Abstract
Papillomaviruses (PVs) are well recognized to cause pre-neoplastic and neoplastic diseases in humans. Similarly, there is increasing evidence that PVs play a significant role in the development of pre-neoplastic and neoplastic diseases of the haired skin of dogs and cats and the mucosa of horses. As the mechanisms by which PVs cause neoplasia are well studied in humans, it is valuable to compare the PV-induced neoplasms of humans with similar PV-associated neoplasms in the companion animal species. In the second part of this comparative review, the pre-neoplastic and neoplastic diseases thought to be caused by PVs in humans, dogs, cats, and horses are described. This includes PV-induced cutaneous plaques, cutaneous squamous cell carcinomas (SCCs) and mucosal SCCs within the four species. The review concludes with a discussion about the potential use of vaccines to prevent PV-induced diseases of dogs, cats, and horses.
Keywords: Papillomavirus, dogs, cats, horses, neoplasia, cancer, oncogenesis, oncogenic viruses, viral plaques, pigmented plaques, Bowenoid in situ carcinoma, squamous cell carcinoma, skin cancer, oral cancer, genital cancer, canine papillomavirus, feline papillomavirus, equine papillomavirus
Introduction
In the second part of this comparative review, the pre-neoplastic and neoplastic diseases caused by papillomaviruses (PVs) in humans, dogs, cats, and horses are described. Unlike the hyperplastic diseases described in part 1, these diseases rarely resolve spontaneously. Diseases included in part 2 include cutaneous plaques, cutaneous squamous cell carcinomas (SCCs), and mucosal SCCs. Although cross-species infection of horses and cats by bovine PV types has been shown to cause equine and feline sarcoids (Chambers et al., 2003; Munday et al., 2015b), neither cross-species PV infections nor PV-induced mesenchymal neoplasia are recognized in people and these will not be discussed in this comparative review. The review ends with a brief discussion on the use of vaccines to prevent diseases caused by PVs in humans and the companion animal species.
Papillomaviral cutaneous plaques
Humans
PV-induced cutaneous plaques are rare in people. They are caused by numerous closely related PV types within the Betapapillomavirus genus (Kremsdorf et al., 1984). Infection by these PVs are ubiquitous, with people being infected in the first few days of life (Antonsson et al., 2000; Antonsson et al., 2003). In an immune competent person, replication of PVs is inhibited by the immune system and the PV causes minimal epithelial hyperplasia and remains asymptomatic. However, if the immune system is unable to mount a normal immune response, increased PV replication results in the development of visible lesions. While plaques are well-recognised to develop in patients that receive immunosuppressive therapy after organ transplantation, the pathogenesis of human PV-induced plaques is best illustrated by the rare genetic disorder epidermodysplasia verruciformis (EV) (Egawa and Doorbar, 2017; Orth, 2006). EV is due to inherited defects in the EVER1, EVER2 or CIB1 genes which code for proteins that contribute to keratinocyte intrinsic immunity (Beziat et al., 2021; Ramoz et al., 2002). As keratinocyte intrinsic immunity inhibits PV replication, loss of this immunity allows greater viral replication and the development of visible cutaneous plaques (Beziat et al., 2021). Interestingly, the impact of mutations within the EVER1, EVER2 and CIB1 genes appears to be specific to the immune response to PVs as people with EV do not show other signs of immunosuppression.
People with EV usually develop flat plaques in early childhood. They are most common on the trunk, neck and extremities and appear as hypo- or hyperpigmented plaques that can coalesce (Cubie, 2013). Larger plaques can appear verrucous, especially if they are exposed to UV light. Histologically, the plaques appear as thickened epidermis covered by increased keratin. PV-induced cellular changes are usually visible in a high proportion of keratinocytes within the plaques (Cubie, 2013).
If possible, restoration of normal immune function will result in lesion resolution. If restoration is not possible (for example in people with EV), plaques are expected to slowly become larger and more numerous throughout life (Sterling, 2016). Progression to SCC occurs in 20–30% of EV patients (Sterling, 2016). However, neoplastic transformation only occurs in plaques that are exposed to UV light (Sterling, 2016), suggesting the Betapapillomaviruses cannot cause neoplasia without a co-factor (McBride, 2022). Although there is some evidence from individual cases that treatment using oral retinol, oral interferon, or topical imiquimod may be beneficial, results have been inconsistent and no treatment is curative if normal immune function cannot be restored (Sterling, 2016).
Dogs
Viral cutaneous plaques (also referred to as pigmented plaques) are rare lesions in dogs. They are caused by a number of closely-related Chipapillomavirus types with CPV4 being the CPV type most often identified in these lesions (Lange et al., 2009a; Lange et al., 2009b; Lange et al., 2012; Luff et al., 2015; Luff et al., 2012a; Luff et al., 2012b; Tobler et al., 2006; Tobler et al., 2008; Yuan et al., 2012; Zhou et al., 2014). Cases are sporadic and contact with other affected dogs has not been reported. The epidemiology of infection by the Chipapillomaviruses has not been studied. However, it is possible that dogs could be frequently asymptomatically infected by Chipapillomaviruses and, as with human cutaneous plaques, the development of a canine cutaneous plaque could be primarily due to an inability to inhibit replication by a PV type that commonly infects the skin. This is supported by rare reports of plaques developing in dogs with possible immunosuppressive conditions (hyperadrenocorticism, putative hypothyroidism, and hypoglobulinemia) and receiving immunosuppressive medication (Stokking et al., 2004; Tobler et al., 2008). Furthermore, certain breeds, such as Vizslas and Pugs, are predisposed to developing plaques (Hansen et al., 2018; Nagata et al., 1995; Tobler et al., 2008) suggesting that it is possible that these dogs inherited an EV-like defect in keratinocyte intrinsic immunity. However, further research is required to determine if there truly is an underlying genetic component in these dogs, and if it parallels that of human EV patients.
Viral plaques have been reported to develop in dogs as young as 2-years-old but are more common in middle-aged dogs (Munday et al., 2022b; Nagata et al., 1995). They are typically dark, multiple, and 1–10mm in diameter. As in humans, they tend to be flat early in the clinical course then become more exophytic as the lesions progress (Munday et al., 2022b). They are most common on the ventrum and medial aspects of the limbs (Gross et al., 2005). In rare cases, lesions can coalesce and involve a large proportion of the body (Hansen et al., 2018; Munday et al., 2022b; Fig. 1). Most canine viral plaques do not significantly impact life and spontaneous regression is possible. However, extensive plaques can cause pruritis or pain (Knight et al., 2016; Munday et al., 2022b). Histology reveals moderately thickened epidermis covered by increased keratin. Lesions typically have a scalloped appearance with prominent epidermal and dermal pigment and keratohyalin granules (Fig. 2). While viral replication has been detected within plaques (Lange et al., 2013a), PV-induced cellular changes are rarely histologically visible (Munday et al., 2017c). This is in contrast to both human and feline plaques that often contain prominent evidence of PV replication.
Figure 1.
Papillomaviral cutaneous plaques, dog. The heavily pigmented plaques on this Chinese crested dog are predominantly confined to the skin of the limbs and ventrum. Larger plaques are mildly exophytic and plaques have coalesced in some areas (Image courtesy of Dr Anne Quain).
Figure 2.
Papillomaviral cutaneous plaques, dog. The plaque consists of heavily pigmented, hyperplastic epidermis covered by increased quantities of keratin. Large quantities of pigment and marked clumping of keratohyalin granules are visible within the thickened epidermis. Neither koilocytosis nor the presence of cells with expanded, blue-grey cytoplasm are visible within these lesions. PCR was used to amplify canine papillomavirus type 18 DNA from this plaque. Scale bar = 25μm. Haematoxylin and eosin.
Canine viral plaques have rarely been reported to progress to SCCs. Some evidence suggests neoplastic transformation is more likely in plaques caused by specific CPV types. Of these types, CPV16 is most frequently associated with neoplastic transformation, although other CPV types have also been identified in plaques that have undergone neoplastic transformation (Alves et al., 2020; Chang et al., 2020; Lange et al., 2009a; Luff et al., 2019; Luff et al., 2016; Munday et al., 2011d; Tobler et al., 2006). Similar to the high-risk human Alphapapillomaviruses, CPV16 has been shown to integrate into the host genome within the plaques, supporting the possibility that this may be a higher-risk CPV type (Luff et al., 2019). Whether or not sun exposure influences neoplastic progression of a canine plaque is currently unknown.
Treatment of dogs with pigmented plaques is often not required. However, if plaques become large and cause irritation to the dog, surgical excision of plaques is possible, although additional plaques usually develop. Other potential treatment options that act by destroying the infected epithelium include laser ablation and tigilanol tiglate gel (Hansen et al., 2018; Knight et al., 2016). Medical treatments that have been tried include oral retinol, topical imiquimod, azithromycin, and interferon alfa-2b although, as in humans with EV, there is no definitive evidence that these therapies are effective. Indeed, if canine plaques are caused by a defect in keratinocyte intrinsic immunity, medical treatments would appear unlikely to be able to restore immunity sufficiently to be curative.
Cats
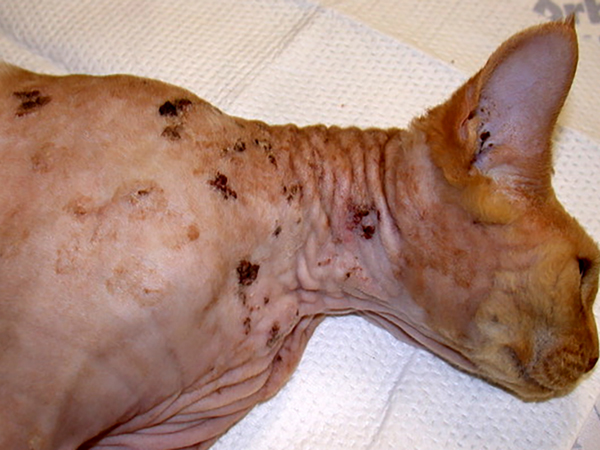
PV-induced cutaneous plaques are rare in cats, typically developing in middle-aged or older animals. Depending on the presence of dysplasia within the epidermal cells these can be classified as feline viral plaques or Bowenoid in situ carcinomas (BISCs). Plaques are most often caused by infection with FcaPV2 (a Dyothetapapillomavirus) although other Taupapillomavirus FcaPV types can also cause these lesions (Demos et al., 2019; Kok et al., 2019; Lange et al., 2009c; Munday et al., 2017a; Munday and Thomson, 2021; Munday et al., 2018; Nespeca et al., 2006; Vascellari et al., 2019). While less is known about the other FcaPV types, infection by FcaPV2 is ubiquitous in cats, with kittens being infected during, or shortly after, birth (Geisseler et al., 2016; Thomson et al., 2015). As in humans, this suggests plaque development is primarily due to an immune dysfunction that allows increased replication by a normally asymptomatic PV type. Sphinx or Devon Rex cats develop plaques more frequently and at a younger age suggesting the likelihood of an inherited defect in the keratinocyte immune system (Munday et al., 2016a; Ravens et al., 2013). The immune defect that allows plaque development appears to be specific for cutaneous PV infection and other signs of immunosuppression have not been reported in affected cats.
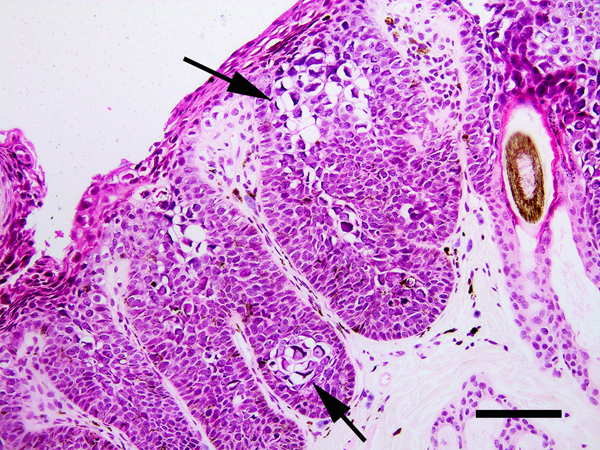
Feline viral plaques present as multiple pigmented or non-pigmented non-painful, non-pruritic, slightly-raised lesions up to 2cm in diameter that are most common on the face, head, and neck (Wilhelm et al., 2006; Fig. 3). Histology reveals mild to moderate hyperplasia of cells within the deeper layers of the epidermis. Unlike human and canine plaques, the epidermal cells within feline plaques are often dysplastic. PV-induced cellular changes can be prominent in smaller plaques (Fig. 4), although these changes become less common in larger, more dysplastic, lesions (Gill et al., 2008; Wilhelm et al., 2006). The frequent dysplasia within feline viral plaques suggests the FcaPV types that cause plaques cause greater dysregulation of epidermal cells than either the human Betapapillomaviruses or the canine Chipapillomaviruses. Additionally, feline viral plaques consistently contain marked accumulation of p16CDKN2A protein (p16) due to PV-induced degradation of the retinoblastoma protein (pRb) (Munday and Aberdein, 2012; Munday et al., 2011a). Such PV-induced changes in cell regulation are observed in human SCCs caused by the high-risk Alphapapillomaviruses, but are not seen in the human SCCs associated with Betapapillomavirus infection (Küsters-Vandevelde et al., 2009).
Figure 3.
Papillomaviral cutaneous plaques, cat. Multifocal pigmented and non-pigmented plaques are visible (Image courtesy of Dr Linda Vogelnest).
Figure 4.
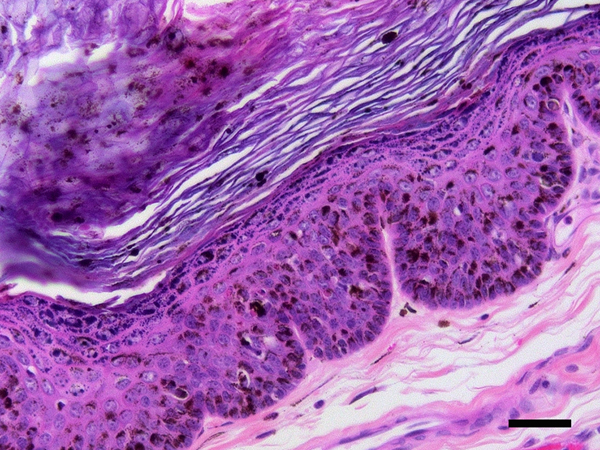
Papillomaviral cutaneous plaque, cat. The epidermis is thickened and contains increased pigment. There is crowding of cells within the basilar layers. Papillomavirus-induced cellular changes are prominent including cells with expanded clear cytoplasm containing perinuclear bodies (arrows). PCR amplified Felis catus papillomavirus type 3 DNA from this plaque. Scale bar = 40μm. Haematoxylin and eosin.
Feline viral plaques can spontaneously regress (Munday JS, personal observation), presumably due to resolution of the immune dysfunction. However, regression appears to be rare and more often plaques persist and can become large and ulcerative, resulting in significant morbidity. Viral plaques can also progress to SCC, especially in Devon Rex and Sphinx cats in which the progression tends to be rapid and result in SCCs with high metastatic potential (Munday et al., 2016a; Ravens et al., 2013). Whether or not sunlight plays a significant role in neoplastic transformation is uncertain.
As in humans and dogs, treatment of feline plaques is local rather than systemic and surgical excision, imiquimod cream, and cryotherapy are all suggested treatment options (Gill et al., 2008). However, considering the likelihood of an underlying inability to control viral replication, development of additional lesions should be expected.
Horses
Horses are currently not recognized to develop widespread PV-induced plaques due to loss of immune control of a normally asymptomatic PV type. However, horses do frequently develop aural plaques on the inner surface of the pinnae (Torres et al., 2010). These plaques are thought to be caused by EcPV types in both the Dyoiotapapillomavirus and Dyorhopapillomavirus genera, different from the EcPV types that cause cutaneous and genital warts (Gorino et al., 2013; Lange et al., 2011a; Mira et al., 2018; Taniwaki et al., 2013; Torres and Koch, 2013). Equine aural plaques may be contagious, with the causative PV types hypothesised to be spread between horses by an insect vector (Fairley et al., 2014). Aural plaques do not spontaneously resolve and there is a single report of progression to SCC (Peters-Kennedy et al., 2020).
Histologically, aural plaques comprise moderately thickened epidermis covered by marked hyperkeratosis. Epidermal folding does not occur so that the thickened epidermis remains plaque-like rather than forming an exophytic wart (Scott and Miller, 2011). Aural plaques contain reduced melanin compared to surrounding skin. Clumped keratohyalin granules and koilocytes can be visible although PV-induced cellular changes are subtle within the plaques. As aural plaques are typically only a cosmetic concern, they are rarely treated. Surgical debulking can be successful although lesion recurrence is common (Peters-Kennedy et al., 2020; Scott and Miller, 2011). Treatment using repeated topical application of imiquimod has been reported to be curative although this treatment usually causes significant pain (Torres et al., 2010; Zakia et al., 2016).
Squamous cell carcinomas of the skin
Humans
As previously discussed in this review, HPVs can cause cutaneous SCCs due to progression of a viral cutaneous plaque or, less commonly, due to progression of a recalcitrant wart (Egawa and Doorbar, 2017; Sterling, 2016). However, both viral plaques and recalcitrant warts develop due to underlying immune defects and it is less clear whether or not PVs are significant causes of SCCs in people with intact immune systems and no pre-existing PV lesions (Smola, 2020). As Betapapillomaviruses asymptomatically infect the skin of all people (Antonsson et al., 2000), it is possible that they could influence regulatory pathways within the infected epidermal cells. For example, PV infection of a keratinocyte could reduce the ability of the cell to undergo apoptosis in response to UV light-induced DNA damage (Akgul et al., 2006). This reduced ability of a cell to undergo apoptosis could allow the accumulation of DNA mutations and subsequent neoplastic transformation. However, evaluating the role of Betapapillomaviuses in skin SCCs is difficult as PV DNA is detectible in both SCCs and normal skin samples (Forslund et al., 2003). Additionally, unlike mucosal SCCs caused by the high-risk Alphapapillomaviruses, cutaneous SCCs associated with Betapapillomaviruses do not consistently contain alterations in p53, pRb, p16 or any other marker that could indicate a PV etiology of the cancer (Küsters-Vandevelde et al., 2009; Smola, 2020). Intriguingly, it has recently been hypothesized that the presence of Betapapillomaviruses could actually protect against skin SCCs by promoting immune surveillance of the skin surface (Strickley et al., 2019). While the high-risk Alphapapillomaviruses are well established as causes of human cancers, these sexually-transmitted PVs have a strong mucosal trophism and are extremely rare causes of SCCs in haired skin (Amiraraghi et al., 2019).
Dogs
There are rare reports of canine cutaneous SCCs developing within viral cutaneous plaques or cutaneous warts (Luff et al., 2016; Thaiwong et al., 2018). Additionally, some studies of canine cutaneous SCCs have detected PV DNA in a proportion of neoplasms (Teifke et al., 1998; Waropastrakul et al., 2012; Zaugg et al., 2005). However, as in other species, asymptomatic infection of canine skin is common (Lange et al., 2011b) making the detection of PV DNA in a SCC difficult to interpret. Overall, there is currently little evidence that PVs are a significant cause of canine cutaneous SCCs.
Cats
Of the four species discussed in this review, there is the strongest evidence of a PV etiology of skin cancer in cats. While PV infections in cats have been associated with basal cell carcinomas and Merkel cell carcinomas (Ito et al., 2022; Munday et al., 2017b), the majority of studies have evaluated cutaneous SCCs. These studies suggest that feline cutaneous SCCs can be subdivided into those that are primarily caused by exposure to UV light and those that appear likely to be caused by PV infection (Munday et al., 2011b; Thomson et al., 2016). The proportion of SCCs associated with PV infection is variable depending on the area of the body. Within UV-protected areas of the body (haired pigmented skin) as many as 75% of SCCs may be caused by PV infection while only around 30% of SCCs from UV-exposed areas of the body (non-pigmented nasal planum, pinnae and eyelids) show evidence of a PV etiology (Munday et al., 2011b). Interestingly, the presence of these two distinct subtypes is similar to human oral SCCs which can be subdivided into those caused by tobacco and alcohol and those caused by infection by PVs. Feline cutaneous SCCs can develop as progression from a viral cutaneous plaque (Munday et al., 2016a; Ravens et al., 2013). However, it is currently uncertain if all PV-associated SCCs develop from plaques or whether some develop within normal skin without progressing through this precursor lesion.
Most studies report that FcaPV2 is the predominant type detectible in feline cutaneous SCCs (Altamura et al., 2018; Munday and Aberdein, 2012; Munday et al., 2009a; Munday et al., 2013; Munday et al., 2008; O’Neill et al., 2011; Thomson et al., 2016; Yamashita-Kawanishi et al., 2021a), although other FcaPV types have also been detected (Carrai et al., 2020; Munday et al., 2011b; Munday et al., 2018; Yamashita-Kawanishi et al., 2021b; Yamashita-Kawanishi et al., 2018). Histological evidence of PV infection is rarely detectible. However, SCCs contain FcaPV2 DNA more frequently, and have higher FcaPV2 loads, than normal skin (Munday et al., 2008; Thomson et al., 2016). In addition, a proportion of SCCs contain detectible FcaPV gene expression (Altamura et al., 2016; Thomson et al., 2016) that can be localized to the neoplastic cells within the SCCs (Hoggard et al., 2018). Additional evidence for a role of PVs in feline skin SCCs is derived from the observation that cats with PV-associated nasal planum SCCs survived longer than cats with nasal planum SCCs that did not contain evidence of PV infection (Munday et al., 2013). This supports the presence of two etiologically different subsets of SCCs and is consistent with human oral SCCs in which patients with PV-induced oral cancers survive longer than those with cancers caused by tobacco and alcohol (Lewis et al., 2010).
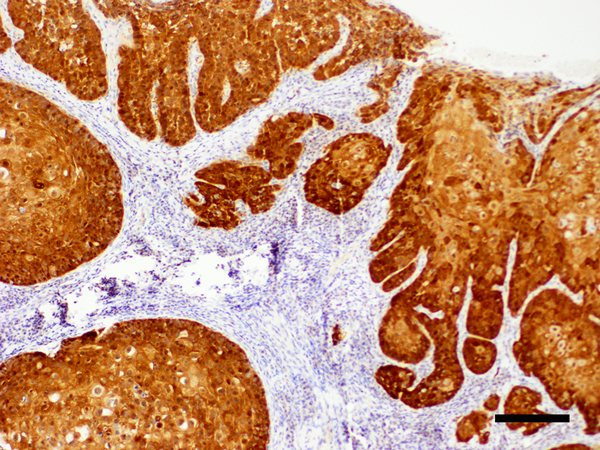
Evidence indicates that FcaPV2 causes neoplasia by the E7 protein degrading pRb and the FcaPV2 E6 protein interfering with normal p53 function (Altamura et al., 2016; Munday and Aberdein, 2012). The loss of pRb removes an important checkpoint preventing cell replication while impaired p53 function reduces the ability of the cell to recognize damaged DNA and undergo apoptosis. Loss of pRb also results in a marked increase in p16 within the cell that can be detected using immunohistochemistry (Munday and Aberdein, 2012) and PV-associated feline cutaneous SCCs consistently contain intense nuclear and cytoplasmic p16 immunostaining (Munday et al., 2013; Munday et al., 2011b; Fig. 5). Similarly, degradation of pRb and p53 are important mechanisms by which the high-risk human Alphapapillomaviruses cause cancer and resultant cancers consistently contain intense p16 immunostaining (Doorbar et al., 2012; Lewis et al., 2017).
Figure 5.
Cutaneous squamous cell carcinoma, cat. Neoplastic cells exhibit intense nuclear and cytoplasmic immunostaining to antibodies against the p16CDKN2A protein. Note the presence of nests of neoplastic cells infiltrating from the overlying epidermis into the dermis. PCR amplified Felis catus papillomavirus type 2 DNA from the lesion. Scale bar = 75μm. Bond Refine Detection staining kit with haematoxylin counterstain.
While FcaPV2 shows some similarities to the high-risk Alphapapillomaviruses, an important difference is that cats are infected with FcaPV2 in the first days of life and infection is ubiquitous and lifelong (Thomson et al., 2015). As most cats do not develop cutaneous SCCs despite being infected, this suggests other factors determine the development of cancer. While these factors are poorly understood, there is variability in the viral loads of FcaPV2 between cats. Furthermore, the viral loads on an individual cat remain constant over an extended period of time (Thomson et al., 2019). This suggests individual variability in the ability of the immune system to control PV replication and it is possible that cats that are less able to prevent replication are predisposed to SCC development due to their chronically higher viral loads. This hypothesis is supported by the key role of the immune system in determining whether or not infection by a human high-risk Alphapapillomavirus will cause cancer (McBride, 2022). Alternatively, SCC development may be more dependent on the presence of co-factors such as UV exposure (Altamura et al., 2016; Munday and Kiupel, 2010). Overall, evidence suggests FcaPV2 (and possibly FcaPV3 and FcaPV4) promotes neoplasia to a much greater extent than the PVs that asymptomatically infect the skin of humans and dogs.
Horses
Non-genital skin SCCs have been rarely reported to develop due to progression from EcPV8-induced skin lesions and from aural plaques (Peters-Kennedy et al., 2020; Peters-Kennedy et al., 2019). However, there is currently little evidence PVs are a common cause of non-genital skin cancers of horses.
Mucosal neoplasia
Humans
The ability of the high-risk Alphapapillomaviruses to cause cancer is well established and these PVs are estimated to cause around 5% of all human cancers (Plummer et al., 2016). This includes almost all cervical and anal SCCs as well as around half of oral, penile, vaginal and vulval SCCs (Brianti et al., 2017). Infections are spread by sexual contact. Viral replication can result in inconspicuous flat plaques, but many infections remain asymptomatic (Doorbar et al., 2012; McBride, 2022). An immune response is generated and the infection is cleared within two years in most people (McBride, 2022). However, in a small proportion, the immune response is unable to resolve the PV infection predisposing to accidental integration of PV DNA into host cell DNA. Integration prevents viral replication but results in increased production of viral proteins and loss of normal pRb and p53 function. This creates a population of rapidly dividing, genetically unstable cells that are at increased risk of developing additional spontaneous DNA mutations that result in neoplastic transformation (Brianti et al., 2017; Doorbar et al., 2012).
While virtually all cervical cancers are considered to be caused by PV infection, oral SCCs can be subdivided into those caused by PV infection and those that are caused by tobacco and alcohol. The two subsets of SCCs have a different clinical presentation, with SCCs caused by PVs generally developing in the oropharynx of younger patients (Economopoulou et al., 2020). As the high-risk Alphapapillomaviruses so consistently cause neoplasia by degrading pRb and loss of pRb causes a marked increase in cell p16, intense nuclear and cytoplasmic p16 immunostaining confirms a PV etiology of a human oral SCC (Lewis et al., 2017).
Dogs
There are rare reports of canine oral SCCs developing within oral warts or plaques. Multiple flat plaques and invasive SCCs developed in the mouth of a dog in association with CPV17 (Munday et al., 2016b). Consistent with human PV-induced oral SCCs, the canine SCCs had marked p16 immunostaining, although unlike in human oral SCCs, PV-induced cellular changes were also prominent within the neoplastic cells (Munday et al., 2015c). There are also rare reports of progression from CPV1-induced oral warts to invasive oral SCCs in dogs (Regalado Ibarra et al., 2018; Thaiwong et al., 2018). However, PVs are not currently thought to be a significant cause of oral, or other mucosal, SCCs in dogs (Munday et al., 2015a; Porcellato et al., 2014).
Cats
Whether or not PVs play a significant role in the development of feline oral SCCs is currently uncertain. Many studies of feline oral SCCs have detected feline PV DNA only sporadically within these neoplasms (Chu et al., 2020; Munday and French, 2015; Munday et al., 2009b; Munday et al., 2011c; O’Neill et al., 2011). Immunohistochemistry revealed variable p16 immunostaining; however, this was not associated with the presence of PV DNA and no oral SCC contained the intense nuclear and cytoplasmic immunostaining that characterises PV-induced oral SCCs in humans (Munday and French, 2015; Munday et al., 2011c). In contrast, FcaPV2 DNA and gene expression were detected in 31% of feline oral SCCs in a recent study (Altamura et al., 2020). However, FcaPV2 DNA was also detected in 36% of non-neoplastic samples making it hard to determine the role of the PV in the SCCs. FcaPV2 DNA was also reported in 43% of a series of feline oral SCCs, although no non-neoplastic samples were included in this study (Yamashita-Kawanishi et al., 2021a). Recently in situ carcinomas of the mouth and third eyelid were reported in a cat (Munday et al., 2022a). Consistent with a PV etiology, these neoplasms contained FcaPV3 DNA and prominent PV-induced changes. Furthermore, like PV-induced oral SCCs in humans, all neoplastic cells contained intense nuclear and cytoplasmic p16 immunostaining (Munday et al., 2022a). Overall, there is evidence that FcaPVs infect the oral and other mucosa of cats (Altamura et al., 2020; Munday and French, 2015). As PVs cause a proportion of SCCs of feline skin, it appears possible they may also cause a proportion of oral SCCs in this species.
Horses
Of the companion animal species, the evidence supporting a role for PVs in the development of mucosal neoplasia is strongest in horses. As in humans, PVs are thought to contribute to penile, vulval and some oropharyngeal SCCs of horses (Sykora et al., 2017; Fig. 6). However, in contrast to humans in which cervical cancers are common and almost always caused by PV infection, SCCs of the internal genitalia are extremely rare in horses and have not been associated with PV infection. The most common location for PV-associated SCCs in horses is the penis and it is generally accepted that a significant proportion of these are caused by EcPV2 (Sykora and Brandt, 2017). Evidence supporting a role of this PV includes the detection of EcPV2 DNA using PCR and in situ hybridization (Bogaert et al., 2012; Greenwood et al., 2020a; Knight et al., 2011; Scase et al., 2010; van den Top et al., 2015; Zhu et al., 2015) and the detection of EcPV2 gene expression in the SCCs (Ramsauer et al., 2019a; Sykora et al., 2012). There are rare reports of detection of other EcPV types in penile neoplasms but the significance of this is unknown (Lange et al., 2013b). In addition to some oropharyngeal SCCs (Hibi et al., 2019; Knight et al., 2013; Sykora et al., 2017), EcPV2 is also present within a proportion of equine gastric SCCs (Alloway et al., 2020). Human gastric cancers have not been associated with PV infection, although this could be due to the absence of squamous epithelium lining the fundus of the human stomach. There is little evidence that EcPV2 significantly contributes to the development of equine periocular SCCs (Newkirk et al., 2014; Greenwood et al., 2020a).
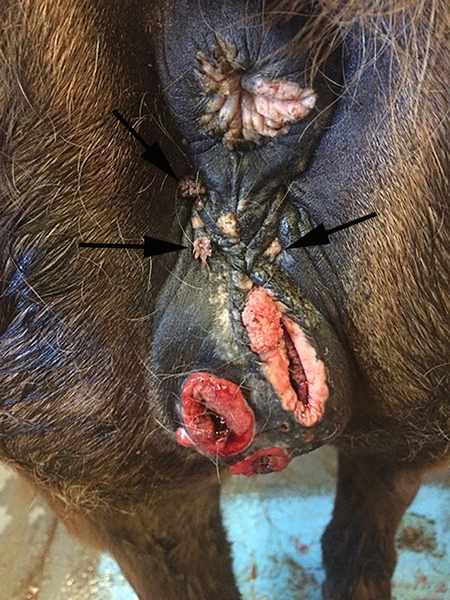
Figure 6.
Vulvar squamous cell carcinoma, horse. Two ulcerated squamous cell carcinomas are visible within the vulva of this horse. Two small exophytic vegetative papillomas are also visible (arrows). PCR amplified Equulis caballus papillomavirus type 2 DNA from one of the SCCs (Image courtesy of Dr Hailey Flemming).
The mechanism of transmission of EcPV2 is unknown. However, asymptomatic infection by EcPV2 is detectable in 10 – 35% of penile samples and 0 – 15% of vulval samples and 36% of horses have serological evidence of previous EcPV2 infection (Bogaert et al., 2012; Fischer et al., 2014; Greenwood et al., 2020b; Knight et al., 2013). In one study, asymptomatic infection by EcPV2 was detected in late term fetuses and 2-day-old foals (Greenwood et al., 2020b) suggesting vertical transmission or infection from the mare during, or shortly after birth. It is possible that, as seen in children with anogenital warts (Padel et al., 1990), an EcPV2 infection acquired at birth could subsequently become localized to the genitals. Alternatively, sexual transmission of EcPV2 could be possible although lesions have been reported in geldings with no history of sexual contact (Bogaert et al., 2012). Additionally, unlike in humans in whom sexual transmission of the causative HPVs causes cancer more frequently of the internal than external genitalia, EcPV2-associated equine genital cancers are confined to the external genitalia. Other possible methods of transmission could include non-sexual direct contact between horses (muzzle to genital) or mechanical transmission by biting flies or human cleaning of the penis and prepuce (Sykora and Brandt, 2017). Such non-sexual transmission would explain the restriction of cancers to the external genitalia.
Like the HPVs that cause cervical cancer, integration of EcPV2 DNA within the cell DNA increases expression of viral E6 and E7 proteins (Ramsauer et al., 2019a). However, unlike the high-risk Alphapapillomaviruses, the EcPV2 proteins do not degrade either p53 (van den Top et al., 2015) or pRb (CG Knight, unpublished observation) and increased expression of p16 is not present within EcPV2-associated SCCs (Ramsauer et al., 2019a). Currently, although EcPV2 has been associated with altered host expression of genes associated with DNA replication, cell cycle replication, ECM-receptor integration and focal adhesion, the precise mechanisms by which EcPV2 promotes neoplasia are unclear (Ramsauer et al., 2019a). Whether co-factors are important in SCC development is also unknown.
In the authors’ experience, equine penile warts can progress to penile SCCs. However, it is currently unknown how often this happens and whether all penile SCCs develop this way or if some develop in clinically normal penile mucosa. The frequency with which penile warts regress or progress to SCC appears to be critical information as this will determine the aggressiveness of the treatment used for a penile wart. The ability of EcPV2 to cause both genital warts and genital SCCs is unexpected as human genital warts are caused by a different subset of PVs than those that cause cervical cancer. This observation adds evidence that EcPV2-induced genital warts less frequently regress, and more commonly progress, than human genital warts, and that equine genital warts may be best considered as pre-neoplastic lesions.
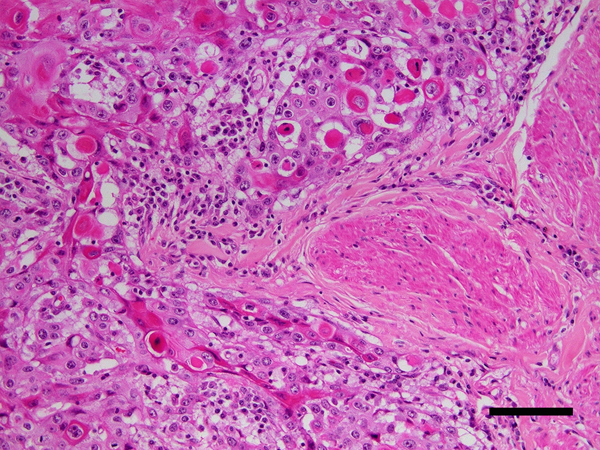
Possibly due to progression from a hyperplastic or pre-neoplastic lesion, equine SCCs tend to be more exophytic than SCCs that develop at other locations, although these can also appear as ulcerative masses on the penis. Histologically, the SCCs appear as typical SCCs with trabeculae and nests of invading epithelial cells with keratin pearls variably present (Fig. 7). While the neoplastic cells do not contain evidence of PV infection, some SCCs are surrounded by hyperplastic mucosa that may contain koilocytosis (Ramsauer et al., 2019b).
Figure 7.
Penile squamous cell carcinoma, horse. Large nests of neoplastic epithelial cells are visible infiltrating adjacent to smooth muscle bundles within the penis (asterisk). Many of the neoplastic cells have brightly eosinophilic cytoplasm consistent with keratinization. Scale bar = 60μm. Haematoxylin and eosin.
Prevention of PV diseases
Multivalent PV vaccines have been successfully used to prevent both genital warts and PV-induced mucosal SCCs in people (Pitisuttithum et al., 2015). However, for a vaccine to be effective it has to be given prior to first infection by the PV (Kreider, 1963). As the more important HPV types are sexually transmitted, this provides ample time to vaccinate people prior to first infection. In contrast, none of the PV types that infect dogs, cats, and horses are known to be sexually transmitted and the mechanism and age of infection is currently less clear for some of the more important PV types that infect the companion animal species.
Dogs are likely to be infected by the PV types that cause canine oral and cutaneous warts within the first 6 to 12 months of age and virus-like particle vaccines have been shown to effectively prevent warts after experiment inoculation of CPV1 (Suzich et al., 1995). However, as warts are self-resolving in the overwhelming majority of cases, it appears unlikely a vaccine would be commercially viable. It is unknown at what age dogs are infected by the Chipapillomavirus types that cause viral plaques. However, due to the similarities between these PVs and the human Betapapillomaviruses, it is possible that dogs could be infected in the first few days of life making it difficult to administer a vaccine prior to first infection.
In cats, an effective vaccine against FcaPV2 would prevent a proportion of cutaneous viral plaques and SCCs (Munday and Thomson, 2021). However, cats are infected by FcaPV2 within the first few days of life (Thomson et al., 2015) making vaccination prior to first infection impossible. It is possible that using a vaccine to stimulate high maternal antibodies could prevent infection of kittens. However, the observation that all kittens are infected despite around 20% of cats have detectible antibody titres against FcaPV2 (Geisseler et al., 2016) suggests maternal antibodies may not fully prevent infection. A study in which adult cats were vaccinated with a virus-like particle FcaPV2 vaccine showed the vaccine significantly increased antibody titres. However, the raised antibody titres did not lower viral loads suggesting that, if viral loads determine which cats develop SCCs, vaccinating adult cats is unlikely to prevent cancer (Thomson et al., 2019).
It is probable that vaccines against cutaneous warts and aural plaques could be administered prior to first infection in horses. However, as both warts and aural plaques generally cause only minor discomfort for the horse, it is uncertain whether such vaccines would be widely used. A vaccine against EcPV2 may be a valuable method to prevent genital and oropharyngeal SCCs in horses (Schellenbacher et al., 2015). However, as EcPV2 infections were detected in late term fetuses and in very young foals in one study (Greenwood et al., 2020b), additional research is required to determine when horses are infected by this PV and therefore the most appropriate way to use vaccines to prevent SCCs caused by EcPV2.
Conclusions
PV-induced cutaneous plaques in humans, dogs and cats are all likely to develop due to a defect in intrinsic keratinocyte immunity. This defect allows increased replication by PV types that normally remain as asymptomatic infections on the skin. The cause of the loss of normal immune function in dogs and cats is unknown, but the susceptibility of some breeds to plaque formation suggests a genetic basis. Feline plaques are unusual as they often contain cell dysplasia. This dysplasia can result in a classification of in situ carcinoma and feline viral plaques appear more likely to progress to neoplasia than plaques in humans and dogs. The best evidence for a significant role of PVs in the development of cutaneous SCCs is seen in cats. Cutaneous SCCs in humans, dogs, and horses could be influenced by the presence of PVs, but there is currently little evidence that PVs are a common cause of SCCs in these species. In contrast, PVs cause most cervical and anal SCCs in people as well as a proportion of oral and genital SCCs. There is similarly good evidence for a role of PVs in equine genital cancer. Oropharyngeal SCCs associated with PV infection have been reported in dogs, cats, and horses but additional evidence is required to determine what role the PVs play and how frequently PVs cause oropharyngeal SCCs in these species.
References
- Akgul B, Cooke JC, Storey A, 2006. HPV-associated skin disease. Journal of Pathology 208, 165–175. [DOI] [PubMed] [Google Scholar]
- Alloway E, Linder K, May S, Rose T, DeLay J, Bender S, Tucker A, Luff J, 2020. A subset of equine gastric squamous cell carcinomas is associated with Equus caballus papillomavirus-2 infection. Veterinary Pathology 57, 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura G, Corteggio A, Pacini L, Conte A, Pierantoni GM, Tommasino M, Accardi R, Borzacchiello G, 2016. Transforming properties of Felis catus papillomavirus type 2 E6 and E7 putative oncogenes in vitro and their transcriptional activity in feline squamous cell carcinoma in vivo. Virology 496, 1–8. [DOI] [PubMed] [Google Scholar]
- Altamura G, Power K, Martano M, Degli Uberti B, Galiero G, De Luca G, Maiolino P, Borzacchiello G, 2018. Felis catus papillomavirus type-2 E6 binds to E6AP, promotes E6AP/p53 binding and enhances p53 proteasomal degradation. Scientific Reports 8, 17529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura G, Cardeti G, Cersini A, Eleni C, Cocumelli C, Bartolomé del Pino LE, Razzuoli E, Martano M, Maiolino P, Borzacchiello G, 2020. Detection of Felis catus papillomavirus type-2 DNA and viral gene expression suggest active infection in feline oral squamous cell carcinoma. Veterinary and Comparative Oncology 18, 494–501. [DOI] [PubMed] [Google Scholar]
- Alves C, Weber MN, Guimarães LLB, Cibulski SP, da Silva FRC, Daudt C, Budaszewski RF, Silva MS, Mayer FQ, Bianchi RM, Schwertz CI, Stefanello CR, Gerardi DG, Laisse CJM, Driemeier D, Teifke JP, Canal CW, 2020. Canine papillomavirus type 16 associated to squamous cell carcinoma in a dog: virological and pathological findings. Brazilian Journal of Microbiology 51, 2087–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiraraghi N, Scott RA, Balaji N, Yaneza MMC, 2019. Human papillomavirus 16 and p16 positive nasal cutaneous squamous cell carcinoma in immunocompetent men in their twenties. The Journal of Laryngology & Otology 133, 348–352. [DOI] [PubMed] [Google Scholar]
- Antonsson A, Forslund O, Ekberg H, Sterner G, Hansson BG, 2000. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. Journal of Virology 74, 11636–11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson A, Karanfilovska S, Lindqvist PG, Hansson BG, 2003. General acquisition of human papillomavirus infections of skin occurs in early infancy. Journal of Clinical Microbiology 41, 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beziat V, Casanova JL, Jouanguy E, 2021. Human genetic and immunological dissection of papillomavirus-driven diseases: new insights into their pathogenesis. Current Opinions in Virology 51, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert L, Willemsen A, Vanderstraeten E, Bracho MA, De Baere C, Bravo IG, Martens A, 2012. EcPV2 DNA in equine genital squamous cell carcinomas and normal genital mucosa. Veterinary Microbiology 158, 33–41. [DOI] [PubMed] [Google Scholar]
- Brianti P, De Flammineis E, Mercuri SR, 2017. Review of HPV-related diseases and cancers. New Microbiology 40, 80–85. [PubMed] [Google Scholar]
- Carrai M, Van Brussel K, Shi M, Li CX, Chang WS, Munday JS, Voss K, McLuckie A, Taylor D, Laws A, Holmes EC, Barrs VR, Beatty JA, 2020. Identification of a novel papillomavirus associated with squamous cell carcinoma in a domestic cat. Viruses 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers G, Ellsmore VA, O’Brien PM, Reid SW, Love S, Campo MS, Nasir L, 2003. Association of bovine papillomavirus with the equine sarcoid. Journal of General Virology 84, 1055–1062. [DOI] [PubMed] [Google Scholar]
- Chang CY, Chen WT, Haga T, Yamashita N, Lee CF, Tsuzuki M, Chang HW, 2020. The detection and association of canine papillomavirus with benign and malignant skin lesions in dogs. Viruses 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, Wylie TN, Wylie KM, Johnson GC, Skidmore ZL, Fleer M, Griffith OL, Bryan JN, 2020. A virome sequencing approach to feline oral squamous cell carcinoma to evaluate viral causative factors. Veterinary Microbiology 240, 108491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubie HA, 2013. Diseases associated with human papillomavirus infection. Virology 445, 21–34. [DOI] [PubMed] [Google Scholar]
- Demos LE, Munday JS, Lange CE, Bennett MD, 2019. Use of fluorescence in situ hybridization to detect Felis catus papillomavirus type 2 in feline Bowenoid in situ carcinomas. Journal of Feline Medicine and Surgery 21, 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA, 2012. The biology and life-cycle of human papillomaviruses. Vaccine 30, F55–70. [DOI] [PubMed] [Google Scholar]
- Economopoulou P, Kotsantis I, Psyrri A, 2020. Special issue about head and neck cancers: HPV positive cancers. International Journal of Molecular Science 21, 3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa N, Doorbar J, 2017. The low-risk papillomaviruses. Virus Research 231, 119–127. [DOI] [PubMed] [Google Scholar]
- Fairley RA, Morley CM, Williams SD, Senior DA, Neill MA, 2014. Aural plaques in two imported horses in New Zealand. New Zealand Veterinary Journal 62, 232–233. [DOI] [PubMed] [Google Scholar]
- Fischer NM, Favrot C, Birkmann K, Jackson M, Schwarzwald CC, Müller M, Tobler K, Geisseler M, Lange CE, 2014. Serum antibodies and DNA indicate a high prevalence of equine papillomavirus 2 (EcPV2) among horses in Switzerland. Veterinary Dermatology 25, 210–e254. [DOI] [PubMed] [Google Scholar]
- Forslund O, Ly H, Reid C, Higgins G, 2003. A broad spectrum of human papillomavirus types is present in the skin of Australian patients with non-melanoma skin cancers and solar keratosis. British Journal of Dermatology 149, 64–73. [DOI] [PubMed] [Google Scholar]
- Geisseler M, Lange CE, Favrot C, Fischer N, Ackermann M, Tobler K, 2016. Geno- and seroprevalence of felis domesticus papillomavirus type 2 (FdPV2) in dermatologically healthy cats. BMC Veterinary Research 12, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill VL, Bergman PJ, Baer KE, Craft D, Leung C, 2008. Use of imiquimod 5% cream (Aldara) in cats with multicentric squamous cell carcinoma in situ: 12 cases (2002–2005). Veterinary and Comparative Oncology 6, 55–64. [DOI] [PubMed] [Google Scholar]
- Gorino AC, Oliveira-Filho JP, Taniwaki SA, Basso RM, Zakia LS, Araujo JP Jr., Borges AS, 2013. Use of PCR to estimate the prevalence of Equus caballus papillomavirus in aural plaques in horses. Veterinary Journal 197, 903–904. [DOI] [PubMed] [Google Scholar]
- Greenwood S, Chow-Lockerbie B, Epp T, Knight C, Wachoski-Dark G, MacDonald-Dickinson V, Wobeser B, 2020a. Prevalence and prognostic impact of Equus caballus papillomavirus type 2 infection in equine squamous cell carcinomas in western Canadian horses. Veterinary Pathology 57, 623–631. [DOI] [PubMed] [Google Scholar]
- Greenwood S, Chow-Lockerbie B, Ramsauer S, Wachoski-Dark G, Knight C, Wobeser B, 2020b. Prevalence of Equus caballus papillomavirus type-2 infection and seropositivity in asymptomatic western Canadian horses. Veterinary Pathology 57, 632–641. [DOI] [PubMed] [Google Scholar]
- Gross TL, Ihrke PJ, Walder EJ, Affolter VK, 2005. Skin diseases of the dog and cat: clinical and histopathologic diagnosis, 2nd edition. Blackwell Science, Oxford, United Kingdom. [Google Scholar]
- Hansen N, Nicholas N, Pack G, Mackie JT, Shipstone M, Munday JS, Reddell P, Orbell G, Malik R, 2018. Progressive cutaneous viral pigmented plaques in three Hungarian Vizslas and the response of lesions to topical tigilanol tiglate gel. Veterinary Medical Science 4, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi H, Hatama S, Obata A, Shibahara T, Kadota K, 2019. Laryngeal squamous cell carcinoma and papilloma associated with Equus caballus papillomavirus 2 in a horse. Journal of Veterinary Medical Science 81, 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggard N, Munday JS, Luff J, 2018. Localization of Felis catus papillomavirus type 2 E6 and E7 RNA in feline cutaneous squamous cell carcinoma. Veterinary Pathology 55 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Chambers JK, Sumi A, Yamashita-Kawanishi N, Omachi T, Haga T, Nakayama H, Uchida K, 2022. Involvement of Felis catus papillomavirus type 2 in the tumorigenesis of feline Merkel cell carcinoma. Veterinary Pathology 59, 63–74. [DOI] [PubMed] [Google Scholar]
- Knight CG, Munday JS, Peters J, Dunowska M, 2011. Equine penile squamous cell carcinomas are associated with the presence of equine papillomavirus type 2 DNA sequences. Veterinary Pathology 48, 1190–1194. [DOI] [PubMed] [Google Scholar]
- Knight CG, Dunowska M, Munday JS, Peters-Kennedy J, Rosa BV, 2013. Comparison of the levels of Equus caballus papillomavirus type 2 (EcPV-2) DNA in equine squamous cell carcinomas and non-cancerous tissues using quantitative PCR. Veterinary Microbiology 166, 257–262. [DOI] [PubMed] [Google Scholar]
- Knight EC, Munday JS, Stone BM, Shipstone MA, 2016. Carbon dioxide laser treatment of extensive pigmented viral plaque lesions in a golden retriever dog. Veterinary Dermatology 27, 442–e117. [DOI] [PubMed] [Google Scholar]
- Kok MK, Yamashita-Kawanishi N, Chambers JK, Haritani M, Ushigusa T, Haga T, Nakayama H, Uchida K, 2019. Pathologic characterization of Felis catus papillomavirus type 5 (FcaPV-5)-associated viral plaques and Bowenoid in situ carcinoma in a Domestic Shorthair cat. Journal of Veterinary Medical Science 81, 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider JW, 1963. Studies on the mechanism responsible for the spontaneous regression of the Shope rabbit papilloma. Cancer Research 23, 1593–1599. [PubMed] [Google Scholar]
- Kremsdorf D, Favre M, Jablonska S, Obalek S, Rueda LA, Lutzner MA, Blanchet-Bardon C, Van Voorst Vader P, Orth G, 1984. Molecular cloning and characterization of the genomes of nine newly recognized human papillomavirus types associated with epidermodysplasia verruciformis. Journal of Virology 52, 1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küsters-Vandevelde HVN, de Koning MNC, Melchers WJG, Quint WGV, de Wilde PCM, de Jong E, van de Kerkhof PCM, Blokx WAM, 2009. Expression of p14ARF, p16INK4a and p53 in relation to HPV in (pre-)malignant squamous skin tumours. Journal of Cellular and Molecular Medicine 13, 2148–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange CE, Tobler K, Ackermann M, Panakova L, Thoday KL, Favrot C, 2009a. Three novel canine papillomaviruses support taxonomic clade formation. Journal of General Virology 90, 2615–2621. [DOI] [PubMed] [Google Scholar]
- Lange CE, Tobler K, Favrot C, Muller M, Nothling JO, Ackermann M, 2009b. Detection of antibodies against epidermodysplasia verruciformis-associated canine papillomavirus 3 in sera of dogs from Europe and Africa by enzyme-linked immunosorbent assay. Clinical Vaccine Immunology 16, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange CE, Tobler K, Markau T, Alhaidari Z, Bornand V, Stockli R, Trussel M, Ackermann M, Favrot C, 2009c. Sequence and classification of FdPV2, a papillomavirus isolated from feline Bowenoid in situ carcinomas. Veterinary Microbiology 137, 60–65. [DOI] [PubMed] [Google Scholar]
- Lange CE, Tobler K, Ackermann M, Favrot C, 2011a. Identification of two novel equine papillomavirus sequences suggests three genera in one cluster. Veterinary Microbiology 149, 85–90. [DOI] [PubMed] [Google Scholar]
- Lange CE, Zollinger S, Tobler K, Ackermann M, Favrot C, 2011b. Clinically healthy skin of dogs is a potential reservoir for canine papillomaviruses. Journal of Clinical Microbiology 49, 707–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange CE, Tobler K, Lehner A, Vetsch E, Favrot C, 2012. A case of a canine pigmented plaque associated with the presence of a Chi-papillomavirus. Veterinary Dermatology 23, 76–80, e18–79. [DOI] [PubMed] [Google Scholar]
- Lange CE, Tobler K, Schraner EM, Vetsch E, Fischer NM, Ackermann M, Favrot C, 2013a. Complete canine papillomavirus life cycle in pigmented lesions. Veterinary Microbiology 162, 388–395. [DOI] [PubMed] [Google Scholar]
- Lange CE, Vetsch E, Ackermann M, Favrot C, Tobler K, 2013b. Four novel papillomavirus sequences support a broad diversity among equine papillomaviruses. Journal of General Virology 94, 1365–1372. [DOI] [PubMed] [Google Scholar]
- Lewis JS Jr., Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q, El-Mofty SK, 2010. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. American Journal of Surgical Pathology 34, 1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JS Jr, Beadle B, Bishop JA, Chernock RD, Colasacco C, Lacchetti C, Moncur JT, Rocco JW, Schwartz MR, Seethala RR, Thomas NE, Westra WH, Faquin WC, 2017. Human papillomavirus testing in head and neck carcinomas: Guideline from the college of American pathologists. Archives of Pathology and Laboratory Medicine 142, 559–597. [DOI] [PubMed] [Google Scholar]
- Luff J, Moore P, Zhou D, Wang J, Usuda Y, Affolter V, Schlegel R, Yuan H, 2012a. Complete genome sequence of canine papillomavirus type 10. Journal of Virology 86, 11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff JA, Affolter VK, Yeargan B, Moore PF, 2012b. Detection of six novel papillomavirus sequences within canine pigmented plaques. Journal of Veterinary Diagnostic Investigation 24, 576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff J, Mader M, Britton M, Fass J, Rowland P, Orr C, Schlegel R, Yuan H, 2015. Complete genome sequence of canine papillomavirus type 16. Genome Announcements 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff J, Rowland P, Mader M, Orr C, Yuan H, 2016. Two canine papillomaviruses associated with metastatic squamous cell carcinoma in two related Basenji dogs. Veterinary Pathology 53, 1160–1163. [DOI] [PubMed] [Google Scholar]
- Luff J, Mader M, Rowland P, Britton M, Fass J, Yuan H, 2019. Viral genome integration of canine papillomavirus 16. Papillomavirus Research 7, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AA, 2022. Human papillomaviruses: diversity, infection and host interactions. National Reviews of Microbiology 20, 95–108. [DOI] [PubMed] [Google Scholar]
- Mira J, Herman M, Zakia LS, Olivo G, Araújo JP Jr., Borges AS, Oliveira-Filho JP, 2018. Frequency of Equus caballus papillomavirus in equine aural plaques. Journal of Veterinary Diagnostic Investigation 30, 565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday JS, Kiupel M, French AF, Howe L, 2008. Amplification of papillomaviral DNA sequences from a high proportion of feline cutaneous in situ and invasive squamous cell carcinomas using a nested polymerase chain reaction. Veterinary Dermatology 19, 259–263. [DOI] [PubMed] [Google Scholar]
- Munday JS, Dunowska M, De Grey S, 2009a. Detection of two different papillomaviruses within a feline cutaneous squamous cell carcinoma: case report and review of the literature. New Zealand Veterinary Journal 57, 248–251. [DOI] [PubMed] [Google Scholar]
- Munday JS, Howe L, French A, Squires RA, Sugiarto H, 2009b. Detection of papillomaviral DNA sequences in a feline oral squamous cell carcinoma. Research in Veterinary Science 86, 359–361. [DOI] [PubMed] [Google Scholar]
- Munday JS, Kiupel M, 2010. Papillomavirus-associated cutaneous neoplasia in mammals. Veterinary Pathology 47, 254–264. [DOI] [PubMed] [Google Scholar]
- Munday JS, French AF, Peters-Kennedy J, Orbell GM, Gwynne K, 2011a. Increased p16CDKN2A protein within feline cutaneous viral plaques, bowenoid in situ carcinomas, and a subset of invasive squamous cell carcinomas. Veterinary Pathology 48, 460–465. [DOI] [PubMed] [Google Scholar]
- Munday JS, Gibson I, French AF, 2011b. Papillomaviral DNA and increased p16CDKN2A protein are frequently present within feline cutaneous squamous cell carcinomas in ultraviolet-protected skin. Veterinary Dermatology 22, 360–366. [DOI] [PubMed] [Google Scholar]
- Munday JS, Knight CG, French AF, 2011c. Evaluation of feline oral squamous cell carcinomas for p16CDKN2A protein immunoreactivity and the presence of papillomaviral DNA. Research in Veterinary Science 90, 280–283. [DOI] [PubMed] [Google Scholar]
- Munday JS, O’Connor KI, Smits B, 2011d. Development of multiple pigmented viral plaques and squamous cell carcinomas in a dog infected by a novel papillomavirus. Veterinary Dermatology 22, 104–110. [DOI] [PubMed] [Google Scholar]
- Munday JS, Aberdein D, 2012. Loss of retinoblastoma protein, but not p53, is associated with the presence of papillomaviral DNA in feline viral plaques, Bowenoid in situ carcinomas, and squamous cell carcinomas. Veterinary Pathology 49, 538–545. [DOI] [PubMed] [Google Scholar]
- Munday JS, French AF, Gibson IR, Knight CG, 2013. The presence of p16 CDKN2A protein immunostaining within feline nasal planum squamous cell carcinomas is associated with an increased survival time and the presence of papillomaviral DNA. Veterinary Pathology 50, 269–273. [DOI] [PubMed] [Google Scholar]
- Munday JS, French AF, 2015. Felis catus papillomavirus types 1 and 4 are rarely present in neoplastic and inflammatory oral lesions of cats. Research in Veterinary Science 100, 220–222. [DOI] [PubMed] [Google Scholar]
- Munday JS, French A, Harvey CJ, 2015a. Molecular and immunohistochemical studies do not support a role for papillomaviruses in canine oral squamous cell carcinoma development. Veterinary Journal 204, 223–225. [DOI] [PubMed] [Google Scholar]
- Munday JS, Thomson N, Dunowska M, Knight CG, Laurie RE, Hills S, 2015b. Genomic characterisation of the feline sarcoid-associated papillomavirus and proposed classification as Bos taurus papillomavirus type 14. Veterinary Microbiology 177, 289–295. [DOI] [PubMed] [Google Scholar]
- Munday JS, Tucker RS, Kiupel M, Harvey CJ, 2015c. Multiple oral carcinomas associated with a novel papillomavirus in a dog. Journal of Veterinary Diagnostic Investigation 27, 221–225. [DOI] [PubMed] [Google Scholar]
- Munday JS, Benfell MW, French A, Orbell GM, Thomson N, 2016a. Bowenoid in situ carcinomas in two Devon Rex cats: evidence of unusually aggressive neoplasm behaviour in this breed and detection of papillomaviral gene expression in primary and metastatic lesions. Veterinary Dermatology 27, 215–e255. [DOI] [PubMed] [Google Scholar]
- Munday JS, Dunowska M, Laurie RE, Hills S, 2016b. Genomic characterisation of canine papillomavirus type 17, a possible rare cause of canine oral squamous cell carcinoma. Veterinary Microbiology 182, 135–140. [DOI] [PubMed] [Google Scholar]
- Munday JS, Dittmer KE, Thomson NA, Hills SF, Laurie RE, 2017a. Genomic characterisation of Felis catus papillomavirus type 5 with proposed classification within a new papillomavirus genus. Veterinary Microbiology 207, 50–55. [DOI] [PubMed] [Google Scholar]
- Munday JS, French A, Thomson N, 2017b. Detection of DNA sequences from a novel papillomavirus in a feline basal cell carcinoma. Veterinary Dermatology 28, 236–e260. [DOI] [PubMed] [Google Scholar]
- Munday JS, Thomson NA, Luff JA, 2017c. Papillomaviruses in dogs and cats. Veterinary Journal 225, 23–31. [DOI] [PubMed] [Google Scholar]
- Munday JS, Thomson NA, Henderson G, Fairley R, Orbell GM, 2018. Identification of Felis catus papillomavirus 3 in skin neoplasms from four cats. Journal of Veterinary Diagnostic Investigation 30, 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday JS, Thomson NA, 2021. Papillomaviruses in domestic cats. Viruses 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday JS, Hardcastle M, Dally N, 2022a. In situ squamous cell carcinoma of the gingiva and nictitating membrane associated with Felis catus papillomavirus type 3 in a cat. Veterinary Pathology 59, 463–466 [DOI] [PubMed] [Google Scholar]
- Munday JS, Lam ATH, Sakai M, 2022b. Extensive progressive pigmented viral plaques in a Chihuahua dog. Veterinary Dermatology 33, 252–254 [DOI] [PubMed] [Google Scholar]
- Nagata M, Nanko H, Moriyama A, Washizu T, Ishida T, 1995. Pigmented plaques associated with papillomavirus infection in dogs: Is this epidermodysplasia verruciformis? Veterinary Dermatology 6, 179–186. [DOI] [PubMed] [Google Scholar]
- Nespeca G, Grest P, Rosenkrantz WS, Ackermann M, Favrot C, 2006. Detection of novel papillomaviruslike sequences in paraffin-embedded specimens of invasive and in situ squamous cell carcinomas from cats. American Journal of Veterinary Research 67, 2036–2041. [DOI] [PubMed] [Google Scholar]
- Newkirk KM, Hendrix DV, Anis EA, Rohrbach BW, Ehrhart EJ, Lyons JA, Kania SA, 2014. Detection of papillomavirus in equine periocular and penile squamous cell carcinoma. Journal of Veterinary Diagnostic Investigation 26, 131–135. [DOI] [PubMed] [Google Scholar]
- O’Neill SH, Newkirk KM, Anis EA, Brahmbhatt R, Frank LA, Kania SA, 2011. Detection of human papillomavirus DNA in feline premalignant and invasive squamous cell carcinoma. Veterinary Dermatology 22, 68–74. [DOI] [PubMed] [Google Scholar]
- Orth G, 2006. Genetics of epidermodysplasia verruciformis: Insights into host defense against papillomaviruses. Seminars in Immunology 18, 362–374. [DOI] [PubMed] [Google Scholar]
- Padel AF, Venning VA, Evans MF, Quantrill AM, Fleming KA, 1990. Human papillomaviruses in anogenital warts in children: typing by in situ hybridisation. British Medical Journal 300, 1491–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters-Kennedy J, Lange CE, Rine SL, Hackett RP, 2019. Equus caballus papillomavirus 8 (EcPV8) associated with multiple viral plaques, viral papillomas, and squamous cell carcinoma in a horse. Equine Veterinary Journal 51, 470–474. [DOI] [PubMed] [Google Scholar]
- Peters-Kennedy J, Lange CE, Ortved K, 2020. Progression of aural plaques to squamous cell carcinoma in a horse. Veterinary Dermatology 31, 397–400. [DOI] [PubMed] [Google Scholar]
- Pitisuttithum P, Velicer C, Luxembourg A, 2015. 9-Valent HPV vaccine for cancers, pre-cancers and genital warts related to HPV. Expert Review of Vaccines 14, 1405–1419. [DOI] [PubMed] [Google Scholar]
- Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S, 2016. Global burden of cancers attributable to infections in 2012: a synthetic analysis. The Lancet Global Health 4, e609–e616. [DOI] [PubMed] [Google Scholar]
- Porcellato I, Brachelente C, Guelfi G, Reginato A, Sforna M, Bongiovanni L, Mechelli L, 2014. A retrospective investigation on canine papillomavirus 1 (CPV1) in oral oncogenesis reveals dogs are not a suitable animal model for high-risk HPV-induced oral cancer. PLoS One 9, e112833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoz N, Rueda L-A, Bouadjar B, Montoya L-S, Orth G, Favre M, 2002. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nature Genetics 32, 579–581. [DOI] [PubMed] [Google Scholar]
- Ramsauer AS, Kubacki J, Favrot C, Ackermann M, Fraefel C, Tobler K, 2019a. RNA-seq analysis in equine papillomavirus type 2-positive carcinomas identifies affected pathways and potential cancer markers as well as viral gene expression and splicing events. Journal of General Virology 100, 985–998. [DOI] [PubMed] [Google Scholar]
- Ramsauer AS, Wachoski-Dark GL, Fraefel C, Tobler K, Brandt S, Knight CG, Favrot C, Grest P, 2019b. Paving the way for more precise diagnosis of EcPV2-associated equine penile lesions. BMC Veterinary Research 15, 356-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravens PA, Vogelnest LJ, Tong LJ, Demos LE, Bennett MD, 2013. Papillomavirus-associated multicentric squamous cell carcinoma in situ in a cat: an unusually extensive and progressive case with subsequent metastasis. Veterinary Dermatology 24, 642–645, e161–642. [DOI] [PubMed] [Google Scholar]
- Regalado Ibarra AM, Legendre L, Munday JS, 2018. Malignant transformation of a canine papillomavirus type 1-induced persistent oral papilloma in a 3-year-old dog. Journal of Veterinary Dentistry 35, 79–95. [DOI] [PubMed] [Google Scholar]
- Scase T, Brandt S, Kainzbauer C, Sykora S, Bijmholt S, Hughes K, Sharpe S, Foote A, 2010. Equus caballus papillomavirus-2 (EcPV-2): an infectious cause for equine genital cancer? Equine Veterinary Journal 42, 738–745. [DOI] [PubMed] [Google Scholar]
- Schellenbacher C, Shafti-Keramat S, Huber B, Fink D, Brandt S, Kirnbauer R, 2015. Establishment of an in vitro equine papillomavirus type 2 (EcPV2) neutralization assay and a VLP-based vaccine for protection of equids against EcPV2-associated genital tumors. Virology 486, 284–290. [DOI] [PubMed] [Google Scholar]
- Scott DW, Miller WH, 2011. Neoplasms, Cysts, Hamartomas and Keratoses, In: Equine Dermatology, 2nd Edition. Saunders, St. Louis, pp. 468–516. [Google Scholar]
- Smola S, 2020. Human papillomaviruses and skin cancer. Advances in Experimental Medical Biology 1268, 195–209. [DOI] [PubMed] [Google Scholar]
- Sterling JC, 2016. Viral infections. Rook’s Textbook of Dermatology, Ninth Edition, 1–124. [Google Scholar]
- Stokking LB, Ehrhart EJ, Lichtensteiger CA, Campbell KL, 2004. Pigmented epidermal plaques in three dogs. Journal of the American Animal Hospital Association 40, 411–417. [DOI] [PubMed] [Google Scholar]
- Strickley JD, Messerschmidt JL, Awad ME, Li T, Hasegawa T, Ha DT, Nabeta HW, Bevins PA, Ngo KH, Asgari MM, Nazarian RM, Neel VA, Jenson AB, Joh J, Demehri S, 2019. Immunity to commensal papillomaviruses protects against skin cancer. Nature 575, 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, Newsome JA, Jenson AB, Schlegel R, 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proceedings of the National Academy of Sciences of the United States of America 92, 11553–11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykora S, Samek L, Schönthaler K, Palm F, Borzacchiello G, Aurich C, Brandt S, 2012. EcPV-2 is transcriptionally active in equine SCC but only rarely detectable in swabs and semen from healthy horses. Veterinary Microbiology 158, 194–198. [DOI] [PubMed] [Google Scholar]
- Sykora S, Brandt S, 2017. Papillomavirus infection and squamous cell carcinoma in horses. Veterinary Journal 223, 48–54. [DOI] [PubMed] [Google Scholar]
- Sykora S, Jindra C, Hofer M, Steinborn R, Brandt S, 2017. Equine papillomavirus type 2: An equine equivalent to human papillomavirus 16? Veterinary Journal 225, 3–8. [DOI] [PubMed] [Google Scholar]
- Taniwaki SA, Magro AJ, Gorino AC, Oliveira-Filho JP, Fontes MR, Borges AS, Araujo JP Jr., 2013. Phylogenetic and structural studies of a novel equine papillomavirus identified from aural plaques. Veterinary Microbiology 162, 85–93. [DOI] [PubMed] [Google Scholar]
- Teifke JP, Lohr CV, Shirasawa H, 1998. Detection of canine oral papillomavirus-DNA in canine oral squamous cell carcinomas and p53 overexpressing skin papillomas of the dog using the polymerase chain reaction and non-radioactive in situ hybridization. Veterinary Microbiology 60, 119–130. [DOI] [PubMed] [Google Scholar]
- Thaiwong T, Sledge DG, Wise AG, Olstad K, Maes RK, Kiupel M, 2018. Malignant transformation of canine oral papillomavirus (CPV1)-associated papillomas in dogs: An emerging concern? Papillomavirus Research 6, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson NA, Dunowska M, Munday JS, 2015. The use of quantitative PCR to detect Felis catus papillomavirus type 2 DNA from a high proportion of queens and their kittens. Veterinary Microbiology 175, 211–217. [DOI] [PubMed] [Google Scholar]
- Thomson NA, Munday JS, Dittmer KE, 2016. Frequent detection of transcriptionally active Felis catus papillomavirus 2 in feline cutaneous squamous cell carcinomas. Journal of General Virology 97, 1189–1197. [DOI] [PubMed] [Google Scholar]
- Thomson NA, Howe L, Weidgraaf K, Thomas DG, Young V, Ward VK, Munday JS, 2019. Felis catus papillomavirus type 2 virus-like particle vaccine is safe and immunogenic but does not reduce FcaPV-2 viral loads in adult cats. Veterinary Immunology and Immunopathology 213, 109888. [DOI] [PubMed] [Google Scholar]
- Tobler K, Favrot C, Nespeca G, Ackermann M, 2006. Detection of the prototype of a potential novel genus in the family Papillomaviridae in association with canine epidermodysplasia verruciformis. Journal of General Virology 87, 3551–3557. [DOI] [PubMed] [Google Scholar]
- Tobler K, Lange C, Carlotti DN, Ackermann M, Favrot C, 2008. Detection of a novel papillomavirus in pigmented plaques of four pugs. Veterinary Dermatology 19, 21–25. [DOI] [PubMed] [Google Scholar]
- Torres SM, Malone ED, White SD, Koch SN, Watson JL, 2010. The efficacy of imiquimod 5% cream (Aldara®) in the treatment of aural plaque in horses: a pilot open-label clinical trial. Veterinary Dermatology 21, 503–509. [DOI] [PubMed] [Google Scholar]
- Torres SM, Koch SN, 2013. Papillomavirus-associated diseases. Veterinary Clinics of North America: Equine Practice 29, 643–655. [DOI] [PubMed] [Google Scholar]
- van den Top JG, Harkema L, Lange C, Ensink JM, van de Lest CH, Barneveld A, van Weeren PR, Gröne A, Martens A, 2015. Expression of p53, Ki67, EcPV2- and EcPV3 DNA, and viral genes in relation to metastasis and outcome in equine penile and preputial squamous cell carcinoma. Equine Veterinary Journal 47, 188–195. [DOI] [PubMed] [Google Scholar]
- Vascellari M, Mazzei M, Zanardello C, Melchiotti E, Albanese F, Forzan M, Croce MF, Alberti A, Abramo F, 2019. Felis catus papillomavirus types 1, 2, 3, 4, and 5 in feline bowenoid in situ carcinoma: An in situ hybridization study. Veterinary Pathology 56, 818–825. [DOI] [PubMed] [Google Scholar]
- Waropastrakul S, Munday JS, French AF, 2012. Infrequent detection of papillomaviral DNA within canine cutaneous squamous cell carcinomas, haemangiosarcomas and healthy skin on the ventrum of dogs. Veterinary Dermatology 23, 197–201. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Degorce-Rubiales F, Godson D, Favrot C, 2006. Clinical, histological and immunohistochemical study of feline viral plaques and bowenoid in situ carcinomas. Veterinary Dermatology 17, 424–431. [DOI] [PubMed] [Google Scholar]
- Yamashita-Kawanishi N, Sawanobori R, Matsumiya K, Uema A, Chambers JK, Uchida K, Shimakura H, Tsuzuki M, Chang CY, Chang HW, Haga T, 2018. Detection of felis catus papillomavirus type 3 and 4 DNA from squamous cell carcinoma cases of cats in Japan. Journal of Veterinary Medical Science 80, 1236–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita-Kawanishi N, Chang CY, Chambers JK, Uchida K, Sugiura K, Kukimoto I, Chang HW, Haga T, 2021a. Comparison of prevalence of Felis catus papillomavirus type 2 in squamous cell carcinomas in cats between Taiwan and Japan. Journal of Veterinary Medical Science 83, 1229–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita-Kawanishi N, Gushino Y, Chang CY, Chang HW, Chambers JK, Uchida K, Haga T, 2021b. Full-genome characterization of a novel Felis catus papillomavirus 4 subtype identified in a cutaneous squamous cell carcinoma of a domestic cat. Virus Genes 57, 380–384 [DOI] [PubMed] [Google Scholar]
- Yuan H, Luff J, Zhou D, Wang J, Affolter V, Moore P, Schlegel R, 2012. Complete genome sequence of canine papillomavirus type 9. Journal of Virology 86, 5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakia LS, Olivo G, Basso RM, Mira J, Herman M, Araujo JP Jr, Borges AS, Oliveira-Filho JP, 2016. Imiquimod treatment for Equus caballus papillomavirus infection in equine aural plaques. Veterinary Dermatology 27, 175–e144. [DOI] [PubMed] [Google Scholar]
- Zaugg N, Nespeca G, Hauser B, Ackermann M, Favrot C, 2005. Detection of novel papillomaviruses in canine mucosal, cutaneous and in situ squamous cell carcinomas. Veterinary Dermatology 16, 290–298. [DOI] [PubMed] [Google Scholar]
- Zhou D, Luff J, Usuda Y, Affolter V, Moore P, Schlegel R, Yuan H, 2014. Complete genome sequence of canine papillomavirus type 11. Genome Announcements 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu KW, Affolter VK, Gaynor AM, Dela Cruz FN Jr., Pesavento PA, 2015. Equine genital squamous cell carcinoma: In situ hybridization identifies a distinct subset containing Equus caballus papillomavirus 2. Veterinary Pathology 52, 1067–1072. [DOI] [PubMed] [Google Scholar]