joint disease in the long term.
Optimal hemophilia management includes other important aspects of treatment, as well as lifestyle choices; for example, regular physical activity/sport can improve muscle strength, protecting joints. 30 Physiotherapy is also key to improve coordination, posture, joint load, and balance. In the past, risk of bleeding because of inadequate hemostatic cover restricted the potential of physiotherapy. With optimal prophylaxis, regular physiotherapy may have a real impact, 31 improving health and reducing bleeds, while containing costs.
Regardless of the treatment patients receive, strategies encouraging adherence will always improve outcomes, 28 and good-quality education and support is effective in addressing this. Whatever resources are available, a multidisciplinary team is key to ensuring coordinated support and care 28 aiming to align clinical and patient-relevant outcomes, with shared decision-making, to achieve health equity.
Improvements in healthcare, as a whole, can heighten the competition for resources. This may exacerbate global health inequities as situations differ between countries, a ‘one-size-fits-all’ approach is not possible. In less-developed economies, with competing healthcare priorities, including infectious diseases and malnutrition, spending on acute disorders may be prioritized over certain chronic conditions. 28 Appropriate government commitment will help allocate resources to rare diseases such as hemophilia, 28 as will manufacturers’ flexibility negotiating products’ price. Affordable payment structures are essential. Efficient supply chain management helps capitalize on available medicines while reducing waste. 29 Resource allocation is also strictly related to epidemiology, and despite the incidence of hemophilia being relatively constant across different populations, diagnosis rates are lower in resource-limited settings. 28
Principle 4: Treatment affordability
Innovative treatments do not have to incur high upfront costs; it may be feasible to implement innovations with sustainable pricing. It is important to look at available pricing options, considering, for example, annual treatment costs and predictability of costs. Moreover, sustainability evaluation should include the spared costs to treat breakthrough bleeds according to demonstrated efficacy of given products (i.e., innovative therapies might offer better protection with less breakthrough bleeds and related cost of care upon prophylaxis).
Certain medicines may have efficacy across a range of approved dose levels; for more expensive medicines, concentration or dosing frequency may be adjusted to reduce costs while maintaining benefit. Ensuring that patients receive optimum levels of therapy may require more advanced understanding of mechanisms of action of innovative treatments.
Long-term benefits (costs over time) should be considered; direct treatment costs may be partially offset by health benefits, as evaluated in Health Technology Assessment; for example, EHL products may enable overall cost savings through improved joint health and lower annualized bleeding rates, while also benefiting health-related quality of life. 32 Costs of uncontrolled disease can include hospitalization and low productivity and participation; ameliorating health problems saves such costs, while providing opportunities for increased productivity and participation.
Resource-constrained countries have seen profound benefits in hemophilia management after receiving innovative treatments free of charge via global humanitarian initiatives. 33 Through national health programs and participation in international clinical trials, gene therapy could also become available in lower socioeconomic countries, providing opportunities to close the gap in hemophilia care globally. 34 To help address health inequity, regulators, payers, and pharmaceutical companies should accept their responsibilities and seek ways to finance innovation and negotiate pricing to allow access for all without disparities, attempting to overcome differences impacting treatment. This should take into account individualized needs and patient-based recommendations.
Efforts to identify and close the gap between those with and without hemophilia provide motivation for improvement. The ‘Treatment for All’ vision promoted by the World Federation of Hemophilia aims to provide care for all those with the condition, with the ‘Theory of Change’ initiative developed to facilitate global stakeholder collaboration. 35
Final thoughts
While factor administration still remains the unique option to manage bleeds, ultimately, all PwH—men and women, of all ages and severities, and worldwide—should have access to treatment that fully prevents bleeding, while allowing personal, social, family, and professional lives of choice. Clearly, we are not there yet, but developing goals/milestones based on the principles described above may help to achieve this, as may consideration of these ideas when developing future guidelines. Identifying appropriate targets can create a pathway to guide the journey, globally, across systems with differing rates of progress, and maybe eventually to those with other congenital bleeding disorders.
Acknowledgments
The authors wish to express gratitude to all those with hemophilia who have enrolled in clinical trials; such participation ultimately helps to improve clinical practice and advance care. Medical writing support for this manuscript was provided by Andy Lockley, PhD (Bioscript Group, Macclesfield, UK), funded by Sobi. Sobi and Sanofi received the manuscript for courtesy review. The authors had full editorial control of the manuscript and provided their final approval of all content. Editorial assistance and support with the submission of this manuscript was provided by Liz Beatty of Bioscript Group and supported by Sobi. The authors have authorized this support and approved the inclusion of all competing interests and funding disclosures.
Footnotes
ORCID iDs: Jan Blatný  https://orcid.org/0000-0001-6261-9157
https://orcid.org/0000-0001-6261-9157
Cédric Hermans  https://orcid.org/0000-0001-5429-8437
https://orcid.org/0000-0001-5429-8437
Katharina Holstein  https://orcid.org/0000-0003-3753-0972
https://orcid.org/0000-0003-3753-0972
Víctor Jiménez-Yuste  https://orcid.org/0000-0003-3937-3499
https://orcid.org/0000-0003-3937-3499
Sébastien Lobet  https://orcid.org/0000-0002-3829-6850
https://orcid.org/0000-0002-3829-6850
Maria Elisa Mancuso  https://orcid.org/0000-0002-7113-4028
https://orcid.org/0000-0002-7113-4028
Contributor Information
Jan Blatný, Hospital Bory, Nemocnica Bory, a.s., Ivana Kadlečíka 2, Bratislava 841 06, Slovakia; University Hospital Brno, Masaryk University, Brno, Czech Republic.
Jan Astermark, Department of Translational Medicine, Lund University, and Department of Hematology, Oncology and Radiation Physics, Skåne University Hospital, Malmö, Sweden.
Cristina Catarino, Immunochemotherapy Department, Congenital Coagulopathies Comprehensive Care Centre, Santa Maria University Hospital, Lisbon University, Lisbon, Portugal.
Gerry Dolan, Centre for Haemostasis and Thrombosis, St Thomas’ Comprehensive Care Centre, London, UK.
Karin Fijnvandraat, Amsterdam UMC, University of Amsterdam, Emma Children’s Hospital, Pediatric Hematology, Amsterdam, Netherlands.
Cédric Hermans, Division of Haematology, Saint-Luc University Hospital, Catholic University of Louvain, Brussels, Belgium.
Katharina Holstein, Department of Haematology and Oncology, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany.
Víctor Jiménez-Yuste, Department of Haematology, La Paz University Hospital-IdiPaz, Autónoma University of Madrid, Madrid, Spain.
Robert Klamroth, Department of Internal Medicine, Vivantes Hospital Friedrichshain, Berlin, Germany, and Institute of Experimental Haematology and Transfusion Medicine, University Hospital Bonn, Medical Faculty, University of Bonn, Bonn, Germany.
Michelle Lavin, Irish Centre for Vascular Biology, School of Pharmacy and Biomedical Sciences, Royal College of Surgeons in Ireland, Dublin, Ireland; National Coagulation Centre, St. James’ Hospital, Dublin, Ireland.
Peter J. Lenting, Université Paris-Saclay, INSERM, Hémostase Inflammation Thrombose HITh U1176, 94276, Le Kremlin-Bicêtre, France
Sébastien Lobet, Service d’ergothérapie et de kinésithérapie, Cliniques universitaires Saint-Luc, Université catholique de Louvain (UCLouvain), Brussels, Belgium; Neuromusculoskeletal Lab (NMSK), Secteur des Sciences de la Santé, Institut de Recherche Expérimentale et Clinique, Université catholique de Louvain (UCLouvain), Brussels, Belgium.
Maria Elisa Mancuso, Center for Thrombosis and Hemorrhagic Diseases, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy and Humanitas University, Pieve Emanuele, Milan, Italy.
Jayashree Motwani, Department of Paediatric Haematology, Birmingham Children’s Hospital, Birmingham, UK.
James S. O’Donnell, Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin, Ireland
Christoph Königs, Goethe University, University Hospital Frankfurt, Department of Paediatrics and Adolescent Medicine, Frankfurt, Germany.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Jan Blatny: Conceptualization; Writing – original draft; Writing – review & editing.
Jan Astermark: Writing – review & editing.
Cristina Catarino: Writing – review & editing.
Gerry Dolan: Writing – review & editing.
Karin Fijnvandraat: Writing – review & editing.
Cedric Hermans: Writing – review & editing.
Katharina Holstein: Writing – review & editing.
Víctor Jiménez-Yuste: Conceptualization; Writing – review & editing.
Robert Klamroth: Writing – review & editing.
Michelle Lavin: Writing – review & editing.
Peter J. Lenting: Writing – review & editing.
Sébastien Lobet: Writing – review & editing.
Maria Elisa Mancuso: Writing – review & editing.
Jayashree Motwani: Conceptualization; Writing – review & editing.
James O’Donnell: Writing – review & editing.
Christoph Königs: Conceptualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This review article is the outcome of a scientific project, the Factor Think Tank, funded by Sobi.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JB has received consultation and/or speaker’s fees from NovoNordisk, Roche, Sobi, Takeda, and CSL Behring. JA has received research grants from Sobi, CSL Behring, Takeda/Shire, and Bayer, and speaker’s fees and consultancy for Octapharma, Novo Nordisk, Pfizer, Bayer, Sobi, CSL Behring, Takeda/Shire, BioMarin, Uniqure, and Spark Therapeutics. CC has received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events, and support for attending meetings and/or travel from Sobi, Bayer, Roche, and Novo Nordisk, and has also participated in data safety monitoring boards or advisory boards for Sobi, Bayer, Roche, and Novo Nordisk. GD has received consulting fees from Pfizer, Biomarin, CSL, Roche, and Sobi, and payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Pfizer, Spark Therapeutics, CSL, Bayer, Takeda, Roche, Chugai, and Sobi. KF has received unrestricted research grants from CSL Behring, Sobi, and Novo Nordisk, as well as consultancy fees from Hoffman-La Roche, Sanofi, Sobi, and Novo Nordisk, with all fees paid to her institution. CH has received research funding from Bayer, BioMarin, CSL Behring, Novo Nordisk, Pfizer, Shire/Takeda, and Sobi, as well as honoraria/speaker’s bureau fees from Bayer, CAF-DCF, CSL Behring, Hoffmann-La Roche, LFB, Novo Nordisk, Octapharma, Pfizer, Shire/Takeda, Sobi, and UniQure. KH has received grants for research or clinical studies (paid to her institution) from Bayer, CSL Behring, Novo Nordisk, Pfizer, Regeneron, Sanofi, and Sobi, as well honoraria or consultancy fees from Bayer, Biomarin, Biotest, CSL Behring, LFB, Novo Nordisk, Pfizer, Roche, Sobi, and Takeda. VJ-Y has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting, and/or funds for research from Takeda, Bayer, BioMarin, CSL Behring, Grifols, Novo Nordisk, Sobi, Roche, Octapharma, and Pfizer. RK has received consultancy fees and honoraria for lectures and advisory boards from Bayer, Biomarin, CSL Behring, Novo Nordisk, Grifols, Octapharma, Pfizer, Roche/Chugai, Sanofi, Sobi, and Takeda. ML has served on an advisory board for CSL Behring, as a consultant for Sobi, CSL Behring, Takeda, and Band Therapeutics, and has received research funding from Takeda and speaker fees from Sobi and Takeda. PJL has received research support to his institute from Sobi, Sanofi, BioMarin, and Roche. SL has acted as a paid consultant to Faust Pharmaceuticals Inc. MEM has acted as a paid speaker/consultant/advisor for Bayer, Biomarin, CSL Behring, Kedrion, LFB, Octapharma, Novo Nordisk, Pfizer, Roche, Sanofi, Sobi, and Takeda. JM has received honoraria and/or educational support from Sobi, Roche, Bayer, and CSL. JSO’D declares no conflicts of interest beyond the support for this manuscript from Sobi. CK has received funding from BFSH, Bayer, CSL Behring, Florio, MSD, Novo Nordisk, Roche/Chugai, Sobi/Sanofi, and Takeda for presentations and/or scientific advice, and his institution has received research funding from Bayer, Biotest, CSL Behring, Intersero, Novo Nordisk, Pfizer, Roche/Chugai, Sobi/Sanofi, and Takeda.
Availability of data and materials: Not applicable.
References
- 1. White GC, II, Rosendaal F, Aledort LM, et al. Definitions in hemophilia. Recommendation of the Scientific Subcommittee on Factor VIII and Factor IX of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost 2001; 85: 560. [PubMed] [Google Scholar]
- 2. Thachil J, Connors JM, Mahlangu J, et al. Reclassifying hemophilia to include the definition of outcomes and phenotype as new targets. J Thromb Haemost 2023; 21: 1737–1740. [DOI] [PubMed] [Google Scholar]
- 3. Young G, Makris M. Time to revisit the classification of hemophilia: if it ain’t broke, don’t fix it! J Thromb Haemost 2023; 21: 1755–1756. [DOI] [PubMed] [Google Scholar]
- 4. Gorman R, Woollard L. “Reclassifying hemophilia to include the definition of outcomes and phenotype as new targets”: comment. J Thromb Haemost 2023; 21: 2977–2979. [DOI] [PubMed] [Google Scholar]
- 5. Castaman G, Matino D. Hemophilia A and B: molecular and clinical similarities and differences. Haematologica 2019; 104: 1702–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nilsson IM, Berntorp E, Löfqvist T, et al. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J Intern Med 1992; 232: 25–32. [DOI] [PubMed] [Google Scholar]
- 7. Scott MJ, Xiang H, Hart DP, et al. Treatment regimens and outcomes in severe and moderate haemophilia A in the UK: the THUNDER study. Haemophilia 2019; 25: 205–212. [DOI] [PubMed] [Google Scholar]
- 8. Négrier C, Mahlangu J, Lehle M, et al. Emicizumab in people with moderate or mild haemophilia A (HAVEN 6): a multicentre, open-label, single-arm, phase 3 study. Lancet Haematol 2023; 10: e168–e177. [DOI] [PubMed] [Google Scholar]
- 9. Mancuso ME, Croteau SE, Klamroth R. Benefits and risks of non-factor therapies: redefining haemophilia treatment goals in the era of new technologies. Haemophilia 2024; 30(Suppl. 3): 39–44. [DOI] [PubMed] [Google Scholar]
- 10. Poon MC, Lee A. Individualized prophylaxis for optimizing hemophilia care: can we apply this to both developed and developing nations? Thromb J 2016; 14: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berntorp E, Hermans C, Solms A, et al. Optimising prophylaxis in haemophilia A: the ups and downs of treatment. Blood Rev 2021; 50: 100852. [DOI] [PubMed] [Google Scholar]
- 12. Castaman G, Jimenez-Yuste V, Gouw S, et al. Outcomes and outcome measures. Haemophilia 2024; 30(Suppl. 3): 112–119. [DOI] [PubMed] [Google Scholar]
- 13. Keam SJ. Concizumab: first approval. Drugs 2023; 83: 1053–1059. [DOI] [PubMed] [Google Scholar]
- 14. Mancuso ME, Holstein K, O’Donnell JS, et al. Synovitis and joint health in patients with haemophilia: statements from a European e-Delphi consensus study. Haemophilia 2023; 29: 619–628. [DOI] [PubMed] [Google Scholar]
- 15. Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007; 357: 535–544. [DOI] [PubMed] [Google Scholar]
- 16. Oldenburg J. Optimal treatment strategies for hemophilia: achievements and limitations of current prophylactic regimens. Blood 2015; 125: 2038–2044. [DOI] [PubMed] [Google Scholar]
- 17. Klamroth R, Windyga J, Radulescu V, et al. Rurioctocog alfa pegol PK-guided prophylaxis in hemophilia A: results from the phase 3 PROPEL study. Blood 2021; 137: 1818–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. von Drygalski A, Chowdary P, Kulkarni R, et al. Efanesoctocog alfa prophylaxis for patients with severe hemophilia A. N Engl J Med 2023; 388: 310–318. [DOI] [PubMed] [Google Scholar]
- 19. Soucie JM, Monahan PE, Kulkarni R, et al. The frequency of joint hemorrhages and procedures in nonsevere hemophilia A vs B. Blood Adv 2018; 2: 2136–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mahlangu J, Kaczmarek R, von Drygalski A, et al. Two-year outcomes of valoctocogene roxaparvovec therapy for hemophilia A. N Engl J Med 2023; 388: 694–705. [DOI] [PubMed] [Google Scholar]
- 21. Pipe SW, Leebeek FWG, Recht M, et al. Gene therapy with etranacogene dezaparvovec for hemophilia B. N Engl J Med 2023; 388: 706–718. [DOI] [PubMed] [Google Scholar]
- 22. Leavitt AD, Mahlangu J, Raheja P, et al. Efficacy and safety of valoctocogene roxaparvovec 4 years after gene transfer in GENEr8-1. In: Presentation OC 30.2 at ISTH 2024, Bangkok, Thailand, 24 June 2024, https://www.isth2024.org/abstracts (accessed 4 July 2024). [Google Scholar]
- 23. von Drygalkski A, Giermasz A, Gomez E, et al. Etranacogene dezaparvovec hemophilia B gene therapy phase 2b trial final results: stable and durable FIX level expression over 5 years. In: Presentation OC 02.3 at ISTH 2024, Bangkok, Thailand, 22 June 2024, https://www.isth2024.org/abstracts (accessed 4 July 2024). [Google Scholar]
- 24. Miesbach W, Mulders G, Breederveld D, et al. A 360-degree perspective on adeno-associated virus (AAV)-based gene therapy for haemophilia: Insights from the physician, the nurse and the patient. Orphanet J Rare Dis 2024; 19: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Skinner MW, Nugent D, Wilton P, et al. Achieving the unimaginable: health equity in haemophilia. Haemophilia 2020; 26: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Srivastava A, Santagostino E, Dougall A, et al. WFH Guidelines for the Management of Hemophilia, 3rd Edition. Haemophilia 2020; 26(Suppl. 6): 1–158. [DOI] [PubMed] [Google Scholar]
- 27. Hermans C, Pierce GF. Towards achieving a haemophilia-free mind. Haemophilia 2023; 29: 951–953. [DOI] [PubMed] [Google Scholar]
- 28. Ndoumba-Mintya A, Diallo YL, Tayou TC, et al. Optimizing haemophilia care in resource-limited countries: current challenges and future prospects. J Blood Med 2023; 14: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ghosh K, Ghosh K. Overcoming the challenges of treating hemophilia in resource-limited nations: a focus on medication access and adherence. Expert Rev Hematol 2021; 14: 721–730. [DOI] [PubMed] [Google Scholar]
- 30. Negrier C, Seuser A, Forsyth A, et al. The benefits of exercise for patients with haemophilia and recommendations for safe and effective physical activity. Haemophilia 2013; 19: 487–498. [DOI] [PubMed] [Google Scholar]
- 31. Chen CM, Lin CH, Kung KY. Effects of physical therapy on joint pain, joint range of motion, joint health, strength, and mobility in patients with hemophilia: a systematic review and meta-analysis. Am J Phys Med Rehabil 2023; 102: 577–587. [DOI] [PubMed] [Google Scholar]
- 32. Bullement A, McMordie ST, Hatswell AJ, et al. Cost-effectiveness analysis of recombinant factor VIII Fc-fusion protein (rFVIIIFc) for the treatment of severe hemophilia A in Italy incorporating real-world dosing and joint health data. Pharmacoecon Open 2020; 4: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lambert C, Meité N, Kouassi GK, et al. Nonreplacement therapy for hemophilia in low-income countries: experience from a prospective study in Ivory Coast. Res Pract Thromb Haemost 2023; 7: 100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reiss UM, Zhang L, Ohmori T. Hemophilia gene therapy-New country initiatives. Haemophilia 2021; 27(Suppl. 3): 132–141. [DOI] [PubMed] [Google Scholar]
- 35. Laliberté J, Coffin D, Haffar A, et al. Theory of change and strategic priorities of the World Federation of Haemophilia. Haemophilia 2023; 29: 45–50. [DOI] [PubMed] [Google Scholar]