ABSTRACT
The New Zealand Genetic Frontotemporal Dementia Study (FTDGeNZ) is an emerging longitudinal study of a large New Zealand pedigree with genetic frontotemporal dementia (FTD). Natural history studies of genetic FTD cohorts provide a unique opportunity to identify biomarkers of pre-symptomatic dementia, as carriers can be identified and studied decades before expected symptom onset. FTDGeNZ was established in 2016 with the aim of identifying the earliest pre-symptomatic biomarkers of FTD, in collaboration with international multi-centre cohorts. We enrolled 25 participants from a single family between April 2016 and August 2018. Participants were genotyped to determine whether they were pre-symptomatic carriers of the mutation (MAPT IVS 10 + 16 C > T), or non-carrier controls. Participants have undergone clinical assessments including neuropsychological and mood assessment; olfactory testing; assessment of social cognition; and blood collection for analyses of microRNA and protein fluid biomarkers annually. We have also performed structural and functional MRI of the brain and assessment of autobiographical memory biennially, and retinal imaging at baseline. Here, we describe the full study protocol and the baseline demographic and clinical characteristics of the FTDGeNZ cohort, and we highlight the latest findings in the field.
KEYWORDS: Behavioural frontotemporal dementia, frontotemporal lobar degeneration, young onset dementia, tau, biomarker
Introduction
Frontotemporal dementia (FTD) is an umbrella clinical term for a heterogeneous group of neurodegenerative disorders characterised by atrophy of the frontal and/or temporal lobes of the brain, accompanied by progressive deficits in behaviour, executive function, and/or language (Bang et al. 2015; Deleon and Miller 2018). FTD is a leading cause of young onset dementia (Teles Vieira et al. 2013), and accounts for approximately 3% of dementia across all ages (Hogan et al. 2016). Importantly, care partner quality of life is more severely affected by FTD than by other dementias (Riedijk et al. 2006; Liu et al. 2017). FTD includes a range of clinical, pathological, and genetic sub-types.
Clinically, FTD presents as one of three major syndromes: behavioural variant FTD (bvFTD), characterised by behavioural change with executive dysfunction (Rascovsky et al. 2011); non-fluent variant primary progressive aphasia (nfvPPA), characterised by agrammatism and motor speech deficits (Gorno-Tempini et al. 2011); or semantic variant PPA (svPPA), characterised by semantic aphasia (Gorno-Tempini et al. 2011). In some cases, symptoms overlap with amyotrophic lateral sclerosis (ALS) and the atypical Parkinsonism disorders progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS) (Lashley et al. 2015).
Pathologically, FTD is characterised by atrophy of cortical grey matter and axons in the frontal and/or anterior temporal lobes, known as frontotemporal lobar degeneration (FTLD), accompanied by neuronal and/or glial inclusion of abnormally folded proteins (Borroni et al. 2019). FTLD subtypes are categorised according to the pathological protein, which is either TAR DNA binding protein 43 (FTLD-TDP; (Mackenzie et al. 2011)); microtubule-associated protein tau (FTLD-Tau; (Dickson et al. 2011)); or RNA-binding protein fused in sarcoma (FTLD-FUS; (Neumann et al. 2009)). Very rarely, inclusions are positive for ubiquitin but negative for TDP-43, tau, and FUS; these cases are known as FTLD-UPS.
FTD is highly heritable: 30-50% of FTD cases have an autosomal dominant family history (Rohrer and Warren 2011). Of these cases, 60% have causative mutations in one of three genes (Olszewska et al. 2016): Chromosome 9 open reading frame 72 (C9orf72) (DeJesus-Hernandez et al. 2011); progranulin (GRN) (Baker et al. 2006); or microtubule-associated protein tau (MAPT) (Hutton et al. 1998). MAPT mutations cause FTLD-Tau; GRN and C9orf72 mutations cause FTLD-TDP. In sporadic FTD cases, particularly sporadic bvFTD, there is poor correlation between phenotype and pathology, hence it is often not possible to determine the underlying molecular pathology using clinical criteria (Josephs et al. 2011). MAPT mutations most commonly cause bvFTD; however, they can also cause PSP, PPA and CBS (Takada 2015). Rarely, patients with MAPT mutations present with an AD phenotype; however, the neuropathological diagnosis was not AD in any of these cases (Takada 2015). In New Zealand it is not common clinical practise for all FTD cases to be genetically screened. In general, FTD cases will not be genetically screened unless a family history is reported or suspected based on questioning.
There is currently no disease-modifying treatment for FTD, but clinical trials for therapies targeting the underlying pathology are underway (Boxer et al. 2020). The ultimate goal of these therapies is to intervene early in the disease process, before symptoms become burdensome. There is strong evidence that disease processes begin decades before symptom onset, presenting an opportunity to intervene during this window of minimal neuronal damage (Meeter et al. 2017). Thus, the major challenge for the field is to identify pre-symptomatic biomarkers of FTD, which would enable clinical trials to enrol appropriate participants at the ideal time, and/or assess therapeutic response (Boxer et al. 2020).
Much of the work on pre-symptomatic biomarkers of FTD involves longitudinal cohort studies of families with genetic FTD, because they are the ideal study population for detecting the earliest changes. These studies include the Genetic Frontotemporal dementia Initiative (GENFI) in Europe and Eastern Canada (genfi.org.uk), the ARTFL-LEFFTDS Longitudinal Frontotemporal Lobar Degeneration Study (ALLFTD) in the United States and Western Canada (www.allftd.org), the Dominantly Inherited Non-Alzheimer Dementias study (DINAD) in Australia, and the multi-partner consortium to expand dementia research in Latin America (ReDLat; Ibanez et al. (2021)). To date, researchers have identified a number of promising pre-symptomatic biomarkers of FTD, including imaging and fluid biomarkers (Table 1; reviewed in Meeter et al. 2017; Chen and Kantarci 2020). These studies have demonstrated the importance of longitudinal analyses, as even small-scale longitudinal studies have proven more sensitive than large cross-sectional studies (Meeter et al. 2017).
Table 1.
Candidate biomarkers identified in studies of pre-symptomatic frontotemporal dementia.
| Biomarker | Example references |
|---|---|
| Fluid biomarkers | |
| Plasma | |
| Progranulin* | Galimberti et al. (2018), Benussi et al. (2019) |
| microRNA | Kmetzsch et al. (2021) |
| Cerebrospinal fluid | |
| Dipeptide repeat proteins** | Lehmer et al. (2017) |
| poly(GP)** | Meeter et al. (2018) |
| Neuroimaging biomarkers | |
| White matter | |
| Decreased FA/increased diffusivity (DTI) | Dopper et al. (2013), Lee et al. (2017), Papma et al. (2017), Bertrand et al. (2018), Jiskoot et al. (2018), Olm et al. (2018), Borroni et al. (2008), Pievani et al. (2014) |
| Increased volume of hyperintensities (T2 FLAIR) | Benussi et al. (2019) |
| Grey matter | |
| Reduced cortical/subcortical volume (T1 Segmentation) | Bertrand et al. (2018), Olm et al. (2018), Rohrer et al. (2015), Popuri et al. (2018), Walhout et al. (2015) |
| Reduced grey matter intensity (T1 VBM) | Benussi et al. (2019), Lee et al. (2017), Papma et al. (2017), Olm et al. (2018), Panman et al. (2019), Spina et al. (2008), Morbelli et al. (2016), Cash et al. (2018) |
| Increased CSF (T1 VBM) | Spina et al. (2008) |
| Cortical thinning (T1 Surface reconstruction) | Pievani et al. (2014), Popuri et al. (2018), Panman et al. (2019), Moreno et al. (2013) |
| Metabolism | |
| Hypometabolism (FDG-PET) | Morbelli et al. (2016), Caroppo et al. (2015) |
| Metabolites (MRS) | Chen et al. (2019) |
| Perfusion | |
| Decreased rCBF (ASL) | Dopper et al. (2013), Dopper et al. (2016) |
| BOLD Signal | |
| Reduced connectivity (rs-fcMRI) | Dopper et al. (2013), Lee et al. (2017), Whitwell et al. (2011), Premi et al. (2014), Premi et al. (2014), Premi et al. (2016), Alexander et al. (2018) |
| Increased connectivity (rs-fcMRI) | Premi et al. (2014), Borroni et al. (2012) |
| Reduced activation (task fMRI – matrix reasoning) | Alexander et al. (2018) |
| Multimodal imaging | |
| Structure-function composite/model/classifier | Lee et al. (2017), Premi et al. (2016), Feis et al. (2019) |
| Neurophysiological biomarkers | |
| Altered intracortical connectivity (TMS) | Benussi et al. (2019) |
| Mood/behaviour biomarkers | |
| CBI-R | Benussi et al. (2019), Rohrer et al. (2015), Tavares et al. (2020) |
| Cognitive biomarkers† | |
| Global cognition | |
| MMSE, MoCA | Benussi et al. (2019), Rohrer et al. (2015), Spina et al. (2008), Barker et al. (2021), Cheran et al. (2019) |
| Language | |
| BNT, SAT, Category fluency, Semantic word-picture matching | Rohrer et al. (2015), Spina et al. (2008), Morbelli et al. (2016), Cheran et al. (2019), Jiskoot et al. (2018) |
| Attention/processing speed | |
| Digit forwards, Digit symbol, LDST, TMT-A, Stroop (word/colour reading) | Benussi et al. (2019), Papma et al. (2017), Rohrer et al. (2015), Spina et al. (2008), Morbelli et al. (2016), Barker et al. (2021), Cheran et al. (2019), Jiskoot et al. (2018) |
| Executive function | |
| COWA/Letter fluency, Design fluency, Digits backwards, FAB, Similarities, Stroop Inhibition, TMT-B, WCST (categories, perseveration) | Papma et al. (2017), Rohrer et al. (2015), Spina et al. (2008), Morbelli et al. (2016), Barker et al. (2021), Cheran et al. (2019), Jiskoot et al. (2018) |
| Memory | |
| Verbal memory (e.g. CVLT delayed, cued, recognition intrusions; Logical memory delayed; RAVLT immediate/delayed/recognition, Selective Reminding Test), Visual memory (e.g. Complex Figure delayed, VAT, Visual Reproduction recall) | Lee et al. (2017), Bertrand et al. (2018), Rohrer et al. (2015), Spina et al. (2008), Morbelli et al. (2016), Barker et al. (2021), Cheran et al. (2019), Jiskoot et al. (2018) |
| Visuospatial | |
| Block design, Complex figure copy, Visual reproduction | Rohrer et al. (2015), Spina et al. (2008), Morbelli et al. (2016) |
| Social Cognition | |
| Happé Cartoons (ToM, non-ToM), Emotion recognition (Ekman faces), Social Norms Questionnaire, IRI-PT, RSMS | Cheran et al. (2019), Jiskoot et al. (2018), Russell et al. (2020) |
| Neuro-ophthalmic biomarkers | |
| Retinal layer thinning | Ward et al. (2014) |
*GRN mutation carriers only; ** C9ORF72 mutation carriers only; †Reduced cognitive performance.
ASL: arterial spin labelling; BOLD: Blood oxygen level dependent; COWA: Controlled Oral Word Association; CBI-R: Cambridge Behavioural Inventory Revised; CSF: Cerebrospinal Fluid; DTI: diffusion tensor imaging; FA: Fractional anisotropy; FAB: Frontal Assessment Battery; FDG-PET: fMRI; functional magnetic resonance imaging; IRI-PT: Interpersonal Reactivity Index – Perspective Taking; LDST: Letter Digit Substitution Test; MMSE: Mini Mental State Examination; MoCA: Montreal Cognitive Assessment; MRI: magnetic resonance imaging; MRS: magnetic resonance spectroscopy; RAVLT: Rey Auditory Verbal Learning Test; rCBF: Regional cerebral blood flow; rs-fcMRI: resting state functional connectivity MRI; RSMS: Revised Self-Monitoring Scale; TMS: transcranial magnetic stimulation; TMT: Trail Making Test; ToM: Theory of Mind; VAT: Visual Association Test; VBM: Voxel based morphometry; WCST: Wisconsin Card Sort Test.
The New Zealand Genetic FTD Study (FTDGeNZ) is a longitudinal cohort study of a New Zealand family that is at risk of familial bvFTD (hereafter referred to as NZ-1). The mutation that causes FTD in this kindred is located in the MAPT gene (MAPT IVS 10 + 16 C > T) and causes FTLD-Tau pathology. This is one of over 60 pathogenic MAPT mutations (Greaves and Rohrer 2019) and is one of the most common, having been identified in at least 27 families worldwide (AD & FTD Mutation Database; www.molgen.vib-ua.be/FTDmutations).
We are prospectively following healthy family members of this kindred to investigate potential pre-symptomatic biomarkers, including imaging and fluid biomarkers, cognition and mood, olfaction, and retinal structure and function. FTDGeNZ is a member of the FTD Prevention Initiative (FPI; (Rohrer and Boxer 2021)), an international consortium of genetic FTD cohort studies.
Here, we present baseline demographic and clinical characteristics of the FTDGeNZ cohort and describe our longitudinal protocol for investigating potential pre-symptomatic biomarkers.
Materials and methods
Participants
Participants were recruited by contacting descendants of the NZ-1 proband’s maternal grandmother via a family liaison. The family had approached researchers at the University of Auckland some years after the proband’s brain was donated to the Neurological Foundation Human Brain Bank. Inclusion criteria included: (1) Biological relative of the NZ-1 proband; (2) Aged 25 or over (age of onset in the NZ-1 kindred ranges from 43–64 years (mean 53), so 25 represents approximately 30 years before expected symptom onset and is the earliest age at which we expect to detect pre-symptomatic changes.); (3) Fluent in English; and (4) Willing to undergo genetic testing to determine carrier status and longitudinal follow-up. Exclusion criteria included: history of (1) moderate-to-severe traumatic brain injury; (2) intellectual disability; (3) cerebral infarct/haemorrhagic stroke; or (4) other significant neurological conditions that could impact findings. Family members will continue to be recruited as they reach 25.
This study was approved by the New Zealand Health and Disability Ethics Committee (16/CEN/74). All participants provided written informed consent at study enrolment. Care was taken to avoid family members feeling pressured to participate because other family members were taking part; the Participant Information Sheet emphasised that decisions regarding participation would be kept confidential from other family members.
Participants nominated a Study Partner who knew them well enough to answer questions about their behaviour; a different Study Partner at each time-point was permitted. Written informed consent was provided by Study Partners by email when joining the study.
Genotyping
All participants are part of a known MAPT family. We specifically sequenced the region harbouring the known MAPT mutation (MAPT IVS 10 + 16 C > T; SNP ID: rs63751011). Genomic DNA was isolated for genotyping from 500 µL buffy coat using the Gentra PureGene Blood Kit (QIAGEN) and eluted in 300 μL DNA hydration solution. A 117-base pair sequence of the MAPT gene flanking the mutation site was amplified using customised primers and 0.1–0.2 µg DNA input. PCR amplicons were sequenced using Big Dye chemistry. Sequence products were run on an Applied Biosystem 3130XL Genetic Analyzer to verify the presence or absence of the mutation. Genotyping was validated using a TaqMan SNP Genotyping Assay specific to SNP ID: rs63751011 (Assay ID C__89363948_10). Data were analysed using QuantStudio Allelic Discrimination software. Clinical investigators were blinded to genetic status. The results of genotyping were not shared with participants. Participants were directed to Genetic Health Services New Zealand if they wished to undergo clinical predictive testing and asked not to disclose their genetic status to researchers. Details regarding mutation status of individuals have intentionally been excluded from this report to maintain confidentiality.
Clinical assessment (baseline; annually)
Standardised clinical assessments (detailed below) were used to determine symptoms of bvFTD according to established clinical criteria (Rascovsky et al. 2011). We defined asymptomatic as any family member who did not meet established clinical criteria for possible bvFTD (Rascovsky et al. 2011), Alzheimer’s disease (AD, (McKhann et al. 2011)), or PSP (Hoglinger et al. 2017); although carriers of the MAPT IVS 10 + 16 C > T mutation generally develop bvFTD, an AD or PSP phenotype has been reported (Morris et al. 2003; Doran et al. 2007; Larner 2008, 2009). Mild cognitive impairment (MCI) was determined according to established criteria for the clinical and cognitive syndrome (Albert et al. 2011).
Clinical and medical history
At baseline, clinical history covered cognitive functioning, social functioning, and behaviour, and medical history included current medications and allergies, substance use, mental health, education, and occupational history. Subsequent assessments focused on areas of change.
Physical and neurological examinations
The physical examination assessed weight, height, body mass index (BMI; kg/m2), supine/standing blood pressure and heart rate, and temperature. The neurological examination assessed cranial nerves, limbs, reflexes, sensation, and gait.
Neuropsychological assessment
The clinical neuropsychologist (C.I.) or consultant neurologist (K.L.B) assessed global cognition using Addenbrooke’s Cognitive Examination III (ACE-III; New Zealand version), a screening tool for cognitive deficits associated with dementia (cut-off score ≤82/100) and validated for use in FTD and AD (Hsieh et al. 2013). Neuropsychological tests assessing executive function, attention and processing speed, memory, and visuospatial abilities are listed in Table 2.
Table 2.
FTDGeNZ neuropsychological test battery.
| Cognitive domain | Measures | Reference |
|---|---|---|
| Global cognition | ACE-III | Hsieh et al. (2013) |
| Executive function | DKEFS Stroop inhibition | Delis et al. (2001) |
| Hayling Sentence Completion | Burgess and Shallice (1997) | |
| TMT Trails B | Reitan (1958) | |
| DKEFS verbal fluency: letter | Delis et al. (2001) | |
| WAIS-IV Digit Span: back | Wechsler et al. (2008) | |
| Attention and processing speed | DKEFS Stroop: colour naming & word reading | Delis et al. (2001) |
| TMT Trails A | Reitan (1958) | |
| WAIS-IV Digit Span: forward | Wechsler et al. (2008) | |
| Language | ||
| Naming, comprehension | SYD BAT naming and comprehension | Savage et al. (2013) |
| Boston Naming Test | Kaplan et al. (1983) | |
| Semantic | SYD BAT semantic | Savage et al. (2013) |
| DKEFS verbal fluency: category | Delis et al. (2001) | |
| Memory | ||
| Learning | RAVLT total | Schmidt (1996) |
| BVMT-R total | Benedict et al. (1996) | |
| Immediate recall | RAVLT immediate | Schmidt (1996) |
| RCFT immediate | Corwin and Bylsma (1993) | |
| Delayed recall | RAVLT delay | Schmidt (1996) |
| BVMT-R delay | Benedict et al. (1996) | |
| RCFT delay | Corwin and Bylsma (1993) | |
| Recognition | RAVLT recognition & false positive | Schmidt (1996) |
| BVMT-R recognition discrimination | Benedict et al. (1996) | |
| RCFT recognition | Corwin and Bylsma (1993) | |
| Prospective | RPA Prospective Memory Test | Radford et al. (2011) |
| Visuospatial | RCFT copy | Corwin and Bylsma (1993) |
ACE-III: Addenbrooke’s Cognitive Examination III; BVMT-R: Brief Visuospatial Memory Test Revised; DKEFS: Delis-Kaplan Executive Function System; RAVLT: Rey Auditory Verbal Learning Test; RCFT: Rey Complex Figure Test; RPA: Royal Prince Alfred; SYD BAT: Sydney Language Battery; TMT: Trail Making Test; WAIS-IV: Wechsler Adult Intelligence Scale Fourth Edition.
Mood and behaviour questionnaires
Participants completed the Hospital Anxiety and Depression Scale (HADS; Zigmond and Snaith 1983), a 14-item measure with anxiety and depression subscales; four items measuring outward irritability from the Irritability, Depression, Anxiety Scale (IDAS; Snaith et al. 1978); and the Apathy Evaluation Scale Self-rated (AES-S; Marin et al. 1991b), an 18-item measure of apathy.
Study partner questionnaires
Study Partners completed the Cambridge Behavioural Inventory Revised (CBI-R; Wear et al. 2008) to measure functional and/or behavioural changes in the previous month; and the Apathy Evaluation Scale Informant (AES-I; Marin et al. 1991a) to measure apathy in the previous month.
Social cognition (baseline; annually)
Toronto empathy questionnaire (TEQ)
This 16-item scale samples a wide range of attributes related to the emotional aspect of empathic responding, including emotional contagion, sensitivity, sympathetic physiological arousal, con-specific altruism and prosocial helping (Spreng et al. 2009).
Facial expressions of emotion task
This task measures the ability to identify emotions from subtle facial expressions (Buxton et al. 2013). For each grayscale photograph, participants selected from seven alternatives the word that best described the actor’s emotional state. Participants complete 52 trials: 8 exemplars of 6 emotions (angry, disgusted, afraid, happy, sad, surprise) at 2 difficulty levels (moderate and difficult) and 4 neutral exemplars.
Reading the mind in the eyes test
This test measures advanced theory of mind (ToM) abilities such as mental state attribution and recognition of complex emotions from grayscale facial photographs cropped to display only the eyes (Baron-Cohen et al. 2001). On each of 36 ToM trials, participants selected from four alternatives the mental state word that best described what the actor was thinking or feeling. Two control tasks were also completed: (1) viewing cropped facial photographs and selecting one of four options that best described the gender and gaze direction of the actor (e.g. female-left; 30 trials); and (2) viewing pictures of animals and selecting one of four adjectives that best described that animal stereotypically (11 trials; Harkness et al. (2005)).
Strange stories task
This advanced ToM task (Happé 1994) assesses the ability to infer the complex mental states and motivations that lead people to say things they do not mean literally (e.g. sarcasm). Participants read 8 ‘strange stories’ (ToM condition) and 8 ‘physical stories’ (control); following each, they answered a comprehension question and a justification question (‘Why did X say that?’). Answers to justification questions were scored for accuracy and usage of mental states versus mechanical/physical causes.
Olfactory testing (baseline; annually)
Olfactory identification was measured using the Alberta Smell Test, which involves naming 10 odorants using one nostril at a time (Heyanka et al. 2014). Impaired olfaction was defined as a score ≤ 2/10 in either nostril. We also measured olfactory identification using the University of Pennsylvania Smell Identification Test (UPSIT), a 40-item self-administered ‘scratch and sniff’ microencapsulated odorant test (Doty et al. 1989). Impaired olfaction was defined as a score corresponding to ‘microsmic’ or ‘anosmic’ according to age- and sex-related norms.
Blood collection, processing, and storage (baseline; annually)
Participants were asked to fast overnight prior to blood collection to avoid the potential effects of food intake on circulating biomarkers; fasting adherence was noted. A 20 mL whole blood sample was collected in 2 × 10 mL EDTA-coated vacutainer tubes. The site of venepuncture was the median cubital vein in the cubital fossa. Venepuncture was performed with the arm in a downward position, using a BD Vacutainer Safety-Lok blood collection set (21G or 23G). EDTA vacutainers were gently inverted 10 times following venepuncture and stored at 6°C for transport. Within 40 min of collection, blood samples were centrifuged at 2000 × g at 4°C for 10 min to isolate plasma. Plasma was stored in 1000 µL aliquots and buffy coat was stored in 750 µL aliquots in 1.7 mL Eppendorf tubes at −80°C. Isolated buffy coat from the first blood sample collected was used for DNA genotyping (as described above). Isolated plasma will be used to investigate potential microRNA and protein biomarkers in whole plasma and isolated extracellular vesicles. This protocol was designed so that an excess of blood is collected, allowing analysis of additional blood-derived markers if required.
Autobiographical memory (baseline; biennially)
Life story task adapted from Negele & Habermas (2010)
This task assesses autobiographical memory and narrative identity by asking participants to tell their life story from when they were born until the present day. Participants narrated their stories uninterrupted (without a time limit). Audio-recorded responses will be transcribed and coded to assess levels of autobiographical memory, autobiographical reasoning, coherence and use of cultural life scripts.
Adapted autobiographical interview
The adapted Autobiographical Interview (AI) assesses episodic abilities by quantifying the number and types of episodic and non-episodic details comprising participants’ descriptions of specific past and future events (Levine et al. 2002; Addis et al. 2008; Addis et al. 2009). In a remote past condition, participants remembered events from time periods sampling the entire lifespan (childhood, teens, early and middle adulthood, and last year); event cues were provided if required. In the near past and near future conditions, participants remembered five past and imagined five future events from the last/next few years in response to cue words. In all conditions, participants described each event for three minutes (audio-recorded for later scoring) with the interviewer using general probes as necessary, before rating aspects of event phenomenology (amount of detail, emotional intensity, personal significance, and visual perspective).
Neuroimaging (baseline; biennially)
Participants underwent a 75-min structural and functional MRI (fMRI) session in a 3-Tesla Siemens Skyra magnet. The following sequences were collected: (1) T1-weighted MP-RAGE; (2) pseudo-continuous Arterial Spin Labelling (pcASL); (3) T2 Flair; (4) T2* EPI (resting state; task-based). Resting state (8-min) was completed while viewing a fixation cross; immediately after this scan, participants rated their level of sleepiness. During six task-based fMRI scans (5.54-min each), participants completed two autobiographical tasks (remembering past or imagining future events from the last/next few years in response to single cue words; Addis et al. 2007) and a control task (sentence/definition task; Addis et al. 2009); 72 trials (24 per task) were interspersed with jittered fixation. On each trial, participants had 20 s to complete the task, and 5 s to rate the amount of detail generated (autobiographical tasks) or task difficulty (control task) using a hand-held response box. Immediately after scanning, participants dated each event generated in the scanner, briefly described it (later used by researchers to determine event specificity), and rated amount of detail, emotional intensity/valence, personal significance and visual perspective; future events were also rated for plausibility and similarity to previous experiences.
In all analyses, we take a whole-brain approach, complemented where appropriate with region-of-interest (ROI) analyses focusing on regions affected in bvFTD (e.g. salience network nodes; Zhang et al. 2022) as well as regions recruited by the autobiographical tasks (e.g. default mode network nodes; Benoit and Schacter 2015). Given the small Ns, a case-study approach will be employed (Streese and Tranel 2021) such that each carrier will be statistically compared to the non-carrier control group using Crawford’s modified t-tests (Crawford and Howell 2010) unless otherwise stated. Standard pre-processing and analysis pipelines will be used for all MRI data using standard software packages. fMRI data will be preprocessed using fmriprep (Esteban et al. 2019). fMRI data will be de-noised using ICA-AROMA (Pruim et al. 2015), and confounds (e.g. motion, signal drift etc.) regressed from fMRI time-series. Resting-state time-series will additionally be band-pass filtered (.01-.1 Hz) to isolate low-frequency signal fluctuations. For resting-state analyses, the conn toolbox (Whitfield-Gabrieli and Nieto-Castanon 2012, implemented in SPM12, https://www.fil.ion.ucl.ac.uk/spm/) will be used to extract time-series from nodes in seven whole-brain networks (Yeo et al. 2011). Correlation coefficients between all nodes within a network (except for self-connections) will be averaged to index within-network connectivity strength, and compared between each carrier and the non-carrier control group. Individual connections will be tested in networks that differ significantly, and/or in a priori ROIs. For task-related fMRI analyses, a multivariate technique – spatiotemporal partial least squares (PLS; McIntosh et al. 2004) – will be used to identify the whole-brain activation patterns associated with the autobiographical tasks (relative to the control task) as well as the regions functionally connected with a priori seed ROIs (e.g. hippocampus) during the tasks (for discussion of application of PLS to autobiographical tasks and patient groups, see Hach et al. 2014). These whole brain patterns related to task and/or seed regions will first be identified in the non-carrier control group. Brain scores – a weighted average denoting the degree to which any given participant expresses the brain pattern – will then be derived and compared between carriers and non-carriers, as will percent signal change extracted from a priori ROIs.
Cortical reconstruction and volumetric segmentation of T1 data will be performed with the Computational Anatomy Toolbox (cat12; Gaser et al. 2022; implemented in SPM12), and measures of volumetrics, cortical thinning and grey matter intensity will be compared between carriers and non-carriers in a priori ROIs. Lesion probability maps of white matter hyperintensities generated from the T2 Flair image using the lesion prediction algorithm (Schmidt et al. 2012; implemented in the LST toolbox, www.statistical-modelling.de/lst.html), and regional cerebral blood flow maps generated from pcASL images using BASIL – Bayesian Inference for Arterial Spin Labelling MRI (Chappell et al. 2009; implemented in FSL, https://fsl.fmrib.ox.ac.uk/) will be compared between carriers and non-carriers.
Lifestyle questionnaires (baseline)
Participants completed a self-reported Food Frequency Questionnaire (FFQ) as well as the Simple Lifestyle Indicator Questionnaire (SLIQ) which assesses various lifestyle factors. Data from these questionnaires will be used to investigate correlations between age of symptom onset and lifestyle factors.
Retinal structure and function (baseline)
Participants underwent a thorough non-invasive neuro-ophthalmic examination, including imaging of the optic nerve and retina with retinal photography, fundus autofluorescence, optical coherence tomography (OCT) and OCT-angiography (OCT-A).
Procedure
On the day of the annual clinical assessment, fasting blood samples were collected before 10 am. Clinical and medical history were assessed by a consultant neurologist (K.L.B.) and/or a registered clinical neuropsychologist (C.I.), both with FTD expertise and blind to genetic status. Participants underwent a physical and neurological examination (K.L.B), a neuropsychological assessment (C.I.), olfactory testing (B.R.), and completed questionnaires. Study Partners were not interviewed and did not attend assessments; questionnaires were emailed to them for completion.
Other study components were not necessarily conducted at the same time as the clinical assessment. The researcher assessing social cognition and autobiographical memory, and conducting the neuroimaging session (A.O.B.) and the registered optometrist conducting the neuro-ophthalmic examination (H.M.K.) were blind to genetic status and the results of the clinical assessments.
Statistical analyses of baseline cohort characteristics
Continuous variables were compared between carrier and non-carrier groups using two-sided Student’s t-tests; categorical variables were compared using Fisher’s exact test. Years from expected symptom onset at baseline was calculated by subtracting the mean age of clinical onset within the family (53 years) from the participant’s age at baseline.
Results
Demographic and clinical data at baseline
Twenty-five participants were recruited from the NZ-1 pedigree between April 2016 and August 2018. None of the family members met the exclusion criteria. Table 3 summarises the baseline demographic and clinical characteristics of the FTDGeNZ cohort. DNA genotyping assigned participants to either the mutation carrier (n = 6) or non-carrier (n = 19) group. At study entry, all participants (aged 25–78 years) were asymptomatic, and none met the criteria for MCI. At baseline, there were no statistically significant differences between mutation carriers and non-carriers regarding age, sex, education, CBI-R score, or ACE-III score (p-values > .061).
Table 3.
FTDGeNZ baseline demographic and clinical data.
| Mutation carriers (n = 6) |
Non-carriers (n = 19) |
p-value | |
|---|---|---|---|
| No. female (%) | 4 (67) | 6 (32) | .175 |
| Age (years) | 41 ± 11.7 | 44 ± 15.9 | .624 |
| Education (years) | 12.7 ± 1.6 | 14.4 ± 2.0 | .061 |
| Asymptomatic (%) | 6 (100) | 19 (100) | >.999 |
| Years from estimated onset | −12.5 ± 11.3 | – | – |
| CBI-R* | 16.0 ± 8.3 | 9.1 ± 8.1 | .152 |
| ACE-III | 91.2 ± 2.9 | 93.5 ± 5.2 | .192 |
*Baseline CBI-R was missing for 1 mutation carrier and 3 non-carriers.
ACE-III: Addenbrooke’s Cognitive Examination III; CBI-R: Cambridge Behavioural Inventory – Revised.
Data shown are count (percentage), or mean ± standard deviation. p-values calculated from Fisher’s exact test or Student’s t-test.
Baseline and follow-up assessments
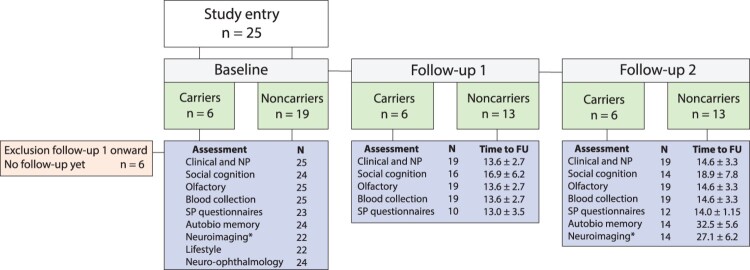
All participants have been followed prospectively with annual assessments (clinical assessment, social cognition, olfactory testing, blood collection). The final sample for the first data freeze (Baseline to Follow-up 2) comprised 19 participants (carriers: n = 6; non-carriers: n = 13; see Figure 1). Six participants have not yet completed follow-up assessments, but have not withdrawn from the study. At Follow-up 2, all participants were asymptomatic and none met clinical MCI criteria.
Figure 1.
FTDGeNZ assessments completed at baseline, follow-up 1 and follow-up 2. Time to follow-up (FU) is presented in months (mean ± SD). *N = 1 contraindicated for MRI. Autobio: autobiographical memory; NP: neurosychology; SP: study partner.
In addition to annual assessments, 24 participants took part in baseline assessment of autobiographical memory, and 22 completed the neuroimaging component (n = 1 was contraindicated for MRI). To date, 14 of these participants have completed biennial follow-up testing for autobiographical memory and neuroimaging. Twenty-four participants have undergone baseline neuro-ophthalmic assessment (Kersten et al. 2019) and follow-up neuro-ophthalmic assessments are planned.
Discussion
FTDGeNZ is a longitudinal cohort study of a New Zealand family with genetic FTD. We have collected data for longitudinal analysis of a variety of potential pre-symptomatic biomarkers, some of which are being investigated by similar international studies, and some of which are novel. Thus far, we have conducted clinical assessments, tested olfaction and social cognition, and collected plasma samples at baseline and two annual follow-up time-points. In addition, autobiographical memory tests and neuroimaging were completed at baseline and a biennial follow-up, and neuro-ophthalmic structure and function was assessed at baseline. Together, these data provide a rich multimodal dataset that will make a unique contribution to the international search for pre-symptomatic biomarkers of FTD.
International studies of genetic FTD cohorts have demonstrated the utility of imaging, cognitive, and fluid biomarkers to detect pre-symptomatic FTD (reviewed in Meeter et al. 2017; Chen and Kantarci 2020). Due to the relative rarity of genetic FTD, harmonisation of international datasets is critical to increase statistical power and validate these findings. FTDGeNZ is therefore collecting longitudinal data for the NZ-1 cohort consistent with international efforts: a comprehensive neuropsychological battery including the Boston Naming Test and CBI-R, which have been shown to detect impairment 10 years before expected symptom onset in MAPT mutation carriers (Rohrer et al. 2015), neuroimaging, and blood plasma collection. Our future analyses of these types of data will allow comparison of FTDGeNZ data to international cohorts.
We are also investigating several novel biomarkers not previously explored in pre-symptomatic MAPT cohorts. One is olfaction, given it is impaired before symptom onset in other neurodegenerative diseases (Fullard et al. 2017; Winchester and Martyn 2020). Surprisingly, it has not yet been studied in pre-symptomatic FTD despite reports that odour identification deficits are prevalent in symptomatic FTD (Pardini et al. 2009; Tonacci and Billeci 2018). Even if the specificity of olfactory impairment as a biomarker is low, it may be improved by the inclusion of additional biomarkers. We are also investigating plasma microRNAs as a potential biomarker. Circulating microRNA profiles can discriminate symptomatic FTD from controls (Denk et al. 2018; Piscopo et al. 2018; Grasso et al. 2019) and specific microRNAs are dysregulated in post-mortem human FTD brain (Kocerha et al. 2011; Chen-Plotkin et al. 2012; Hebert et al. 2013; Gascon et al. 2014) and iPSC-derived neurons from FTD patients (Zhang et al. 2013; Gascon et al. 2014). Dysregulation of plasma microRNAs was only recently reported in a cross-sectional analysis of C9ORF72 mutation carriers, in both the symptomatic and pre-symptomatic stage (Kmetzsch et al. 2021), suggesting its sensitivity as a biomarker.
Moreover, we have collected longitudinal social cognition data that may provide markers of early subtle changes in socioemotional functioning. Although one of the most prominent impairments in symptomatic FTD (Gregory et al. 2002; Adenzato et al. 2010; Kumfor and Piguet 2012), pre-symptomatic deficits are either not evident or not specific to socioemotional conditions (Jiskoot et al. 2016; Jiskoot et al. 2018), or only evident at a late pre-symptomatic stage (Russell et al. 2020). Our use of moderate-to-difficult emotional expressions rather than prototypical ones, as well as advanced ToM abilities, therefore holds promise for detecting subtle changes at even earlier pre-symptomatic stages.
Data collected at future time-points in the FTDGeNZ study will allow longitudinal analyses of additional novel biomarkers. Behavioural performance on episodic autobiographical memory tasks will be combined with functional neuroimaging to build on emerging evidence of hippocampal atrophy in pre-symptomatic disease (Rohrer et al. 2015) as well as episodic deficits in both pre-symptomatic (Barker et al. 2021) and symptomatic FTD (Irish et al. 2013), with the aim of determining whether episodic deficits are mediated by prefrontal and/or medial temporal dysfunction. Other forms of episodic memory have been investigated in pre-symptomatic FTD (Jiskoot et al. 2016; Cheran et al. 2019; Barker et al. 2021; Poos et al. 2020). However, autobiographical memory, a more ecologically valid form of episodic memory, has not been explored. The advantage of assessing autobiographical memory is that it enables a more sensitive examination of episodic memory: autobiographical memory tasks require more effortful cognitive coordination to successfully retrieve the personal event and recombine it with associated temporal, sensory, and emotional content (Conway and Pleydell-Pearce, 2000).
Neuro-ophthalmic findings are potentially useful as biomarkers of neurodegenerative disease (Kersten et al. 2014). OCT and OCT-A assessment allow for the non-invasive visualisation and quantitative analysis of retinal layers and retinal vasculature respectively. Based on evidence of symptomatic (Ferrari et al. 2017; Kim et al. 2017, 2019) and pre-symptomatic (Ward et al. 2014) retinal thinning in FTD we have collected data on neuro-ophthalmic structure and function in this cohort, including the first investigation of retinal perfusion in a symptomatic or pre-symptomatic FTD cohort. Participants undergo a complete neuro-ophthalmic assessment including visual acuity, pupil assessment, colour vision, perimetry, slit-lamp biomicroscopy, intraocular pressure, and dilated fundus examination so that potentially confounding ocular pathology can be detected, and only patient eyes with no evidence of ocular disease are included. One eye from each participant is used in analyses. At baseline, we have previously reported that there were no statistically significant differences between carriers and controls for macular or peripapillary OCT or OCT-A measures, including retinal nerve fibre layer thickness, ganglion cell complex (ganglion cell layer + inner plexiform layer) thickness, total retinal thickness and retinal vessel density (Kersten et al. 2019). Although we did not detect neuro-ophthalmic changes at baseline, our planned longitudinal analyses may detect change over time. It is interesting to note that OCT findings in FTD have been variable, with reduced global, outer or inner retinal layer thickness measurements all being reported across published studies. This could be due to differences in demographics, diagnosis, OCT-methodology, or image processing techniques.
In addition to novel biomarkers, another major strength of FTDGeNZ is the focus on a single mutation in a single cohort, with the inclusion of biologically related non-carrier controls allowing us to control for potential genetic and environmental confounders. Further, we have established a cohort of participants willing to undergo repeated assessments, and none have withdrawn from the study. FTDGeNZ is limited by a relatively small sample size, but nevertheless our findings can still identify candidate biomarkers to validate in larger cohorts.
In summary, FTDGeNZ is an emerging longitudinal study that is well-placed to contribute to the international search for pre-symptomatic biomarkers of FTD. These biomarkers are critical to the success of future clinical trials aimed at preventing or treating FTD.
Acknowledgements
The authors would like to thank the Neurological Foundation Human Brain Bank, and the participants and their families for taking part in the FTDGeNZ study.
Funding Statement
This work was supported by the Health Research Council of New Zealand [grant number 18/382], the Auckland Medical Research Foundation [grant number 3715182], Brain Research New Zealand Rangahau Roro Aotearoa [grant numbers 3709946, 3709932 and 3717693], the Kelliher Charitable Trust [grant number 3716229] and the University of Auckland Faculty Research Development Fund [grant number 3714135]. D.R.A. is supported by the Canada 150 Research Chairs Program.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL.. 2009a. Constructive episodic simulation of the future and the past: distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 47(11):2222–2238. [DOI] [PubMed] [Google Scholar]
- Addis DR, Sacchetti DC, Ally BA, Budson AE, Schacter DL.. 2009b. Episodic simulation of future events is impaired in mild Alzheimer's disease. Neuropsychologia. 47(12):2660–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL.. 2007. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 45(7):1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL.. 2008. Age-related changes in the episodic stimulation of future events. Psychol Sci. 19(1):33–41. [DOI] [PubMed] [Google Scholar]
- Adenzato M, Cavallo M, Enrici I.. 2010. Theory of mind ability in the behavioural variant of frontotemporal dementia: an analysis of the neural, cognitive, and social levels. Neuropsychologia. 48(1):2–12. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, et al. . 2011. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander C, Zeithamova D, Hsiung GR, Mackenzie IR, Jacova C.. 2018. Decreased prefrontal activation during matrix reasoning in predementia progranulin mutation carriers. J Alzheimers Dis. 62(2):583–589. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, et al. . 2006. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 442(7105):916–919. [DOI] [PubMed] [Google Scholar]
- Bang J, Spina S, Miller BL.. 2015. Frontotemporal dementia. The Lancet. 386(10004):1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker MS, Manoochehri M, Rizer SJ, Appleby BS, Brushaber D, Dev SI, Devick KL, Dickerson BC, Fields JA, Foroud TM, et al. . 2021. Recognition memory and divergent cognitive profiles in prodromal genetic frontotemporal dementia. Cortex. 139:99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I.. 2001. The “reading the mind in the eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psych. 42(2):241–251. [PubMed] [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B.. 1996. Revision of the brief visuospatial memory test: studies of normal performance, reliability, and validity. Psychol Assess. 8(2):145–153. [Google Scholar]
- Benoit RG, Schacter DL.. 2015. Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia. 75:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi A, Gazzina S, Premi E, Cosseddu M, Archetti S, Dell'Era V, Cantoni V, Cotelli MS, Alberici A, Micheli A.. 2019. Clinical and biomarker changes in presymptomatic genetic frontotemporal dementia. Neurobiol Aging. 76:133–140. [DOI] [PubMed] [Google Scholar]
- Bertrand A, Wen J, Rinaldi D, Houot M, Sayah S, Camuzat A, Fournier C, Fontanella S, Routier A, Couratier P, et al. . 2018. Early cognitive, structural, and microstructural changes in presymptomatic C9orf72 carriers younger than 40 years. JAMA Neurol. 75(2):236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B, Alberici A, Buratti E.. 2019. Review: molecular pathology of frontotemporal lobar degenerations. Neuropathol Appl Neurobiol. 45(1):41–57. [DOI] [PubMed] [Google Scholar]
- Borroni B, Alberici A, Cercignani M, Premi E, Serra L, Cerini C, Cosseddu M, Pettenati C, Turla M, Archetti S, et al. . 2012. Granulin mutation drives brain damage and reorganization from preclinical to symptomatic FTLD. Neurobiol Aging. 33(10):2506–2520. [DOI] [PubMed] [Google Scholar]
- Borroni B, Alberici A, Premi E, Archetti S, Garibotto V, Agosti C, Gasparotti R, Di Luca M, Perani D, Padovani A.. 2008. Brain magnetic resonance imaging structural changes in a pedigree of asymptomatic progranulin mutation carriers. Rejuvenation Res. 11(3):585–595. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Gold M, Feldman H, Boeve BF, Dickinson SL, Fillit H, Ho C, Paul R, Pearlman R, Sutherland M, et al. . 2020. New directions in clinical trials for frontotemporal lobar degeneration: Methods and outcome measures. Alzheimers Dement. 16(1):131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Shallice T.. 1997. The Hayling and Brixton tests. Thurston: Thames Valley Test Company. [Google Scholar]
- Buxton SL, MacDonald L, Tippett LJ.. 2013. Impaired recognition of prosody and subtle emotional facial expressions in Parkinson’s disease. Behav Neurosci. 127(2):193–203. [DOI] [PubMed] [Google Scholar]
- Caroppo P, Habert MO, Durrleman S, Funkiewiez A, Perlbarg V, Hahn V, Bertin H, Gaubert M, Routier A, Hannequin D, et al. . 2015. Lateral temporal lobe: an early imaging marker of the presymptomatic GRN disease? J Alzheimers Dis. 47(3):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash DM, Bocchetta M, Thomas DL, Dick KM, van Swieten JC, Borroni B, Galimberti D, Masellis M, Tartaglia MC, Rowe JB, et al. . 2018. Patterns of gray matter atrophy in genetic frontotemporal dementia: results from the GENFI study. Neurobiol Aging. 62:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell MA, Groves AR, Whitcher B, et al. . 2009. Variational Bayesian inference for a nonlinear forward model. IEEE Transactions on Signal Processing. 57:223–236. [Google Scholar]
- Chen Q, Boeve BF, Tosakulwong N, Lesnick T, Brushaber D, Dheel C, Fields J, Forsberg L, Gavrilova R, Gearhart D, et al. . 2019. Frontal lobe (1)H MR spectroscopy in asymptomatic and symptomatic MAPT mutation carriers. Neurology. 93(8):e758–e765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Kantarci K.. 2020. Imaging biomarkers for neurodegeneration in presymptomatic familial frontotemporal lobar degeneration. Front Neurol. 11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Plotkin AS, Unger TL, Gallagher MD, Bill E, Kwong LK, Volpicelli-Daley L, Busch JI, Akle S, Grossman M, Van Deerlin V.. 2012. TMEM106B, the risk gene for frontotemporal dementia, is regulated by the microRNA-132/212 cluster and affects progranulin pathways. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 32(33):11213–11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheran G, Wu L, Lee S, Manoochehri M, Cines S, Fallon E, Lynch T, Heidebrink J, Paulson H, Goldman J, et al. . 2019. Cognitive indicators of preclinical behavioral variant frontotemporal dementia in MAPT carriers. J Int Neuropsychol Soc. 25(2):184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW.. 2000. The construction of autobiographical memories in the self-memory system. Psychol Rev. 107(2):261–288. [DOI] [PubMed] [Google Scholar]
- Corwin J, Bylsma FW.. 1993. Psychological examination of traumatic encephalopathy. Clinical Neuropsychologist. 7(1):3–21. [Google Scholar]
- Crawford JR, Howell DC.. 2010. Comparing an individual’s test score against norms derived from small samples. Clinical Neuropsychologist. 12:482–486. [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J.. 2011. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 72(2):245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleon J, Miller BL.. 2018. Frontotemporal dementia. Handb Clin Neurol. 148:409–430. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH.. 2001. The Delis-Kaplan executive function system: examiner’s manual. San Antonio: The Psychological Corporation. [Google Scholar]
- Denk J, Oberhauser F, Kornhuber J, Wiltfang J, Fassbender K, Schroeter ML, Volk AE, Diehl-Schmid J, Prudlo J, Danek A, et al. . 2018. Specific serum and CSF microRNA profiles distinguish sporadic behavioural variant of frontotemporal dementia compared with Alzheimer patients and cognitively healthy controls. PLoS One. 13(5):e0197329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Kouri N, Murray ME, Josephs KA.. 2011. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci. 45(3):384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopper EG, Chalos V, Ghariq E, den Heijer T, Hafkemeijer A, Jiskoot LC, de Koning I, Seelaar H, van Minkelen R, van Osch MJ.. 2016. Cerebral blood flow in presymptomatic MAPT and GRN mutation carriers: A longitudinal arterial spin labeling study. Neuroimage Clin. 12:460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopper EGP, Rombouts SARB, Jiskoot LC, den Heijer T, de Graaf JRA, Hammerschlag AR, Seelar H, Seeley WW, Veer IM, van Buchem MA.. 2013. Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology. 80:814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran M, du Plessis DG, Ghadiali EJ, Mann DMA, Pickering-Brown S, Larner AJ.. 2007. Familial early-onset dementia with tau intron 10 + 16 mutation with clinical features similar to those of Alzheimer disease. Arch Neurol. 64(10):1535–1539. [DOI] [PubMed] [Google Scholar]
- Doty RL, Frye RE, Agrawal Y.. 1989. Internal consistency reliability of the fractionated and whole University of Pennsylvania smell identification test. Perception & Psychophysics. 45(4):381–384. [DOI] [PubMed] [Google Scholar]
- Esteban O, Markiewicz CJ, Blair RW.. 2019. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 16:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feis RA, Bouts M, de Vos F, Schouten TM, Panman JL, Jiskoot LC, Dopper EGP, van der Grond J, van Swieten JC, Rombouts S.. 2019. A multimodal MRI-based classification signature emerges just prior to symptom onset in frontotemporal dementia mutation carriers. J Neurol Neurosurg Psychiatry. 90(11):1207–1214. [DOI] [PubMed] [Google Scholar]
- Ferrari L, Huang SC, Magnani G, Ambrosi A, Comi G, Leocani L.. 2017. Optical coherence tomography reveals retinal neuroaxonal thinning in frontotemporal dementia as in Alzheimer’s disease. J Alzheimers Dis. 56(3):1101–1107. [DOI] [PubMed] [Google Scholar]
- Fullard ME, Morley JF, Duda JE.. 2017. Olfactory dysfunction as an early biomarker in Parkinson’s disease. Neurosci Bull. 33(5):515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti D, Fumagalli GG, Fenoglio C, Cioffi SMG, Arighi A, Serpente M, Borroni B, Padovani A, Tagliavini F, Masellis M, et al. . 2018. Progranulin plasma levels predict the presence of GRN mutations in asymptomatic subjects and do not correlate with brain atrophy: results from the GENFI study. Neurobiol Aging. 62(245):e249–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon E, Lynch K, Ruan H, Almeida S, Verheyden JM, Seeley WW, Dickson DW, Petrucelli L, Sun D, Jiao J, et al. . 2014. Alterations in microRNA-124 and AMPA receptors contribute to social behavioral deficits in frontotemporal dementia. Nat Med. 20(12):1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Dahnke R, Thompson PM.. 2022. CAT – a computational anatomy toolbox for the analysis of structural MRI data. bioRxiv. [DOI] [PMC free article] [PubMed]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve B, et al. . 2011. Classification of primary progressive aphasia and its variants. Neurology. 76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso M, Piscopo P, Talarico G, Ricci L, Crestini A, Tosto G, Gasparini M, Bruno G, Denti MA, Confaloni A.. 2019. Plasma microRNA profiling distinguishes patients with frontotemporal dementia from healthy subjects. Neurobiol Aging. 84(240):e241–e240. [DOI] [PubMed] [Google Scholar]
- Greaves CV, Rohrer JD.. 2019. An update on genetic frontotemporal dementia. J Neurol. 266(8):2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, Hodges JR.. 2002. Theory of mind in patients with frontal variantfrontotemporal dementia and Alzheimer’s disease: theoretical and practical implications. Brain. 125:725–764. [DOI] [PubMed] [Google Scholar]
- Hach S, Tippett LJ, Addis DR.. 2014. Neural changes associated with the generation of specific past and future events in depression. Neuropsychologia. 65:41–55. [DOI] [PubMed] [Google Scholar]
- Happé FGE. 1994. An advanced test of theory of mind: understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. Autism Dev Disord. 24(2):129–154. [DOI] [PubMed] [Google Scholar]
- Harkness K, Sabbagh M, Jacobson J, Chowdrey N, Chen T.. 2005. Enhanced accuracy of mental state decoding in dysphoric college students. Cognition & Emotion. 19(7):999–1025. [Google Scholar]
- Hebert SS, Wang WX, Zhu Q, Nelson PT.. 2013. A study of small RNAs from cerebral neocortex of pathology-verified Alzheimer’s disease, dementia with Lewy bodies, hippocampal sclerosis, frontotemporal lobar dementia, and non-demented human controls. J Alzheimers Dis. 35(2):335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyanka DJ, Golden CJ, McCue RB, Scarisbrick DM, Linck JF, Zlatkin NI.. 2014. Olfactory deficits in frontotemporal dementia as measured by the Alberta Smell test. Applied Neuropsychology Adult. 21(3):176–182. [DOI] [PubMed] [Google Scholar]
- Hogan DB, Jette N, Fiest KM, Roberts JI, Pearson D, Smith EE, Roach P, Kirk A, Pringsheim T, Maxwell CJ.. 2016. The prevalence and incidence of frontotemporal dementia: a systematic review. Can J Neurol Sci. 43(Suppl. 1):S96–S109. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Muller U, Nilsson C, Whitwell JL, et al. . 2017. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 32(6):853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR.. 2013. Validation of the Addenbrooke’s cognitive examination III in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord. 36(3-4):242–250. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, et al. . 1998. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 393:702–705. [DOI] [PubMed] [Google Scholar]
- Ibanez A, Yokoyama JS, Possin KL, Matallana D, Lopera F, Nitrini R, Takada LT, Custodio N, Sosa Ortiz AL, Avila-Funes JA, et al. . 2021. The multi-Partner consortium to expand dementia Research in Latin America (ReDLat): driving multicentric research and implementation science. Front Neurol. 12:631722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish M, Devenney E, Wong S, Dobson-Stone C, Kwok JB, Piguet O, Hodges JR, Hornberger M.. 2013. Neural substrates of episodic memory dysfunction in behavioural variant frontotemporal dementia with and without C9ORF72 expansions. Neuroimage Clin. 2:836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiskoot LC, Bocchetta M, Nicholas JM, Cash DM, Thomas D, Modat M, Ourselin S, Rombouts S, Dopper EGP, Meeter LH, et al. . 2018a. Presymptomatic white matter integrity loss in familial frontotemporal dementia in the GENFI cohort: a cross-sectional diffusion tensor imaging study. Ann Clin Transl Neurol. 5(9):1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiskoot LC, Dopper EGP, Den Heijer T, Timman R, Van Minkelen R, van swieten JC, Papma JM.. 2016. Presymptomatic cognitive decline in familial frontotemporal dementia: a longitudinal study. Neurology. 87:384–391. [DOI] [PubMed] [Google Scholar]
- Jiskoot LC, Panman JL, van Asseldonk L, Franzen S, Meeter LHH, Donker Kaat L, van der Ende EL, Dopper EGP, Timman R, van Minkelen R, et al. . 2018b. Longitudinal cognitive biomarkers predicting symptom onset in presymptomatic frontotemporal dementia. J Neurol. 265(6):1381–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, Mann DM, Dickson DW.. 2011. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol. 122(2):137–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S.. 1983. The Boston naming test. Philadelphia: Lea & Fibiger. [Google Scholar]
- Kersten H, Ryan B, Brickell KL, Ilse C, Vaghefi E, Addis DR, Tippett L, Curtis MA, Danesh-Meyer H.. 2019. The New Zealand Genetic Frontotemporal Dementia Study (FTDGeNZ): baseline retinal characteristics. Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO).
- Kersten HM, Roxburgh RH, Danesh-Meyer HV.. 2014. Ophthalmic manifestations of inherited neurodegenerative disorders. Nat Rev Neurol. 10(6):349–362. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Grossman M, Song D, Saludades S, Pan W, Dominguez-Perez S, Dunaief JL, Aleman TS, Ying GS, Irwin DJ.. 2019. Persistent and progressive outer retina thinning in frontotemporal degeneration. Front Neurosci. 13:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Irwin DJ, Song D, Ebenezer D, Leveque J, Raquib AR, Pan W, Ying G-S, Aleman TS, Dunaief JL, et al. . 2017. Optical coherence tomography identifiesouter retina thinning in frontotemporaldegeneration. Neurology. 89:1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmetzsch V, Anquetil V, Saracino D, Rinaldi D, Camuzat A, Gareau T, Jornea L, Forlani S, Couratier P, Wallon D, et al. . 2021. Plasma microRNA signature in presymptomatic and symptomatic subjects with C9orf72-associated frontotemporal dementia and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 92(5):485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocerha J, Kouri N, Baker M, Finch N, DeJesus-Hernandez M, Gonzalez J, Chidamparam K, Josephs KA, Boeve BF, Graff-Radford NR, et al. . 2011. Altered microRNA expression in frontotemporal lobar degeneration with TDP-43 pathology caused by progranulin mutations. BMC Genomics. 12:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfor F, Piguet O.. 2012. Disturbance of emotion processing in frontotemporal dementia: a synthesis of cognitive and neuroimaging findings. Neuropsychol Rev. 22(3):280–297. [DOI] [PubMed] [Google Scholar]
- Larner AJ. 2008. Mutation negative early-onset familial Alzheimer disease: consider screening for tau gene mutations. Alzheimer Dis Assoc Disord. 22(2):194–195. [DOI] [PubMed] [Google Scholar]
- Larner AJ. 2009. A 50-year-old man with deteriorating cognitive function and impaired movement. PLoS Medicine. 6(1):e1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley T, Rohrer JD, Mead S, Revesz T.. 2015. Review: an update on clinical, genetic and pathological aspects of frontotemporal lobar degenerations. Neuropathol Appl Neurobiol. 41(7):858–881. [DOI] [PubMed] [Google Scholar]
- Lee SE, Sias AC, Mandelli ML, Brown JA, Brown AB, Khazenzon AM, Vidovszky AA, Zanto TP, Karydas AM, Pribadi M, et al. . 2017. Network degeneration and dysfunction in presymptomatic C9ORF72 expansion carriers. Neuroimage Clin. 14:286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmer C, Oeckl P, Weishaupt JH, Volk AE, Diehl-Schmid J, Schroeter ML, Lauer M, Kornhuber J, Levin J, Fassbender K, et al. . 2017. Poly-GP in cerebrospinal fluid links C9orf72-associated dipeptide repeat expression to the asymptomatic phase of ALS/FTD. EMBO Mol Med. 9(7):859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M.. 2002. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychology and Aging. 17(4):677–689. [PubMed] [Google Scholar]
- Liu S, Jin Y, Shi Z, Huo YR, Guan Y, Liu M, Liu S, Ji Y.. 2017. The effects of behavioral and psychological symptoms on caregiver burden in frontotemporal dementia, Lewy body dementia, and Alzheimer's disease: clinical experience in China. Aging Ment Health. 21(6):651–657. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM.. 2011. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 122(1):111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S.. 1991a. Reliability and validity of the apathy evaluation scale. Psychiatry Research. 38:143–162. [DOI] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S.. 1991b. Reliability and validity of the apathy evaluation scale. Psychiat Res. 38(2):143–162. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Chau WK, Protzner AB.. 2004. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 23:764–775. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R.. 2011. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeter LH, Kaat LD, Rohrer JD, van Swieten JC.. 2017. Imaging and fluid biomarkers in frontotemporal dementia. Nat Rev Neurol. 13(7):406–419. [DOI] [PubMed] [Google Scholar]
- Meeter LHH, Gendron TF, Sias AC, Jiskoot LC, Russo SP, Donker Kaat L, Papma JM, Panman JL, van der Ende EL, Dopper EG, et al. . 2018. Poly(GP), neurofilament and grey matter deficits in C9orf72 expansion carriers. Ann Clin Transl Neurol. 5(5):583–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morbelli S, Ferrara M, Fiz F, Dessi B, Arnaldi D, Picco A, Bossert I, Buschiazzo A, Accardo J, Picori L, et al. . 2016. Mapping brain morphological and functional conversion patterns in predementia late-onset bvFTD. Eur J Nucl Med Mol Imaging. 43(7):1337–1347. [DOI] [PubMed] [Google Scholar]
- Moreno F, Sala-Llonch R, Barandiaran M, Sanchez-Valle R, Estanga A, Bartres-Faz D, Sistiaga A, Alzualde A, Fernandez E, Marti Masso JF, et al. . 2013. Distinctive age-related temporal cortical thinning in asymptomatic granulin gene mutation carriers. Neurobiol Aging. 34(5):1462–1468. [DOI] [PubMed] [Google Scholar]
- Morris HR, Osaki Y, Holton J, Lees AJ, Wood NW, Revesz T, Quinn N.. 2003. Tau exon 10 + 16 mutation FTDP-17 presenting clinically as sporadic young onset PSP. Neurology. 61:102–104. [DOI] [PubMed] [Google Scholar]
- Negele A, Habermas T.. 2010. Self-continuity across developmental change in and of repeated life narratives. In: Mclean K, Pasupathi M, editors. Narrative Development in Adolescence. Advancing Responsible Adolescent Development. Springer. [Google Scholar]
- Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IR.. 2009. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 132(Pt 11):2922–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olm CA, McMillan CT, Irwin DJ, Van Deerlin VM, Cook PA, Gee JC, Grossman M.. 2018. Longitudinal structural gray matter and white matter MRI changes in presymptomatic progranulin mutation carriers. Neuroimage Clin. 19:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewska DA, Lonergan R, Fallon EM, Lynch T.. 2016. Genetics of frontotemporal dementia. Curr Neurol Neurosci Rep. 16(12):107. [DOI] [PubMed] [Google Scholar]
- Panman JL, Jiskoot LC, Bouts M, Meeter LHH, van der Ende EL, Poos JM, Feis RA, Kievit AJA, van Minkelen R, Dopper EGP, et al. . 2019. Gray and white matter changes in presymptomatic genetic frontotemporal dementia: a longitudinal MRI study. Neurobiol Aging. 76:115–124. [DOI] [PubMed] [Google Scholar]
- Papma JM, Jiskoot LC, Panman JL, Dopper EG, Den Heijer T, Kaat LD, Pijnenburg YAL, Meeter LH, Van Minkelen R, Rombouts SARB, et al. . 2017. Cognition and gray and white matter characteristics of presymptomatic C9orf 72 repeat expansion. Neurology. 89:1256–1264. [DOI] [PubMed] [Google Scholar]
- Pardini M, Huey ED, Cavanagh AL, Grafman J.. 2009. Olfactory function in corticobasal syndrome and frontotemporal dementia. Arch Neurol. 66(1):92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pievani M, Paternico D, Benussi L, Binetti G, Orlandini A, Cobelli M, Magnaldi S, Ghidoni R, Frisoni GB.. 2014. Pattern of structural and functional brain abnormalities in asymptomatic granulin mutation carriers. Alzheimers Dement. 10(Suppl. 5):S354–S363. [DOI] [PubMed] [Google Scholar]
- Piscopo P, Grasso M, Puopolo M, D'Acunto E, Talarico G, Crestini A, Gasparini M, Campopiano R, Gambardella S, Castellano AE, et al. . 2018. Circulating miR-127-3p as a potential biomarker for differential diagnosis in frontotemporal dementia. J Alzheimers Dis. 65(2):455–464. [DOI] [PubMed] [Google Scholar]
- Poos JM, Jiskoot LC, Leijdesdorff SMJ, Seelaar H, Panman JL, van der Ende EL, Mol MO, Meeter LHH, Pijnenburg YAL, Donker Kaat L, et al. . 2020. Cognitive profiles discriminate between genetic variants of behavioral frontotemporal dementia. J Neurol. 267(6):1603–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popuri K, Dowds E, Beg MF, Balachandar R, Bhalla M, Jacova C, Buller A, Slack P, Sengdy P, Rademakers R, et al. . 2018. Gray matter changes in asymptomatic C9orf72 and GRN mutation carriers. Neuroimage Clin. 18:591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premi E, Cauda F, Costa T, Diano M, Gazzina S, Gualeni V, Alberici A, Archetti S, Magoni M, Gasparotti R, et al. . 2016. Looking for neuroimaging markers in frontotemporal lobar degeneration clinical trials: a multi-voxel pattern analysis study in Granulin disease. J Alzheimers Dis. 51(1):249–262. [DOI] [PubMed] [Google Scholar]
- Premi E, Cauda F, Gasparotti R, Diano M, Archetti S, Padovani A, Borroni B.. 2014a. Multimodal FMRI resting-state functional connectivity in granulin mutations: the case of fronto-parietal dementia. PLoS One. 9(9):e106500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premi E, Formenti A, Gazzina S, Archetti S, Gasparotti R, Padovani A, Borroni B.. 2014b. Effect of TMEM106B polymorphism on functional network connectivity in asymptomatic GRN mutation carriers. JAMA Neurol. 71(2):216–221. [DOI] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, et al. . 2015. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 112:267–277. [DOI] [PubMed] [Google Scholar]
- Radford KA, Lah S, Say MJ, Miller LA.. 2011. Validation of a new measure of prospective memory: the Royal Prince Alfred prospective memory test. Clin Neuropsychol. 25(1):127–140. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, et al. . 2011. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 134(Pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. 1958. Validity of the Trail Making Test and an indicator. Percept Motor Skill. 8:271–276. [Google Scholar]
- Riedijk SR, De Vugt ME, Duivenvoorden HJ, Niermeijer MF, Van Swieten JC, Verhey FR, Tibben A.. 2006. Caregiver burden, health-related quality of life and coping in dementia caregivers: a comparison of frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord. 22(5-6):405–412. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Boxer AL.. 2021. The frontotemporal dementia prevention initiative: linking together genetic frontotemporal dementia cohort studies. Adv Exp Med Biol. 1281:113–121. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Nicholas JM, Cash DM, van Swieten J, Dopper E, Jiskoot L, van Minkelen R, Rombouts SA, Cardoso MJ, Clegg S, et al. . 2015. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the genetic frontotemporal dementia initiative (GENFI) study: a cross-sectional analysis. The Lancet Neurology. 14(3):253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD.. 2011. Phenotypic signatures of genetic frontotemporal dementia. Curr Opin Neurol. 24(6):542–549. [DOI] [PubMed] [Google Scholar]
- Russell LL, Greaves CV, Bocchetta M, Nicholas J, Convery RS, Moore K, Cash DM, van Swieten J, Jiskoot L, Moreno F, et al. . 2020. Social cognition impairment in genetic frontotemporal dementia within the GENFI cohort. Cortex. 133:384–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage S, Hsieh S, Leslie F, Foxe D, Piguet O, Hodges JR.. 2013. Distinguishing subtypes in primary progressive aphasia: application of the Sydney language battery. Dement Geriatr Cog. 35:208–218. [DOI] [PubMed] [Google Scholar]
- Schmidt M. 1996. Rey auditory and verbal learning test: a handbook. Los Angeles: Western Psychological Services. [Google Scholar]
- Schmidt P, Gaser C, Arsic M, et al. . 2012. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage. 59:3774–3783. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Constantopoulos AA, Jardine MY, McGuffin P.. 1978. A clinical scale for the self-assessment of irritability. Brit J Psychiat. 132(2):164–171. [DOI] [PubMed] [Google Scholar]
- Spina S, Farlow MR, Unverzagt FW, Kareken DA, Murrell JR, Fraser G, Epperson F, Crowther RA, Spillantini MG, Goedert M, et al. . 2008. The tauopathy associated with mutation +3 in intron 10 of Tau: characterization of the MSTD family. Brain. 131(Pt 1):72–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, McKinnon MC, Mar RA, Levine B.. 2009. The Toronto Empathy Questionnaire: scale development and initial validation of a factor-analytic solution to multiple empathy measures. Journal of Personality Assessment. 91(1):62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streese C, Tranel D.. 2021. Combined lesion-deficit and fMRI approaches in single-case studies: unique contributions to cognitive neuroscience. Curr Opin Behav Sci. 40:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada LT. 2015. The genetics of gonogenic frontotemporal dementia. Dement Neuropsychol. 9(3):219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares TP, Mitchell DGV, Coleman KK, Coleman BL, Shoesmith CL, Butler CR, Santana I, Danek A, Gerhard A, de Mendonca A, et al. . 2020. Early symptoms in symptomatic and preclinical genetic frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 91(9):975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles Vieira R, Caixeta L, Machado S, Silva AC, Nardi AE, Arias-Carrion O, Carta MG.. 2013. Epidemiology of early-onset dementia: a review of the literature. Clinical Practice & Epidemiology in Mental Health. 9:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonacci A, Billeci L.. 2018. Olfactory testing in frontotemporal dementia: a literature review. Am J Alzheimers Dis Other Demen. 33(6):342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhout R, Schmidt R, Westeneng H-J, Verstraete E, Seelen M, van Rheenen W, de Reus MA, van Es MA, Hendrikse J, Veldink JH, et al. . 2015. Brain morphologic changes in asymptomatic C9orf72 repeat expansion carriers. Neurology. 85(1):1780–1788. [DOI] [PubMed] [Google Scholar]
- Ward ME, Taubes A, Chen R, Miller BL, Sephton CF, Gelfand JM, Minami S, Boscardin J, Martens LH, Seeley WW, et al. . 2014. Early retinal neurodegeneration and impaired Ran-mediated nuclear import of TDP-43 in progranulin-deficient FTLD. J Exp Med. 211(10):1937–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wear HJ, Wedderburn CJ, Mioshi E, Williams-Gray CH, Mason SL, Barker RA, Hodges JR.. 2008. The Cambridge behavioural inventory revised. Dementia and Neuropsychologia. 2(2):102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, Coalson DL, Raiford SE.. 2008. WAIS-IV: Wechsler adult intelligence scale. San Antonio: Pearson. [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A.. 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2:125–141. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA, Avula R, Tosakulwong N, Weigand SD, Senjem ML, Vemuri P, Jones DT, Gunter JL, Baker M, et al. . 2011. Altered functional connectivity in asymptomatic MAPT subjects: a comparison to bvFTD. Neurology. 77(9):866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester RL, Martyn K.. 2020. Could early identification of changes in olfactory function be an indicator of preclinical neurodegenerative disease? A systematic review. Neurol Ther. 9(2):243–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, et al. . 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Flagan TM, Chu SA.. 2022. Presymptomatic and symptomatic MAPT mutation carriers feature functional connectivity alterations. Alzheimer’s & Dementia. 17:e054128. [Google Scholar]
- Zhang Z, Almeida S, Lu Y, Nishimura AL, Peng L, Sun D, Wu B, Karydas AM, Tartaglia MC, Fong JC, et al. . 2013. Downregulation of microRNA-9 in iPSC-derived neurons of FTD/ALS patients with TDP-43 mutations. PloS one. 8(10):e76055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP.. 1983. The hospital anxiety and depression scale. Acta Psychiatr Scand. 67(6):361–370. [DOI] [PubMed] [Google Scholar]