ABSTRACT
We describe the New Zealand Parkinson’s Progression Programme (NZP3), its goals, findings, and future plans. To date, 354 people with Parkinson’s disease and 89 healthy older controls have participated over a 14-year period. A major focus of the programme has been the characterisation of current cognitive impairment, and the identification of biomarkers for its future emergence in people with Parkinson’s. The programme has made significant contributions to the concept of mild cognitive impairment (MCI) in Parkinson’s and the development and validation of standardised criteria for it. Brain imaging, both MRI and PET, has also been a focus, showing associations between increasing brain pathology and declining cognitive function. Additional biomarkers such as genetics, fluid biomarkers, eye movement, speech, and quantitative electroencephalography (EEG) are also under investigation. The programme has become a platform supporting many other avenues of research, from investigating the personal impacts of caregiver burden through to national-level epidemiology. To date, the programme has led to multiple journal publications and 17 completed and 9 ongoing PhDs, and many other postgraduate theses. It has led to the development of a skilled core of early-career through to senior researchers and clinicians. We discuss the future directions for the programme.
KEYWORDS: Parkinson’s disease, cognitive impairment, dementia, longitudinal assessment, neuropsychology
Introduction: context, motivation, and clinico-scientific Whakapapa
Parkinson’s disease is the second most common neurodegenerative condition, after Alzheimer’s. Assuming the continuing lack of an effective cure or prevention, the number of people with Parkinson’s in New Zealand (having increased from 7000 in 2006 to currently over 11,000) is expected to double by 2040 (Myall et al. 2017). The increasing prevalence has largely been driven by rapidly increasing lifespans in the past few decades. This is due to not only more people surviving to an age where they develop the disorder, but also an increased duration of disease. Longer disease duration has also led to a changed appreciation of what Parkinson’s is. Traditionally viewed primarily as a movement disorder (evidenced by its cardinal symptoms of slow movement, tremor, and rigidity), it is now known to evolve to a complex multi-system disease. The many non-motor symptoms can become more disabling than the primary motor signs, and are the focus of the longitudinal project described in this paper.
Early studies of Parkinson’s in New Zealand were medically-oriented, with investigations of pharmaceutical interventions carried out both before (Gallagher and Palmer 1950; Palmer and Gallagher 1950) and after (Jorgensen et al. 1971; Wallis 1988) the introduction of dopamine replacement therapy (Birkmayer and Hornykiewicz 1962), which remains the front-line treatment to this day. A particularly important clinical/epidemiological study was carried out in Wellington by Pollock and Hornabrook (1966). This was one of the early substantial papers to highlight the significance of dementia in Parkinson’s and the role of cerebrovascular risk factors. Prof. Martin Pollock continued his clinical Parkinson’s research in Dunedin, with a focus on cognitive impairment, and also led the first Parkinson’s neuroimaging study in New Zealand (Lichter 1988). That study noted the inadequacies of assessing dementia in Parkinson’s using the then-current DSM-III psychiatric criteria, as the pattern of cognitive impairment was clearly different to that seen in primary dementia conditions such as Alzheimer’s.
Broadly speaking, Parkinson’s-related research in New Zealand has subsequently developed along three directions, with a focus on neuropathology at the University of Auckland, on basal ganglia models and single-cell physiology at the University of Otago in Dunedin, and on clinical studies in Christchurch, the latter pioneered by Prof. Ivan Donaldson. He returned from studying at Queen Square in London with ‘the father of movement disorders’, David Marsden (Donaldson 2014), with whom he subsequently worked to produce a definitive text on the topic (Donaldson et al. 2012). Ivan mentored a local movement disorders successor in Prof. Tim Anderson, who in turn also worked with Marsden in London (Anderson et al. 1994, 2008; Steiger et al. 1995). Meanwhile, Prof. John Dalrymple-Alford arrived from the UK to take up a position at the Department of Psychology at the University of Canterbury, with twin interests in the neuropsychology of Parkinson’s and in animal model behavioural neuroscience. Early Parkinson’s projects in Christchurch included clinical trials (Anderson et al. 1992; Dalrymple-Alford et al. 1995) as well as investigations of the perceptual (R. D. Jones et al. 1992; R. D. Jones and Donaldson 1995), motor (Muir et al. 1995; Myall, MacAskill, Anderson, et al. 2008; Myall, MacAskill, Davidson, et al. 2008) and oculomotor (MacAskill et al. 2002a, 2002b; Le Heron et al. 2005) impacts of the disorder, but even then an emphasis on cognitive impairment and neuropsychological performance was prominent (Waddell and Donaldson 1990; Dalrymple-Alford et al. 1994, 1995).
A major advance came with the establishment of the Van der Veer Institute for Parkinson’s and Brain Research in 2004. This was created as an independent research organisation to host research and clinical staff and postgraduate students from the Universities of Canterbury and Otago and the Canterbury District Health Board. The goal was to bring people together from multiple institutions and disciplines in a facility that combined both research and clinical services. Research into Parkinson’s has continued to be the major focus of the organisation, notwithstanding its subsequent renaming as the New Zealand Brain Research Institute (NZBRI) in 2011. The Van der Veer bequest enabled the establishment of a Chair in Movement Disorders, held by Tim Anderson (Department of Medicine, University of Otago, Christchurch). The creation of the Institute enabled a strengthened multidisciplinary collaboration centred around his specialisation in clinical neurology and John Dalrymple-Alford’s expertise in neuropsychology. In 2006, the country’s first 3 tesla, research-grade, MRI scanner was installed below the Institute, in a partnership with Pacific Radiology Group (and particularly neuroradiologist Dr Ross Keenan) and Dr Richard Watts (Department of Medical Physics, University of Canterbury), a physicist specialising in magnetic resonance imaging (Watts 2005).
The first NZBRI project to combine the disciplines of neurology, neuropsychology, and neuroimaging began in 2007, funded by the Neurological Foundation of New Zealand. This was a cross-sectional study of 45 people with Parkinson’s: 15 each with normal cognition (PDN), mild cognitive impairment (PD-MCI), or Parkinson’s dementia (PDD, see Figure 1), and a group of 30 age-similar controls. Although appearing relatively modest in scope now, this would have been one of the earliest projects to target the nascent diagnostic category of PD-MCI for neuroimaging. This initial cross-sectional study formed the nucleus of what would become a major longitudinal study, the New Zealand Parkinson’s Progression Programme (NZP3), with the original participants being followed-up and supplemented by ongoing recruitment. This has centred on tracking changes in cognitive status over time, while linking these with neuroimaging, motor and psychiatric symptoms, and biosampling. NZP3 has also provided a platform for focussed sub-studies arising directly from participant, caregiver or clinician demand, such as investigations into hallucinations, speech changes, and apathy.
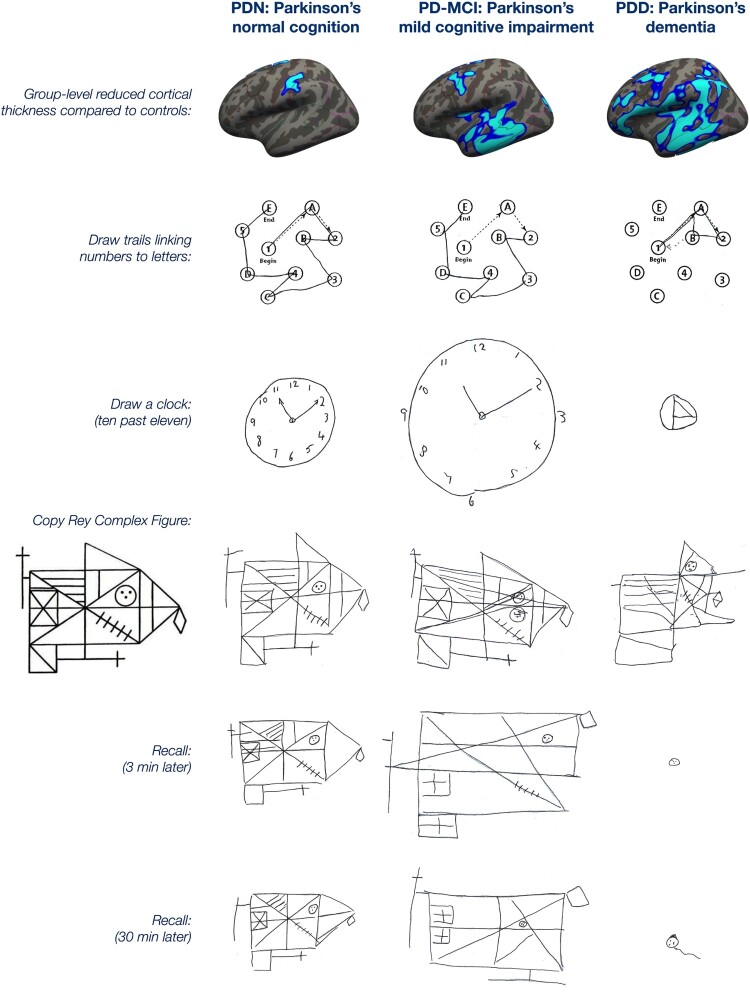
Figure 1.
Examples of data collected in the programme from Parkinson’s participants with normal cognition (PDN), mild cognitive impairment (PD-MCI), and dementia (PDD). The top row shows group-level progression across groups in the extent of cortical thinning relative to controls. The consequences of this are shown in this selection of neuropsychological tests from three representative male participants (PDN: age 68, 8 years symptom duration; PD-MCI: age 72, 6 years duration; PDD: age 60, 5 years duration; all with 10–11 years of education). The PDN participant shows good performance in the trail-making test of executive function, and the visuo-spatial tests of drawing a clock and copying a complex figure. The memory ability that allows drawing that figure again after delays of 3 and 30 min is also relatively preserved. The PD-MCI participant shows good trail making and only mildly disorganised clock drawing and figure copying. The ability to redraw the complex figure from memory, however, is somewhat degraded. The participant with dementia is severely impaired on all of the tasks. Figure available under an open CC-BY licence (MacAskill and Horne 2021).
Design and methods
Participants
As at November 2021, 354 people with Parkinson’s and 89 age-similar controls have been assessed at least once in the study. A total of 258 participants are currently being followed up (190 Parkinson’s and 68 controls). As shown in Figure 2, recruitment of new participants has occurred in several discrete waves, contingent on major funding injections. Ongoing longitudinal assessments of existing participants has, however, been relatively continuous. The central core of the study is the dataset arising from their 1586 full neuropsychology assessments (1242 from PD cases and 344 from Controls), typically taken at 1–2 year intervals (Figures 1–4). An additional 359 abbreviated neuropsychology assessments have been conducted to allow more frequent (six-monthly) monitoring in some sub-studies.
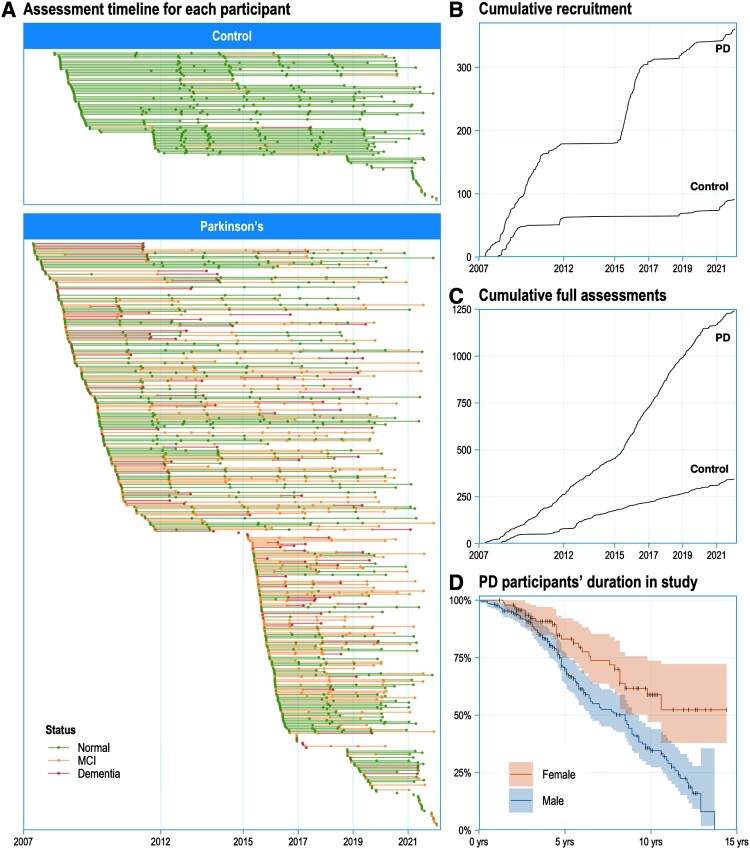
Figure 2.
A, Individual assessment timelines through the duration of the study, with most participants assessed multiple times. B, Recruitment of new participants has occurred in a pulsatile fashion, depending on commencement of discrete major sub-studies. C, Assessments have been continuous, however, as efforts are made to continually track existing participants (with recent interruptions due to COVID-19). D, Survival curves showing the study duration of Parkinson’s participants: ongoing recruitment is required to balance drop-outs due to mortality and other factors. Figure available under an open CC-BY licence (MacAskill 2022).
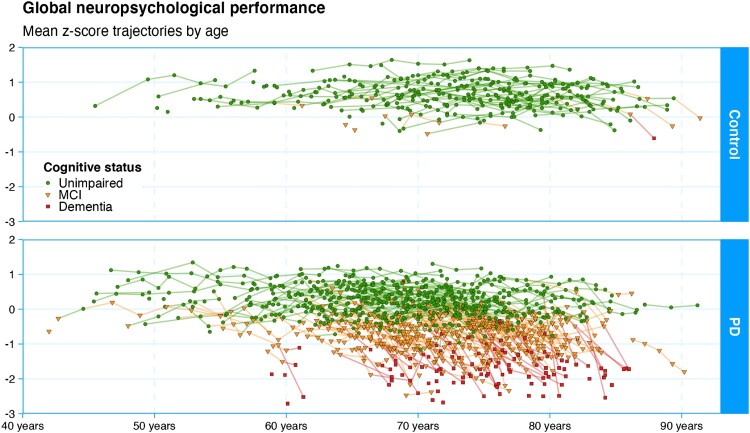
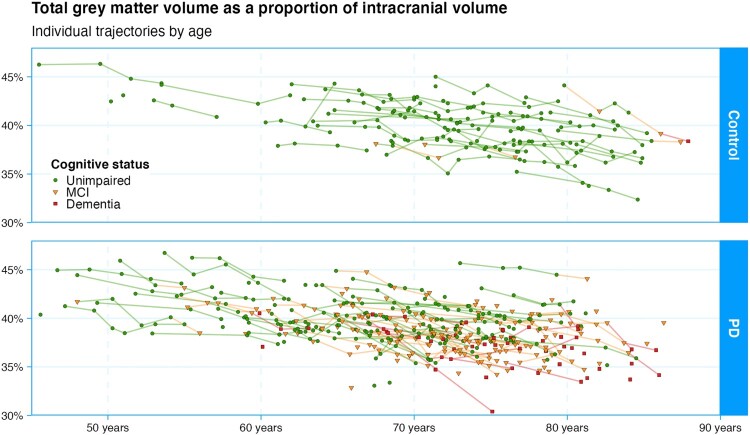
Figure 3.
Here we depict the core dataset of the study: individual trajectories of cognitive status from all participants. Each point represents a summary measure of one participant’s comprehensive neuropsychological assessment (the mean z-score of the component tests grouped within each of five cognitive domains). Dementia is the end-point for gathering this measure, as administering the full test battery becomes infeasible beyond that point. The diagnostic categories are represented by green circles (normal functioning), yellow triangles (mild cognitive impairment), or red squares (dementia). Controls show a pattern of relatively stable cognitive functioning over a wide age range, with only a few showing mild cognitive impairment, and one declining to dementia to date. While the younger Parkinson’s participants also show relatively stable functioning, a large proportion eventually develop mild cognitive impairment and many progress to dementia. A proportion, however, retains good functioning even into very old age. Understanding this heterogeneity in the course of disease is the main goal of the research programme. Figure available under an open CC-BY licence (MacAskill 2021a).
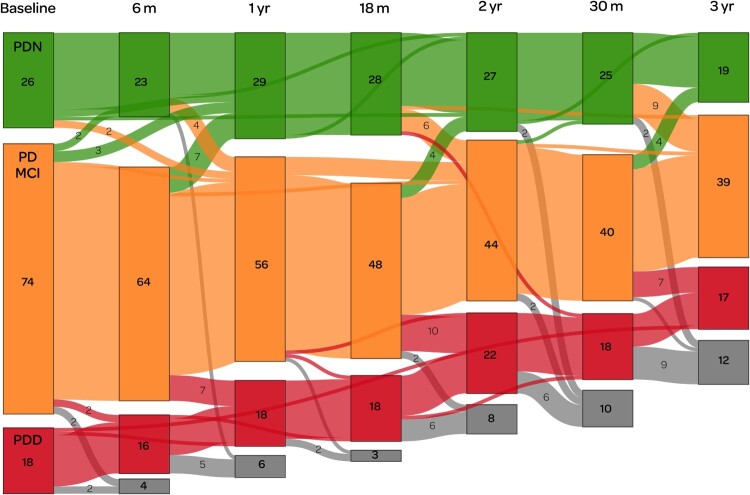
Figure 4.
Depiction of a sub-study in which we used positron emission tomography (PET) imaging to measure cerebral amyloid in Parkinson’s (Melzer et al. 2019). This illustrates the complexity of longitudinal research in patients who can change (not always unidirectionally) between diagnostic categories, who do not always attend all scheduled sessions, and who are also at substantial risk of dropping out due to mortality and morbidity. We began with a sub-sample enriched to over-represent PD-MCI (74 participants at baseline, shown in orange), with smaller reference groups of PDN (green, n = 26) and PDD (red, n = 18). Coloured boxes show the number of participants within each category at (ideally) 6-monthly assessment intervals over a three year period. Grey boxes indicate those who were lost to follow-up at each assessment, due to death or other factors. Numbers in a lighter-weight font indicate transitions between different categories. Only one person appeared to transition directly (between assessments at 18 and 30 months) from PDN to PDD: otherwise, all conversions to dementia were detected via the intermediate stage of PD-MCI. Some PD-MCI participants, meanwhile, reverted to being classified as PDN, indicating the inherent difficulty in determining the boundary between the two states. Although the number of people with PDD appears to have been relatively constant throughout the study, only one person who entered the study with PDD actually survived to completion: the remainder developed dementia during the study. Figure available under an open CC-BY licence (MacAskill 2021b).
The samples reflect the male bias of the disorder (PD: 69% male, Controls: 54%) and that this is primarily a disorder of old age, with PD mean age at study entry 68.8 years (SD 7.9, range 42–87); Control mean 67.3 (SD 7.7, range 45–80). The overall sample is overwhelmingly New Zealand European/Pākehā (93%), with only 1% Māori. Although this is to some extent reflective of this age cohort in the city of Christchurch, and that Parkinson’s is much more common in Pākehā than other ethnicities in New Zealand (Pitcher et al. 2018), a residual sampling bias is likely.
Design
The Programme evolved from the initial cross-sectional neuroimaging study of n = 45 PD and 30 Controls. Funding was secured to follow up those participants two years after their initial assessments and scans. In parallel, other projects funded the recruitment and follow-up of additional participants, which is ongoing. The core of the study is detailed neuropsychological assessment (protocol described in Dalrymple-Alford et al. 2011; Wood et al. 2016), with neuroimaging (generally MRI but also PET) incorporated as resources permit. We generally aim to re-assess participants on the neuropsychological battery and motor measures, and funding permitting, with MR imaging, every two years. With interleaved complementary projects, however, full assessments could occur for subsets of participants at yearly intervals, and, with abbreviated assessments, even six-monthly (Figure 4). The end point for participants was originally at their progression to dementia, although drop-outs also occur due to mortality or morbidity or otherwise being lost to follow-up. In the later phases of the study, however, we have continued to follow up participants beyond the onset of dementia. The rapid progression of disability at this stage necessitates relatively short interval (six-monthly) follow-ups, with an abbreviated neuropsychological battery and assessment of functional and motor status.
Statistical approaches
A number of factors complicate the analysis of the data and hence statistical rigour has been a defining aspect of the programme. The convenience nature of the sample entails that participants can enter the study at varying ages and disease durations, and have differing durations of follow-up and intervals between follow-ups. Data can also be missing in ways that can not necessarily be assumed to be random (such as the level of disability precluding some but not all items collected at a given assessment). Changes in some variables can be highly non-linear, and non-independence is common. At a minimum, multi-level models have been critical, as they are suited to the practicalities of missing data amongst a large clinical study, while handling varying numbers of longitudinal assessments within subjects. More capable and computationally-intensive Bayesian models are now being applied, to allow for more sophisticated modelling of missing data and the non-independence of measures. The local data has also been used to train probabilistic epidemiological models using national-level big data sources (Myall et al. 2017; Pitcher et al. 2018; Le Heron et al. 2021).
Careful modelling is required because so many clinical variables are correlated with each other. For example, it is received wisdom that hallucinations in Parkinson’s are a predictor of future dementia. We replicated that association in 202 non-demented Parkinson’s participants followed up over four years, of whom 51 developed dementia. We could show, however, through Bayesian model comparison, that neuropsychiatric symptoms did not add useful independent predictive information beyond that simply provided by age and current cognitive functioning (Horne et al. 2021). It seems that hallucinations evolve in parallel as part of the deterioration process towards dementia, rather than being predictive of it.
The study has introduced new statistical approaches to the Parkinson’s fields as required. For example, an early major outcome (Dalrymple-Alford et al. 2010) was to establish cut-off values of the Montreal Cognitive Assessment (Nasreddine et al. 2005) as a brief screening tool for cognitive status in Parkinson’s. The outcome is an ordered trichotomy (normal functioning vs MCI vs dementia) rather than a dichotomy, and hence the traditional method of assessing the area under an ROC (receiver operating characteristic) curve (AUC) curve cannot capture the overall performance of the test. Instead, we applied a then recently-developed generalisation to assess the volume under the three-dimensional ROC surface (VUS; Nakas and Yiannoutsos 2004; Nakas and Alonzo 2007), and have continued to contribute to the development of the technique (Nakas et al. 2012; Bantis et al. 2017). More recently we have begun to apply latent class trajectory analyses (Proust-Lima et al. 2017), which allow for modelling the heterogeneity of longitudinal patterns of change across data-driven clusters of individual subjects, without the mixed-model assumption that individuals within a specified group will randomly vary around a common trajectory.
Results
Cognitive impairment and dementia
By the time the study commenced in 2007, the importance of dementia in Parkinson’s was established but diagnostic criteria for it had only just been promulgated by an International Movement Disorder Society (MDS) Task Force (Dubois et al. 2007; Emre et al. 2007). These Parkinson’s-specific criteria were a great advance upon those designed for Alzheimer’s or other dementias (for example, the memory impairments that are the sine qua non of Alzheimer’s (Dubois et al. 2014) are not necessarily a feature of Parkinson’s dementia). Hence the new criteria were incorporated into the protocol for the programme from its inception. Still to be demonstrated was whether the concept of mild cognitive impairment (MCI), a transitional state between normal functioning and dementia, was as useful a concept in Parkinson’s as it had become in Alzheimer’s. The utility of a diagnosis of ‘PD-MCI’ would inherently be tied to its operational definition, which at that stage had not been agreed upon. An overly conservative threshold would lead to low sensitivity to predict eventual PDD, but an excessively liberal one would result in poorer specificity, and lead to unnecessary stress to patients. Multiple degrees-of-freedom existed in the competing psychometric definitions of PD-MCI, from the number of the five neuropsychological domains that needed to be impaired (ranging from one to two), the number of individual tests within a domain needing to be impaired (one to two), the threshold for declaring impaired performance on an individual test (from 1.0 to 2.0 SD below norms), and what determined the basis for comparison (which could be against established population test norms, or at an individual level, based on either previous test performance or estimated premorbid functioning). In a contribution to that debate, we compared the results of applying twelve different criteria in 119 PD participants and 50 controls. Performance across the criteria ranged from classifying 89% of PD and 70% of controls as MCI (clearly too liberal) through to 14% of PD and 0% of controls (too conservative). We advocated classifying MCI on the basis of impairment in at least one of the five domains, on the basis of at least two impaired tests within a domain, at > 1.5SD below norms. This detected a plausible 30% of PD and 6% of controls as MCI. These results (Dalrymple-Alford et al. 2011) helped inform the development of subsequent MDS Task Force guidelines for the diagnosis of MCI in Parkinson’s (Litvan et al. 2012). Those guidelines still allow for leeway in setting the cut-off threshold, but subsequent papers have tended to settle on 1.5 SD (Aarsland et al. 2021). As the study evolved longitudinally, we were then able to assess the effectiveness of various PD-MCI criteria at actually predicting subsequent dementia (Wood et al. 2016). In 121 PD participants followed longitudinally over 4 years, we were able to show that our proposed criteria were optimal for predicting the development of dementia, at a relative risk of 7.2 times that of those who did not meet the criteria (CI 3.4–16.6, p < 0.0001). Another substantial contribution was the previously-mentioned study of the effectiveness of the Montreal Cognitive Assessment (MoCA, Nasreddine et al. 2005) as a brief screening tool to discriminate MCI and dementia from normal cognitive functioning in Parkinson’s (Dalrymple-Alford et al. 2010). We showed that it performed acceptably and was psychometrically superior to the established Mini-mental Status Exam. This again informed the MDS Task Force guidelines (Litvan et al. 2012), and the paper has been cited over 700 times.
Neuroimaging
With access to New Zealand’s first 3 tesla MRI (General Electric HDxt), the first NZP3 scans were acquired in 2007. Having an MR-specialised medical physicist enabled recognition by GE as a research site, granting more control over the scanner configuration than is possible in regular clinical facilities. In particular, this meant the ability to install and trial WIPs (‘works in progress’): cutting-edge sequences that had not yet been released commercially. Our first major MRI paper arose from that process: the first time that arterial spin labelling (ASL) was applied in Parkinson’s disease (Melzer et al. 2011; Seppi 2011). Previously, such perfusion imaging could only be obtained by infusing radioactive tracers. ASL has the advantage of being non-invasive but is also quantified in absolute units (in ml/100 g/min), rather than the relative measures usually derived from radionuclide scans. Previous PET & SPECT studies had shown a characteristic Parkinson’s-related spatial pattern of perfusion changes, in essence consistently revealing decreased cortical perfusion and apparently increased perfusion subcortically (see Eidelberg 2009). We could demonstrate that the latter surprising finding was in fact a mathematical artefact. With access to absolute rather than relative quantification, we found that the true pattern was more aptly interpreted physiologically as overall lowered grey matter perfusion (with sub-regions of particularly intense hypoperfusion), while the subcortical regions previously said to be hyper-perfused in fact showed preserved levels of blood flow (Melzer et al. 2011). The extent to which a given individual’s brain exhibited the characteristic Parkinson’s perfusion pattern can be expressed by a scalar network score. This score acted well as a biomarker, increasing monotonically across the Control, PD-N, PD-MCI and PDD groups, and within the Parkinson’s sample, correlating well with continuous measures of both motor and cognitive disease severity. In a comparison of dementia due to either Parkinson’s or Alzheimer’s (Le Heron et al. 2014), both showed similar patterns of cortical hypoperfusion compared to controls but could be distinguished from each other by relative hypoperfusion in medial temporal lobes (Alzheimer’s) vs frontal cortex (PDD).
We have applied more established imaging sequences such as T1-weighted structural imaging with a focus on cognitive status in Parkinson’s. Both cortical grey matter atrophy (Melzer et al. 2012) and striatal volume loss (Pitcher et al. 2012) were shown to be related to the degree of cognitive impairment. Diffusion tensor imaging (DTI) also showed that white matter tracts were extensively disrupted even in the mild stage of cognitive impairment (Melzer et al. 2013): with rapidly improving technical advances, this imaging modality is becoming increasingly useful (Melzer 2013). We also applied the non-image-forming technique of MR spectroscopy to measure ratios of metabolites (N-acetylaspartate, choline, creatine, and myo-inositol) that are sensitive to neuronal loss, axonal damage, and impaired metabolism. In a longitudinal study we found that this did not appear to be useful in our Parkinson’s sample (Almuqbel et al. 2016), and subsequently dropped spectroscopy from the protocol, in favour of adding resting-state BOLD (to assess functional connectivity) and quantitative susceptibility mapping (to measure iron deposition). Another approach to molecular imaging is via PET, using a radioligand to target a particular substance. In 118 Parkinson’s participants (Figure 4), we used 18F-Florbetaben (FBB) to measure the distribution of amyloid accumulation throughout the brain. Abnormal deposits of misfolded amyloid protein are characteristic of Alzheimer’s, and a natural question was whether they are also associated with the dementia due to Parkinson’s. We found that FBB binding was indeed higher on average in the PDD group compared to PDN and PD-MCI, but this was accounted for by the older age of that group. There were no associations between amyloid levels and other cognitive or disease severity measures. On this basis, we posit that amyloid does not play a major role in advanced idiopathic Parkinson’s. Developing a PET tracer for alpha-synuclein has not yet been possible (Korat et al. 2021), but is keenly awaited to allow in vivo rather than post mortem characterisation of the pathological process specific to Parkinson’s.
The initial MRI protocol was designed and implemented on the 3T GE scanner in 2007, and 657 scanning sessions were acquired over the following decade (Figure 5): 476 from 239 unique PD participants (mean 2.0 scans each, range 1–6) and 181 from 53 Controls (mean 3.4 scans each, range 1–6). In 2018 we ceased collecting MR images from that scanner when it was relocated to a new facility across the city. This provided a unique opportunity to quantify the impact upon reproducibility of measures before and after the shift (Melzer et al. 2020). Although we showed there would likely have been negligible impact upon data comparability across sites, we decided to retire the GE system from the longitudinal study. By 2018, the available sequences were no longer ‘state-of-the-art’ research MRI scans, and development of new capabilities had ceased on that platform. In 2019, we developed and commenced a new MRI protocol on a 3T Siemens Skyra system, leveraging the latest developments in both hardware and software (both within Siemens and in collaborating research groups internationally). The new sequences utilise simultaneous multislice (multiband) acquisition, allowing faster imaging (by up to a factor of 8). This has enabled a combination of reduced individual sequence duration, improved spatial resolution, and an increased total amount of data acquired in the same session time. The revised protocol has a stronger focus on cerebrovascular health, with sequences investigating blood flow, microbleeds, white matter integrity, resting-state functional connectivity, iron deposition, and tissue biomechanics. To date, 169 participants (125 Parkinson’s and 44 Controls) have been scanned once, with the intention for this to also form the baseline for ongoing longitudinal studies. The previous series of scans has shown that advanced MR is a sensitive tool for characterising the disease process and distinguishing between groups, but it has not proved useful as a biomarker predictive of outcome at an individual-level – we hope that a greater focus on cerebrovascular factors may advance further in that direction.
Figure 5.
Longitudinal measurement of grey matter volume in the NZP3 study. The control participants show a typical age-related linear decline of 0.22 percentage points per year. The Parkinson’s participants declined at a slightly but statistically significantly faster rate (by an additional 0.06% per year). Figure available under an open CC-BY licence (MacAskill and Melzer 2021).
Motor/oculomotor control
Parkinson’s has characteristic impacts on oculomotor control (Anderson and MacAskill 2013), which mirror to some extent the somatomotor symptoms. In saccades (rapid eye movements), especially in tasks with a cognitive component, hypometria (reduced amplitude) is clearly evident, as is increased reaction time (corresponding to akinesia). In a large video-oculography study of visually-guided saccades, we showed that the velocity of movement is, however, preserved, once the reduced amplitude is accounted for (MacAskill et al. 2012). In that study, with cognitively well-characterised participants, we could also show that some of the conflict between previous studies may have been due to the considerable heterogeneity in performance introduced by the spectrum of cognitive impairment that occurs in Parkinson’s. With a substantial collection of oculographic recordings, we were also able to bring evidence to bear on the controversial topic of ‘ocular tremor’, which has been claimed to be an early and universal diagnostic sign of Parkinson’s (Gitchel et al. 2012). We showed that this phenomenon is a technological artefact arising from somatomotor tremor: the eyes in Parkinson’s are themselves quite steady and exhibit no tremulousness (MacAskill et al. 2013; MacAskill, Myall, Pitcher, et al. 2020; Bronstein et al. 2022). The longitudinal study also provided the platform for a number of sub-projects carefully examining attentional processes in the control of eye movements (e.g. MacAskill et al. 2012; Pascoe et al. 2018; Alamri et al. 2020), including investigating the apparently paradoxical finding that saccades can also exhibit facilitated rather than delayed reaction times in Parkinson’s (van Stockum et al. 2008, 2012, 2013; van Stockum, MacAskill, and Anderson, 2011; van Stockum, MacAskill, Myall, et al. 2011).
Biosampling and genetics
Biosampling commenced in 2012, with collection of peripheral blood for DNA extraction from the then-current 215 participants. From 2016, a new round of biosampling included non-fasting plasma, serum, and urine, with follow-ups beginning in August 2020 to allow for longitudinal analysis. By August 2021, initial samples had been given by 312 participants. Biosampling is not an internal capability of NZBRI and was enabled through collaborations with the local Cancer Society Tissue Bank for sample processing and storage, and geneticist Prof. Martin Kennedy for DNA-based analyses. The Kennedy lab pioneered the use of a nanopore technique as a high-throughput method to identify known and novel variants of the glucocerebrosidase beta (GBA) gene (Graham et al. 2020). Variants within this gene are the most common genetic susceptibility in Parkinson’s but it is technically challenging to sequence, due to the presence of a pseudogene. Approximately 9% of our Parkinson’s sample had at least one disease-specific mutation in the GBA gene, consistent with expectations from predominantly European populations. Further investigations of GBA are underway, which is exciting because there are potential therapeutic interventions that can be targeted at this specific sub-population.
Biosampling has led to many new local, national, and international collaborations, with DNA and related clinical data being contributed to a number of international consortia. The international PD-MCI consortium investigated specific mutations within the SNCA (alpha-synuclein) gene (Guella et al. 2016), to which we contributed 10% of the samples. We also joined groups from Australia to form the Systems Genomics of Parkinson’s Disease (SGPD) consortium. This resulted in an analysis of DNA methylation (Vallerga et al. 2020) and led to inclusion in larger international analyses (Blauwendraat et al. 2019; Nalls et al. 2019; Reynolds et al. 2019). We contributed to the ENIGMA-3 analysis (Grasby and Jahanshad 2020), the first time ENIGMA (Enhancing Neuroimaging Genetics through Meta-Analysis) used Parkinson’s as a group of interest, and are contributors to the ongoing ENIGMA-PD working group (Laansma et al. 2021). This is currently imaging-based, but will move towards combined imaging and genetic analyses. We have also been accepted as a contributing site to GP2 (The Global Parkinson’s Genetics Program 2021).
A collaboration with researchers at the US National Institutes of Health is focussed on alpha-synuclein and other neurodegeneration-associated proteins from neuron-derived extracellular vesicles (in preparation), while our plasma samples have been used to investigate the association between vitamin C (Spencer et al. 2020) and cGP (Fan et al. 2020) in disease progression and cognitive decline. Currently we are undertaking a pilot project to assess the feasibility of measuring alpha-synuclein from tear samples. In the future it would be advantageous to add CSF sample collection to the programme, however this is more invasive, and the cost and risks to ongoing study retention need to be balanced against the value of the data.
Discussion
Study limitations
The duration of the study has resulted in some key measures themselves changing during its course. For example, the gold standard for clinical assessment at study commencement was the Unified Parkinson’s Disease Rating scale (UPDRS; Fahn et al. 1987), which in 2008 was revised by the Movement Disorder Society (MDS-UPDRS; Goetz et al. 2008). For the first round of follow-up assessments, we shifted to the new scale due to its better performance and to keep up with the international best practice. Although conversion factors are available to harmonise the motor scores (Goetz et al. 2012), this came at the cost of introducing some incompatibility between the first set of participant sessions and subsequent ones. As noted above, ASL was a new imaging capability at the start of the study, and we pioneered its application in Parkinson’s. When the sequence became commercially available, we lost access to the development version. The improvements to the sequence introduced inconsistencies with the previous data. Long-term imaging studies are challenged by such upgrades to scanner software or hardware (Melzer et al. 2020). The evolution of new sequences over the time scale of a longitudinal study can offer greatly improved quality (Melzer 2013), at the cost of loss of comparability with previous scans.
A tertiary care clinic-derived convenience sample is seldom representative of a national Parkinson’s population. For example, the median age of diagnosis in our sample (63.5) is lower than we have found in the general New Zealand population (72.5, Myall et al. 2017). A more significant issue is the limited representation of Māori, Pasifika and Asian New Zealanders (Pitcher et al. 2018). In part a limitation of the demographics of the city of Christchurch, we are working to counteract this by establishing a larger, population-representative national sample for a risk factor study, which will also provide a platform for future projects.
Being a prevalence sample means that people can enter the study at different stages of the disease. This has allowed us to enrich the sample in favour of those with MCI and dementia, at the cost of not necessarily being representative of typical disease progression. At times a limitation, this design can also provide advantages. For example, by contrasting our data to the incident-design PPMI study (Marek et al. 2018), we could disprove previously-reported associations between the number of pregnancies and later disease onset in women (MacAskill, Myall, Shoorangiz, et al. 2020). We showed that this association was an artefact of incident samples, in which age of onset is necessarily confounded with cohort secular effects.
Challenges and lessons learned
The most crucial requirement for any longitudinal study is effective participant retention. Perhaps the most important contributor for that in our study has been attracting and retaining skilled and engaged assessment staff and graduate students. In particular, our most senior study coordinator has been with the project since its inception and has a strong personal knowledge of and connection with all of the participants. This has been critical in maintaining good response levels and maintaining ongoing periodic assessments in the main longitudinal study, as well as selective recruitment into sub-projects. Internal training sessions have acted to maintain standards across the many staff and graduate students who have carried out assessments over time, with staff and students expected to perform to the same level. Participants appreciate an improved level of healthcare, with frequent research monitoring allowing for early detection of issues and referral to appropriate services. Another important element in maintaining high levels of retention has been engagement and communications with the participants outside of the research sessions. This has ranged from newsletters and frequent social media content through to organising large and well-attended events where participants gather for a morning tea to interact with each other and to engage in question-and-answer sessions with researchers and clinicians on the latest findings. In recent years, with the appointment of a communications manager, these outreach activities have been professionalised and increased in frequency and scale, and given high priority among the Institute’s overall communications plan.
The greatest challenge to the success of the project has been the retention of both junior and senior externally-funded researchers. The majority of academic staff working on the programme are reliant on unstable ‘soft-money’. The research funding environment in New Zealand is always challenging, but coupling senior researcher salaries with support staff salaries and expensive measures such as MRI and PET imaging, and biosample collection and analysis, make it more so. Key to maintaining the momentum of the programme has been that it is hosted in an organisation that is structured as an independent charity. The Institute has provided bridging funding to key staff and research-related costs during interruptions in external grant funding, offering the programme a level of resilience. Diversification of income, both philanthropic and commercial, is being sought to continue that support in a more ambitious manner.
The programme has been successful in attracting graduate students (with 17 completed and 9 ongoing PhD theses), although these numbers would likely be higher if more of the academic staff had teaching roles.
Future directions
In a sub-study of caregiver burden (A. J. Jones et al. 2017), the majority self-identified that the hardest thing about providing care and support to someone with Parkinson’s was the impact of reduced personal and social communication (A. J. Jones 2013). Communication is accordingly becoming a growing area within the programme, with studies focusing on speech, communicative participation, and neuropsychiatric influences. Speech studies will go beyond assessment of well-known dysarthric features such as changes in prosody and volume. For example, investigating the onset and characteristics of acquired stuttering following Parkinson’s disease will allow for improved diagnosis of speech problems and the development of specific behavioural speech therapy approaches. Focusing on communicative participation will enable us to look beyond directly observable speech characteristics to identify speaker, listener and environmental features that can be modified to maximise successful life participation (a similar approach has proven successful in aphasia treatment (The RELEASE Collaborators 2022)).
A new avenue of research is investigating swallowing pathology (Yiu et al. 2020; Perry et al. 2021; Perry and Troche 2021), an issue in Parkinson’s that is problematic but which also shows promise for targeted behavioural therapeutic interventions. We will use both instrumented measures to examine cognitive influences on airway protection (swallowing and cough), as well as screening tools to facilitate the earlier identification of those at risk of swallowing disorders. This will inform future clinical management decisions – such as earlier referral for swallowing assessment – as well as the development of novel therapeutic rehabilitative strategies for airway protection. We are also collaborating with researchers in Canada and the United States to validate a novel screening tool for identifying caregiver burden in people with swallowing disorders (Shune et al. 2020), with the goal of improving clinical outcomes and quality of life for both people with Parkinson’s and their families. Meanwhile, cough has traditionally received little attention in the clinical management of Parkinson’s, but by detecting and removing aspirated material, it plays a vital role in preventing respiratory illness in those with compromised swallowing. We are currently investigating how cough is affected during adverse conditions such as cognitive or motor distraction (i.e. dual tasking), to inform new strategies for rehabilitating cough.
A crucial determinant of function and quality of life in people with Parkinson’s are behavioural disturbances such as apathy (pathological loss of motivation) and impulsivity, and neuropsychiatric disturbances including anxiety, depression, and hallucinations. Treatment options for these are limited – in large part because of poor understanding of their underlying mechanisms. There is a growing base within the group with expertise in applying current neuroscientific understanding of normal brain function to these issues, to ultimately identify treatments (Le Heron et al., 2018, 2018, 2019, 2020). We have developed a cognitive framework for understanding apathy grounded in the neuroscience of cost–benefit decision making: broadly, we systematically manipulate and dissociate rewards and costs to measure how they are weighed as people act to pursue goals (Le Heron et al. 2018). We aim to use this information to predict the development of apathy in Parkinson’s, and to guide specific therapeutic targets. For example, those with apathy associated with reward insensitivity may benefit from medications such as dopamine agonists, while those with increased sensitivity to effort costs may benefit from noradrenergic modulators. Such approaches offer similar utility for understanding and treating other behavioural disturbances such as impulsivity (Morris et al. 2022) as well as hallucinations, anxiety and depression, with the promise of guiding individualised treatment.
We retain an interest in the traditional motor symptoms of Parkinson’s, however, and are currently applying deep learning computer vision approaches to quantify somatomotor symptoms from simple video recordings (Nath et al. 2019), as an alternative to laboratory-centred methods involving accelerometry or other motion tracking systems (Figure 6). This has clear telehealth applications, which within our own programme we intend to apply with online data gathering and hence wider engagement of participants across the country, including currently under-represented populations.
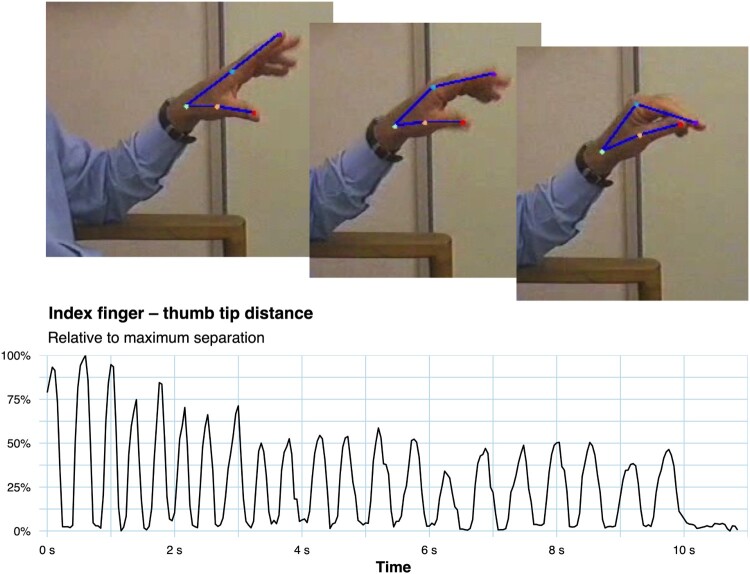
Figure 6.
A new direction for motor control research in the programme is applying advanced deep-learning pose estimation techniques (Nath et al. 2019) from video recordings in non-laboratory settings. Here the algorithm automatically identifies and tracks hand joints in a repetitive finger-tapping task. The extracted quantitative data shows progressive bradykinesia, i.e. fatiguing (decreasing movement amplitude over time) and an increased interval between successive movements. Figure available under an open CC-BY licence (Marshall and MacAskill 2022).
Conclusion
The NZP3 study was built upon strong clinico-scientific collaborations, particularly between neurology and neuropsychology, and neuroradiology and medical physics. It has benefited from being conducted within a collaborative multi-disciplinary and cross-institutional facility, and sustained by philanthropic and commercial revenue that can supplement unstable competitive research funding streams. We anticipate that it will continue to provide a strong platform for improving knowledge of Parkinson’s disease.
Funding Statement
The New Zealand Parkinson’s Progression Programme has been primarily funded and sustained by the Health Research Council, Neurological Foundation, Rangahau Roro Aotearoa, Canterbury Medical Research Foundation, New Zealand Brain Research Institute, Lottery Health Research, University of Otago, and the Orr family. The study would not have been possible without the long-standing support of our research participants and their whānau.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K, Weintraub D.. 2021. Parkinson disease-associated cognitive impairment. Nature Reviews Disease Primers. 7(1):47. doi: 10.1038/s41572-021-00280-3. [DOI] [PubMed] [Google Scholar]
- Alamri Y, Dalrymple-Alford J, MacAskill M, Anderson TJ.. 2020. Exploring eye movements of Parkinson’s disease patients performing the judgement of line orientation test. Journal of Clinical Neuroscience. 76:183–188. doi: 10.1016/j.jocn.2020.04.031. [DOI] [PubMed] [Google Scholar]
- Almuqbel M, Melzer TR, Myall DJ, MacAskill MR, Pitcher TL, Livingston L, Wood K-LL, Keenan RJ, Dalrymple-Alford JC, Anderson TJ.. 2016. Metabolite ratios in the posterior cingulate cortex do not track cognitive decline in Parkinson’s disease in a clinical setting. Parkinsonism and Related Disorders. 22:54–61. doi: 10.1016/j.parkreldis.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Ewer TC, Gilchrist NL, Donaldson IM.. 1992. Trial of Sinemet CR4 in patients with Parkinson’s disease. New Zealand Medical Journal. 105(929):81–82. [PubMed] [Google Scholar]
- Anderson TJ, Jenkins IH, Brooks DJ, Hawken MB, Frackowiak RSJ, Kennard C.. 1994. Cortical control of saccades and fixation in man: a PET study. Brain. 117:1073–1084. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Luxon L, Quinn N, Daniel S, David Marsden C, Bronstein A.. 2008. Oculomotor function in multiple system atrophy: clinical and laboratory features in 30 patients. Movement Disorders. 23(7):977–984. doi: 10.1002/mds.21999. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, MacAskill MR.. 2013. Eye movements in patients with neurodegenerative disorders. Nature Reviews Neurology. 9:74–85. doi: 10.1038/nrneurol.2012.273. [DOI] [PubMed] [Google Scholar]
- Bantis LE, Nakas CT, Reiser B, Myall D, Dalrymple-Alford JC.. 2017. Construction of joint confidence regions for the optimal true class fractions of receiver operating characteristic (ROC) surfaces and manifolds. Statistical Methods in Medical Research. 26(3):1429–1442. doi: 10.1177/0962280215581694. [DOI] [PubMed] [Google Scholar]
- Birkmayer W, Hornykiewicz O.. 1962. Der L-Dioxyphenylalanin (=L-DOPA)-Effekt beim Parkinson-Syndrom des Menschen: Zur Pathogenese und Behandlung der Parkinson-Akinese. Archiv für Psychiatrie und Nervenkrankheiten Vereinigt mit Zeitschrift für die Gesamte Neurologie und Psychiatrie. 203(5):560–574. doi: 10.1007/BF00343235. [DOI] [PubMed] [Google Scholar]
- Blauwendraat C, Heilbron K, Vallerga CL, Bandres-Ciga S, von Coelln R, Pihlstrøm L, Simón-Sánchez J, Schulte C, Sharma M, Krohn L, et al. . 2019. Parkinson’s disease age at onset genome-wide association study: defining heritability, genetic loci, and α-synuclein mechanisms. Movement Disorders. 34(6):866–875. doi: 10.1002/mds.27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein AM, Anderson TJ, Kaski D, MacAskill MR, Shaikh A.. 2022. Oculomotor and visuo-vestibular function in Parkinson’s disease. In: Gálvez-Jiménez N, Korczyn AD, Lugo-Sanchez R, editors. Non-motor Parkinson’s disease. Cambridge: Cambridge University Press; p. 115–129. [Google Scholar]
- Dalrymple-Alford JC, Jamieson CF, Donaldson IM.. 1995. Effects of selegiline (deprenyl) on cognition in early Parkinson's disease. Clinical Neuropharmacology. 18(4):348–359. doi: 10.1097/00002826-199508000-00007. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, Kalders AS, Jones RD, Watson RW.. 1994. A central executive deficit in patients with Parkinson’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 57(3):360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, Livingston L, MacAskill MR, Graham C, Melzer TR, Porter R, Watts R, Anderson TJ.. 2011. Characterizing mild cognitive impairment in Parkinson’s disease. Movement Disorders. 26(4):629–636. doi: 10.1002/mds.23592. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, Melzer TR, Kirwan J, Keenan R, Wells S, et al. . 2010. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 75:1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- Donaldson IM. 2014. The truant from medicine: how a decent young doctor was seduced to the dark side. Auckland: Random House. [Google Scholar]
- Donaldson IM, Marsden CD, Schneider S.. 2012. Marsden’s book of movement disorders. Oxford: Oxford University Press. [Google Scholar]
- Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, Dickson D, Duyckaerts C, Cummings J, Gauthier S.. 2007. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Movement Disorders. 22(16):2314–2324. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, et al. . 2014. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. The Lancet Neurology. 13(6):614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Eidelberg D. 2009. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends in Neurosciences. 32(10):548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, et al. . 2007. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement Disorders. 22(12):1689–1707. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, Committee, members of U . 1987. Unified Parkinson’s disease rating scale. In: Fahn S., Marsden C. D., Goldstein M., Calne D. M., editors. Recent developments in Parkinson’s disease (2). New York: Macmillan; p. 153–163. [Google Scholar]
- Fan D, Pitcher T, Dalrymple-Alford J, MacAskill M, Anderson T, Guan J.. 2020. Changes of plasma cGP/IGF-1 molar ratio with age is associated with cognitive status of Parkinson disease. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 12(1). doi: 10.1002/dad2.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher DJA, Palmer H.. 1950. A comparative study of the use of artane and lysivane in the treatment of Parkinsonism. The New Zealand Medical Journal. 49(273):531–536. http://europepmc.org/abstract/MED/14806877. [PubMed] [Google Scholar]
- Gitchel GT, Wetzel PA, Baron MS.. 2012. Pervasive ocular tremor in patients with Parkinson disease. Archives of Neurology. 69(8):1011–1017. doi: 10.1001/archneurol.2012.70. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Stebbins GT, Tilley BC.. 2012. Calibration of unified Parkinson’s disease rating scale scores to movement disorder society-unified Parkinson’s disease rating scale scores. Movement Disorders. 27(10):1239–1242. doi: 10.1002/mds.25122. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, et al. . 2008. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement Disorders. 23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Graham OEE, Pitcher TL, Liau Y, Miller AL, Dalrymple-Alford JC, Anderson TJ, Kennedy MA.. 2020. Nanopore sequencing of the glucocerebrosidase (GBA) gene in a New Zealand Parkinson’s disease cohort. Parkinsonism & Related Disorders. 70:36–41. doi: 10.1016/j.parkreldis.2019.11.022. [DOI] [PubMed] [Google Scholar]
- Grasby KL, Jahanshad N.. 2020. The genetic architecture of the human cerebral cortex. Science. 367:eaay6690. doi: 10.1126/science.aay6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guella I, Evans DM, Szu-Tu C, Nosova E, Bortnick SF, Group SCS, Goldman JG, Dalrymple-Alford J, Geurtsen GJ, Litvan I, et al. . 2016. α-synuclein genetic variability: a biomarker for dementia in Parkinson’s disease. Annals of Neurology. 79:991–999. doi: 10.1002/ana.24664. [DOI] [PubMed] [Google Scholar]
- Horne K, MacAskill MR, Myall DJ, Livingston L, Grenfell S, Pascoe MJ, Young B, Shoorangiz R, Melzer TR, Pitcher TL, et al. . 2021. Neuropsychiatric symptoms are associated with dementia in Parkinson’s disease but not predictive of it. Movement Disorders Clinical Practice. mdc3.13151. doi: 10.1002/mdc3.13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AJ. 2013. Parkinson’s disease, cognitive status and caregiver outcomes [MA, University of Canterbury]. https://ir.canterbury.ac.nz/handle/10092/8717.
- Jones AJ, Kuijer RG, Livingston L, Myall D, Horne K, MacAskill MR, Pitcher T, Barrett PT, Anderson TJ, Dalrymple-Alford JC.. 2017. Caregiver burden is increased in Parkinson’s disease with mild cognitive impairment (PD-MCI). Translational Neurodegeneration. 6(1). doi: 10.1186/s40035-017-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RD, Donaldson IM.. 1995. Fractionation of visuoperceptual dysfunction in Parkinson’s disease. Journal of the Neurological Sciences. 131(1):43–50. doi: 10.1016/0022-510X(95)00043-2. [DOI] [PubMed] [Google Scholar]
- Jones RD, Donaldson IM, Timmings PL.. 1992. Impairment of high-contrast visual acuity in Parkinson’s disease. Movement Disorders. 7(3):232–238. doi: 10.1002/mds.870070308. [DOI] [PubMed] [Google Scholar]
- Jorgensen P, Bergin J, Haas L, Cuningham J, Morah D, Pollock M, Robinson R, Spears G.. 1971. Controlled trial of amantadine hydrochloride in Parkinson’s disease. The New Zealand Medical Journal. 73(468):263–267. http://europepmc.org/abstract/MED/4932146. [PubMed] [Google Scholar]
- Korat Š, Bidesi NSR, Bonanno F, Di Nanni A, Hoàng ANN, Herfert K, Maurer A, Battisti UM, Bowden GD, Thonon D, et al. . 2021. Alpha-synuclein PET tracer development—an overview about current efforts. Pharmaceuticals. 14(9):847. doi: 10.3390/ph14090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laansma MA, Bright JK, Al-Bachari S, Anderson TJ, Ard T, Assogna F, Baquero KA, Berendse HW, Blair J, Cendes F, et al. . 2021. International multicenter analysis of brain structure across clinical stages of Parkinson’s disease. Movement Disorders. 36:2583–2594. doi: 10.1002/mds.28706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Heron CJ, Apps MAJ, Husain M.. 2018. The anatomy of apathy: a neurocognitive framework for amotivated behavior. Neuropsychologia. 118:54–67. doi: 10.1016/j.neuropsychologia.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Heron CJ, Holroyd CB, Salamone J, Husain M.. 2019. Brain mechanisms underlying apathy. Journal of Neurology, Neurosurgery & Psychiatry. 90(3):302–312. doi: 10.1136/jnnp-2018-318265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Heron CJ, Kolling N, Plant O, Kienast A, Janska R, Ang Y-S, Fallon S, Husain M, Apps MAJ.. 2020. Dopamine modulates dynamic decision-making during foraging. The Journal of Neuroscience. 40(27):5273–5282. doi: 10.1523/JNEUROSCI.2586-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Heron CJ, MacAskill M, Mason D, Dalrymple-Alford J, Anderson T, Pitcher T, Myall D.. 2021. A multi-step model of Parkinson’s disease pathogenesis. Movement Disorders. 36(11):2530–2538. doi: 10.1002/mds.28719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Heron CJ, MacAskill MR, Anderson TJ.. 2005. Memory-guided saccades in Parkinson’s disease: long delays can improve performance. Experimental Brain Research. 161(3):293–298. [DOI] [PubMed] [Google Scholar]
- Le Heron CJ, Plant O, Manohar S, Ang Y-S, Jackson M, Lennox G, Hu MT, Husain M.. 2018. Distinct effects of apathy and dopamine on effort-based decision-making in Parkinson’s disease. Brain. 141(5):1455–1469. doi: 10.1093/brain/awy110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Heron CJ, Wright SL, Melzer TR, Myall DJ, MacAskill MR, Livingston L, Keenan RJ, Watts R, Dalrymple-Alford JC, Anderson TJ.. 2014. Comparing cerebral perfusion in Alzheimer’s disease and Parkinson’s disease dementia – an ASL-MRI study. Journal of Cerebral Blood Flow & Metabolism. 34(6):964–970. doi: 10.1038/jcbfm.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichter DG. 1988. Cognitive and motor dysfunction in Parkinson’s disease: clinical, performance, and computed tomographic correlations. Archives of Neurology. 45(8):854. doi: 10.1001/archneur.1988.00520320040013. [DOI] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, et al. . 2012. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement disorder society task force guidelines. Movement Disorders. 27(3):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill MR. 2021a. Trajectories of cognitive status with age in Parkinson’s disease. Figshare.com. doi: 10.6084/M9.FIGSHARE.17046305. [DOI]
- MacAskill MR. 2021b. Transitions between cognitive diagnoses during a longitudinal study in Parkinson’s disease. Figshare.com. doi: 10.6084/M9.FIGSHARE.17046320. [DOI]
- MacAskill MR. 2022. NZP3 study description plots. Figshare.com. doi: 10.6084/M9.FIGSHARE.20382591. [DOI]
- MacAskill MR, Anderson TJ, Jones RD.. 2002a. Adaptive modification of saccade amplitude in Parkinson’s disease. Brain. 125(7):1570–1582. doi: 10.1093/brain/awf168. [DOI] [PubMed] [Google Scholar]
- MacAskill MR, Anderson TJ, Jones RD.. 2002b. Saccadic adaptation in neurological disorders. Progress in Brain Research. 140:419–433. [DOI] [PubMed] [Google Scholar]
- MacAskill MR, Graham CF, Pitcher TL, Myall DJ, Livingston L, van Stockum S, Dalrymple-Alford JC, Anderson TJ.. 2012. The influence of motor and cognitive impairment upon visually-guided saccades in Parkinson’s disease. Neuropsychologia. 50:3338–3347. doi: 10.1016/j.neuropsychologia.2012.09.025. [DOI] [PubMed] [Google Scholar]
- MacAskill MR, Horne K.. 2021. Example neuropsychological test impairments in Parkinson’s disease. Figshare.com. doi: 10.6084/M9.FIGSHARE.17046290. [DOI]
- MacAskill MR, Melzer TR.. 2021. Longitudinal trajectories of grey matter volume in Parkinson’s disease. Figshare.com. doi: 10.6084/M9.FIGSHARE.17046338. [DOI]
- MacAskill MR, Myall DJ, Anderson TJ.. 2013. “Ocular tremor” in Parkinson's disease: a technology-dependent artifact of universal head motion? Movement Disorders. 28(8):1165–1166. doi: 10.1002/mds.25602. [DOI] [PubMed] [Google Scholar]
- MacAskill MR, Myall DJ, Pitcher TL, Watanabe M, Anderson TJ.. 2020. “Pervasive ocular tremor of Parkinson’s” is not pervasive, ocular, or uniquely parkinsonian [Preprint]. Open Science Framework. doi: 10.31219/osf.io/s8rwt. [DOI]
- MacAskill MR, Myall DJ, Shoorangiz R, Anderson TJ, Pitcher TL.. 2020. Childbirth and delayed Parkinson’s onset: a reproducible nonbiological artifact of societal change. Movement Disorders. 35(7):1268–1271. doi: 10.1002/mds.28135. [DOI] [PubMed] [Google Scholar]
- Marek K, Chowdhury S, Siderowf A, Lasch S, Coffey CS, Caspell-Garcia C, Simuni T, Jennings D, Tanner CM, Trojanowski JQ, et al. . 2018. The Parkinson’s progression markers initiative (PPMI) – establishing a PD biomarker cohort. Annals of Clinical and Translational Neurology. 5(12):1460–1477. doi: 10.1002/acn3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall ET, MacAskill MR.. 2022. DeepLabCut finger tapping bradykinesia. Figshare.com. doi: 10.6084/M9.FIGSHARE.20382471. [DOI]
- Melzer TR. 2013. The evolution of diffusion tensor imaging in Parkinson’s disease research. Movement Disorders. 28(9):1316. doi: 10.1002/mds.25566. [DOI] [PubMed] [Google Scholar]
- Melzer TR, Keenan RJ, Leeper GJ, Kingston-Smith S, Felton SA, Green SK, Henderson KJ, Palmer NJ, Shoorangiz R, Almuqbel MM, Myall DJ.. 2020. Test-retest reliability and sample size estimates after MRI scanner relocation. NeuroImage. 211:116608. doi: 10.1016/j.neuroimage.2020.116608. [DOI] [PubMed] [Google Scholar]
- Melzer TR, Stark MR, Keenan RJ, Myall DJ, MacAskill MR, Pitcher TL, Livingston L, Grenfell S, Horne K-L, Young BN, et al. . 2019. Beta amyloid deposition is not associated with cognitive impairment in Parkinson’s disease. Frontiers in Neurology. 10:391. doi: 10.3389/fneur.2019.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer TR, Watts R, MacAskill MR, Pearson JF, Rüeger S, Pitcher TL, Livingston L, Graham C, Keenan R, Shankaranarayanan A, et al. . 2011. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson’s disease. Brain. 134(3):845–855. doi: 10.1093/brain/awq377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan R, Dalrymple-Alford JC, Anderson TJ.. 2012. Grey matter atrophy in cognitively impaired Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 83(2):188–194. doi: 10.1136/jnnp-2011-300828. [DOI] [PubMed] [Google Scholar]
- Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan R, Dalrymple-Alford JC, Anderson TJ.. 2013. White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology. 80:1841–1849. doi: 10.1212/WNL.0b013e3182929f62. [DOI] [PubMed] [Google Scholar]
- Morris L-A, O’Callaghan C, Le Heron C.. 2022. Disordered decision making: a cognitive framework for apathy and impulsivity in Huntington’s disease. Movement Disorders. 37(6):1149–1163. doi: 10.1002/mds.29013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir SR, Jones RD, Andreae JH, Donaldson IM.. 1995. Measurement and analysis of single and multiple finger tapping in normal and Parkinsonian subjects. Parkinsonism & Related Disorders. 1(2):89–96. doi: 10.1016/1353-8020(95)00001-1. [DOI] [PubMed] [Google Scholar]
- Myall DJ, MacAskill MR, Anderson TJ, Jones RD.. 2008. Submovements in visually-guided and memory-guided reaching tasks: changes in Parkinson’s disease. Proceedings of the 30th Annual International Conference of IEEE Engineering in Medicine and Biology Society. 30:1761–1764. [DOI] [PubMed] [Google Scholar]
- Myall DJ, MacAskill MR, Davidson PR, Anderson TJ, Jones RD.. 2008. Design of a modular and low-latency virtual-environment platform for applications in motor adaptation research, neurological disorders, and neurorehabilitation. IEEE Transactions on Neural Systems & Rehabilitation Engineering. 16(3):298–309. doi: 10.1109/TNSRE.2008.922676. [DOI] [PubMed] [Google Scholar]
- Myall DJ, Pitcher TL, Pearson JF, Dalrymple-Alford JC, Anderson TJ, MacAskill MR.. 2017. Parkinson’s in the oldest old: impact on estimates of future disease burden. Parkinsonism & Related Disorders. 42:78–84. doi: 10.1016/j.parkreldis.2017.06.018. [DOI] [PubMed] [Google Scholar]
- Nakas CT, Alonzo TA.. 2007. ROC graphs for assessing the ability of a diagnostic marker to detect three disease classes with an umbrella ordering. Biometrics. 63(2):603–609. [DOI] [PubMed] [Google Scholar]
- Nakas CT, Dalrymple-Alford JC, Anderson TJ, Alonzo TA.. 2012. Generalization of Youden index for multiple-class classification problems applied to the assessment of externally validated cognition in Parkinson disease screening. Stat Med. doi: 10.1002/sim.5592. [DOI] [PubMed] [Google Scholar]
- Nakas CT, Yiannoutsos CT.. 2004. Ordered multiple-class ROC analysis with continuous measurements. Stat Med. 23(22):3437–3449. doi: 10.1002/sim.1917. [DOI] [PubMed] [Google Scholar]
- Nalls MA, Blauwendraat C, Vallerga CL, Heilbron K, Bandres-Ciga S, Chang D, Tan M, Kia DA, Noyce AJ, Xue A, et al. . 2019. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. The Lancet Neurology. 18(12):1091–1102. doi: 10.1016/S1474-4422(19)30320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H.. 2005. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nath T, Mathis A, Chen AC, Patel A, Bethge M, Mathis MW.. 2019. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nature Protocols. 14(7):2152–2176. doi: 10.1038/s41596-019-0176-0. [DOI] [PubMed] [Google Scholar]
- Palmer H, Gallagher DJA.. 1950. Lysivane therapy for Parkinsonism. British Medical Journal. 2(4678):558–559. doi: 10.1136/bmj.2.4678.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe M, Alamri Y, Dalrymple-Alford J, Anderson T, MacAskill M.. 2018. The symbol-digit modalities test in mild cognitive impairment: evidence from Parkinson’s disease patients. European Neurology. 79(3–4):206–210. doi: 10.1159/000485669. [DOI] [PubMed] [Google Scholar]
- Perry SE, Borders JC, Dakin AE, Troche MS.. 2021. Characterizing quality of life in caregivers of people with Parkinson’s disease and dysphagia. Dysphagia. doi: 10.1007/s00455-021-10299-z. [DOI] [PubMed] [Google Scholar]
- Perry SE, Troche MS.. 2021. Dual tasking influences cough reflex outcomes in adults with Parkinson’s disease: a controlled study. Dysphagia. doi: 10.1007/s00455-020-10223-x. [DOI] [PubMed] [Google Scholar]
- Pitcher TL, Melzer TR, MacAskill MR, Graham CF, Livingston L, Keenan RJ, Watts R, Dalrymple-Alford JC, Anderson TJ.. 2012. Reduced striatal volumes in Parkinson’s disease: a magnetic resonance imaging study. Translational Neurodegeneration. 1(1):17. doi: 10.1186/2047-9158-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher TL, Myall DJ, Pearson JF, Lacey CJ, Dalrymple-Alford JC, Anderson TJ, MacAskill MR.. 2018. Parkinson’s disease across ethnicities: a nationwide study in New Zealand. Movement Disorders. 33(9):1440–1448. doi: 10.1002/mds.27389. [DOI] [PubMed] [Google Scholar]
- Pollock M, Hornabrook RW.. 1966. The prevalence, natural history and dementia of Parkinson’s disease. Brain. 89(3):429–448. doi: 10.1093/brain/89.3.429. [DOI] [PubMed] [Google Scholar]
- Proust-Lima C, Philipps V, Liquet B.. 2017. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. Journal of Statistical Software. 78(2):1–56. doi: 10.18637/jss.v078.i02. [DOI] [Google Scholar]
- Reynolds RH, Botía J, Nalls MA, Hardy J, Gagliano Taliun SA, Ryten M.. 2019. Moving beyond neurons: the role of cell type-specific gene regulation in Parkinson’s disease heritability. NPJ Parkinson’s Disease. 5(1):6. doi: 10.1038/s41531-019-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppi K. 2011. The role of arterial spin labeling, a noninvasive MRI perfusion method, in identifying an abnormal cerebral perfusion pattern in Parkinson’s disease. Movement Disorders. 26(7):1197. doi: 10.1002/mds.23803. [DOI] [Google Scholar]
- Shune SE, Resnick B, Zarit SH, Namasivayam-MacDonald AM.. 2020. Creation and initial validation of the caregiver analysis of reported experiences with swallowing disorders (CARES) screening tool. American Journal of Speech-Language Pathology. 29(4):2131–2144. doi: 10.1044/2020_AJSLP-20-00148. [DOI] [PubMed] [Google Scholar]
- Spencer ES, Pitcher T, Veron G, Hannam T, MacAskill M, Anderson T, Dalrymple-Alford J, Carr AC.. 2020. Positive association of ascorbate and inverse association of urate with cognitive function in people with Parkinson’s disease. Antioxidants. 9(10):906. doi: 10.3390/antiox9100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger MJ, El-Debas T, Anderson T, Findley LJ, Marsden CD.. 1995. Double-blind study of the activity and tolerability of cabergoline versus placebo in Parkinsonians with motor fluctuations. Journal of Neurology. 243(1):68–72. doi: 10.1007/BF00878534. [DOI] [PubMed] [Google Scholar]
- The Global Parkinson’s Genetics Program . 2021. GP2: the global Parkinson’s genetics program. Movement Disorders. 36(4):842–851. doi: 10.1002/mds.28494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The RELEASE Collaborators . 2022. Dosage, intensity, and frequency of language therapy for aphasia: a systematic review–based, individual participant data network meta-analysis. Stroke. 53(3):956–967. doi: 10.1161/STROKEAHA.121.035216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallerga CL, Zhang F, Fowdar J, McRae AF, Qi T, Nabais MF, Zhang Q, Kassam I, Henders AK, Wallace L, et al.. 2020. Analysis of DNA methylation associates the cystine–glutamate antiporter SLC7A11 with risk of Parkinson’s disease. Nature Communications. 11(1):1238. doi: 10.1038/s41467-020-15065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stockum S, MacAskill MR, Anderson TJ.. 2011. Bottom-up effects modulate saccadic latencies in well-known eye movement paradigm. Psychological Research. 75:272–278. doi: 10.1007/s00426-010-0305-4. [DOI] [PubMed] [Google Scholar]
- van Stockum S, MacAskill MR, Anderson TJ.. 2012. Impairment of voluntary saccades and facilitation of reflexive saccades do not co-occur in Parkinson’s disease. Journal of Clinical Neuroscience. 19:1119–1124. doi: 10.1016/j.jocn.2011.10.014. [DOI] [PubMed] [Google Scholar]
- van Stockum S, MacAskill MR, Anderson TJ, Dalrymple-Alford JC.. 2008. Don’t look now or look away: two sources of saccadic disinhibition in Parkinson’s disease? Neuropsychologia. 46:3108–3115. doi: 10.1016/j.neuropsychologia.2008.07.002. [DOI] [PubMed] [Google Scholar]
- van Stockum S, MacAskill MR, Myall D, Anderson TJ.. 2013. A perceptual discrimination task results in greater facilitation of voluntary saccades in Parkinson’s disease patients. European Journal of Neuroscience. 37(1):163–172. doi: 10.1111/ejn.12033. [DOI] [PubMed] [Google Scholar]
- van Stockum S, MacAskill MR, Myall DJ, Anderson TJ.. 2011. A perceptual discrimination task abnormally facilitates reflexive saccades in Parkinson’s disease. European Journal of Neuroscience. 33:2091–2100. doi: 10.1111/j.1460-9568.2011.07697.x. [DOI] [PubMed] [Google Scholar]
- Waddell PA, Donaldson IM.. 1990. Behind the iron mask: the mental changes of Parkinson’s disease. New Zealand Medical Journal. 103(899):478–479. [PubMed] [Google Scholar]
- Wallis W. 1988. A progress report on the New Zealand multicentre Parkinson’s disease trial. A comparison of low-dose treatment with bromocriptine or L-dopa. European Neurology. 28(Suppl 1):9–10. http://europepmc.org/abstract/MED/3288479. [PubMed] [Google Scholar]
- Watts R. 2005. Advanced neurological magnetic resonance imaging. New Zealand Science Review. 62:26–27. [Google Scholar]
- Wood K-L, Myall DJ, Livingston L, Melzer TR, Pitcher TL, MacAskill MR, Geurtsen GJ, Anderson TJ, Dalrymple-Alford JC.. 2016. Different PD-MCI criteria and risk of dementia in Parkinson’s disease: 4-year longitudinal study. Npj Parkinson’s Disease. 2:15027. doi: 10.1038/npjparkd.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu Y, Curtis JA, Perry SE, Troche MS.. 2020. Relationship of vocal fold atrophy to swallowing safety and cough function in Parkinson’s disease. The Laryngoscope. 130(2):303–308. doi: 10.1002/lary.28158. [DOI] [PubMed] [Google Scholar]