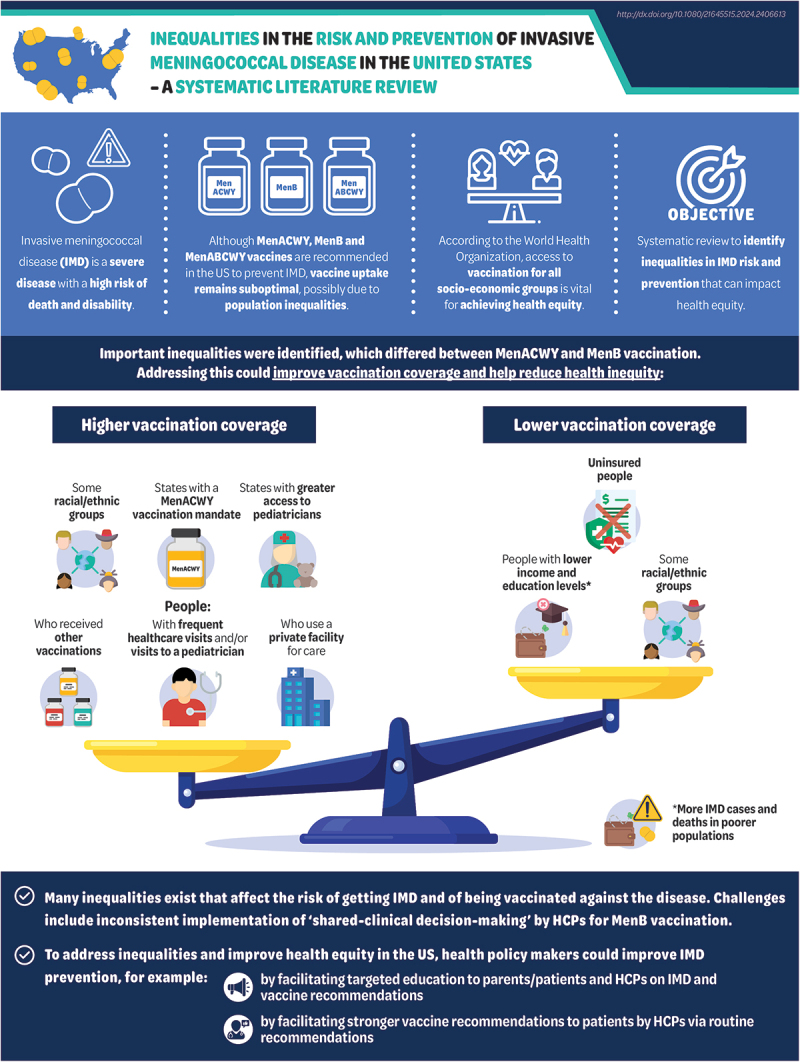
ABSTRACT
Vaccination remains the most effective strategy to prevent invasive meningococcal disease (IMD), with MenACWY, MenB and MenABCWY recommended for adolescents/young adults in the United States (US). However, vaccination coverage remains suboptimal, which could be related to population inequalities. To understand the impact of IMD risk, prevention and control inequalities, a global systematic literature review (Medline, Embase, 2012–2022) was conducted on individual, socioeconomic, and environmental inequalities associated with IMD risk, prevention and control in all ages. Studies on IMD risk (n = 15) and prevention (n = 14) inequalities were identified. IMD incidence proportions were higher in Medicaid versus commercially insured populations, and IMD mortality was higher in poorer neighborhoods. White adolescents, adolescents from lower income families, and with lower maternal education were more likely to receive MenB vaccination; while Black and Hispanic adolescents, and adolescents with higher family incomes, were more likely to receive MenACWY vaccination. Meningococcal vaccination was associated with being up-to-date with other vaccinations, having multiple healthcare/well child visits, having a pediatrician as healthcare provider (HCP), and attending private facilities; while being uninsured was associated with lower vaccination. States with a MenACWY vaccination mandate and higher pediatrician-to-children ratios had higher vaccination rates. Important inequalities were due to individual differences, socioeconomic, and environmental factors. IMD prevention is suboptimal, especially among adolescents/young adults. To improve health equity, health policy makers could ameliorate meningococcal vaccination coverage across the US, with simplified and stronger meningococcal vaccine recommendations from public health authorities, and initiatives to enhance parental/patient and HCP knowledge of IMD and vaccine recommendations.
KEYWORDS: Invasive meningococcal disease, inequality, bacterial meningitis, prevention, health equity, United States
Plain Language Summary
(1) What is the context?
Invasive meningococcal disease (IMD) is a severe disease with a high risk of death and long-term sequelae in survivors. Three types of vaccines are recommended in the United States (US) to prevent IMD among adolescents and young adults: MenACWY, MenB, and MenABCWY. According to the World Health Organization, access to vaccination, regardless of socioeconomic status, is one of the most important ways to achieve equitable health standards. However, US vaccine coverage is suboptimal, especially among older adolescents and young adults, possibly because of population-based inequalities. This study investigated the impact of inequalities on IMD incidence, mortality, and vaccination in the US.
(2) What is new?
A systematic literature review identified several studies reporting on inequalities for IMD risk and prevention.
IMD cases and deaths were more likely in poorer populations. Vaccination coverage varied according to race/ethnicity, income, and education levels. Vaccination was more likely in people with frequent healthcare visits, those who received other vaccinations, those who visit a pediatrician, and those who go to a non-public/private facility for care. Vaccination was less likely in uninsured people. States with a MenACWY vaccination mandate and with greater access to pediatricians had better vaccination rates.
(3) What is the impact?
Many inequalities exist in relation to the risk of getting IMD and the chances of getting vaccinated against IMD. To improve IMD prevention, health policy makers need to strengthen and simplify current meningococcal vaccine recommendations, and introduce/support initiatives that increase parental/patient and HCP awareness of IMD and vaccine recommendations.
GRAPHICAL ABSTRACT
Background
Invasive meningococcal disease (IMD), caused by the Neisseria meningitidis bacterium, is an unpredictable and devastating life-threatening disease. IMD has a high mortality rate within hours of symptom onset (ranging from 10% to 40% worldwide),1 and a risk of disabling sequelae in up to 40% of survivors.2 IMD sequelae include physical, neurological, and psychological complications, such as limb amputations, skin scars, hearing loss, learning disabilities, anxiety and depression.2 Long-term sequelae significantly impact health-related quality of life in patients and their families,3 and place a financial burden on healthcare systems and society.1 Of the twelve serogroups identified, six meningococcal serogroups (A, B, C, W, Y, and X) cause most cases;4 in the United States (US), serogroup B was responsible for around 60% of cases in children <5 years (incidence 0.56 in infants <1 year) and most cases in adolescents, while serogroups A, C, W and Y IMD was more prevalent among older adults.5
Vaccination is currently the most effective strategy to prevent IMD. The US Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination with a MenACWY vaccine with a primary dose in adolescents aged 11–12 years and a booster at 16 years. A two-dose MenB vaccine is recommended for 16–23-year-olds (preferred age 16–18 years), based on shared clinical decision-making (SCDM) among parents and healthcare providers (HCPs).6 Both MenACWY and MenB vaccines are recommended (in different age groups and schedules than those for healthy adolescents) for patients at increased risk for IMD (e.g., asplenia, complement deficiency, and MenACWY only for human immunodeficiency virus [HIV]).6 In October 2023, the ACIP recommended a MenABCWY vaccine when both MenACWY and MenB vaccines are indicated at the same visit (i.e., routine schedule for healthy individuals aged 16–23 years when SCDM indicates MenB vaccination; and in individuals aged ≥10 years at increased risk of IMD due for both vaccines).7 MenACWY vaccination coverage in adolescents aged 13–17 years was 88.6% for ≥1 dose and 60.80% for ≥2 doses as reported in the 2022 National Immunization Survey (NIS)-Teen data, while only 29.4% and 11.9% of 17-year-olds received ≥1 dose and ≥2 doses of the MenB vaccine, respectively.8 Despite vaccine availability, suboptimal vaccination coverage limits protection against IMD, which may be related to population inequalities affecting healthcare access, awareness of these vaccines, and the complexity of current meningococcal vaccine recommendations. Data from the Centers for Disease Prevention and Control (CDC) show that there are individual (e.g., age, sexual orientation, HIV status), socioeconomic (e.g., homelessness), and environmental characteristics (e.g., living at college) that may affect the risk of acquiring IMD and of being vaccinated against it. Indeed, previous US-focused research has shown that lower vaccination coverage is related to lower income, insurance status, region, and uptake of other routinely recommended vaccines.9,10
Health inequalities are defined as differences in health linked to individual, social, economic, and environmental disadvantages, such as race and ethnicity, disability, gender, socioeconomic status, and geographic location.11,12 Health inequity is defined as the “unfair and avoidable inequalities that are not inevitable or natural, but are the product of human behavior” by the World Health Organization (WHO).11 Thus, not all inequalities are unfair and avoidable, or lead to inequity. Policies to reduce health inequity in IMD risk, prevention, and control require an understanding of health inequalities and their underlying individual, socioeconomic, and environmental determinants (see Figure S1 for a breakdown of determinants of health).13 Health equity is achieved when everyone has the opportunity to “attain his or her full health potential” and no one is at a disadvantage “because of social position or other socially determined circumstances.”12 Due to the severe impact of IMD, the WHO developed a road map to 2030 to defeat the main causes of bacterial meningitis worldwide. With equity as the guiding framework, the road map outlines a goal to achieve equal access and high coverage of existing and new vaccines by 2030.14
In this context, this study set out to understand the impact of social, economic, environmental, and other inequalities on a) IMD risk (e.g., incidence, mortality, nasopharyngeal carriage); b) IMD prevention (e.g., vaccination access); and c) control of IMD and its sequelae (e.g., healthcare resource use and access to healthcare services). The results from a global systematic literature review (SLR) are presented here, focusing on US studies. The SLR aimed to identify inequalities and associated knowledge gaps, to potentially help health policy/decision-makers to address potential sources of health inequity in IMD risk, prevention, and control in the US.
Methods
The SLR methods followed the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions15 and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).16
Search strategy
Searches were conducted in Medline, Embase, Cochrane Library and EconLit on August 23rd, 2022, for studies published from January 1st, 2012, to August 23rd, 2022, in English, French, Spanish, Italian and Portuguese. Search terms (both subject heading and free text) for meningococcal disease were combined with terms for inequalities associated with a range of individual, socioeconomic, and environmental characteristics (see Table S1 for Embase search strategy).
The gray literature was searched (i.e., the two most recent meetings from six key conferences): European Congress of Clinical Microbiology & Infectious Diseases (ECCMID); International Society for Infectious Diseases (ISID); Annual Congress on Vaccinology Research (ACVR); World Vaccine Congress (WVC); International Society for Pharmacoeconomics and Outcomes Research (ISPOR) EU and US; and Epidemics, using similar terms to those used in the database searches.
Selection criteria and process
Duplicates were removed (in EndNote X9), and unique records were evaluated against predetermined PECOS (Population, Exposures and Comparators, Outcomes and Study design) criteria (Table S2), in Distiller Systematic Review software (DistillerSR; Evidence Partners, Ottawa, Ontario, Canada).
The study population included IMD cases, carriers, and controls of all ages, excluding viral and other bacterial causes of IMD. Exposures included inequalities of health related to the individual (e.g., ethnicity, sex/sexual orientation, religion, and physical disability); to socioeconomic factors (e.g., social deprivation, insurance, education, and access to affordable health services); to environmental factors (e.g., geographic location, and housing); and to any other health inequalities. Exposures not attributed to health inequalities were excluded (such as genetic mutations). Comparisons included those within exposures (e.g., inequalities by rural versus urban, levels of education, employed versus unemployed). Outcomes were the association between exposures and IMD risk, prevention or control. Studies reporting outcomes of clinical efficacy/effectiveness, safety, and clinical burden were excluded. Study designs included observational studies (i.e., cohort, case control, cross-sectional, case series), database studies, modeling studies, economic evaluations, and literature reviews (for hand searching of references only). Clinical trials, animal studies and narrative reviews were excluded. While the SLR included studies conducted worldwide, only US-specific publications were included here.
For article selection using the DistillerSR platform, screening of titles and abstracts (step one) and full texts (step two) was performed independently by two reviewers, with disagreements resolved by a third researcher. Screening questions were developed and tested based on the selection criteria, to align decisions across team members.
Data extraction
Data were extracted into a pre-specified Microsoft Excel® template by one researcher and independently validated by a second researcher for accuracy and consistency. Data extraction included citation, study design, methods (e.g., data source, sample size), demographics, IMD exposures and outcomes of interest, and meningococcal vaccine type and uptake data.
Quality assessment
The quality of evidence was assessed using the Newcastle Ottawa Scale, which evaluates the risk of bias in observational studies by considering three domains: selection (four questions), comparability (one question) and exposure or outcome (up to three questions).17,18 Different versions of the scale were used according to the type of observational study (e.g., cross-sectional, cohort or case-control study). Each question was assessed and a score of 0, 1 or 2 was applied, with a maximum total score of nine.
Qualitative analysis
A qualitative assessment was conducted of the inequalities in IMD risk and prevention attributable to the various factors identified in the SLR. No quantitative statistical analysis was performed e.g. pooling of results via meta-analytical approaches, or adjustment for confounding factors. However, these factors and their impact were captured and discussed where possible, as reported in the primary study.
Results
Study characteristics and quality assessment
The SLR reports studies for IMD risk (n = 15) and IMD prevention (n = 14) (Figure 1). At the time of the search, MenABCWY was not yet recommended in the US; therefore, no studies reported on IMD prevention with MenABCWY. Two studies reported outcomes for IMD control but did not identify any inequalities.19,20 Due to the limited evidence on inequalities for IMD control, the results reported here focus on IMD risk and prevention. These studies included IMD patients, susceptible populations, the general population (vaccinated and unvaccinated), parents/caregivers of IMD patients, and HCPs; from 34 IMD patients19 to 32,9 million commercially insured adults patients;21 from a wide range of national and regional data sources. Six studies mentioned confounders for which the model/analysis were adjusted,20–25 mainly demographic characteristics (e.g., age, sex, race, and ethnicity). Multivariable regression analyses adjusted for clustering,23 for individual- and state-level determinants,26 and for parents’ demographic characteristics and child’s age.27 A summary of study characteristics is provided in Table S3.
Figure 1.
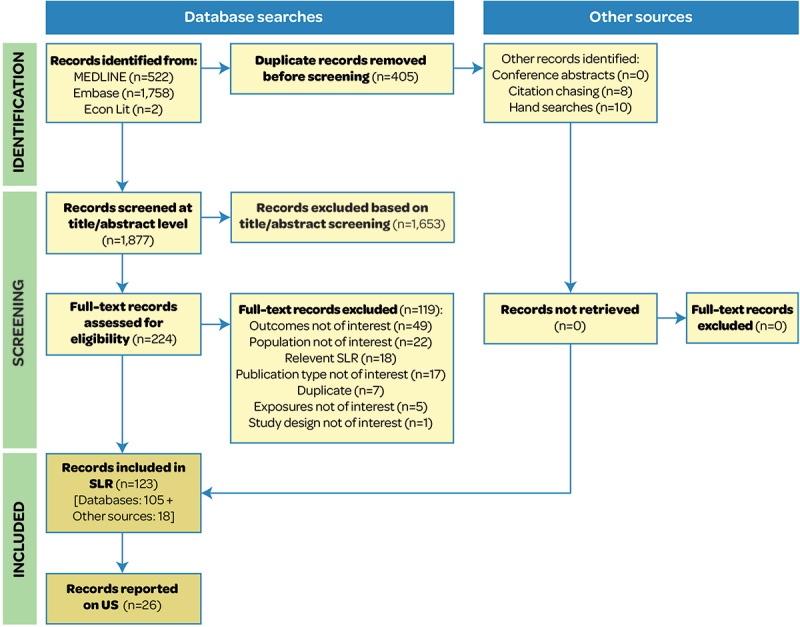
PRISMA flow diagram.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SLR: systematic literature review; US: United States.
All studies were of good quality, scoring ≥ 5 on the Newcastle Ottawa Scale. Three studies21,22,24 were of the highest quality, scoring nine (Table S4).
Population characteristics
The median age of the study population ranged from 15 to 52 years;19,23,28 however, age was not reported in nine studies. The proportion of females ranged from 15% to 74%.21,22 Three studies reported on sexual orientation, specifically men-who-have-sex-with-men (MSM).24,25,29 Nineteen studies evaluated the ethnic or racial group distribution, including Hispanic, non-Hispanic, White, and Black individuals. HIV status was reported in six studies19,20,24,25,28,29 and one study20 reported other comorbidities (hepatitis C, diabetes, and cancer).
The proportion of participants expressing serogroup B ranged from 6.8% to 100%,29,30 serogroup C from 3.9% to 100%,25,31 serogroup W from 1.3% to 21%19,31 and serogroup Y from 3.4% to 35%.19,32 Serogroup A was reported in one study19 in four cases who were unvaccinated or of unknown vaccination status.
Inequalities in IMD risk and prevention from individual characteristics and behaviors
The following section describes the results of inequalities in IMD risk and prevention by individual characteristics and behaviors (Figure 2).
Figure 2.
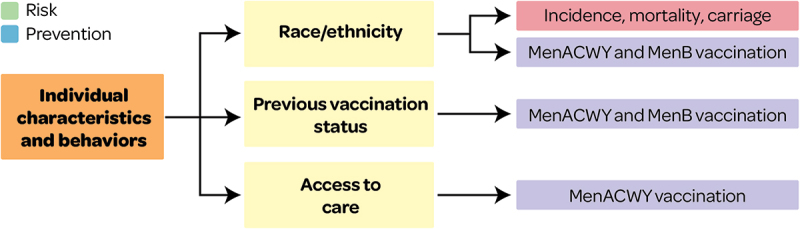
Overview of individual characteristics associated with IMD risk and prevention outcomes.
This figure and section present factors associated with IMD risk and prevention and that may lead to health inequity (see Supplementary file S1 for all other factors).
Race/ethnicity
Inequalities in IMD risk by race/ethnicity
Incidence
A comparison of IMD incidence in adult men in the US (2012–2015) found that 50.0% and 39.2% of MSM were White and Black, respectively, compared with 59.2% and 20.3% in non-MSM; and 67.6% of MSM versus 60.9% of non-MSM were non-Hispanic.29 Among MSM, being Black was associated with serogroup C IMD in 58.8% of cases vs. 10% of controls (matched OR adjusted for HIV status 8.0 [95% CI 1.6–63.7]), during an outbreak in NYC (2012–2013). However, the authors concluded that race is not likely to be a biological risk factor but rather a proxy for a social risk factor.25
Mortality
Inequalities in IMD mortality by ethnicity were reported in a population aged ≥15 years in NYC (2008–2016). Hispanic ethnicity was associated with lower risk of death compared with non-Hispanic White populations (aRR 0.43 [95% CI 0.19–0.96]).20
Carriage
Meningococcal carriage was associated with White race (8%) compared with other ethnicities (2.1%) (OR 3.2, [95% CI 2.1–4.9]) across eight high schools in Maryland and Georgia in 2006–2007.23
Inequalities in IMD prevention by race/ethnicity
MenACWY
Figure 3a shows that White adolescents versus other races/ethnicities had a lower likelihood of receiving MenACWY vaccines (from analyses of large healthcare claims databases, Commercial Claims and Encounters [CCAE] and Medicaid),34 and White adolescents aged 17 years were less likely to complete MenACWY primary and booster doses (based on NIS-Teen data 2011–2016)26 (Figure 3a).
Figure 3.
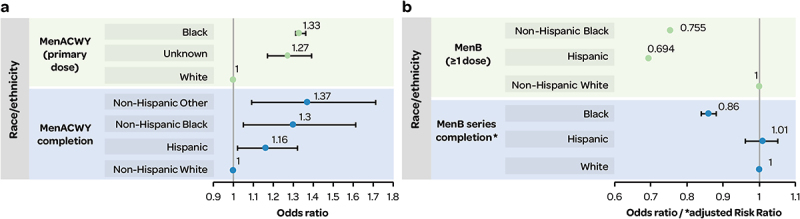
a) inequalities in race/ethnicity associated with MenACWY vaccination; b) inequalities in race/ethnicity associated with MenB vaccination.
Figure 3b: OR (p values) were as follows: Non-Hispanic Black OR 0.755 (0.026); Hispanic OR 0.694 (0.006).33
MenB
From a web HCP survey, non-Hispanic Black and Hispanic adolescents/young adults were less likely to receive MenB vaccination versus their non-Hispanic White counterparts (Figure 3b), while no difference was observed for Asian (OR 0.775) and other/unknown (OR 0.788) versus non-Hispanic White.33 In addition, MenB series completion rates were less likely in Black (aRR 0.86 [95% CI 0.84–0.88]), and comparable in Hispanic (aRR 1.01 [95% CI 0.98–1.05]), versus White 16–23-year-olds with Medicaid insurance35 (Figure 3b).
A survey to assess factors associated with MenB vaccine awareness found that White non-Hispanic parents/guardians were more aware of MenB vaccines (aware vs. not aware: OR 2.2, 95% CI 1.09–4.46), but Hispanic parents/guardians were more interested in vaccination (unaware but interested vs. unaware and not interested: OR 5.05, 95% CI 1.13–22.63), compared with Black and other non-Hispanic groups.36
Previous vaccination status: MenB/MenACWY/Others
Inequalities in IMD prevention by previous vaccination status
MenACWY
MenACWY primary and booster dose completion and compliance among adolescents aged 17 years (NIS-Teen 2011–2016) was more likely in adolescents who were up-to-date with other vaccinations (Figure 4a).26 Similarly, individuals were more likely to receive ≥ 1 dose of MenACWY vaccine if they had received pneumococcal vaccines PCV13/PPSV23 (hazard ratio HR 26.02; 95% CI 21.01–32.22).38
Figure 4.
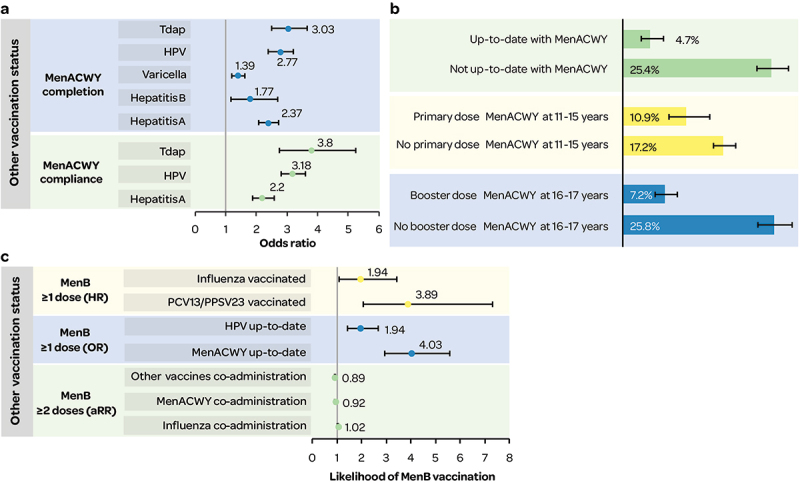
a) MenACWY (primary and booster dose) completion and compliance (OR) by other vaccination status; b) MenB (≥1 dose) coverage by MenACWY vaccination status;10,37 c) likelihood of MenB (≥1 dose and ≥2 doses) receipt by other vaccination status.
aRR: adjusted relative risk; HPV: human papillomavirus; HR: hazard ratio; OR: odds ratio; PCV13/PPSV23: 13-valent pneumococcal conjugate vaccine/23-valent pneumococcal polysaccharide vaccine; Tdap: tetanus-diphtheria-acellular pertussis vaccine.
MenB
Data from the NIS-Teen in 2017–2018 showed lower MenB vaccination coverage (≥1 dose) in individuals who were not up to date with MenACWY vaccinations10,37 (Figure 4b).
Adolescents had a higher likelihood of receiving ≥1 dose of MenB vaccine if they were up-to-date with their MenACWY and human papillomavirus (HPV) vaccinations10,37 (Figure 4c). Individuals were more likely to receive MenB (≥1 dose) if they had received pneumococcal vaccines PCV13/PPSV23 or influenza vaccination38 (Figure 4c). MenB series completion in 16–23-year-old Medicaid populations was less likely if MenB vaccines were co-administered with other vaccines or with MenACWY versus with influenza35 (Figure 4c).
Access to care: healthcare visits/check-ups
Inequalities in IMD prevention by healthcare access
MenACWY
Higher odds of MenACWY primary and booster dose completion were observed in adolescents aged 17 years who had ≥1 visits to an HCP in the past year versus none (OR for 1 visit 1.36 [1.11–1.67]; 2–5 visits 1.52 [1.25–1.85]; ≥6 visits 1.44 [1.10–1.88]]); and in adolescents who had an 11–12-year-old well-child visit compared to none (OR 1.41 [1.14–1.76]) (NIS-Teen data 2011–2016).26 Similarly, a study that assessed MenACWY vaccination coverage using data from the CCAE and Medicaid MarketScan Databases (2011–2016) reported an increased likelihood of receiving MenACWY vaccination in adolescents was associated with the number of preventive care/well-child visits (aOR: 1.63 [1.62–1.64]).34
Attendance at a well-care visit was also associated with an increased uptake of the MenACWY vaccine (HR 3.67 [1.11–12.12]) in people aged ≥2 years with a new diagnosis of HIV eligible for MenACWY.22 Similarly, in patients with asplenia; those who attended ≥1 well-care visit had a higher likelihood of receiving meningococcal vaccines (MenACWY: HR 6.63 [4.84–9.09]; MenB: HR 11.17 [3.02–41.26]).38
A higher odds of MenACWY primary and booster dose completion was observed in adolescents aged 17 years with married versus unmarried mothers (OR 1.14 [1.01–1.28]) (NIS-Teen data).26
MenB
Parental vaccine awareness influenced willingness to vaccine,27 and HCP relationship with adolescents influenced vaccine awareness36 (Table 1)
Table 1.
Parental willingness to vaccinate and vaccine awareness.
| Parents of teenagers1 who were aware of at least one of the MenB vaccines (reference) compared with those who were not, were: | ||
|---|---|---|
| Willing to vaccinate their child27 | MenB OR: 3.8; 95% CI: 1.2, 12.2 |
MenACWY OR: 6.3; 95% CI: 1.3, 29.4 |
| With enough doses to fully vaccinate their child27 | OR: 1.9; 95% CI: 1.1, 3.2 | |
| MenB vaccine awareness among parents/guardians of US adolescents, for parents who felt their HCP knew their child well (reference, 1) versus did not: | ||
| MenB vaccine awareness when HCP did not know child well36 | OR 0.53, 95% CI 0.30–0.96 | |
1attending high school in 2017 to 2018 in Minnesota; 95% CI: 95% confidence interval; HCP: healthcare provider; OR: odds ratio.
Inequalities in IMD risk and prevention from socioeconomic factors
Insurance status and type
Inequalities in IMD risk by insurance type/status
Incidence
IMD incidence proportions (per 100,000 people) in adults were significantly higher in Medicaid-insured versus commercially insured populations in 2010 and in 2006–2010 (Figure 6).21 Based on five-year data on incidence proportions across the US, populations less able to afford private health insurance were at increased risk of IMD.21
Figure 6.
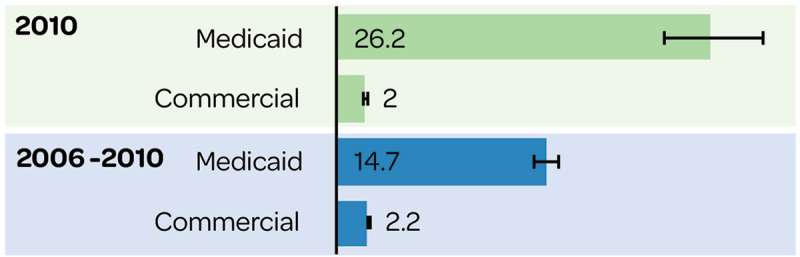
IMD incidence proportion (per 100,000) with Medicaid and commercial insurance.
Inequalities in IMD prevention by insurance type/status
MenACWY
MenACWY vaccine (≥1 dose) uptake among adolescents was more likely in all health plans, except Comprehensive and Point of Service, compared with Health Maintenance Organization34 (Figure 7a). Uninsured adolescents had lower MenACWY vaccination coverage (for ≥1 dose and ≥2 doses, Figure 7b,39 and lower compliance i.e., adherence to the ACIP-recommended schedule (based on adolescents aged 17 years, NIS-Teen data 2011–2016, Figure 7a26 compared with adolescents with private, Medicaid and other health insurance.
Figure 7.
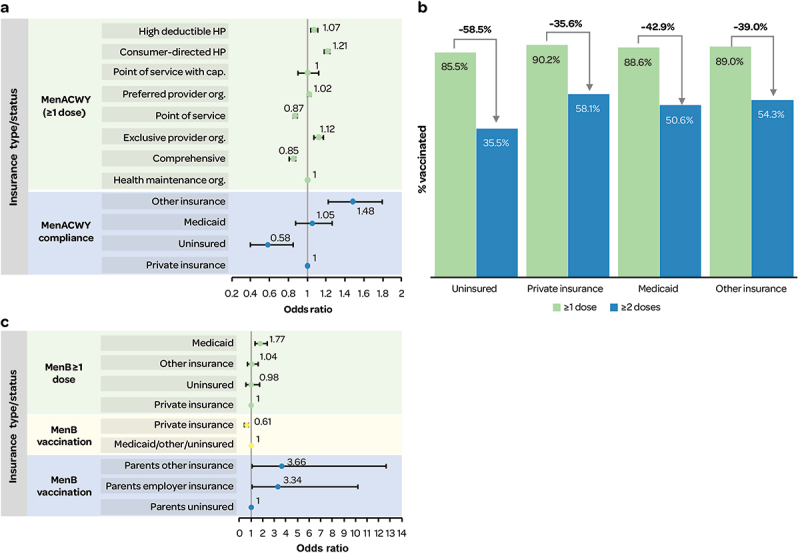
a) likelihood of MenACWY (≥1 dose) vaccination and compliance by insurance type/status; b) MenACWY vaccination by dose and insurance; c) likelihood of MenB vaccination by insurance type/status.
Cap.: capitation; HP: health plan; org.: organization; Note Figure 7c: For ‘MenB vaccination’ number of doses was not specified.
MenB
MenB vaccination coverage (NIS-Teen data 2017–2018) in adolescents aged 17 years was lower in uninsured adolescents (10.8 [7.1–16.0]) versus in adolescents with private insurance (13.3 [11.7–15.2]), Medicaid (21.9 [18.1–26.2]), and other insurance (12.5 [9.0–17.2]).10 Multivariate models found a lower likelihood of MenB vaccination for private versus Medicaid insurance10; and for private versus Medicaid/other/no insurance37 (Figure 7c). However, in states with a MenACWY vaccine mandate, health insurance was no longer significantly associated with MenB vaccine uptake in adolescents.37 Higher vaccination coverage was also observed in adolescents with continuity of health insurance coverage since age 11 years (never uninsured since age 11 years, 16.2 [14.4–18.2]; uninsured at some point since age 11 years, 14.3 [11.0–18.5]).10 Adolescents of parents/guardians (of ≥ 1 dependent aged 16 to 19 years) with insurance versus without were significantly more likely to receive MenB vaccine36 (Figure 7c).
MenB series completion rates in 16–23-year-olds were more likely in commercially insured (56.7%) versus Medicaid (44.7%) populations, and higher with MenB-4C vs. MenB-FHbp vaccines (Commercial: 61.1% versus 49.8%; Medicaid: 47.8% versus 33.9%, respectively).35 Similarly, from a web survey of US HCPs, most adolescents who were prescribed MenB only or MenB and MenACWY, respectively, had private insurance: private/commercially insured: 69.4% and 68.6% versus student healthcare plan 7.3% and 5.4%; Medicaid 18% and 21.8%; Government/Veterans Affairs hospital 2.4% and 1.3%; and not insured: 1.4% and 2.3%, respectively.33
Social deprivation
Inequalities in IMD risk by social deprivation
Mortality
In a NYC population aged ≥15 years during 2008 to 2016, a higher neighborhood poverty level of 30% to 100% (percentage of population living below 100% federal poverty level at census tract level) was associated with IMD mortality, compared with a neighborhood poverty level of 0% to 10% (adjusted RR 2.57 [1.00–6.61]).20
Inequalities in IMD prevention by social deprivation
MenACWY
In adolescents aged 17 years (NIS-Teen data 2011–2016), a higher odds of MenACWY primary and booster dose completion was associated with a family income >$75,000 compared with ≤$30,000 (OR 1.21 [1.02–1.45]).26 Similarly, in commercially insured patients with asplenia, MenACWY vaccine uptake (≥1 dose) was less likely in individuals with a household income of <$40,000 vs. ≥ $100,000 (HR 0.62 [0.47–0.83]).38
Coverage of MenACWY vaccination (≥2 doses) in adolescents (13–17 years) tended to be higher in adolescents living at/above the federal poverty level versus below, especially in residents of metropolitan statistical area (MSA) non-principal cities (Figure 8a) (2020 NIS Teen data).39
Figure 8.
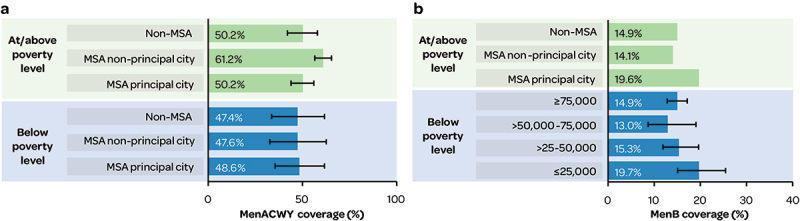
a) poverty level & metropolitan residence and MenACWY coverage (≥2 doses); b) Income/poverty level and MenB (≥1 dose) coverage.
MSA: metropolitan statistical area.
MenB
Higher MenB vaccination (≥1 dose) coverage was reported in adolescents aged 17 years from families with lower incomes (≤$25,000 per year) versus higher incomes, and in families living below the poverty level versus above (NIS-Teen data) (Figure 8b).10
Education level
Inequalities in IMD risk by education level
Incidence
Incidence rate (IR, per 100,000 population) among 18–24-year-olds (2014–2016) was higher in college students versus non-college students for MenB IMD (IR 0.167 vs. 0.049, RR 3.54 [2.21–5.41]); and lower for MenACWY IMD (IR 0.028 vs. 0.050, RR 0.56 [0.27–1.14].31
Carriage
In students from eight high schools in Maryland and Georgia, IMD carriage was higher in students in grades 11 or 12 (8.0%) versus grades 9 or 10 (3.4%).23
Inequalities in IMD prevention by education level
MenB
MenB vaccination coverage among adolescents aged 17 years was highest for those with lower maternal educational attainment (<12 years) (Figure 9).10
Figure 9.
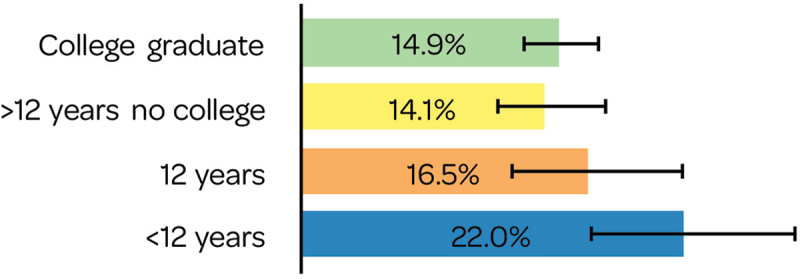
Adolescent MenB coverage by maternal education.
MenACWY/MenB in chronic conditions
In newly diagnosed asplenia patients, those with a high school diploma or lower level were less likely to receive ≥1 dose of MenACWY vaccine versus those with college/associate degrees (HR 0.78 [0.64–0.94]), and those with a bachelors/graduate degree/professional degree were more likely to receive ≥1 dose of MenB vaccine versus those with a college/associate degree (HR 2.21 [1.23–4.09]) (Optum Research Database 2005–2018).38
Homelessness
Inequalities in IMD risk by homelessness
Incidence
In IMD outbreak cases in 45 states across the US (2016–2019), the incidence of IMD was found to be higher in people experiencing homelessness (PEH) than in non-PEH (incidence per 100,000 people: 2.12 vs. 0.11, RR 19.8 [95% CI 14.8–26.7]); and serogroup C was more prevalent among PEH (68% vs 26.4%). Among adults only, a higher incidence was seen in PEH compared with same-aged non-PEH (RR 24.6 [95% CI 18.1–33.3]).28
Household characteristics
Inequalities in IMD prevention by household characteristics
MenACWY
MenACWY primary and booster dose completion and compliance were more likely in adolescents aged 17 years with 2–3 children (<18 years) in their household versus one other child in the household (completion, 2–3 children OR 1.14 [1.03–1.27]; ≥4 children OR 1.14 [0.97–1.34]; and compliance, 2–3 children; OR 1.12 [1.01–1.24]; ≥4 children; OR 1.05 [0.86–1.28]) (NIS-Teen data 2011–2016). Adolescents had a lower odds of MenACWY primary and booster dose compliance if household members had any high-risk health conditions (compliance OR 0.84 [0.76–0.94]).26 Inequalities in MenACWY primary and booster dose completion were also found, with higher odds of completion in adolescents who had married mothers vs. unmarried mothers (OR 1.14 [1.01–1.28]).26
MenB
Participants living in on-campus dormitory/shared living were more likely to be vaccinated with MenB vaccine than those living with parents, alone, other or unknown category (OR 2.094, 95% CI not reported).33
Health care professional (provider type)
Inequalities in IMD prevention by provider type
MenACWY
Adolescents with provider types other than pediatricians had a decreased likelihood of receiving MenACWY vaccine (≥1 dose), based on healthcare claims database analyses (CCAE and Medicaid).34
MenACWY primary and booster dose completion and compliance were more likely in adolescents whose vaccine provider was a private facility, hospital, or other/mixed/unknown facilities compared to a public facility (Figure 10). MenACWY primary and booster dose completion was more likely in adolescents aged 17 years whose vaccine provider reported vaccinations to an immunization registry (OR 1.31 [1.11–1.55]). Adolescents who used Immunization Information Systems (IIS) had an increased likelihood of MenACWY primary and booster dose compliance (per 10%-unit increase, 1.09 [1.02–1.17]) (NIS-Teen data 2011–2016).26
Figure 10.
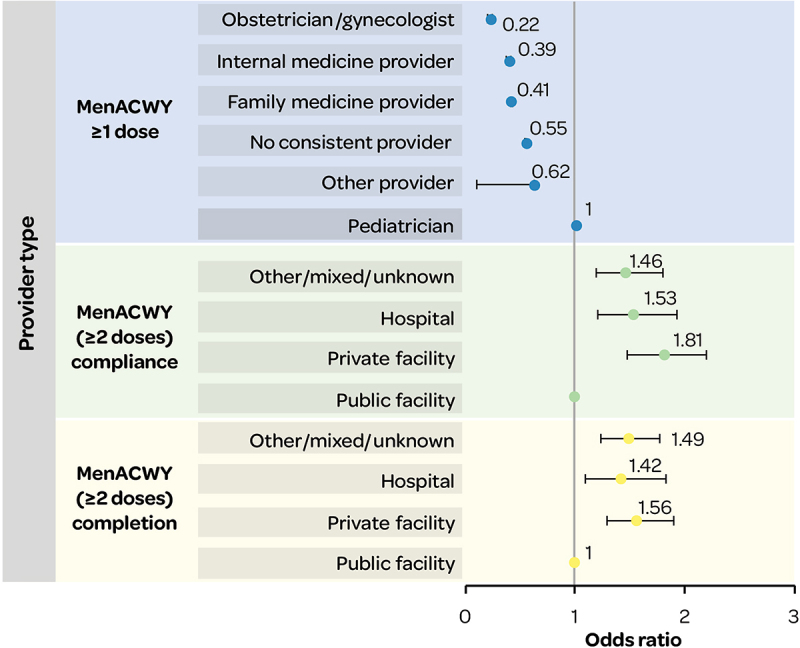
MenACWY (≥1 dose) receipt, and MenACWY (≥2 dose) completion and compliance, by provider type.
Inequalities in IMD risk and prevention from environmental factors
Geographic location/region
Inequalities in IMD risk by geographic location
Incidence
Comparable trends in IMD incidence were reported across all of the US for MSM (with and without HIV) versus non-MSM, suggesting that geographic location does not result in inequalities in incidence in this group of individuals29 (Figure 12).
Figure 12.
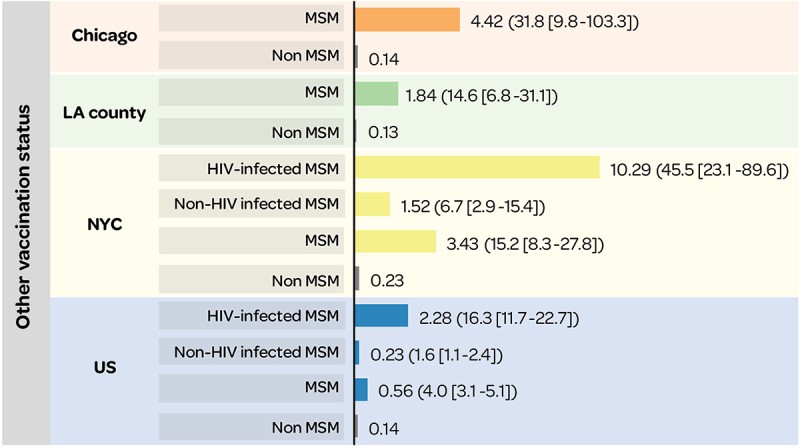
Annualized IMD incidence rate (per 100,000) in MSM versus non-MSM (RR [95% CI]).29
95% CI: 95% confidence interval; HIV: human immunodeficiency virus; LA: Los Angeles; MSM: men-who-have-sex-with-men; NYC: New York City; RR: relative risk; US: United States.
Inequalities in IMD prevention by geographic location
MenACWY
MenACWY primary and booster dose completion in adolescents aged 17 years (NIS-Teen data 2011–2016), was more likely in those residing in states with a high pediatrician-to-children ratio of 11.8 to <56.5 (4th quartile) (the number of pediatricians per 10,000 population aged <18 years) versus 0 to <7.6 (1st quartile) (OR 1.69 [1.16–2.46]); and in those residing in states with versus without a booster dose vaccination mandate by age 17 years (OR 2.08 [1.48–2.93]).26
MenACWY vaccination coverage (≥1 dose) in adolescents aged 13–17 years was 85.7% for those living outside an MSA versus 89.4% and 90.2% for those in MSA non-principal and principal cities, respectively (2020 NIS-Teen data 2020).39
In subgroups with asplenia38 or newly diagnosed HIV,22 MenACWY vaccine (≥1 dose) coverage differed by geographic location (Figure 13).
Figure 13.
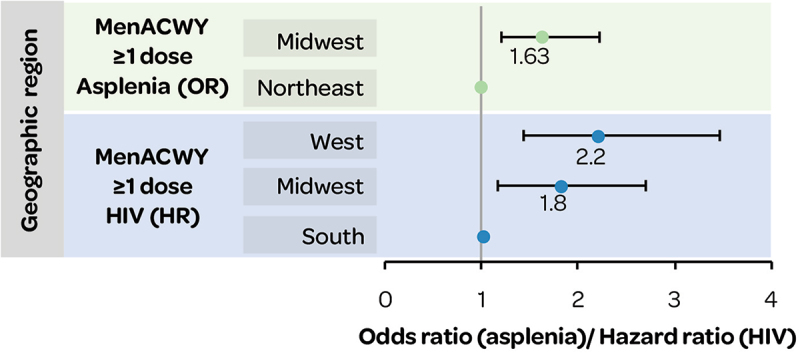
MenACWY vaccination in subgroups with asplenia or HIV by geographic region.
HIV: human immunodeficiency virus; HR: hazard ratio; OR: odds ratio.
MenB
MenB vaccination coverage, in adolescents aged 17 years (NIS-Teen data 2017–2018), varied by region (see data by Census division, Figure 14), with the highest coverage rates (≥1 dose) seen in the Middle Atlantic (19.7%), South Atlantic (17.6%) and Pacific (17.4%) census divisions.10 Multivariate models showed associations between receipt of ≥1 dose of MenB vaccine and adolescents residing in the South Atlantic (OR 1.90 [1.24–2.92]) or the Mountain (OR 1.64 [1.03–2.62]) Census divisions.10 MenB series completion, in 16–23-year-olds with commercial insurance, varied from 49.3% (Mountain) to 66.4% (New England), with significantly lower rates of completion in all locations versus New England (Figure 14).35
Figure 14.
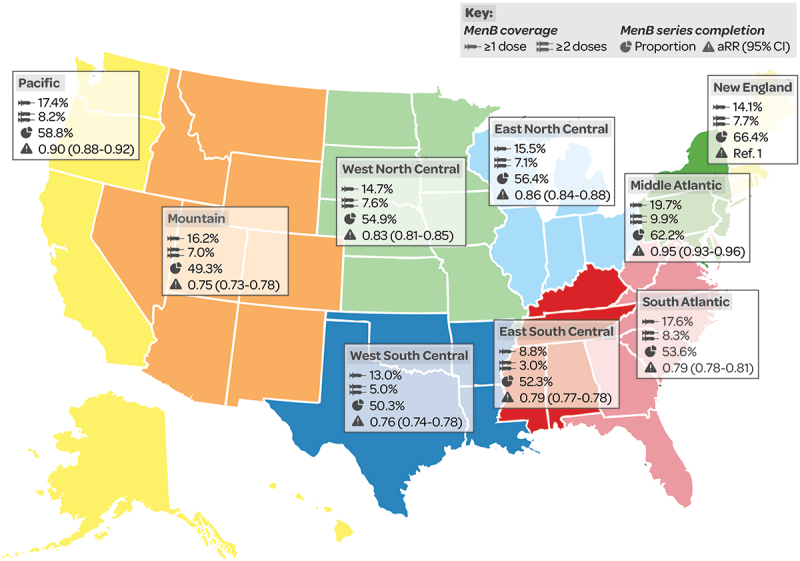
MenB vaccination coverage (% with ≥1 dose/≥2 doses) in 17-year-olds10 and MenB series completion (%, aRR [95% CI] vs New England) in 16–23-year-olds35 by census division.
95% CI: 95% confidence interval; aRR: adjusted relative risk.
MenB (MenB-4C and MenB-FHbp) vaccine series completion rates in 16–23-year-olds were less likely in rural versus urban locations among commercially insured populations (commercial aRR 0.96 [95% CI 0.94–0.98] and Medicaid aRR 0.96 [0.94–0.99]).35
In subgroups with asplenia, MenB (≥1 dose) coverage was significantly more likely in the Midwest (OR 2.53 [1.19–5.41]) and West (OR 2.57 [1.13–5.86]) versus the South.38
Organization/Community cases
Inequalities in IMD risk by organization/community cases
Incidence
Overall, 4.9% of IMD cases in the US (January 2009 to December 2013) were part of 36 outbreaks. Outbreak cases were either organization-based (i.e., had a common affiliation of cases other than a shared geographic area, such as a university) or community-based outbreaks (i.e., no common affiliation other than a shared geographic area)32 with different serogroup distributions (Figure 15).
Figure 15.
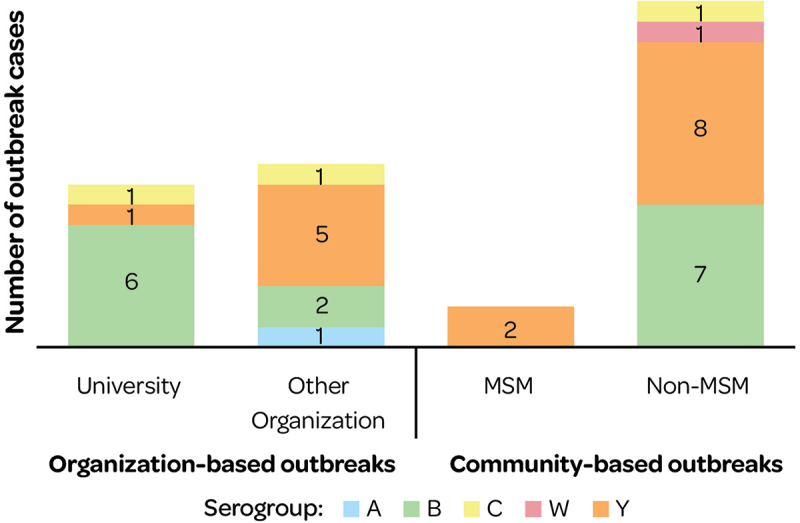
Organization and community-based outbreak cases (2009–2013) by serogroup.
MSM: men-who-have-sex-with-men.
Mortality
More deaths were reported in outbreak cases contracted in the community (27.3%) versus related to organizations (19%).32
Housing and household size
Inequalities in IMD risk by housing/household size
Incidence
Among MSM in NYC (2012–2013), living in a household with >1 other person was associated with IMD outbreaks (56.3% cases vs. 19.6% controls, matched OR 5.6 [1.5–27.0]).25
Discussion
This systematic literature review provides a comprehensive overview of several quantifiable inequalities associated with IMD risk and prevention in the US. Inequalities that may be linked to health inequity (Figures 2, 5 and 11) were identified for IMD incidence (differences by race/ethnicity, insurance status and homelessness), mortality (race/ethnicity and poverty) and carriage (race/ethnicity). Inequalities in IMD prevention (e.g., uptake and adherence of MenACWY and MenB vaccines) that may be linked to health inequity were also identified i.e., differences in race/ethnicity, wealth, education status, insurance status, access to healthcare (including healthcare provider, receipt of other recommended vaccines, pediatricians per 10,000 population, and geographic region), and parental vaccine awareness. These factors may be considered surrogates for socioeconomic status and could be indicative of higher IMD risks in socially deprived groups. Research published in 2023 (after the cutoff date in this SLR) also confirmed that IMD risk was higher in people with Medicaid versus commercial insurance, and considerably higher in PEH versus non-PEH among Medicaid insured persons;40 and that IMD risk was higher among college students, particularly the risk of MenB IMD, and was associated with living on campus/in residence halls, Greek life, and socializing.41 The findings on IMD prevention inequalities are in line with a recent SLR which found that prevention was impacted by inequalities in race/ethnicity, socioeconomic factors, geography and insurance status.42 Another recent US review found that factors associated with healthcare access improved coverage and adherence.43
Figure 5.
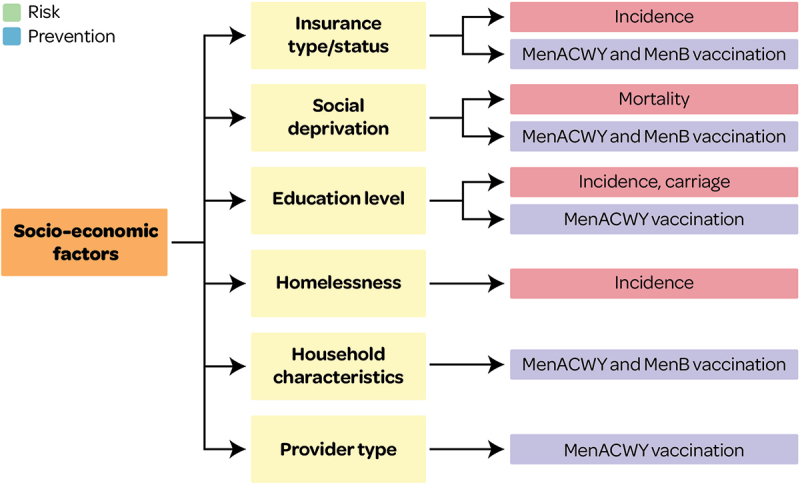
Overview of socioeconomic factors associated with IMD risk and prevention outcomes.
This figure and section present factors associated with IMD risk and prevention and that may lead to health inequity (see Supplementary file S1 for all other factors).
Figure 11.
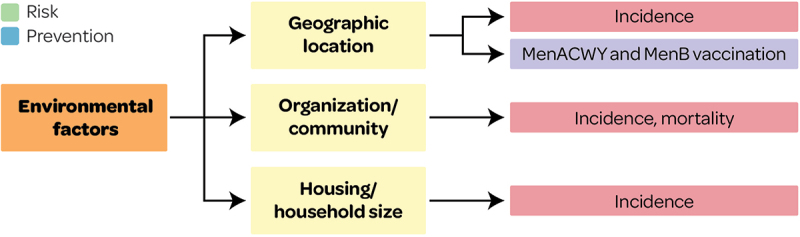
Overview of environmental factors associated with IMD risk and prevention outcomes.
This figure and section present factors associated with IMD risk and prevention and that may lead to health inequity (see Supplementary file S1 for all other factors).
The following sections focus on possible indicators of underlying health inequity, followed by limitations, recommendations, and overall conclusions.
Race/ethnicity
Overall, there were some inequalities by racial/ethnic groups in IMD incidence, carriage, mortality and prevention, which differed for MenACWY and MenB vaccination. Findings were also inconsistent across racial/ethnic groups (e.g., Black MSM associated with serogroup C IMD,44 White race associated with meningococcal carriage).23 White adolescents were less likely to receive MenACWY vaccines, whereas non-Hispanic Black and Hispanic individuals were less likely to receive MenB vaccination).33
Vaccination coverage is potentially influenced by vaccine recommendations and awareness in certain race and ethnic groups, however, the studies identified did not adequately describe the exact reasons for racial differences in vaccine access and coverage.
These racial trends could be due to underlying inequities, or race may be a proxy for social mixing or other unclear factors (e.g. health literacy, education and awareness). Furthermore, the underlying reason for the lower MenACWY coverage in White adolescents is unclear, and may be partly attributed to programs such as Vaccines for Children (VFC), which aim to bridge gaps in inequalities in healthcare access, by providing free recommended vaccinations for children ≤18 years of age who are uninsured/under insured, with Medicaid, or American Indian or Alaska Native.45 There has been an increase in MenB vaccination in the overall population (from 17.2% of 17-year-olds receiving ≥1 dose in 201846 to 29.4% in 20228 with a positive public health impact). However, despite an increase in the total population, disparities remain in MenB uptake and completion and47 non-Hispanic White individuals were more likely to be aware of the MenB vaccine.36 These findings could also be indicative of racial differences in awareness, and therefore utilization, of vaccines. A previous review supports the racial differences in MenB vaccine access and awareness, reflecting poor interpretation and implementation of ACIP’s recommendations.48
Social deprivation, income and insurance status
IMD incidence, vaccine coverage and mortality were associated with insurance status and income. IMD incidence was generally found to be higher in the Medicaid population versus commercially insured populations, while vaccination coverage (particularly with MenB vaccines) tended to be lower in families with lower incomes and uninsured populations. In some instances, however, individuals from poorer families were reported to have better vaccine uptake than their wealthier counterparts. It is likely that programs like VFC that aim to reduce inequalities may have played a role in this. This needs to be investigated further to understand the relationship between socioeconomic deprivation and vaccination coverage. Both MenACWY and MenB vaccines are covered in the US by the VFC program for individuals under the age of 19 years; however, this review found that inadequate insurance coverage resulted in inequities in MenACWY and MenB vaccination rates. Hansen, 2021 found contrasting evidence of a lower likelihood of MenB vaccine uptake in adolescents with private insurance than in uninsured/Medicaid adolescents,37 which needs to be further investigated.
Another socially deprived group found to be at risk of health inequities was people experiencing homelessness (PEH). The elevated incidence in homeless individuals is likely due to people living in crowded shelters and other factors, such as a high prevalence of underlying conditions, thereby increasing their risk of infectious diseases.49 There are numerous challenges in defining and reaching out to this target population, which are also reflected in the potential misclassification of PEH cases in the single study28 identified on this topic. Currently, the CDC recommends routine vaccinations for various at-risk groups spanning age, medical conditions, and certain occupational groups,50 but broader recommendations would also benefit other groups with socioeconomic risk factors. This review highlights the inequalities in risk leading to inequity in PEH populations, who would benefit from routine vaccination recommendations.
Finally, a higher risk of death due to IMD was reported in poorer neighborhoods than in neighborhoods with <10% poverty.20 These inequalities could be indicators of deprivation, which in turn result in inequities in healthcare access and poorer health outcomes.51
Geographical location
Inequalities in vaccine uptake for geographic regions were interpreted to be linked to health inequities. MenACWY vaccine uptake was higher in individuals who resided in the West or Midwest versus the South or other locations in the US. Higher vaccination coverage was reported for residents in states with a high pediatrician-to-child ratio, with booster dose mandates by age 17, and from East South Central and New England Census divisions. Individuals living on university campuses were more aware of vaccine recommendations due to direct outreach from vaccinated peers, and were more likely to be vaccinated for MenB than those living with parents or alone. A recent review of stocking of MenACWY and MenB vaccine doses found disparities across US counties by SES and school mandates, leading to inequality in IMD prevention.52
Access to care
Inequality in access to healthcare was reflected by disparities in uptake e.g. uptake of meningococcal vaccines tended to be higher in adolescents who were up-to-date with other vaccines, such as HPV or MenACWY, consistent with previous studies.10,26,37,38 Similarly, many studies report that having a healthcare visit/annual preventive well-child visit in the past year increased the likelihood of receiving MenACWY,22,26,34,38 highlighting the importance of preventive care visits and well-child exams as opportunities to vaccinate. These findings may reflect underlying health inequity in populations who are unable to regularly access healthcare.
Key HCP characteristics that influenced inequalities in vaccination coverage included type of provider, provider recommendations, individuals seeking care mainly from non-pediatric providers, providers reporting vaccinations to immunization registries, provider facility and frequency of HCP contact. Providers were more likely to recommend MenB vaccine at pre-college visits rather than routine health visits in 16–18-year-olds, possibly due to awareness around college outbreaks of serogroup B meningococcal disease, and potentially due to MenACWY vaccination mandates where co-administration with MenB vaccine could occur. Approximately 10% of pediatricians and one-third of family physicians reported recommending MenB vaccine to healthy 11–12-year-olds, demonstrating confusion with MenACWY primary dose timing and misinterpretation of MenB SCDM recommendations.53 In addition, 19% of pediatricians and 43% of family physicians reported making no recommendation regarding MenB vaccine for children aged ≥10 years at increased risk for meningococcal disease.53 The understanding around SCDM and timing appears to be unclear,33,48 contributing to a lack of strong and precise vaccination recommendations to parents. These findings suggest that socioeconomic inequalities coupled with provider characteristics, including prescribing behavior, provider type and provider knowledge gaps,54 impact vaccination coverage.
Education
Inequality in education status impacting vaccine coverage points to possible health inequities. Individuals with some college/associate degrees tended to have a higher likelihood of vaccination coverage for both the MenB and MenACWY vaccines.38 The reason for this is not clearly described, it may be that HCPs focus more on college attendance as risk factor for IMD, along with greater awareness due to MenACWY mandates for school/college enrollment. Thus, individuals who do not go to college may miss out on HCP recommendations for meningococcal vaccination. Another reason is that inequalities by education status may result from health illiteracy, where individuals with higher qualifications are more aware of vaccination recommendations than individuals with lower qualifications, leading to higher coverage rates.52
Strengths and limitations
This SLR provides robust evidence on inequalities in IMD risk and prevention in the US. A limitation of this review is the heterogeneity across studies with regards to reported age groups, geography, study design, specific subgroups, and exposure categories. This can limit the potential for conducting a meta-analysis in future, and for generalizability of the findings. Several studies reported differences in risk or prevention as trends rather than providing statistical analyses. In addition, studies which do not report differences (e.g., findings only reported for the total population and do not fulfil the inclusion criteria), may have been excluded. Evidence for inequalities in IMD control and long-term IMD sequelae was sparse, thus limiting further interpretation. While the definitions of inequality and inequity were based on the literature, the review lacked a framework to measure these inequalities and inequities. Many studies were not designed to explore inequalities, and study authors rarely used this terminology. Lastly, factors affecting the whole population may also indirectly impact IMD risk and prevention (e.g., economic conditions, socio-political factors, infrastructure and healthcare systems, epidemiology, demographics, culture) but these were out of scope of this study.
Recommendations and future research
Several data gaps were identified, as well as opportunities for future research and to reduce inequalities.
The current evidence suggests that differences in uptake and adherence to existing vaccines do not impact all populations equally, but the impact on health inequities remain unclear. Recent additions to the US meningococcal vaccine recommendations include a MenABCWY combination vaccine, under SCDM, when both MenACWY and MenB are indicated at the same visit.7 The increasing meningococcal vaccine options will provide greater flexibility in immunization regimens and could improve public health impact. However, SCDM is associated with inconsistent implementation of MenB vaccination; therefore, the real-world implications of these recommendations may increase schedule complexity for HCPs (e.g., stocking multiple vaccines, inconsistent use with vaccine label, HCP lack of clarity on how to recommend). Instead, providing clear and routine recommendations for all five disease-causing serogroups could improve uptake and series completion and help to reduce inequities in socially deprived groups.
Furthermore, factors that influenced inequalities in IMD risk varied from the factors that influenced IMD prevention. The risk of IMD needs to be investigated from an equity perspective in certain racial/ethnic, MSM, uninsured, and PEH populations, to understand what further action is needed to reduce inequalities in IMD risk. Further investigation is needed to determine the factors that lead to IMD mortality in poor neighborhoods, and to implement optimal prevention strategies. Suboptimal uptake of and adherence to meningococcal vaccines may relate to inequities in healthcare access, as inequalities were reported for race/ethnicity, geographical factors, income/poverty level and health insurance status. Further studies exploring vaccination coverage and regional-, state- and/or local-level geographical variables and the connection to socioeconomic factors are needed. Findings also highlight the need for further analyses to establish the association between education level and vaccination, controlled for confounding factors.
To better inform priorities for policy development/intervention, an evidence-based conceptual framework is needed, to elucidate the interactions between IMD-related inequalities quantified in this study and their impact on health equity, in terms of IMD risk, prevention and control. The association with risk from individual biological and behavioral characteristics potentially emanating from broader health inequalities needs to be investigated. Several inequalities were identified which may not be reflective of inequities (e.g., vaccine uptake by age and chronic conditions). Inequalities relating to social mixing (>1 kissing partners, group social activities, and visits to pubs/bars) are not systemic inequalities that lead to health inequity, but social and behavioral factors that increase the risk of IMD transmission. These behaviors and individual choices may be underlying factors in these populations, such as health literacy, which may be linked to societal inequities as well. A clearer understanding is needed of the relationship between health inequity and behavioral factors or individual choices, which in turn may be associated with social deprivation, and thus, with underlying health inequity (e.g., lack of means to attain health literacy). Countries are increasingly trying to incorporate equity considerations in their assessment of vaccination programs, for instance, leading to the expansion of the HPV vaccination program in the United Kingdom to include vaccination of 12–13-year-old boys.55 Similarly, in Canada56 and the US,57 guidelines now recommend including the impact on health equity of new vaccination programs, and provide a framework to do so. Experts in the field recently provided practical examples of how to potentially quantify the impact of vaccination on equity, including an example showing that including equity benefits results in a more cost-effective IMD vaccination program.58 Lastly, IMD prevention efforts would benefit from focusing on reducing missed opportunities for vaccination,59 assessing provider characteristics that influence vaccine uptake and implementation of recommendations, barriers to vaccine access and geographical variations influencing vaccination coverage in the US.
Conclusion
IMD risk and prevention in the US is associated with inequalities by individual characteristics, socioeconomic, and environmental factors. Differences in incidence and carriage were reported, demonstrating inequalities in the risk of IMD. Furthermore, several barriers to vaccine coverage and access were reported, including lack of health literacy and awareness, indicating IMD prevention is suboptimal in the US. This suggests health policy makers could improve IMD prevention by a) simplifying current vaccine recommendations (e.g., facilitating stronger vaccine recommendations to patients by HCPs via routine recommendations, or better integrating current vaccines to streamline administration/improve implementation), and b) providing or facilitating targeted education to parents, patients and HCPs to improve disease awareness and vaccine recommendations, including clear guidance on implementation. These actions would help to improve vaccination coverage and health equity in the US.
Supplementary Material
Acknowledgments
The authors would like to thank Business & Decision Life Sciences Medical Communication Service Center for editorial assistance and manuscript coordination, on behalf of GSK. Kavi Littlewood provided medical writing support.
Biography
Zeki Kocaata PhD, is the Senior Director, Global Value Evidence Portfolio Lead for Neisseria and Gonococcus Vaccines at GSK. He earned his PhD in Economics from the University of Bonn. Dr. Kocaata has significantly contributed to the literature on health policy, economics, and real-world evidence, focusing on infectious disease burden, vaccination policies, treatment effectiveness, and healthcare preferences. He has collaborated with multiple stakeholders, ranging from academia and the private sector to international policy organizations and think tanks. His work is published in esteemed journals such as the European Journal of Health Economics, The STD Journal, and PharmacoEconomics-Open. His research has been showcased at numerous scientific congresses and has informed policy decisions to enhance the societal benefits of healthcare interventions.
Funding Statement
GSK funded this study and was involved in all stages of study conduct, including analysis of the data. GSK also took in charge all costs associated with the development and publication of this manuscript.
Disclosure statement
Shahina Begum, Oscar Herrera-Restrepo, Anar Andani, Hiral Shah, Zeki Kocaata, are employed by and hold financial equities in GSK. Catherine Rolland, Sneha Purushotham received fees during the conduct of the study. The authors declare no other financial and non-financial relationships and activities.
Authors’ contributions
All authors have been included in Conception or design of the work, Data analysis and interpretation, Critical revision of the article, Final approval of the version to be published.
Availability of data and materials
Data are available under reasonable request by contacting author Shahina Begum (shahina.x.begum@gsk.com).
Consent for publication
Authors give consent for publication.
List of abbreviations
- MenABCWY
Meningococcal A, B, C, W, Y vaccine
- ACIP
Advisory Committee on Immunization Practices
- MenACWY
Meningococcal A, C, W, Y vaccine
- MenB
Meningococcal B vaccine
- CCAE
Commercial Claims and Encounters
- CDC
Centers for Disease Prevention and Control
- CFR
Case-fatality rate
- CI
Confidence interval
- COVID-19
Coronavirus disease 2019
- ER
Emergency room
- EU
European Union
- HCP
Healthcare provider
- HCRU
Healthcare resource use
- HIV
Human immunodeficiency virus
- HPV
Human papillomavirus
- HR
Hazard ratio
- ICU
Intensive care unit
- IIS
Immunization Information Systems
- IMD
Invasive meningococcal disease
- IR
Incidence rate
- MSM
Men-who-have-sex-with-men
- NIS-Teen
National Immunization Survey-Teen
- NNDSS
National Notifiable Diseases Surveillance System
- NOS
Newcastle Ottawa Scale
- NYC
New York City
- OR
Odds ratio
- PCV13
Pneumococcal conjugate vaccine 13
- PECOS
Population Exposure Comparator Outcomes Study
- PEH
People experiencing homelessness
- PPSV23
Pneumococcal polysaccharide vaccine 23
- RCT
Randomized controlled trial
- SCDM
Shared clinical decision-making
- SLR
Systematic literature review
- Tdap
Tetanus-diphtheria-acellular pertussis
- UK
United Kingdom
- US/USA
United States/United States of America
- WHO
World Health Organization
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2406613
References
- 1.Martinón-Torres F. Deciphering the burden of meningococcal disease: conventional and under-recognized elements. J Adolesc Health. 2016;59(2):S12–18. doi: 10.1016/j.jadohealth.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 2.Shen J, Begum N, Ruiz-Garcia Y, Martinon-Torres F, Bekkat-Berkani R, Meszaros K. Range of invasive meningococcal disease sequelae and health economic application – a systematic and clinical review. BMC Public Health. 2022;22(1):1078. doi: 10.1186/s12889-022-13342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olbrich KJ, Muller D, Schumacher S, Beck E, Meszaros K, Koerber F. Systematic review of invasive meningococcal disease: sequelae and quality of life impact on patients and their caregivers. Infect Dis Ther. 2018;7(4):421–438. doi: 10.1007/s40121-018-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh SR, Campbell H, Bettinger JA, Harrison LH, Marshall HS, Martinon-Torres F, Safadi MA, Shao Z, Zhu B, von Gottberg A, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. The J Infect. 2020;81(4):483–498. doi: 10.1016/j.jinf.2020.05.079. [DOI] [PubMed] [Google Scholar]
- 5.Centers for disease control and prevention (CDC) meningococcal disease: surveillance. [accessed 2023 Apr 7]. https://www.cdc.gov/meningococcal/surveillance/index.html.
- 6.Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, MacNeil JR. Meningococcal vaccination: recommendations of the advisory committee on immunization practices, United States, 2020. MMWR Recomm Rep. 2020;69(9):1–41. doi: 10.15585/mmwr.rr6909a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Advisory Committee on Immunization Practices (ACIP) ACIP Recommendations . 2023. Oct 25–26 [accessed 2023 Dec 11]. https://www.cdc.gov/vaccines/acip/recommendations.html.
- 8.Pingali C, Yankey D, Elam-Evans LD, Markowitz LE, Valier MR, Fredua B, Crowe SJ, DeSisto CL, Stokley S, Singleton JA. Vaccination coverage among adolescents aged 13–17 years — national immunization survey–teen, United States, 2022. MMWR Morb Mortal Wkly Rep. 2023;72(34):912–919. doi: 10.15585/mmwr.mm7234a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taha MK, Martinon-Torres F, Kollges R, Bonanni P, Safadi MAP, Booy R, Smith V, Garcia S, Bekkat-Berkani R, Abitbol V. Equity in vaccination policies to overcome social deprivation as a risk factor for invasive meningococcal disease. Expert Rev Vacc. 2022;21(5):659–674. doi: 10.1080/14760584.2022.2052048. [DOI] [PubMed] [Google Scholar]
- 10.La EM, Garbinsky D, Hunter S, Poston S, Novy P, Ghaswalla P. Meningococcal B vaccination coverage among older adolescents in the United States. Vaccine. 2021;39(19):2660–2667. doi: 10.1016/j.vaccine.2021.03.071. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) . Social determinants of health. [accessed 2023 Apr 7]. https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1.
- 12.Braveman P. What are health disparities and health equity? We need to be clear. Public Health Rep. 2014;129(Suppl 2):5–8. doi: 10.1177/00333549141291S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahlgren G, Whitehead M. The Dahlgren-Whitehead model of health determinants: 30 years on and still chasing rainbows. Public Health. 2021;199:20–24. doi: 10.1016/j.puhe.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO) . Defeating meningitis by 2030: a global road map (2020). [accessed 2023 Sep 26]. https://www.who.int/publications/i/item/9789240026407.
- 15.The cochrane collaboration cochrane handbook for systematic reviews of interventions. [accessed 2023 Apr 7]. https://training.cochrane.org/handbook.
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(1):b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. [accessed 2023 Apr 7]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 18.Herzog R, Alvarez-Pasquin MJ, Diaz C, Del Barrio JL, Estrada JM, Gil A. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Publ Health. 2013;13:154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blain AE, Reese HE, Marjuki H, Topaz N, Mbaeyi S, McNamara LA. Serogroup A, C, W, and Y meningococcal disease in persons previously vaccinated with a serogroup ACWY meningococcal vaccine – United States, 2014–2018. Vaccine. 2021;39(52):7541–7544. doi: 10.1016/j.vaccine.2021.11.035. [DOI] [PubMed] [Google Scholar]
- 20.Bloch D, Murray K, Peterson E, Ngai S, Rubinstein I, Halse TA, Ezeoke I, Miller L, Arakaki L, Ramautar A, et al. Sex difference in meningococcal disease mortality, New York City, 2008–2016. Clin Infect Dis. 2018;67(5):760–769. doi: 10.1093/cid/ciy183. [DOI] [PubMed] [Google Scholar]
- 21.Krishnarajah G, Carroll C, Priest J, Arondekar B, Burstin S, Levin M. Burden of vaccine-preventable disease in adult Medicaid and commercially insured populations: analysis of claims-based databases, 2006-2010. Hum Vaccin Immunother. 2014;10(8):2460–2467. doi: 10.4161/hv.29303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghaswalla PK, Marshall GS, Bengtson LGS, Buikema AR, Bancroft T, Koep E, Novy P, Hogea CS. Meningococcal vaccination rates among people with a new diagnosis of HIV infection in the US. JAMA Netw Open. 2022;5(4):e228573. doi: 10.1001/jamanetworkopen.2022.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison LH, Shutt KA, Arnold KE, Stern EJ, Pondo T, Kiehlbauch JA, Myers RA, Hollick RA, Schmink S, Vello M, et al. Meningococcal carriage among Georgia and Maryland high school students. J Infect Dis. 2015;211(11):1761–1768. doi: 10.1093/infdis/jiu679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holloway IW, Wu ESC, Gildner J, Fenimore VL, Tan D, Randall L, Frew PM. Quadrivalent meningococcal vaccine uptake among men who have sex with men during a meningococcal outbreak in Los Angeles County, California, 2016-2017. Public Health Rep. 2018;133(5):559–569. doi: 10.1177/0033354918781085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridpath A, Greene SK, Robinson BF, Weiss D. Meningococcal investigation T. Risk factors for serogroup C meningococcal disease during outbreak among men who have sex with men, New York City, New York, USA. Emerg Infect Dis. 2015;21(8):1458–1461. doi: 10.3201/eid2108.141932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng WY, Chang R, Novy P, O’Connor C, Duh MS, Hogea CS. Determinants of meningococcal ACWY vaccination in adolescents in the US: completion and compliance with the CDC recommendations. Hum Vaccin Immunother. 2020;16(1):176–188. doi: 10.1080/21645515.2019.1632679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basta NE, Becker AB, Li Q, Nederhoff D. Parental awareness of meningococcal B vaccines and willingness to vaccinate their teens. Vaccine. 2019;37(4):670–676. doi: 10.1016/j.vaccine.2018.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudmann KC, Brown NE, Rubis AB, Burns M, Ramsey A, De Las Nueces D, Martin T, Barnes M, Davizon ES, Retchless AC, et al. Invasive meningococcal disease among people experiencing homelessness-United States, 2016-2019. J Infect Dis. 2022;226(Suppl 3):322–326. doi: 10.1093/infdis/jiac230. [DOI] [PubMed] [Google Scholar]
- 29.Folaranmi TA, Kretz CB, Kamiya H, MacNeil JR, Whaley MJ, Blain A, Antwi M, Dorsinville M, Pacilli M, Smith S, et al. Increased risk for meningococcal disease among men who have sex with men in the United States, 2012-2015. Clin Infect Dis. 2017;65(5):756–763. doi: 10.1093/cid/cix438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandal S, Wu HM, MacNeil JR, Machesky K, Garcia J, Plikaytis BD, Quinn K, King L, Schmink SE, Wang X, et al. Prolonged university outbreak of meningococcal disease associated with a serogroup B strain rarely seen in the United States. Clin Infect Dis. 2013;57(3):344–348. doi: 10.1093/cid/cit243. [DOI] [PubMed] [Google Scholar]
- 31.Mbaeyi SA, Joseph SJ, Blain A, Wang X, Hariri S, MacNeil JR. Meningococcal disease among college-aged young adults: 2014–2016. Pediatrics. 2019;143(1):e20182130. doi: 10.1542/peds.2018-2130. [DOI] [PubMed] [Google Scholar]
- 32.Mbaeyi SA, Blain A, Whaley MJ, Wang X, Cohn AC, MacNeil JR. Epidemiology of meningococcal disease outbreaks in the United States, 2009–2013. Clin Infect Dis. 2019;68(4):580–585. doi: 10.1093/cid/ciy548. [DOI] [PubMed] [Google Scholar]
- 33.Huang L, Goren A, Lee LK, Li VW, Dempsey A, Srivastava A. Disparities in healthcare providers’ interpretations and implementations of ACIP’s meningococcal vaccine recommendations. Hum Vaccin Immunother. 2020;16(4):933–944. doi: 10.1080/21645515.2019.1682845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurosky SK, Esterberg E, Irwin DE, Trantham L, Packnett E, Novy P, Whelan J, Hogea C. Meningococcal vaccination among adolescents in the United States: a tale of two age platforms. J Adolesc Health. 2019;65(1):107–115. doi: 10.1016/j.jadohealth.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Packnett ER, Zimmerman NM, Kim G, Novy P, Morgan LC, Chime N, Ghaswalla P. A real-world claims data analysis of meningococcal serogroup b vaccine series completion and potential missed opportunities in the United States. Pediatr Infect Dis J. 2022;41(4):158–165. doi: 10.1097/INF.0000000000003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srivastava A, Dempsey A, Galitsky A, Fahimi M, Huang L. Parental awareness and utilization of meningococcal serogroup B vaccines in the United States. BMC Public Health. 2020;20(1):1109. doi: 10.1186/s12889-020-09181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen CE, Niccolai LM. Factors associated with receipt of meningococcal b vaccine among United States adolescents, National Immunization Survey-Teen, 2017-2018. J Adolesc Health. 2021;69(5):769–773. doi: 10.1016/j.jadohealth.2021.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghaswalla PK, Bengtson LGS, Marshall GS, Buikema AR, Bancroft T, Schladweiler KM, Koep E, Novy P, Hogea CS. Meningococcal vaccination in patients with newly diagnosed asplenia in the United States. Vaccine. 2021;39(2):272–281. doi: 10.1016/j.vaccine.2020.11.068. [DOI] [PubMed] [Google Scholar]
- 39.Pingali C, Yankey D, Elam-Evans LD, Markowitz LE, Williams CL, Fredua B, McNamara LA, Stokley S, Singleton JA. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(35):1183–1190. doi: 10.15585/mmwr.mm7035a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isenhour CJ, Crowe SJ, McNamara LA, Ma S. Differences in meningococcal disease incidence by health insurance type and among persons experiencing homelessness—United States, 2016–2019. PLOS ONE. 2023;18(10):e0293070. doi: 10.1371/journal.pone.0293070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weil LM, Crowe SJ, Rubis AB, Soeters HM, Meyer SA, Hariri S, McNamara LA. Risk factors for serogroup B meningococcal disease among college students. Open Forum Infect Dis. 2023;10(12):ofad607. doi: 10.1093/ofid/ofad607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masaquel C, Schley K, Wright K, Mauskopf J, Parrish RA, Presa JV, Hewlett D. The impact of social determinants of health on meningococcal vaccination awareness, delivery, and coverage in adolescents and young adults in the United States: a systematic review. Vaccines. 2023;11(2):256. doi: 10.3390/vaccines11020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herrera-Restrepo O, Kuang Y, D’Angelo J, Bekkat-Berkani R, Clements DE, Uyei J. Determinants of meningococcal vaccination coverage and adherence: a targeted literature review supporting a 16-year-old healthcare visit. Infect Dis Ther. 2023;12(5):1265–1282. doi: 10.1007/s40121-023-00793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips G 2nd, Johnson AK, Adames CN, Mustanski B. Meningitis vaccination, knowledge, and awareness among YMSM in Chicago. Health Educ Behav. 2018;45(4):607–615. doi: 10.1177/1090198117752786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for disease control and prevention (CDC) vaccines for children (VFC) eligibility criteria. [accessed 2023 Oct 31]. https://www.cdc.gov/vaccines/programs/vfc/providers/eligibility.html#SnippetTab.
- 46.Walker TY, Elam-Evans LD, Yankey D, Markowitz LE, Williams CL, Fredua B, Singleton JA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(33):718–723. doi: 10.15585/mmwr.mm6833a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrera-Restrepo O, Kwiatkowska M, Ndegwa N, Poston S, Ganz M. 1143. Meningococcal vaccination coverage disparities in the United States: an analysis with 2016-2021 national immunization survey-teen data. Open Forum Infect Dis. 2023;10(Supplement_2):10. doi: 10.1093/ofid/ofad500.984. [DOI] [Google Scholar]
- 48.Fergie J, Howard A, Huang L, Srivastava A. Implementation experience with meningococcal serogroup b vaccines in the United States: impact of a nonroutine recommendation. Pediatr Infect Dis J. 2021;40(3):269–275. doi: 10.1097/INF.0000000000003033. [DOI] [PubMed] [Google Scholar]
- 49.Doroshenko A, Hatchette J, Halperin SA, MacDonald NE, Graham JE. Challenges to immunization: the experiences of homeless youth. BMC Public Health. 2012;12(1):338. doi: 10.1186/1471-2458-12-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for disease control and prevention (CDC) recommended vaccines by age (special situations). 2023. [accessed 2023 Nov 1]. https://www.cdc.gov/vaccines/vpd/vaccines-age.html.
- 51.Butler DC, Petterson S, Phillips RL, Bazemore AW. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv Res. 2013;48(2pt1):539–559. doi: 10.1111/j.1475-6773.2012.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schley K, Jodar E, Presa JV, Willis SJ, Prener CG. The impact of regional disparities on the availability of meningococcal vaccines in the US. BMC Public Health. 2024;24(1):1771. doi: 10.1186/s12889-024-19081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kempe A, Allison MA, MacNeil JR, O’Leary ST, Crane LA, Beaty BL, Hurley LP, Brtnikova M, Lindley MC, Albert AP. Adoption of serogroup b meningococcal vaccine recommendations. Pediatrics. 2018;142(3):e20180344. doi: 10.1542/peds.2018-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herrera-Restrepo O, Sweeney C, Mond T, Davenport E, Wang J, Marshall GS. Nurse practitioners’ and physician assistants’ knowledge, attitudes, and practices regarding meningococcal vaccination for healthy adolescents and young adults in the United States. The J For Nurse Practitioners. 2023;20(1):104793. doi: 10.1016/j.nurpra.2023.104793. [DOI] [Google Scholar]
- 55.Joint Committee on Vaccination and Immunisation (JCVI) Statement on HPV vaccination . 2018. [accessed 2024 Sep 2]. https://assets.publishing.service.gov.uk/media/5b4e0a34e5274a73119d7614/JCVI_Statement_on_HPV_vaccination_2018.pdf.
- 56.Government of Canada National Advisory Committee on Immunization (NACI) . Guidelines for the economic evaluation of vaccination programs in Canada. [accessed 2024 Aug 27]. https://www.canada.ca/en/public-health/programs/guidelines-economic-evaluation-vaccine-programs-canada-stakeholder-consultation/guidelines-document.html.
- 57.Centers for Disease Control and Prevention (CDC)- Advisory Committee on Immunization Practices (ACIP) . ACIP evidence to recommendations framework. [accessed 2024 Aug 27]. https://www.cdc.gov/vaccines/acip/recs/grade/downloads/acip-evidence-recs-framework.pdf.
- 58.Biundo E, Dronova M, Chicoye A, Cookson R, Devlin N, Doherty TM, Garcia S, Garcia-Ruiz AJ, Garrison LP, Nolan T, et al. Capturing the value of vaccination within health technology assessment and health economics-practical considerations for expanding valuation by including key concepts. Vaccines (Basel). 2024;12(7):773. doi: 10.3390/vaccines12070773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herrera-Restrepo O, Zhou Z, Krishnan A, Conley WJ, Oladele E, Multani JK, Tuly R, Shi L, Chen C-C, Preiss S, et al. Awareness, attitudes, and practices on meningococcal serogroup B vaccination in the United States among parents of older adolescents and among young adults. Curr Med Res And Opin. 2023;40(1):125–140. doi: 10.1080/03007995.2023.2285366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available under reasonable request by contacting author Shahina Begum (shahina.x.begum@gsk.com).


